AbstractIn this study, a cerium-Metal-Organic Framework/Graphene Oxide (Ce-MOF/GO) composite material with highly efficient phosphate removal was synthesized via a solvothermal method. The physiochemical properties and removal mechanism of phosphate on composite material were analyzed by Fourier-transform infrared spectroscopy (FT-IR), scanning electron microscopy (SEM), X-ray diffraction (XRD), and Brunauer-Emmett-Teller (BET). The influencing parameters such as, effect of reaction time, solution pH, initial concentration, adsorbent dosage, and temperature on the adsorption capacity of the adsorbent were investigated. The kinetic and isothermal adsorption data were consistent with the pseudo-second-order model and the Langmuir adsorption isotherm model. The result shows that the maximum adsorption capacity of Ce-MOF/GO-2% composite for phosphate removal was 308. 64 mg g-1, which is higher than that of Ce-MOF. The adsorbent shows excellent reusability and after four adsorption/regeneration cycles, still maintains (92%) adsorption capacity.
Graphical Abstract
1. IntroductionPhosphorus is an essential component for both humans and plants that humans have used in various processes such as fertilizer, mineral process, agriculture, detergents, pigment formation, and mineral processing. However, excessive use of phosphorus leads to deterioration in water quality, and it contributes to the overgrowth of algae, bacteria, and other harmful aquatic plants in water bodies, threatening the health of aquatic life and causing eutrophication [1–3]. Many lakes and reservoirs face eutrophication problems as effluents from industrial factories, municipalities, and agricultural land that contain extensive amounts of phosphorus are discharged into water bodies, that accelerate the ecological crisis [4]. According to Luo et al. [3] it is estimated that over 6 million metric tons of phosphorus are released into water bodies every year. In contrast, a concentration of 50 μg L−1 of phosphorus could result in the eutrophication phenomenon. Meanwhile, over 40% of the United States, rivers and streams are contaminated with phosphorus, and mitigating eutrophication in the US costs over 2.2 billion dollars a year [5, 6].
Henceforth, to protect the ecological environment and remove excess phosphate from water bodies, numerous methods, which are generally categorized as chemical and physical-chemical, biological treatment, and biological-chemical methods [7], have been applied, including chemical precipitation [8], crystallization [9], biological treatment [10], membrane separation [11], photocatalysis [12], electrolysis [13], adsorption [14] etc. However, the application of these methods (except adsorption) is limited due to their high cost, generation of secondary pollution, unsatisfactory removal rate, and complex use. For instance, chemical precipitation [8] was applied to remove phosphate from semiconductor wastewater. The result indicates that a pilot-scale study removed 97% of the phosphate in a two-stage precipitation process, which cost approximately 1.58 $/m3. Although the removal rate seems satisfactory, this method is not eco-friendly since it requires a variety of chemicals and produces a lot of secondary sludge. Even though the vivianite (Fe3(PO4)2.8H2O) crystallization method [9] seems a promising technique for phosphate removal, it is a complex process that can produce dischargeable sludge into the environment. The results of this study indicate that, due to the slow crystallization and low vivianite supersaturation, only 30–40% of the phosphate was removed at pH 5. At pH 6 and 7, the removal rate significantly increased (>90%). However, when Fe2+ oxidation became more severe at dissolved oxygen, phosphate removal and recovery efficiency decreased by 10–20 percent. Biological treatment [10] was used to remove phosphorus from wastewater, and the results showed that the phosphorus release rate and uptake in the biochemical tank was relatively low. It was due to the low activity of phosphate-accumulating organisms (PAO) and the poor effectiveness of biological phosphate removal. Although the contribution percentage of phosphate removal increased by up to 18% and the dosage of chemicals decreased by up to 60 percent following enhanced biological phosphate removal. However, biological phosphorus removal in full-scale wastewater treatment plants is a challenging problem. Overall, this method is costly, requires a unique environment, uses more chemicals, and has a lower phosphate removal rate. Through membrane separation[ 11], about 96.73 % phosphate rejection was achieved. However, this method uses considerable energy and requires more money to invest, which limits its practical application. Another commonly used method is photocatalysis[12], which results in a 94% and 95% removal rate of nitrate and phosphate, respectively. Electrolysis [13] is also used to treat eutrophic wastewater; the findings show that at a concentration of 0.1 mM, the removal rate of phosphate could still reach 99.7%. However, when the initial concentration was raised to 5 mM, the removal rate of phosphate decreased to 34.3%. Although this method appears to be effective for eutrophic wastewater treatment, its practical application is limited by the equipment’s high initial cost. Among the methods used to remove phosphate from aqueous solutions, adsorption [14] is recognized as the most effective technique. It has attracted much attention because of its high removal efficiency, wide pH range, comparatively low cost, simplicity, and environmental friendliness. This study shows a maximum adsorption capacity of 278.8 mg g−1 and 128 mg g−1 for phosphate and fluoride, respectively, and high selectivity toward phosphate removal.
Similarly, various traditional porous adsorbents, including activated carbon [15], graphene [16], carbon nanotubes [17], etc., have been used to remove pollutants from wastewater. Still the application of these materials has been limited due to their low adsorption capacity and reusability.
Recently, Metal-Organic Frameworks (MOFs) have received much attention as a novel porous material for removing water and wastewater contaminants due to their high specific surface area, tunable pore structure, easy surface modification, many active sites, crystalline structure, and various functionalities [3, 18]. MOFs, a hybrid organic-inorganic nanomaterial, are assembled by coordination bonds from organic ligands and metal ions or metal clusters to form an infinite crystalline network [19]. MOFs are mainly synthesized by solo evaporation [20], solvothermal [21], hydrothermal [22], electrochemical [23], sonochemical [24], microwave [25], spray drying [26], flow chemistry [27], ionothermal [28], and ultrasound [29] methods. Solo evaporation was applied to prepare novel MOF-type compounds with higher thermal stability using polysulfonic acids as linkers. The result shows that light green single crystals of [Cu2(B4S) (H2O)8]0.5H2O were produced by slow evaporation of H4B4S and Cu2(OH)2(CO3). The Cu2+ ions are coordinated by four water molecules and two monodentate sulfonate anions, creating a tetragonally distorted [CuO6] octahedron. The anions are linked to additional copper ions, resulting in ladder-shaped chains [20]. The solvothermal method [21] was applied to synthesize MIL-101 (Fe), MIL-101 (Fe, Cu), and GO/MIL-101 (Fe, Cu) composite. The results indicate good properties, such as higher adsorption capacity and a specific surface area of 778.11 m2 g−1, compared to that of MIL-101 (Fe) and MIL-101 (Fe, Cu). A copper diphenylamine MOF was prepared via the hydrothermal method [22], and the result shows higher adsorption capacities of 97.6%, 99.5% and 99.5%, for Cd, Cr, and Pb, respectively. The higher removal efficiency of the synthesized MOF was due to its high specific surface area and excellent porosity.
Electrochemical synthesis [23] is a promising and eco-friendly method MOFs preparation method. A Cu3(BTC)2 MOF microstructure of was prepared via this method and used for CO2 and CH4 sorption. The results showed a higher adsorption of CO2 and CH4 at 298 K. It was discovered that as pressure and composition of the CO2 component increased, the selectivity of MOF towards CO2 over CH4 increased. UiO-66 (Ce) MOFs were prepared via sonochemical method [24]. The results demonstrated that, in contrast to MOF obtained under static conditions, structures with more developed surfaces and fewer linker defects could be produced by agitating the reacting mixture during synthesis. In the context of CO2 adsorption, the contribution of Ce3+ ions linked to the concentration of linker defects was more significant than the specific surface area. This method led to higher adsorption of irreversible CO2 due to the smallest particle size and strong Ce3+ accessible for CO2 adsorption. Through microwave [25] synthesis, highly stable and nanoscale Hf and Zr-based MOFs were fabricated quickly with small particle sizes and high yields. The prepared materials (Hf and Ze-MOFs) exhibited high porosity, and good structure compared to the original framework and showed higher adsorption capacity (463.02 and 466.39 mg g−1) toward drugs containing curcumin’s overdose. The spray drying method [26] was used to synthesize high-quality ZIF-8, ZIF-67, and bimetallic Zn/Co-ZIF MOFs. The results demonstrated that the spray drying is an efficient, simple, and continuous synthetic method for producing MOFs (ZIFs) in greater quantities with high-quality properties with minimal effort and time, which is unusual in other MOF synthesis techniques. CPO-27-Ni MOF was prepared via flow chemistry [27] through tubular reactor techniques in less time. The resulting MOF has a powder X-ray diffraction pattern consistent with the known structure of CPO-27-Ni and a specific surface area of 1085 m2 g−1. The tubular reactor method yields crystallite sizes around 40 nm. Ionothermal synthesis [28] was applied to fabricate a series of Co–BDC MOFs with eight kinds of 1-methyl-3-alkyl imidazolium halide [RMI]+. The eight [RMI]2[Co3 (BDC)3X2] frameworks showed the same negative 2D [Co3(BDC)3 X2]2– skeletons with RMI+ positioning in the interlayer space. [RMI]+ cations serve as a model for MOF structure. High properties [PCN-222(M)-U] were synthesized by ultrasonic methods [29]. The result showed that this method yielded a good quantitative (PCN-222(M)-U) MOF with desirable properties, including high water stability, excellent yields, short reaction times, high adsorption performance, and good recyclability.
Abdelmoaty et al.[30] synthesized UiO-66 MOF by the hydrothermal method by dissolving 0.480 g of zirconium tetrachloride (ZrCl4) and 3.66 g of terephthalic acid (H2BDC) in 45 ml of N, N-dimethylformamide (DMF). The mixture was heated in an oven at 140 °C for 36 h, after cooling to room temperature the solid was washed by DMF and chloroform. After the solvent was removed from the vial bottles through decantation, the solid was dried at 60 °C under a vacuum. Then, the prepared MOF was modified with melamine to obtain melamine-coated UiO-66 MOF. The results showed enhancing crystallinity, active sites, and surface area. The removal efficiency was considerably high for Pb and Cd (II) removal.
