AbstractRecently, electrochemically self-doped TiO2 nanotubes (R-TiO2 NTs) have emerged as promising anodes in the electrochemical oxidation process for wastewater treatment, owing to their high degradation capability of organic compounds. However, the degradation mechanism of organic compounds on R-TiO2 NTs has not been well demonstrated, and therefore, industrial practices have been limited. This study aimed to elucidate the oxidation mechanism of organic compounds on R-TiO2 NTs by investigating phenol degradation. The performance of phenol degradation on R-TiO2 NTs was comparable to that on the boron-doped diamond (BDD) electrode that is well-known as an excellent anode for phenol degradation. In particular, phenol degradation on R-TiO2 NTs was completely achieved at the electrode potential of 5.0 V while negligible degradation was found at the electrode potential of 2.0 V. In addition, no peaks associated with phenol oxidation in cyclic voltammograms were noticed on R-TiO2 NTs at the potential range between 1.0–2.5 V. Considering direct oxidation is a fundamental factor for phenol degradation at low electrode potential, it contributes less to phenol degradation on R-TiO2 NTs. Therefore, phenol degradation on R-TiO2 NTs is mostly owing to indirect oxidation mediated by hydroxyl radicals.
Graphical Abstract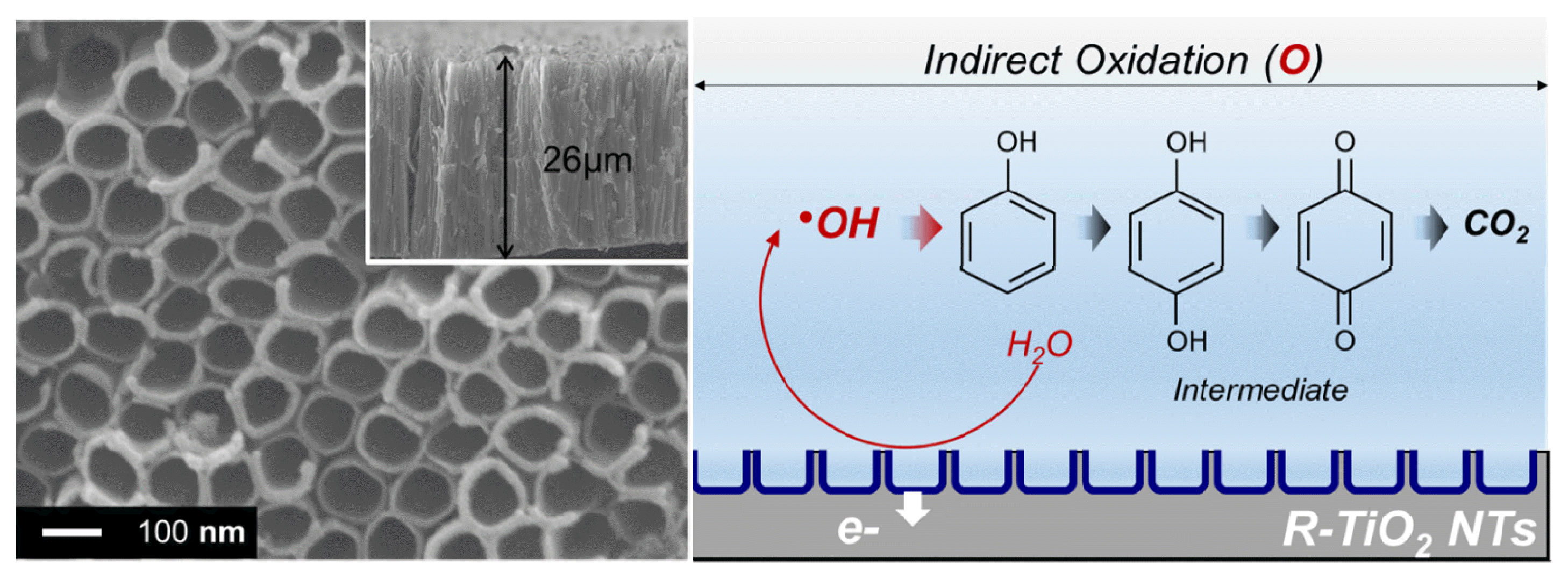
1. IntroductionFor a couple of decades, highly aligned TiO2 nanotubes (TiO2 NTs) have extensively gained interest in various fields, such as dye-sensitized solar cells, photo-electrolysis cells, and sensors, owing to their photochemical properties, biocompatibility, and simple and cheap preparation for fabricating nanostructure [1–4]. In particular, TiO2 NTs are promising electrode materials for oxidant generation and supercapacitors owing to their chemical stability and high accessibility of solvated ions associated with the open-end nanotube structure [5–7]. However, unfortunately, studies on TiO2 NTs as electrode materials has been extremely limited because of their low electrocatalytic activity in aqueous solution caused by their semi-conductive nature and self-limiting process, which shows a progressive decrease in the electric field with increasing oxide thickness [8, 9]. To enhance the limited conductivity and electrocatalytic activity of TiO2 NTs, doping has been persistently studied using various methods such as metallic or nonmetallic doping as a donor and acceptor and thermal hydrogenation [10–13].
