AbstractThe mass production, continual usage, and improper disposal of plastic products have resulted in significant environmental pollution. The larger plastic polymers gradually break down into smaller particles called microplastics (<5 mm). Existing studies on the occurrence and ecological impact of microplastics have focused on the aquatic ecosystems, with very little attention given to the soil environment. The soil represents a natural sink for microplastics from sources such as sewage sludge, landfills, plastic mulch from agricultural activities, fertilizers, and municipal wastewater effluent. The current study, therefore, provides an overview of existing knowledge on soil microplastic pollution focusing on the impact of microplastics on soil microbial community and microbial degradation of microplastics in soil to systematically identify knowledge gaps to be filled with further research. Future research challenges to be addressed include detailed monitoring of the sources and distribution of microplastics in soil under different land uses, exploring diverse microorganisms in their natural environments for their microplastic biodegradation potential using cultivation-dependent and independent approaches, understanding the mechanism of ecological impacts of microplastics and contributions of microplastic additives, degradation products, and other adsorbed environmental pollutants on soil microbial community.
Graphical Abstract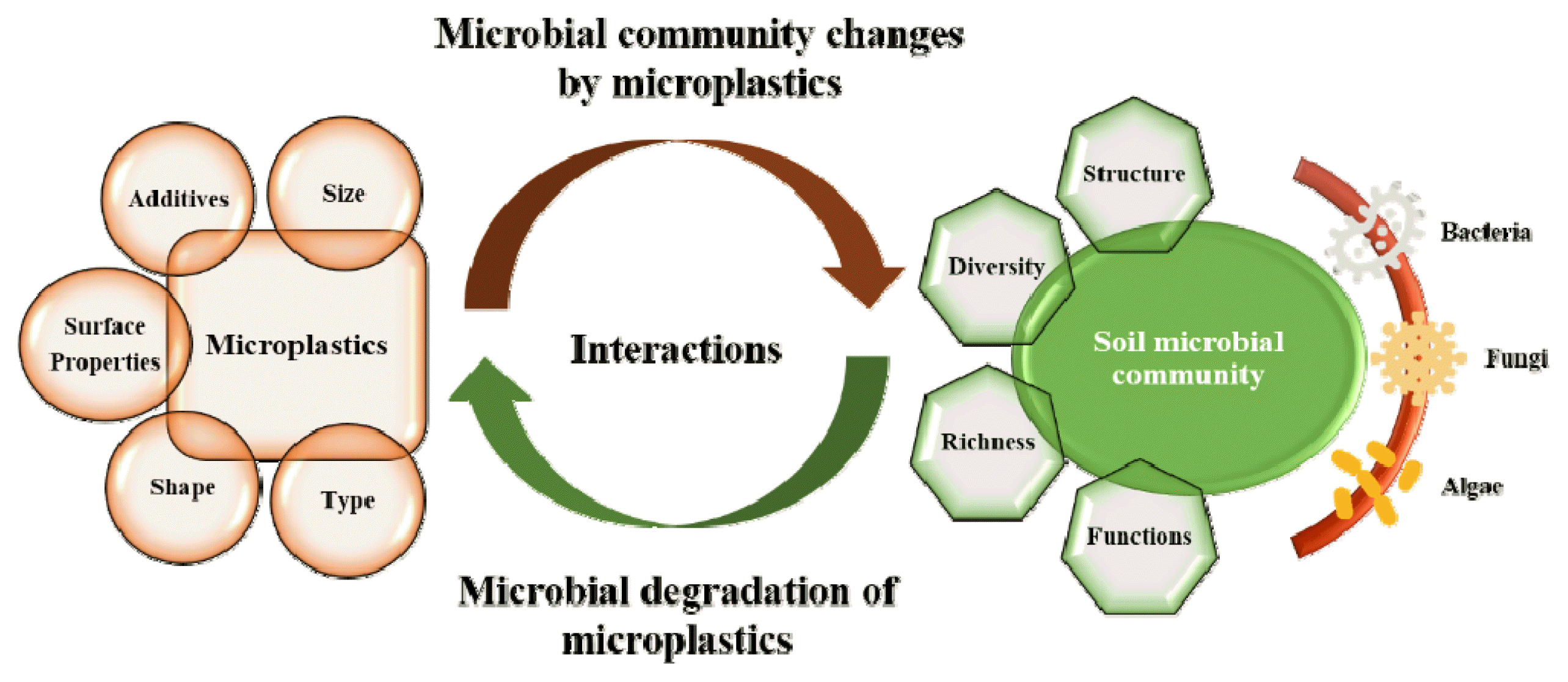
1. IntroductionDue to their durability, lightweight, and cost-effectiveness, plastics are widely and frequently used synthetic materials [1]. Almost every aspect of human activities has found synthetic plastics useful, and it can be concluded that, in recent years, plastic materials have grown to become indispensable, replacing other materials such as wood, metals, and glass in various applications. Different synthetic plastics have been used in the production of various materials such as polyethylene (PE) for shopping bags, plastic bottles, and toiletry bottles; polystyrene (PS) for food containers, packaging foams, and disposable cups; polyurethane (PU) for sealants, adhesives, and extrusion and injection-molded parts; polyvinyl chloride (PVC) for plumbing pipes and guttering, window frames, and shower curtains; polypropylene (PP) for microwavable containers, drinking straws, and plastic pressure pipe systems; polyethylene terephthalate (PET) for plastic films, engineering components, and carbonated drinks bottles; nylon for gears, bushings, and plastic bearings; polycarbonate for automobile components, plastic lenses, riot shields; and polytetrafluoroethylene (PTFE) for hookup wire, coaxial cables, and gaskets [2].
Since 1950, the production of plastics has rapidly increased on a global scale due to their extensive use. Between 1950 and 2019, the annual global production increased dramatically from 2 million tons to 368 million tons, with about 40% going toward single-use applications [3]. Virgin plastics produced are largely single-use convenience products that are discarded within a short period after use, and they result in a rapid and massive accumulation in the natural environments [4]. It is predicted that, by 2050, up to 26 billion tons of plastic waste will be generated and more than 50% will be discarded into landfills and eventually enter natural environments like oceans, lakes, rivers, cultivated lands, etc., thereby resulting in serious environmental pollution [5]. Undoubtedly, as a result of its wide distribution across the world [6–8], plastic pollution has become a global scale issue, and the fate of plastic waste in the environment is now a subject of increasing concern.
The term “microplastic (MP)” is proposed to be first used by the African scientists named Ryan and Moloney in 1990 in their research article titled “Plastic and other artefacts on South African beaches: temporal trends in abundance and composition” [9]. This term, however, became widely recognized among researchers after a report published by Thompson et al. [10] who examined the abundance of microplastics (MPs) in the sediment of beaches, estuarine, and subtidal around Plymouth in the United Kingdom. Since then, the term “microplastics” has been generally used to describe small particles of plastics [11]. The defining characteristics of MPs are still under debate [12], but it is agreed by most researchers that plastic particles ranging between 100 nm to <5 mm in size are regarded as MPs.
In the past years, most studies on MP pollution have focused on the marine environment, with very little attention drawn to the terrestrial environment, although between 2018 and 2021, more researchers have studied the effect of MPs on the soil ecosystem [13, 14]. Due to the critical role that the soil plays in regulating nutrient cycling, maintaining the biodiversity of organisms, and providing food [15], it is necessary to evaluate the ecological effect of MP pollution in the terrestrial environment, especially the soil [14, 16]. After the findings of Rillig [17] on the detrimental effects of MPs in soil and terrestrial ecosystems, more research interests have been drawn to plastic pollution in soil. Studies have shown that the soil environment receives much more plastic waste than the marine environment [18] and researchers have warned about the ecological effects of plastics and small plastic particles in soil and terrestrial environments [17, 19]. The main effects of MPs in soil are likely to occur at the interface between soil particles and plastic polymers (i.e., plastisphere). Similar to interactions in the plant rhizosphere, the physicochemical properties of MPs could stimulate the diversity and activities of soil microbial communities at the soil-plastic interface. These interactions can favor the proliferation and activities of specific microbial taxa, and lead to the formation of microbial hotspots in the soil [20]. With the increasing rate of MP contamination in most agricultural soils [21], the specific microbial niches at the soil-MP interface (i.e., microplastisphere) are of ecological importance. However, the effect of MPs on soil microbial communities and its corresponding impact on biodegradation in soil remains largely unclear.
Because of their physicochemical properties, which enhance their resistance to degradation, plastics can accumulate in natural ecosystems. High crystallinity, high molecular weight, and absence of functional groups that favor oxidative reaction processes are contributing factors to the non-biodegradability of plastics [22]. Current methods for managing plastic waste including incineration, landfilling, and recycling have associated demerits that could lead to further environmental issues. For instance, more toxic and volatile waste materials such as nitrogen oxides, furans, heavy metals, sulfides, and dioxins, which are considered to have potential carcinogenic effects, are produced when different synthetic plastics are incinerated [23]. Also, cost-ineffectiveness [24] and down-cycling [25] are undesirable consequences associated with recycling synthetic plastics. Landfilling, a widely used method for plastic waste disposal, especially in developing countries, results in a huge accumulation of plastic waste occupying a vast amount of land. Due to these, efforts are recently being made by researchers to explore other environmentally friendly and sustainable approaches to manage plastic waste and decrease environmental pollution caused by plastic waste. Microorganisms have been explored and reported as promising alternative for the degradation MPs. A number of research reports have identified different microbial species capable of degrading MPs. Although most reports available focused mainly on the biodegradation of a single kind of plastic such as PET [26], PE [27], PU [28], PS [29], and PP [30]. More recently, insects in their larva form including waxworm, mealworm, and superworm have also demonstrated the ability to eat, degrade, and mineralize various MPs, though not without the support of the microorganisms residing in their guts [31]. Research ideas focusing on the biodegradation of all main types of MPs are necessary as well as the biological upcycling of plastic waste [32].
Therefore, the aim of this study is to review previous literatures on the impact of MPs on soil microbial communities and biodegradation of MPs in soil. Also, extensively described are the current knowledge and knowledge gaps regarding the interactions between MPs and microbial communities in soil. Additionally, an effort has been made to outline the MP-related factors that affect the structural composition, diversity, and functionality of soil microbial communities. Finally, the challenges that need to be addressed in order to fill the knowledge gaps are itemized, along with future research prospects.
