1. Introduction
With the rapid pace of industrialization, heavy metal pollution in the environment has become an increasingly serious issue throughout the world [1]. Cadmium (Cd) is one of the most general and toxic metals which presents in the environment [2]. Ionic liquids (ILs) have emerged as a new type of organic solvent and are widely used in industry [3–4]. Their usage has the potential to yield functional materials for green and sustainable chemistry, such as energy, electronics, medicine, food, cosmetics, and more [5]. However, the industrial usage of ILs could mean that the release of ILs into the environment may be inevitable. Furthermore, there are also an enormous number of possible combinations of anions and cations that can form ILs [6]. Jing et al. [7] studied the effect of 1-octyl-3-methylimidazolium chloride [C8mim][Cl], as an ionic liquid, on cell replication and membrane permeability of Escherichia coli DH5α, and found that [C8mim][Cl], can decrease the viability of Escherichia coli DH5α and inhibite bacterial cell replication. Pernak and Docherty chose different ionic liquids to have an experiment on fungi and bacteria, and found that the organic cations were inhibitory to the microorganisms, with long chain length resulting in greater toxicity [8]. Studying on the toxicity of ionic liquids on plants has led to similar conclusions [9–10]. Liu et al. [11] found that 1-methyl-3-octylimidazolium bromide ([C8mim]Br) can inhibit the photosynthesis and increase the oxidative stress on wheat seedlings. These findings suggest that ILs is toxic to the environment. When ILs get into the environment polluted by cadmium, an unknown combination of reaction can occur. Chen investigated the joint effect of 0.5 mmol/L Cd2+ and various concentrations (50–400 mg/L) of the ionic liquid [C2mim][OAc] (1-ethyl-3-methyl-imidazolium acetate) and [C4mim][OAc] (1-butyl-3-methyl-imidazolium acetate) on the growth and photosynthetic performance of wheat seedlings in hydroponic culture and found that lower concentrations (50–200 mg/L) of [C2mim][OAc] and [C4mim][OAc] can alleviate the toxicity exerted on wheat seedlings by 0.5 mmol/L Cd2+ [12–13]. However, the relationship between different concentrations of ionic liquid and different concentrations of Cd2+ under the joint effect on the growth of wheat seedlings’ has not been reported. In the presence of ionic liquids, Marina et al. studied on effects of different alkyl chains lengths on barley seedlings [14], and reached the conclusion that the toxic effect on the plant increases as the length of the carbon chain of the ionic liquid increases. And the effects of different carbon chain length ionic liquids and Cd on higher plants have not been reported. As a continuation of our previous investigations [15], the current study investigated the effect of different concentrations of [C3mim][OAc] plus different concentrations of Cd2+ on various aspects of wheat seedlings. The aim of this experiment is to investigate whether there is a dynamic equilibrium relationship between the effects of different concentrations of ionic liquids and Cd on plants, which it not only compares the difference between this experimental results and [C2mim][OAc] experiments in future, but also provides an approach to explore the joint effect of different chains of ionic liquids and different concentration of Cd.
2. Materials and Methods
2.1. Synthesis of [C3mim][OAc]
[C3mim][OAc] was synthesized as shown in Fig. 1. 1-propyl-3-methyl-imidazolium bromide ([C3mim]Br) was synthesized according to the method reported by Wilkes et al. (1982). The intermediate, 1-propyl-3-methy-imidazolium hydroxide ([C3mim]OH), was then synthesized by the anion exchange reaction. Finally, the desired product, [C3mim][OAc], was synthesized by the neutralization reaction.
2.2. Wheat Culture
Wheat seeds, Liao Chun No. 18 (Triticum aestivum), used in the experiments were provided by the Liaoning Academy of Agricultural Sciences.
Wheat seeds were firstly disinfected with 0.1% HgCl2 for 10 min, and then thoroughly rinsed in distilled water. After that, the seeds were placed on nylon net wrapped over the top of a plastic beaker (inner diameter: 20 cm; height: 15 cm). The beaker had been prefilled to the top with Hoagland solution. The Hoagland solution is a hydroponic nutrient that is developed by Hoagland [16] in 1938, and the Hoagland is one of the most popular solution compositions for growing plants. It provides every necessary nutrient for plant growth and so it is appropriate for the growth of a large variety of plant species. Different concentrations of Cd2+ (0, 0.05, 0.125, 0.250, 0.375 mmol/L) and [C3mim][OAc] (0, 50, 100, 200, 300, 400 mg/L) were used in the culture preparation. The various combinations of Cd2+ plus [C3mim][OAc] are shown in Table 1. Fifty seeds were planted in per beaker. The experiment was carried out under natural light at room temperature. Three replicate were conducted for each treatment and three seedlings from each treatment were used for subsequent analysis.
