AbstractThe aim of this study was to synthesize novel and highly efficient functionalized material (BNMPTS) for selective elimination of Cu2+ and Cd2+ from aqueous waste. The detailed insights of solid/solution interactions were investigated by X-Ray photoelectron spectroscopic analyses. The grafting of silane caused for significant decrease in specific surface area of bentonite from 41.14 to 4.65 m2/g. The functionalized material possessed significantly high sorption capacity (12.59 mg/g for Cu2+ and 13.19 mg/g for Cd2+) and selectivity for these cations. The material showed very high elimination efficiency at a wide range of pH ~2.0 to 7.0 for Cu2+, ~3.0 to 10.0 for Cd2+ and concentration (1.0 to 25.0 mg/L) for Cu2+ and Cd2+. A rapid uptake of these two cations achieved an apparent equilibrium within 60 minutes of contact. The increased level of background electrolyte concentrations (0.0001 to 0.1 mol/L) did not affect the elimination efficiency of these two cations by BNMPTS. Moreover, the common coexisting ions did not inhibit the removal of these toxic ions. Furthermore, high breakthrough volumes i.e., 1.4 and 3.69 L for Cu2+, 2.6 and 6.64 L for Cd2+ was obtained using 0.25 and 0.50 g of BNMPTS respectively in a fixed-bed column operations.
1. IntroductionWater is a basic requirement for the survival of human beings and the supply of clean water is greatly required for healthy life; however, the shortage of clean water is a serious concern worldwide. The contamination of water bodies with several toxic heavy metal ions is a widespread concerns for environmentalists due to its distinctive physico-chemical properties such as non-biodegradability, high toxicity, and persistence in nature. The natural phenomena such as soil and rock erosions are partly responsible for contamination of aquatic environment; however, the anthropogenic activities are greatly contaminating the water bodies by variety of toxic heavy metal ions [1–5]. Among the toxic heavy metals, Cr, Pb, Fe, Cu, Ni, etc are excessively discharged into the environment through human activities.
Copper is an essential element in the human body at trace level but becomes toxic at higher concentration levels. The toxicity of copper includes liver and kidney dysfunction, abdominal and intestinal anxiety, anaemia, etc. [6, 7]. The presence of copper generates free reactive oxygen species in blood which seemingly damages DNA, proteins and lipids [8]. Therefore, because of its toxic nature the regulatory bodies demonstrated that the maximum contamination level of Cu in potable water is 1.5 mg/L [9]. On the other hand, cadmium (Cd) is another potential heavy metal often occurs in wastewaters. Cadmium toxicity causes nausea, itai itai disease, salivation, diarrhoea, respiratory problems, skeletal malformation, hypertension and renal calculi, etc. In some enzymes, zinc (II) ions are readily replaced by cadmium (II) ions, thereby disturbing the enzyme activity in bio-systems. Additionally, the cadmium is known to be a potential carcinogen hence, the IARC has classified Cd2+ as category 1 human carcinogen [10, 11]. The maximum contamination level of cadmium in drinking water was prescribed as low as 0.003 mg/L [9].
The decontamination of water bodies from heavy metal toxic ions is proposed by different methods which greatly includes with adsorption, membrane filtration, coagulation, reverse osmosis etc. These methods are having its own merits and demerits in terms of the operational cost, procedure, subsequent environmental impact etc. However, among these methods, the adsorption is considered to be simple and cost-effective since it offers the possible utilization of naturally abundant materials for the removal of various water pollutants [12, 13].
Clay minerals are natural filter media eliminating a variety of pollutants from aqueous media. Clay minerals possessed a large number of exchangeable cations which could play an important role in the adsorption process [14, 15]. These materials are ubiquitous and relatively low in cost as compared to the activated carbon commonly used as filter media for water purification [16]. Previous reports indicated that clay and clay-based materials are highly effective in removing toxic heavy metals from aquatic environments [17–19]. Bentonite is abundant clay having layered phyllosilicates. Similar to other clay minerals, bentonite possesses exchangeable cations and distinct porous structure which makes bentonite a suitable and efficient adsorbent material [20, 21]. The adsorption ability of bentonite is primarily defined by its pore structure and the cation exchange capacity (CEC) [22]. However, the use of pristine bentonite in real water treatment showed inherent limitations including the selectivity of materials, the swelling nature of clay and most importantly the settling capacity of solids. Therefore, the intended modifications of the clay materials were previously reported which overcome partly the limitations of clay minerals for its greater use in water decontamination studies [23–29]. Surfactants are commonly employed for the surface modification of clay minerals; however, the surfactant loaded clay materials showed its limitations since the surfactant molecules easily desorb and making secondary pollution of water bodies [30]. Therefore, grafting/incorporation of organosilanes with the clay minerals showed an impetus for researchers since the silane grafted composite materials possessed significantly enhanced stability. Moreover, the selective introduction of functional group through specific silanes enables the selectivity of materials for targeted pollutants in aqueous wastes that enhances greater applicability of materials for wider applications in various waste water treatment strategies [11, 24]. It was reported previously that the reaction conditions, surface hydroxyl group concentration and silane characteristics play a vital role in successful sialylation of clay minerals [24]. Therefore, the present communication is intended to functionalize the bentonite with the thiol group (-SH) using the 3-mercaptopropyltrimethoxysilane (MPTS) molecules using a simple one step process. Further, the functionalized material was employed for the removal of copper (II) and cadmium (II) under batch and column reactor studies. The mechanistic aspects involved in the sorption of copper (II) and cadmium (II) by the surface of functionalized bentonite was extensively studied. Further, the efficient use of functionalized natural clay in the dynamic reactor operations could possibly enable the scaling up of the technology for real treatment of aqueous wastes.
2. Materials and Methods2.1. MaterialsBentonite (BN) as mined at Bhuj, Gujarat, India was collected and utilized in the studies. The pristine bentonite (particle size of 0.15 mm) was thoroughly washed using purified water and then employed for further use. 3-mercaptopropyletrimethoxy silane (Sigma Aldrich, USA), CdSO4, and CuSO4.5H2O (Kanto Chemical Co. In., Japan), oxalic acid glycine (Daejung Chemicals and Metals Co. Ltd., Korea) were utilized. Moreover, manganese (II) chloride, ethylenediaminetetraacetic acid, nickel chloride and sodium phosphate are procured from Duksan Pure Chemicals Co. Ltd., Korea. Magnesium sulfate heptahydrate and calcium chloride dihydrate powder were purchased from Samchun Pure Chem. and Metals Co. Ltd., Korea.
2.2. Methodology2.2.1. Grafting of silane with bentonite3-mercaptopropyletrimethoxy silane was grafted with the bentonite network using slightly modified method [31, 32]. Briefly, 30 g of bentonite was dispersed in 600 mL of toluene. The suspension was refluxed under stirring for 30 min at 60°C under nitrogen atmosphere. 30 mL of 3-mercaptopropyletrimethoxy silane was then added dropwise to this suspension and it was further refluxed for 48 h. The suspension was carefully filtered and the solid was thoroughly washed by toluene (20 mL) followed by ethanol (50 mL). The material was dried at 100°C for 24 h. The silane grafted bentonite was carefully stored in a sealed polyethylene bottle and named as BNMPTS.
2.2.2. CharacterizationsThe FT-IR data of pristine bentonite (BN) and functionalized bentonite (BNMPTS) were obtained by using FT-IR (Affinity-1 Shimadzu, Japan) machine. The surface images of BN and BNMPTS were taken by the SEM (FE-SEM; S-4700, Hitachi, Japan). Energy-dispersive X-ray spectroscopic analysis (EDAX) was employed to obtain the elemental composition of BN and BNMPTS. The X-ray diffraction results were obtained using the XRD machine (PANalytical, Netherland). BET Analyzer (Model-1201)) was utilized to measure the specific surface area determination of silids. Moreover, the X-ray photoelectron spectroscopy (XPS) (Model: ESCALAB-250Xi+) analyses were conducted to obtain greater insights of the interaction of sorbate ions with BNMPTS. Further, the pHPZC of bentonite and BNMPTS were obtained following the known method which was reported previously [33].