Recently, electrochemical self-doping has emerged as an alternative approach to control the electronic state of TiO2 NTs [14]. In electrochemical self-doping, the bandgap of TiO2 NTs can be narrowed by producing trivalent titanium [Ti(III)] as a dopant via electrochemical reduction [15–19]. Thereby, a small structural distortion on TiO2 NTs is noticed since no foreign impurities are required [20]. The high potential of R-TiO2 NTs as photocatalysts and energy storage materials with highly improved photocatalytic activity and capacitive properties has been reported [4, 21–24]. In particular, electrochemically self-doped TiO2 NTs (R-TiO2 NTs) exhibit high electrochemical conductivity and durability; therefore, they are considered promising anode materials [5]. R-TiO2 NTs show high oxygen overpotential and produce hydroxyl radicals through electrochemical oxidation of water [20, 25]. The degradation of organic pollutants and disinfection in wastewater treatment have been extensively studied [26–28]. For example, the high production potential of chlorine and hydroxyl radicals on R-TiO2 NTs has been successfully proved [5]. In addition, peroxodisulfate (S2O82−) production as a strong oxidant has been examined [25]. Moreover, R-TiO2 NTs effectively minimize chemical oxygen demand and total organic carbon with low energy consumption during wastewater treatment [26, 27].
Studies on electrochemical oxidation for wastewater treatment have mostly focused on the performance of degradation of organic compounds on R-TiO2 NTs, and reports on elucidating their degradation mechanism on the surface of R-TiO2 NTs are scanty. Therefore, this study aimed to investigate the oxidation mechanism of organic compounds on R-TiO2 NTs via phenol degradation. In particular, the direct and indirect oxidation mechanisms (i.e., direct electron transfer on the electrode surface and hydroxyl radical-mediated oxidation, respectively) were elucidated by examining the production of hydroxyl radicals and cyclic voltammetry (CV) measurements compared to those using the boron-doped diamond (BDD) electrode that is the most well-known electrode material for phenol degradation.
2. Experimental Methods2.1. Preparation of R-TiO2 NTs
R-TiO2 NTs were prepared by electrochemical self-doping of pristine TiO2 NTs. First, pristine TiO2 NTs were synthesized via electrochemical anodization. Titanium foil (apparent geometric area, 2 cm × 3 cm) and Pt foil were immersed in a solution of ethylene glycol with H2O (2.5 wt%) and NH4F (0.2 wt%), and the Ti foil was anodized with a constant potential of 45 V for 16 h (UDP-150I, Unicorn Tech Co.) at room temperature (~25°C). The anodized Ti foil (amorphous TiO2 NTs) was cleaned and annealed at 450 °C for 1 h in air. Annealed TiO2 NTs (pristine TiO2 NTs, anatase) were treated with a constant cathodic current of 17 mA/cm2 for 90 s in 0.1 M KH2PO4 with NaOH (pH 7.2). The surface morphologies were examined by scanning electron microscopy (SEM, JSM-6700F, JEOL), field emission transmission electron microscopy (FE-TEM, JEM-F200, JEOL), high-resolution X-ray diffraction (XRD, Bruker D8 DISCOVER), and X-ray photoelectron spectroscopy (XPS, Sigma Probe, ThermoVG).
2.2. Phenol Degradation TestPhenol degradation was conducted using a three-electrode system. R-TiO2 NTs or BDD electrodes (apparent geometric area, 2 cm × 3 cm, CONDIAS Gmbh) were employed as the working electrodes. Pt foil and Ag/AgCl (KCl sat’d) were selected as the counter and reference electrodes (Potentiostat, Princeton Applied Research), respectively. The distance between working and counter electrode was approximately 1 cm. Phenol was electrochemically oxidized at a constant potential (2.0 V or 5.0 V vs. Ag/AgCl), or constant current (50 mA/cm2) in 100 mM KNO3 solution containing 1 mM phenol. The total volume of electrolyte was 150 mL. Degradation was expressed with the assumption of a pseudo-first-order reaction (Eq. (1)–(2)).
By integrating Equation (1),
where [phenol] = concentration of phenol, [•OH]ss = steady-state concentration of •OH, and kobs = k[•OH]ss.
To elucidate the oxidation mechanism, CV was carried out at a scan rate of 100 mV/s with a potential range of 0–3.0 V in the same solution (1 mM phenol + 100 mM KNO3).
2.3. N,N-dimethyl-p-nitrosoaniline (RNO) Bleaching TestTo measure the production of hydroxyl radicals on R-TiO2 NTs and BDD electrode, RNO was used as a probe [29], and RNO bleaching was carried out at a constant current of 50 mA/cm2 in 100 mM KNO3 containing 20 μM RNO. RNO content was spectrophotometrically measured at 440 nm (Agilent 8453, Agilent Technologies). The observed pseudo-first-order rate constant of the reaction between RNO and hydroxyl radicals was calculated by the slope in a semi-log plot (ln([RNO]/[RNO]0)/Time).