2. Sources of MP Pollution in the Natural EnvironmentBased on source points and their formation pathway, MPs in the environment can be categorized into two, namely; primary MPs and secondary MPs [8]. Primary MPs are synthesized from manufacturing activities and designed for commercial purposes, such as manufacture of personal care and cosmetic products (e.g., microbeads in hand and facial cleansers, toothpaste, shower gel), appliance manufacturing, industrial abrasives (e.g., air blasting), and textile fibers in clothing (e.g., acrylic fibers) [8, 33]. Primary MPs can be produced from the air-blasting industry as a result of the abrasion of materials during the process of preproduction of resin pellets [34]. Production of secondary MPs, on the other hand, is from physical (e.g., wave strike, abrasion, and water disturbance), chemical (e.g., UV radiation, the freeze-thaw cycle), and biological (e.g., biodegradation) activities, which involve the degradation and fragmentation of larger plastic materials into micro-sized particles [34].
The sources of MPs in the soil include vinyl mulch commonly used in agricultural activities [35], domestic sewage water containing MP beads from biosolids, personal care products, and fibers from clothing materials [36], landfills from industrial and urban centers [37], fertilizers [18], illegal waste dumping, irrigation with wastewater, littering roads and lake water flooding [38], atmospheric MP particles transported over long distances [39], and tire abrasion [40]. These MP particles settle on the soil surface and can penetrate into subsoils via physical and environmental activities. Recently, increasing attention has been drawn to MP pollution in the soil ecosystem from various pathways [41]. Studies have not revealed the transfer or presence of MPs in groundwater; however, there is growing concern over the potential distribution and transportation of MPs into the groundwater system and the hyporheic zone based on previous investigations on the mechanism of transport of MPs [38].
As more attention is drawn to MP pollution in the soil environment, it is necessary to understand the interaction and response of soil microbial communities to MPs in the soil and its effect on MP biodegradation. The process of microbial degradation of MPs in soil is governed by the diverse soil microbial communities that are able to colonize the MP surface, form biofilms, and establish a microenvironment that facilitates MP degradation [42] in the soil environment.
3. Microplastic Pollution and Soil Microbial Community3.1. Microbial Interactions with MPs in SoilMicroorganisms interact with MPs in different environments, including the soil [43]. As more MPs are introduced in the soil, there is an increasing concern about their ecological effect, especially their effect on soil microbiota. For example, the worldwide use of plastic mulch films in agricultural activities increased from around 4.4 million tons in 2012 to about 7.4 million tons in 2019 [44], and this represents a significant source of MP contamination in the terrestrial environment [45]. Investigations have revealed that several groups of microorganisms including bacteria, fungi, and algae are found attached to MP surfaces [46, 47]. Previous studies have shown that different types of MPs can selectively stimulate the proliferation of specific soil microbial taxa in a microbial community (Table 1). For example, specific soil bacterial and fungal taxa were enriched when exposed to different plastic types (Table 1). It is interesting to note that, in the soil environment, the microbial richness and diversity on MP surfaces are unique and less diverse than those found colonizing natural materials such as wood [48] and the surrounding rhizospheric soil [49]. Due to their uniqueness, the microbial communities on MPs have been referred to as microplastisphere/plastisphere [50].
A few studies have investigated the effect of MPs on the composition and diversity of soil microbial communities; however, their reports remain inconsistent. While some studies have reported that no significant effect was observed on soil microbial community structure [51–53], others reported considerable changes in the abundance and diversity of soil microbial communities exerted by MPs which affect the overall soil function [54]. Yi et al. [55] found that the alpha diversity and soil microbial communities changed significantly with membrane-like PE and fibrous PP, and this resulted in the abundance of Acidobacteria and Bacteroidetes, while Deinococcus-Thermus and Chloroflexi decreased in abundance. In another report, the substrate-induced respiration rate was reduced and significant changes in the root colonization rate of arbuscular mycorrhizal fungi were observed with the addition of MPs, and these suggest that the presence of MPs caused changes in microbial functions [53].
The soil treated with 0.007% low-density polyethylene (LDPE) resulted in three times higher specie turnover rate of bacterial communities than the untreated soil, and the divergence of soil microbial communities increased continually as the LDPE exposure time prolonged [56]. Ng et al. [57] also observed that the addition of LDPE (3% w/w) and PET (0.2% and 0.4% w/w) to the growth medium affected the even distribution and richness of soil microorganisms and soil microbial functions, and 14 unique bacterial genera were predominant and enriched. Liu et al. [58] reported that PP (7% and 28%) had a positive effect on the activities of soil microbiota, while de Souza Machado et al. [59] and Awet et al. [60] observed that polyester (0.05–0.4%), polyacrylic (0.05–0.4%) and PS (1 mg kg−1) showed significant negative effects on soil microbial activities. From these studies, it is quite difficult to draw a general conclusion on the effect of MPs on soil microbial communities as the types, sizes, shapes, and concentrations of MPs, as well as environmental conditions varied in these investigations. The different physical, chemical, and biological properties of the soil types used in these investigations might also have played a role in their findings. However, further research is required to gain deeper insight and validate the effect of co-interaction of soil properties and MP properties on soil microbial communities.
3.2. Factors Influencing the Interaction and Response of Soil Microbial Community to MPsThe structure, diversity, and richness of soil microbial communities can be significantly impacted by MP-related properties. The size of MPs might influence the microbial attachment and colonization as well as the distribution and diversity of soil microbial communities, although existing information on this remains scarce. For example, LDPE powder (150–250 μm) at 2% and 7% (w/w) showed differential tolerance on the bacterial diversity at the genus level [71], but LDPE particles (30 μm) at 0.2% (w/w) had no effect on the microbial community [72]. These findings corroborated the earlier report of Frère et al. [73] who found that MP size, regardless of the MP type, had no significant effect on the composition and diversity of microbial communities attached to them. These, however, contradict the report of Guo et al. [74] who found a significant increase in the richness of soil microbial communities in the presence of PE microfiber of <2 mm long. It was also observed that Actinobacteria was significantly enriched on PE, PS, and PP MPs when compared with their macroplastic forms [75]. These findings indicate that the MP size might play a role in microbial diversity and richness; however, more investigations are needed for a better understanding of the effect of MP size on soil microbial communities.
Apart from the size of MPs, the type of MPs could affect the composition, richness, and diversity of soil microbial communities. The soil samples treated with PE, PS, and PP of the same size (150 μm) and concentration (1% w/w) showed that the microbial communities of the soils treated with PP and PE responded differently from the PS-treated soil, although no major effect was observed in the bacterial diversity among the different MP types [76]. Similarly, the treatment of soil with LDPE particles (150–250 μm) and PS particles (0.33–0.64 μm) did not show significant changes in bacterial diversity [71, 77], whereas the treatment of soil with LDPE particles (678 μm) and PVC particles (15 μm) of the same concentration resulted in a decrease in the Shannon diversity indices of microbial communities [61]. Also, the increases in the relative abundances of Bacteroidetes and Gemmatimonadetes in the PP- and PE-treated soils were greater than that observed in the PS-treated soil [55]. The disparities in these findings can be attributed to the vast and robust microbial diversity in different soils with varying capacities to respond to disturbances [78] and responses of soil microorganisms to single artificial pressure are not always straightforward [79]. The hydrophobicity and roughness of MP surfaces are important parameters that could influence the microbial attachment and colonization of MPs and the resulting microbial communities [80]. Microorganisms colonizing MP surfaces are found to secret several extracellular polymeric substances (EPS), which act as bio-adhesives to enhance their attachment to MP surfaces [80]. Information on the relationship between MP surface hydrophobicity and soil microbial community and their interaction is still limited. However, it was reported that during the early colonization stage, marine bacteria adhere to the hydrophilic carrier interface for biofilm formation [81]. It was observed that hydrophilic groups such as C-O and C=O increased on PE surface in seawater, leading to a significant decrease in the MP hydrophobic properties [82].
As biofilms form and mature on MP surfaces, the bacterial hydrophobicity positively enhances the adhesion of the microbial communities to the MPs [83]. During the MP aging process, MP properties such as surface topography, polarity, surface area, and roughness could change [84]. These physical and structural changes could exert a direct influence on the composition and diversity of the associated microbial communities. For example, Betaproteobacteria was dominant on the smooth PS surface, while Gammaproteobacteria was dominant on the rough PE surface [85]. A rougher MP surface was found to have a positive correlation with the growth rate and density of the adhered microorganisms [83]. During the early biofilm colonization stage, Vibrio crassostreae colonized the smooth PS surface faster (<10 h) than the rough PS surface (6 d); however, rapid decolonization was also observed with the smooth PS surface (i.e., zero after 24 h) [86]. Furthermore, the surface charge on MPs plays a significant role on the abundance and diversity of microbial communities; plastic surface energy of 31–43 mN m−1 was discovered to be ideal for microbial colonization [87].
Plastic additives such as citrate esters, phthalate esters, fatty acid esters, glycerides, and polyhydric alcohols have the potential to leach out during the plastic weathering process [88], and their concentrations might increase in soil over time and cause significant changes to the abundance, diversity, and activities of soil microbial communities. Few studies have investigated the effect of plastic additives on soil microbial community and microcosm activity [89–91]. For instance, soil treated with diethyl phthalate (DEP) and di (2-ethyl hexyl) phthalate (DEHP) at 0.1 mg g−1 had no significant effect on the structural and functional diversity of the soil microbial community [91]. Also, the increasing dibutyl phthalate (DBP) concentration in soil decreased the bacterial diversity and the relative structure, composition, and abundance of soil bacterial communities reflected a successional change as DBP undergoes degradation [90]. Although plastic additives are not often observed to have a profound impact on soil microbiomes at concentrations of environmental relevance [91], more studies on the impact of different categories of plastic additives on soil microbial communities could throw more insight into understanding the microbial colonization pattern of MPs in the natural environment.