2.2.3. Batch reactor operationsThe flow-chart showing the experimental design of the present research work is shown in Fig. S1. The stock solutions (100.0 mg/L) of each copper (II) sulphate pentahydrate and cadmium (II) sulphate were prepared by dissolving an appropriate amount of copper (II) and cadmium (II) in purified water. Further, the required experimental concentrations of copper (II) and cadmium (II) were obtained by successive dilution of stock solutions. Batch reactor operations in the removal of copper (II) or cadmium (II) by solids were carried out using 60 mL polyethylene bottles. The pH dependent adsorption studies was performed by taking 50.0 mL of 10.0 mg/L copper (II) and 5.0 mg/L cadmium (II) solutions in different polyethylene bottles and the pH of each solutions were adjusted with drop wise addition of 0.1 mol/L HCl/NaOH solutions. Further, 0.1 g of solid (BN or BNMPTS) was introduced in each bottles and bottles were tightly closed. These experimental bottles were kept in the shaking incubator (KUJKE, Shaking Incubator, Korea; Model 36-SIN-125) and solution mixtures were shaken for 24 h at 25±1°C. The solution mixtures were then filtered by syringe filters (0.45 μm). Filtrates were subjected for measurement of sorbate concentrations using the atomic absorption spectrometer (AAS) (Model AA240FS, Varian, Australia). The blank sorbate concentration was also obtained and hence, the percentage removal of these cations was computed.
Similarly, the concentration dependence studies were conducted varying the metal ion concentrations from 1.0 to 25.0 mg/L at pH 4.0 and 5.0 for Cu(II) and Cd(II), respectively. The remaining adsorption experiments were performed as described previously and the percentage removal of these ions as a function of sorbate concentration is computed. The time dependent adsorption was carried out using Cu (II) and Cd(II) concentrations 10.0 mg/L (pH 4.0) and 5.0 mg/L (pH 5.0), respectively. The removal of Cu(II) and Cd(II) as a function of time is obtained. The detailed procedure was described previously [15]. The Background electrolyte dependent elimination of these two cations was studied by adding accurate amounts of NaCl in sorptive solution as to obtain the NaCl concentrations of 0.0001, 0.001, 0.01 and 0.1 mol/L. The pH of Cu(II) and Cd(II) was maintained to 4.0 and 5.0, respectively. The percentage removals of these two cations were obtained as a function of background electrolyte conecntrations. The competitive sorption was extensively studied in presence of several co-anions (ethylenediaminetetraacetic acid, glycine, oxalic acid and phosphate) and co-cations (Mg2+, Mn2+, Ni2+ and Ca2+). The adsorption experiments were conducted using the co-ions concentrations of 50.0 mg/L at 25°C by keeping Cu2+ and Cd2+ concentrations 10.0 mg/L at pH 4.0 and 5.0 mg/L at pH 5.0, respectively.
2.2.4. Fixed bed column reactor operationsColumn studies were conducted employing a glass column (length 30 cm and inner diameter 1 cm). The glass beads were used having the diameter of 1 mm. The column was packed with 0.25 g or 0.5 g of BNMPTS solid. The BNMPTS material was safely placed in the middle of the column and 1.0 g of sand was kept below and above to the BNMPTS solid. The remaining space in the column was packed with glass beads. Cu2+ (10.0 mg/L) or Cd2+ (5.0 mg/L) solution was pumped upward using the peristaltic pump having a flow rate of 1.0 mL/min. A fraction collector was used to collect the effluent volume. Effluent was filtered by 0.45 μm filter and the filtrate was subjected for the analysis of Cu(II) or Cd(II) concentrations using the AAS. The loading capacity of BNMPTS was obtained by modelling the breakthrough data with non-linear Thomas equation which is reported previously [7].
3. Results and Discussions3.1. Characterization of the MaterialsThe FT-IR spectra of pristine bentonite and functionalized bentonite (BNMPTS) are illustrated in Fig. S2(a). A prominent vibrational peak appeared at wavenumber 3,365 cm−1 which was due to the stretching vibrations of O-H of the silanol group. Weak vibrational bands appeared at 2913 and 2,837 cm−1 were assigned as stretching vibrations of −CH3 and −CH2, respectively [32, 34]. The methoxy group (−OCH3) of MPTS possessed a characteristic vibration at 2,914 cm−1 [32, 35]. A stretching vibrational peak at 2,550 cm−1 was due to the sulfhydryl group of MPTS [36]. Further, C-H deformation and C-S stretching vibrations of the sulfhydryl group of MPTS were observed at 682 and 1,402 cm−1, respectively [37]. These results confirmed that MPTS is bonded with the bentonite network. Further, the XRD peaks of bentonite and BNMPTS solids are given in Table S1. There is no significant change in the peak positions after the incorporation of organosilane. The prominent peaks obtained at 2 theta values of 20.95, 26.78, 36.68, 50.26, 60, and 68.36 indicated the presence of quartz in the clay sample [38, 39].
The BET method is employed to obtain the N2 adsorption/desorption isotherms for BN and BNMPTS solids (Fig. S2(b)). Fig. S2(b) exhibited type IV isotherm having H3 type hysteresis loop which indicates the mesoporous structure of materials [40]. Further, the pristine bentonite showed relatively a wider hysteresis loop and the pore size and pore volume were 80.92 Å and 0.070 cm3/g, respectively. The pore size and pore volume of bentonite are notably changed and BNMPTS material possessed 237.91 Å and 0.027 cm3/g of pore size and pore volumes, respectively. The BET specific surface area of BN and BNMPTS were obtained as 41.14 to 4.65 m2/g, respectively. The surface area of bentonite clay was considerably decreased after the grafting of MPTS since the silane appeared to occupy the places within the galleries of bentonite. A significant reduction in the surface area of smectite after functionalization with MPTS was reported previously [34]. Moreover, a similar decrease in surface area of bentonite was observed with the modification of bentonite by hexadecyl trimethyl ammonium (HDTMA) [39]. The immobilization of MPTS onto sepiolite caused for a significant decrease in surface area of clay [36, 41] and the surface area of SBA-15 modified with mercapto- or amino-propyl had resulted in the reduction of specific surface area of the material [42].
The SEM micrograph of BN is displayed in Fig. 1(a) whereas that of BNMPTS is shown in Fig. 1(b). The BN and BNMPTS showed heterogeneous and disordered surface structures. Moreover, it is clearly visible from the SEM images that the pristine bentonite possessed more porosity and the porosity is drastically reduced after the grafting of MPTS molecules with the bentonite. This observation is in line with the textural properties obtained by the BET surface area results. Furthermore, EDX analyses were performed for BN (Fig. 1(c)) and BNMPTS (Fig. 1(d)). The EDX analytical graph showed that the major elemental composition of bentonite included with Si, Al, O and Fe along with Na and K elements at lesser extent. It is noteworthy to observe that the EDX spectrum of BNMPTS was contained with a considerable amount of sulphur which was not observed in pristine bentonite. This indicated that the mercapto (−SH) group was successfully incorporated to bentonite and possibly formed a stable bonding on bentonite surface. Similar results were reported in which the sulphur composition corresponding to the mercapto (−SH) group were observed with kaolinite [32, 35, 43].
3.2. Batch Reactor Studies3.2.1. pH dependence studiesPercentage elimination of Cu2+ and Cd2+ by pristine bentonite and BNMPTS material at various pH values is shown in Fig. 2(a) and 2(b). It is interesting to observe that the grafting of MPTS onto bentonite clay has enabled to increase the percentage removal of these two toxic ions and almost 100% of Cu2+ was removed within the pH range ~2.0 to 7.0 using BNMPTS. Moreover, a high percentage (Ca 99%) of Cd2+ was successfully removed within pH ~3.0 to 10.0. The pH dependent study revealed that BNMPTS possessed high affinity towards Cu2+ and Cd2+ which showed potential applicability of the material in the remediation of aqueous waste containing these toxic contaminants. The pHpzc of the BNMPTS was determined and found to be 7.73. This indicated that BNMPTS possessed net positive charge at pH lower than 7.73 and it is having net negative charge above pH 7.73. Besides, the speciation of Cu2+ (Fig. S3(a)) shows that copper exists as Cu2+ species up to pH 5.5. Between pH 5.0–7.0, Cu2+ exists as insignificant of Cu(OH)+ species with a maximum of 1.4% only. Further, Cu2+ starts to form insoluble tenorite species at pH > 5.5. Almost 100% of Cu2+ was removed beyond pH 5.5 and this could be due to both adsorption and co-precipitation of Cu2+onto the surfaces of BNMPTS material [15]. On the other hand, the speciation of Cd2+ (Fig. S3(b)) shows that Cd2+ (ionic species) exists up to pH 8.8 and insoluble Cd(OH)2(s) primarily exists above this pH. In between Ca pH 8.7, about 5% of Cd(OH)+ species exists. Therefore, cadmium (II) is predominantly present as cationic (Cd2+) species within the studied pH region. The high percentage of Cu2+ and Cd2+ are removed even at acidic conditions or below the pHpzc of the BNMPTS. Therefore, all the remaining sorption experiments were performed at pH 4.0 and 5.0 for Cu2+ and Cd2+, respectively. The results indicated that the Cu2+/or Cd2+ are bound with strong chemical forces rather than the weak physical forces. The available −SH or −OH groups, perhaps, take part predominantly in the bond formation onto the surface. Additionally, the dense brushes of the silanes are present on the surface of BNMPTS which traps the Cu2+ and Cd2+ efficiently and this significantly enhances the sorptive elimination of these pollutants in aqueous wastes at a wide range of pH (Fig. 2(c)).