Based on the study by Chen et al. [13] which are explored the effect of combination Cd2+ and ionic liquid on the growth of wheat seedlings, four different concentrations (0.05, 0.125, 0.250, and 0.375 mmol/L) of Cd2+ and six concentrations (0, 50, 100, 200, 300, 400 mg/L) of the ionic liquid [C3mim][OAc] were chosen in this study. For the seeds treated with 500mg/L [C3mim][OAc] and different concentrations of Cd2+ had no significant difference on the germination rate in the pre-experiment, and higher concentrations of [C3mim][OAc] was not used in this study. The combinations of these Cd2+ and [C3mim][OAc] were summarized in Table 1.
2.3. Growth and Physiology Indexes
For each treatment, the measurements were repeated three times and the mean experimental data were obtained. Experiments were carried out under natural light at room temperature.
After 8 days of growth, the length and dry weight of the shoot and root of the seedlings were measured to assess the extent of growth under the different Cd2+ and [C3mim][OAc] conditions. 10 seedlings were picked up randomly, and plant height from the bottom to highest of stem was measured by a ruler. The longest root length of each seedling was determined and the average root length was calculated, after that, the shoot and root biomass was measured. The fresh weight was measured by the electronic balance, and then they were put into the dryer at 80°C for some time until the weigh did not change.
The activity of superoxide dismutase (SOD) and content of malondialdehyde (MDA) of the leaves of the seedlings were determined after 8, 13 and 18 days of growth, according to the growth of seedlings respectively. SOD activity was determined with the NBT Photo reduction method by using spectrophotometry (type: 722, determine wavelength: 560 nm) [17]. MDA content was obtained via the Thiobarbituric Acid (TBA) test (type: 722, determine wavelength: 534 nm, 600 nm) [18].
Leaf tissue (0.1 g) of wheat seedling was extracted with 10 mL of ethanol-acetone (1:1 v/v) according to the method described by Hao and Liu [19]. The Cd2+ content in the extract was then determined by inductively coupled plasma mass spectroscopy (Agilent 7500a; limit of detection: 0.06 μg/L; RF power: 1280 w; carrier gas flow: 1.14 L/min; sample depth: 7 mm; nebulizer type: Babington; Sampling cone: Ni). Plant samples were weighed into the digestion vessels, and then HNO3 and H2O2 were added to each sample. Samples were pre-digested overnight in a fume hood, after the entire digest were transferred into plastic bottles and diluted with deionized water, and filtered. And then configured the standard solution, finally, the parameters of the spectrometer were set up and the Cd content of leaf tissue was determined [20].