Other factors beyond MP-related properties could also play significant roles in the structural composition, diversity, and activities of soil microbial communities. Exposure duration of soil microorganisms to MPs could impact the composition and diversity of the microbial communities associated with MPs [92, 93]. Molecules adsorbed to MPs such as sugars, nucleic acids, proteins, fatty acids, and lipids have also been shown to improve the primary colonization process of microorganisms [94]. Environmental conditions such as nutrient availability (organic and inorganic nutrients), presence/absence of other pollutants, temperature, pH, light intensity, ionic strength, salinity, and biotic factors are critical to the composition, richness, diversity, and activities of microbial communities associated with MPs [48, 95]. Similarly, seasonal variations and geographical locations have been demonstrated to have significant influence on the diversity of microbial communities associated with MPs [95, 96]. So far, the mechanism of colonization of microbial communities on MPs is still largely unknown. When plastic is introduced to the soil, a series of physicochemical changes occur as a result of natural weathering activities [97], which could impact the soil microbial communities. Virgin plastics are normally smooth with a uniform structure and nearly no surface charge [98]. When exposed to sunlight, photooxidation occurs where solar photons with wavelengths within the UV and blue spectrum strike chromophores in the plastic polymer to initiate photooxidative decomposition [97]. The photooxidative weathering process creates radicals that are responsible for chain scissions in the plastic polymer and their reaction with oxygen oxidizes the polymer surface. This results in an increase in hydrophilic groups [30] and carbonyl groups that enhance the primary attachment and colonization of microorganisms on plastic surfaces for microbial community formation and biodegradation [87, 99, 100]. Plastic additives such as plasticizers, UV stabilizers, heat stabilizers, flame retardants, and pigment agents are also partially degraded during photooxidation [97] and might play a critical role in shaping the composition, abundance, and diversity of microbial communities associated with MPs in the soil [52, 99]. Similar to photooxidation, thermal weathering processes also generate radicals through chain scissions at high temperatures, which enhance microbial colonization and biofilm formation on MP surfaces, and biodegradation [101]. Also, MP fragmentation by mechanical stresses in the soil and biogeochemical attacks reduce the hydrophobicity, brittleness, and stiffness of MPs, thereby, enhancing microbial colonization and the formation of plastic-associated microbial communities [27, 97].
4. Microbial Degradation of MPsGenerally, synthetic plastics can resist degradation and persist in the environment for a long period of time. In the natural environment, the degradation of MPs is an integrated process that in corporates physical, chemical, and biological elements [102]. Despite MPs being less susceptible to degradation as compared to other biodegradable materials, a number of microbial strains have been identified with the potential to biodegrade MPs in the natural environment, including soils of plastic-dumping sites, waste of mulch films, marine water, crude oil-contaminated soils, landfills, sewage sludge, and guts of plastic-eating insects [103]. Microorganisms are found everywhere in nature, and they are able to adapt to a variety of environmental conditions, including extreme conditions. Their complex enzymatic system, ability to utilize various organic and inorganic substrates for growth, and ability to adapt to extreme environmental conditions are factors that contribute to their capacity to break down a wide variety of environmental contaminants. Therefore, they represent a sustainable and eco-friendly alternative to the management of plastic pollution in the environment. MPs form a novel ecological niche for microorganisms by providing a support system for colonization and growth, and a source of nutrient for microbial nutritional needs. The attachment of different groups of microorganisms (e.g. bacteria, actinomycetes, fungi, protists, algae, and viruses) to MP surfaces provides them an enabling environment to form biofilms [104]. The result of the microbial enzymatic activities leads to the structural deformation and loss of properties of the MPs [105]. Different categories of microorganisms capable of degrading MPs have been isolated from various environments [106]; however, there is still a knowledge gap about microbial interactions with MPs, which has contributed to the lesser adoption of plastic biodegradation strategies [107]. There are four stages involved in biofilm formation and biofilm-mediated MP degradation [104], and these include 1) microorganism adhesion to MP surface and modification of their surface properties, 2) MP deterioration, in which microbial enzymes speed up degradation and liberate monomers and additives from the MPs, 3) MP fragmentation, where the MPs lose their mechanical stability and become fragile as a result of microbial attack, and 4) assimilation of MPs, which involves the penetration of microbial filaments and water and the subsequent microbial decomposition and use of MPs as a nutrient source.
4.1. Actinomycetes in MPs BiodegradationActinomycetes, a group of gram-positive, filamentous bacteria, can be found in various ecological habitats including soil, plant tissues, freshwater, and marine environments [108]. Actinomycetes are recognized for their metabolic versatility and can perform different functions in the environment. They are explored for numerous biotechnological applications such as the production of enzymes, anticancer drugs, antibiotics, and other bioactive metabolites [109]. Some strains of actinomycetes have also been used for the bioremediation of toxic materials, breakdown of resistant carbohydrates (e.g. chitin, cellulose), and recycling of organic carbons [110]. Their ability to produce hydrolytic enzymes and bioactive metabolites enables them to grow on and degrade many complex polymers [111] including MPs [112]. Actinomycetes are known to produce extracellular biopolymers like levan, glycogen, dextran, and N-acetylglucosamine-rich slime polysaccharides, which are assumed to enhance their attachment to MP surfaces for subsequent microbial activities [113]. Actinomycetes such as the genera Streptomyces, Actinomadura, Rhodococcus, and thermophilic species of Thermoactinomycetes have been isolated from different environments with significant plastic degradation potential [112, 114]. Other actinomycetes species that have been reported to be associated with MP degradation are listed in Table 2. From the list, it is evident that limited actinomycetes genera have so far been identified to have MP-degradation potential, predominantly members of Streptomyces, Rhodococcus, and Micrococcus. Reports have also shown that some actinomycetes can form biofilm, similar to other bacterial strains, which is important for their survival and colonization on MPs [115].
4.2. Other Bacteria in MPs BiodegradationBacteria are the most abundant group of microbes. They are well recognized for their ubiquity and can be found in various environments such as water, soil, and atmosphere. Numerous bacterial species have been extensively studied for their roles in the biodegradation of complex polymers and bioremediation of environmental pollutants such as metal compounds, crude oil, antibiotics, plastic, and other compounds of environmental concern [136]. With their diversity and metabolic activities, bacterial strains are able to adsorb, desorb, and break down MPs [114, 137]. Different bacterial strains have been reported to utilize MPs as their main carbon source in minimal medium, and with their metabolic activities, they induced significant weight loss and changes to the morphological and chemical structure of MPs (Table 3).
Plastic-degrading bacteria have been isolated from different environments such as cold marine environment [138], dumpsites [139], landfills [140], recycling sites [141], and insects’ guts [142]. Most studies on the bacterial degradation of MPs have used pure bacterial cultures isolated from different environments or obtained from culture collections for the degradation of MPs under laboratory conditions. The use of pure bacterial strains can be advantageous when investigating specific metabolic pathways, evaluating different conditions, and/or closely monitoring the process of MP degradation [143]. However, in nature, many bacterial strains act in synergy, forming consortium and constituting a stable microbial community, which ultimately enhances their survival and degradation potential. So far, few studies have focused on MP biodegradation by bacterial consortia [129], it is, therefore, essential that more investigations be conducted to explore the potential of different bacterial consortia in MP biodegradation, as this might lead to greater efficiency due to the metabolic synergism between different bacterial strains.
4.3. Algae in MPs BiodegradationIn the last decades, algae have been extensively studied for their biotechnological applications, especially in the production of biofuels [155, 156]. The ability of different photosynthetic and heterotrophic algae to degrade environmental pollutants, both organic and inorganic, has also been well studied and established [157]. They are able to degrade environmental pollutants by adsorbing, accumulating, or metabolizing them into safer levels [156, 157]. Most studies on the environmental biodegradation of MPs have focused on the potentials of other groups of microorganisms such as bacteria and fungi, and only a few studies have investigated the potential of algae for biodegradation of MPs. Rather, recent studies have focused on the potential of algae in green plastic production [158].
Algal species including Oscillatoria, Spirogyra, Anabaena, Spirulina, and Chlorella have been reported to be found colonizing MP surfaces in terrestrial environments, but it is still inconclusive to confirm that these algal species are able to metabolize the MPs [159, 160]. In a study by Khoironi et al. [161], Spirulina sp. was able to biodegrade PP and PET during 112 d incubation. The authors reported that the tensile strength of PP and PET decreased by 0.1977 MPa d−1 and 0.9939 MPa d−1, respectively. Kumar et al. [162] also reported that Navicula pupula, Scenedesmus dimorphus, and Anabaena spiroides showed degradation potentials on both LDPE and high-density PE (HDPE). From their findings, A. spiroides was able to degrade 8.18% of LDPE after 30 d, representing the most promising algal strain in their study. The reported degradation rate is considerably lower than that of some investigations where bacteria and fungi were used to degrade PE. For instance, Brevibacillus borstelensis, a thermophilic bacteria isolated from soil, was able to degrade 30% of PE films after 30 d incubation [163]. A marine fungus, Zalerion maritumum, was similarly able to degrade 43% of PE pellets after 28 d incubation [164]. Generally, the low MP degradation rate by algae in comparison with bacteria and fungi is quite understandable. Unlike bacteria and fungi, most algae are considered to be photoautotrophic organisms that use atmospheric CO2 as their main source of carbon, and their main energy source is derived from sunlight [165]. Some algae are able to grow under heterotrophic conditions where external carbon and energy sources are utilized under dark conditions [166]. Even though they are able to colonize and assimilate MPs, algae are not metabolically inclined to mineralize MPs [162]; therefore, there is the possibility of plastic accumulation in algae which might be introduced to the food chain [167].
4.4. Fungi in MPs BiodegradationFungi are known to possess vast metabolic potentials, including the production of extracellular multienzyme complexes [168], thereby making them microorganisms of interest in MP biodegradation research. Different fungal strains can be found in different natural environments and they play significant roles in maintaining biogeochemical cycles and promoting the transformation of different substances [169]. As highlighted in Table 4, different fungal species and consortia have been reported with MP-degrading potential based on their ability to utilize MPs as their sole carbon or energy source, predominantly members of the genus Aspergillus, Penicillium, Fusarium, Trichoderma, Mucor, and Cladosporium. Some edible fungal species such as Agaricus bisporus, Pleurotus abalones, and Pleurotus ostreatus have also been reported to utilize PE and PS for growth, with changes in laccase activity [170]. Similar to bacteria, fungi are able to adhere to and utilize MPs [171]. Through their metabolic activities, they have the potential to decrease the hydrophobicity of MPs by promoting the formation of chemical bonds like ester, carbonyl, and carboxyl functional groups in MPs [104].