3.2.2. Time dependent elimination of Cu2+ and Cd2+Time dependent elimination of Cu2+ and Cd2+ using BNMPTS are illustrated graphically in Fig. S4(a). BNMPTS showed very fast and rapid initial uptake of these two cations and about 95% of these two toxic ions were removed within 5 minutes of contact. The percentage removal was gradually slowed down and apparent sorption equilibrium was reached within 30 minutes of contact for both the metals cations. The results confirmed that the BNMPTS possessed high affinity towards the Cu2+ and Cd2+ and the active sites on BNMPTS solid were readily accessible to these toxic ions [36, 44]. This further reaffirmed that the dense brushes of silanes available on BNMPTS material forms strong chemical bonds with these cations and rapid removal of Cu2+ and Cd2+ were occurred using the BNMPTS material. Further, the kinetics was conducted for pseudo-first order (PFO) and pseudo-second order (PSO) models using the known kinetic equations as given elsewhere [45]. Fig. S4(b) and (c) showed apparent simulation of PFO and PSO models employing experimental results. The uptake capacity and rate constants are optimized for possible minimum value of least square sums. The results are returned in Table S2. PSO model relatively fitted well to kinetic data for the sorption of Cu2+ and Cd2+ by BNMPTS solid. Further, the removal capacity was found to be 4.13 and 2.03 mg/g respectively for Cu2+ and Cd2+ using BNMPTS solid. The well-fitting of PSO model suggested that the chemical interactions are predominantly involved at the solid/solution interface [46]. The removal of Cu2+ using bentonite coated with Fe3O4 magnetite nanoparticles and Cd2+ using 3-MPA@PMNPs were also followed PSO model than PFO model and showed that the interaction of sorbates species with adsorbent is chemical in nature [38, 47].
3.2.3. Influence of initial metal concentrationsThe elimination efficiency of Cu2+ and Cd2+ by BNMPTS at varied concentrations of adsorbates are given in Fig. S5. It is observed that BNMPTS material was able to attain very high removal efficiency for Cu2+ and Cd2+ even at high concentrations of respective ions. Further, increasing the concentrations of metals from 1.0 to 25.0 mg/L did not show significant impact on the percentage removal of these ions. It is noted that at the concentration of 25.0 mg/L, BNMPTS was able to remove 96 and 93% of Cu2+ and Cd2+, respectively. These findings revealed that the high uptake capacity of BNMPTS for Cu2+ and Cd2+ and affinity of these cations towards the composite material.
It is further observed that increasing the concentration of Cu2+ and Cd2+ from 1.0 to 25.0 has caused to increase the amount of Cu2+ from 0.493 to 11.955 mg/g and Cd2+ from 0.418 to 11.592 mg/g, respectively.
The concentration dependent data are utilized to obtain the Langmuir and Freundlich adsorption isotherms [15]. The Langmuir monolayer sorption capacity (qo), Langmuir constant (b), Freundlich constants (Kf and 1/n) for BN and BNMPTS are obtained and given in Table S3. It is observed that the sorption of Cu2+ and Cd2+ by the BNMPTS material followed Langmuir sorption isotherm model better than Freundlich adsorption isotherm since higher R2 value is obtained for Langmuir adsorption isotherm. Similar results are reported in which the removal of Cd(II) by dopamine-functionalized meso-structured silica (DMOS) and Cu(II) by mercapto functionalized palygorskite (MPAL) followed Langmuir adsorption isotherm [48, 49]. Previously, it was also reported that the adsorption of Cu(II) and Zn(II) onto natural bentonite (NB) and modified bentonite (MB) followed Langmuir adsorption isotherm indicated homogenous distribution of adsorption sites on the solid surfaces and the formation of monolayer adsorbate species onto the sorbent surface [50] Furthermore, the affinity of BNMPTS towards Cu2+ and Cd2+ was shown by higher values of Langmuir constant (b). The Freundlich constant (1/n) has fractional values which showed the heterogeneous surface of the material and the available sites for adsorption is distributed exponentially. Similarly, the higher level of adsorbate removal is indicated by Kf values and good adsorption intensity is denoted by lesser value of 1/n [51]. Comparison for maximum adsorption capacity of various adsorbents for Cd(II) and Cu(II) are given in Table 1, respectively. The adsorption capacity of various sorbents are different due to the variation in physico-chemical properties of adsorbents as well as the experimental factors such as pH, temperature, ionic strength, concentration range, etc [11]. However, it is interesting to observe that BNMPTS showed fairly high adsorption capacity for Cu(II) and Cd(II) which is comparable or even higher than several similar adsorbents reported previously.
3.2.4. Effect of Background electrolyte concentrationsRemoval of pollutants from water is generally impacted by the presence of background electrolytes present in aqueous wastes. However, this relies on the concentration and nature of the electrolytes [11]. Therefore, the elimination of Cu2+ and Cd2+ were performed at varied concentrations of background electrolyte (NaCl) and results are shown in Fig. 3. Fig. 3 showed that a thousand times increase in background electrolyte concentrations could not affect the elimination efficiency of Cu2+ and Cd2+ by BNMPTS. These findings again showed that the modified bentonite has a strong affinity towards these metal ions and suggests that Cd2+ions are aggregated onto BNMPTS solid through the strong chemical bonds, possibly, through the −SH or −OH functional groups which leads the ‘inner-sphere’ complexation at solid surface [10, 11]. Similarly, the percent removal of Cu2+ by walnut (Juglans regia), hazelnut (Corylus avellana), and almond (Prunus dulcis) is negligible when the concentration of KNO3 was increased from 0.01 to 0.1 mol/L [63].
3.2.5. Effect of Co-existing ionsThe percent elimination of Cu2+ and Cd2+ using BNMPTS was studied in presence of the selected cations (Mg2+, Mn2+, Ca2+ and Ni2+) and anions (oxalic acid, glycine and phosphate) and the results are given in Fig. 4(a) and 4(b). The presence of EDTA significantly suppressed the removal of Cu2+ and Cd2+ by BNMPTS. This is because EDTA is forming stable complexes with metal ions and the complex species are not bound with the solid surface [64, 65]. On the other hand, other cations or anions did not affect the elimination efficiency of Cu2+ and Cd2+. Therefore, the findings further showed the selectivity and the efficiency of BNMPTS towards Cu2+ and Cd2+ in presence of these co-existing cations/anions. Tamez et al. [66], found that the presence of sodium, potassium and calcium or magnesium did not affect significantly the binding of Cu2+ and Cd2+ onto the Fe3O4 solid. Hence, only a small decrease in percentage removal of these ions was recorded in presence of Mg2+ (3,000 mg/L). Similarly, the elimination of Cu(II) and Pb(II) by non-living green algae was not affected significantly in the presence of several anions except EDTA. The presence of 1.0 mmol/L EDTA had caused to suppress significantly the removal efficiency of copper and lead by the non-living green algae [67]. Similarly, nitrate salts of Na, K and Mg showed insignificant influence on the elimination of Cd(II) by brown, green, and red seaweeds. But uptake of cadmium was suppressed at calcium level of 1.62 mmol/L to 80% and further decreased to 65% at 3.24 mmol/L [68]. The removal of Cd2+, Cu2+, Pb2+ and Hg2+ by ZnS and FeS nanomaterials was 99% in mixed metal cation solutions in presence of 60 fold concentrations of Ca2+ and Mg2+. Hence the presence of other cations have negligible or no effect on the toxic metal removal using metal-sulfide nanomaterials [69]. In other study, the elimination of Cd2+ by MnO2 loaded D301 showed no significant effect in presence of several cations (Na+, K+ and Ca2+, or Mg+2) and anions ( Cl−, NO3−, SO42− and PO43−) except of PO43− [70]. The presence of EDTA on the sorption of Cd2+ and Zn2+ by apatite showed a decrease in the uptake of Cd2+ and Zn2+ due to the formation of [CdEDTA]2− and [ZnEDTA]2− complexes [71].
3.3. Fixed Bed Reactor OperationsThe breakthrough column results are shown in Fig. 5. The functionalized material showed complete breakthrough volume at 1.4 and 3.69 L for Cu2+, 2.6 and 6.64 L for Cd2+ while using 0.25 g and 0.50 g of BNMPTS, respectively. Furthermore, the known Thomas equation was exploited in order to estimate the loading capacity of BNMPTS by performing the non-linear least square fitting of breakthrough column results. The constants KT and loading capacity (qo) are presented in Table 2. The loading capacity obtained for BNMPTS are relatively high at least for the Cu2+ and Cd2+. This eventually infers the useful applicability of BNMPTS for efficient elimination of aqueous waste containing copper and cadmium [39, 45].