3. Results and Discussion
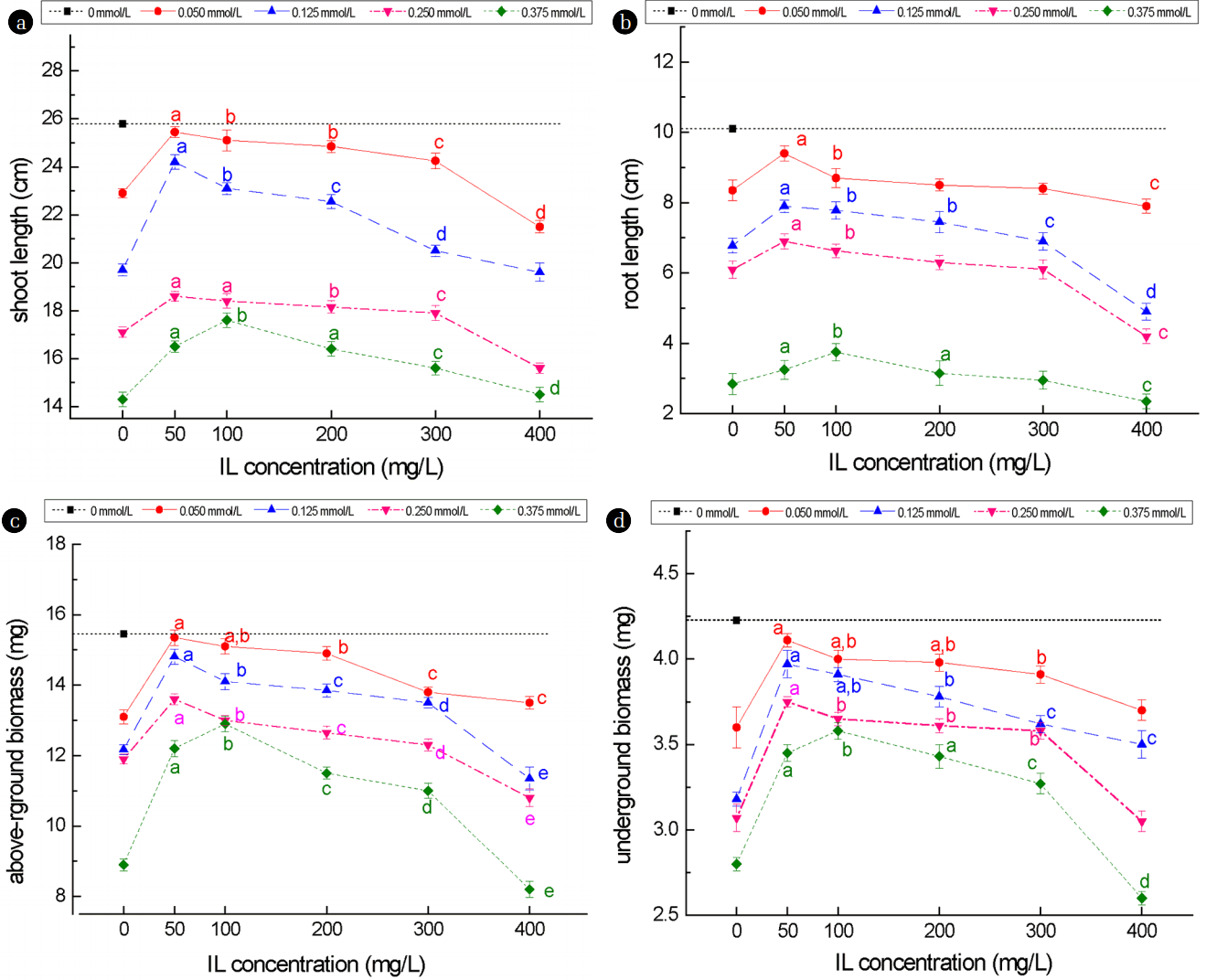
Existing research has shown that Cd2+ could hinder the root growth, inhibit the absorption of water and nutrients, and reduce the intensity of photosynthesis and respiration [21]. In this study, greater toxicity to the growth of wheat seedlings was observed for higher concentrations of Cd2+, as shown by more severe decreases in shoot and root lengths as well as in shoot and root biomasses (Fig. 2 & Fig. 3). In the case of shoot and root lengths, compared to seedlings grown in the absence of stress (represented by a single black square dot in each graph), seedlings grown in the presence of Cd2+ showed significant (p < 0.05) reduction in shoot length (17% to 40%) (Fig. 3(a)) and root length (17% to 40%) (Fig. 3(b)), and higher Cd2+ concentrations appeared to result in greater reduction. When the wheat seedlings were cultured in the presence of both Cd2+ plus [C3mim][OAc], some but significant improvements in shoot and root lengths were observed at lower concentrations of [C3mim][OAc], which ranged from 5.9% to 12.6%, with 50 mg/mL [C3mim][OAc] giving the highest improvement across the first three Cd2+ concentrations, although at the maximum Cd2+ used, 100 mg/mL [C3mim][OAc] appeared to give the highest improvement (Figs. 3(a) & 3(b)). At higher concentrations (≥ 300 mg/mL) of [C3mim][OAc], no real improvement in shoot length was observed, and root length appeared to be inhibited compared to no [C3mim][OAc].
