AbstractCoagulation, a fundamental and crucial operation in water treatment, has a long history of application in water/wastewater treatment processes. The efficiency of coagulation depends primarily on the quality, type, and quantity of coagulants used. Although aluminum or iron-based coagulants are the most commonly employed, polymeric silicate coagulants (PSCs) are less frequently utilized. However, the PSCs are promising candidates that deserve further exploration and examination of their potential applications. This review presents an examination of the advantages, synthesis theory, coagulation mechanism, and structure of PSCs. It also delves into the current limitations of these coagulants in research and the challenges that lie ahead for future studies. Finally, it offers some suggestions for future research directions. These discussions are intended to aid readers in comprehending the fundamental characteristics of these coagulants, enabling them to grasp their design methodologies and application area. This understanding can contribute to overcoming the limitations of this type of flocculants, further enhancing their effectiveness.
Graphical Abstract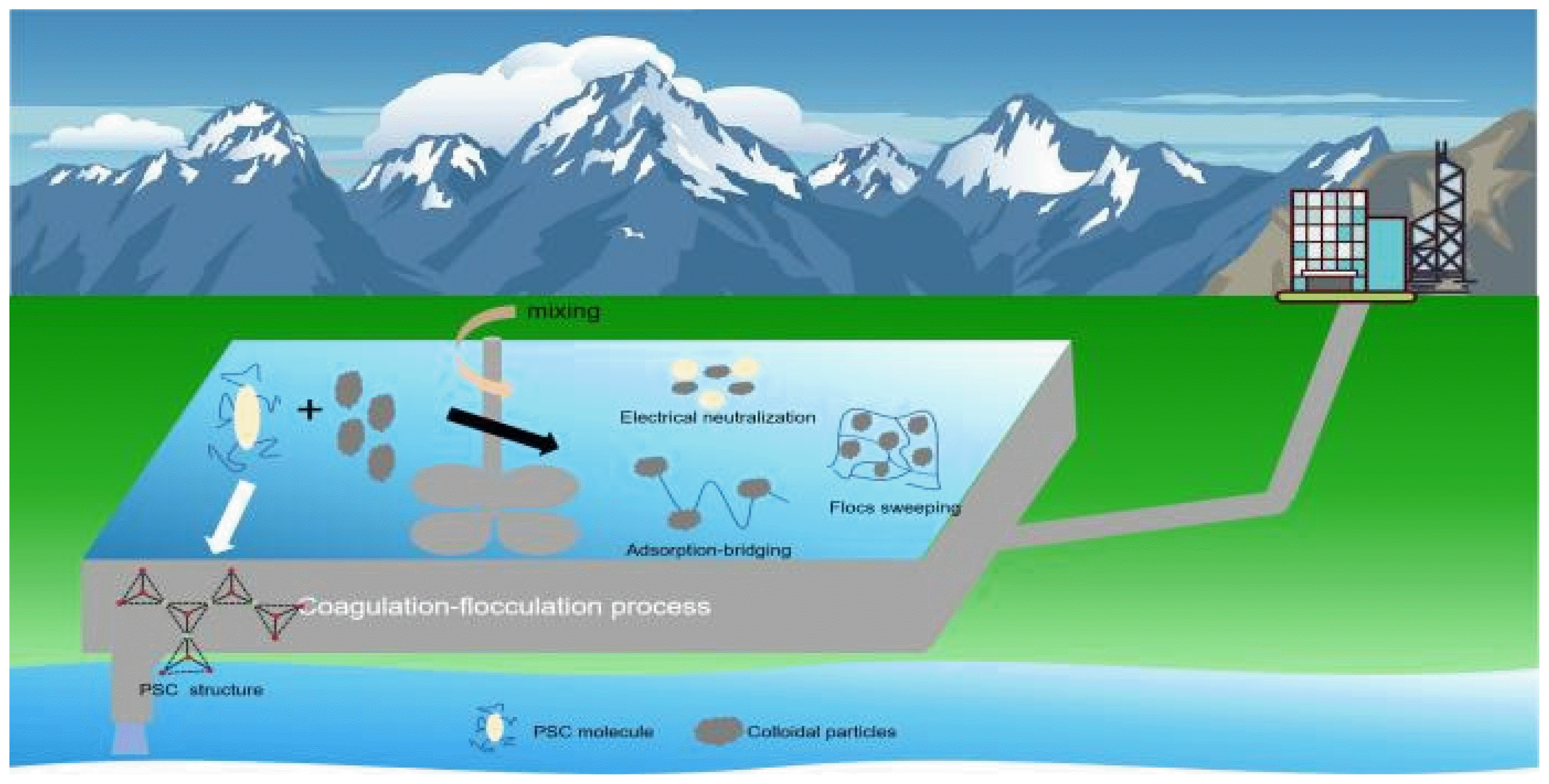
1. IntroductionWater is not only an important basis for human life, but also a necessary in development of industry. Many rivers in China are experiencing varying degrees of pollution, and its tendency is continuously worsening. It has posed a serious threat to the safety of drinking water for residents, and long-term consumption or use of contaminated water can jeopardize human health. Presently, water for Chinese residents mainly comes from rivers, lakes and groundwater. Within the seven major river systems, up to 40% of river sections are unsuitable for use as drinking water sources, particularly in industrially developed towns where 78% of urban river sections are unsuitable for use as drinking water sources [1]. The main reason for this issue is due to the discharge of domestic sewage and industrial and agricultural wastewater. When the pollutants in wastewater exceed self-purification capacity, water pollution arise and ultimately threaten the safety of drinking water and, consequently, human health [2, 3]. A survey report by the World Health Organization reveals that water pollution is linked to 80% of human diseases, and waterborne diseases caused by microbial contamination of drinking water, which can spread various infectious diseases such as diarrhea, cholera, dysentery, typhoid, and poliomyelitis, causing 485,000 deaths annually due to diarrhea [4]. Therefore, ensuring water resource security and addressing water pollution issues have become crucial tasks.
There are various methods for water treatment, such as coagulation, filtration, and adsorption[5–10]. Among them, coagulation is widely used as an effective water treatment method due to its simple and efficient process. Traditional coagulation methods can effectively remove 80% to 95% of suspended solids and 65% to 95% of colloidal substances from water, with a significant impact on reducing chemical oxygen demand (COD) [11]. However, despite the numerous types of coagulants available on the market, their effectiveness varies. As water resources become increasingly polluted, the treatment effects of many coagulants are unable to meet the required standards. Therefore, it is imperative to investigate new types of coagulants that can overcome the limitations of traditional coagulants in water treatment.
Polysilicic acid-based coagulant (PSC) is a kind of typical metal-based coagulant. Its development is far less than that of aluminum and ferric based coagulants, and it is rarely used in industry. One of the main reasons is due to its poor stability [12]. It also has some better functions over conventional coagulants, such as larger flocs size and better algae removal effect, so it is of great significance to solve the deficiency of this kind of flocculant [13]. In order to better understand the development of this kind of flocculant, to lay a foundation for the modification of this kind of flocculant. This paper provides a review on the relevant theories and technology to coagulation behaviors of the PSC, which includes synthesis method, structure, and coagulation mechanism. Finally, suggestions are given in order to support the development of the PSC in future.
2. Literature ResearchThe data in Fig. 1 was collected from the core database of Web of Science, including SCI-EXPANDED, SSCI, CPCI-S, CCR-EXPANDED, IC. It retrieved a total of 33 papers related to PSCs from 1998 to 2023. The search keywords included polymeric silicate, coagulant, water treatment, etc. The data analysis software used for this study was CiteSpace. Fig. 1(a) shows the detailed distribution results of PSC study in various countries. In recent years, the researches in China are very active, and a large number of research papers have been published. Other countries such as Japan, Malaysia, the United States, India and Canada have also made significant contributions, although not as extensive as China. Fig. 1(b) presents a keyword analysis of papers. The figure illustrates the appearance duration on the timeline for the top thirteen keywords. For instance, around 2015, “ferric” first appeared in large numbers in PSC-involved papers in the core database of Web of Science. The latest keywords, such as “decolonization,” began to appear in large numbers in papers in 2020 and continue to be popular in PSC papers until 2023. Fig. 1(c) displays the occurrence of keywords in papers on the research of PSC. The rightmost column represents cluster labels, generated by an algorithm to summarize common themes in the keywords along the left line. This label serves as a representation of commonalities in papers associated with these keywords. For example, the first keyword “efficiency” is associated with other high-frequency keywords on the left axis, along with their appearance timelines. The most frequently appearing keyword selected through a threshold, “removal,” indicates a high occurrence in the “efficiency” domain around 2013. Additionally, this keyword is found to co-occur with other keywords under the same cluster label in the keywords of the same paper.
A comprehensive analysis unearthed distinctive patterns and crucial characteristics in international PSC research. To begin, China has emerged as the most active and productive nation in this domain, exerting a significant impact. Furthermore, the evolving trends of keywords from “ferric” to “decolonization” suggest a shift in research priorities across different timeframes. Lastly, cluster labels reveal connections between keywords, aiding in the comprehension of common themes and collaborative relationships within the field. These findings offer valuable insights into the dynamics of PSC action and serve as a valuable primer for researchers entering the field.