3.4. Adsorption MechanismPrevious reports have shown that an enhanced uptake of Cu2+ was achieved using mercapto functionalized silica aerogel [72], Poly(vinyl alcohol)/silica (PVA/silica) membranes [73], electrospun CeO2 nanofiber [74] and palygorskite [49]. Similarly, the removal of Cd2+ was successfully succeeded with mercapto functionalized silica [75, 76], palygorskite [77], attapulgite [78], sepiolite [79], zinc based metal organic framework [80] and coal gangue [81]. It was assumed that the copper/cadmium may undergo chemisorption on the surface of mercapto functionalized surface and consequently forming an ‘inner-sphere complexes’ at adsorbent surface. Several researchers have reported that the incorporated mercapto group participates in chemisorption of copper and cadmium [74, 80, 81]; whereas few researchers reported that the sorption of toxic ions proceeds through hydroxyl group instead mercapto group [49, 78]. Hence, in order to ascertain the mechanistic pathway of Cu2+ and Cd2+ sorption onto synthesized hybrid, the BNMPTS was analysed by XPS results as obtained before and after the adsorption of these cations. The wide scan XPS spectra are shown in Fig. S6 (a)–(c). The XPS results of BNMPTS loaded with Cu2+ and Cd2+ confirmed the distinctive electron peaks of Cu2p and Cd3d. The doublet peaks at 933.3 and 957.3 eV binding energies are due to the Cu2p(1/2) and Cu2p(3/2) electrons. Similarly, the binding energy peaks at 405.7 and 412.4 eV are due to the Cd3d(3/2) and Cd3d(5/2) electrons. This evidently showed that Cu2+ and Cd2+ are sorbed onto the surface of BNMPTS material.
Further, the alteration in binding energy of O1s with and without loading of Cu2+ and Cd2+ with BNMPTS are illustrated as in Fig. 6(a). It is shown that the O1s peak was slightly shifted from 530.0 to 531.2 eV and 530 to 531.3 eV with the loading of Cu2+ and Cd2+, respectively. This inferred that Cu2+ and Cd2+ are bound through the −OH group of BNMPTS [82, 83]. Moreover, the binding energy of S2p electrons was obtained at 163.14 eV (Fig. 6(b)) and this peak is attributed to the disulfide bond [49]. This binding energy remains almost the same after the loading of Cu2+/or Cd2+ which suggests that the mercapto group is not participating in the sorption of these two toxic ions. Previously, Wang et al. [78] showed the role of disulfide in sorption of cadmium by mercapto-functionalized attapulgite (MATP) using density functional theory (DFT) calculations and found that disulfide did not play significant role in Cd adsorption. Therefore, insight mechanistic study revealed that adsorption of copper (II) or cadmium (II) mainly proceeded through hydroxyl groups present on the functionalized material and consequently forming an ‘inner sphere complexes’ at the surface of solid. Further, the XPS results indicated that Cu2+ and Cd2+ are substantially sorbed onto BNMPTS and atomic percentage of Cu and Cd in the used BNMPTS solids were recorded as 0.87 and 0.45% respectively.
4. Conclusions3-mercaptopropyltrimethoxy silane (MPTS) was grafted onto the bentonite surface and it was reaffirmed with the FT-IR and SEM/EDX analyses. The structural and textural properties of clay (BN) and functionalized clay (BNMPTS) were characterized by the XRD and BET analyzers. The silane grafting caused a significant decrease in the specific surface area i.e., from 41.14 to 4.65 m2/g obtained for BN and BNMPTS, respectively. The functionalized bentonite (BNMPTS) was enabled to remove a very high percentage (almost 100%) of Cu2+ and Cd2+ at a wide range of pH. The maximum sorption capacities were found to be 12.59 and 13.19 mg/g for Cu2+ and Cd2+, respectively. The sorption reaction is rapid and an apparent equilibrium is reached within 30 minutes of contact and the kinetic data is fitted well to the PSO model. Increasing the concentration of background electrolyte and the presence of co-ions generally did not affect the removal efficiency of these two cations by BNMPTS except the presence of EDTA. Furthermore, the maximum loading capacities of Cu2+ and Cd2+ on to the column packed with BNMPTS were found to be 32.03 and 35.09 mg/g, respectively. The insight mechanism study performed using XPS analyses revealed that sorption of Cu2+ and Cd2+ mainly proceeded via the −OH group present at the terminal of BNMPTS. The study therefore, implied that MPTS functionalized bentonite is a potential solid material to be employed efficiently in the elimination of Cu2+ and Cd2+ in aqueous wastes.
References2. Glatstein DA, Francisca FM. Influence of pH and ionic strength on Cd, Cu and Pb removal from water by adsorption in Na-bentonite. Appl Clay Sci. 2015;118:61–67.
3. Sari A, Tuzen M, Citak D, Soylak M. Equilibrium, kinetic and thermodynamic studies of adsorption of Pb(II) from aqueous solution onto Turkish kaolinite clay. J Hazard Mater. 2007;149:283–291.
4. Qin F, Wen B, Shan X-Q, et al. Mechanisms of competitive adsorption of Pb, Cu, and Cd on peat. Environ Pollut. 2006;144:669–680.
5. Usman ARA. The relative adsorption selectivities of Pb, Cu, Zn, Cd and Ni by soils developed on shale in New Valley, Egypt. Geoderma. 2008;144:334–343.
6. Gündoğan R, Acemioğlu B, Alma MH. Copper (II) adsorption from aqueous solution by herbaceous peat. J Colloid Interface Sci. 2004;269:303–309.
7. Lee S-M, Laldawngliana C, Tiwari D. Iron oxide nano-particles-immobilized-sand material in the treatment of Cu(II), Cd(II) and Pb(II) contaminated waste waters. Chem Eng J. 2012;195–196:103–111.
8. Hossain MA, Ngo HH, Guo WS. Removal of copper from water by adsorption onto banana peel as bioadsorbent. Int J Geomate. 2012;2(2)227–234.
9. World Health Organization. Guidelines for drinking-water quality: first addendum to the third edition, volume 1 reccommendations. Geneva: WHO; 2006.
10. Lalchhingpuii , Tiwari D, Lalhmunsiama , Lee SM. Chitosan templated synthesis of mesoporous silica and its application in the treatment of aqueous solutions contaminated with cadmium( II) and lead(II). Chem Eng J. 2017;328:434–444.
11. Lalhmunsiama , Tiwari D, Lee S-M. Surface-functionalized activated sericite for the simultaneous removal of cadmium and phenol from aqueous solutions: Mechanistic insights. Chem Eng J. 2016;283:1414–1423.
12. Burham N, Sayed M. Adsorption Behavior of Cd2+ and Zn2+ onto natural Egyptian bentonitic clay. Minerals. 2016;6:129.
13. Han H, Rafiq MK, Zhou T, Xu R, Mašek O, Li X. A critical review of clay-based composites with enhanced adsorption performance for metal and organic pollutants. J Hazard Mater. 2019;369:780–796.
14. Ikhsan J, Johnson BB, Wells JD. A Comparative Study of the Adsorption of Transition Metals on Kaolinite. J Colloid Interface Sci. 1999;217:403–410.
15. Tiwari D, Kim H-U, Lee S-M. Removal behavior of sericite for Cu(II) and Pb(II) from aqueous solutions: Batch and column studies. Sep Purif Technol. 2007;57:11–16.
16. Bhattacharyya KG, Gupta SS. Adsorption of a few heavy metals on natural and modified kaolinite and montmorillonite: A review. Adv Colloid Interface Sci. 2008;140:114–131.
17. El-Maghrabi H, Mikhail S. Removal of heavy metals via adsorption using natural clay material. Environ Earth Sci. 2014;4:19.
18. Gu S, Kang X, Wang L, Lichtfouse E, Wang C. Clay mineral adsorbents for heavy metal removal from wastewater: a review. Environ Chem Lett. 2019;17:629–654.
19. Lee SM, Tiwari D. Organo and inorgano-organo-modified clays in the remediation of aqueous solutions: An overview. Appl Clay Sci. 2012;59–60:84–102.
20. Bergaya F, Lagaly G. General introduction: clays, clay minerals, and clay science. Dev Clay Sci. 2006;1:1–18.
21. Prabhu P, Prabhu B. A Review on Removal of Heavy Metal Ions from Waste Water using Natural/ Modified Bentonite. MATEC Web of Conferences. 2018;144:02021.
22. Kul AR, Koyuncu H. Adsorption of Pb(II) ions from aqueous solution by native and activated bentonite: Kinetic, equilibrium and thermodynamic study. J Hazard Mater. 2010;179:332–339.