3. Results and Discussion3.1. Surface Properties of R-TiO2 NTs
Fig. 1 shows the surface properties of R-TiO2 NTs examined by SEM (A) and XRD (B). As shown in Fig. 1A, R-TiO2 NTs were well-aligned with an inner diameter of approximately 80 nm and tube thickness of approximately 10 nm, as supported by the TEM image (Fig. 1B). The length of nanotubes was approximately 26 nm (inset of panel A). In addition, in the XRD pattern (Fig. 1C), the peak positions of R-TiO2 NTs were nearly identical to those of anatase TiO2 (PDF84-1286), indicating the polycrystalline structure of R-TiO2 NTs in the anatase phase. The grain size was approximately 32 nm, which can be calculated by the Scherrer equation with the (101) peak [20]. Moreover, no significant differences in the peaks of the XPS spectra of Ti 2P3/2 and Ti 2P1/2 were noticed between R-TiO2 NTs and pristine TiO2 NTs. This is attributed to the fact that Ti(III) as a dopant has negligible impact on the crystal structure and bonding environment in pristine TiO2 NTs, as reported in a previous study [14].
3.2. Phenol Degradation on R-TiO2 NTs with Constant CurrentThe electrochemical degradation of phenol and RNO bleaching (inset) on R-TiO2 NTs and BDD electrodes are presented in Fig. 2. A linear slope in the semi-log plot was observed for phenol degradation on R-TiO2 NTs and BDD electrode. This implies that phenol degradation on the electrodes follows the pseudo-first-order kinetics [5]. In addition, the rate constant (kobs) of R-TiO2 NTs for phenol degradation was approximately 3.3 × 10−2 min−1. This is approximately 1.3-times higher than that of the BDD electrode (kobs = 2.5 × 10−2 min−1). In particular, this trend of phenol degradation matched well with hydroxyl radical generation in the RNO bleaching test (inset). This indicates that hydroxyl radicals play an important role in phenol degradation on the electrodes. Furthermore, the results suggest that R-TiO2 NTs are excellent candidates as a feasible anode material for generating hydroxyl radicals, considering that the BDD electrode is an eminent electrode material for degrading organic pollutants [30, 31].
3.3. Analysis of Oxidation Mechanism of R-TiO2 NTsTo better understand phenol degradation on R-TiO2 NTs, the experiment was conducted at constant electrode potentials of 2.0 and 5.0 V. From the results of Fig. 3, approximately 97% of phenol was degraded on R-TiO2 NTs at a constant potential of 5.0 V, which is much higher than that on the BDD electrode (approximately 80% degradation). In particular, R-TiO2 NTs revealed a lower current density of 38 ± 3.5 mA/cm2 than did the BDD electrode (current density of 89 ± 9.3 mA/cm2). The energy consumption of R-TiO2 NTs and BDD was calculated to be approximately 7.8 kWh/mol and 22.3 kWh/mol, respectively. It indicates that R-TiO2 NTs are more effective in degrading phenol at high electrode potentials. This is in good agreement with the results shown in Fig. 2. However, at a low electrode potential of 2.0 V, phenol degradation on R-TiO2 NTs was not observed, whereas approximately 55% phenol was degraded on the BDD electrode. This can be explained by the effect of direct oxidation on the BDD electrode at low electrode potential because hydroxyl radical generation by indirect oxidation requires > 2.7 V [32]. In contrast, for R-TiO2 NTs, direct oxidation did not significantly contribute to phenol degradation. Therefore, the two electrodes have different characteristics of phenol degradation.
This assumption was supported by further investigation with cyclic voltammetry measurements in the presence and absence of 1 mM phenol in a 100 mM KNO3 solution (Fig. 4). As shown in Fig. 4A, R-TiO2 NTs exhibited a typical shape of cyclic voltammograms (CVs) with high capacitive properties in the low potential regime and a large overpotential for oxygen evolution at a potential of approximately 2.5 V. No peaks of phenol oxidation on R-TiO2 NTs was observed, which can be found with identical CVs in the potential range of 1.0–2.5 V (inset of panel A). Therefore, the direct oxidation of phenol via electron transfer did not take place on R-TiO2 NTs. In contrast, on the BDD electrode, significant peaks of phenol oxidation was observed at the potential range of 1.0–2.5 V (inset of panel B) owing to the direct electron transfer reaction of phenol on the surface of BDD electrodes [32]. Therefore, unlike the BDD electrode, R-TiO2 NTs showed no direct oxidation of phenol.
