AbstractDesiccated coconut waste (DCW) is an agricultural waste that originates from the coconut milk processing industry. In this study, it was utilized for the removal of copper (Cu(II)) and nickel (Ni(II)) via adsorption process. The physicochemical characterization of the DCW adsorbent shows that the adsorbent have a surface area of 6.63 m2/g, have high elemental carbon content and existences of important functional groups on its surface. The adsorptive capability of DCW adsorbent in removing the heavy metal were conducted in batch studies. DCW adsorbent performed highest Ni(II) and Cu(II) adsorption capacity at pH 6, where equilibrium is achieved at 450 minutes. The kinetic analysis showed the adsorption agreed with pseudo-second order kinetic model, indicating the Cu(II) and Ni(II) adsorption were a chemical adsorption, limited by the film diffusion. The DCW adsorbent still retained its effective adsorption capacity after 2 adsorption-desorption cycles, which is one of the excellent criteria of a good adsorbent for an adsorption process.
1. IntroductionIn nature, copper Cu(II) and nickel Ni(II) are among the heavy metals that can be found in magmatic rocks, metamorphic rocks, ocean sediments, and groundwater [1]. These heavy metals are also found in environments where they are released from various industrial activities, such as pharmaceutical industry [2], paint processing industry [3], rubber and leather production industry [4], battery manufacturing [5, 6], paper industry [7], electroplating industry [8] and petroleum refinery industry [9]. Exposure to these heavy metals can cause detrimental problems to human health and also the environment as these heavy metals are not biodegradable and highly persistent [10–12]. Copper exposure can cause insomnia problem and also Wilson’s disease which damages the brains and liver [13]. On the other hand, exposure to nickel cause severe coughing, skin problem or dermatitis, cancer of lung, bone and noses and also encephalopathy [10, 14, 15].
The maximum permissible limit for Cu(II) and Ni(II) limit regulated by the Environmental Protection Agency is approximately 1.3 mg/L and 0.1 mg/L, respectively [16]. High concentration of these heavy metals in the environment has led to the study and development of various heavy metal removal techniques, such as coagulation and flocculation [17], reverse osmosis [18], electrochemical treatment [19], ion exchange [20], adsorption [21], ultrafiltration [22] and chemical precipitation [23]. However, some of these methods are not suitable because the process can be expensive, low removal rate of heavy metals and possible production of harmful transformational byproducts [24].
Among the removal technologies, adsorption prevails as the most suitable method to be used in removing heavy metals [25]. Adsorption process is widely employed for this purpose due to its simplicity and flexibility, relatively low cost as compared to other methods, no possible production of harmful byproducts, and high removal and binding capacity with heavy metals [18, 20]. Typically, the application of adsorption process to remove Cu(II) and Ni(II) adsorbents, such as activated carbon [26, 27], silica [28], bentonite [29], and zeolite [30] were used. However, these conventional adsorbents are very expensive and complex to prepare, and thus a cheaper and more efficient adsorbent alternatives are required for this process [31].
An adsorbent can be considered cheap depending on the characteristic of the raw materials used, such as abundant availability, a by-product of another industry, and require only minimal processing before it can be utilized as an adsorbent [32]. Recently, agricultural wastes (AWs) are the potential precursors that have gained interest in research. Many studies reported that with only minimal processing methods or treatment processes, AWs can be efficiently used as precursors of adsorbent in removing heavy metals. In applications for Cu(II) and Ni(II) removal, tomato waste [33], sesame straw [34], rice straw [35] marine algae [36], and pomegranate peel [37] have been utilized and proven good potential and low-cost adsorbents.
Besides that, utilization of coconut waste such as coconut pith [38], coconut shells [39], coconut leaves [40] and coconut fiber [41] received much attention for the application of heavy metal removal. However, to the best of the researchers’ knowledge, only a few studies are available on the utilization of desiccated coconut waste (DCW) as an adsorbent precursor for heavy metal adsorption. Therefore, the objectives of this study is to investigate feasibilities of DCW as adsorbent precursor for removal of Cu(II) and Ni(II). In addition, as to evaluate the adsorptive capabilities of the DCW adsorbent, studies using parameters such as initial solution pH and contact time were employed. The experimental data obtained were further analyzed to study the Cu(II) and Ni(II) adsorption kinetics as to investigates its rate of heavy metal adsorption as well as its mechanism.
2. Materials and Methods2.1. Materials and ChemicalsThe desiccated coconut waste (DCW) was obtained from a coconut milk processing market in Perak, Malaysia. Sodium hydroxide (NaOH, 99.99%), nitric acid (HNO3, 99.99%), copper nitrate trihydrate (Cu(NO3)2.3H2O, 99.50%) and nickel nitrate hexahydrate (Ni(NO3)2.6H2O, 99.50%) were purchased from Merck (Germany). Distilled water that was used for preparing and diluting all solutions was obtained from the laboratory water distiller (Divine Pure Water Drinking System).
2.2. Preparation of AdsorbentsIn the coconut milk processing industry, the coconut inner flesh is grated, and undergoes a moisture extraction process to produce coconut milk with DCW as the byproduct. The DCW obtained was washed with distilled water for a few times and sun dried. Then, it was ground into fine particles (150 μm – 250 μm), washed with distilled water, and oven dried. The dried DCW sample was then stored in a laboratory desiccator before being used in further experiments.
2.3. Characterization of AdsorbentsSurface area and porosity of the DCW adsorbent were measured using Surface Area and Porosimetry (SAP) analyzer (Micromeritics ASAP 2020, USA). Elemental carbon, hydrogen, nitrogen and sulfur composition of the DCW adsorbent were ascertain by CHNS analyzer (Elementar Variomicro Cube, UK). Fourier-transform Infrared spectroscopy, FTIR (Pelkin Elmer, Spectrum One, USA) were used to identify existence of functional group on the DCW surface. The surface morphology of the DCW adsorbent were observed using the Scanning Electron Microscopy, SEM (Zeiss EVO LS15, Canada).