3. Advantages and ApplicationPSCs are a new type of coagulant formed by polymerizing metal salt with silicate. The introduction of different metal salts into the silicate can impart different water treatment effects to the resulting coagulant. Silicate itself has a mesh structure and high molecular weight, providing it with good flocculation capabilities. As a coagulant, it exhibits outstanding water treatment effects. However, its poor stability leads to gel formation and deactivation thus limiting its widespread application. Currently, there are two main types of coagulant products in the market, namely aluminum salt coagulant and ferric salt coagulant [14]. However, both types of coagulants have their own advantages and disadvantages. Ferric salt coagulant possesses excellent settling performance but often results in high residual color. In contrast, aluminum salt coagulant produces smaller and looser flocs particles, which may cause potential issues such as exceeding residual aluminum content [15–22]. Faced with the complex and variable water environment, the application of traditional aluminum and ferric salt coagulants is not enough perfect [23]. It is necessary to study trajectory flocculants, because they have larger molecular weight and stable function, such as algae removal and heavy metal ions [24–30].Introducing metal salt solution into silicate improves the drawbacks of easy gel formation and deactivation of silicate, Some studies have also achieved this effect by making such flocculants solid [31]. Additionally, metal salts can fully exert their coagulation properties during coagulation. Therefore, PSC has become a research area of great interest with broad application prospects.
The addition of silicate gives the PSCs excellent adsorption bridging ability and a mesh structure [32, 33], which may result in a large surface area. In actual coagulation processes, an appropriate increase in dosage may enhance the coagulant’s charge neutralization ability, and moderate stirring makes it easier for the coagulant’s mesh structure to capture pollutants. It is for these advantages that PSC has a broad development space and prospect in the research and practice of treating water pollution and ensuring the safety of drinking water. Research on the application of PSC has mainly focused on the treatment of dye wastewater, algae, heavy metals, organic compounds, and so on, experimental results have demonstrated excellent coagulation effects on laboratory finding [24–30, 34–41]. The type of water that is actually available for treatment is shown in Fig. 2.
In the treatment of dye wastewater, the ratio of each component in PSC has a significant impact on the color removal of dye wastewater. Gao et al. [34] used polymeric silicate magnesium to treat dye wastewater and found that the highest color removal rate was achieved at a Mg/Si molar ratio of 1.36. Li et al. [35] used polymeric silicate titanium ferric zinc to treat Congo Red dye wastewater and found that the molar ratios of Si/Fe, Si/Zn, and Si/Ti at 1, 3.03, and 1.64, respectively, resulted in a removal rate of 99.16% for Congo Red. Tong et al. [36] combined polymeric silicate aluminum ferric (PSAF) with carboxymethyl chitosan for treating sulfur black dye wastewater. When the mass ratio of PSAF to carboxymethyl chitosan was three, the removal rate of color reached 88.6%.
The amount of coagulant is an important factor affecting algae removal. Lv and Qiao [24] use chitosan modified PSAF for algae removal. When the chitosan dosage was 0.5 mg/L and the PSAF was 2–8 mg/L, the removal rates for algae suspension absorbance and chlorophyll-a are both above 90%. Yan [25] used polymeric silicate-modified clay coagulant for removal of simulated algae, and the experimental results showed that the removal rate of algae could be up to 89% at the dosage of 10 mg/L of coagulant. Yang [26] applied self-made polymeric silicate zinc ferric sulfate to simulate algae-containing water, and the test results indicated that at a dosage of 25 mg/L, the removal rate of chlorophyll a reached 98.7%. Wang et al. [27] prepared a novel polymeric silicate aluminum chloride zinc ferric coagulant, and at the dosage of 14 mg/L, it exhibited the best coagulation and algae removal effect, with a removal rate of 98.97%.
Guo et al. [28] used optimized polysilicate aluminum ferric sulfate (PAFSS) for mining wastewater treatment, resulting in post-treatment concentrations of As, Be, and Pb at 34, 0.2, and 13 μg/L, respectively, meeting the first-level standards for wastewater. Xiao and Feng [29] used a novel coagulant, polymeric silicate ferric aluminum, prepared with sodium silicate, ferrous sulfate, and aluminum sulfate, for treating cadmium-contaminated wastewater. The results showed that the concentration of hexavalent chromium in the water was below 0.05 mg/L after treatment, and in comparison experiments with poly-aluminum chloride and polymeric sulfate ferric, polymeric silicate ferric aluminum exhibited significantly better chromium removal. Liu et al. [30] added surfactants to PSAF coagulants for the treatment of heavy metal flue gas wastewater, achieving a removal rate of 80.5% for Cd2+. Yu et al. [37] used polysilicate aluminum ferric sulfate coagulant (PAFS) for treating refinery wastewater and the removal of COD and oil reached 91.5% and 93.2%, respectively, at the dosage of 90 mg/L. Huang and Luo [38] found that when PSAF coagulant was used to re-treat urban sewage, the turbidity removal rate was around 92%, color removal rate around 88%, and the removal of CODCr could reach about 82%. Zhang et al. [39] used PSAF for coagulation treatment of late-stage leachate from garbage landfill, and with the addition of polyacrylamide as a coagulant aid, the removal rate of COD reached 62%. Ma et al. [40] used a composite of polymeric silicate aluminum magnesium (PMAS) and cationic polyacrylamide for the step-by-step coagulation treatment of drinking water, achieving good results. Liu et al. [41] used polymeric silicate aluminum calcium grafted with alginate for treating drinking water. Experimental results showed that the new inorganic alginate coagulant had higher coagulation efficiency compared to conventional PSC. Based on the aforementioned research findings, it is evident that PSC, as novel coagulants, demonstrate effective treatment of wastewater pollutants.
4. Preparation TheoryThere are various methods for preparing PSC, which can be categorized as either hybridization or copolymerization techniques. When preparing single-metal polymerized silicate coagulants, the hybrid method is typically used. However, if two or more metal ions are to be polymerized with PSC, the primary difference in the preparation process lies in whether the metal ions are polymerized first and then mixed with the polymerized silicate [42].
4.1. Hybridization MethodThe hybrid method involves mixing the solution of the metal salt needed for coagulant preparation in advance and then adding it to the polymeric silicate solution for mixing. In the preparation of a single metal polymeric silicate coagulant, the metal salt is added to the polymeric silicate solution in specific proportions and polymerized with stirring under specific conditions as shown in Fig. 3(a). Then, maturation is performed. Sun [43] prepared polymeric silicate titanium coagulant by dripping polymeric titanium chloride solution into polymeric silicate solution according to a specific Si/Ti molar ratio. The coagulant achieved removal rates of 98.3% and 96.2% for turbidity and UV254 of simulated wastewater, respectively. When the metal ions polymerized with PSC are two or more, the hybriding methods requires pre-polymerization of the metal salt solution before adding it to the polymeric silicate solution for polymerization, as shown in Fig. 3(b). Shi et al. [44]introduced aluminum ferric salt mixture into matured polymeric silicate to prepare PSAF coagulant. It was found that the optimal molar ratio of n(Fe) / n(Al) / n(Si) was 1:1:1, and the alkalinity was around 0.8, resulting in the best coagulation effect. Li [45] prepared PSAF coagulant by the hybriding methods and used it to treat dye wastewater. The results showed that the optimal mass ratio of Al / Fe was 4:1, and the coagulant concentration was 16 mg/L. The decolorization rate was above 90% at a water sample pH of 7.5. Qiu et al. [46] used the hybriding methods to prepare PSAF coagulant. In the preparation process, many controllable factors, among which pH adjustment is crucial, were identified. Qi et al. [47] used the hybriding methods to prepare polymeric silicate ferric titanium coagulant, and the optimal ratio was n(Fe) / n(Ti) was 7, n(Fe+Ti) / n(Si) was 6, showing excellent removal effects for coking wastewater. A limitation of the hybriding methods used in the preparation of bimetallic PSC coagulants is that they often exhibit relatively low polymerization and hydrolysis degrees. This is due to the pre-polymerization of the metal salt during the process.
4.2. Copolymerization MethodThe copolymerization method involves combining the metal salts required for coagulant preparation with polymeric silicate and then stirring the mixture under specific conditions. This method has eliminated the need for the pre-polymerization step of the metal salt solution, as illustrated in Fig. 3(c). Coagulants prepared by this method have high degrees of polymerization and hydrolysis and show excellent coagulation effects. However, it cannot be used to prepare a single metal polymeric silicate coagulant. Wang and Yi [48] found that PMAS prepared by the copolymerization method exhibited better adsorption bridging and mesh capture effects. Wang and Ren [49] used the copolymerization method to prepare PMAS coagulant, obtaining the optimal parameters of n(Mg) / n(Al) / n(Si) was 1:1:1. Under these conditions, the turbidity and CODcr removal rates of sewage reached 91.98% and 82.85%, respectively. Liang [50] dripped aluminum salt and magnesium salt into polymeric silicate solution to prepare PMAS coagulant. Through experimental verification, the optimal parameters were determined to be n(Mg) / n(Al) was 2, n(Mg+Al) / n(Si) was 4. Fan et al. [51] prepared PMAS coagulant using the copolymerization method by adding aluminum sulfate and magnesium sulfate solution into sodium silicate solution with adjusted pH. The coagulant with Mg/Si ratio of 3:1 was found to be the most effective for COD removal. Liu and Wang [52] prepared PMAS coagulant by the copolymerization method, and the best preparation process parameters were: polymeric silicate activation for 120 minutes, silicate concentration of 3%, n(Si) / n(Al) was 1:3, and maturation time of 150 minutes.