23. Abate G, Masini JC. Sorption of atrazine, propazine, deethylatrazine, deisopropylatrazine and hydroxyatrazine onto organovermiculite. J Braz Chem Soc. 2005;16:936–943.
24. He H, Tao Q, Zhu J, Yuan P, Shen W, Yang S. Silylation of clay mineral surfaces. Appl Clay Sci. 2013;71:15–20.
25. Kónya J, Nagy NM. Sorption of dissolved mercury (II) species on calcium-montmorillonite: an unusual pH dependence of sorption process. J Radioanal Nucl Chem. 2011;288:447–454.
26. Najafi H, Farajfaed S, Zolgharnian S, Mosavi Mirak SH, Asasian-Kolur N, Sharifian S. A comprehensive study on modified-pillared clays as an adsorbent in wastewater treatment processes. Process Saf Environ Prot. 2021;147:8–36.
27. Orta M, del M, Martín J, Santos JL, Aparicio I, Medina-Carrasco S, Alonso E. Biopolymer-clay nanocomposites as novel and ecofriendly adsorbents for environmental remediation. Appl Clay Sci. 2020;198:105838.
28. Otunola BO, Ololade OO. A review on the application of clay minerals as heavy metal adsorbents for remediation purposes. Environ Technol Innov. 2020;18:100692.
29. Tohdee K, Kaewsichan L, Asadullah . Potential of BCDMACl modified bentonite in simultaneous adsorption of heavy metal Ni (II) and humic acid. J Environ Chem Eng. 2018;6:5616–5624.
30. Qin Z, Yuan P, Yang S, Liu D, He H, Zhu J. Silylation of Al13-intercalated montmorillonite with trimethylchlorosilane and their adsorption for Orange II. Appl Clay Sci. 2014;99:229–236.
31. Marjanović V, Lazarević S, Janković-Častvan I, Potkonjak B, Janaćković Đ, Petrović R. Chromium (VI) removal from aqueous solutions using mercaptosilane functionalized sepiolites. Chem Eng J. 2011;166:198–206.
32. Şahan T, Erol F, Yılmaz Ş. Mercury(II) adsorption by a novel adsorbent mercapto-modified bentonite using ICP-OES and use of response surface methodology for optimization. Microchem J. 2018;138:360–368.
33. Rivera-Utrilla J, Bautista-Toledo I, Ferro-García MA, Moreno-Castilla C. Activated carbon surface modifications by adsorption of bacteria and their effect on aqueous lead adsorption. J Chem Technol Biotechnol. 2001;76:1209–1215.
34. Tonle IK, Ngameni E, Njopwouo D, Carteret C, Walcarius A. Functionalization of natural smectite-type clays by grafting with organosilanes: physico-chemical characterization and application to mercury(II) uptake. Phys Chem Chem Phys. 2003;5:4951–4961.
35. Yılmaz Ş, Şahan T, Karabakan A. Response surface approach for optimization of Hg(II) adsorption by 3-mercaptopropyl trimethoxysilane-modified kaolin minerals from aqueous solution. Korean J Chem Eng. 2017;34:2225–2235.
36. Liang X, Han J, Xu Y, Wang L, Sun Y, Tan X. Sorption of Cd2+ on mercapto and amino functionalized palygorskite. Appl Surf Sci. 2014;322:194–201.
37. Carvalho WA, Vignado C, Fontana J. Ni(II) removal from aqueous effluents by silylated clays. J Hazard Mater. 2008;153:1240–1247.
38. Mohammed AA, Samaka IS. Bentonite coated with magnetite Fe3O4 nanoparticles as a novel adsorbent for copper (II) ions removal from water/wastewater. Environ Technol Innov. 2018;10:162–174.
39. Thanhmingliana , Tiwari D. Efficient use of hybrid materials in the remediation of aquatic environment contaminated with micro-pollutant diclofenac sodium. Chem Eng J. 2015;263:364–373.
40. Cychosz KA, Thommes M. Progress in the Physisorption Characterization of Nanoporous Gas Storage Materials. Engineering. 2018;4:559–566.
41. Celis R, Hermosín MC, Cornejo J. Heavy Metal Adsorption by Functionalized Clays. Environ Sci Technol. 2000;34:4593–4599.
42. Liu AM, Hidajat K, Kawi S, Zhao DY. A new class of hybrid mesoporous materials with functionalized organic monolayers for selective adsorption of heavy metal ions. Chem Commun. 2000;1145–1146.
43. Moraes DS, Miranda LCR, Angélica RS, et al. Functionalization of Bentonite and Vermiculite after the Creation of Structural Defects through an Acid Leaching Process. J Brazilian Chem Soc. 2018;29:320–327.
44. Ouakouak A, Rihani K, Youcef L, Hamdi N, Guergazi S. Adsorption characteristics of Cu (II) onto CaCl2 pretreated algerian bentonite. Mater Res Express. 2020;7:025045.
45. Lalhmunsiama , Lee S-M, Lalchhingpuii , Tiwari D. Functionalized hybrid material precursor to chitosan in the efficient remediation of aqueous solutions contaminated with As(V). J Environ Chem Eng. 2016;4:1537–1544.
46. Foo KY, Hameed BH. Insights into the modeling of adsorption isotherm systems. Chem Eng J. 2010;156:2–10.
47. Ali I, Peng C, Naz I. Removal of lead and cadmium ions by single and binary systems using phytogenic magnetic nanoparticles functionalized by 3-marcaptopropanic acid. Chin J Chem Eng. 2019;27:949–964.
48. Chen Y, Gao J, Wen X, Wu W. Efficient removal of cadmium using facile functionalized of mesoporous silica via a biomimetic coating. RSC Adv. 2016;6:18340–18347.
49. Han J, Liang X, Xu Y, Xu Y. Removal of Cu2+ from aqueous solution by adsorption onto mercapto functionalized palygorskite. J Ind Eng Chem. 2015;23:307–315.
50. Tohdee K, Kaewsichan L, Asadullah . Enhancement of adsorption efficiency of heavy metal Cu(II) and Zn(II) onto cationic surfactant modified bentonite. J Environ Chem Eng. 2018;6:2821–2828.
51. Raji C, Anirudhan TS. Batch Cr(VI) removal by polyacrylamide-grafted sawdust: Kinetics and thermodynamics. Water Res. 1998;32:3772–3780.
52. Lalhmunsiama , Gupta PL, Jung H, Tiwari D, Kong S-H, Lee S-M. Insight into the mechanism of Cd(II) and Pb(II) removal by sustainable magnetic biosorbent precursor to Chlorella vulgaris. J Taiwan Inst Chem Eng. 2017;71:206–213.
53. Wang FY, Wang H, Ma JW. Adsorption of cadmium (II) ions from aqueous solution by a new low-cost adsorbent—Bamboo charcoal. J Hazard Mater. 2010;177:300–306.
54. Zhang X, Sun C, Zhang L, et al. Adsorption studies of cadmium onto magnetic Fe3O4@FePO4 and its preconcentration with detection by electrothermal atomic absorption spectrometry. Talanta. 2018;181:352–358.
55. Li C, Duan H, Wang X, Meng X, Qin D. Fabrication of porous resins via solubility differences for adsorption of cadmium (II). Chem Eng J. 2015;262:250–259.
56. Lalchhingpuii , Lee SM, Tiwari D. Synthesis and Characterization of Chitosan templated Mesoporous Silica: Efficient use of Mesoporous Silica in the removal of Cu(II) from Aqueous Solutions. STJ. 2016;4:105–112.
57. Pawar RR, Lalhmunsiama , Bajaj HC, Lee S-M. Activated bentonite as a low-cost adsorbent for the removal of Cu(II) and Pb(II) from aqueous solutions: Batch and column studies. J Ind Eng Chem. 2016;34:213–223.
58. Grisdanurak N, Akewaranugulsiri S, Futalan CM, et al. The study of copper adsorption from aqueous solution using cross-linked chitosan immobilized on bentonite. J Appl Polym Sci. 2012;125:E132–42.
59. Olu-Owolabi BI, Popoola DB, Unuabonah EI. Removal of Cu2+ and Cd2+ from Aqueous Solution by Bentonite Clay Modified with Binary Mixture of Goethite and Humic Acid. Water Air Soil Pollut. 2010;211:459–474.
60. Zhao Y, Zhan L, Xue Z, Yusef KK, Hu H, Wu M. Adsorption of Cu (II) and Cd (II) from Wastewater by Sodium Alginate Modified Materials. J Chem. 2020;2020:1–13.
61. Yessica Bendezu Roca, Fuentes Walter S. Use of Nanoclay as an Adsorbent to Remove Cu(ii) from Acid Mine Drainage (amd). Chem Eng Trans. 2019;73:241–246.