5. Knowledge Gaps and Future ProspectsThe majority of research on MP pollution and its ecological effects has focused on the marine environment and other aquatic ecosystems, and there have been limited investigations on MP pollution in soil thus far. Unlike water, the soil is a unique media with relatively complex and distinctive physical, chemical, and biological characteristics; as a result, it is challenging to investigate the ecological effect of MPs, particularly with regard to soil microorganisms, which are crucial to nutrient cycling and maintenance of soil functions. From this study, it is evident that, although research on MPs in soil is increasing as more attention is drawn to this area, there is still a substantial gap in understanding the interactions of soil microorganisms with MPs as well as the mechanisms by which microorganisms degrade MPs in the soil. The ecological effect of MPs on the structural composition, diversity, and activities of soil microbial communities are far from being fully understood. Therefore, future studies should focus on addressing the following issues. Firstly, it is crucial to monitor the sources and distribution of MPs in the soil. The extent of MP pollution under different land uses and natural environments should be understood. Also, further research is needed to better understand the ecological impact of MPs on soil microbiota and microbial communities. Critical questions to be answered with multiple lines of evidence would include i) what is the mechanism by which MPs affect soil microbial communities?, ii) how do MP properties affect the structural composition, richness, diversity, and activities of soil microbial communities in MP-polluted soils?, iii) would the co-interactions of MP properties and soil properties have a significant impact on soil microbial communities?, iv) do intermediates and products of MP degradation impact the diversity and activities of soil microbial communities?, v) what ecological impact do MP additives and adsorbed environmental pollutants have on the microbial communities in the soil?. Lastly, current literature revealed that information on microbial degradation of MPs has centered on specific groups of microorganisms of limited genera, majorly pure microbial cultures, with a few studies on microbial consortium. This amplifies the necessity to explore the potential of various microbes in their natural environments for MP degradation. As most microorganisms exhibit synergistic interactions in their natural environments, it is suggested that the use of different microbial strains in consortium will result in greater efficiency in MP degradation. Furthermore, the application of omic tools such as metagenomics, genomics, metabolomics, proteomics, and transcriptomics will aid in understanding the biological activities that take place at the genetic and metabolic levels, and the influence of environmental factors during MP biodegradation.
AcknowledgmentsThis study was funded by the National Research Foundation of Korea (NRF-2021R1A2C4001746).
References1. Chae Y, An YJ. Current research trends on plastic pollution and ecological impacts on the soil ecosystem: A review. Environ. Pollut. 2018;240:387–395.
https://doi.org/10.1016/j.envpol.2018.05.008
2. Amobonye A, Bhagwat P, Singh S, Pillai S. Plastic biodegradation: Frontline microbes and their enzymes. Sci. Total Environ. 2021;759:1–16.
https://doi.org/10.1016/j.scitotenv.2020.143536
3. Plastic Europe. Plastics - the Facts 2020 [Internet]. Brussels: Plastics Europe; c2022. [cited 31 October 2022]. Available from: https://plasticseurope.org/knowledge-hub/plastics-thefacts-2020/
4. Nielsen T, Hasselbalch J, Holmberg K, Stripple J. Politics and the plastic crisis: A review throughout the plastic life cycle. WIREs Energy Environ. 2019;9:1–18.
https://doi.org/10.1002/wene.360
5. Jambeck JR, Geyer R, Wilcox C, et al. Marine pollution: Plastic waste inputs from land into the ocean. Science. 2015;347:768–771.
https://doi.org/10.1126/science.1260352
6. Van Cauwenberghe L, Devriese L, Galgani F, Robbens J, Janssen CR. Microplastics in sediments: A review of techniques, occurrence and effects. Mar. Environ. Res. 2015;111:5–17.
https://doi.org/10.1016/j.marenvres.2015.06.007
7. E erkes-Medrano D, Thompson RC, Aldridge DC. Microplastics in freshwater systems: a review of the emerging threats, identification of knowledge gaps and prioritisation of research needs. Water Res. 2015;75:63–82.
https://doi.org/10.1016/j.watres.2015.02.012
8. Duis K, Coors A. Microplastics in the aquatic and terrestrial environment: sources (with a specific focus on personal care products), fate and effects. Environ. Sci. Eur. 2016;28:1–25.
https://doi.org/10.1186/s12302-015-0069-y
9. Ryan P, Moloney C. Plastic and other artefacts on South African beaches: Temporal trends in abundance and composition. S. Afr. J. Sci. 1990;86:450–452.
10. Thompson RC, Olsen Y, Mitchell RP, et al. Lost at sea: where is all the plastic? Science. 2004;304(5672)838.
https://doi.org/10.1126/science.1094559
11. Alimi OS, Fadare OO, Okoffo ED. Microplastics in African ecosystems: Current knowledge, abundance, associated contaminants, techniques, and research needs. Sci. Total Environ. 2021;755:1–42.
https://doi.org/10.1016/j.scitotenv.2020.142422
12. Othman A, Abu Hasan H, Muhamad M, Ismail NI, Abdullah S. Microbial degradation of microplastics by enzymatic processes: A review. Environ. Chem. Lett. 2021;19:1–17.
https://doi.org/10.1007/s10311-021-01197-9
13. Monkul MM, Ozhan HO. Microplastic contamination in soils: A review from geotechnical engineering view. Polymers. 2021;13:1–24.
https://doi.org/10.3390/polym13234129
14. Hur J, Jho EH. Current research trends on the effects of microplastics in soil environment using earthworms: Mini-review. J. Korean Soc. Environ. Eng. 2021;43:299–306.
https://doi.org/10.4491/KSEE.2021.43.4.299
15. Zhu F, Zhu C, Wang C, Gu C. Occurrence and ecological impacts of microplastics in soil systems: A review. Bull. Environ. Contam. Toxicol. 2019;102:741–749.
https://doi.org/10.1007/s00128-019-02623-z
16. Hasan MM, Jho EH. Effect of microplastics on the germination and growth of terrestrial plants. J. Korean Soc. Environ. Eng. 2022;44:375–382.
https://doi.org/10.4491/KSEE.2022.44.10.375
17. Rillig MC. Microplastic in terrestrial ecosystems and the soil? Environ. Sci. Technol. 2012;46:6453–6454.
https://doi.org/10.1021/es302011r
18. Horton AA, Walton A, Spurgeon DJ, Lahive E, Svendsen C. Microplastics in freshwater and terrestrial environments: Evaluating the current understanding to identify the knowledge gaps and future research priorities. Sci. Total Environ. 2017;586:127–141.
https://doi.org/10.1016/j.scitotenv.2017.01.190
19. Rodriguez-Seijo A, Lourenço J, Rocha-Santos TAP, et al. Histopathological and molecular effects of microplastics in Eisenia andrei Bouché. Environ. Pollut. 2017;220:495–503.
https://doi.org/10.1016/j.envpol.2016.09.092
20. Kuzyakov Y, Blagodatskaya E. Microbial hotspots and hot moments in soil: Concept and review. Soil Biol. Biochem. 2015;83:184–199.
https://doi.org/10.1016/j.soilbio.2015.01.025
21. Steinmetz Z, Wollmann C, Schaefer M, et al. Plastic mulching in agriculture. Trading short-term agronomic benefits for long-term soil degradation? Sci. Total Environ. 2016;550:690–705.
https://doi.org/10.1016/j.scitotenv.2016.01.153
22. Rajendran S, Kannan V, Natarajan K, et al. The role of microbes in plastic degradation. Chandra R, editorEnvironmental Waste Management. 1st edFlorida: CRC Press; 2015. p. 341–370.
23. Verma R, Shankarappa V, Papireddy M, Gowda ANS. Toxic pollutants from plastic waste-A review. Procedia Environ. Sci. 2016;35:701–708.
https://doi.org/10.1016/j.proenv.2016.07.069
24. Gradus R, Nillesen P, Dijkgraaf E, Koppen R. A cost-effectiveness analysis for incineration or recycling of Dutch household plastic waste. Ecol. Econ. 2017;135:22–28.
https://doi.org/10.1016/j.ecolecon.2016.12.021
25. Rahimi A, Garcia J. Chemical recycling of waste plastics for new materials production. Nat. Rev. Chem. 2017;1:1–11.
https://doi.org/10.1038/s41570-017-0046
26. T aniguchi I, Yoshida S, Hiraga K, Miyamoto K, Kimura Y, Oda K. Biodegradation of PET: Current status and application aspects. ACS Catal. 2019;9:4089–4105.
https://doi.org/10.1021/acscatal.8b05171
27. Restrepo-Flórez J-M, Bassi A, Thompson M. Microbial degradation and deterioration of polyethylene – A review. Int. Biodeterior. Biodegradation. 2014;88:83–90.
https://doi.org/10.1016/j.ibiod.2013.12.014
28. Magnin A, Pollet E, Phalip V, Avérous L. Evaluation of biological degradation of polyurethanes. Biotechnol. Adv. 2020;39:1–24.
https://doi.org/10.1016/j.biotechadv.2019.107457
29. Ho BT, Roberts TK, Lucas S. An overview on biodegradation of polystyrene and modified polystyrene: the microbial approach. Crit. Rev. Biotechnol. 2018;38:308–320.
https://doi.org/10.1080/07388551.2017.1355293
30. Arutchelvi J, Muniyasamy S, Arkatkar A, Doble M, Bhaduri S, Uppara P. Biodegradation of polyethylene and polypropylene. Indian J. Biotechnol. 2008;7:9–22.
31. Zhang J, Gao D, Li Q, et al. Biodegradation of polyethylene microplastic particles by the fungus Aspergillus flavus from the guts of wax moth Galleria mellonella
. Sci. Total Environ. 2020;704:1–8.
https://doi.org/10.1016/j.scitotenv.2019.135931
32. Blank LM, Narancic T, Mampel J, Tiso T, O’Connor K. Biotechnological upcycling of plastic waste and other non-conventional feedstocks in a circular economy. Curr. Opin. Biotechnol. 2020;62:212–219.
https://doi.org/10.1016/j.copbio.2019.11.011
33. Cincinelli A, Martellini T, Guerranti C, Scopetani C, Chelazzi D, Giarrizzo T. A potpourri of microplastics in the sea surface and water column of the Mediterranean Sea. TrAC Trends Anal. Chem. 2019;110:321–326.
https://doi.org/10.1016/j.trac.2018.10.026
34. Khalid N, Aqeel M, Noman A, et al. Linking effects of microplastics to ecological impacts in marine environments. Chemosphere. 2021;264:1–13.
https://doi.org/10.1016/j.chemosphere.2020.128541
35. Farmer J, Zhang B, Jin X, Zhang P, Wang J. Long-term effect of plastic film mulching and fertilization on bacterial communities in a brown soil revealed by high through-put sequencing. Arch. Agron. Soil Sci. 2016;63:230–241.
https://doi.org/10.1080/03650340.2016.1193667
36. Carr SA, Liu J, Tesoro AG. Transport and fate of microplastic particles in wastewater treatment plants. Water Res. 2016;91:174–182.
https://doi.org/10.1016/j.watres.2016.01.002
37. Nizzetto L, Bussi G, Futter MN, Butterfield D, Whitehead PG. A theoretical assessment of microplastic transport in river catchments and their retention by soils and river sediments. Environ. Sci: Processes Impacts. 2016;18:1050–1059.