A large number of MOFs have been applied in waste-water treatment processes, including photocatalysis [31], membrane separation [32], adsorption [33], etc. For example, Shooto and Dikio [34] used a highly porous cadmium MOF to remove heavy metal ions such as Cu (II), Pb (II), and Ni (II) from wastewater. The results showed a high degree of stability in aqueous media and excellent adsorption performance due to the reinforcement of oxygen-containing functional groups on its surface and its high porosity, as well as high adsorption capacities of 183.43, 171.42 and 120.31 mg g−1 for Cu (II), Pb (II), and Ni (II), respectively. Yu et al. [35] fabricated a 3D porous MOF with the oxygen group and the N=N unit, using Zn(NO3)2.6H2O as a metal source and H2ADB as an organic linker through solvothermal method. The results indicate a high adsorption capacity (463.52 mg g−1) for Pb2+ removal from aqueous solutions, which is due to the availability of many active sites of the oxygen group and the N=N units. In addition, the prepared MOF showed excellent selectivity for Pb2+ removal among other coexistence ions in aqueous media.
There are few reported literatures regarding the application of Ce-MOF for phosphate removal. In a study, Ce(III) nanocomposite MOFs were applied to remove phosphate, and the maximum adsorption capacity reached 189.4 mg g−1 with a wide applicable pH ranging (2–12) and great selectivity for phosphate in the presence of competing anions [36]. Likewise, Hassan et al. [2] used cerium-based metal-organic framework as a high-capacity adsorbent for phosphate removal from eutrophic waters. In particular, the Ce (IV)-based UiO-66 analogue (Ce-BDC), has good water stability, high surface area, microporous structure, and the high binding affinity of phosphate as well as high selectivity for phosphate removal in presence of common anions (Cl−, Br−, I−, NO3−, HCO3−, SO42−). Furthermore, the sorbent showed a fast removal rate (4 min) with a maximum adsorption capacity of 179 mg g−1. Stanton et al. [37] investigated the impact of defects and linker exchange in MOF for the removal of phosphate using the node structure M6(OH)4(O)4 for M = Hf, Zr, and Ce. According to the findings, linker exchange at two defect sites during phosphate anions adsorption led to significant phosphate anions adsorption than at the pristine one. This study revealed that by changing the organic linker and substituting the metal center, one can prepare MOF with high-capacity, tunable structure, and readily synthesizable sorbents for the removal of various water contaminants.
Rego et al, [38]. used Ce-UiO-66 MOF with similar framework topologies exist in Zr-UiO-66, to remove a number of potentially harmful water contaminants. The Zr metal center was replaced with Ce in Zr-UiO-66 MOF, resulting in creation of a MOF with outstanding multipollutant adsorption capabilities that can be prepare quickly with less energy. According to the study, the maximum adsorption capacity of Ce-UiO-66 can reach to 793.7, 110, 66.1, 30, and 485.4 mg g−1 for Congo red (CR), methylene blue (MB), fluoride (F−), Cr6+, and diclofenac sodium, respectively. Li and his co-worker [39], investigated the introduction of defects into hierarchical porous metal-organic frameworks (HP-MOFs) to control the type and number of defects in HP-UiO-66(Zr) and improve their adsorption performance. After the template was removed, a variety of defect-rich HP-UiO-66(Zr) derivatives were produced using monocarboxylic acids by varying chain lengths as the template. Compared to the original UiO-66 (Zr), the prepared HP-UiO-66 (Zr) showed a higher adsorption capacity and a faster sorption performance. In particular, the octanoic acid-modulated UiO-66 (Zr) has a high adsorption capacity of 186.6 mg g−1 and an intraparticle diffusion rate of 6.19 mg g−1 min−1, which is respectively 4.8 times and 1.9 times higher than that of pure UiO-66 (Zr). Through defect engineering, this work offers a promising method for modifying the adsorption performance of MOF-based adsorbents. To effectively remove phosphate from water. Wang et al. [40] successfully synthesized an isoreticular metal-organic framework (Fe-IRMOF-3-10) with a bimetallic center and double ligand from an isoreticular metal-organic framework (IRMOF-3) with a single metal center and single ligand. Among the four IRMOFs, FeIRMOF-3-10 had a faster phosphate adsorption and a higher adsorption capacity of 177 mg g−1. The phosphate removal mechanism of Fe-IRMOF-3-10 involved not only the affinity of the amine group and Fe3+ for phosphate, but also the ion exchange between the hydroxyl group and the phosphate.
Although MOFs have a higher adsorption capacity towards wastewater pollutants, they generally suffer from low stability in aqueous solutions, which limits their practical application in wastewater treatment. Therefore, many MOF composite materials have been fabricated and applied to removal wastewater pollutants. The results confirmed the excellent adsorption performance and stability [41, 42]. In particular, the MIL series [21], zinc-based MOFs [43], and zirconium-based MOFs [44] are widely used for the removal of different wastewater pollutants due to their excellent adsorption performances. Moreover, carbonaceous materials such as GO widely used as a suitable adsorbent for the adsorption of various wastewater pollutants [45, 46] since it has good chemical, mechanical, and thermal stability, a high specific surface area, and plenty of oxygen-rich functional groups, including epoxy, hydroxy, and carboxyl groups on its surface. In addition, it has excellent interfacial contact with other materials and is low in cost, making it an ideal material for removing various wastewater pollutants. However, the mechanical strength of GO in monolayer form is weak, and it can easily aggregate in most solvents, making the equal dispersion of GO very difficult.
Therefore, many researchers combined MOF with GO to overcome its shortcomings and prepare promising adsorbents with high adsorption capacity and robust stability to remove wastewater pollutants [42, 47, 21]. Specifically, Dai et al. [48] prepared Cu-BTC/GO composites via an ultrasonically assisted hydrothermal and ethanol activation process for toluene adsorption, exhibiting a maximum adsorption capacity of 183 mg g−1, which is three times more than Cu-TBC (62.7 mg g−1). In addition, the Cu-BTC/GO composite maintains its high adsorption performance (82.1%) after five adsorption-regeneration cycles. Similarly, Zhu et al. [49] successfully fabricated MIL-68/GO composites to remove uranium (VI). The result shows that the introduction of GO into MIL-68 remarkably improved the adsorption capacity of the composite material. The excellent properties of MIL-68 MOF and the dense layer of GO lead to the synergistic effects of the MOF and GO in removing various organic contaminants from the water.
In this study, Ce (IV)-MOF was chosen as the base material due to its high affinity and selectivity towards phosphate removal [2], as well as other excellent properties[38, 50]. On the other hand, when GO is combined with MOF, it can change the distance between GO layers and improve the transfer of molecules between layers [51], leading to higher adsorption performance and stability of the composite material. Furthermore, when GO is combined with MOF, it can interact with the metal ions in the MOF and improve the water stability of the composite material and its physiochemical properties. Considering above, a Ce-MOF/GO composite was synthesized through a solvothermal reaction, and its adsorption performance, synergistic effect, and water stability were studied. The structure and morphology of the composite materials were characterized by scanning electron microscopy (SEM), X-ray diffraction (XRD), Fourier transform infrared spectroscopy (FTIR), and X-Ray photoelectron spectroscopy (XPS). The adsorption kinetics, isotherms, and the influencing factors for phosphate removal from aqueous solutions were investigated.
2. Material and Methods2.1. Chemicals and ReagentsTerephthalic acid (H2BDC), ammonium ceric nitrate (NH4)2 [Ce (NO3)6], acetic acid (CH3COOH) and N, N-dimethylformamide (DMF) were purchased from Fuchen and Tianjin Kermel Chemical Reagent Co. Ltd. Other chemicals, such as monopotassium phosphate (KH2PO4), potassium antimony tartrate (C8H4K2O12Sb2), and ascorbic acid (C6H8O6), were supplied by Tianjin Kermel Chemical Reagent Co. Ltd. All the chemical reagents were of analytically pure, and aqueous solutions were prepared with deionized water.
2.2. Preparation of Ce -MOF and Ce -MOF/GO CompositeGO was synthesized by Hummer’s method [52], and Ce-MOF was prepared based on Zhou et al. reported work with some modifications [53]. To summarize, (NH4)2[Ce (NO3)6] (5.482 g) and H2BDC (0.831g) was completely dissolved in (30/6 ml) DMF/DI water, and 10 mL of acetic acid (HAc) was added the solution. The mixture was reacted at 100 °C for 24 h and the precipitate was washed several times with DMF and ethanol, respectively. The product was then oven-dried for 12 h at 120 °C. The Ce-MOF/GO composite was prepared in the same way as Ce-MOF, except a certain amount of GO was added by ultrasonic treatment to the Ce-MOF mixture.
2.3. Characterization InstrumentsThe phosphate concentration was measured with a UV-visible spectrophotometer (TU-1810SPC, Universal Analysis, Beijing) at a wavelength of 710 nm. X-ray powder diffraction (XRD) (Universal Analysis, Beijing) examined the product’s structure and composition. The measurement condition was the copper target Kα rays, the scanning step is 0.02º, and the scanning range 2q is 10 ~ 80º (40 kV, 40 mA, λ1/4 0.15418 nm). The morphology of fabricated material was verified by scanning electron microscopy (FE-SEM S4800 Hitachi, Japan), and a Fourier transform infrared (FT-IR) spectrometer (Nicolet iS50, Thermo Fisher Technology Co. Ltd.) was used to scan the samples and observe the chemical properties. A N2 physical adsorption analyzer (JW-BK122W, Beijing Jingwei Gaobo Science and Technology Co., Ltd.) with Brunauer-Emmett-Teller (BET) was applied to study the specific surface area, pore-volume, and pore size of the adsorbent. X-ray photoelectron spectroscopy analysis was conducted by ESCALAB250Xi (Thermo Fisher, USA) photoelectron spectrometer under Al Kα radiation. The zeta potential of the sample was measured by the JS94H microelectrophoresis instrument.
2.4. Batch Adsorption ExperimentThe batch experiments were conducted at room temperature at 140 rpm on a water bath shaker. To compare the adsorption performances of the adsorbents, 0.02 g of adsorbents (GO, Ce-MOF, and Ce-MOF/GO-2% composite) were added to 50 ml of phosphate solution with a concentration of 100 mg L−1 at pH»6, shaken for180 min. In the adsorption isotherm experiment, 50 ml of phosphate solution with various concentrations (50, 100, 200, 300, 400, and 500 mg L−1) were measured. In kinetic adsorption, 50 ml of phosphate solution at a concentration of 130 mg L−1, were measured at different time interval, such as 10, 20, 40, 60, 120, 180, 240, 300, and 360 min, respectively. To determine the best pH value, 50 ml of phosphate solution (100 mg L−1) with various pH values (2–12) were placed in a constant temperature water bath shaker and oscillated for 180 min. The phosphate concentration was determined at equilibrium by ammonium molybdate spectrophotometry at 710 nm, according to the Chinese standard test method, and the standard curve. The adsorption capacity of the adsorbents was calculated according to Eq. (1):
where qe (mg g−1) represent the amount of phosphate adsorbed per unit mass of adsorbent, C0 (mg L−1), and Ce (mg L−1) represent the pre- and post-adsorption of the phosphate concentration, V(ml) represents the sample volume, and m (g) represents the adsorbent mass. The percentage of phosphate removal by the adsorbents was calculated using Eq. (2):
where η is the removal percentage of phosphate, C0 (mg L−1), and Ct (mg L−1) are the phosphate concentration before and after adsorption. The selectively of our adsorbent toward phosphate removal was examined in the presence of common competing ions with various concentrations of competing ions using 0.02 g of the adsorbents.