62. Kamari A, Yusoff SNM, Abdullah F, Putra WP. Biosorptive removal of Cu(II), Ni(II) and Pb(II) ions from aqueous solutions using coconut dregs residue: Adsorption and characterization studies. J Environ Chem Eng. 2014;2:1912–1929.
63. Altun T, Pehlivan E. Removal of Copper(II) Ions from Aqueous Solutions by Walnut-, Hazelnut- and Almond-Shells. Clean (Weinh). 2007;35:601–606.
64. Chen B, Zhao H, Chen S, et al. A magnetically recyclable chitosan composite adsorbent functionalized with EDTA for simultaneous capture of anionic dye and heavy metals in complex wastewater. Chem Eng J. 2019;356:69–80.
65. Ezzeddine Z, Batonneau-Gener I, Pouilloux Y, Hamad H, Saad Z, Kazpard V. Divalent heavy metals adsorption onto different types of EDTA-modified mesoporous materials: Effectiveness and complexation rate. Microporous Mesoporous Mater. 2015;212:125–136.
66. Tamez C, Hernandez R, Parsons JG. Removal of Cu (II) and Pb (II) from aqueous solution using engineered iron oxide nanoparticles. Microchem J. 2016;125:97–104.
67. Deng L, Su Y, Su H, Wang X, Zhu X. Biosorption of copper (II) and lead (II) from aqueous solutions by nonliving green algae Cladophora fascicularis: Equilibrium, kinetics and environmental effects. Adsorption. 2006;12:267–277.
68. Hashim MA, Chu KH. Biosorption of cadmium by brown, green, and red seaweeds. Chem Eng J. 2004;97:249–255.
69. Wang C, Yin H, Bi L, et al. Highly efficient and irreversible removal of cadmium through the formation of a solid solution. J Hazard Mater. 2020;384:121461.
70. Zhu Z, Ma H, Zhang R, Ge Y, Zhao J. Removal of cadmium using MnO2 loaded D301 resin. J Environ Sci. 2007;19:652–656.
71. Viipsi K, Sjöberg S, Tõnsuaadu K, Shchukarev A. Cd2+ and Zn2+ sorption on apatite in the presence of EDTA and humic substance. E3S Web of Conferences. 2013;1:01008.