https://doi.org/10.1039/c6em00206d
38. Bläsing M, Amelung W. Plastics in soil: Analytical methods and possible sources. Sci. Total Environ. 2018;612:422–435.
https://doi.org/10.1016/j.scitotenv.2017.08.086
39. Dris R, Gasperi J, Saad M, Mirande C, Tassin B. Synthetic fibers in atmospheric fallout: A source of microplastics in the environment? Mar. Pollut. Bull. 2016;104:290–293.
https://doi.org/10.1016/j.marpolbul.2016.01.006
40. Foitzik M-J, Unrau H-J, Gauterin F, Dörnhöfer J, Koch T. Investigation of ultra fine particulate matter emission of rubber tires. Wear. 2018;394–395:87–95.
https://doi.org/10.1016/j.wear.2017.09.023
41. Ng EL, Huerta Lwanga E, Eldridge SM, et al. An overview of microplastic and nanoplastic pollution in agroecosystems. Sci. Total Environ. 2018;627:1377–1388.
https://doi.org/10.1016/j.scitotenv.2018.01.341
42. Debroy A, George N, Mukherjee G. Role of biofilms in the degradation of microplastics in aquatic environments. J. Chem. Technol. Biotechnol. 2021;97:3271–3282.
https://doi.org/https://doi.org/10.1002/jctb.6978
43. Mammo FK, Amoah ID, Gani KM, et al. Microplastics in the environment: Interactions with microbes and chemical contaminants. Sci. Total Environ. 2020;743:1–21.
https://doi.org/10.1016/j.scitotenv.2020.140518
44. Batista T, Cansado IP, Tita B, et al. Dealing with plastic waste from agriculture activity. Agronomy. 2022;12:1–12.
https://doi.org/10.3390/agronomy12010134
45. Huang Y, Liu Q, Jia W, Yan C, Wang J. Agricultural plastic mulching as a source of microplastics in the terrestrial environment. Environ. Pollut. 2020;260:1–6.
https://doi.org/10.1016/j.envpol.2020.114096
46. Masó M, Garcés E, Pagès F, Camp J. Drifting plastic debris as a potential vector for dispersing Harmful Algal Bloom (HAB) species. Sci. Mar. 2003;67:107–111.
https://doi.org/10.3989/scimar.2003.67n1107
47. De Tender C, Devriese LI, Haegeman A, et al. Temporal dynamics of bacterial and fungal colonization on plastic debris in the North sea. Environ. Sci. Technol. 2017;51:7350–7360.
https://doi.org/10.1021/acs.est.7b00697
48. Oberbeckmann S, Kreikemeyer B, Labrenz M. Environmental factors support the formation of specific bacterial assemblages on microplastics. Front. Microbiol. 2017;8:1–12.
https://doi.org/10.3389/fmicb.2017.02709
49. Yu H, Zhang Y, Tan W. The “neighbor avoidance effect” of microplastics on bacterial and fungal diversity and communities in different soil horizons. Environ. Sci. Ecotechnol. 2021;8:1–9.
https://doi.org/https://doi.org/10.1016/j.ese.2021.100121
50. Zettler ER, Mincer TJ, Amaral-Zettler LA. Life in the “plastisphere”: Microbial communities on plastic marine debris. Environ. Sci. Technol. 2013;47:7137–7146.
https://doi.org/10.1021/es401288x
51. Huang Y, Zhao Y, Wang J, Zhang M, Jia W, Qin X. LDPE microplastic films alter microbial community composition and enzymatic activities in soil. Environ. Pollut. 2019;254:1–10.
https://doi.org/10.1016/j.envpol.2019.112983
52. Yan Y, Chen Z, Zhu F, Zhu C, Wang C, Gu C. Effect of polyvinyl chloride microplastics on bacterial community and nutrient status in two agricultural soils. Bull. Environ. Contam. Toxicol. 2021;107:602–609.
https://doi.org/10.1007/s00128-020-02900-2
53. Judy JD, Williams M, Gregg A, et al. Microplastics in municipal mixed-waste organic outputs induce minimal short to long-term toxicity in key terrestrial biota. Environ. Pollut. 2019;252:522–531.
https://doi.org/10.1016/j.envpol.2019.05.027
54. Gao B, Yao H, Li Y, Zhu Y. Microplastic addition alters the microbial community structure and stimulates soil carbon dioxide emissions in vegetable-growing soil. Environ. Toxicol. Chem. 2021;40:352–365.
https://doi.org/10.1002/etc.4916
55. Yi M, Zhou S, Zhang L, Ding S. The effects of three different microplastics on enzyme activities and microbial communities in soil. Water Environ. Res. 2021;93:24–32.
https://doi.org/10.1002/wer.1327
56. Wang J, Huang M, Wang Q, Sun Y, Zhao Y, Huang Y. LDPE microplastics significantly alter the temporal turnover of soil microbial communities. Sci. Total Environ. 2020;726:1–8.
https://doi.org/10.1016/j.scitotenv.2020.138682
57. Ng EL, Lin SY, Dungan AM, et al. Microplastic pollution alters forest soil microbiome. J. Hazard. Mater. 2021;409:1–10.
https://doi.org/10.1016/j.jhazmat.2020.124606
58. Liu H, Yang X, Liu G, et al. Response of soil dissolved organic matter to microplastic addition in Chinese loess soil. Chemosphere. 2017;185:907–917.
https://doi.org/10.1016/j.chemosphere.2017.07.064
59. de Souza Machado AA, Lau CW, Till J, et al. Impacts of microplastics on the soil biophysical environment. Environ. Sci. Technol. 2018;52:9656–9665.
https://doi.org/10.1021/acs.est.8b02212
60. Tsegai A, Kohl Y, Meier F, et al. Effects of polystyrene nanoparticles on the microbiota and functional diversity of enzymes in soil. Environ. Sci. Eur. 2018;30:1–10.
https://doi.org/10.1186/s12302-018-0140-6
61. Fei Y, Huang S, Zhang H, et al. Response of soil enzyme activities and bacterial communities to the accumulation of microplastics in an acid cropped soil. Sci. Total Environ. 2020;707:1–9.
https://doi.org/10.1016/j.scitotenv.2019.135634
62. Zhang X, Li Y, Lei J, et al. Time-dependent effects of microplastics on soil bacteriome. J. Hazard. Mater. 2023;447:1–13.
https://doi.org/https://doi.org/10.1016/j.jhazmat.2023.130762
63. Wang Q, Feng X, Liu Y, et al. Effects of microplastics and carbon nanotubes on soil geochemical properties and bacterial communities. J. Hazard. Mater. 2022;433:1–13.
https://doi.org/https://doi.org/10.1016/j.jhazmat.2022.128826
64. Dong Y, Gao M, Qiu W, Song Z. Effect of microplastics and arsenic on nutrients and microorganisms in rice rhizosphere soil. Ecotoxicol. Environ. Saf. 2021;211:1–12.
https://doi.org/https://doi.org/10.1016/j.ecoenv.2021.111899
65. Fan P, Tan W, Yu H. Effects of different concentrations and types of microplastics on bacteria and fungi in alkaline soil. Ecotoxicol. Environ. Saf. 2022;229:1–9.
https://doi.org/https://doi.org/10.1016/j.ecoenv.2021.113045
66. Li H-Q, Shen Y-J, Wang W-L, Wang H-T, Li H, Su J-Q. Soil pH has a stronger effect than arsenic content on shaping plastisphere bacterial communities in soil. Environ. Pollut. 2021;287:1–8.
https://doi.org/https://doi.org/10.1016/j.envpol.2021.117339
67. Qian H, Zhang M, Liu G, et al. Effects of soil residual plastic film on soil microbial community structure and fertility. Water Air Soil Pollut. 2018;229:1–11.
https://doi.org/10.1007/s11270-018-3916-9
68. Ren X, Tang J, Liu X, Liu Q. Effects of microplastics on greenhouse gas emissions and the microbial community in fertilized soil. Environ. Pollut. 2020;256:1–11.
https://doi.org/https://doi.org/10.1016/j.envpol.2019.113347
69. Temporiti ME, Nicola L, Girometta CE, Roversi A, Daccò C, Tosi S. The analysis of the mycobiota in plastic polluted soil reveals a reduction in metabolic ability. J. Fungi. 2022;8:1–20.
https://doi.org/10.3390/jof8121247
70. Zhou Y, Sun Y, Liu J, Ren X, Zhang Z, Wang Q. Effects of microplastics on humification and fungal community during cow manure composting. Sci. Total Environ. 2022;803:1–10.
https://doi.org/https://doi.org/10.1016/j.scitotenv.2021.150029
71. Rong L, Zhao L, Zhao L, et al. LDPE microplastics affect soil microbial communities and nitrogen cycling. Sci. Total Environ. 2021;773:1–11.
https://doi.org/10.1016/j.scitotenv.2021.145640
72. Li HZ, Zhu D, Lindhardt JH, Lin SM, Ke X, Cui L. Long-term fertilization history alters effects of microplastics on soil properties, microbial communities, and functions in diverse farmland ecosystem. Environ. Sci. Technol. 2021;55:4658–4668.
https://doi.org/10.1021/acs.est.0c04849
73. Frère L, Maignien L, Chalopin M, et al. Microplastic bacterial communities in the Bay of Brest: Influence of polymer type and size. Environ. Pollut. 2018;242:614–625.
https://doi.org/10.1016/j.envpol.2018.07.023
74. Guo QQ, Xiao MR, Ma Y, Niu H, Zhang GS. Polyester microfiber and natural organic matter impact microbial communities, carbon-degraded enzymes, and carbon accumulation in a clayey soil. J. Hazard. Mater. 2021;405:1–10.
https://doi.org/10.1016/j.jhazmat.2020.124701
75. Zhang K, Xiong X, Hu H, et al. Occurrence and characteristics of microplastic pollution in Xiangxi Bay of Three Gorges Reservoir, China. Environ. Sci. Technol. 2017;51:3794–3801.
https://doi.org/10.1021/acs.est.7b00369
76. Sun Y, Duan C, Cao N, et al. Effects of microplastics on soil microbiome: The impacts of polymer type, shape, and concentration. Sci. Total Environ. 2022;806:1–11.
https://doi.org/https://doi.org/10.1016/j.scitotenv.2021.150516
77. Xu M, Du W, Ai F, et al. Polystyrene microplastics alleviate the effects of sulfamethazine on soil microbial communities at different CO2 concentrations. J. Hazard. Mater. 2021;413:1–11.