Wheat seedling biomass was determined by measuring the dry weight of the shoot and root. Seedlings germinated and grown under Cd2+ stress alone showed significant (p < 0.05) decrease in both shoot and root, which ranged from 15.21% – 42.39% and 14.96% – 33.85%, respectively, compared to those of seedlings grown in the absence of stress (Fig. 3(c) & Fig. 3(d)). When the seedling was germinated and grown under both Cd2+ and [C3mim][OAc] stresses, shoot and root dry weights increased with increasing concentrations of [C3mim][OAc] up to 200 mg/mL, but appeared to peak at [C3mim][OAc] concentration of 50 mg/L in the case of 0.05 mmol/L Cd2+. However, at 0.375 mmol/L Cd2+, shoot and root dry weights peaked at 100 mg/L [C3mim][OAc]. Overall, the biomass profile of wheat seedlings under both Cd2+ and [C3mim][OAc] stresses exhibited similar trends, with some increases over Cd2+ stress alone for the lower range of [C3mim][OAc], regardless of Cd2+ and no improvement to reduction at the higher range (300 to 400 mg/mL) of [C3mim][OAc]. This showed that the higher concentration of [C3mim][OAc] aggravated the inhibitory effect of Cd2+ on wheat seedlings. This phenomenon may due to the excessive complex or [C3mim][OAc] damaging the cell membrane of wheat seedling and other subcellular structures, thus promoting the stress of Cd2+ on wheat seedlings [22–23].
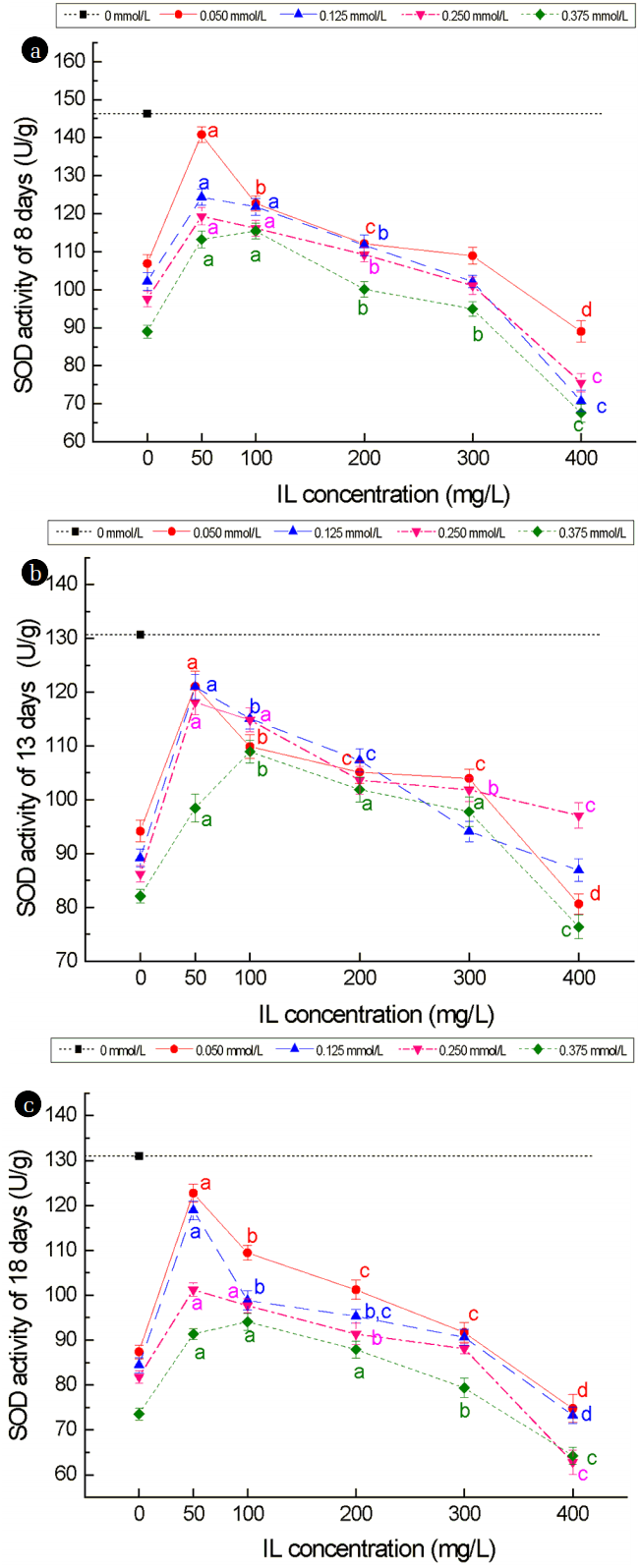
Fig. 4 shows the effects of Cd2+ and [C3mim][OAc] on the trend of SOD activity for wheat seedlings. The levels of SOD activity detected for wheat seedlings under the Cd2+ stress alone were significantly (p < 0.05) lower in all stages tested compared to that of seedlings grown in the absence of stress, with the reduction ranging from 27.16% to 43.79% (Fig. 4). Comparison of the SOD activity levels of seedlings grown under the same concentration of [C3mim][OAc], different concentrations of Cd2+ showed that higher concentrations of Cd2+ resulted in lower SOD activity levels. This indicated that Cd2+ exerted a more prominent effect of SOD activity than [C3mim][OAc]. Overall, the trend in SOD activity showed similar characteristic as those observed for growth, in which some improvements in SOD activity levels were obvious at the lower range of [C3mim][OAc] concentrations, which peaked at 50 mg/mL for the different Cd2+ concentrations, except for 0.375 mmol/L, whereby the peak occurred at 100 mg/mL [C3mim][OAc]. At the maximum [C3mim][OAc] concentration, the level of SOD was much lower compared to no [C3mim][OAc].