This can be further supported by CVs obtained with a narrow potential range of 1.0–1.7 V (vs. Ag/AgCl) (Fig. 5). As can be seen in Fig. 5, no significant changes in CVs were observed on R-TiO2 NTs, although the cycle number of CVs was increased. Only capacitive properties (rectangular shape) were noticed. Meanwhile, the oxidation peaks on the BDD electrode were noticed at approximately 1.5 V vs. Ag/AgCl, and the peaks decreased as the number of cycles increased because the residues resulting from direct oxidation of phenol were deposited on the surface of the BDD electrode, which hindered direct electron transfer. Consequently, the oxidation current gently decreased on the BDD electrode with increasing cycle numbers. These results suggest that the direct oxidation of phenol does not occur on R-TiO2 NTs, and that the hydroxyl radical is a fundamental factor in electrochemical degradation of phenol on R-TiO2 NTs, as shown in Fig. 2. Although the phenol oxidation reaction of the two electrodes has different electron transfer paths, it is expected that the intermediate product generated before being degraded into CO2, which is the final oxide, would be similar to Catechol (C6H4(OH)2), Benzoquinone (C6H4O2), etc (Fig. 6). Therefore, we conclude that phenol degradation capability of R-TiO2 NTs is comparable to that of the BDD electrode; hydroxyl radicals may have a fundamental role in phenol degradation on R-TiO2 NTs; and indirect oxidation via hydroxyl radicals is the major mechanism of phenol degradation on R-TiO2 NTs, whereas BDD electrochemically degrades phenol via both direct and indirect mechanisms.
4. ConclusionsIn this study, we examined the electrochemical degradation of phenol on R-TiO2 NTs and elucidated the mechanism using electrochemical oxidation tests and cyclic voltammetry measurements. When a constant current of 50 mA/cm2 was applied, the R-TiO2 NTs exhibited pseudo-first-order kinetics with a rate constant (kobs) of 3.3 × 10−2 min−1 for phenol degradation, which was 1.3-times higher than that of the BDD electrode (kobs =2.5 × 10−2 min−1). On both R-TiO2 NTs and BDD electrode, hydroxyl radicals played an important role in phenol degradation, as confirmed by RNO bleaching. Phenol was completely degraded on R-TiO2 NTs at the electrode potential of 5.0 V, and negligible degradation was noticed at the electrode potential of 2.0 V. Moreover, considering no oxidation peaks in cyclic voltammograms on R-TiO2 NTs (i.e., no direct oxidation), indirect oxidation may be the major mechanism of phenol degradation on R-TiO2 NTs. The results obtained in this study provide novel knowledge, possibly leading to the further development of R-TiO2 NTs for many applications, including as substrate for supercapacitors and Li+ batteries, photo-catalyst activated by visible light, and oxidants-generating anodes for water treatment. It provides knowledge of electrochemical oxidation with R-TiO2 NTs and may contribute to further advancement in electrochemical treatment of wastewater.
AcknowledgmentsThis study was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (NRF-2021R1I1A3040360) and by a research grant from the Waste to Energy Recycling Human Resource Development Project of the Korean Ministry of Environment (ME).
References1. Roy P, Berger S, Schmuki P. TiO2 nanotubes: synthesis and applications. Angew. Chem. Int. Ed. 2011;50:2904–2939.
https://doi.org/10.1002/anie.201001374
2. Mor GK, Shankar K, Paulose M, Varghese OK, Grimes CA. Use of highly-ordered TiO2 nanotube arrays in dye-sensitized solar cells. Nano Lett. 2006;6:215–218.
https://doi.org/10.1021/nl052099j
3. Hou Y, Li X, Zhao Q, Quan X, Chen G. Electrochemically assisted photocatalytic degradation of 4-chlorophenol by ZnFe2O4–modified TiO2 nanotube array electrode under visible light irradiation. Environ. Sci. Technol. 2010;44:5098–5103.
https://doi.org/10.1021/es100004u
4. Zhu H, Zhao M, Zhou J, et al. Surface states as electron transfer pathway enhanced charge separation in TiO2 nanotube water splitting photoanodes. Appl. Catal. B: Environ. 2018;234:100–108.
https://doi.org/10.1016/j.apcatb.2018.04.040
5. Kim C, Kim S, Choi J, et al. Blue TiO2 nanotube array as an oxidant generating novel anode material fabricated by simple cathodic polarization. Electrochim Acta. 2014;141:113–119.
https://doi.org/10.1016/j.electacta.2014.07.062
6. Jun Y, Park JH, Kang MG. The preparation of highly ordered TiO2 nanotube arrays by an anodization method and their applications. Chem. Commun. 2012;48:6456–6471.
https://doi.org/10.1039/C2CC30733B
7. Grimes CA. Synthesis and application of highly ordered arrays of TiO2 nanotubes. J. Mater. Chem. 2007;17:1451–1457.
https://doi.org/10.1039/B701168G
8. Macak JM, Gong BG, Hueppe M, Schmuki P. Filling of TiO2 nanotubes by self-doping and electrodeposition. Adv. Mater. 2007;19:3027–3031.
https://doi.org/10.1002/adma.200602549
9. Macak JM, Tsuchiya H, Ghicov A, et al. TiO2 nanotubes: Self-organized electrochemical formation, properties and applications. Curr. Opin. Solid State Mater. Sci. 2007;11:3–18.