2.4. General Experimental MethodologyThe Cu(II) and Ni(II) removal from aqueous phase was conducted by using monometal batch adsorption process. The desired concentrations of Cu(NO3)2 and Ni(NO3)2 solutions were prepared by diluting the stock solution of 1,000 mmol/L. 25 mL of the heavy metal solution was prepared in a 125 mL Erlenmeyer flask and the pH was regulated by using HNO3 and NaOH as they provide better control on regulating the pH of the solution. The adsorption process of the heavy metal ions was instigated by mixing the solution with 25 mg of DCW adsorbent. Then, the mixture was agitated on a mechanical agitator of 200 rpm at 25 ± 1.0°C. The adsorption process was carried out for approximately 24 h, which was an ample time to ensure that adsorption equilibrium was achieved. The mixture was then filtered by using a filter syringe and the filtrate was collected and analyzed by using Atomic Absorption Spectrophotometer (AAS), Shimadzu Model, Japan. The adsorption capacity, Q (mmol/g) was calculated by using Eq. (1).
Where, Ci and Cf are the initial and final heavy metal concentrations (mmol/L), respectively, V is the volume of heavy metal solution (L) and m is the mass of DCW adsorbent used (g). The experiments were repeated with different parameters: i) effect of heavy metals solution pH (pH 2 – pH 6), ii) effect of agitation time (1 min – 1,440 min).
2.5. Regeneration StudyThe spent DCW from the experiment was subjected to another of adsorption-desorption cycle of heavy metal ions. The desorption of the DCW adsorbent was carried out by oven drying the spent adsorbent at 80 ± 0.5°C for one day, followed with agitating in a volume of mass-equivalent of 0.1 M HNO3 solution. The mixture was then filtered, and oven dried again at 80 ± 0.5°C for another one day. The desorbed DCW was then subjected to another cycle of adsorption in Cu(II) and Ni(II) solutions for about one day. The adsorption capacity of the DCW adsorbent was compared to evaluate the adsorbent regenerative properties.
3. Results and Discussions3.1. Characterization of DCW AdsorbentThe DCW adsorbent have a specific surface area of 6.63 m2/g. The obtained surface area was comparable to a similar adsorbent utilized in a Hg(II) adsorption study where the recorded specific surface area was 9.67 m2/g [42]. The SAP analysis shows that the adsorbent has a pore size of 4.64 nm with pore volume recorded at 77.03 × 10−4 cm3/g. The elemental composition of the DCW adsorbent are as follows: C (48.33%), H (7.46%), N (1.20 %), S (0.21%) and O (42.82%). Fig. 1 represented the surface morphology of the DCW adsorbent at a magnification of ×300 and ×500. The imaging shows that the surface of the adsorbent have a smooth appearance with minimal pore formation that are irregular in shapes and sizes. The FT-IR spectrum of the DCW adsorbent were represented by Fig. 2. Generally, the spectrum shows the existence of several main functional groups which represents the content of cellulose, hemicellulose and lignin which are very typically found in coconut waste materials. The peaks in the wavelength region of 3,500 – 3,300 cm−1 and 3,000 – 2,800 cm−1 represent the existence of stretching hydroxyl (O-H) and stretching alkane (C-C) group. The groups are attributed to the presence of water and hemicellulose on the adsorbent surface. Peaks in 1,745 cm−1 and 1,644 cm−1 represented the stretching of C=O and stretching of C=C coming from esters and alkene, respectively. At the peaks of 1,463 cm−1 and 1,200 – 1,100 cm−1 are assigned to the bending of alkane (C-C) and stretching of secondary alcohol (C-O).
3.2. Effect of Heavy Metals Initial Solution pHThe pH condition of the solution greatly affected the adsorption process of Cu(II) and Ni(II) by the DCW adsorbent. The solution pH influenced the DCW adsorbent surface charges and the species of heavy metal ions in the solution [31, 32]. In this study, the effect of initial pH of heavy metal ion solutions on the adsorption capacity of the DCW adsorbent was tested at pH range 2 – 10. Fig. 3 shows the effect of the initial solution pH towards the adsorption capacity of DCW adsorbent. The experimental results indicated that the adsorption capacity of DCW adsorbent in removing Cu(II) and Ni(II) increased as the pH of the heavy metal solutions increased. At pH 2, the adsorption capacity of DCW towards Ni(II) and Cu(II) were low, recorded at 0.05 mmol/g and 0.01 mmol/g. This was because in strong acidic condition, H+ ions were dominant in the solution, resulting in a repulsion between H+ ions and the Cu(II) or Ni(II) ions in the solution [43]. The repulsion effect between the ions resulted in a decrease in probability of the heavy metal ions to be adsorbed onto the surface of DCW adsorbent, resulting in lower adsorption capacity with lower pH condition. As the pH of heavy metal solution increases, the adsorption capacity increases up until pH 6 where the recorded adsorption capacity towards Ni(II) and Cu(II) are 0.31 mmol/g and 0.154 mmol/g, respectively. The increase in adsorption capacity is attributed to the decrease of H+ ions in the solution, leading to less repulsion effect, and higher DCW adsorbent adsorption capacity.
However, as the solution pH is further increased, adsorption capacity of DCW adsorbent decreases for both Ni(II) and Cu(II) cases. The adsorbent have low adsorption capacity as the Ni(II) and Cu(II) existed predominantly as complex hydroxide precipitate, Ni(OH)2 [44, 45] and Cu(OH)2 [46]. In addition, the pHpzc of the DCW adsorbent is 5.44 in which at pH below and above this value, the adsorbent is negatively and positively charged [42]. Hence, based on the findings of this study, it can be concluded that the adsorption of both Cu(II) and Ni(II) by DCW adsorbent were best conducted at pH 6. Similar findings were also obtained by studies conducted on the removal of Pb(II) through adsorption by using coconut dregs [47] and coconut coir pith [38].