MDA is a product of the plant’s membrane lipid oxidation reaction when the plants are under stress, and it is toxic and can damage the cell membrane [23]. Wheat seedlings grown under Cd2+ stress without or with [C3mim][OAc] stress all exhibited higher levels of MDA (69.70 to 172.69%) more than those grown under no stress (Fig. 5). Again, in the presence of Cd2+, lower concentrations of [C3mim][OAc] could significantly decrease the level of MDA compared to no [C3mim][OAc], whereas higher (300 and 400 mg/mL) had no effect, although 400 mg/mL [C3mim][OAc] seemed to result in significant increase in MDA for seedlings grown in the presence of highest Cd2+ concentration at days of growth. The data of MDA was consistent with that of growth, demonstrating that damage to cell membrane may be one of the factors that led to growth inhibition for seedlings under Cd2+ stress.
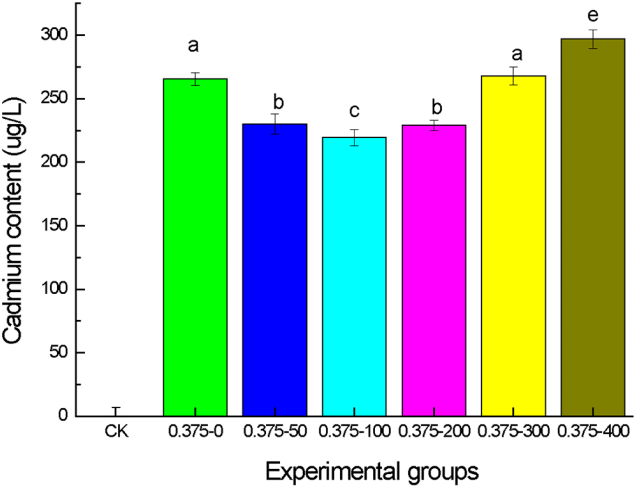
The joined effect of [C3mim][OAc] and Cd2+ stress on the growth and two physiological indexes of wheat seedlings observed in the above experiments suggested that 100–300 mg/L [C3mim][OAc] could partly relieve the toxicity of Cd2+ on wheat seedlings. To further explore this experimental result, the content of Cd2+ in the leaf tissues was determined by inductively coupled plasma mass spectroscopy for seedlings grown in the presence of 0.375 mmol/L Cd2+ plus 100 mg/L, 200 mg/L and 300 mg/L [C3mim][OAc] and compared to the Cd2+ content in the leaf tissues of the control (Fig. 6). Seedlings grown under both Cd2+ and [C3mim][OAc] stresses exhibited significant reduction in Cd2+ of the leaf tissues compared to seedlings grown under Cd2+ only. The biggest reduction was almost 60% and this was achieved with 100 mg/mL [C3mim][OAc]. At 300 mg/mL there was no obvious reduction in Cd2+ in the whereas at 400 mg/mL, the content of Cd2+ in the leaf tissues was even higher than that in the absence of [C3mim][OAc]. The result of this experiment also is paralleled with those obtained for growth, SOD activity level and MDA content.