3. Result and Discussion3.1. Material Characterization3.1.1. Effect of HAc as a modulatorHAc is commonly used as a modulator synthesizing and developing MOFs with a chemically tunable structure such as UiO-66, UiO-67, and MIL-88A [54, 55]. Moreover, many studies show that using HAc as a modulator can enhance textural properties, including surface area and pore volume, and improve adsorption performance compared to the pristine material [56, 57]. Fig. 1 (a–c) shows that the addition of 10 ml of HAc in Ce-MOF and Ce-MOF/GO synthesis led to the formation of a well-shaped octahedral crystals with an ordered structure as well as some small rod-like structure. While increasing the HAc concentration can result in the formation of Ce-MOF/GO-2% composite with larger particles and rob-like shaped crystals [Fig. 1 (d–i)], which is consistent with previously published literature [58]. Furthermore, when the proper amount of HAc was used, it made the prominent diffraction of the XRD patterns become shaper, along with an increase in the intensity of the peaks, illustrating that HAc has a positive role in forming higher crystallinity in the Ce-MOF and Ce-MOF/GO composite with good octahedral morphology. However, with further addition of HAc (>15 ml), a new peak appeared at 2θ= 19°, indicating the formation of a new phase cerium phase. It is in accordance with the observation of XRD patterns of the samples in Fig. S8.
3.1.2. Effect of GO adding proportionTo study the influence of GO on the structure of Ce-MOF, different amounts of GO (1, 2, 5, 10, and 15 wt%) were used for synthesize of Ce-MOF/GO composite depending on the molar ratio of Ce-MOF. The adsorption experiment revealed that Ce-MOF/GO-2% showed the highest adsorption capacity compared to other ratios, as shown in (Fig. S1). Hence, the Ce-MOF/GO-2% composite was fixed for the rest of the experiment. As can be seen from the XRD diffraction result, the peak intensity of the Ce-MOF/GO-2% composite material is sharper than that of others. As the amount of GO increases, the XRD peaks of the samples become weaker because the GO mixture has a monolayer structure, and adding more GO prevents the composite bonding and crystal growth in Ce-MOF, which causes most of the peaks in the composite material to disappear as shown in (Fig. 2).
3.1.3. SEM analysisThe surface morphological structure of the material was analyzed at 1 and 100 mm, respectively. The Ce-MOF, Ce-MOF/GO-1% and Ce-MOF/GO-2% confirm the well-shaped octahedral morphology of the particles, along some small rod-like crystalline shapes as shown in Fig. 1(a–c), showing that the addition of a small amount of GO did not change the shape and crystallinity of the Ce-MOF, that might be due to the monolayer structure of ultrasonically processed GO and the small amount of GO mass. While adding more GO Fig. 1(d–f) can alter the shape and crystallinity of the adsorbent, resulting in irregular shapes, rod-like structures, and even the aggregation of GO. Some microcrystal structures were observed on the Ce-MOF and the Ce-MOF/GO composite surface. It should be mentioned that adding more HAc (>20 ml) leads to the formation of long, irregular rod-like structures and even damage the crystalline structure of Ce-MOF/GO composite (Fig. 1g–j).
3.1.4. XRD analysisX-ray diffraction patterns were used to examine the crystal structure of the samples. As seen in (Fig. 2), GO has a strong diffraction peak at 2q of 11.2, which corresponds to the (002) crystal plane, confirming that GO was synthesized successfully. The positions of the Ce-MOF peaks 2q= 16.7º, 23º, 29º, 33.6º, 41º, 44.9º, 48.2º corresponding to the (111), (200), (220), (311), (222), (400), and (331) crystals planes [59]. This is consistent with the observation of rod-shaped crystal [Fig. 1 (f, g)], which is in agreement with previous reports in the literature [36, 53] indicating the successful synthesis of the adsorbent and formation of a highly crystalline structure. As seen in (Fig. 2) where most of Ce forms Ce-MOF with an organic chain; hence no cerium oxide crystal phase was found. The strong peak of GO disappears in the structure of the composite material, which might be due to the monolayer structure and the ultrasonic dispersion of GO. Compared to the other samples, the intensity of the diffraction peaks in the Ce-MOF/GO-2% composite material increased and showed higher crystallinity, due to the strong Ce (VI)-O bond formation in Ce6 nodes. The XRD peaks did not change significantly when a small amount of GO was added, as shown in (Fig. 2), which could be attributed to the monolayer structure of the GO, that was ultrasonically processed. However, as shown in (Fig. 2), as the amount of GO percentage increases in Ce-MOF/GO composite the position of the diffraction peaks and the strength of the characteristic peaks of Ce-MOF/GO become weak, which could be attributed to the GO sheet influence that blocks the binding and reaction of precursor materials [21]. It is worth noting that adding more HAc changes the diffraction peaks of the samples, and the new peaks were revealed at 19° which might due to the formation of a new phase cerium phase (Fig. S8).
3.1.5. FT-IR analysisThe FTIR spectra shown in (Fig. 3) illustrate that the peaks at 1632-1572, 1500-1389, and 746 cm−1 are corresponding to carboxylic linker groups and the stretching vibrations of Ce-O, Ce-O-C, respectively, confirming the successful interaction of carboxylic groups with C=O groups in Ce-MOF and Ce-MOF/GO composites, which is consistent with the previously reported literature [38, 58]. The peak at 512 cm−1 corresponds to the Ce-O stretching vibrations. The -OH groups are present at 3400–3550 cm−1. All the characteristic peaks of the bands at 1033, 1619, and 3146 cm−1 of GO correspond to the carboxyl -(COOH), carbonyl (C=O), and hydroxyl -(OH) functional groups of GO [42, 60]. The bands at (1033–1166) and 1619 cm−1 belong to carboxylic groups and other oxygen-containing functionals of GO, which is associated with the vibration of sp2 and sp3 carbon atoms [42, 61]. When GO was added to the Ce-MOF, the adsorption peaks of the Ce-MOF slightly shifted from 1656 to 1632 cm−1, because the surface of GO contains plenty of oxygen-rich functional groups that can react with the carboxyl groups of the Ce-MOF, generating irregular C=O chains, and reduce the number of free carbonyl C=O bonds, which indicates that GO was satisfactorily synthesized and interacted with the Ce-MOF.
When the phosphate was absorbed by the adsorbent, the strong peak at 1632 cm−1 vanished, associating with the Ce-O stretching vibration, and the peak at 512 cm−1 for the stretching vibration of Ce-O was assigned to Ce-O-P bending mode. These observations indicate that phosphate and cerium interact chemically [62]. In addition, after phosphate was adsorbed by the sorbent, the peak intensity at 812 cm−1 increased slightly to 824 cm−1, indicating that hydrogen bonding (O-H…O) might be formed between phosphate and the composite material. Furthermore, the 154 cm−1 peak is attributed to the –OH groups of Ce, indicating that the hydroxyl groups play an important role in phosphate adsorption [63]. A new bank appears at 548 cm−1 after phosphate adsorption, which is associated to the presence of O-P-O band vibrations in PO43− [64]. As can be seen in (Fig. S9) further addition of GO in the Ce-MOF/GO composite makes the peaks of the samples become weak and even some peaks disappeared.
3.1.6. Nitrogen adsorption–desorption analysisThe textural properties of the three samples, including pore volume, average pore size, and specific surface area, were examined by N2 adsorption-desorption isotherm (BET) analysis indicating that the composite material has a mesoporous structure with a typical IV-type curve, and the pore size range between 6.75 and 13.9 nm, as shown in [Fig. 4 (a, b)]. The mesoporous structure of the composite material exposes more active sites for pollutants adsorption resulting in better adsorption performance of the adsorbent. The result of the BET investigations is presented in Table S1, and the specific surface areas of the three samples (GO, Ce-MOF, and Ce-MOF/GO-2%) were found to be 3.98, 257.49, and 46.67 m2 g−1, respectively. Compared with UiO-66 MOF, the specific area of Ce-MOF decreases as the particle size increases, which is consistent with previous reports [19]. On the other hand, partial coverage of Ce-MOF particles by GO may reduce the BET surface area of Ce-MOF/GO-2% composite, which is in agreement with the previous report [65]. As the amount of GO in the composite material increased, the surface area of the adsorbent decreased, which might be due to the effect of GO, which covers the structure of Ce-MOF and prevents the formation of a high crystalline structure in the composite material.
3.1.7. XPS analysisThe XPS spectrum of Ce-MOF/GO-2% is shown in (Fig. S12), revealing useful information about the local chemical environment before and after the phosphate removal. As shown in (Fig. S12 a), the Ce 3d, Ce2p, O1s, N1s, and C1s peaks were present before adsorption, indicating that cerium was successfully synthesized. After phosphate adsorption, the peak of P 2p was observed, indicating that phosphate was effectively adsorbed. As can be seen in (Fig. 12Sc), the peak of Ce 3d was resolved into three peaks before phosphate adsorption, and the corresponding Ce 3d3/2 and Ce 3d5/2 peaks were located at 905.85 eV, 903.59 eV 900.17eV, 885.57 eV, and 881.90 eV, respectively. After phosphate adsorption, the Ce 3d3/2 and Ce 3d5/2 peaks shifted to 905.53 eV, 903.53 eV 900.49 eV, 885.06 eV, and 881.96, respectively, which contributed to the generation of inner-sphere complexes of Ce–O–P from cerium and phosphate [14, 66]. In addition, after phosphate uptake, a new peak appeared at 886.78 which might be corresponding to Ce-O-P. The deconvoluted O 1s spectra before and after phosphate adsorption by Ce-MOF/GO-2% is shown in (Fig. S12 d). Before adsorption, two subpeaks were observed, namely, Ce-O-OH (531.45 eV), and O–C=O (532.15 eV). After adsorption, a peak attributed to P–O-H appeared at 531.80 eV. Meanwhile, the peak intensity related to the Ce–O-H content decreased to 531.25 eV, and the peak related to the O-C=O reduced to 531.41 eV. These results indicate that the metal hydroxyl groups are the possible active sites that were substituted by phosphate during the uptake reaction, leading to a substantial decrease in the metal hydroxyl content, which demonstrates that by inner-sphere complexes the metal hydroxyl groups contributed to the removal of phosphate.