3.3. Effect of Contact TimeIn addition, study on the effect of agitation time on the adsorption capacity of DCW adsorbent in aqueous Cu(II) and Ni(II) solutions was conducted over a period of 24 h. Fig. 4 shows that the adsorption capacity of DCW adsorbent in removing both heavy metals increased with increase in agitation time. It was also observed that the removal of Ni(II) ion was generally higher than that of Cu(II) ion. The adsorption rate of Cu(II) and Ni(II) ions towards DCW adsorbent increased rapidly until 60 min, followed by a minimal increase in the adsorption capacity until 450 min. From the time of 450 min to 1,440 min, the adsorption trend for Cu(II) and Ni(II) showed a plateau trend, which indicated that the adsorption process of both heavy metal ions had achieved adsorption equilibrium. This result showed that the adsorption of DCW adsorbent towards Cu(II) and Ni(II) achieved equilibrium at a period of 450 min (7.5 h). A similar finding was reported by a study that utilized DCW adsorbent to remove Hg(II) from its aqueous solution [42].
3.4. Adsorption Kinetic Model StudyTo further understand the mechanism involved in the adsorption of Cu(II) and Ni(II), the obtained experimental data were further analyzed by using several adsorption kinetic models, namely pseudo-first order (PFO) model, pseudo-second order (PSO) model and Elovich kinetic model. The PFO kinetic model assumes that the adsorption process happens on site that are localized and absence of interactions between the adsorbed ions [48], which is a characteristic of the physical adsorption process. The PSO kinetic model assumes that the adsorption process is a chemical adsorption process, where the adsorbent has covalently bonded with the surface of the adsorbent. The Elovich model assumes that the adsorption process happens on localized sites with the presence of interaction between the adsorbed ions [48]. Table 1 summarizes the linearized model of the adsorption kinetic models, their respective parameters and calculated values, whereas Fig. S1 and Fig. S2 show the fitting of experimental data with the adsorption kinetic models.
Table 1 shows that the data fitted best into the PSO kinetic model. In order to justify the finding, the determination coefficient of the plots (R2) was compared. Table 1 shows that the R2 values for Cu(II) and Ni(II) adsorption process were nearer to 1 as compared to the other adsorption kinetic models. On top of that, comparisons on the obtained experimental adsorption capacity, Qexp were also done. The PSO adsorption kinetic model gave closer Qexp and Qtheory values for the Cu(II) and Ni(II) adsorption process. In addition, the fitting of the Cu(II) and Ni(II) adsorption experimental data with pseudo-second order kinetic model is well represented in Fig. S1 and Fig. S2, respectively. Fitting into the PSO model indicated that the adsorption process of Cu(II) and Ni(II) ions into the DCW adsorbent was a chemical adsorption process, whereby the ions covalently bonded with the surface of the adsorbent. DCW adsorbent had heterogeneous adsorption sites where the driving force was found to be proportional to the available fraction of the DCW active sites [42]. Therefore, the overall rate of adsorption and desorption of the active sites must be the same at equilibrium.
3.5. Diffusion KineticsOver the period of the adsorption process, the kinetics of the adsorption process was either controlled by surface reaction process or diffusion. For diffusion process, there were three main steps involved, which were bulk diffusion, film diffusion, and intraparticle diffusion [49]. Bulk diffusion is the diffusion from the surrounding adsorbate solution to the adsorbent surface. Film diffusion is the diffusion of the adsorbate across the liquid film around the adsorbent particle [50]. Intraparticle particle is the diffusion of the adsorbate from the external adsorbent surface to the internal adsorbent surface. The Weber-Morris plot was used to analyze the diffusion process and it is depicted by Eq. (2).
The adsorption capacity represents Qt (mmol/g) at given time t. The kid represents the intraparticle diffusion constant. The Weber-Morris plot was explained in three steps. In the first step, the external surface adsorption or instantaneous adsorption took place. The second step involved the gradual adsorption step, whereby the intraparticle diffusion was maintained. Lastly, the third step was the final equilibrium step, where a slower rate of adsorption occurred. The Weber-Morris plot will determine if the plot passed through the origin. Therefore, the intraparticle diffusion was the rate-limiting step of the process and vice versa. Based on Fig. S3(a), the Weber-Morris plot did not pass through the origin. Therefore, the intraparticle diffusion was not the rate-limiting step of the adsorption process. It was observed that there were two-line regions for Cu(II) and Ni(II) adsorption. These were indications that there were more than one diffusion steps involved in the adsorption of Cu(II) and Ni(II). The rate-limiting step was determined by comparing the coefficients for film diffusion and effective diffusion, whereby the lower diffusivity constant was the rate-limiting step.
To determine the film diffusion coefficient, the data were analyzed by using the Fick’s Law equation, which is given by Eq. (3).
The data were plotted by using Qt/Qe vs. t0.5, based on Equation (3), Qt/Qe represent the ratio of adsorbed metal ion at time t to the amount adsorbed at equilibrium, t represents time and RP is the radius of DCW adsorbent, which was 0.015 cm. The plot of Fick’s Law is represented by Fig. S3(b). The film diffusion constant (Df) obtained for Cu(II) and Ni(II) was 1.872 × 10−8 cm2/min and 1.160 × 10−8 cm2/min, respectively. In order to calculate the effective diffusivity constant, Deff, Boyd plot equation represented by Eq. (4) and Eq. (5) were used:
where, F = Qt/Qe. The data were plotted by using Bt vs. t, to determine the value of Deff. The coefficient of effective diffusion for Cu(II) and Ni(II) adsorption was calculated by taking into account the slope of the Boyd plot, which is represented by Eq. (6).
where, s is the slop of the Boyd plot and Rp is the radius of DCW particle, which was 0.015 cm. Based on the calculation from the Boyd’s plot represented by Fig. S4, Deff for Cu(II) adsorption was 1.819 × 10−7 cm2/min, while for Ni(II) adsorption was 2.102 × 10−7 cm2/min.
