1. Introduction
Gallic acid (3,4,5-trihydroxybenzoic acid) is a naturally abundant plant phenolic compound [1]. Gallic acid and its derivatives have been used to prevent and treat a variety of diseases [2, 3], it has beneficial effect such as antitumor [4], trypanocidia [5], also it can protect liver [6], which has caused widespread interest. Gallic acid can be produced from Chinese nutgall, Turkey trough, Tara pod, pomegranate, sumac, or Cotinus coggygria. The methods for producing gallic acid include acid hydrolysis, alkali hydrolysis, biological or enzymatic processes [7]. Generally, manufacturers use the alkali hydrolysis process to produce gallic acid, with Chinese nutgall as the raw material in China [8]. The production of 1 ton gallic acid will generate 6.5m3 wastewater. The major components and concentrations in the wastewater are: Gallic acid 16.96 g·L−1, CODCr 64,356.5 mg·L−1, BOD5 2,414.0 mg·L−1, salinity 100 g·L−1, and the pH value is 0.58.
The wastewater generated by the alkaline hydrolysis process contains a certain amount of gallic acid, excess HCl and NaCl produced during the neutralization reaction [9], which cannot be discharged until meeting the emission guidelines. However, currently the existing industrial applications for the treatment of Chinese nutgall processing wastewater are not effective enough, and the problem of severe environmental pollution is hindering the development of the Chinese nutgall processing industry. Because the environmental regulations have become increasingly stringent, the Chinese nutgall processing industry is facing a significant challenge. Therefore, research and development toward efficient and practical technologies for the treatment of wastewater produced from the gallic acid production process is of significant importance and urgency.
The economic value of gallic acid is high, relinquishing the recovery of gallic acid and treating it directly would lead to a waste of resource. In addition, gallic acid is a component of the chemical oxygen demand (CODCr) and five day biochemical oxygen demand (BOD5); the recovery of gallic acid can decrease the CODCr concentration and improve the biodegradability (BOD5/CODCr ratio) of the wastewater, reducing the difficulty of the biological treatment. Compared with traditional evaporation and crystallization process [10], the recovery efficiency by the extraction process is high, and the operation process is simple, providing an effective means for the recovery and enrichment of gallic acid. In addition, as the extractant can be recycled, the operational cost is much lower. We can not only recover gallic acid from wastewater by the extraction process, but can also reduce the CODCr content as gallic acid is an organic matter, improving the biodegradability of the wastewater, making it conducive for the subsequent biological treatment.
The existing researches have focused on the extraction of trace-scale gallic acid in aqueous solutions [11, 12], which cannot be used for the separation of high gallic acid concentration in wastewater. In our previous work, methyl isobutyl ketone, ethyl acetate, butanol and 30% tributyl phosphate (TBP)/kerosene were used as extractant to recover gallic acid from actual gallic acid production wastewater [13, 14]. We have found that TBP/kerosene was the best extractant which had the advantages of high extraction yield, good selectivity, rapid separation, and large capacity among others. The reported researches on the recovery of organic acid by extraction were of laboratory scale and the runtime was short [15, 16], it was still unknown how to design industrial production process and what might happen during the long time continuous operation.
The object of this study was to verify the reliability of the continuous recovery of gallic acid by TBP/kerosene extraction system from actual Chinese nutgall processing wastewater. Firstly, we examined the theoretical stages for extraction and stripping, and then designed a complete set of industrial scale extraction and stripping devices based on the theoretical stage study. The devices were operated continuously. In addition, the removal efficiencies of gallic acid and CODCr were studied.
2. Materials and Methods
2.1. Chemicals and Reagents
Analytical grade gallic acid, HCl, NaOH, phosphoric acid, and methanol were used in this study. Actual gallic acid processing wastewater was obtained from a biotechnology company in Hunan Province, China.