4.3. Discussion on PreparationIn order to achieve industrial-scale and practical treatment applications, both the hybridization method and copolymerization method are considered ideal synthesis methods for polysilicate coagulants (PSCs). These methods yield PSCs with excellent coagulation effects, but they also have their own advantages and limitations. The hybridization method can produce polymeric silicate monometallic coagulants with excellent coagulation effects. However, when more than one metal salt is involved in the polymerization, it is necessary to pre-polymerize the metal salt solution used. This pre-polymerization step may impact the subsequent polymerization with silicate, leading to lower polymerization and hydrolysis degrees of polymeric silicate metal salt coagulants, thereby affecting their coagulation performance in practical applications. Moreover, the pre-polymerization step can increase the preparation cost of coagulants in industrial scale-up. The copolymerization method simplifies the pre-polymerization step in the hybridization method. It has simple operating steps, and the PSCs prepared by copolymerization exhibit good polymerization and hydrolysis degrees. However, the copolymerization method has limitations in that it cannot produce polymeric silicate monometallic coagulants. In practical preparation, when there is an excessive amount of metal salt solution for copolymerization, the different properties of metal salt solutions and silicate may result in different reaction environments. This makes it challenging to find the optimal preparation conditions in actual production. Both of the above synthesis methods can be flexibly applied. Researchers can choose the preparation method that best suits their own research needs during the study.
In the research on the preparation of PSCs, various factors can influence the synthesis of coagulants, such as pH, polymerization temperature, the ratio of metal salts to polysilicate, and the ratio between metal salts. Xie [53] found that the most ideal removal efficiency of pollutants in river water was achieved when the ratio of Al / Fe / Si was 1:1:2. Gao et al. [54] studied the impact of polymeric silicate aluminum-iron coagulants on the phosphorus removal rate in simulated phosphorus-containing wastewater. The results indicated that the best coagulation effect was obtained when (Al+Fe) / Si was 3 and Al / Fe was 2. Li [55] conducted experiments demonstrating that, under a pH of 6, the removal rate of Cr (VI) in wastewater reached 99.77% when the mass concentration of the prepared polymeric silicate aluminum-iron coagulant was 6 g/L. Canizares et al. [56] found that the solution pH decreased with an increase in the dosage of the coagulant, affecting the types of hydrolysis products and the surface charge of the precipitate, leading to a reduction in the coagulation efficiency. Therefore, in research, it is essential to thoroughly consider multiple factors to identify the optimal preparation conditions.
5. ClassificationPSCs encompass several typical preparation methods and are widely utilized for water treatment, including single-metal, bimetallic composites, and trimetallic coupling [57–59]. These methods are also suitable for the production of aluminum- or iron-based coagulants. Consequently, acquiring this knowledge provides a fundamental understanding of PSC preparation and is crucial for its further enhancement.
5.1. Single MetalJapan pioneered the study of polymeric silicate ferric (PFS) coagulants [60]. It was found experimentally that in the actual coagulation process, obvious flocs would appear in the rapid stirring stage, and the flocs were stable and not easy to be dispersed [61]. Synthesis studies of PFS coagulants were also carried out in China. Zheng et al. [62] studied the preparation of polysilicate ferric sulfate (PFSS) and found that the optimal preparation conditions were w(SiO2) was 1.4% to 2.0%, n(Fe) / n(Si) was 0.8 to 1.0, pH was 1.5 to 1.8, and silicate activation time of 1 to 18 hours. Gao et al. [63] prepared PFSS, and the coagulation results showed that the optimal n(Fe) / n(SiO2) was around 1.5 for the best coagulation and turbidity removal effect. In polymeric silicate aluminum (PAS) coagulants, polymeric silicate has a certain complexation and adsorption effect on Al3+, and there exists a non-ionic bond between polymeric silicate ions and polymeric aluminum ions [64–66]. In the actual preparation, the concentration of polymeric silicate solution directly affects the coagulation effect and stability of PAS. Within a certain aluminum-silicon ratio range, the stability of the coagulant increases as the aluminum-silicon ratio decreases[ 67]. Some studies are dissatisfied with the use of pharmaceutical reagents in the production of PAS coagulants, and instead focus on optimizing the raw materials to reduce costs and pave the way for industrial applications. Ning et al. [68] obtained the optimal process parameters for preparing polymeric silicate aluminum chloride from gangue through experiments. Zhang et al. [69] extracted effective components from yellow furnace slag to prepare PAS coagulant for treating polluted water, achieving a turbidity removal rate of 99.3% and a COD reduction rate of 75.8%. Besides introducing ferric and aluminum salts, the introduction of some other metal salts into polymeric silicate solutions has also shown good coagulation performance. Yu and Rong [70] found through experiments that under conditions of pH ≥ 12, the removal rate of polymeric silicate magnesium [n(Mg) / n(Si) was 1] could reach over 95%. Gao et al. [71] demonstrated that polymeric silicate zinc has superior neutralization, sweep flocculation, and curling and sweeping abilities compared to PFS and PAS coagulants.
5.2. Bimetallic CompositeIntroducing two kinds of metal ions into polymeric silicate solution simultaneously can combine various effective components and complement the deficiencies of a single coagulant [72]. This type of bimetallic ion composite coagulant has significantly better coagulation effect than PSC with a single metal [73]. Nowadays, the most common coagulant of this type is PSAF coagulant. Studies have found that different aluminum-ferric-to-silicon ratios and aluminum-to-ferric ratios have a significant impact on the coagulation performance of the coagulant [74]. It has a wide applicable pH range, high floc density, fast floc rate, simple preparation, wide raw material sources, and strong stability, making it highly valuable and widely applicable [75]. The success of PSAF coagulant has led to the birth of other bimetallic PSC. Zhuang et al. [76] synthesized a novel inorganic composite coagulant, polymeric silicate zirconium aluminum sulfate, using water glass and aluminum/zirconium salts as raw materials, showing high coagulation performance. Tang et al. [77] synthesized a new composite coagulant, polymeric silicate ferric manganese (PSFM), using sodium silicate, ferrous sulfate, and potassium permanganate as raw materials. The effectiveness of PSFM was superior to conventional coagulants like PFS. The research of PSC bimetallic salt is not only the preparation, but also the optimization and modification. Li et al. [78] used boron-modified polymeric silicate aluminum titanium and found that the modified coagulant was a compact amorphous polymer with good coagulation performance. The optimal preparation conditions were found to be n(Al+Ti) / n(Si) was 0.6, n(Al) / n(Ti) was 8:2, n(B) / n(Si) was 0.05, and pH was 2.5. Ma et al. [79] prepared a novel magnetic coagulant by using polymeric silicate aluminum zinc, chitosan, and Fe3O4 nanoparticles. The rough surface of the coagulant enhances its adsorption and sweep flocculation capabilities, effectively transforming it into a novel magnetic composite polymer.
5.3. Trimetallic CompositeThe trimetallic salts of PSC exhibit better performance than the monometallic or bimetallic salts of PSC. The different proportions of various metal salts in the coagulant lead to different coagulation performances [80]. Some PSC trimetallic salts have an internal network structure with rod-shaped particles intertwining and stacking layer by layer, resulting in a high degree of polymerization. This structure enhances the adsorption bridging ability of the coagulant [81]. Other coagulants’ crystals present a branched long-chain form, with branch chains connecting into longer chains. This structure is favorable for exerting adsorption bridging and sweep flocculation and curling and sweeping effects [82]. In the actual coagulation processes, the trimetallic salt of PSC also showed good coagulation effects. Li et al. [83] prepared the coagulant polymeric silicate ferric magnesium zinc, which, when used to treat simulated humic acid wastewater, achieved a turbidity of 0.913 NTU, a color removal rate of 99.2%, and a UV254 removal rate of 81% at the optimum dosage. Chen et al. [84] used an orthogonal experiment to prepare polymeric silicate aluminum ferric magnesium (PAFMS) coagulant, which showed good treatment effects on dyeing wastewater in the pH range of 11 to 12, with removal rates of 84.2% for COD, 87.9% for SS, and 95.7% for color.
6. Characterization of StructureThe mesh-like structure and high molecular weight of the polymeric silicate give the PSC a distinctive morphological structure, clearly distinguishing it from conventional coagulants. Examining the structure under a microscope provides a deeper understanding of coagulation mechanisms and characteristics, such as functional groups and chemical bonds. This critical information is essential for further enhancing and optimizing coagulants. The primary methods used to analyze the morphological structure, chemical groups, and species of coagulants are Scanning Electron Microscopy (SEM), Fourier Transform Infrared Spectroscopy (FTIR), and Ferron Complexation Timed Spectrophotometry (Ferron method).