Table S1 summarizes the values obtained for Df and Deff based on the plot that was constructed. In both Cu(II) and Ni(II) adsorption process, Deff value was lower than Df value. This low coefficient value shows that film diffusion was the rate limiting step in the adsorption process of Cu(II) and Ni(II) by the DW adsorbent. Film diffusion is the diffusion steps that governed the movement of the Cu(II) and Ni(II) adsorbate during the adsorption process.
3.6. Regeneration StudiesThe spent DCW adsorbent that was used for the adsorption of Cu(II) and Ni(II) were subjected to another cycle of adsorption-desorption. The focal objective of conducting a regeneration study was to evaluate and determine whether the DCW adsorbent was feasible to be used for several adsorption process cycles. Fig. 5 shows the adsorption capacity of DCW adsorbent from Cycle 1 – 3 of the adsorption-desorption process. In Cycle 1, the adsorption capacity of DCW towards Cu(II) and Ni(II) was 0.050 mmol/g and 0.150 mmol/g, respectively. However, in Cycle 2, the adsorption capacity of DCW towards Cu(II) and Ni(II) decrease to 0.032 mmol/g and 0.090 mmol/g while in Cycle 3, 0.022 mmol/g and 0.075 mmol/g for Cu(II) and Ni(II), respectively. A decrease trend in the adsorption capacity of the DCW adsorbent towards both heavy metal ions was observed. The possible explanation for the reduction of the adsorption capacity of the DCW adsorbent is that there are possibility that the heavy metals ions are not fully desorbed during the desorption process. This leads to reduction in pores that are available for the heavy metal ions to bind in the next cycle of adsorption process. Another study reported a similar finding where the DCW adsorbent were able to be regenerated for few cycles of adsorption-desorption in which the adsorption capacity of the adsorbent was almost constant [42]. In conclusion, the DCW has excellent regenerative properties and it is a good raw material that can be used as a low-cost adsorbent in removing heavy metal ions, such as Cu(II) and Ni(II). However, after the adsorbent have reached exhaustion, the spent adsorbent can be disposed into the landfill where it can be biologically degraded or incinerated in a incinerator which will save landfill spaces.
3.7. Adsorbent Cost EvaluationCost of adsorbents has a significant impact on the capital required for operating heavy metal ions adsorption process. Commercialized adsorbents, such as activated carbon, zeolite, and bentonite are widely utilized for adsorption application. However, these adsorbents are expensive due to the complex and costly manufacturing processes. In addition, high cost is also incurred from the utilization of high energy (electricity) in manufacturing the adsorbent. Currently, the costs of commercially activated carbon, zeolite and bentonite are RM 461.00/kg, RM 1, 268.00/kg and RM 346.00/kg, respectively [51]. For industrial applications, the high cost of these commercialized adsorbents can be a financial burden for the corporation as the adsorbents are used in huge quantities.
Therefore, various studies were conducted to identify low-cost adsorbent alternative for heavy metal ions removal. In this study, desiccated coconut waste (DCW) was used as the precursor adsorbent for heavy metal ions. The DCW adsorbent is a waste-by-product originated from the coconut milk processing industry. This industry is very common in Malaysia as coconut milk is widely used in cooking among Malaysians. However, DCW has no significant commercial or industrial value [42], which lead to the disposal of DCW to the landfill, causing a significant waste management problem.
DCW that was used in this study was obtained from the local coconut-milk processing market without charge. Then the DCW underwent a simple adsorbent preparation method before it was utilized in the adsorption study. Based on the employed preparation method and the result from the regeneration study of the DCW adsorbent, the adsorbent can be categorized as a low-cost adsorbent. This is because the adsorbent only needs a simple preparation method which requires the use of minimal energy (electricity) and it can be used for several cycles of adsorption process, which are the criteria of an excellent low-cost adsorbent. The results from these experiments showed that DCW adsorbent has potential as a precursor for low-cost adsorbent.
In addition, tabulated in Table 2 are several adsorbent materials that have been utilized in studies for the adsorbent of Cu(II) and Ni(II) from aqueous solution. The adsorbent materials listed in Table 2 which include various agriculture waste such as pomegranate peel [52], rice hull [53], sawdust [53], and coconut waste e.g. coir pith [14], copra [56] and husk [57] were utilized in their raw form i.e. not subjected to chemical or physical pre-treatment. The maximum adsorption capacity of DCW adsorbent utilized in this study were 0.31 mmol/g (18.19 mg/g) for Ni(II) and 0.154 mmol/g (9.78 mg/g) for Cu(II). Through comparisons, DCW performed better Cu(II) and Ni(II) removal compared to the other listed adsorbent. This shows that DCW adsorbent not only of cheaper raw materials but also, it is able to performed high Cu(II) and Ni(II) adsorption compared to other adsorbent reported in literature.
4. ConclusionsThe present results showed that the desiccated coconut waste (DCW) adsorbent was a good adsorbent material in removing Cu(II) and Ni(II) from its aqueous solution. The adsorbent characterization shows that the adsorbent have relatively high surface area (6.63 m2/g) and porosity with minimal pore formation which are irregular in shape and size. The spectrum confirm the existence of functional groups representing the lignin, cellulose and hemicellulose in the adsorbent materials. The adsorption capacity of the DCW adsorbent was found to increase with the increase in pH where the optimum pH for Cu(II) and Ni(II) adsorption is pH 6 where the adsorption by DCW towards Cu(II) and Ni(II) reached equilibrium 450 minutes. The maximum adsorption capacity for Cu(II) and Ni(II) was 0.154 mmol/g and 0.306 mmol/g, respectively. The data were fitted into pseudo-first order, pseudo-second order and Elovich kinetic model and the analysis perform best fitting into pseudo-second order kinetic model which indicate the adsorption of Cu(II) and Ni(II) is a chemical adsorption process. The DCW adsorbent was proven to be an excellent adsorbent due to its high adsorption capacity, good regenerative properties and economical compared to existing commercialized adsorbent.
AcknowledgmentThe authors would like to thank the Department of Chemical Engineering, Faculty of Engineering, Universiti Teknologi PETRONAS (UTP), Malaysia for providing their facilities and support throughout the research project.