On the basis of the above analysis, the possible phosphate removal mechanisms by Ce-MOF/GO-2% are electrostatic attractions and ligand exchange through the formation of an inner-layer complex. An inner-layer complex formed between the phosphate and Ce–OH groups via ligand exchange, resulting in an excellent uptake efficiency [14, 66]. Furthermore, the presence of GO functional and amino groups in Ce-BDC provides additional absorption sites in Ce-MOF/GO-2% composite material that improve the adsorption capacity of the sorbent [21].
3.2. Influencing Factors on Adsorption of Phosphate by Ce-MOF/GO Composite3.2.1. Effect of contact timeThe adsorption capacity of the fabricated materials depends on the contact time. Therefore, to study the phosphate adsorption capacity of the adsorbents, 50 ml of phosphate solution with an initial concentration of 130 mg L−1, 0.02 g of the sorbents, was placed in 100 ml of an iodine flask and shaken for 10, 20, 40, 60, 120, 180, 240, 300, and 360 min, respectively. The phosphate solution was then centrifuged/filtered, and 5 ml of the supernatant was placed in a 50 ml colorimetric tube; it was diluted about 40 ml with DI water, and then 2 ml of ammonium molybdate solution and 1 ml of ascorbic acid solution were added, shaken well, and allowed to stand at room temperature for 10 min. The absorbance was then measured using a cuvette by a spectrophotometer at 710 nm. The results show that within 10 min of the first contact time, Ce-MOF and Ce-MOF/GO-2% composite removed 195.1 and 224.2 mg g−1 of phosphate, respectively, as shown in (Fig. 5). The adsorption capacity of the adsorbent reached equilibrium at 180 min. The Ce-MOF/GO-2% composite showed higher adsorption capacity, which might be due to the presence of many oxygens containing functional groups such as hydroxyl, carboxy, and epoxy on the surface of GO, which can react with the metal ions in MOF through coordination bonding and improve the physiochemical properties of Ce-MOF/GO composite material.
3.2.2. Effect of adsorbent dosageThe influence of adsorbent dosage on phosphate adsorption was evaluated by changing the adsorbent dosage (0.01, 0.02, 0.03, 0.04, and 0.05 g) with a phosphate initial concentration of 100 mg L−1. The result shows that as the adsorbent dosage increases, the adsorption capacity of the sorbent also gradually increased and reaches equilibrium when 0.02 g of the adsorbents were used. After this dosage, the phosphate adsorption becomes stable and there was no further change in the adsorption capacity of the adsorbent that could be attributed to the saturation of active sites on the sorbent surface compared to the initial phosphate concentration (Fig. 6). Therefore, 0.02 g of the adsorbent dosage was fixed for the rest of the phosphate adsorption experiment, while for GO, by increasing the adsorbent dosage, the removal rate of phosphate gradually increased.
3.2.3. Effect of initial concentrationTo investigate the influence of initial phosphate concentration, 0.02 g of the sorbents (GO, Ce-MOF, and Ce-MOF/GO-2%) with different phosphate initial concentrations (50, 100, 200, 300, 400, and 500 mg L−1) were placed in 100 ml iodine flask and oscillated for 180 min, after which the adsorption capacity of the sorbents was measured. The result shows that a greater phosphate concentration increases the adsorption performance of the sorbents and can reach the maximum adsorption capacity of 308. 64 mg g−1 at 180 min according to the Langmuir adsorption isotherm data (see Fig. S3 and Table S3).
3.2.4. Effect of pH on adsorption performanceThe pH of the solution is one of the critical factors that greatly affects the adsorption performance of the sorbent, as it can change the surface charge of the sorbents via protonation and deprotonation of the surface functional groups. Accordingly, the chemical stability of the sorbents was tested over a wide pH range (2–12). Based on (Fig. 7), the phosphate removal capacity of the sorbent almost remained at a high level throughout the entire pH range (2–8), indicating a broad use. As seen when the pH increases from 2 to 3, the adsorption capacity of the Ce-MOF/GO-2% composite material significantly increased, and is maintained its high adsorption capacity up to pH 6 and above the pH 6, its adsorption capacity slightly decreased, which is due to the existence of phosphate in different ionic species at dissociate form (H2PO4−, HPO42−, and PO43−) which depends on the pH of the solution. Generally, under lower pH values (2.12) the protonated adsorbent has difficulty capturing neural H3PO4, which reduces the composite material’s sorption performances. Over 2.12, the phosphate exists in a negatively charged forms such as H2PO4− (pH=2.12.–7.2), HPO42− (pH=7.3–12.3), and PO43− (pH= >12.3), [36] which is favorable to be adsorbed by the sorbent due to electrostatic interaction and ligand exchange. Whereas phosphate can be removed by ligand exchange over a wide pH range, even when the adsorbent’s surface is negatively charged, however phosphate removal by electrostatic interaction depends on pH values which decreases with increasing pH values. As a result, the electrostatic attraction between the positively charged composite material and the negatively charged H2PO−/HPO42− was the dominant force between pH 2.2 and 6. Over pH > 6.5 the adsorption of phosphate gradually decreased, which might be due to the repulsion between negatively charged composite material and phosphate. However, under the strong alkaline condition, the adsorption capacity of the sorbent was reduced which might be attributed to negative surface charge and vie the binding sites for phosphate ions by excessive OH−. It’s worth noting, the adsorbent showed a good phosphate removal rate even under strong acidic and alkaline condition, which proves that our prepared sorbent has good stability.
In contrast, the adsorption efficiency of GO over a wide pH range (2–12) was poor because the surface of GO was negatively charged, causing repulsion between the adsorbent and the pollutant. Furthermore, competition between negatively charged phosphate ions and OH− ions decreased phosphate removal by GO. In addition, in comparison to the phosphate concentration, the amount of GO was lower, resulting in poor adsorption effectiveness. The effects of phosphate adsorption capacity with an initial concentration of 100 mg L−1 at different pH values on the adsorbents are shown in (Fig. 7). The zeta potentials of three samples (GO, Ce-MOF, and Ce-MOF/GO) were measured at different pH values at room temperature. (Fig. 8) shows that the zeta potential of (Ce-MOF, and Ce-MOF/GO-2%) was positively charged in the pH range (2–6) and above pH 6.6 it was negatively charged, while GO was negatively charged in the pH range (2–12). The zeta potential of the three samples at pH= 6 was −37.1 mV, 0.59 mV, and 5.57 mV, respectively. As a result of electrostatic interaction, positively charged adsorbents are beneficial for the adsorption of negatively charged phosphate and other anionic pollutants [67].
3.2.5. Effect of competing ionsMany coexisting ions found in eutrophic waters may compete with phosphate removal for the same adsorption sites in the Ce-MOF/GO composite, resulting in reduced phosphate uptake efficiency. To fully explore the potential of using Ce-MOF/GO composite for the decontamination of phosphate-rich sites, the selectivity of Ce-MOF/GO was tested through batch experiments in the presence of anions like chloride, nitrate, bicarbonate, and sulfate, which are frequently found on surface water and in wastewater (Fig. S7). The concentration of coexisting ions was set to (0–80 mg L−1) and mixed with 100 mg L−1 phosphate solution. As shown in (Fig. S7) even if the concentration of coexisting ions is 80 mg. L−1, the phosphate removal efficiency of the adsorbent hardly changes. This result indicates that the binding capacity between the phosphate and the prepared Ce-MOF/GO-2% composite is stronger than that between the studied interfering ions. The Ce-MOF/GO-2% composite formed inner-sphere complexes with the phosphate through ligand exchange, which is in agreement with the results of the similar phosphate removal material. Furthermore, this result is also in agreement with our XPS analysis report [68, 69]. Although the dominant phosphate species were H2PO4− and HPO42− at pH > 2.2 to 5.5. However, SO42− had no effect on phosphate adsorption (Fig. S7c) whereas SO42− slightly reduced the Ce-MOF adsorption capacity. The results reveal the high selectivity of our composite material toward phosphate removal and the negligible effect of interferents on the adsorbent’s performance.
3.3. Adsorption Characteristics3.3.1. Adsorption kineticsThe phosphate uptake on GO, Ce-MOF, and Ce-MOF/GO-2% and its adsorption mechanism were investigated. The experimental data were evaluated with pseudo-first order and pseudo-second order kinetic models, and the data were analyzed according to Eqns. (3) and (4):
where qt and qe (mg g−1) are the adsorption capacities of the Ce-MOF/GO-2% composite at time t, and at equilibrium, respectively. k1 (min−1) is the pseudo-first order adsorption rate constant, and k2 (g/mg min−1) is the equilibrium pseudo-second-order rate constant. Fig. S2 (a, b). illustrates the linear plots of pseudo-first and pseudo-second order models for the adsorption of phosphate on three adsorbents (GO, Ce-MOF, and Ce-MOF/GO-2%). The number of linear coefficients of regression (R2) in the pseudo second-order model is the most suitable kinetic model for the adsorption process. In addition, according to the (Table S2), the equilibrium adsorption capacities (qe,cal) calculated from pseudo-second order model are close to the experimental values (qe,exp) compared to that of pseudo-first order model.
Furthermore, by comparing the determination coefficients (R2) of both models, it was determined that the phosphate adsorption onto Ce-MOF/GO-2% fit better with the pseudo-second-order model. The adsorption process of phosphate on the Ce-MOF/GO-2% composite is a three-stage process. The first stage (rapid adsorption) is where the phosphate molecules are transferred from the bulk to the Ce-MOF/GO-2% composite surface, where all the adsorption sites are available (Fig. 5). In the first 10 min of contact time, 75% of the phosphate was removed by the adsorbent. In the second stage, phosphate molecules enter the internal pores of the Ce-MOF/GO-2% composites, and after saturating its adsorption sites, the adsorption process becomes slower than that of the first stage. The third stage is the adhesion of adsorbate molecules to the adsorbent active sites via physical and chemical attractive forces. At this stage, the adsorption decreases slightly, showing that the adsorption reaches equilibrium at 180 min. From (Fig. 5) it can be seen that the adsorption capacity of Ce-MOF/GO-2% was superior to that of GO, and Ce-MOF, which shows that the addition of 2% GO in the synthesis of Ce-MOF, significantly increased the phosphate removal capacity. When the concentration of the phosphate solution was 130 mg L−1, the adsorption capacity of the composite material reached 264.66 64 mg g−1.