2.2. Analysis Methods
High-performance liquid chromatography (HPLC) (Agilent 1100 LC, Agilent Corp.) with a UV detector was used to analyze the gallic acid concentrations in the aqueous phase [17, 18]. The separation was performed on a Diamonsil C18 column (250 mm × 4.0 mm, 5 μm), the column temperature was 298 K, and the injection volume of sample was 5.0 μL. The mobile phase was methanol and 0.05% phosphoric acid (5:95) which was operated at the flow rate of 1.0 mL·min−1.
The extraction yield (η) was calculated by Eq. (1), meanwhile, the gallic acid concentration (CO) in the organic phase was calculated by Eq. (2), and the distribution ratio (D) was calculated by Eq. (3), the results of which were used as the design parameter in the determination of theoretical stages:
Where CO represents the gallic acid content in the organic phase; CW and CR represent the gallic acid in the wastewater before pre-treatment and in the raffinate, respectively, in mg·L−1; VO and VA represent the volumes of organic phase and aqueous phase, respectively, in L−1.
The CODCr levels were determined by rapid digestion and spectrophotometry (UV-1801, Ruili Corp) [19]. The pH value of the wastewater and raffinate were measured with a pH meter using a combined electrode (320-S, Mettler Toledo), and the pH values of the three-stage strip liquor were measured using online pH meters with combined electrodes (OPM 253, Amer & Innovative Sensors, Inc.).
2.3. Experimental Setup
The extraction tank was 4,300 mm long, 4,200 mm wide and 1,400 mm in height. The stripping tank was 3,250 mm long, 1,900 mm wide and 1,400 mm in height. The extraction and stripping tanks were constructed of polypropylene (PP).
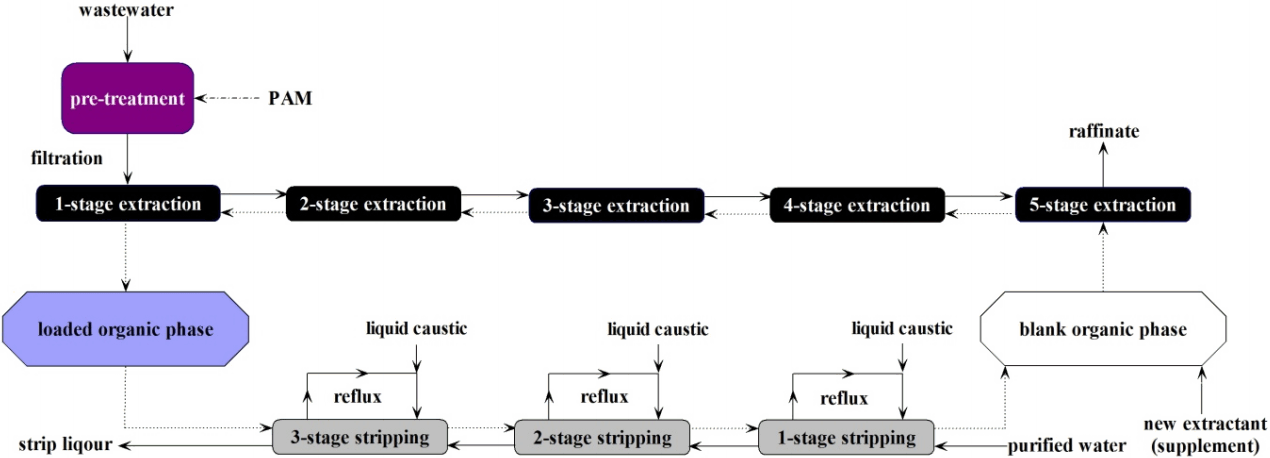
Before pumped into the extraction tank, the wastewater was pre-treated by cationic polyacrylamide (PAM), and was filtered to remove the impurities such as suspended powder activated carbon particles and pectin impurities, among others. The flow rate of the aqueous phase and organic phase in the extraction tank was 1.0 m3·h−1. To obtain a high gallic acid level in the strip liquor, the flow rate of the loaded organic phase in the stripping tank was controlled at 1.0 m3·h−1, and the aqueous phase, which was purified water was controlled at 0.1 m3·h−1. Because the velocity between the loaded organic phase and the aqueous phase was significant different, in each stage of the stripping tank, the aqueous phase was pumped from the clarification chamber to the stirred tank to increase the contact time of the two phases, the reflux flow rate was set at 1.5 m3·h−1. The caustic soda was pumped into the reflux port to adjust the pH value (Fig. 1), pH values of the three-stage strip liquor was maintained at approximately 10.