NotesAuthor Contributions A.R.A.R (MSc student) conducted experiments, analysis of data and write up research article. I.S (BSc student) conducted experiments, analysis of data, and write up of research article. N.S (MSc student) analysis of data and write up research article. K.J (Ph.D.) supervising experiments and methodology, review drafts. N.S (Ph.D.) review drafts. H.M (Ph.D.) review drafts References2. Antosz FJ, Xiang Y, Diaz AR, et al. The use of total reflectance X-ray fluorescence (TXRF) for the determination of metals in the pharmaceutical industry. J Pharm Biomed Anal. 2012;62:17–22.
3. Malakootian M, Nouri J, Hossaini H. Removal of heavy metals from paint industry’s wastewater using Leca as an available adsorbent. Int J Environ Sci Technol. 2009;6:183–190.
4. Lasindrang M, Suwarno H, Tandjung SD, et al. Adsorption pollution leather tanning industry wastewater by chitosan coated coconut shell active charcoal. Agric Sci Procedia. 2015;3:241–247.
5. Hegazi HA. Removal of heavy metals from wastewater using agricultural and industrial wastes as adsorbents. Hous Build Natl Res Cent J. 2013;9:276–282.
6. Mobasherpour I, Salahi E, Ebrahimi M. Removal of divalent nickel cations from aqueous solution by multi-walled carbon nano tubes: Equilibrium and kinetic processes. Res Chem Intermed. 2012;38:2205–2222.
7. Wajima T, Sugawara K. Adsorption behaviors of mercury from aqueous solution using sulfur-impregnated adsorbent developed from coal. Fuel Process Technol. 2011;92:1322–1327.
8. Igberase E, Osifo P, Ofomaja A. The adsorption of Pb, Zn, Cu, Ni, and Cd by modified ligand in a single component aqueous solution : Equilibrium, kinetic, thermodynamic, and desorption studies. 2017;Epub ahead of print 201710.1155/2017/6150209.
9. Vukelic D, Boskovic N, Agarski B, et al. Eco-design of a low-cost adsorbent produced from waste cherry kernels. J Clean Prod. 2018;174:1620–1628.
10. Sobhanardakani S. Assessment of heavy metal content of atmospheric dry deposition, a case study: Kermanshah, Iran. Biol Trace Elem Res. 2019;187:602–610.
11. Sobhanardakani S, Tayebi L, Hosseini SV. Health risk assessment of arsenic and heavy metals (Cd, Cu, Co, Pb, and Sn) through consumption of caviar of Acipenser persicus from Southern Caspian Sea. Environ Sci Pollut Res. 2018;25:2664–2671.
12. Akar S, Lorestani B, Sobhanardakani S, et al. Surveying the efficiency of Platanus orientalis bark as biosorbent for Ni and Cr(VI) removal from plating wastewater as a real sample. Environ Monit Assess. 191. Epub ahead of print 201910.1007/s10661-019-7479-z.
13. World Health Organization. Trace elements in human nutrition and health. Geneva: 1996.
14. Swarnalatha K, Ayoob S. Adsorption studies on coir pith for heavy metal removal. Int J Sustain Eng. 2016;9:259–265.
15. Sobhanardakani S, Zandipak R. 2,4-Dinitrophenylhydrazine functionalized sodium dodecyl sulfate-coated magnetite nanoparticles for effective removal of Cd(II) and Ni(II) ions from water samples. Environ Monit Assess. 187. Epub ahead of print 201510.1007/s10661-015-4635-y.
16. Inyang MI, Gao B, Yao Y, et al. A review of biochar as a low-cost adsorbent for aqueous heavy metal removal. Crit Rev Environ Sci Technol. 2016;46:406–433.
17. Lakherwal D. Adsorption of heavy metals : A review. 2014;4:41–48.
18. Linnan Z, Yanjun WU, Xiaoyan QU, et al. Mechanism of combination membrane and electro-winning process on treatment and remediation of Cu2+ polluted water body. J Environ Sci. 2009;21:764–769.
19. Tran T, Chiu K, Lin C, et al. Electrochemical treatment of wastewater : Selectivity of the heavy metals removal process. Int J Hydrogen Energy. 2017;42:27741–27748.
20. Azizi S, Kamika I, Tekere M. Evaluation of heavy metal removal from wastewater in a modified packed bed biofilm reactor. PLoS One. 2016;11:1–13.
21. Fu F, Wang Q. Removal of heavy metal ions from wastewaters: A review. J Environ Manage. 2011;92:407–418.
22. Barakat MA, Schmidt E. Polymer-enhanced ultrafiltration process for heavy metals removal from industrial wastewater. DES. 2010;256:90–93.
23. Matlock MM, Howerton BS, Atwood DA. Chemical precipitation of heavy metals from acid mine drainage. Water Res. 2002;36:4757–4764.
24. Ihsanullah , Abbas A, Al-Amer AM, et al. Heavy metal removal from aqueous solution by advanced carbon nanotubes: Critical review of adsorption applications. Sep Purif Technol. 2016;157:141–161.
25. Dixit R, Wasiullah , Malaviya D, et al. Bioremediation of heavy metals from soil and aquatic environment: An overview of principles and criteria of fundamental processes. Sustain. 2015;7:2189–2212.
26. Yu F, Li Y, Han S, et al. Adsorptive removal of antibiotics from aqueous solution using carbon materials. Chemosphere. 2016;153:365–385.
27. Xu J, Cao Z, Zhang Y, et al. A review of functionalized carbon nanotubes and graphene for heavy metal adsorption from water : Preparation, application, and mechanism. Chemosphere. 2018;195:351–364.
28. Sheet I, Kabbani A, Holail H. Removal of heavy metals using nanostructured graphite oxide, silica nanoparticles and silica/graphite oxide composite. Energy Procedia. 2014;50:130–138.
29. Tohdee K, Kaewsichan L. Enhancement of adsorption efficiency of heavy metal Cu (II) and Zn (II) onto cationic surfactant modified bentonite. J Environ Chem Eng. 2018;6:2821–2828.