https://doi.org/10.1016/j.cossms.2007.08.004
10. Rani S, Garg A, Singh N. Efficient degradation of doxycycline and ofloxacin in an aqueous environment using Fe and Cu doped TiO2-SiO2 photocatalyst under sunlight. Environ. Eng. Res. 2022;27:210282.
https://doi.org/10.4491/eer.2021.282
11. Zeng X, Sun X, Wang Y. Photocatalytic degradation of flume-quine by N-doped TiO2 catalysts under simulated sunlight. Environ. Eng. Res. 2021;26:200524.
https://doi.org/10.4491/eer.2020.524
12. Park JH, Kim S, Bard AJ. Novel carbon-doped TiO2 nanotube arrays with high aspect ratios for efficient solar water splitting. Nano Lett. 2006;6:24–28.
https://doi.org/10.1021/nl051807y
13. Nah YC, Paramasivam I, Schmuki P. Doped TiO2 and TiO2 nanotubes: synthesis and applications. ChemPhysChem. 2010;11:2698–2713.
https://doi.org/10.1002/cphc.201000276
14. Hong SP, Kim S, Kim N, Yoon J, Kim C. A short review on electrochemically self-doped TiO2 nanotube arrays: Synthesis and applications. Korean J. Chem. Eng. 2019;36:1753–1766.
https://doi.org/10.1007/s11814-019-0365-0
15. Kim C, Kim S, Lee J, Kim J, Yoon J. Capacitive and oxidant generating properties of black-colored TiO2 nanotube array fabricated by electrochemical self-doping. ACS Appl. Mater. Interfaces. 2015;7:7486–7491.
https://doi.org/10.1021/acsami.5b00123
16. Kim C, Lee S, Kim S, Yoon J. Effect of annealing temperature on the capacitive and oxidant-generating properties of an electrochemically reduced TiO2 nanotube array. Electrochim. Acta. 2016;222:1578–1584.
https://doi.org/10.1016/j.electacta.2016.11.143
17. Zhou H, Zhang Y. Electrochemically self-doped TiO2 nanotube arrays for supercapacitors. J. Phys. Chem. C. 2014;118:5626–5636.
https://doi.org/10.1021/jp4082883
18. Zhu WD, Wang CW, Chen JB, Li Y, Wang J. Enhanced field emission from Ti3+ self-doped TiO2 nanotube arrays synthesized by a facile cathodic reduction process. Appl. Surf. Sci. 2014;301:525–529.
https://doi.org/10.1016/j.apsusc.2014.02.116
19. Du K, Liu G, Li M, Wu C, Chen X, Wang K. Electrochemical reduction and capacitance of hybrid titanium dioxides—nanotube arrays and “nanograss”. Electrochim. Acta. 2016;210:367–374.
https://doi.org/10.1016/j.electacta.2016.05.027
20. Kim C, Kim S, Hong SP, Lee J, Yoon J. Effect of doping level of colored TiO2 nanotube arrays fabricated by electrochemical self-doping on electrochemical properties. Phys. Chem. Chem. Phys. 2016;18:14370–14375.
https://doi.org/10.1039/C6CP01799A
21. Yang Y, Liao J, Li Y, et al. Electrochemically self-doped hierarchical TiO2 nanotube arrays for enhanced visible-light photo-electrochemical performance: an experimental and computational study. RSC adv. 2016;6:46871–46878.
https://doi.org/10.1039/C6RA05805A
22. Zhou H, Zhang Y. Enhancing the capacitance of TiO2 nanotube arrays by a facile cathodic reduction process. J. Power Sources. 2013;239:128–131.
https://doi.org/10.1016/j.jpowsour.2013.03.114
23. Vellacheri R, Zhao H, Mühlstädt M, et al. All-solid-state cable-type supercapacitors with ultrahigh rate capability. Adv. Mater. Technol. 2016;1:1600012.
https://doi.org/10.1002/admt.201600012
24. Radjenovic J, Sedlak DL. Challenges and opportunities for electrochemical processes as next-generation technologies for the treatment of contaminated water. Environ. Sci. Technol. 2015;49:11292–11302.
https://doi.org/10.1021/acs.est.5b02414
25. Kim J, Lee C, Yoon J. Electrochemical peroxodisulfate (PDS) generation on a self-doped TiO2 nanotube array electrode. Ind. Eng. Chem. Res. 2018;57:11465–11471.
http://dx.doi.org/10.1021/acs.iecr.8b01208
26. Yang Y, Hoffmann MR. Synthesis and stabilization of blue-black TiO2 nanotube arrays for electrochemical oxidant generation and wastewater treatment. Environ. Sci. Technol. 2016;50:11888–11894.
https://doi.org/10.1021/acs.est.6b03540
27. Yang Y, Kao LC, Liu Y, et al. Cobalt-doped black TiO2 nanotube array as a stable anode for oxygen evolution and electrochemical wastewater treatment. ACS Catal. 2018;8:4278–4287.