3. Results and Discussion
3.1. The Determination of the Extraction and Stripping Stages
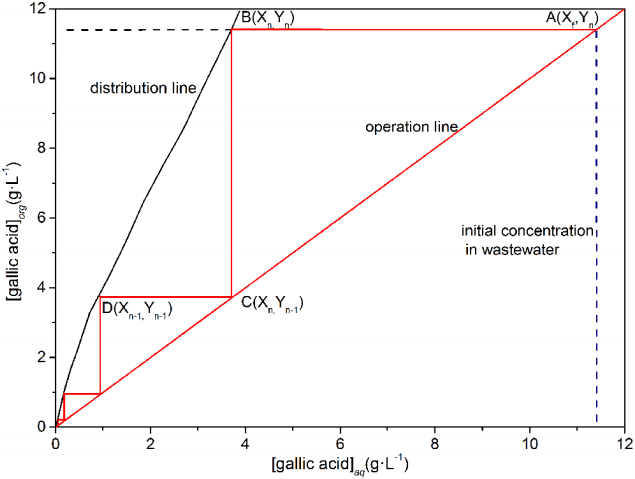
The theoretical stages of multistage countercurrent extraction and stripping were determined by the McCabe-Thiele diagram [20], using the equilibrium line and operation line. The theoretical stages were studied with actual wastewater as the object, with the phase ratio was 1:1. The process of the multistage counter-current extraction or stripping was shown in Fig. 2.
The coordinate of point A (Xf, Yn) was the gallic acid in the aqueous phase Xf entering the n-stage and the gallic acid in the organic phase Yn leaving the n-stage. The gallic acid content in the aqueous phase Xf and the gallic acid content in organic phase Yn were in equilibrium in the n-stage. The coordinate of point B (Xn, Yn) was the horizontal line passed through point A and intersected the distribution line. The coordinate of point C (Xn, Yn−1) was the vertical line across point B and intersecting the operation line, which was the gallic acid content in the aqueous phase Xn entering the n−1-stage and the gallic acid content in the organic phase Yn−1 leaving the n−1-stage. The coordinate of D (Xn−1, Yn−1) was the intersection point of the horizontal line from point C and intersecting the equilibrium line, which represented the gallic acid content in the aqueous phase Xn−1 entering the n−1-stage and the gallic acid content in the organic phase Yn−1 leaving the n−1-stage. This process continued until the gallic acid in the outlet of the aqueous phase was close to X1. The result was the number of steps, which was also the theory stage.
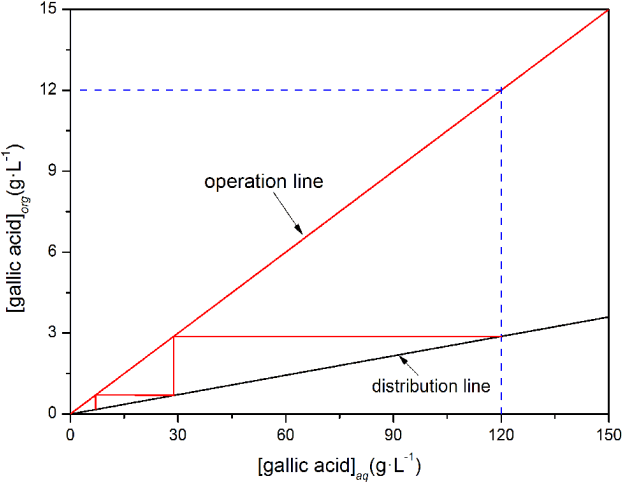
The number of the steps outlined in Fig. 3 was four, which indicated that the theoretical stage for extraction was four. In the same way, we determined that the number of steps for stripping was two (Fig. 4), which indicated that the number of theoretical stages of stripping was two. When we were designing the industrial scale extraction and stripping device, an additional stage for both the extraction and stripping process was added because redundancy design was an effective way to improve the reliability and availability of the extraction - stripping system. If the extraction and stripping yield were not ideal after the theoretical four-stage extraction or two-stage stripping, the additional stage would play the role making the outcome meeting the design requirements. Therefore, the actual number of extraction stages was five and stripping stages was three in this study.