30. Yurekli Y. Removal of heavy metals in wastewater by using zeolite nano-particles impregnated polysulfone membranes. J Hazard Mater. 2016;309:53–64.
31. Bhatnagar A, Vilar VJP, Botelho CMS, et al. Coconut-based biosorbents for water treatment-A review of the recent literature. Adv Colloid Interface Sci. 2010;160:1–15.
32. Khoo E, Ong S. Utilization of agricultural waste as a biosorbent for the removal of dyes from aqueous solution. In : 1st World Sustain Forum; 2011; p. 1–9.
33. Yargiç AS, Yarbay Şahin RZ, Özbay N, et al. Assessment of toxic copper(II) biosorption from aqueous solution by chemically-treated tomato waste. J Clean Prod. 2015;88:152–159.
34. Park JH, Ok YS, Kim SH, et al. Competitive adsorption of heavy metals onto sesame straw biochar in aqueous solutions. Chemosphere. 2016;142:77–83.
35. Wu Y, Fan Y, Zhang M, et al. Functionalized agricultural biomass as a low-cost adsorbent: Utilization of rice straw incorporated with amine groups for the adsorption of Cr(VI) and Ni(II) from single and binary systems. Biochem Eng J. 2016;105:27–35.
36. Barquilha CER, Cossich ES, Tavares CRG, et al. Biosorption of nickel(II) and copper(II) ions in batch and fixed-bed columns by free and immobilized marine algae Sargassum sp. J Clean Prod. 2017;150:58–64.
37. Ben-Ali S, Jaouali I, Souissi-Najar S, et al. Characterization and adsorption capacity of raw pomegranate peel biosorbent for copper removal. J Clean Prod. 2017;142:3809–3821.
38. Ratan S, Singh I, Sarkar J, et al. The removal of nickel from waste water by modified coconut coir pith. Chem Sci J. 2016;7:3–8.
39. Sousa FW, Oliveira AG, Ribeiro JP, et al. Green coconut shells applied as adsorbent for removal of toxic metal ions using fixed-bed column technology. J Environ Manage. 2010;91:1634–1640.
40. Gowda R, Nataraj AG, Rao NM. Coconut leaves as a low cost adsorbent for the removal of nickel from electroplating effluents. Int J Sci Eng Res. 2012;2:1–5.
41. Saman N, Abdul Aziz A, Johari K, et al. Adsorptive efficacy analysis of lignocellulosic waste carbonaceous adsorbents toward different mercury species. Process Saf Environ Prot. 2015;96:33–42.
42. Johari K, Saman N, Song ST, et al. Utilization of coconut milk processing waste as a low-cost mercury sorbent. Ind Eng Chem Res. 2013;52:15648–15657.
43. Basu M, Guha AK, Ray L. Biosorptive removal of lead by lentil husk. J Environ Chem Eng. 2015;3:1088–1095.
44. Srivastava NK, Majumder CB. Novel biofiltration methods for the treatment of heavy metals from industrial wastewater. J Hazard Mater. 2008;151:1–8.
45. Kabra K, Chaudhary R, Sawhney RL. Effect of pH on solar photocatalytic reduction and deposition of Cu(II), Ni(II), Pb(II) and Zn(II): Speciation modeling and reaction kinetics. J Hazard Mater. 2007;149:680–685.
46. Liu H, Feng S, Zhang N, et al. Removal of Cu(II) ions from aqueous solution by activated carbon impregnated with humic acid. Front Environ Sci Eng. 2014;8:329–336.
47. Putra WP, Kamari A, Najiah S, et al. Biosorption of Cu(II), Pb(II) and Zn(II) ions from aqueous solutions using selected waste materials: Adsorption and characterisation studiesw. J Encapsulation Adsorpt Sci. 2014;4:25–35.
48. Largitte L, Pasquier R. A review of the kinetics adsorption models and their application to the adsorption of lead by an activated carbon. Chem Eng Res Des. 2016;109:495–504.
49. Saman N, Rashid MU, Lye JWP, et al. Recovery of Au(III) from an aqueous solution by aminopropyltriethoxysilane-functionalized lignocellulosic based adsorbents. React Funct Polym. 2018;123:106–114.
50. Saman N, Johari K, Song ST, et al. High removal efficacy of Hg(II) and MeHg(II) ions from aqueous solution by organo-alkoxysilane-grafted lignocellulosic waste biomass. Chemosphere. 2017;171:19–30.
51. Sigma Aldrich. Activated charcoal.
https://www.sigmaaldrich.com/catalog/product/sigald/292591?lang=en®ion=MY
accessed 15 January 2019
52. El-Ashtoukhy ESZ, Amin NK, Abdelwahab O. Removal of lead (II) and copper (II) from aqueous solution using pomegranate peel as a new adsorbent. Desalination. 2008;223:162–173.
53. Asadi F, Shariatmadari H, Mirghaffari N. Modification of rice hull and sawdust sorptive characteristics for remove heavy metals from synthetic solutions and wastewater. J Hazard Mater. 2008;154:451–458.
54. Kariuki Z, Kiptoo J, Onyancha D. Biosorption studies of lead and copper using rogers mushroom biomass ‘Lepiota hystrix’. South African J Chem Eng. 2017;23:62–70.
55. Shroff KA, Vaidya VK. Kinetics and equilibrium studies on biosorption of nickel from aqueous solution by dead fungal biomass of Mucor hiemalis. Chem Eng J. 2011;171:1234–1245.