https://doi.org/https://doi.org/10.1016/j.jhazmat.2021.125286
78. Lee S-H, Sorensen JW, Grady KL, Tobin TC, Shade A. Divergent extremes but convergent recovery of bacterial and archaeal soil communities to an ongoing subterranean coal mine fire. ISME J. 2017;11:1447–1459.
https://doi.org/10.1038/ismej.2017.1
79. Rillig M, Ryo M, Lehmann A, et al. The role of multiple global change factors in driving soil functions and microbial biodiversity. Science. 2019;366:886–890.
https://doi.org/10.1126/science.aay2832
80. Ogonowski M, Motiei A, Ininbergs K, et al. Evidence for selective bacterial community structuring on microplastics. Environ. Microbiol. 2018;20(8)2796–2808.
https://doi.org/10.1111/1462-2920.14120
81. Pinto M, Langer TM, Hüffer T, Hofmann T, Herndl GJ. The composition of bacterial communities associated with plastic biofilms differs between different polymers and stages of biofilm succession. PLoS One. 2019;14:1–20.
https://doi.org/10.1371/journal.pone.0217165
82. Tu C, Chen T, Zhou Q, et al. Biofilm formation and its influences on the properties of microplastics as affected by exposure time and depth in the seawater. Sci. Total Environ. 2020;734:1–9.
https://doi.org/10.1016/j.scitotenv.2020.139237
83. Pompilio A, Piccolomini R, Picciani C, D’Antonio D, Savini V, Di Bonaventura G. Factors associated with adherence to and biofilm formation on polystyrene by Stenotrophomonas maltophilia: the role of cell surface hydrophobicity and motility. FEMS Microbiol. Lett. 2008;287:41–47.
https://doi.org/10.1111/j.1574-6968.2008.01292.x
84. Mao R, Lang M, Yu X, Wu R, Yang X, Guo X. Aging mechanism of microplastics with UV irradiation and its effects on the adsorption of heavy metals. J. Hazard. Mater. 2020;393:1–9.
https://doi.org/https://doi.org/10.1016/j.jhazmat.2020.122515
85. Parrish K, Fahrenfeld N. Microplastic biofilm in fresh- and wastewater as a function of microparticle type and size class. Environ. Sci.: Water Res. Technol. 2019;5:1–25.
https://doi.org/10.1039/C8EW00712H
86. Foulon V, Le Roux F, Lambert C, Huvet A, Soudant P, Paul-Pont I. Colonization of polystyrene microparticles by Vibrio crassostreae: Light and electron microscopic investigation. Environ. Sci. Technol. 2016;50:10988–10996.
https://doi.org/10.1021/acs.est.6b02720
87. Nauendorf A, Krause S, Bigalke NK, et al. Microbial colonization and degradation of polyethylene and biodegradable plastic bags in temperate fine-grained organic-rich marine sediments. Mar. Pollut. Bull. 2016;103:168–178.
https://doi.org/10.1016/j.marpolbul.2015.12.024
88. Hahladakis JN, Velis CA, Weber R, Iacovidou E, Purnell P. An overview of chemical additives present in plastics: Migration, release, fate and environmental impact during their use, disposal and recycling. J. Hazard. Mater. 2018;344:179–199.
https://doi.org/10.1016/j.jhazmat.2017.10.014
89. Kong S, Ji Y, Liu L, et al. Diversities of phthalate esters in suburban agricultural soils and wasteland soil appeared with urbanization in China. Environ. Pollut. 2012;170:161–168.
https://doi.org/10.1016/j.envpol.2012.06.017
90. Kong X, Jin D, Jin S, et al. Responses of bacterial community to dibutyl phthalate pollution in a soil-vegetable ecosystem. J. Hazard. Mater. 2018;353:142–150.
https://doi.org/10.1016/j.jhazmat.2018.04.015
91. Cartwright C, Thompson I, Burns R. Degradation and impact of phthalate plasticizers on soil microbial communities. Environ. Toxicol. Chem. 2000;19:1253–1261.
https://doi.org/10.1002/etc.5620190506
92. Kirstein IV, Wichels A, Gullans E, Krohne G, Gerdts G. The Plastisphere - Uncovering tightly attached plastic “specific” microorganisms. PLoS One. 2019;14:1–17.
https://doi.org/10.1371/journal.pone.0215859
93. Xu X, Wang S, Gao F, et al. Marine microplastic-associated bacterial community succession in response to geography, exposure time, and plastic type in China’s coastal seawaters. Mar. Pollut. Bull. 2019;145:278–286.
https://doi.org/10.1016/j.marpolbul.2019.05.036
94. Keswani A, Oliver DM, Gutierrez T, Quilliam RS. Microbial hitchhikers on marine plastic debris: Human exposure risks at bathing waters and beach environments. Mar. Environ. Res. 2016;118:10–19.
https://doi.org/10.1016/j.marenvres.2016.04.006
95. Kesy K, Oberbeckmann S, Kreikemeyer B, Labrenz M. Spatial environmental heterogeneity determines young biofilm assemblages on microplastics in Baltic Sea mesocosms. Front. Microbiol. 2019;10:1–18.
https://doi.org/10.3389/fmicb.2019.01665
96. Curren E, Leong SCY. Profiles of bacterial assemblages from microplastics of tropical coastal environments. Sci. Total Environ. 2019;655:313–320.
https://doi.org/10.1016/j.scitotenv.2018.11.250
97. Pickett JE. Weathering of Plastics. Kutz M, editorHandbook of environmental degradation of materials. 3rd edOxford; William Andrew Publishing: 2018. p. 163–184.
98. Fotopoulou KN, Karapanagioti HK. Surface properties of beached plastics. Environ. Sci. Pollut. Res. 2015;22:11022–11032.
https://doi.org/10.1007/s11356-015-4332-y
99. Wei R, Zimmermann W. Microbial enzymes for the recycling of recalcitrant petroleum-based plastics: how far are we? Microb. Biotechnol. 2017;10:1308–1322.
https://doi.org/10.1111/1751-7915.12710
100. Dong M, Zhang Q, Xing X, Chen W, She Z, Luo Z. Raman spectra and surface changes of microplastics weathered under natural environments. Sci. Total Environ. 2020;739:1–9.
https://doi.org/https://doi.org/10.1016/j.scitotenv.2020.139990
101. Volke T, Saucedo-Castañeda G, Gutiérrez-Rojas M, Manzur A, Favela-Torres E. Thermally treated low density polyethylene biodegradation by Penicillium pinophilum and Aspergillus niger
. J. Appl. Polym. Sci. 2001;83:305–314.
https://doi.org/10.1002/app.2245
102. Ammalaa A, Batemana S, Deana K, et al. An overview of degradable and biodegradable polyolefins. Prog. Polym. Sci. 2010;36:1015–1049.
https://doi.org/10.1016/j.progpolymsci.2010.12.002
103. Krueger M, Harms H, Schlosser D. Prospects for microbiological solutions to environmental pollution with plastics. Appl. Microbiol. Biotechnol. 2015;99:8857–8874.
https://doi.org/10.1007/s00253-015-6879-4
104. Yuan J, Ma J, Sun Y, Zhou T, Zhao Y, Yu F. Microbial degradation and other environmental aspects of microplastics/plastics. Sci. Total Environ. 2020;715:1–9.
https://doi.org/10.1016/j.scitotenv.2020.136968
105. Miao L, Wang P, Hou J, et al. Distinct community structure and microbial functions of biofilms colonizing microplastics. Sci. Total Environ. 2019;650:2395–2402.
https://doi.org/10.1016/j.scitotenv.2018.09.378
106. Park SY, Kim CG. Biodegradation of micro-polyethylene particles by bacterial colonization of a mixed microbial consortium isolated from a landfill site. Chemosphere. 2019;222:527–533.
https://doi.org/10.1016/j.chemosphere.2019.01.159
107. Alshehrei F. Biodegradation of synthetic and natural plastic by microorganisms. J. Appl. Environ. Microbiol. 2017. 5:8–19.
http://pubs.sciepub.com/jaem/5/1/2
108. Valan Arasu M, Asha K, Duraipandiyan V, Ignacimuthu S, Agastian P. Characterization and phylogenetic analysis of novel polyene type antimicrobial metabolite producing actinomycetes from marine sediments: Bay of Bengal India. Asian Pac. J. Trop. Biomed. 2012;2:803–810.
https://doi.org/10.1016/S2221-1691(12)60233-0
109. Omidoyin KC, Femi-Ola TO. Isolation and screening of Streptomyces spp from soil samples of Ekiti State University Nigeria for antibacterial activity. Int. J. Res. Innovation Entrepreneurship. 2020;1:48–54.
https://doi.org/10.15294/ijrie.v1i1
110. Gohain A, Manpoong C, Saikia R, De Mandal S. Actinobacteria: diversity and biotechnological applications. De Mandal S, Bhatt P, editorsRecent Advancements in Microbial Diversity. Amsterdam: Academic Press; 2020. p. 217–231.
111. Sharma M, Dangi P, Choudhary M. Actinomycetes: Source, identification, and their applications. Int. J. Curr. Microbiol. Appl. Sci. 2014;3:801–832.
112. Jabloune R, Khalil M, Ben Moussa IE, et al. Enzymatic degradation of p-Nitrophenyl esters, polyethylene terephthalate, cutin, and suberin by Sub1, a suberinase encoded by the plant pathogen Streptomyces scabies
. Microbes Environ. 2020;35:1–7.
https://doi.org/10.1264/jsme2.ME19086
113. Petar P, Beaman B, Ravalison M, Boiron P, Rodriguez-Nava V. Nocardia and Actinomyces. Yi-Wei T, Andrew S, editorsMolecular Medical Microbiology. 2nd ed; Amsterdam: Academic Press; 2015. p. 731–752.