3.3.2. Adsorption isothermThe adsorption of phosphate on Ce-MOF/GO-2% was investigated at different concentrations (50, 100, 200, 300, 400, and 500 mg L−1) and the experimental data clarified the adsorption behavior of the sorbent using the Langmuir and Freundlich methods, which show the relationship between the adsorption of pollutants on the adsorbent and its equilibrium concentration in solution. The Langmuir adsorption model illustrates a monolayer adsorption process that takes place on a homogeneous surface that has a specific number of active sites. When all the active sites were filled with the pollutant, the adsorption reached its equilibrium, and no adsorption took place. The Freundlich model describes a multi-layered adsorption process over a heterogeneous surface with various chemical and physical adsorption energies. The Langmuir models can be calculated according to Eq. (5).
In this formula, Q0 (mg g−1) is the unit saturated adsorption capacity when the monolayer adsorption is formed; Ce (mg L−1) describes the amount of phosphate adsorbed and the phosphate concentration in the solution at equilibrium; qe(mg g−1) is the equilibrium adsorption capacity, b is the Langmuir equilibrium constant. The Freundlich isotherm Eq. (6).
where Kf and n are the adsorption constants, which correspond to factors such as temperature and the specific surface area of the sorbent. The n value describes the type of isotherm, 1/n <1 (favorable), 1/n > 2 (unfavorable). According to (Fig. S3) and Table S3, the result of the experimental data best fits the Langmuir isotherm with a maximum adsorption capacity of 308.64 mg g−1, showing that our composite material has excellent adsorption performance toward phosphate removal in comparison to that of other sorbents reported in the literature (Table S4), which might be due to the high affinity of cerium toward phosphate, and the synergetic effect between Ce-MOF and GO, Since GO has plenty of oxygen-rich functional groups on its surface, like hydroxyl groups, carboxyl groups, and pyridine, etc., which can coordinate metal cations in a MOF competing with organic linkers. This results in more defects in the resulting crystalline structure, and more unsaturated bonds, and provides new active centers that can strongly interact with adsorbate molecules.
3.3.3. Adsorption thermodynamic
Fig. S4 (a, b) shows the TGA curve of the material at various temperatures in the range (25°C to 65°C) and its thermodynamic adsorption onto three samples. The result shows that the Ce-MOF/GO-2% composite material has not changed much with temperature increases (Fig. S4). However, the adsorption capacity of GO increased slightly as temperature increased, whereas the adsorption capacities of Ce-MOF and Ce-MOF/GO-2% decreased relatively as temperature increased, indicating that the sorbent adsorption process is an exothermic reaction, and that temperature had no effect on the adsorption process. The corresponding thermodynamic parameters are calculated by the following Eq. (7):
where ΔG°/kJmol−1 shows the Gibbs free energy change, ΔH°/kJ.mol−1 is the adsorption enthalpy change, ΔS°/J.mol−1 K−1 is the adsorption entropy change, and T(K) is unit of the thermodynamic temperature, which calculated according to the following Eqns (8) and (9):
where Kd is the distribution coefficient and R is the thermodynamic constant, which is 8.314 JK−1mol−1. The corresponding thermodynamic parameters for phosphate adsorption on three adsorbents (GO, Ce-MOF, and Ce-MOF/GO-2%) are presented in (Table S5), and reveal that for (Ce-MOF and Ce-MOF/GO-2%) all DH values are positive, indicating that phosphate adsorption onto two samples is an endothermic reaction process; DS values were positive, showing that after phosphate adsorption on the adsorbent surface, the internal reaction disorder occurred on the adsorption process, while DH values for GO are negative, indicating that the adsorption is an exothermic reaction process. The DS value is negative, which describes that the disorder at the solid-liquid interface was reduced during the adsorption process.
3.3.4. ReusabilityRegeneration and reusability of the sorbent is a pivotal factor for the fabrication of a renewable and cost-effective adsorbent. Thus, four adsorption/regeneration cycles were conducted to examine the regeneration and reusability of the sorbents. For this purpose, a certain amount of GO, Ce-MOF, and Ce-MOF/GO-2% composite material were taken in 100 mg L−1 of phosphate solution and after the phosphate adsorbed, the composite material was soaked into NaOH (0.1 mol L−1) solution for 3h. Following, washed with deionized water and ethanol in a ratio of (9:1), then dried at 80 °C for 1h and activated in a vacuum dryer at 120°C for 12 h. Fig. S5 shows that after four adsorption/regeneration cycles, the composite material adsorption capacity reduced from 90% and 100% to 72% and 92%, respectively for Ce-MOF and Ce-MOF/GO-2%, which indicated that Ce-MOF/GO-2% composite after four adsorption-desorption cycles still maintains its high adsorption capacity toward phosphate removal. Based on the XRD patterns and SEM images of the sample (Fig. S10 and Fig. S11), after four adsorption-desorption our prepared composite still maintain its good crystalline structure, which proves that adding GO to Ce-MOF improve the physio-chemical properties of the composite material. It’s worth noting that after the first phosphate adsorption cycles a new peak appeared at 18.1, and the intensity of peaks belonging to 220 crystal plans significantly increased, which shows phosphate was successfully adsorbed by the composite material. After the first adsorption cycles the peaks belonging to 111 and 200 crystal plans disappeared and the intensity of the peak attributed to 220 crystal plans significantly increased. It’s worth noting that the ICP test result proved that adding GO can improve the Ce-MOF water stability. The reusability of GO and GO composite sorbents are shown in Table S6.
3.4. Adsorption MechanismPhosphate can be removed by the Ce-MOF, and the Ce-MOF/GO-2% composite in several ways, such as electrostatic interaction and hydrogen bonding, and ligand exchange might also attribute phosphate removal. (Fig. 3) shows the FTIR spectra of Ce-MOF and Ce-MOF/GO-2% before and after phosphate adsorption. The results illustrate that the peaks at 1632 cm−1 disappeared after phosphate adsorption, associating with the Ce-O stretching vibration, and the peak at 512 cm−1 for the stretching vibration of Ce-O was assigned to the Ce-O-P bending mode. These observations suggest that phosphate and cerium interact chemically [63]. The peaks before adsorption at 1682–1383 cm−1 correspond to the O=-C-O symmetric and asymmetric stretching vibrations of the carboxylic ions [36], indicating the successful coordination between cerium (IV) ions and organic linkers, which exhibit excellent structural integrity even after phosphate adsorption with insignificant changes.
Electrostatic interaction plays a key role in phosphate adsorption, which depends on the pH of the solutions, affecting the surface charge of the sorbent and the ionization of the pollutant. (Fig. 8) shows that phosphate adsorption is negatively affected under strongly acidic and highly alkaline conditions, since under strong acidic condition phosphate exist in neutral form (H3PO4), and under the strong alkaline condition the OH− ions increases which result in repulsion between the sorbent and the pollutant. However, at a pH range (3–6), the Ce-MOF/GO-2% composite material demonstrated a higher adsorption capacity toward phosphate removal. This is because the phosphate is an anionic pollutant, and the zeta potential indicates that at pH (3–6), our adsorbent surface is positively charged, which is conducive to electrostatic interaction. These results indicate that electrostatic interaction can play a crucial role in the adsorption of phosphate in an aqueous solution. Another proposed mechanism for phosphate adsorption is hydrogen bonding, in which cerium elements in Ce-BDC exhibit a strong tendency in binding with -OH groups because the peak 1157 cm−1 was attributed to the –OH groups from Ce, indicating that the hydroxyl groups play an important role in phosphate adsorption [63]. Following phosphate adsorption, a new weak band at 610, cm−1 appeared, which is associated with the presence of O-P-O band vibration in PO43− [64], as well as a weak new adsorption band at 1055 cm−1, which corresponds to the typical P-O asymmetric vibration of H2PO4− or HPO42− [62]. The XPS spectrum of Ce-MOF/GO-2% before and after the phosphate removal is shown in (Fig. S12). As can be seen in (Fig. 12sc), the peak of Ce 3d was resolved into three peaks before phosphate adsorption, and the corresponding Ce 3d3/2 and Ce 3d5/2 peaks were located at 905.85 eV, 903.59 eV 900.17eV, 885.57 eV, and 881.90 eV, respectively. After phosphate adsorption, the Ce 3d3/2 and Ce 3d5/2 peaks shifted to 905.53 eV, 903.53 eV 900.49 eV, 885.06 eV, and 881.96, respectively, which contributed to the generation of inner-sphere complexes of Ce–O–P from cerium and phosphate [14, 66]. The deconvoluted O 1s spectra before and after phosphate adsorption by Ce-MOF/GO-2% is shown in (Fig. S12d). Before adsorption, two subpeaks were observed, namely, Ce-O-OH (531.45 eV), O–C=O (532.15 eV), and after phosphate adsorption, a peak attributed to P–O-H appeared at 531.80 eV. Meanwhile, the peak intensity related to the Ce–O-H content decreased to 531.25 eV, and the peak related to the O-C=O reduced to 531.41 eV. These results indicate that the metal hydroxyl groups are the possible active sites that were substituted by phosphate during the uptake reaction, and through inner-sphere complexes, the metal hydroxyl groups contributed to the removal of phosphate.
Based on the above analysis, another possible phosphate removal mechanism by Ce-MOF/GO-2% ligand exchange is through the formation of an inner-layer complex. Furthermore, the presence of GO functional and amino groups in Ce-BDC provides additional absorption sites in Ce-MOF/GO-2% composite material that improve the adsorption capacity of the sorbent [21]. (Fig. S6) shows the possible adsorption mechanism of phosphate by the adsorbent.
To sum up, the possible adsorption mechanism was through hydrogen bonding, electrostatic interaction, and inner-layer complex between phosphate and Ce-MOF/GO-2% at the missing ligand site, which enabled phosphate ions to disperse into the MOF channels and many available binding sites.