6.1. MorphologySEM allows for the observation of the original surface of coagulants in the microscopic domain. This provides insights into the surface morphology features of coagulants, facilitating the analysis of the surface morphology of coagulants prepared under different conditions. The optimal ratio for preparing coagulants can be clarified. During observation, the dried and powdered samples need to be coated on a conductive adhesive and undergo gold spraying pretreatment before observation. Analysis of SEM results for PSC reveals that the introduction of metal ions makes the mesh-like structure of polymeric silicate rougher, significantly increasing its surface area. Some metal ions attach to the surface of polymeric silicate, forming chain structures, further enhancing the coagulation performance of PSC. The branched structure of polymeric silicate ferric magnesium promotes adsorption bridging during the coagulation process, and metal ions at the chain ends prevent the deactivation of polymeric silicate gel [85]. In some coagulants, the presence of polymeric silicate increases the polymerization degree of PSAF, resulting in a mesh-like structure on the coagulant’s surface, enhancing its adsorption bridging and sweep flocculation and curling and sweeping capabilities [86]. The coagulation units of fly ash-based PSAF coagulant are large plate-shaped aggregates, with aluminum, ferric, and their hydrolysis products adsorbed and chelated on the surface of polymeric silicate particles [87]. The surface of polymeric silicate aluminum ferric magnesium coagulant is rough, exhibiting a three-dimensional, porous structure. This characteristic increases the surface area and adsorption capacity of the coagulant, demonstrating good coagulation ability [88].
When PSC is combined with other materials, a comparison of their SEM results with conventional or non-combined coagulants reveals that the combined coagulants have rougher surface. For example, polymeric silicate aluminum ferric-sodium alginate shows a rougher surface with more fine gaps, facilitating the adsorption of colloidal particles [89]. The particles of polymeric silicate aluminum zinc-starch composite are smaller and more closely connected than polymeric silicate alone, and the particle surface is rougher, enhancing the adsorption bridging effect and improving the ability to capture suspended particles [90].
6.2. Chemical GroupsFTIR is based on the absorption of infrared radiation by molecules, causing energy level transitions in chemical bonds or functional groups. This method helps identify the chemical bonds and functional groups present in the molecule, providing clarity on the structure of coagulants. For coagulants, infrared spectroscopy can visually distinguish whether the preparation process involves simple mixing of raw materials or the generation of new substances through mutual reactions. In the mid-infrared spectrum of PSAF, significant functional groups such as Al-OH, Fe-OH, Si-O-Al, Si-O-Fe, can be observed [91]. The infrared spectrum of fly ash-based PSAF coagulant shows hydroxyl groups and aluminum ion-connected polymers, suggesting that the coagulant is a complex of aluminum ions, ferric ions, and polymeric silicate formed through chelation reactions [92]. In the infrared spectrum of silica sol-polymeric silicate aluminum zinc, the formation of new bonds such as Si—O—Zn and Si—O—Zn can be observed [93]. According to the infrared spectrum of polymeric silicate ferric zinc coagulant, it can be inferred that hydroxylated ferric chelates and hydroxylated zinc chelates are formed. During actual observation of the infrared spectrum, a conventional coagulant can be selected for comparison with PSC. This allows for a more intuitive demonstration of the generation of new chemical bonds or functional groups during the preparation of PSC.
6.3. SpeciesThe Ferron method can analyze the polymeric species of ions in PSC. Different polymeric species of ions often exist in coagulants. Within a certain concentration range, different species of ions and their complexes react with Ferron reagent at different rates. By distinguishing the reaction rates, the polymeric species of ions can be determined, and the optimal ion ratio can be established.
Taking aluminum ions as an example, the actual working curve for Al is shown in Fig. 5(a) [94]. During the actual working curve measurement, 20 μL of the coagulant is taken into the colorimetric tube, and the timing starts immediately. A dual-beam UV spectrophotometer measures the absorbance of the solution at a wavelength of 370 nm, with the absorbance values recorded hourly. In PAS coagulants, aluminum generally exists in three polymeric species, and the higher the polymerization degree, the slower the reaction with Ferron. In PAS coagulants, different species of aluminum exhibit different coagulation mechanisms. For example, the Alb component destabilizes colloidal particles through adsorption-neutralization. The Alc component mainly functions through adsorption bridging and sweep flocculation mechanisms [94]. In general, the main structural species of aluminum in polymeric silicate sulfuric acid aluminum is Alc. As the pH increases, the amounts of Ala and Alb relatively decrease, and the amount of Alc relatively increases [95]. As shown in table 1. For both PAC and PAS, the content of Ala gradually decreased, and the content of Alb gradually increased with the increase of alkalization degree. The difference is that in PAC, the content of Alc will increase slowly with the increase of the degree of alkalinity, while in PAS, under the condition of specific n(Si) / n(Al), the content of Alc first increases with the increase of the degree of alkalinity, and then gradually decreases with the increase of the degree of alkalinity [96]. In polysilicate aluminum sulfate (PASS), polyaluminum ferric silicate (PAFS) and polyphosphor aluminum chloride (PPAC), under the same alkalinity condition, Al species is also affected by ionic molar ratio, pH and other conditions. It can be seen that no matter the traditional aluminum salt or aluminum polysilicate flocculant, the hydrolysis types of Al are inconsistent and completely affected by external conditions [97, 98].
Taking ferric ions as an example, Fe3+ undergoes hydrolysis-polymerization-precipitation in water, similar to the coagulation process of coagulants. Like aluminum ions, the species of ferric in coagulants are also divided into Fea, Feb, and Fec [101], the actual working curve for Fe shown in Fig. 5(b) [102]. The measurement method is consistent with the testing of Al species, with the wavelength range set at 598 nm. As the coagulant matures, the Fea and Feb contents gradually decrease, while the Fec content increases, indicating rapid changes in the hydrolysis species and structure of Fe in the early stages of maturation [103]. The hydrolysis species of ions in coagulants are closely related to the coagulation effect of coagulants. The amount of Feb in PFC is small, indicating that the Fea species is rapidly transformed into Fec species during the preparation process, the intermediate transition state Feb is small, the proportion of Fec is large, and the Fec species is relatively stable. As shown in Table 1. Comparing the hydrolyzed species of Fe in PFSS and polyferric titanium sulfate (PTFS), it can be found that with the increase of n(Ti) / n(Fe), the contents of Fea and Feb in polyferric titanium sulfate are gradually reduced, and the content of Fec is gradually increased. The situation of polyferric silicate sulfate is completely different, with the increase of n(Fe)/n(Si), Fea content gradually increases, and the content of Feb and Fec gradually decreases. But in the same n(Fe) / n(Si), with the increase of alkalization degree, Fea content gradually decreases, Fec content gradually increases, and Feb content first decreases and then increases. In addition, in aluminum and iron polysilicates flocculants, n(Fe) / n(Al) also affects the hydrolysis species of Fe in the flocculants, so whether it is traditional iron salt or polyferric silicate flocculants, the change law of the hydrolysis species of Fe will be affected by external conditions [98–100].
When Al or Fe is combined in PAS flocculant, its hydrolytic species will be affected by external conditions of ionic molar ratio, thus affecting the flocculation performance. Therefore, external conditions should be fully considered in the study of PAS preparation and its actual flocculation to achieve the best flocculation effect, which also provides a flexible method and mechanism for PAS regulation and treatment of specific polluted water bodies. But it also brings its own complexity. Determining the polymeric species of ions in PSC through the ferron complexation timed spectrophotometry may facilitate a better understanding of the coagulation mechanisms of coagulants under different conditions.
7. Coagulation MechanismThe exploration of coagulation mechanisms transitions from colloidal to microscopic domains [104, 105]. The investigation of these mechanisms not only contributes to the development of more effective coagulants, but also brings practical benefits to people’s lives. The extensively studied coagulation mechanisms include compressive double-layer action, adsorption-electrostatic neutralization, adsorption bridging action, and sweep flocculation [106].
The compressive bilayer effect is the introduction of an active electrolyte with a high valence counterion into the colloidal system, which leads to a decrease in the Zeta potential (ζ) and changes the primary repulsive force between colloidal particles to an attractive one [107]. The adsorption layer of ions species appears on the colloid’s surface with an outer diffuse layer. The combined layers are termed the “double layer” [108, 109]. A study on PSAF coagulant by Sun and Xu [110] revealed that aluminum and ferric ions precisely opposed the charge of colloidal particles. During coagulation, an exchange between colloidal particles and aluminum-ferric ions occurs, leading to a thinning of the diffuse layer, a reduction in the charge count, a subsequent decrease in the ζ, increased attraction between colloidal particles, and aggregation settling. The reduction in ζ is correlated with other factors, such as coagulation and ion concentration. Zouboulis A I [111] found a correlation between the decrease in ζ and a decrease in polymerization degree during the laboratory synthesis of PFSS. While the compressive double-layer action can explain coagulation mechanisms to some extent, its practical applications are limited by factors like ion concentration.
Adsorption-electrostatic neutralization involves the attraction between oppositely charged ions, colloidal particles, or polymers on the surface of colloidal particles. This neutralizes the surface charge, lowers the potential, and induces destabilization, aggregation, and settling of colloids [112]. This attraction process is accompanied by electrostatic forces, van der Waals forces, covalent bonds, and hydrogen bonds, with electrostatic forces being the primary force. Zhu et al. [113] found that the initial ζ of the supernatant in coagulation experiments changed from negative to positive, demonstrating the significant role of adsorption-electrostatic neutralization in coagulation reactions. This mechanism explains the hydrolysis species of various metal salt coagulants and their role in destabilizing and aggregating colloidal particles. PSC exhibits strong electrostatic neutralization ability [114, 115], but their internal chemical bonds and structures, such as the presence status of certain functional groups, can influence adsorption, thereby affecting coagulant performance. This is consistent with the characteristics of aluminum- and ferric-based flocculants[33]. The difference is that silicon contains a higher valence state, which would result in a better neutralization role.