The results of four growth indexes and two physiology indexes showed that moderate concentration of [C3mim][OAc] could alleviate the effect of Cd2+ stress partially. The toxicity exerted by 0.05–0.25 mmol/L Cd2+ could be effectively reduced by 50 mg/L [C3mim][OAc], whereas 100 mg/L [C3mim][OAc] was needed to reduce the toxicity exerted by 0.375 mmol/L Cd2+. This means that 0.050–0.250 mmol/L Cd2+ can reach the dynamic equilibrium with 50 mg/L [C3mim][OAc], and 0.375 mmol/L Cd2+ needs 100 mg/L [C3mim][OAc]. This was due to the formation of large molecular complexes with the ionic liquid and Cd2+ [13]. They can thereby promote or inhibit the uptake or accumulation of metal ions in plants [24]. The concentration of free Cd2+ declined in the culture medium because of forming of large complexes, resulting in the decrease of Cd2+ content in the leaves of the plant. The impact on the cell membrane was less affected by the joint of low concentration of ionic liquid (50–200 mg/L) and Cd, whereas the higher concentration (≥ 300 mg/L) of [C3mim][OAc] and Cd2+ can make the cell membrane seriously damaged, resulting in large molecular complexes can enter into the plants and have a great toxic effect on the normal growth of plants [24].
When plants are under the stress of heavy metal, they will produce more reactive oxygen species (ROS), which can induce antioxidant enzymes activity and disrupt protein, nucleic acid and lipids [25]. The peak value of the curve at 0.050–0.250 mmol/L Cd2+ and 50mg/L [C3mim][OAc] is due to the dynamic balance of the large molecular complex formation, and it is the same at 0.375 mmol/L Cd2+ and 100 mg/L [C3mim][OAc]. Liu et al. studied the phytotoxicity and oxidative stress effect of 1-octyl-3-methyl-imidazolium chloride ionic liquid on rice seedlings, finding that ILs could change the structure of organelle, decrease of Hill reaction activity and root system activity, and the increase of root membrane permeability [26]. This phenomenon due to the structure of imidazolium ionic liquids is similar to that of the cationic surfactant [27], which has a hydrophobic portion and a lipophilic one. This characteristic of the ILs can cause the destruction of membrane lipid structure and the change of composition [28] (Gong et al. 2004). 400 mg/L [C3mim][OAc] offered no real benefits to the seedlings under Cd2+ stress, this phenomenon due to the toxic effect of excessive ionic liquids on the seedlings, increasing the permeability of membrane, and this lead to the increase of cadmium content in plants, and the conclusions are proved by the determination of Cd2+ in wheat leaf cell organelles.
4. Conclusions
Compared with no stress, the shoot, root length, the aboveground, underground biomass and SOD activity of wheat seedlings were significantly decreased under the stress of Cd2+ only, while MDA content was increased. On the joint effect of [C3mim][OAc] and Cd2+, the shoot, root length, the aboveground, underground biomass and SOD activity of wheat seedlings under low concentration (50–200 mg/L) of [C3mim][OAc] was higher than that under the Cd2+ stress only, while the MDA content was decreased, though there was no significant difference on the 300 mg/L concentration of [C3mim][OAc]. When the concentration of [C3mim][OAc] reached 400 mg/L, the shoot, root length, the aboveground, underground biomass and SOD activity of wheat seedlings were decreased, while the MDA content was increased. Compared to Cd2+ stress only, the Cd content in leaf tissues was less under the joint of low concentration (50–200 mg/L) of [C3mim][OAc] combined with Cd2+, while, it was more under the joint of high concentration (≥ 300 mg/L) of [C3mim][OAc] combined with Cd2+ than that under Cd2+ stress only. Hence, it shows that low concentration (50–200 mg/L) of [C3mim][OAc] could reduce the toxicity on the wheat seedlings.
A result was also be found, that is, when it could reach a dynamic equilibrium: Low Cd2+ concentration (0.05–0.250 mmol/L) requiring 50 mg/L [C3mim][OAc] or high Cd2+ concentration (0.375 mmol/L) requiring 100 mg/L [C3mim][OAc], the reduction of toxicity was optimal.