Fig. 3Effect of initial solution pH on Cu(II) and Ni(II) adsorption. (Experimental conditions: initial concentration = 0.3 mmol/L, DCW dosage = 1 mg/mL, contact time = 24 h, temperature = 25°C) 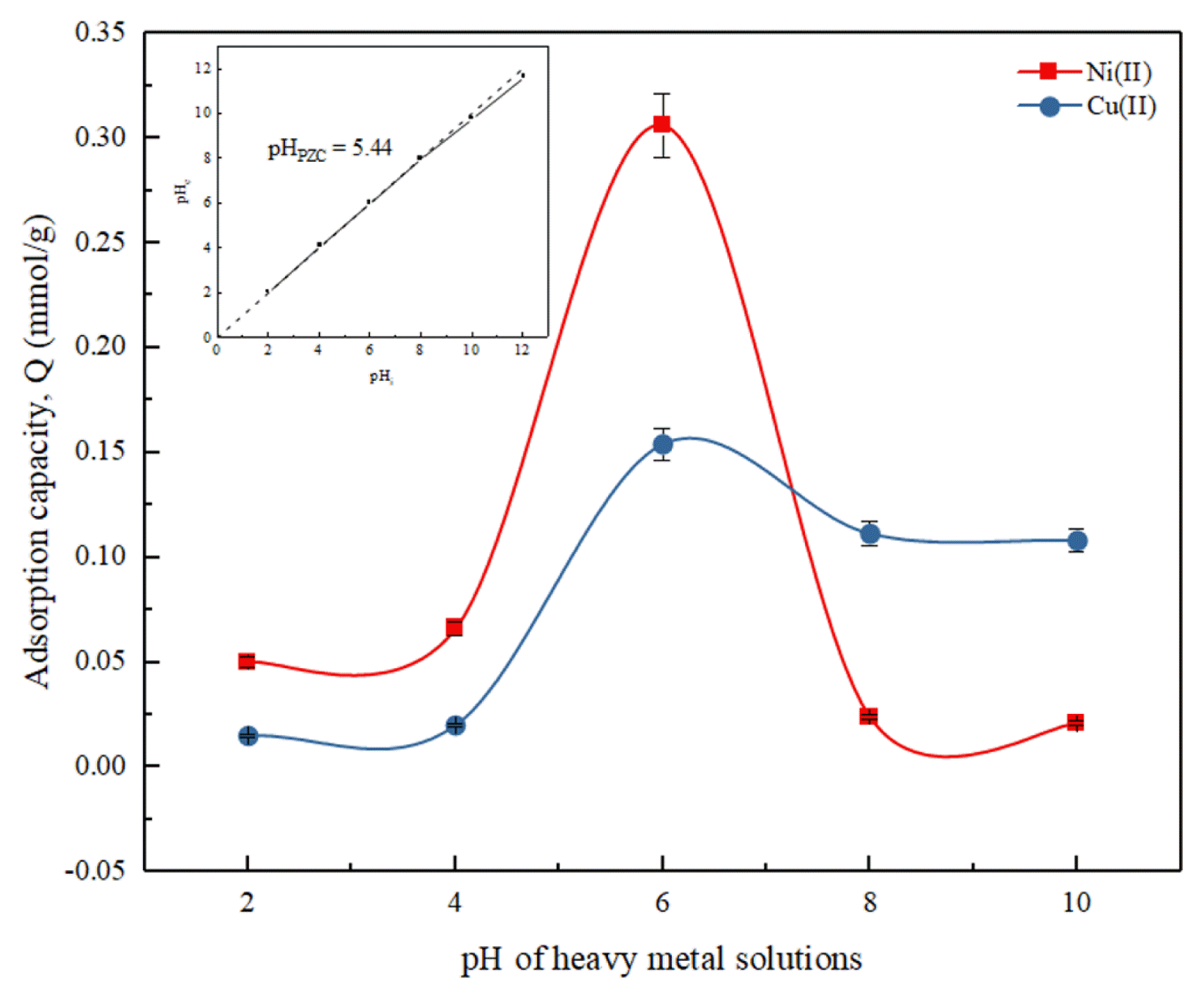
Fig. 4Effect of contact time on Cu(II) and Ni(II) adsorption. (Experimental condition: initial concentration = 0.3 mmol/L, DCW dosage = 1 mg/mL, solution pH = pH 6, temperature = 25°C) 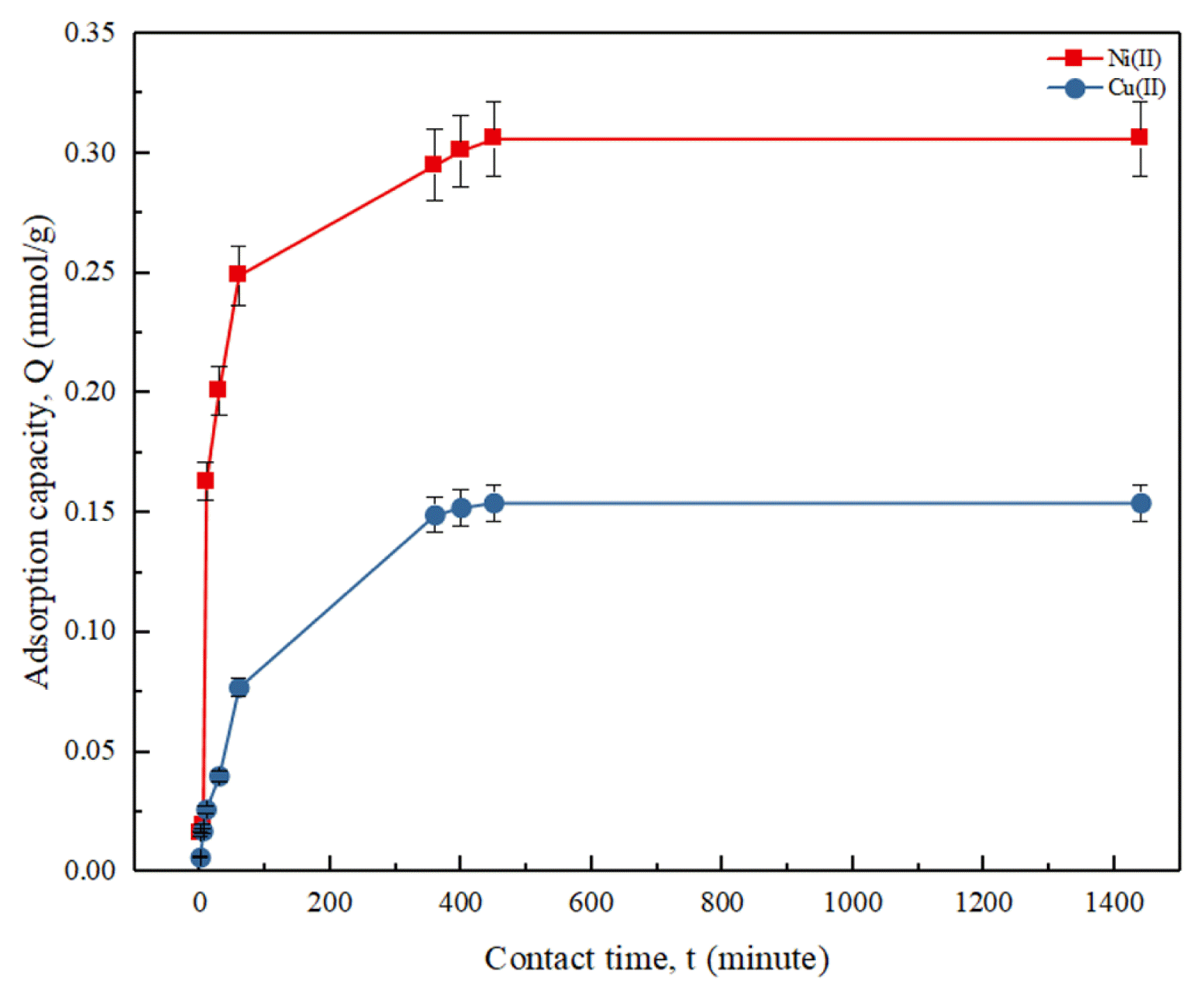