Adsorption bridging action refers to mutual adsorption between PSC and colloids, creating bridges between particles and forming large aggregates for destabilization and settling [116]. During coagulation, PSC synthesize branched long-chain structures that extend in various directions, enhancing their adsorption bridging action [117–119]. However, although the conventional aluminum salt and ferric salt flocculants also form long chain structures during the flocculation process, due to the low polymerization degree and poor adsorption performance, the adsorption bridging effect is not as good as the PSC with higher polymerization degree and more three-dimensional spatial structure. Chen et al. [120] used PAS and poly-aluminum chloride to treat bleached pulp wastewater. The analysis of raw water and two aluminum salt precipitates proved that adsorption bridging was the main mechanism of their reaction. The outstanding adsorption bridging ability of PSC is attributed to the addition of highly polymerized silica, which acts as an aggregating agent and exhibits excellent adsorption bridging ability itself [121, 122]. Additionally, the introduction of metal salts can increase the polymerization degree of coagulants, thereby enhancing their adsorption bridging ability [123]. Natural coagulants, such as proteins, starch and cellulose with long-chain structures, can enhance the adsorption bridging ability of PSC when combined with them. Wang et al. [124] demonstrated through experiments that the microstructure of three composite coagulants with carboxymethyl cellulose sodium, chitosan, and starch, in combination with PAS, was rougher and had a larger surface area than PAS alone. This formation facilitated a surface structure conducive to bridging and trapping, positively influencing coagulation effectiveness.
Sweep flocculation refers to the precipitation of hydrated metal oxides formed after the hydrolysis of coagulants. These precipitates have a three-dimensional structure and capture colloidal particles in water, causing settling [107]. This mechanical action is relatively simple, Therefore, the efficiency of impurity removal is not high, the polymerization degree of conventional aluminum salt and ferric salt flocculants is not high, the long chain structure formed in the adsorption bridge is not long enough, the subsequently-formed floc is not stable enough, and the sweep flocculation is weak, but PSC have high polymerization degrees, forming stable flocs during coagulation, the PSC sweep flocculation is stronger [125]. Wang and Yi [48] found that polymeric silicate species a cross-linked network during polymerization, resulting in a higher degree of aluminum-silicon polymerization and improved sweep flocculation. Chen et al. [126] prepared PSAF coagulants and increased sweep flocculation action by adding lime when treating coal washing wastewater. To further increase sweep flocculation, introducing natural coagulants into coagulants is also a crucial task for future research. Wang et al. [127] introduced carboxymethyl cellulose sodium, with its hydroxyl groups and other active groups on the molecular chain, and found that the combined coagulants had rougher microstructures and larger surface areas than PAS alone, enhancing their sweep flocculation.
In practical wastewater coagulation processes, the above four coagulation mechanisms do not act independently but are interconnected. The dominant mechanism varies under different conditions, and these mechanisms complement each other to achieve coagulation and settling.
8. Limitations and ChallengesAddressing the limitations and challenges in the study of PSCs is crucial. The instability of PSCs poses a significant challenge, resulting in a loss of coagulation activity and affecting their performance in water environments. Therefore, it is essential to improve the stability of PSCs to enhance their performance in water treatment. To enhance the stability of PSCs and improve their performance in water treatment, it is essential to conduct further studies on their molecular structure. While PSCs demonstrate good coagulation performance and advantages compared to traditional coagulants, comprehensive validation studies are necessary to fully understand their potential.
Investigating the relationship between structure and performance during the polymerization process can enhance our understanding of the coagulation mechanism, but it is not sufficient. Improving the adaptability and versatility of PSCs in various water quality environments is essential. When treating complex water bodies and wastewater, exploring innovative and cost-effective preparation methods can expand the practical usability of PSCs. Additionally, since the production cost of PSCs is relatively higher compared to traditional aluminum and iron-based coagulants, it is necessary to develop cost-effective preparation technologies to facilitate large-scale industrial applications of PSCs and reduce production costs.
9. ConclusionsPolymeric silicate coagulants (PSCs) possess numerous significant advantages, such as the formation of large and stable flocs, a shorter coagulation reaction time, and a noteworthy coagulation effect. Furthermore, the wide range of materials used for their preparation and their cost-effectiveness provide a solid foundation for their future industrial applications. However, current research on PSC stability remains limited, posing a challenge for their broader implementation. Therefore, it is essential to investigate novel coupling mechanisms to enhance their stability for further development. This paper presents an overview of the preparation and application of PSCs, emphasizing the need for a cost-effective method for removing specific contaminants using these coagulants. Additionally, the mechanisms of coagulation require further exploration. The recycling and utilization of industrial waste and other resources to reduce production costs, while ensuring treatment safety, should be considered.
AcknowledgementThis work was financially supported by the Hunan Provincial Educational Commission (No. 21A0324), the Hunan Provincial Natural Science Foundation (No. 2021JJ30272), and China Railway Urban Construction Group First Engineering Co., Ltd. Foundation (No. D123ED).
References1. Shunze W, Qing X, Hongliang L. Analysis of water pollution in Chinese watersheds. Environ. Sci. Tech. 2000;02:1–6.
https://doi.org/10.19672/j.cnki.1003-6504.2000.02.0011
2. Qian L. Analysis of water pollution control measures based on environmental protection. Heilongjiang Environmental Journal. 2022;35(02)71-72+75.
3. Qiang Z, Tangchun W. The Status of Water Pollution in China and Its Health Hazards and Countermeasures. Chin. J. Dis. Control. Prev. 2023;27(05)503-507+521.
https://doi.org/10.16462/j.cnki.zhjbkz.2023.05.002
4. WHO. Drinking-water [Internrt]. World Health Organization; c2023. [cited 13 September 2023]. Available from: https://www.who.int/news-room/fact-sheets/detail/drinking-water
5. Soobin J, Sangmin L, Heejin K, Intae S, Hyungjun K. Evaluation of the adsorption characteristics of dissolved organic matter using granular activated carbon (GAC) and the regeneration efficiency of denitrification, Electro-Fenton, and Sono-Fenton oxidation. Environ. Eng. Res. 2024;29(2)230177–230170.
https://doi.org/10.4491/eer.2023.177
6. Ali ZM, Zabihollah Y, Esmaeil B, Alireza A. Evaluation of combined efficiency of conventional coagulation-flocculation process with advanced oxidation process (sulfate-hydroxyl radical) in leachate treatment. Environ. Eng. Res. 2024;29(3)230548–230540.
https://doi.org/10.4491/eer.2023.548
7. Xiaogang H, Zhuannian L, Fahim BM, Junnan L, Luncong D. Preparation of novel Ce (IV)-based MOF/GO composite and its highly effective phosphate removal from aqueous solution. Environ. Eng. Res. 2023;28(6)220647–220640.
https://doi.org/10.4491/eer.2022.647
8. Manikandan S, Saraswathi R. Electrocoagulation technique for removing Organic and Inorganic pollutants (COD) from the various industrial effluents: An overview. Environ. Eng. Res. 2023;28(4)220231–220230.
https://doi.org/10.4491/eer.2022.231
9. Sushvanth Reddy A, Kalla S, Murthy ZVP. Textile wastewater treatment via membrane distillation. Environ. Eng. Res. 2022;27(5)210228–210220.
https://doi.org/10.4491/eer.2021.228
10. AEE , LMM , FSA , AAF . Using nano-magnesium oxide/bentonite composite for cadmium removal from industrial wastewater. Environ. Eng. Res. 2023;28(2)210545–210540.
https://doi.org/10.4491/eer.2021.545
11. Jin X, Yongliang Q. The Current Situation and Countermeasures of Flocculant Development in China. Mod. Chem. Ind. 1997. 12:6–9.
https://doi.org/10.16606/j.cnki.issn0253-4320.1997.12.002
12. Xiguang L, zining P, Junli N. Research progress on properties and stability of aluminum polysilicate flocculants. In : Environmental Engineering 2017 Supplement 2; 30 Aug 2017; China. p. 105–109.