4. ConclusionIn this study, to improve the physio-chemical properties of Ce-MOF, different amounts of GO were synthesized via a solvothermal method, which led the Ce-MOF to grow on the surface of GO, resulting in the formation of a well-shaped octahedral high-crystalline structure with good water stability and a high phosphate adsorption capacity. The Ce-MOF/GO-2% composite material exhibits higher adsorption performance with a maximum adsorption capacity of 308. 64 mg g−1, which is significantly better than that of Ce-MOF. The adsorption isotherm was best fitted with the Langmuir isotherm model, which indicates that it is a monolayer adsorption process, and the adsorption kinetic data was in agreement with a second-order kinetic model. The adsorption mechanism was mainly via hydrogen bonding, electrostatic interaction, and inner-layer complex, The composite material showed excellent adsorption performance, reusability, and regeneration potential, which make it a promising adsorbent for phosphate uptake from polluted water.
AcknowledgementsThe research was supported by the Shaanxi Provincial Water Conservancy Science and Technology Project (2022slkj-5) and Key R&D plan of Shaanxi province(2022SF-578).
NotesData Availability Statement All data generated or used during the study appear in the submitted article. Author Contribution X. H. (Ph.D. student) conducted the experiment and wrote the first draft, Z. L. (Professor) directed the research and revised the manuscript. M. F. B. (Ph.D. Student) helped in developing the conceptualization, methodology, and writing the manuscript, L. J. (Master student) helped in drafting the manuscript, and L. D. (Master student) helped in material characterization and publishing. All the co-authors commented on the first drafted manuscript and approved the final manuscript. References1. Bakshi S, Laird DA, Smith RG, Brown R. Capture and Release of Orthophosphate by Fe-Modified Biochars: Mechanisms and Environmental Applications. ACS. Sustain. Chem. Eng. 2021;9(2)658–668.
https://doi.org/10.1021/acssuschemeng.0c06108
2. Hassan M, Stanton R, Secora J, Trivedi D, Andreescu S. Ultrafast Removal of Phosphate from Eutrophic Waters Using a Cerium-Based Metal-Organic Framework. ACS. Appl. Mater. Interfaces. 2020;12(47)52788–52796.
https://doi.org/10.1021/acsami.0c16477
3. Luo F, Feng X, Li Y, Zheng G, Xie P, Wang Z, et al. Magnetic amino-functionalized lanthanum metal-organic framework for selective phosphate removal from water. Colloids Surf. A: Physicochem. Eng. Asp. 2021;611:125906.
https://doi.org/10.1016/j.colsurfa.2020.125906
4. Guan T, Li X, Fang W, Wu D. Efficient removal of phosphate from acidified urine using UiO-66 metal-organic frameworks with varying functional groups. Appl. Surf. Sci. 2020;501:1444074.
https://doi.org/10.1016/j.apsusc.2019.144074
5. Lu Y, Xie A, Hu M, Wu J, Zhang D, Lan Y. Assembly of Two Mesoporous Anionic Metal-Organic Frameworks for Fluorescent Sensing of Metal Ions and Organic Dyes Separation. Inorg. Chem. 2021;60(1)167–174.
https://doi.org/10.1021/acs.inorgchem.0c02760
6. Othman A, Dumitrescu E, Andreescu D, Andreescu S. Nanoporous Sorbents for the Removal and Recovery of Phosphorus from Eutrophic Waters: Sustainability Challenges and Solutions. ACS Sustain. Chem. Eng. 2018;6(10)12542–12561.
https://doi.org/10.1021/acssuschemeng.8b01809
7. Ruzhitskaya O, Gogina E. Methods for Removing of Phosphates from Wastewater. MATEC Web Conf. 2017;106:6.
https://doi.org/10.1051/MATECCONF/201710607006conferencepaper
8. Huang H, Liu J, Zhang P, Zhang D, Gao F. Investigation on the simultaneous removal of fluoride, ammonia nitrogen and phosphate from semiconductor wastewater using chemical precipitation. Chem. Eng. J. 2017;307:696–706.
https://doi.org/10.1016/j.cej.2016.08.134
9. Yang X, Zhang C, Zhang X, Deng S, Cheng X, Waite T. Phosphate Recovery from Aqueous Solutions via Vivianite Crystallization: Interference of Fe(II) Oxidation at Different DO Concentrations and pHs. Environ. Sci. Technol. 2023;57(5)2105–2117.
https://doi.org/10.1021/acs.est.2c06668
10. Hei S, Xu H, Liu Y, Liu B, Zhang S, Zhu X, et al. Redox environment inducing strategy for enhancing biological phosphorus removal in a full-scale municipal wastewater treatment plant. J. Clean. Prod. 2022;376:134237.
https://doi.org/10.1016/j.jclepro.2022.134237
11. Shalaby M, Abdallah H, Cenian A, Solowski G, Sawczak M, Shaban A, et al. Laser synthesized gold-nanoparticles, blend NF membrane for phosphate separation from wastewater. Sep. Purif. Technol. 2020;247:116994.
https://doi.org/10.1016/j.seppur.2020.116994
12. Velu M, Balasubramanian B, Velmurugan P, Kamyab H, Ravi A, Chelliapan S, et al. Fabrication of nanocomposites mediated from aluminium nanoparticles/Moringa oleifera gum activated carbon for effective photocatalytic removal of nitrate and phosphate in aqueous solution. J. Clean. Prod. 2021;281:124553.
https://doi.org/10.1016/j.jclepro.2020.124553
13. Chen M, Li X, Zhang Q, Wang C, Hu H, Wang Q, et al. Phosphate removal from aqueous solution by electrochemical coupling siderite packed column. Chemosphere. 2021;280:130805.
https://doi.org/10.1016/j.chemosphere.2021.130805
14. He J, Xu H, Xiong Z, Lai B, Sun Y, Yang Y, et al. The enhanced removal of phosphate by structural defects and competitive fluoride adsorption on cerium-based adsorbent. Chemosphere. 2020;256:127056.
https://doi.org/10.1016/j.chemosphere.2020.127056
15. Xiao W, Garba Z, Sun S, Lawan I, Wang L, Lin M, et al. Preparation and evaluation of an effective activated carbon from white sugar for the adsorption of rhodamine B dye. J. Clean. Prod. 2020;253:119989.
https://doi.org/10.1016/j.jclepro.2020.119989
16. Faysal M, Akther N, Zhou Y. Recent advancements in graphene adsorbents for wastewater treatment: Current status and challenges. Chin. Chem. Lett. 2020;31(10)2525–2538.
https://doi.org/10.1016/j.cclet.2020.05.011
17. Mashkoor F, Nasar A, Inamuddin . Carbon nanotube-based adsorbents for the removal of dyes from waters: A review. Environ. Chem. Lett. 2020;18:605–629.
https://doi.org/10.1007/s10311-020-00970-6
18. Jun B, Heo J, Taheri-Qazvini N, Park C, Yoon Y. Adsorption of selected dyes on Ti3C2Tx MXene and Al-based metal-organic framework. Ceram. Int. 2020;46(3)2960–2968.
https://doi.org/10.1016/j.ceramint.2019.09.293
19. Rego R, Kuriya G, Kurkuri M, Kigga M. MOF based engineered materials in water remediation: Recent trends. J. Hazard. Mater. 2021;403:123605.
https://doi.org/10.1016/j.jhazmat.2020.123605
20. Muesmann TWT, Mietrach A, Christoffers J, Wickleder MS. Synthesis of 1,2,4,5-Benzenetetrasulfonic Acid (H4B4S) and its Implementation as a Linker for Building Metal-Organic Frameworks on the Example of [Cu2(B4S) (H2O)8].0.5H2O. Inorg. Chem. 2010;636(7)1307–1312.
https://doi.org/10.1002/ZAAC.201000056
21. Wu Y, Liu Z, Bakhtari M, Luo J. Preparation of GO/MIL-101(Fe, Cu) composite and its adsorption mechanisms for phosphate in aqueous solution. Environ. Sci. Pollut. Res. 2021;28(37)51391–51403.
https://doi.org/10.1007/s11356-021-14206-9
22. Haso H, Dubale A, Chimdesa M, Atlabachew M. High Performance Copper Based Metal Organic Framework for Removal of Heavy Metals from Wastewater. Front. Mater. 2022;9:840806.
https://doi.org/10.3389/fmats.2022.840806
23. Pirzadeh K, Ghoreyshi A, Rahimnejad M, Mohammadi M. Electrochemical synthesis, characterization and application of a microstructure Cu3(BTC)2 metal organic framework for CO2 and CH4 separation. Korean J. Chem. Eng. 2018;35(4)974–983. 10.1007/s11814-017-0340-6
24. Stawowy M, Róziewicz M, Szczepańska E, Silvestre-Albero J, Zawadzki M, et al. The Impact of Synthesis Method on the Properties and CO2 Sorption Capacity of UiO-66(Ce). Catalysts. 2019;9(4)309.
https://doi.org/10.3390/catal9040309
25. Dang Y, Huang H, Dong H, Bui K, Nguyen L, Phan T, et al. Microwave-assisted synthesis of nano Hf- and Zr-based metal-organic frameworks for enhancement of curcumin adsorption. Microporous Mesoporous Mater. 2020;298:110064.
https://doi.org/10.1016/j.micromeso.2020.110064
26. Chaemchuen S, Zhou K, Mousavi B, Ghadamyari M, Heynderickx P, Zhuiykov S, et al. Spray drying of zeolitic imidazolate frameworks: investigation of crystal formation and properties. Cryst. Eng. Comm. 2018;20(25)3601–3608.
https://doi.org/10.1039/C8CE00392K
27. Didriksen T, Spjelkavik A, Blom R. Continuous synthesis of the metal-organic framework CPO-27-Ni from aqueous solutions. J. Flow Chem. 2017;7(1)13–17.
https://doi.org/10.1556/1846.2016.00040
28. Zhang Z, Xu L, Jiao H. Ionothermal synthesis, structures, properties of cobalt-1,4-benzenedicarboxylate metal–organic frameworks. J. Solid State Chem. 2016;238:217–222.
https://doi.org/10.1016/j.jssc.2016.03.028
29. Gangu KM, Assila S, Mukkamala S, Jonnalagadda B. A review on contemporary Metal–Organic Framework materials. Inorganica Chim. Acta. 2016;446:61–74.
https://doi.org/10.1016/j.ica.2016.02.062
30. Abdelmoaty AS, El-Wakeel ST, Fathy N, Hanna AA. High Performance of UiO-66 Metal–Organic Framework Modified with Melamine for Uptaking of Lead and Cadmium from Aqueous Solutions. J. Inorg. Organomet. Polym. Mater. 2022;32(7)2557–2567.
https://doi.org/10.1007/s10904-022-02326-9
31. Cheng P, Wang C, Kaneti Y, Eguchi M, Lin J, Yamauchi Y, et al. Practical MOF Nanoarchitectonics: New Strategies for Enhancing the Processability of MOFs for Practical Applications. Langmuir. 2020;36(16)4231–4249.