114. Auta HS, Emenike CU, Jayanthi B, Fauziah SH. Growth kinetics and biodeterioration of polypropylene microplastics by Bacillus sp. and Rhodococcus sp. isolated from mangrove sediment. Mar. Pollut. Bull. 2018;127:15–21.
https://doi.org/10.1016/j.marpolbul.2017.11.036
115. Gilan I, Sivan A. Effect of proteases on biofilm formation of the plastic-degrading actinomycete Rhodococcus ruber C208. FEMS Microbiol. Lett. 2013;342:18–23.
https://doi.org/10.1111/1574-6968.12114
116. Sivan A, Szanto M, Pavlov V. Biofilm development of the polyethylene-degrading bacterium Rhodococcus ruber
. Appl. Microbiol. Biotechnol. 2006;72:346–352.
https://doi.org/10.1007/s00253-005-0259-4
117. Farzi A, Dehnad A, Fotouhi A. Biodegradation of polyethylene terephthalate waste using Streptomyces species and kinetic modeling of the process. Biocatal. Agric. Biotechnol. 2018;17:25–31.
https://doi.org/10.1016/j.bcab.2018.11.002
118. Bonhomme S, Cuer A, Delort A-M, Lemaire J, Sancelme M, Scott G. Environmental biodegradation of polyethylene. Polym. Degrad. Stab. 2003;81:441–452.
https://doi.org/10.1016/S0141-3910(03)00129-0
119. Kathiresan K. Polythene and plastic-degrading microbes in an Indian mangrove soil. Rev. Biol. Trop. 2003;51:629–633.
120. Nowak B, Pająk J, Drozd-Bratkowicz M, Rymarz G. Microorganisms participating in the biodegradation of modified polyethylene films in different soils under laboratory conditions. Int. Biodeterior. Biodegradation. 2011;65:757–767.
https://doi.org/10.1016/j.ibiod.2011.04.007
121. Farzi A, Dehnad A, Shirzad N, Norouzifard F. Biodegradation of high density polyethylene using Streptomyces species. J. Coastal Life Med. 2017;5:474–479.
https://doi.org/10.12980/jclm.5.2017J7-94
122. Rajandas H, Parimannan S, Sathasivam K, Ravichandran M, Lee S. A novel FTIR-ATR spectroscopy based technique for the estimation of low-density polyethylene biodegradation. Polym. Test. 2012;31:1094–1099.
https://doi.org/10.1016/j.polymertesting.2012.07.015
123. Midhun K, Kalyani L, Guntuku G, Dandu S. Biodegradation of low density polyethylene (LDPE) by a new biosurfactant-producing thermophilic Streptomyces coelicoflavus NBRC 15399T. Afr. J. Biotechnol. 2015;14:327–340.
https://doi.org/10.5897/AJB2014.14224
124. Pometto AL, Lee BT, Johnson KE. Production of an extracellular polyethylene-degrading enzyme(s) by Streptomyces species. Appl. Environ. Microbiol. 1992;58:731–733.
https://doi.org/10.1128/aem.58.2.731-733.1992
125. Abraham J, Ghosh E, Mukherjee P, Gajendiran A. Microbial degradation of low density polyethylene. Environ. Prog. Sustain. Energy. 2016;36:1–8.
https://doi.org/10.1002/ep.12467
126. Mor R, Sivan A. Biofilm formation and partial biodegradation of polystyrene by the actinomycete Rhodococcus ruber: biodegradation of polystyrene. Biodegradation. 2008;19:851–858.
https://doi.org/10.1007/s10532-008-9188-0
127. Fontanella S, Bonhomme S, Brusson J-M, et al. Comparison of biodegradability of various polypropylene films containing additives based on Mn, Mn/Fe or Co. Polym. Degrad. Stab. 2013;98:875–884.
https://doi.org/10.1016/j.polymdegradstab.2013.01.002
128. Usha R, Sangeetha T, Muthusamy P. Screening of polyethylene degrading microorganisms from garbage soil. Libyan Agric. Res. Cen. J. Int. 2011;2:200–2014.
129. Shah A, Hasan F, Akhter J, Hameed A, Ahmed S. Degradation of polyurethane by novel bacterial consortium isolated from soil. Ann. Microbiol. 2008;58:381–386.
https://doi.org/10.1007/BF03175532
130. El-Shafei H, Alborki AN, Kansoh A, Ali A. Biodegradation pf disposable polyethylene by fungi Streptomyces species. Polym. Degrad. Stab. 1998;62:361–365.
https://doi.org/10.1016/S0141-3910(98)00019-6
131. Jarerat A, Tokiwa Y, Tanaka H. Production of poly(L-lactide)-degrading enzyme by Amycolatopsis orientalis for biological recycling of poly(L-lactide). Appl. Microbiol. Biotechnol. 2006;72:726–731.
https://doi.org/10.1007/s00253-006-0343-4
132. Jarerat A, Tokiwa Y. Poly(L-lactide) degradation by Saccharothrix waywayandensis
. Biotechnol. Lett. 2003;25:401–404.
https://doi.org/10.1023/a:1022450431193
133. Jarerat A, Tokiwa Y, Tanaka H. Poly(L-lactide) degradation by Kibdelosporangium aridum
. Biotechnol. Lett. 2003;25:2035–2038.
https://doi.org/10.1023/b:bile.0000004398.38799.29
134. Pranamuda H, Tokiwa Y, Tanaka H. Polylactide Degradation by an Amycolatopsis sp. Appl. Environ. Microbiol. 1997;63:1637–1640.
https://doi.org/10.1128/aem.63.4.1637-1640.1997
135. Sukkhum S, Tokuyama S, Kitpreechavanich V. Development of fermentation process for PLA-degrading enzyme production by a new thermophilic Actinomadura sp. T16–1. Biotechnol. Bioprocess Eng. 2009;14:302–306.
https://doi.org/10.1007/s12257-008-0207-0
136. Bakir A, Rowland SJ, Thompson RC. Enhanced desorption of persistent organic pollutants from microplastics under simulated physiological conditions. Environ. Pollut. 2014;185:16–23.
https://doi.org/10.1016/j.envpol.2013.10.007
137. Auta HS, Emenike CU, Fauziah SH. Screening of Bacillus strains isolated from mangrove ecosystems in Peninsular Malaysia for microplastic degradation. Environ. Pollut. 2017;231:1552–1559.
https://doi.org/10.1016/j.envpol.2017.09.043
138. Urbanek AK, Rymowicz W, Mirończuk AM. Degradation of plastics and plastic-degrading bacteria in cold marine habitats. Appl. Microbiol. Biotechnol. 2018;102:7669–7678.
https://doi.org/10.1007/s00253-018-9195-y
139. Muhonja CN, Makonde H, Magoma G, Imbuga M. Biodegradability of polyethylene by bacteria and fungi from Dandora dumpsite Nairobi-Kenya. PLoS One. 2018;13:1–17.
https://doi.org/10.1371/journal.pone.0198446
140. Gaytán I, Sánchez-Reyes A, Burelo M, et al. Degradation of recalcitrant polyurethane and xenobiotic additives by a selected landfill microbial community and its biodegradative potential revealed by proximity ligation-based metagenomic analysis. Front. Microbiol. 2019;10:1–19.
https://doi.org/10.3389/fmicb.2019.02986
141. Yoshida S, Hiraga K, Takehana T, et al. A bacterium that degrades and assimilates poly(ethylene terephthalate). Science. 2016;351:1196–1199.
https://doi.org/10.1126/science.aad6359
142. Ren L, Men L, Zhang Z, et al. Biodegradation of polyethylene by Enterobacter sp. D1 from the guts of wax moth Galleria mellonella
. Int. J. Environ. Res. Public Health. 2019;16:1–11.
https://doi.org/10.3390/ijerph16111941
143. Janssen PH, Yates PS, Grinton BE, Taylor PM, Sait M. Improved culturability of soil bacteria and isolation in pure culture of novel members of the divisions Acidobacteria, Actinobacteria, Proteobacteria, and Verrucomicrobia. Appl. Environ. Microbiol. 2002;68:2391–2396.
https://doi.org/10.1128/aem.68.5.2391-2396.2002
144. Mahdi M, Ameen R, Ibrahim H. Study on degradation of nylon 6 by thermophilic bacteria Anoxybacillus rupiensis Ir3 (JQ912241). Int. J. Adv. Res. Biol. Sci. 2016;3:200–209.
http://dx.doi.org/10.22192/ijarbs.2016.03.09.027
145. Ambika Devi K, Lakshmi BKM, Hemalatha KPJ. Degradation of low density polythene by Achromobacter denitrificans strain S1, a novel marine isolate. Int. J. Rec. Sci. Res. 2015;6:5454–5464.
146. Yang J, Yang Y, Wu WM, Zhao J, Jiang L. Evidence of polyethylene biodegradation by bacterial strains from the guts of plastic-eating waxworms. Environ. Sci. Technol. 2014;48:13776–13784.
https://doi.org/10.1021/es504038a
147. Pramila R, Ramesh KV. Potential biodegradation of low density polyethylene (LDPE) by Acinetobacter baumannii
. Afr. J. Bacteriol. Res. 2015;7:24–28.
https://doi.org/10.5897/JBR2015.0152
148. Syranidou E, Karkanorachaki K, Amorotti F, et al. Development of tailored indigenous marine consortia for the degradation of naturally weathered polyethylene films. PLoS One. 2017;12:1–21.
https://doi.org/10.1371/journal.pone.0183984
149. Shahnawaz M, Sangale MK, Ade AB. Analysis of the plastic degradation products. Shahnawaz M, Sangale MK, Ade AB, editorsBioremediation technology for plastic waste. Singerpore: Springer; 2019. p. 93–101.
150. Novotný C, Malachová K, Adamus G, et al. Deterioration of irradiation/high-temperature pretreated, linear low-density polyethylene (LLDPE) by Bacillus amyloliquefaciens
. Int. Biodeterior. Biodegradation. 2018;132:259–267.
https://doi.org/10.1016/j.ibiod.2018.04.014
151. Umamaheswari S, Subramani M. GCMS Analysis of Pseudomonas sp., mediated degradation of polystyrene. Ann. Biol. Res. 2017;8:8–11.