13. Lixin G, Guangyong L, Daquan Z. Research progress of inorganic polymer flocculants containing silicon. Environ. Technol. 2003. 05:36-38+42.
https://doi.org/10.3969/j.issn.1004-7204.2003.05.010
14. Zhengan Z, Huaili Z, Yongjun S, et al. A combined process of chemical precipitation and flocculation for treating phosphating wastewater. Desalin. Water Treat. 2016;57(53)25520–25531.
https://doi.org/10.1080/19443994.2016.1157707
15. Guoxiang X, Fuchang R. Performance Analysis of Ferric-based and Aluminum-based Inorganic Coagulants. Environmental Impact Assessment. 2001. 03:52-55+72.
https://doi.org/10.3969/j.issn.1674-2842.2001.03.016
16. Satyawali Y, Balakrishnan M. Effect of PAC addition on sludge properties in an MBR treating high strength wastewater. Water Res. 2009;43(6)1577–1588.
https://doi.org/10.1016/j.watres.2009.01.003
17. Moussavi G, Talebi S. Comparing the efficacy of a novel waste-based adsorbent with PAC for the simultaneous removal of chromium (VI) and cyanide from electroplating wastewater. Chem. Eng. Res. Des. 2012;90(7)960–966.
https://doi.org/10.1016/j.cherd.2011.10.014
18. Guocheng Z, Huaili Z, Wenyuan C, Wei F, Peng Z, Tiroyaone T. Preparation of a composite coagulant: Polymeric aluminum ferric sulfate (PAFS) for wastewater treatment. Desalination. 2012;285:315–323.
https://doi.org/10.1016/j.desal.2011.10.019
19. Ern LK, Tow TT, Norhashimah M, Teik PB, Mohanapriya M. Flocculation activity of novel ferric chloride–polyacrylamide (FeCl3-PAM) hybrid polymer. Desalination. 2011;266(1)108–113.
https://doi.org/10.1016/j.desal.2010.08.009
20. Panyue Z, HHH , Erhard H, Guangming Z. Influence of some additives to aluminium species distribution in aluminium coagulants. Chemosphere. 2004;57(10)1489–1494.
https://doi.org/10.1016/j.chemosphere.2004.08.066
21. Ping G, Xueming C, Feng S, Guohua C. Removal of chromium( VI) from wastewater by combined electrocoagulation–electroflotation without a filter. Sep. Purif. Technol. 2005;43(2)117–123.
https://doi.org/10.1016/j.seppur.2004.10.008
22. Lihong W. Study on the Preparation and Treatment of Eutrophic Water by Polymerized Aluminum Ferric Chloride [dissertation]. Baoding: Hebei University; 2009.
23. RSB , CDN , CMU . Flocculants—an Ecofriendly Approach. J. Polym. Environ. 2006;14(2)195–202.
https://doi.org/10.1007/s10924-006-0011-x
24. Liping L, Junlian Q. Chitosan combined with polysilicate aluminum ferric coagulation for removal of algal blooms and copper green microcapsules. In : The 13th National Water Treatment Chemistry Conference and Cross Strait Water Treatment Chemistry Seminar;; 22 April 2016; Nanjing. p. 66.
25. Quanxiang Y. Preparation and treatment of simulated algal blooms using polysilicate ferric modified clay flocculant. In : 2011 Academic Conference on Environmental Pollution and Public Health; 16 September 2011; Wuhan. p. 1001–1005.
26. Qingqing Y. Preparation of Polysilicate Zinc Ferric Sulfate Flocculant and Its Application in Algae Containing Water [dissertation]. Chongqing: Chongqing University; 2015.
27. Jinglei W, Ting X, Tao H, Qinkai W. Preparation and Algae Removal Effect of a Novel Polymeric Silicate Aluminum Chloride Zinc Ferric Flocculant. Water Purif. Technol. 2018;37(05)12–19.
https://doi.org/10.15890/j.cnki.jsjs.2018.05.003
28. Chaohui G, Shanshan Y, Xiyuan X, Yanan L, Zhichao J. Polysilicate aluminum sulfate ferric complex and its application in tungsten bismuth beneficiation wastewater. J. Cent. South Univ. 2013;44(02)461–468.
29. Guangyu X, Jing F. Preparation of Polyferrous Aluminum Silicate and Its Efficiency and Mechanism for Chromium Removal. Low Carbon World. 2019;9(01)276–277.
https://doi.org/10.16844/j.cnki.cn10-1007/tk.2019.01.170
30. Xueying L, Dongmei X, Wei C, Liping Q. Application of polysilicate aluminum ferric flocculant with surfactant addition in the treatment of heavy metafume wastewater. J. Guilin Univ. Technol. 2013;33(01)136–140.
31. Guocheng Z, Junfei L, Tengzhi Z, et al. Preparation and structural characterization of a new solid polysiferric sulfate coagulant. Spectrosc. Spectral Anal. 2016;36(08)2455–2461.
https://doi.org/10.3964/j.issn.1000-0593(2016)08-2455-07
32. Xin H, Yang W, Baoyou S, Jian S, Huan C, Huikai L. Characterization and application of poly-ferric-titanium-silicate-sulfate in disperse and reactive dye wastewaters treatment. Chemosphere. 2020;249:126129.
https://doi.org/10.1016/j.chemosphere.2020.126129
33. Guocheng Z, Qian W, Jun Y, et al. Toward a better understanding of coagulation for dissolved organic nitrogen using polymeric zinc-ferric-phosphate coagulant. Water Res. 2016;100:201–210.
https://doi.org/10.1016/j.watres.2016.05.035
34. Lixin G, Cong C, Beibei L, Yanjiao Z. Application of magnesium polysilicate in the treatment of printing and dyeing wastewater. Dyeing Finish. 2008;20:33–35.
35. Xiao L, Cuiling Z, Weitao L, Yu Z. Optimization preparation of polysilicate titanium ferric zinc and its flocculation performance for azo dye wastewater. Appl. Chem. Ind. 2023;52(07)2053–2058.
https://doi.org/10.16581/j.cnki.issn1671-3206.20230714.020
36. Haijuan T, Huixiang O, Bingfang S, Weiyuan Z. Study on the Treatment of Sulfide Black Dye Wastewater with Polysilicate Aluminum Ferric/Carboxyl Chitosan Composite Flocculant. Industrial Safety and Environmental Protection. 2020;46(01)73-75+102.
https://doi.org/10.3969/j.issn.1001-425X.2020.01.017
37. Le Y, Shihong W, Jinzhao L, Juan T, Jie W. Treatment of Refinery Wastewater with Composite Polysilicate Aluminum Ferric Sulfate Coagulant. J. Tiangong Univ. 2019;38(02)32–37.
38. Peng H, Daocheng L. Research on the application of polysilicate ferric aluminum in advanced treatment of urban sewage. Chem. Eng. Des. Commun. 2006. 01:44-46+62.
https://doi.org/10.3969/j.issn.1003-6490.2006.01.015
39. Chaowei Z, Liqiu Z, Shugeng L, Hong L, Jiajun X, Xiaoling L. Study on Flocculation of Refractory Organic Compounds in Late Stage Leachate by Polyaluminum Ferric Silicate. Guangdong Chem. Ind. 2019;46(22)4-6+42.
https://doi.org/10.3969/j.issn.1007-1865.2019.22.003
40. Jie M, Runnan W, Xiyue W, et al. Drinking water treatment by stepwise flocculation using polysilicate aluminum magnesium and cationic polyacrylamide. J. Environ. Chem. Eng. 2019;7(3)103049.
https://doi.org/10.1016/j.jece.2019.103049
41. Yimin L, Jie M, Lili L, et al. Flocculation performance of alginate grafted polysilicate aluminum calcium in drinking water treatment. Process Saf. Environ. 2021;155:287–294.
https://doi.org/10.1016/j.psep.2021.09.012
42. Fu Y, Yu S. Characterization and phosphorus removal of poly-silicic-ferric coagulant. Desalination. 2009;247(1)442–455.
https://doi.org/10.1016/j.desal.2009.02.053
43. Pu S. Preparation of titanium polysilicate and its application in coking wastewater treatment [dissertation]. Shanghai: Shanxi University; 2019.
44. Wenzhong S, Lingzhi L, Guozhong Y. Preparation and Flocculation Performance of Polymerized Aluminum Ferric Silicate (PSAF). Environmental Impact Assessment. 2003. 0429-32+60-61.
https://doi.org/10.3969/j.issn.1674-2842.2003.04.010
45. Dagang L. Application of polysilicate aluminum ferric in the treatment of printing and dyeing wastewater. Guangdong Chem. Ind. 2009;36(02)68-69+75..
https://doi.org/10.3969/j.issn.1007-1865.2009.02.023
46. Zumin Q, Wentian J, Zongjian H. Post-treatment of banknote printing wastewater using polysilicate ferro-aluminum sulfate (PSFA). J. Hazard. Mater. 2009;166(2)740–745.
https://doi.org/10.1016/j.jhazmat.2008.11.128
47. Wenhao Q, Shujun W, Xuming W, Zhiping D. Preparation of polysilicate ferric titanium flocculant and coagulation treatment test of coking wastewater. Water Purif. Technol. 2021;40(04)101-105+120.
48. Xuejiao W, Shouzhi Y. Preparation and flocculation performance of polysilicate aluminum magnesium chloride. Environ. Prot. Chem. Ind. 2013;33(02)171–174.
https://doi.org/10.3969/j.issn.1006-1878.2013.02.018
49. Xingang W, Chaohua R. Research on the Application of New Flocculant PAMSS. Leather Sci. Eng. 2004. 02:53–56.
https://doi.org/10.3969/j.issn.1004-7964.2004.02.014
50. Wan L. Study on the treatment of simulated dye wastewater containing H-acid using polysilicate metal salt flocculant [dissertation]. Xian: Northwestern University; 2009.