Fig. 5Regeneration of DCW adsorbent using 0.1 M HNO3. (Experimental conditions: initial concentration: 0.3 mmol/L, DCW dosage: 1 mg/mL, solution pH= pH 6, temperature = 25°C) 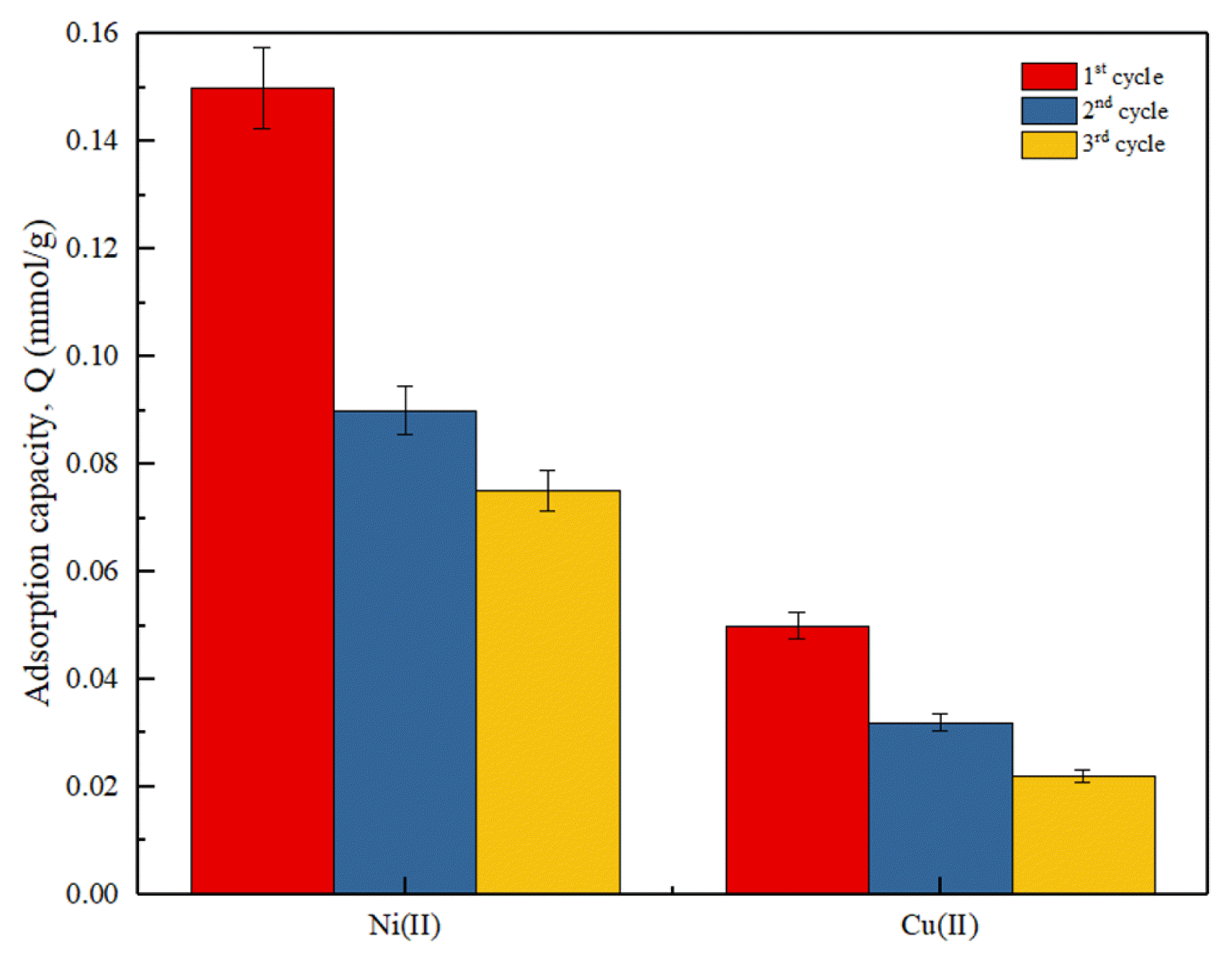
Table 1The Adsorption Equilibrium and Constants for Adsorption of Cu(II) and Ni(II) onto DCW Adsorbent Table 2List of Agriculture Waste Materials Utilized for Cu(II) and Ni(II) Removal
|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||