152. Uscátegui YL, Arévalo FR, Díaz LE, Cobo MI, Valero MF. Microbial degradation, cytotoxicity and antibacterial activity of polyurethanes based on modified castor oil and polycaprolactone. J. Biomater. Sci. Polym. Ed. 2016;27:1860–1879.
https://doi.org/10.1080/09205063.2016.1239948
153. Shimpi N, Borane M, Mishra S, Kadam M. Biodegradation of polystyrene (PS)-poly(lactic acid) (PLA) nanocomposites using Pseudomonas aeruginosa
. Macromol. Res. 2012;20:181–187.
https://doi.org/10.1007/s13233-012-0026-1
154. Jeon H, Kim M. Biodegradation of poly(L-lactide) (PLA) exposed to UV irradiation by a mesophilic bacterium. Int. Biodeterior. Biodegradation. 2013;85:289–293.
https://doi.org/10.1016/j.ibiod.2013.08.013
155. Kathirvel B, Mathimani T, Rene E, Shanmugam S, Chi N, Pugazhendhi A. Impact of cultivation conditions on the biomass and lipid in microalgae with an emphasis on biodiesel. Fuel. 2020;284:1–8.
https://doi.org/10.1016/j.fuel.2020.119058
156. Correa D, Beyer H, Possingham H, Fargione J, Hill J, Schenk P. Microalgal biofuel production at national scales: Reducing conflicts with agricultural lands and biodiversity within countries. Energy. 2020;215:1–12.
https://doi.org/10.1016/j.energy.2020.119033
157. Mustafa S, Bhatti HN, Maqbool M, Iqbal M. Microalgae biosorption, bioaccumulation and biodegradation efficiency for the remediation of wastewater and carbon dioxide mitigation: Prospects, challenges and opportunities. J. Water Process Eng. 2021;41:1–15.
https://doi.org/https://doi.org/10.1016/j.jwpe.2021.102009
158. Shanmugam S, Hari A, Kumar D, et al. Recent developments and strategies in genome engineering and integrated fermentation approaches for biobutanol production from microalgae. Fuel. 2021;285:1–9.
https://doi.org/10.1016/j.fuel.2020.119052
159. Sarmah P, Rout J. Algal colonization on polythene carry bags in a domestic solid waste dumping site of Silchar town in Assam. Phykos. 2018;48:67–77.
160. Wang F, Zhang X, Zhang S, Zhang S, Sun Y. Interactions of microplastics and cadmium on plant growth and arbuscular mycorrhizal fungal communities in an agricultural soil. Chemosphere. 2020;254:1–10.
https://doi.org/10.1016/j.chemosphere.2020.126791
161. Khoironi A, Anggoro S, Sudarno S. Evaluation of the interaction among microalgae Spirulina sp, plastics polyethylene terephthalate and polypropylene in freshwater environment. J. Ecol. Eng. 2019;20:161–173.
https://doi.org/10.12911/22998993/108637
162. Gopal R. Biodegradation of polyethylene by green photosynthetic microalgae. J. Bioremediat. Biodegradation. 2017;8:1–8.
https://doi.org/10.4172/2155-6199.1000381
163. Hadad D, Geresh S, Sivan A. Biodegradation of polyethylene by the thermophilic bacterium Brevibacillus borstelensis
. J. Appl. Microbiol. 2005;98:1093–1100.
https://doi.org/10.1111/j.1365-2672.2005.02553.x
164. Paço A, Duarte K, da Costa JP, et al. Biodegradation of polyethylene microplastics by the marine fungus Zalerion maritimum
. Sci. Total Environ. 2017;586:10–15.
https://doi.org/10.1016/j.scitotenv.2017.02.017
165. Dineshbabu G, Uma V, Mathimani T, Prabaharan D. Elevated CO2 impact on growth and lipid of marine cyanobacterium Phormidium valderianum BDU 20041–towards microalgal carbon sequestration. Biocatal. Agric. Biotechnol. 2020;25:1–7.
https://doi.org/10.1016/j.bcab.2020.101606
166. Jareonsin S, Pumas C. Advantages of heterotrophic microalgae as a host for phytochemicals production. Front. Bioeng. Biotechnol. 2021;9:1–17.
https://doi.org/10.3389/fbioe.2021.628597
167. Hoffmann L, Eggers SL, Allhusen E, Katlein C, Peeken I. Interactions between the ice algae Fragillariopsis cylindrus and microplastics in sea ice. Environ. Int. 2020;139:1–9.
https://doi.org/10.1016/j.envint.2020.105697
168. Matavuly M, Peter M. Marine fungi: Degraders of poly-3-hydroxyalkanoate based plastic materials. Zb. Matice Srp. Prir. Nauke. 2009;116:253–265.
https://doi.org/10.2298/ZMSPN0916253M
169. Chen Y, Stemple B, Kumar M, Wei N. Cell surface display fungal laccase as a renewable biocatalyst for degradation of persistent micropollutants bisphenol A and sulfamethoxazole. Environ. Sci. Technol. 2016;50:8799–8808.
https://doi.org/10.1021/acs.est.6b01641
170. Ong H, Ling Shing W. The growth and laccase activity of edible mushrooms involved in plastics degradation. Curr. Top. Toxicol. 2019;15:57–62.
171. Mitik-Dineva N, Wang J, Truong VK, et al.
Escherichia coli, Pseudomonas aeruginosa, and Staphylococcus aureus attachment patterns on glass surfaces with nanoscale roughness. Curr. Microbiol. 2009;58:268–273.
https://doi.org/10.1007/s00284-008-9320-8
172. da Luz JMR, da Silva MdCS, dos Santos LF, Kasuya MCM. Plastics polymers degradation by fungi. Blumenberg M, Shaaban M, Elgaml A, editorsMicroorganisms. London: IntechOpen; 2019. p. 261–270.
173. Yamada-Onodera K, Mukumoto H, Katsuyaya Y, Saiganji A, Tani Y. Degradation of polyethylene by a fungus, Penicillium simplicissimum YK. Polym. Degrad. Stab. 2001;72:323–327.
https://doi.org/10.1016/S0141-3910(01)00027-1
174. Sowmya HV, Ramalingappa , Krishnappa M, Thippeswamy B. Degradation of polyethylene by Trichoderma harzianum-SEM, FTIR, and NMR analyses. Environ. Monit. Assess. 2014;186:6577–6586.
https://doi.org/10.1007/s10661-014-3875-6
175. Gajendiran A, Krishnamoorthy S, Abraham J. Microbial degradation of low-density polyethylene (LDPE) by Aspergillus clavatus strain JASK1 isolated from landfill soil. 3 Biotech. 2016;6:1–6.
https://doi.org/10.1007/s13205-016-0394-x
176. Chaudhary AK, Vijayakumar R. Studies on biological degradation of polystyrene by pure fungal cultures. Environ. Dev. Sustain. 2020;22:4495–4508.
https://doi.org/10.1007/s10668-019-00394-5
177. Yanto D, Krishanti N, Ardiati F, et al. Biodegradation of styrofoam waste by ligninolytic fungi and bacteria. IOP Conf. Ser.: Earth Environ. Sci. 2019;308:1–11.
https://doi.org/10.1088/1755-1315/308/1/012001
178. Brunner I, Fischer M, Rüthi J, Stierli B, Frey B. Ability of fungi isolated from plastic debris floating in the shoreline of a lake to degrade plastics. PLoS One. 2018;13:1–14.
https://doi.org/10.1371/journal.pone.0202047
179. Álvarez-Barragán J, Domínguez-Malfavón L, Vargas-Suárez M, González-Hernández R, Aguilar-Osorio G, Loza-Tavera H. Biodegradative activities of selected environmental fungi on a polyester polyurethane varnish and polyether polyurethane foams. Appl. Environ. Microbiol. 2016;82:5225–5235.
https://doi.org/10.1128/aem.01344-16
180. Cosgrove L, McGeechan PL, Robson GD, Handley PS. Fungal communities associated with degradation of polyester polyurethane in soil. Appl. Environ. Microbiol. 2007;73:5817–5824.
https://doi.org/10.1128/aem.01083-07
181. Khan S, Nadir S, Shah ZU, et al. Biodegradation of polyester polyurethane by Aspergillus tubingensis
. Environ. Pollut. 2017;225:469–480.
https://doi.org/10.1016/j.envpol.2017.03.012
182. Ali M, Perveen Q, Ahmad B, et al. Studies on biodegradation of cellulose blended polyvinyl chloride films. Int. J. Agric. Biol. 2009;11:577–580.
183. Sumathi T, Viswanath B, Sri Lakshmi A, SaiGopal DV. Production of laccase by Cochliobolus sp. isolated from plastic dumped soils and their ability to degrade low molecular weight PVC. Biochem. Res. Int. 2016;2016:1–10.
https://doi.org/10.1155/2016/9519527
184. Jarerat A, Tokiwa Y. Degradation of Poly(L-lactide) by a fungus. Macromol. Biosci. 2001;1:136–140.
https://doi.org/10.1002/1616-5195(20010601)1:43.0.CO;2-3
185. Karamanlioglu M, Houlden A, Robson GD. Isolation and characterisation of fungal communities associated with degradation and growth on the surface of poly(lactic) acid (PLA) in soil and compost. Int. Biodeterior. Biodegradation. 2014;95:301–310.
https://doi.org/10.1016/j.ibiod.2014.09.006
186. Lipsa R, Tudorachi N, Darie-Nita RN, Oprică L, Vasile C, Chiriac A. Biodegradation of poly(lactic acid) and some of its based systems with Trichoderma viride
. Int. J. Biol. Macromol. 2016;88:515–526.
https://doi.org/10.1016/j.ijbiomac.2016.04.017
Table 1Response of Soil Microbial Communities to Different Plastics
Table 2Plastic Degradation by Actinomycetes, Biodegradation Assay Conditions, and Applied Detection Methods
PE: Polyethylene; PET: Polyethylene terephthalate; HDPE: High-density polyethylene; LDPE: Low-density polyethylene; PS: Polystyrene; PP: Polypropylene; PU: Polyurethane; PLA: Polylactic acid; SEM: Scanning electron microscope; FTIR: Fourier-transform infrared spectroscopy; GPC: Gel permeation chromatography; AFM: Atomic force microscopy; NMR: Nuclear magnetic resonance spectroscopy; ADP: Adenosine diphosphate; ATP: Adenosine triphosphate Table 3Plastic degradation by bacteria, biodegradation assay conditions, and applied detection methods
PE: Polyethylene; PET: Polyethylene terephthalate; PS: Polystyrene; PP: Polypropylene; PU: Polyurethane; PLA: Polylactic acid; HPLC: High-performance liquid chromatography; FTIR: Fourier-transform infrared spectroscopy; SEM: Scanning electron microscope; NMR: Nuclear magnetic resonance spectroscopy; XRD: X-Ray diffraction analysis; GC-MS: Gas chromatography–mass spectrometry; GPC: Gel permeation chromatography; DSC: Differential scanning calorimetry; TGA: Thermogravimetric analysis; ESI-MS: Electrospray ionization mass spectrometry, Table 4Plastic degradation by fungi, biodegradation assay conditions, and applied detection methods
PE: Polyethylene; PS: Polystyrene; PU: Polyurethane; PVC: Polyvinyl chloride; PLA: Polylactic acid; FTIR: Fourier-transform infrared spectroscopy; SEM: Scanning electron microscope; GC-MS: Gas chromatography–mass spectrometry; GPC: Gel permeation chromatography; NMR: Nuclear magnetic resonance spectroscopy; AFM: Atomic force microscopy |
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||