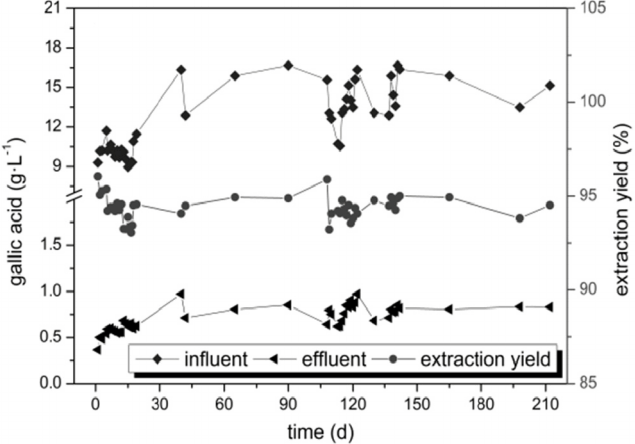
3.2. Continuous Separation of Gallic Acid by Five-stage Extraction
The gallic acid content in the influent wastewater ranged from 8.95 g·L−1 to 16.67 g·L−1 (Fig. 5) during the continuous operation of the industrial scale extraction, but the change of the influent content had no significant effect on the extraction efficiency. The gallic acid levels in the raffinate increased from 0.37 g·L−1 to 0.80 g·L−1 during the initial 17 d, and subsequently did not change after 17 d. During 17 d to 212 d, the average gallic acid concentration in the raffinate was 0.85 g·L−1, and the average extraction yield was 94.14%, no down trend was shown, which indicated that the separation efficiency was good, the ideal recovery efficiency could be obtained and the five-stage countercurrent extraction was stable for long-term and continuous operation.
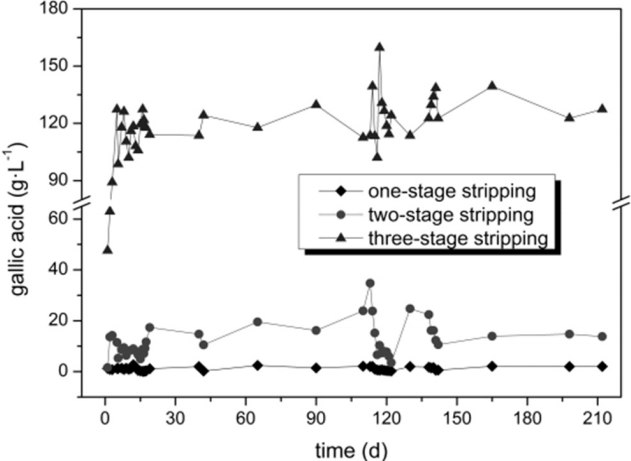
3.3. Continuous Recovery of Gallic Acid by Three-stage Reflux Stripping
The gallic acid concentration in the strip liquor of the three-stage stripping was approximately 120 g·L−1 (Fig. 6), as gallic acid content in the wastewater produced by the hydrolysis process was 16.96 g·L−1 as shown in Table 1, it was 7.5 times higher than the gallic acid content in the wastewater. The gallic acid level in the aqueous phase of the two-stage stripping ranged from 4.94 to 34.76 g·L−1 during operation, which was caused by occasionally incomplete stripping in the third stage stripping tank. The gallic acid concentration in the aqueous phase of the first stage stripping tank was less than 2 g·L−1; thus, we could deduce that the gallic acid level in the organic phase was less than 0.6 g·L−1 based on the stripping isotherms [14]. The production yield of gallic acid by traditional alkali hydrolysis was about 80% [21]. During 9-Oct-2014 to 27-Dec-2014, when the strip liquor was used as raw material, the average production yield was 88.64% (n = 17, SD = 0.42), therefore, the product yield was 8.64% higher.