https://doi.org/10.1021/acs.langmuir.0c00236
32. Li T, Liu L, Zhang Z, Han Z. Preparation of nanofibrous metal-organic framework filter for rapid adsorption and selective separation of cationic dye from aqueous solution. Sep. Purif. Technol. 2020;237:116360.
https://doi.org/10.1016/j.seppur.2019.116360
33. Shan S, Tang H, Zhao Y, Wang W, Cui F. Highly porous zirconium-crosslinked graphene oxide/alginate aerogel beads for enhanced phosphate removal. Chem. Eng. J. 2019;359:779–789.
https://doi.org/10.1016/j.cej.2018.10.033
34. Shooto ND, Dikio ED. Highly Porous MOF Adsorbent for Wastewater Treatment. Asian J. Chem. 2018;30(8)1723–1730.
https://doi.org/10.14233/ajchem.2018.21275
35. Yu C, Wang K, Li X, Liu D, Ma L, Liu L. Highly Efficient and Facile Removal of Pb2+ from Water by Using a Negatively Charged Azoxy-Functionalized Metal–Organic Framework. Cryst. Growth Des. 2020;20(8)5251–5260.
https://doi.org/10.1021/acs.cgd.0c00437
36. He J, Xu Y, Wang W, Hu B, Wang Z, Yang X, et al. Ce (III) nanocomposites by partial thermal decomposition of Ce-MOF for effective phosphate adsorption in a wide pH range. Chem. Eng. J. 2020;379:122431.
https://doi.org/10.1016/j.cej.2019.122431
37. Stanton R, Trivedi D. Influence of Defects and Linker Exchange on Removal of Phosphate Using MOFs with the Node Structure M6(OH)4(O)4 for M = Hf, Zr, or Ce. Chem. Mater. 2021;33(14)5730–5737.
https://doi.org/10.1021/acs.chemmater.1c01521
38. Rego R, Sriram G, Ajeya K, Jung H, Kurkuri M, Kigga M. Cerium based UiO-66 MOF as a multipollutant adsorbent for universal water purification. J. Hazard. Mater. 2021;416:125941.
https://doi.org/10.1016/j.jhazmat.2021.125941
39. Li M, Liu Y, Li F, Shen C, Kaneti Y, Yamauchi Y, et al. Defect-Rich Hierarchical Porous UiO-66(Zr) for Tunable Phosphate Removal. Environ. Sci. Technol. 2021;55(19)13209–13218.
https://doi.org/10.1021/acs.est.1c01723
40. Wang J, Xia Y. Fe-Substituted Isoreticular Metal–Organic Framework for Efficient and Rapid Removal of Phosphate. ACS Appl. Nano Mater. 2019;2(10)6492–6502.
https://doi.org/10.1021/acsanm.9b01429
41. Li Y, Xie Q, Hu Q, Li C, Huang Z, Yang X, et al. Surface modification of hollow magnetic Fe3O4@NH2-MIL-101(Fe) derived from metal-organic frameworks for enhanced selective removal of phosphates from aqueous solution. Sci. Rep. 2016;6(1)30651.
https://doi.org/10.1038/srep30651
42. Liu Z, He W, Zhang Q, Shapour H, Bakhtari MF. Preparation of a GO/MIL-101(Fe) Composite for the Removal of Methyl Orange from Aqueous Solution. ACS Omega. 2021;6:4597–4608.
https://doi.org/10.1021/acsomega.0c05091
43. Tao Z, Jin X, Owens G, Chen Z. Remediation of malachite green in wastewater by ZIF-8@Fe/Ni nanoparticles based on adsorption and reduction. J. Colloid Interface. Sci. 2021;594:398–408.
https://doi.org/10.1016/j.jcis.2021.03.065
44. Tambat S, Sane P, Suresh S, Varadan O, Pandit A, Sontakke S. Hydrothermal synthesis of NH2-UiO-66 and its application for adsorptive removal of dye. Adv. Powder Technol. 2018;29(11)2626–2632.
https://doi.org/10.1016/j.apt.2018.07.010
45. Bhattacharya S, Banerjee P, Das P, Bhowal A, Majumder SK, Ghosh P. Removal of aqueous carbamazepine using graphene oxide nanoplatelets: process modelling and optimization. Sustain. Environ. Res. 2020;30:17.
https://doi.org/10.1186/s42834-020-00062-8
46. Wernke G, Shimabuku-Biadola Q, Santos T, Silva M, Fagundes-Klen M, Bergamasco R. Adsorption of cephalexin in aqueous media by graphene oxide: kinetics, isotherm, and thermodynamics. Environ. Sci. Pollut. Res. Int. 2020;27(5)4725–4736.
https://doi.org/10.1007/s11356-019-07146-y
47. Wei F, Zhang H, Ren Q, Chen H, Yang L, Ding B, et al. Removal of organic contaminants from wastewater with GO/MOFs composites. PLOS One. 2021;16(7)e0253500.
https://doi.org/10.1371/journal.pone.0253500
48. Dai Y, Li M, Liu F, Xue M, Wang Y, Zhao C. Graphene oxide wrapped copper-benzene-1,3,5-tricarboxylate metal organic framework as efficient absorbent for gaseous toluene under ambient conditions. Environ. Sci. Pollut. Res. 2019;26:2477–2491.
https://doi.org/10.1007/s11356-018-3657-8
49. Zhu J, Zhang H, Liu Q, Wang C, Sun Z, Li R, et al. Metal-organic frameworks (MIL-68) decorated graphene oxide for highly efficient enrichment of uranium. J. Taiwan Inst. Chem. Eng. 2019;99:45–52.
https://doi.org/10.1016/j.jtice.2019.01.008
50. Lammert M, Wharmby M, Smolders , Bueken B, Lieb A, Lomachenko K, et al. Cerium-based metal organic frameworks with UiO-66 architecture: synthesis, properties and redox catalytic activity. Chem. Commun. 2015;51:12578–12581.
https://doi.org/10.1039/c5cc02606g
51. Haeri Z, Ramezanzadeh B, Ramezanzadeh M. Recent progress on the metal-organic frameworks decorated graphene oxide (MOFs-GO) nano-building application for epoxy coating mechanical-thermal/flame-retardant and anti-corrosion features improvement. Prog. Org. Coat. 2022;163:106645.
https://doi.org/10.1016/j.porgcoat.2021.106645
52. Rattana S, Witit-anun N, Nuntawong N, Chindaudom P, Oaew S, Kedkeaw C, et al. Preparation and characterization of graphene oxide nanosheets. Procedia Eng. 2012;32:759–764.
https://doi.org/10.1016/j.proeng.2012.02.009
53. Zhou J, HAO L, Lin Y, Zhou C, Huang A. Synthesis of well-shaped and high-crystalline Ce-based metal organic framework for CO2/CH4 separation. Microporous Mesoporous Mater. 2020;302:110224.
https://doi.org/10.1016/j.micromeso.2020.110224
54. Bagherzadeh E, Zebarjad S, Hosseini H. Morphology Modification of the Iron Fumarate MIL-88A Metal–Organic Framework Using Formic Acid and Acetic Acid as Modulators. Eur. J. Inorg.Chem. 2018;2018(18)1909–1915.
https://doi.org/10.1002/ejic.201800056
55. Qiu J, Feng Y, Zhang X, Jia M, Yao J. Acid-promoted synthesis of UiO-66 for highly selective adsorption of anionic dyes: Adsorption performance and mechanisms. J. Colloid Interface Sci. 2017;499:151–158.
https://doi.org/10.1016/j.jcis.2017.03.101
56. Jiao Y, Liu Y, Hungerford J, Bhattacharyya S, Lively R, Sholl D, Walton K. Heat-Treatment of Defective UiO-66 from Modulated Synthesis: Adsorption and Stability Studies. J. Phys. Chem. C. 2017;121(42)23471–23479.
https://doi.org/10.1021/acs.jpcc.7b07772
57. Liu N, Shi L, Meng X. Tuning the adsorption properties of UiO-66 via acetic acid modulation. J. Chem. Sci. 2019;131:50.
https://doi.org/10.1007/s12039-019-1628-3
58. Xiong Y, Chen S, Ye F, Su L, Zhang C, Shen S, Zhao S. Synthesis of a mixed valence state Ce-MOF as an oxidase mimetic for the colorimetric detection of biothiols. Chem. Comm. 2015;51:4635–4638.
https://doi.org/10.1039/c4cc10346g
59. Khan U, lqbal N, Tayyaba Noor, Ahmad R, Ahmad A. Cerium based metal organic framework derived composite with reduced graphene oxide as efficient supercapacitor electrode. J. Energy Storage. 2021;41:102999.
https://doi.org/10.1016/j.est.2021.102999
60. Çiplak Z, Yildiz N, Çalimli A. Investigation of Graphene/Ag Nanocomposites Synthesis Parameters for Two Different Synthesis Methods. Fuller. Nanotub. Carbon nanostructures. 2014;23(4)361–370.
https://doi.org/10.1080/1536383X.2014.894025
61. Luo S, Xu X, Zhou G, Liu C, Tang Y, Liu Y. Amino siloxane oligomer-linked graphene oxide as an efficient adsorbent for removal of Pb (II) from wastewater. J. Hazard. Mater. 2014;274:145–55.
https://doi.org/10.1016/j.jhazmat.2014.03.062
62. Liu M, Li S, Tang N, Wang Y, Yang X, Wang S. Highly efficient capture of phosphate from water via cerium-doped metal-organic frameworks. J. Clean. Prod. 2020;265:121782.
https://doi.org/10.1016/j.jclepro.2020.121782
63. Min X, Wu X, Shao P, Ren Z, Ding L, Luo X. Ultra-high capacity of lanthanum-doped UiO-66 for phosphate capture: Unusual doping of lanthanum by the reduction of coordination number. Chem. Eng. J. 2019;358:321–330.
https://doi.org/10.1016/j.cej.2018.10.043
64. He J, Xu Y, Shao P, Yang L, Sun Y, Yang Y, et al. Modulation of coordinative unsaturation degree and valence state for cerium-based adsorbent to boost phosphate adsorption. Chem. Eng. J. 2020;394:124912.
https://doi.org/10.1016/j.cej.2020.124912
65. Xiao Y, Guo B, Zhang J, Hu C, Ma R, Wang D, et al. A bimetallic MOF@graphene oxide composite as an efficient bifunctional oxygen electrocatalyst for rechargeable Zn-air batteries. Dalton Trans. 2020;49(17)5730–5735.