51. Wenyu F, Yuanjie C, Qi T, Ye Q. Preparation and application of polysilicate aluminum magnesium flocculant. J. Shenyang Univ. Chem. Technol. 2003. 02:89–91.
https://doi.org/10.3969/j.issn.2095-2198.2003.02.003
52. Baoyu L, Xuejiao W. Preparation and Performance Study of Polysilicate Aluminum Magnesium Chloride Flocculant. Tianjin Construction Science and Technology. 2013;23(03)69–71.
https://doi.org/10.3969/j.issn.1008-3197.2013.03.031
53. Juan X. Preparation and application of composite flocculant polyaluminum ferric silicate. Industrial Safety and Environmental Protection. 2006. 09:18–19.
https://doi.org/10.3969/j.issn.1001-425X.2006.09.009
54. Hongli G, Hongtao L, Lei G, Junhui Z, Yuhan W. Study on the treatment effect of fly ash polyaluminum-ferrosilicate flocculant on wastewater containing phosphorus. Henan Science. 2017;35(06)985–989.
https://doi.org/10.3969/j.issn.1004-3918.2017.06.025
55. Chen L. Experimental study on the treatment of wastewater containing chromium by polysilicate aluminum ferric acid. Plat. Finish. 2013;35(08)44–46.
https://doi.org/10.3969/j.issn.1001-3849.2013.08.010
56. Pablo C, Carlos J, Fabiola M, ARM , Cristina S. The pH as a key parameter in the choice between coagulation and electrocoagulation for the treatment of wastewaters. J. Hazard. Mater. 2009;163(1)158–164.
https://doi.org/10.1016/j.jhazmat.2008.06.073
57. Stumm W, O Melia CR. Stoichiometry of coagulation. J. Am. Water Workes Ass. 1968;60(5)514–539.
https://doi.org/10.1002/j.1551-8833.1968.tb03579.x
58. La Mer VK. Coagulation symposium introduction. 19:Academic Press; 1964. p. 291–293.
59. James IK. The scientific basis of flocculation. 27:Springer Science & Business Media; 2012. p. 26–27.
60. TH , KH , TO , Koki G, NT . Characteristics of metal-polysilicate coagulants. Water Sci. Technol. 1991;23(7–9)1713–1722.
https://doi.org/10.2166/wst.1991.0626
61. KO , MU , MS , TK , YM . Practical design of flocculator for new polymeric inorganic coagulant-PSI. Water Sci. Tech-w. Sup. 2004;4(1)67–75.
https://doi.org/10.2166/ws.2004.0008
62. Huaili Z, Zongxuan Y, Yingkun G. Preparation and Performance Study of Inorganic Polymer Composite Flocculant PFSS. Fine Chem. 2002. 08:450-452+462.
https://doi.org/10.3321/j.issn:1003-5214.2002.08.006
63. Baoyu G, Yonghui S, Qinyan Y. Study on the performance of polysilicate ferric sulfate coagulant. Environ. Sci. 1997;02:48-50+96..
64. Baoyu G, Qinyan Y, Shuren W. Polysilicic acid flocculant containing aluminum ions. Environ. Sci. 1990. 05:37-41+95.
https://doi.org/10.13227/j.hjkx.1990.05.009
65. Baoyu G, Qinyan Y, Jishun W, Shuren W. Performance and application of polysilicate flocculant containing aluminum ions. Ind. Water Treat. 1993;01:17–19.
66. Baoyu G, Cuiping L, Qinyan Y, Ziping A, Shuren W. The interaction between aluminum ions and polysilicic acid. Environ. Chem. 1993;04:268–273.
67. Jinchuan F, Minqiang F. Study on the Flocculation and Stability of Polyaluminum Silicate. J. Taiyuan Univ. Technol. 2003. 02:158-160+165.
https://doi.org/10.16355/j.cnki.issn1007-9432tyut.2003.02.016
68. Qin N, Caixia L, Xin L, Bangguo W, Yan H, Xingfeng W. Preparation of polysilicate aluminum chloride from coal gangue and its flocculation performance. J. Chin. Silic. Soc. 2023;51(01)204–214.
https://doi.org/10.14062/j.issn.0454-5648.20220375
69. Qiong Z, Guobin L, Yi S, Fengshi Y. Experimental study on preparation of polysilicate aluminum flocculant from yellow phosphorus furnace slag. Non-Metallic Mines. 2014;37(05)22-24+45.
https://doi.org/10.3969/j.issn.1000-8098.2014.05.007
70. Xiaoyong Y, Aiyun R. Study on the decolorization of printing and dyeing wastewater using polysilicate magnesium salt flocculant. Guangdong Chem. Ind. 2011;38(01)125-126-115.
https://doi.org/10.3969/j.issn.1007-1865.2011.01.058
71. Lixin G, Quan C, Daquan Z. Study on the flocculation effect of zinc polysilicate. Ind. Water Treat. 2006. 07:50–53.
https://doi.org/10.3969/j.issn.1005-829X.2006.07.016
72. Ern LK, Norhashimah M, Tow TT, Teik PB. Development, characterization and the application of hybrid materials in coagulation/flocculation of wastewater: A review. Chem. Eng. J. 2012;203:370–386.
https://doi.org/10.1016/j.cej.2012.06.109
73. Yanhui L. Study on the flocculation effect of polysilicate aluminum calcium flocculant. Chemical Engineering & Equipment. 2021. 02:18–19.
https://doi.org/10.19566/j.cnki.cn35-1285/tq.2021.02.008
74. Lei G, Di Z, Hongli G, Junzhou H, Hongtao L. Study on the Treatment Performance of Beverage Wastewater by the Ratio of Polysilicate Aluminum and Ferric Elements. Environ. Sci. Technol. 2017;40(S1)211–214.
75. Huaihong X, Shancheng M, Guqing P, Dingliang T. Preparation and Performance Study of Polysilicate Aluminum Ferric Flocculant. J. Huaiyin Inst. Technol. 2000;04:12–14.
76. Jiayan Z, Yaohui Q, Huizhu Y, Haoyu L, Taihong S. Preparation of polyaluminum zirconium silicate coagulant and its performance in water treatment. J. Water Process Eng. 2021;41:102023.
https://doi.org/10.1016/j.jwpe.2021.102023
77. Lipeng T, Qunshan W, Qiang L, Relaxation Ra, Yanan L, Jianshe L. Optimization of the Treatment of Dye Wastewater with New Ecological Polysilicate Ferric Manganese. Environ. Sci. 2019;40(01)318–326.
https://doi.org/10.13227/j.hjkx.201805094
78. Yuying L, Hao Z, Xiyue W, Jie M, Lili L, Dawei L. Preparation and Flocculation Performance of Boron Modified Polyaluminum Titanium Silicate. Chem. Reagents. 2020;42(08)921–925.
https://doi.org/10.13822/j.cnki.hxsj.2020007369
79. Honghe M, Hao Z, Runnan W, Dawei L. Preparation and Flocculation Performance of Magnetic Polyaluminum Zinc Silicate Chitosan Composite Flocculant. J. Jilin Inst. Chem. Technol. 2022;39(05)36–42.
https://doi.org/10.16039/j.cnki.cn22-1249.2022.05.007
80. KDS . Application of the precipitation-charge neutralization model of coagulation. Environ. Sci. Technol. 1988;22(7)825–832.
https://doi.org/10.1021/es00172a013
81. Xiao L, Cuiling Z, Weitao L, Yu Z. Optimization of preparation of polymeric silicate titanium ferric zinc and its coagulation performance on azo dye wastewater. Appl. Chem. Ind. 2023;52(07)2053–2058.
https://doi.org/10.16581/j.cnki.issn1671-3206.20230714.020
82. Yuanhong W, Qunshan W, Youzhi J, Jianshe L. Preparation of Polyaluminum Magnesium Zinc Silicate Coagulant and Preliminary Study on Its Antibiotic Removal Performance and Mechanism. J. Saf. Environ. 2017;17(03)1057–1063.
https://doi.org/10.13637/j.issn.1009-6094.2017.03.048
83. Liang L, Yanzhen Y, Juan T, Heping L. Preparation of polysilicate ferric magnesium zinc coagulant with warm adhesive and its treatment of humic acid wastewater. Water & Wastewater Engineering. 2017;53(06)75–80.
https://doi.org/10.13789/j.cnki.wwe1964.2017.0143
84. Ling C, Charlene , Zhenghong Z, Caiping Z, Peirong Q, Qing Z. Preparation of polysilicate aluminum ferric (II) magnesium coagulant and its treatment of printing and dyeing wastewater. Technol. Water Treat. 2011;37(12)42[-45+50.
https://doi.org/10.16796/j.cnki.1000-3770.2011.12.010
85. Yanjie W. Preparation and Performance Study of Polysilicate Metal Salt Flocculant [dissertation]. Beijing: Beijing University of Chemical Technology; 2012.
86. Shuang C, Baoyue S, Annika , Xuesong Z. Preparation of Polysilicate Aluminum Ferric Flocculant from Coal Gangue for the Treatment of Fluorine Chemical Wastewater. Environ. Prot. Chem. Ind. 2023;43(04)506–513.
87. Hongli G, Junzhou H, Lei G, Hongtao L, Guanwei Z, Di Z. Study on the stability of fly ash based polysilicate aluminum ferric flocculant. Coal Sci. Technol. 2020;48(S1)236–241.
88. Mingzhe G, Spring C, Shuangchun Y, Yi P, Haiyan Z. Research and development of Al-Fe-Mg trication polysilicate flocculant. Chem. Res. Appl. 2017;29(10)1558–1563.
https://doi.org/10.3969/j.issn.1004-1656.2017.10.018
89. Honghe M, Hao Z, Runnan W, Dawei L. Preparation of polyaluminum ferric silicate sodium alginate composite flocculant. Environ. Prot. Chem. Ind. 2023;43(01)126–131.