https://doi.org/10.1021/acscatal.7b04340
28. Ahmadi A, Wu T. Inactivation of E. coli using a novel TiO2 nanotube electrode. Environ. Sci. Water Res. Technol. 2017;3:534–545.
https://doi.org/10.1039/C6EW00319B
29. Simonsen ME, Muff J, Bennedsen LR, Kowalski KP, Søgaard EG. Photocatalytic bleaching of p-nitrosodimethylaniline and a comparison to the performance of other AOP technologies. J. Photochem. Photobiol. 2010;216:244–249.
https://doi.org/10.1016/j.jphotochem.2010.07.008
30. McBeath ST, Wilkinson DP, Graham NJ. Application of boron-doped diamond electrodes for the anodic oxidation of pesticide micropollutants in a water treatment process: a critical review. Environ. Sci. Water Res. Technol. 2019;5:2090–2107.
https://doi.org/10.1039/C9EW00589G
31. Clematis D, Panizza M. Application of boron-doped diamond electrodes for electrochemical oxidation of real wastewaters. Curr. Opin. Electrochem. 2021;30:100844.
https://doi.org/10.1016/j.coelec.2021.100844
32. Iniesta J, Michaud P, Panizza M, Cerisola G, Aldaz A, Comninellis C. Electrochemical oxidation of phenol at boron-doped diamond electrode. Electrochim. Acta. 2001;46:3573–3578.
https://doi.org/10.1016/S0013-4686(01)00630-2
Fig. 1(a) SEM and (b) TEM images, (c) XRD pattern, and (d) XPS spectra of R-TiO2 NTs. The inset of panel A shows the length of nanotubes on R-TiO2 NTs (cross-sectional SEM image). The XRD pattern and XPS spectra were indexed with reference to the JCPDS file (PDF84-1286) and pristine TiO2 NTs, respectively. 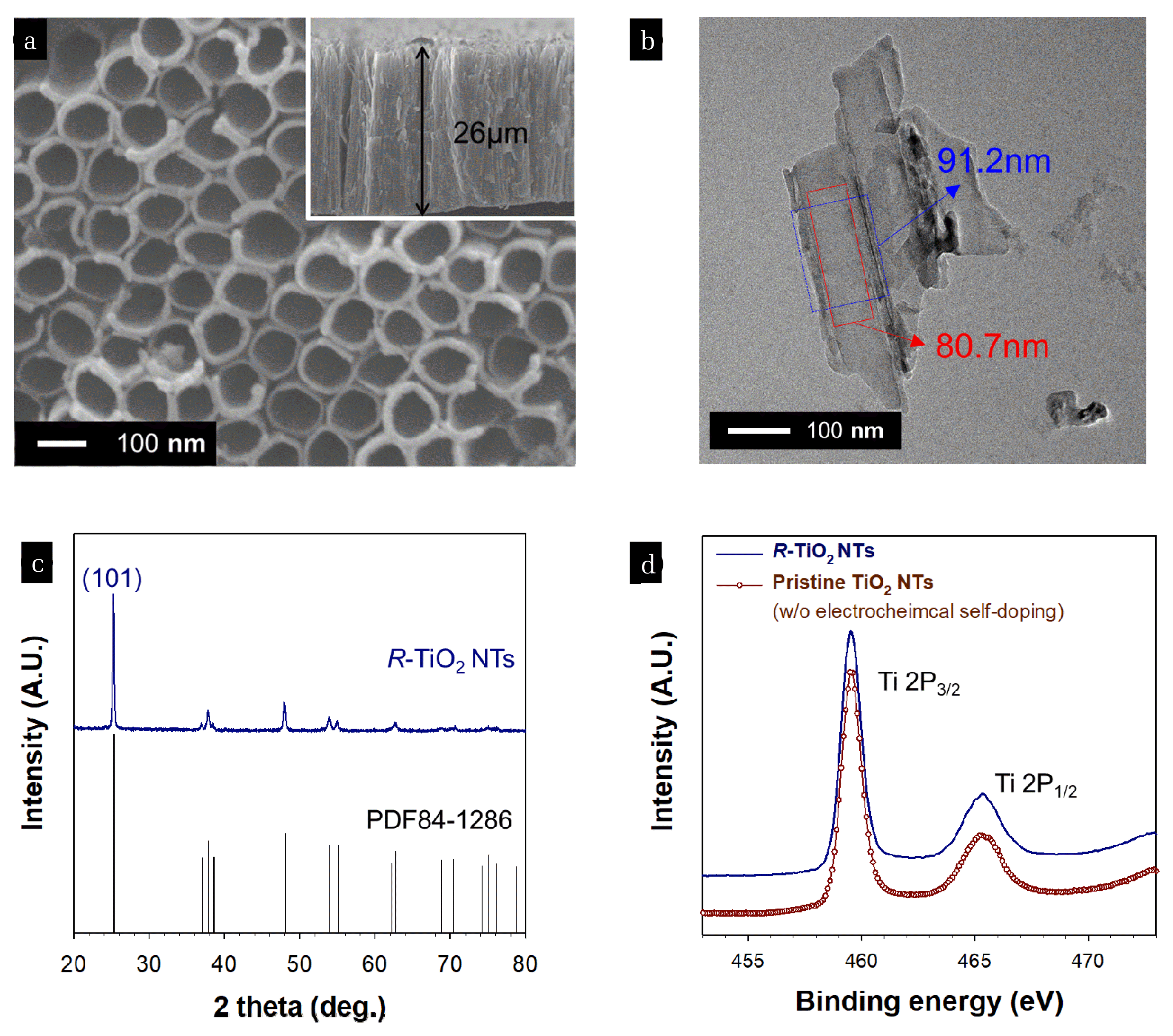
Fig. 2Electrochemical degradation of phenol and RNO bleaching (inset) on R-TiO2 NTs examined by the semi-log plot with an assumption of pseudo-first-order kinetics. The experiments were conducted in 100 mM KNO3 containing 1 mM phenol for phenol