The extraction and stripping tanks were connected in series and operated continuously. After stripping, the loaded organic phase changed into blank organic phase and re-entered into the extraction tank, which was a closed loop system. During operation, we found that the low-level gallic acid concentration remaining in the organic phase had no obvious effect on the extraction process, which indicated that the recovery of gallic acid by the three-stage reflux stripping was feasible.
3.4. Separation Efficiency of Gallic Acid among Different Extraction Stages
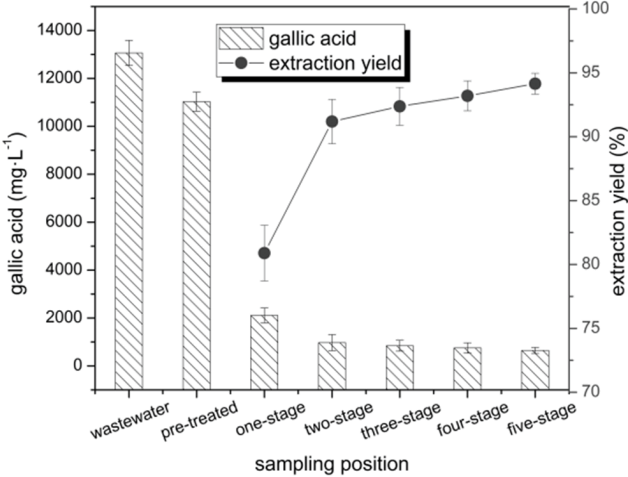
The gallic acid content in the influent, pre-treatment and effluent of the different stages of extraction (Fig. 7) was analyzed on 20 d, 70 d, 105 d, 125 d and 144 d, respectively. From the results we could see that after the five-stage extraction, the total extraction yield was 94.11%, and the gallic acid concentration remaining in the raffinate was 0.83 g·L−1, which indicated that the ideal extraction effect could be obtained by the five-stage countercurrent extraction.
3.5. CODCr Removal Efficiency among Different Extraction Stages
The days when CODCr samples taken were the same as the time of gallic acid samples taken: 20 d, 70 d, 105 d, 125 d and 144 d. After the pre-treatment and five-stage extraction (Fig. 8), the CODCr content in wastewater decreased by 38.20% on average. The small portion of CODCr removed by the pre-treatment was because of the removal of suspended solids, gum, and organic matters adsorbed on powdered activated carbon (loaded with gallic acid) by the polyacrylamide (PAM)-based flocculent. The primary reason for the reduction of CODCr by the extraction process was that the organic matter gallic acid was separated from wastewater and transferred into the organic phase. The CODCr concentration decreased with the increasing stages of extraction, and the effluent CODCr in the raffinate reduced to approximately 35,000 mg·L−1. After extraction, the BOD5 level in the wastewater decreased to 2,106.4 mg·L−1, and the BOD5/CODCr ratio was 1.61 times than before extraction, also because gallic acid which could inhibit the growth and metabolism of microorganisms [22, 23] was separated, the biodegradability of the wastewater was significantly improved.
4. Conclusions
The continuous recovery of gallic acid by TBP/kerosene extraction system from actual Chinese nutgall processing wastewater was a reliable method. The use of 30% TBP/kerosene as an extractant resulted in excellent recovery of gallic acid from Chinese nutgall processing wastewater. The extraction efficiency was high, the operation conditions were mild, and the separation of the product was simple. The process route was a closed loop system, which could run continuously. In addition to the recovery of the valuable gallic acid component obtaining economic value from the waste-water by the extraction process, the biodegradability of the waste-water was also significantly improved, which provided favorable conditions for the smooth performance of the sub-sequent biological treatment.