72. Štandeker S, Veronovski A, Novak Z, Knez Ž. Silica aerogels modified with mercapto functional groups used for Cu(II) and Hg(II) removal from aqueous solutions. Desalination. 2011;269:223–230.
73. Keshtkar AR, Irani M, Moosavian MA. Comparative study on PVA/silica membrane functionalized with mercapto and amine groups for adsorption of Cu(II) from aqueous solutions. J Taiwan Inst Chem Eng. 2013;44:279v286.
74. Yari S, Abbasizadeh S, Mousavi SE, Moghaddam MS, Moghaddam AZ. Adsorption of Pb(II) and Cu(II) ions from aqueous solution by an electrospun CeO2 nanofiber adsorbent functionalized with mercapto groups. Process Saf Environ Prot. 2015;94:159–171.
75. Košak A, Lobnik A, Bauman M. Adsorption of Mercury(II), Lead(II), Cadmium(II) and Zinc(II) from Aqueous Solutions using Mercapto-Modified Silica Particles. Int J Appl Ceram Technol. 2015;12:461–472.
76. Machida M, Fotoohi B, Amamo Y, Ohba T, Kanoh H, Mercier L. Cadmium(II) adsorption using functional mesoporous silica and activated carbon. J Hazard Mater. 2012;221–222:220–227.
77. Liang X, Xu Y, Tan X, et al. Heavy metal adsorbents mercapto and amino functionalized palygorskite: Preparation and characterization. Colloids Surf A Physicochem Eng Asp. 2013;426:98–105.
78. Wang L, Shi Y, Yao D, et al. Cd complexation with mercapto-functionalized attapulgite (MATP): Adsorption and DFT study. Chem Eng J. 2019;366:569–576.
79. Liang X, Xu Y, Sun G, et al. Preparation and characterization of mercapto functionalized sepiolite and their application for sorption of lead and cadmium. Chem Eng J. 2011;174:436–444.
80. Zhang J, Xiong Z, Li C, Wu C. Exploring a thiol-functionalized MOF for elimination of lead and cadmium from aqueous solution. J Mol Liq. 2016;221:43–50.