https://doi.org/10.1039/d0dt00976h
66. Liu M, Huang Q, Li L, Zhu G, Yang X, Wang S. Cerium-doped MIL-101-NH(2)(Fe) as superior adsorbent for simultaneous capture of phosphate and As(V) from Yangzonghai coastal spring water. J. Hazard. Mater. 2022;423(Pt A)126981.
https://doi.org/10.1016/j.jhazmat.2021.126981
67. Wu S, Ge Y, Wang Y, Chen X, Li F, Xuan H, et al. Adsorption of Cr (VI) on nano Uio-66-NH2 MOFs in water. Environ. Technol. 2018;39(15)1937–1948.
https://doi.org/10.1080/09593330.2017.1344732
68. Li S, Liu M, Yin C, Chen J, Yang X, Wang S. Tuning the structure flexibility of metal-organic frameworks via adjusting precursor anionic species for selective removal of phosphorus. Process Saf. Environ. Prot. 2020;143:322–331.
https://doi.org/10.1016/j.psep.2020.05.054
69. Liu R, Chi L, Wang X, Sui Y, Xie T, Arandiyan A. Effective and selective adsorption of phosphate from aqueous solution via trivalent-metals-based amino-MIL-101 MOFs. Chem. Eng. J. 2019;357:159–168.
https://doi.org/10.1016/j.cej.2018.09.122
70. Cheng Y, Zhang J. Facile design of UiO-66-NH2@La(OH)3 composite with enhanced efficiency for phosphate removal. J. Environ. Chem. Eng. 2021;9(1)104632.
https://doi.org/10.1016/j.jece.2020.104632
71. Li S, Lei T, Jiang F, Liu M, Wang Y, Wang S, et al. Tuning the morphology and adsorption capacity of Al-MIL-101 analogues with Fe3+ for phosphorus removal from water. J. Colloid Interface Sci. 2020;560:321–329.
https://doi.org/10.1016/j.jece.2020.104632
72. Shams M, Dehghani M, Nabizadeh R, Mesdaghinia A, Alimohammadi M, Najafpoor A. Adsorption of phosphorus from aqueous solution by cubic zeolitic imidazolate framework-8: Modeling, mechanical agitation versus sonication. J. Mol. Liq. 2016;224:151–157.
https://doi.org/10.1016/j.molliq.2016.09.059
73. Liu H, Zhong Hua L, Li X, Wang R. Effective adsorption of phosphate from aqueous solution by La-based metal–organic frameworks. RSC Adv. 2016;6(107)105282–105287.
https://doi.org/10.1039/C6RA24568D
74. Lu B, Wang S, Zhao L, Zhou D, Dong S, Wang G. Selective and superior capture of phosphate by using bimetallic bismuth-based metal-organic frameworks. Chem. Eng. J. 2021;425:131514.
https://doi.org/10.1016/j.cej.2021.131514
75. Yang Z, Zhu T, Xiong M, Sun A, Xu Y, Wu Y, et al. Tuning adsorption capacity of metal–organic frameworks with Al3+ for phosphorus removal: Kinetics, isotherm and regeneration. Inorg. Chem. Commun. 2021;132:108804.
https://doi.org/10.1016/j.inoche.2021.108804
76. Li S, Zhang Q, Yin C, Chen J, Yang X, Wang S. Tuning microscopic structure of Al-based metal-organic frameworks by changing organic linkers for efficient phosphorus removal. J. Clean. Prod. 2021;292:125998.
https://doi.org/10.1016/j.jclepro.2021.125998
77. Eltaweil A, lbrahimm K, El-Monaem E, El-Subruiti G, Omer A. Phosphate removal by Lanthanum-doped aminated graphene oxide@aminated chitosan microspheres: Insights into the adsorption mechanism. J. Clean. Prod. 2022;385:135640.
https://doi.org/10.1016/j.jclepro.2022.135640
78. Vicente-Martinez Y, Caravaca M, Soto-Meca A, Francisco-Ortíz Ó, Gimeno F. Graphene oxide and graphene oxide functionalized with silver nanoparticles as adsorbents of phosphates in waters. A comparative study. Sci. Total Environ. 2020;709:136111.
https://doi.org/10.1016/j.scitotenv.2019.136111
79. Mazloomi S, Yousefi M, Nourmoradi H, Shams M. Evaluation of phosphate removal from aqueous solution using metal organic framework; isotherm, kinetic and thermodynamic study. J. Environ. Health Sci. Eng. 2019;17(1)209–218.
https://doi.org/10.1007/s40201-019-00341-6
80. Bai L, Yuan L, Ji Y, Yan H. Effective Removal of Phosphate from Aqueous by Graphene Oxide Decorated with α-Fe2O3: Kinetic, Isotherm, Thermodynamic and Mechanism Study. Arab. J. Sci. Eng. 2018;43(7)3611–3620.
https://doi.org/10.1007/s42452-020-3074-8
81. Aswin Kumar I, Naushad M, Viswanathan N. Microfabrication of Triazine Functionalized Graphene Oxide Anchored Alginate Bead System for Effective Nutrients Removal. J. Chem. Eng. Data. 2020;65(5)2712–2724.
https://doi.org/10.1021/acs.jced.0c00066
82. Wang H, Zhang P, Liu J. Triethylene Tetramine Functionalized Magnetic Graphene Oxide Chitosan Composite with Superior Capacity for the Removal of Phosphate. J. Chem. Eng. Data. 2017;62(10)3341–3352.
https://doi.org/10.1021/acs.jced.7b00417
83. El-Maghrabi N, Fawzy M, Mahmoud A. Efficient Removal of Phosphate from Wastewater by a Novel Phyto-Graphene Composite Derived from Palm Byproducts. ACS Omega. 2022;7(49)45386–45402.
https://doi.org/10.1021/acsomega.2c05985
84. Lai Y, Huang Y, Chen C, Lin Y, Jeng H, Chang M, et al. Green Treatment of Phosphate from Wastewater Using a Porous Bio-Templated Graphene Oxide/MgMn-Layered Double Hydroxide Composite. iScience. 2020;23(5)101065.
https://doi.org/10.1016/j.isci.2020.101065
Fig. 1SEM images of Ce-MOF (a), Ce-MOF/GO with 1%, 2%, 5% 10%, and 15% GO (b–f)+10ml HAc, respectively. Ce-MOF/GO-2% (g–j) with 15, 20, 25 and 30 ml HAc respectively. 
Fig. 3FT-IR spectra images of the samples, GO(a); Ce-MOF(b); Ce-MOF/GO-2% before and after phosphate adsorption (c, d) 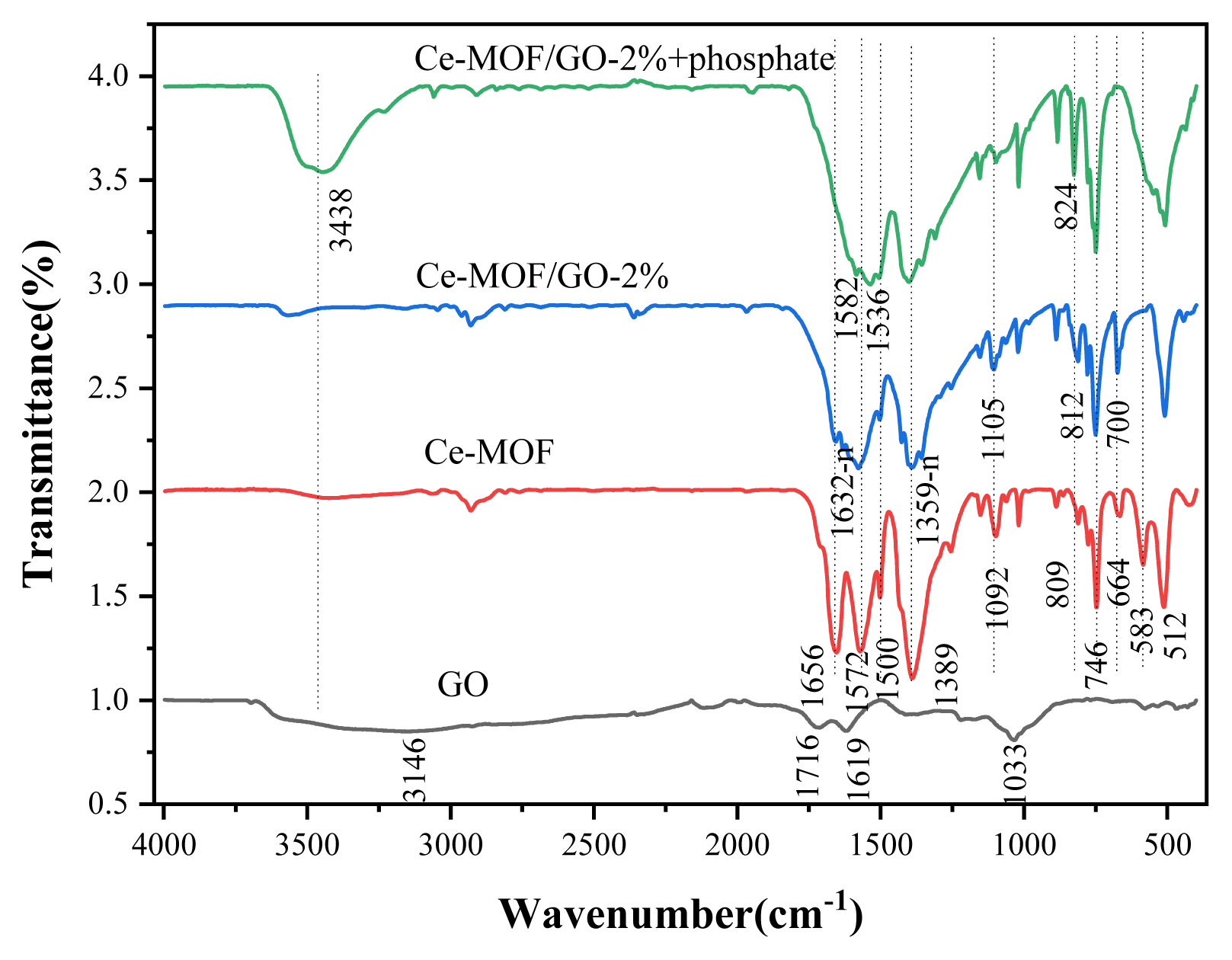
|
|
|||||||||||||||||||||||||||||||||||