degradation and in 100 mM KNO3 containing 20 μM RNO for RNO bleaching (inset) at a constant current density of 50 mA/cm2 (counter electrode, Pt; reference electrode, Ag/AgCl). 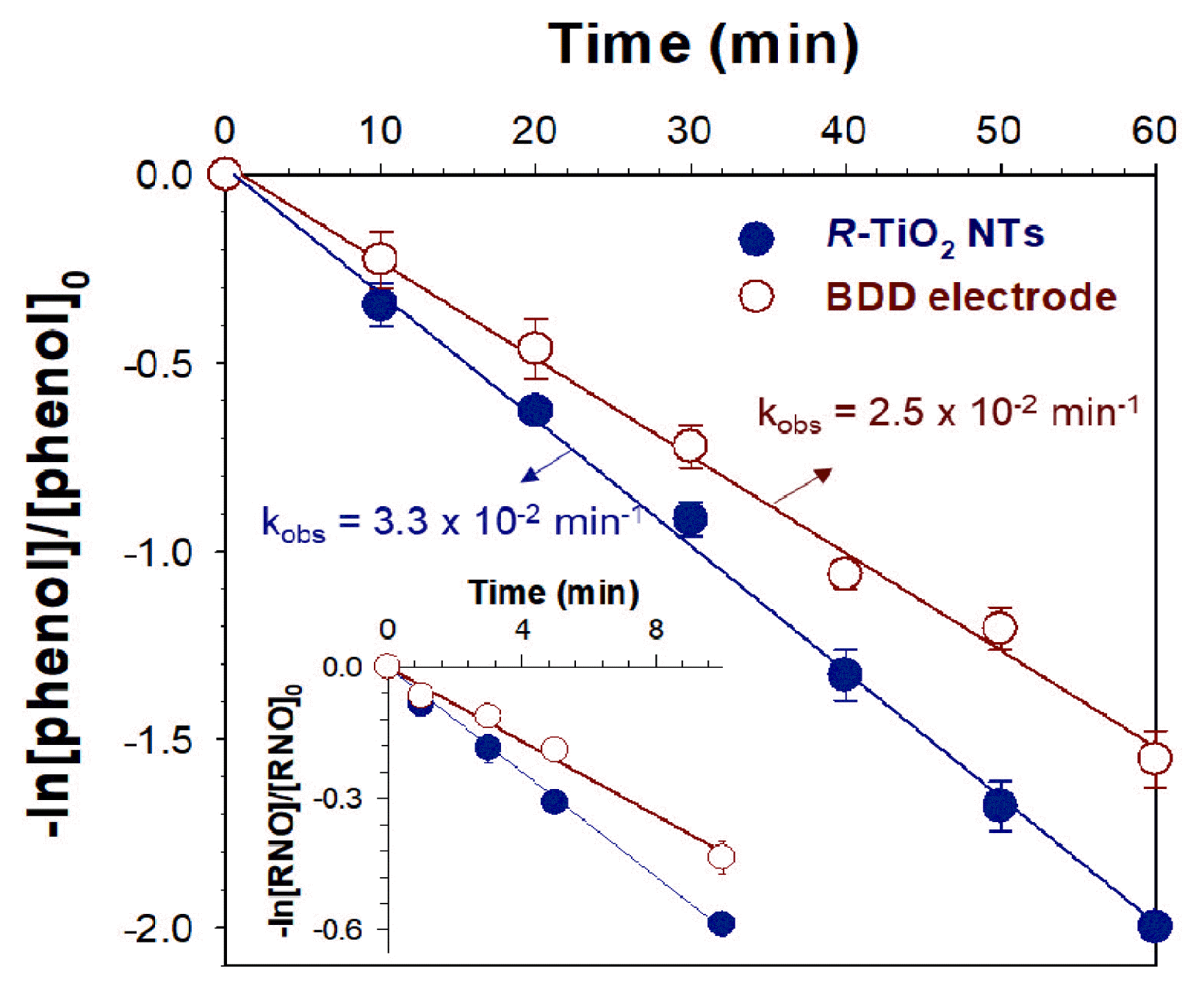
Fig. 3Phenol removal by electrochemical oxidation on R-TiO2 NTs and BDD electrode at constant electrode potentials of 2.0 V and 5.0 V in 100 mM KNO3 containing 1 mM phenol (counter electrode, Pt; reference electrode, Ag/AgCl; duration of electrolysis, 60 min). ND, -not detected. 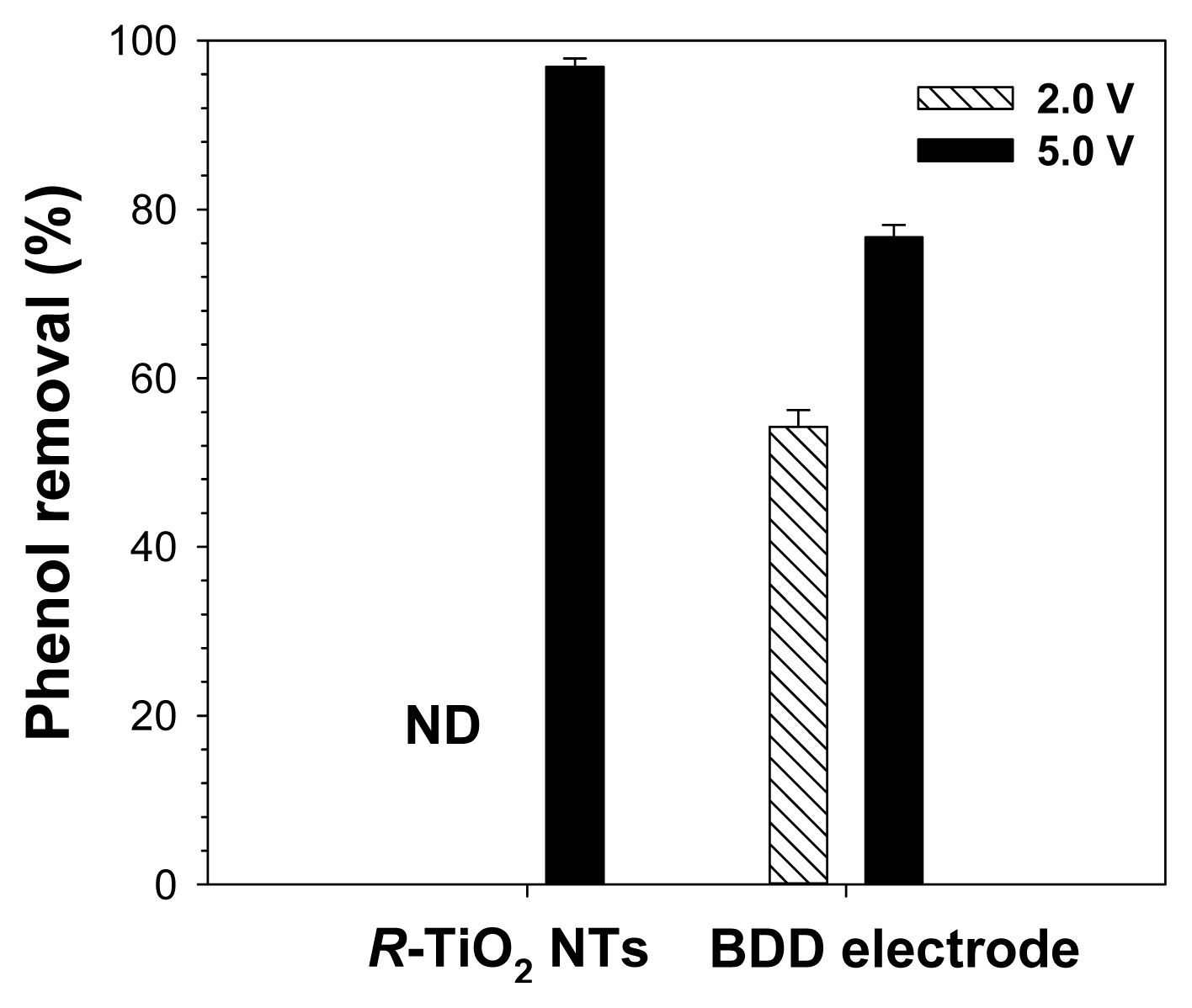
Fig. 4CVs on R-TiO2 NTs (a) and BDD electrode (b) in 100 mM KNO3 in the presence of and absence of 1 mM phenol (a scan rate of 100 mV/s in the potential range of 0 3.0 V vs. Ag/AgCl). The insets of panels A and B are enlarged CVs on R-TiO2 NTs and BDD electrode in the potential range of 1.0 2.5 V, respectively. 
|
|
|||||||||||||||||||||||||||||||||||