81. Shang Z, Zhang L, Zhao X, Liu S, Li D. Removal of Pb(II), Cd(II) and Hg(II) from aqueous solution by mercapto-modified coal gangue. J Environ Manage. 2019;231:391–6.
Fig. 1Scanning electron microscopic images of (a) pristine bentonite (BN); and (b) BNMPTS and EDX analysis of (c) pristine bentonite (BN); and (d) BNMPTS. 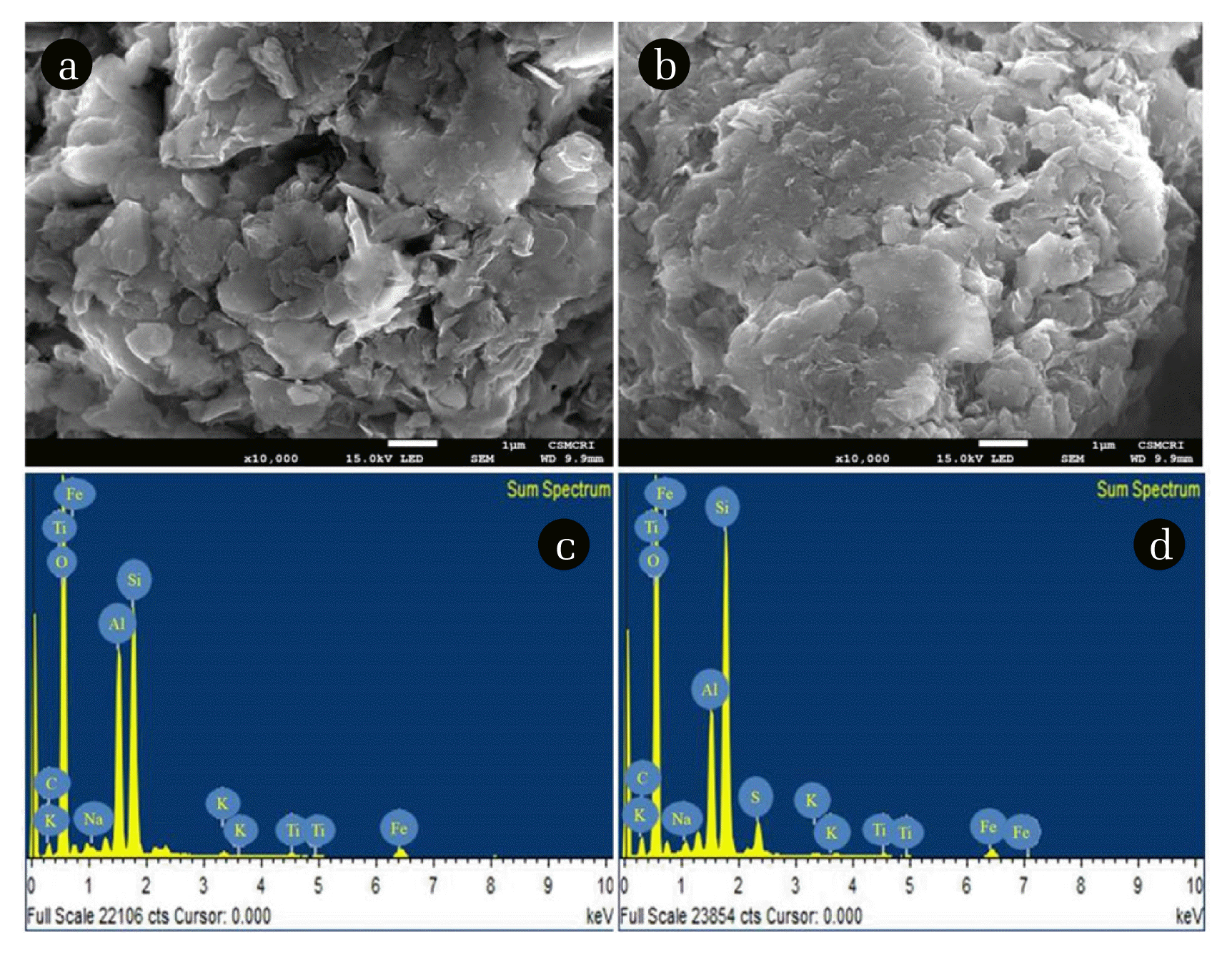
Fig. 2Sorptive removal of (a) Cu2+; (b) Cd2+ as a function of pH using the BN and BNMPTS ([Cu2+]: 10.0 mg/L; [Cd2+]: 5.0 mg/L at 25°C); (c) Schematic of sorption of Cu2+ and Cd2+ by the BNMPTS solid. 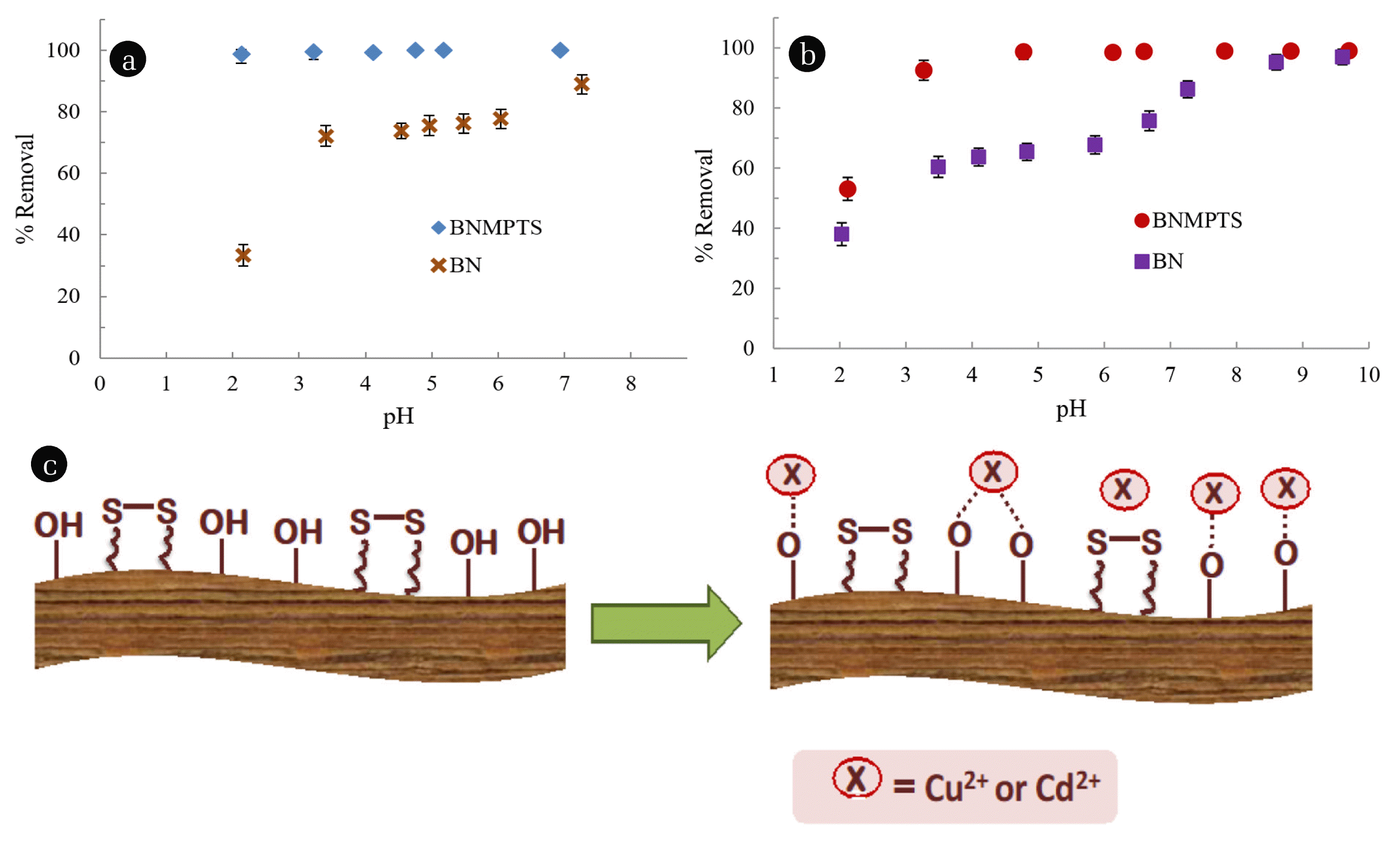
Fig. 3Removal efficiency of Cu2+ and Cd2+ at different concentrations of NaCl using BNMPTS ([Cu2+)]: 10.0 mg/L (pH: 4.0); [Cd2+)]: 5.0 mg/L (pH: 5.0) at 25°C]. 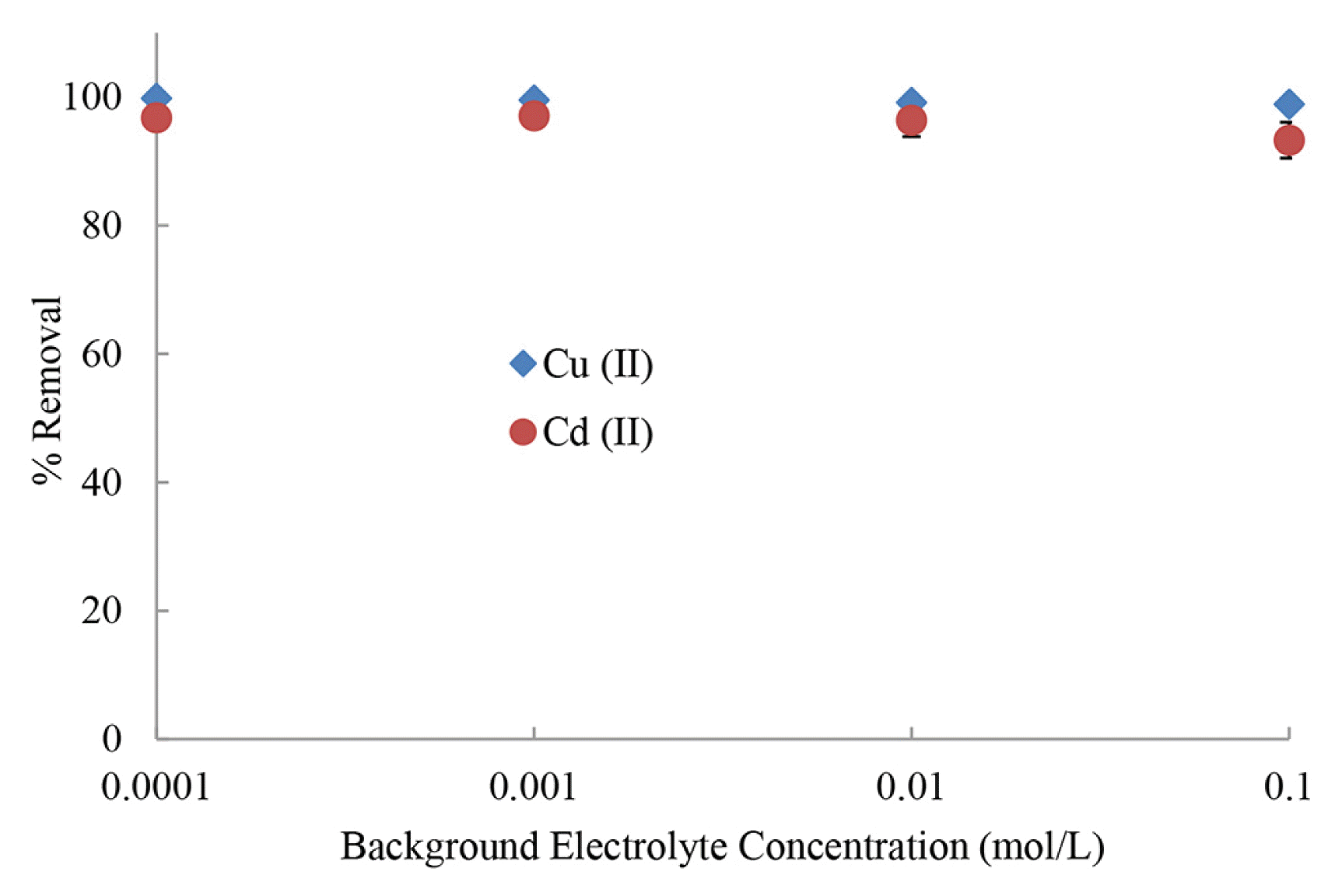
Fig. 4Cu2+ and Cd2+ removal by BNMPTS solid in presence of several co-existing (a) cations; and (b) anions ([Cu2+]: 10.0 mg/L (pH: 4.0); [Cd2+]: 5.0 mg/L (pH: 5.0); [Co-existing ions]: 50.0 mg/L at 25°C]. 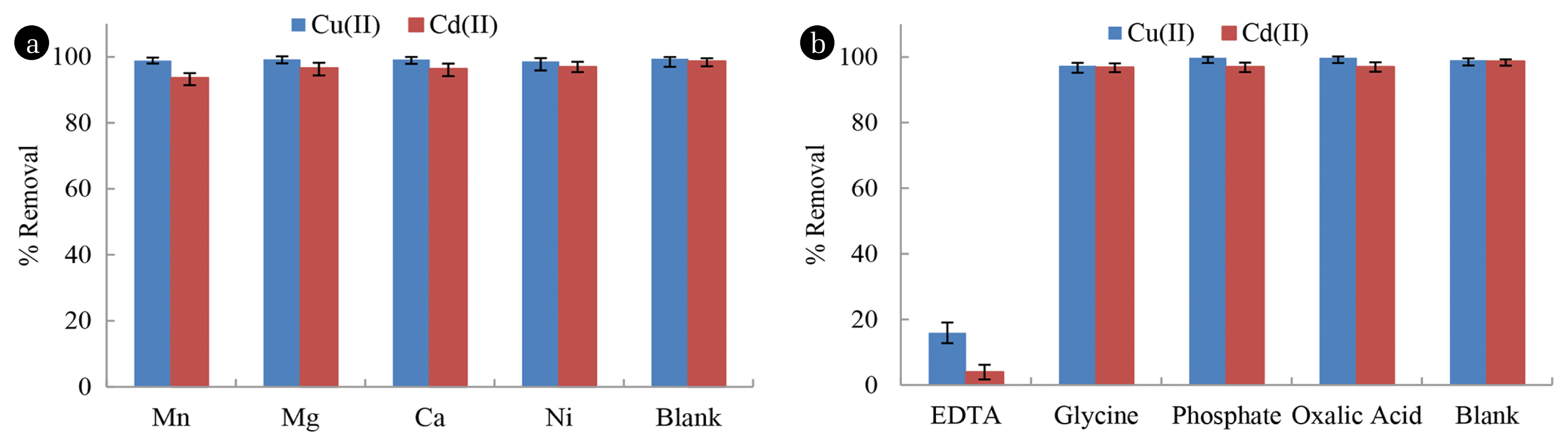
Fig. 5Breakthrough curves obtained for the removal of Cu2+ and Cd2+ using BNMPTS material ([Cu2+]: 10.0 mg/L (pH: 4.0); [Cd2+]: 5.0 mg/L (pH: 5.0), Flow rate: 1 mL/min]. 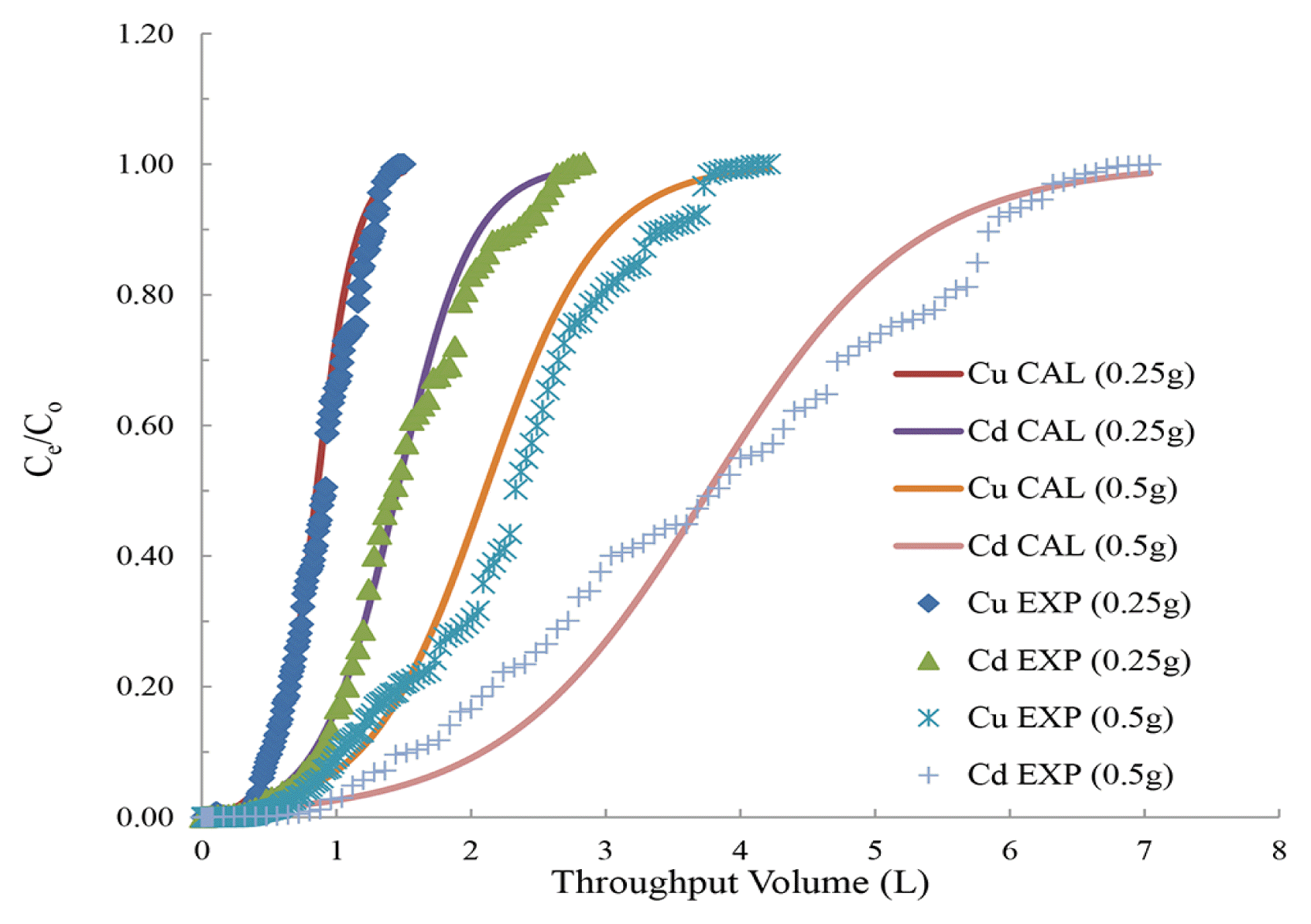
Fig. 6XPS spectra of (a) O1s scan of BNMPTS before and after the sorption of Cu2+ and Cd2+, (b) S(2p) scan of BNMPTS before and after the sorption experiment. 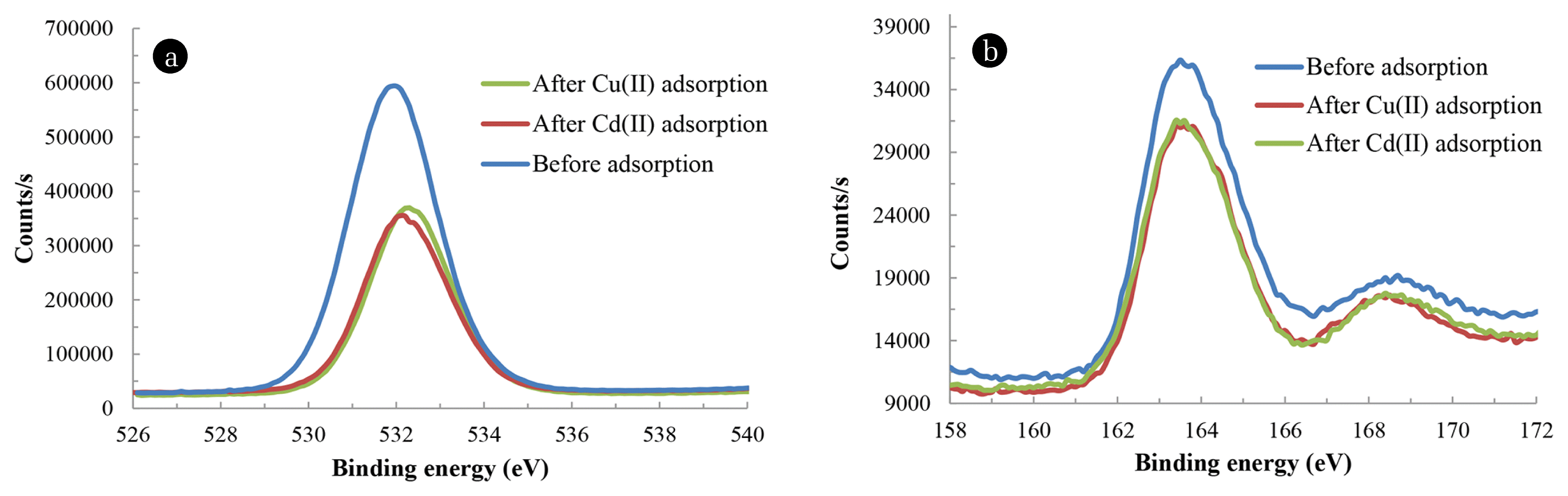
Table 1Maximum Adsorption Capacity of Various Adsorbents for Cd (II) and Cu(II)
Table 2The Loading Capacity and Thomas Rate Constants in the Elimination of Cu2+ and Cd2+ by BNMPTS |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||