90. Runnan W, Hao Z, Lili L, Xiyue W, Bo Z, Dawei L. Preparation and characterization of a new type of polysilicate aluminum zinc starch composite flocculant. Bull. Chin. Silic. Soc. 2017;36(09)3119–3124.
https://doi.org/10.16552/j.cnki.issn1001-1625.2017.09.041
91. Xinyuan L. Study on the synthesis and enhanced treatment of coking wastewater using polysilicate aluminum ferric flocculant [dissertation]. Taiyuan: Shanxi University; 2017.
92. Yufei W, Long Y, Na X, Jian L, Bi C. Study on the treatment of coal washing wastewater with fly ash based polysilicate aluminum feric flocculant. Non-Metallic Mines. 2018;41(06)80–82.
https://doi.org/10.3969/j.issn.1000-8098.2018.06.025
93. Li G, Lili X, Chao Z, Gang Y, Weihong X. Preparation and application of composite flocculant of silica sol-aluminum and zinc polysilicate. J. Nanjing Tech Univ. Ed. 2018;40(02)65–70.
https://doi.org/10.3969/j.issn.1671-7627.2018.02.011
94. Baoyu G, Qinyan Y, Yan W, Hui Y. The hydrolytic polymerization form of aluminum in PACS was studied by Al-Ferron time by time complexation colorimetry. Environ. Chem. 1996;03:234–239.
95. Wenxia L, Spring L, Qichang W, Laisu X. Study on the Structural Morphology of Polyaluminum Silicate Sulfate by Al-Ferron Chronological Complexation Colorimetry. Trans. China Pulp Pap. 2001. 01:59–66.
https://doi.org/10.3321/j.issn:1000-6842.2001.01.011
96. Baoyu G, Qinyan Y, Zhansheng W, Hongxiao T. The morphological distribution and transformation rule of aluminum in PASC solution were studied by Al-Ferron time by time complexation colorimetry. Environ. Chem. 2000;01:1–7.
97. Ying L. Research of morphology and flocculation mechanism of new flocculant polymerized aluminum silicate sulfate [dissertation]. Chongqing: Chongqing University; 2003.
98. Jia S. Research of preparation and morphology distribution of polysilicate aluminum-ferric flocculant [dissertation]. Beijing: North China Electric Power University; 2012.
99. Shuang L. Preparation of polyferric titanium sulfate compound coagulant and experimental study on treatment of printing and dyeing wastewater [dissertation]. Chongqing: Chongqing University; 2021.
100. Guang G, Feng Q, Linshi J, Honglin Z, Shuyi W. Synthesis and morphology of ferrosilicon flocculant (PFSS). Technol. Water Treat. 2005. 03:35–38.
https://doi.org/10.16796/j.cnki.1000-3770.2005.03.010
101. Baozhen T, Hongxiao T. Determination of polymer morphology in Fe (III) solution by ferron hourly complexation colorimetry. Environ. Chem. 1989;04:27–34.
102. Baoyu G, Bingjian W, Qinyan Y. Morphological distribution and transformation of iron in polymerized aluminum iron silicate flocculant. Res. Environ. Sci. 2002. 01:13=15+37.
https://doi.org/10.13198/j.res.2002.01.15.gaoby.004
103. Dongsheng W, Hongxiao T, Zhaokun Y. Analysis method of morphology of Fe(III) solution containing silicate by combining Fe-Ferron with β-Silicomolybdate. Environ. Chem. 1998;03:225–230.
104. Yujiang L, Guiying X, Tao W. Magnesium-aluminum composite active silica for decolorization of dye wastewater. J. Shandong Univ. Eng. Sci. 1996;04:484–489.
105. KDS . Coagulant control in water treatment. Crit. Rev. Env. Sci. Tec. 1991;21(1)41–135.
https://doi.org/10.1080/10643389109388409
106. Chengjin W, Naiyun G, Shigu Z. Research progress of polysilicate aluminum ferric flocculant. Sichuan Environment. 2009;28(04)119-122+140.
https://doi.org/10.14034/j.cnki.schj.2009.04.001
107. Guangyu X. Study on the preparation of polyferroaluminum silicate flocculant and its effect of removing chromium [dissertation]. Changsha: Hunan University; 2014.
108. Zhaokun L, Yonghui S. Preparation and flocculation performance of polysilicate metal salt flocculants. Environ. Chem. 1997;06:534–540.
109. Jianhui S, Siqing X, Ruixia S. Preparation and Performance Study of Flocculant PFCS. Environ. Sci. 1996;04:59-61+94..
110. Jianhui S, Yi X. Research Progress of Polysilicate Flocculants. Ind. Water Treat. 2000. 03:4–7.
https://doi.org/10.3969/j.issn.1005-829X.2000.03.002
111. IZA , AMP . Polyferric silicate sulphate (PFSiS): Preparation, characterisation and coagulation behaviour. Desalination. 2008;224(1)307–316.
https://doi.org/10.1016/j.desal.2007.06.012
112. YGB , YYQ , JWB , BCY . Poly-aluminum-silicate-chloride (PASiC)—a new type of composite inorganic polymer coagulant. Colloid. Surface. A. 2003;229(1)121–127.
https://doi.org/10.1016/j.colsurfa.2003.07.005
113. Li Z, Yanqin L, Ying Y. Study on the decolorization of direct red dye wastewater using polysilicate ferric zinc chitosan composite flocculant. Technol. Water Treat. 2016;42(01)75–78.
https://doi.org/10.16796/j.cnki.1000-3770.2016.01.016
114. Baoyu G, Qinyan Y, Zhansheng W, Hongxiao T. The coagulation performance of polyaluminum silicate chloride (PASC) coagulant. Environ. Sci. 2000. 02:46–49.
https://doi.org/10.13227/j.hjkx.2000.02.011
115. Gang Z, Xianyuan F, Xiaoli Z, Luying J. Study on the performance of polysilicate aluminum ferric composite flocculant. Environ. Sci. Technol. 2012;35(08)185–188.
116. Huaili Z, Kewan L. Research progress and development trend of inorganic polymer composite flocculants. Environ. Sci. Technol. 2004. 06:315–319.
https://doi.org/10.16796/j.cnki.1000-3770.2004.06.001
117. Qiong Z, Guobin L, Yi S, Huping L. Study on the Morphology, Structure and Properties of Composite Polysilicate Aluminum Ferric Flocculant. Non-Metallic Mines. 2015;38(01)1–4.
https://doi.org/10.3969/j.issn.1000-8098.2015.01.001
118. HYS , ANBK , HC . Process sequence optimization based on a new cost–tolerance model. J. Intell. Manuf. 1998;9(1)29–37.
https://doi.org/10.1023/A:1008895224256
119. Hong L, Yuemei F, Guanghui W, Huimin X. Electron microscopic analysis of the morphology of polysilicate metal salt composite flocculants. Environ. Chem. 2006. 01:45–49.
https://doi.org/10.3321/j.issn:0254-6108.2006.01.011
120. Yuancai C, Jin X, Huaiyu Z. Study on Coagulation Characteristics of Two Aluminum Salts in Treating Pulp CEH Bleaching Wastewater. Technol. Water Treat. 2000. 03:165–168.
https://doi.org/10.16796/j.cnki.1000-3770.2000.03.009
121. Hongxiao T. Development trends of inorganic polymer composite flocculants. China Water Wastewater. 1999. 02:2-5+1.
https://doi.org/10.3321/j.issn:1000-4602.1999.02.001
122. Yonghui S, Zhaokun L. Preparation and Performance Study of Novel Polyaluminosilicate Composite Flocculant. Environ. Chem. 1997;06:541–545.
123. Baoyu G, Bingjian W, Qinyan Y. Distribution and transformation of ferric in poly aluminum silicate ferric coagulants. Res. Environ. Sci. 2002. 01:13-15+37.
https://doi.org/10.13198/j.res.2002.01.15.gaoby.004
124. Runnan W, Hao Z, Lili L, Xiyue W, Bo Z, Dawei L. Performance Comparison of Several Polysilicate Aluminum Polysaccharide Composite Flocculants. Technol. Water Treat. 2017;43(11)73–76.
https://doi.org/10.16796/j.cnki.1000-3770.2017.11.016
125. Tong S, Chunhua S, Guoli Z, et al. Preparation and coagulation performance of poly-ferric-aluminum-silicate-sulfate from fly ash. Desalination. 2011;268(1)270–275.
https://doi.org/10.1016/j.desal.2010.10.023
126. Xuemei C, Yue Z, Yang C. Study on the treatment of high concentration coal washing wastewater with polysilicate aluminum ferric. Journal of Northeast Electric Power University. 2015;35(02)53–56.
https://doi.org/10.19718/j.issn.1005-2992.2015.02.010
127. Runnan W, Hao Z, Lili L, Xiyue W, Bo Z, Dawei L. Preparation and application of polysilicate aluminum magnesium carboxymethyl cellulose sodium composite flocculant. Fine Chem. 2017;34(09)1044–1050.
https://doi.org/10.13550/j.jxhg.2017.09.013
Fig. 1(a) National distribution of PSC research results, (b) Ranking scores of the top 8 keywords in PSC research results, and (c) Keywords appearing in PSC research papers. 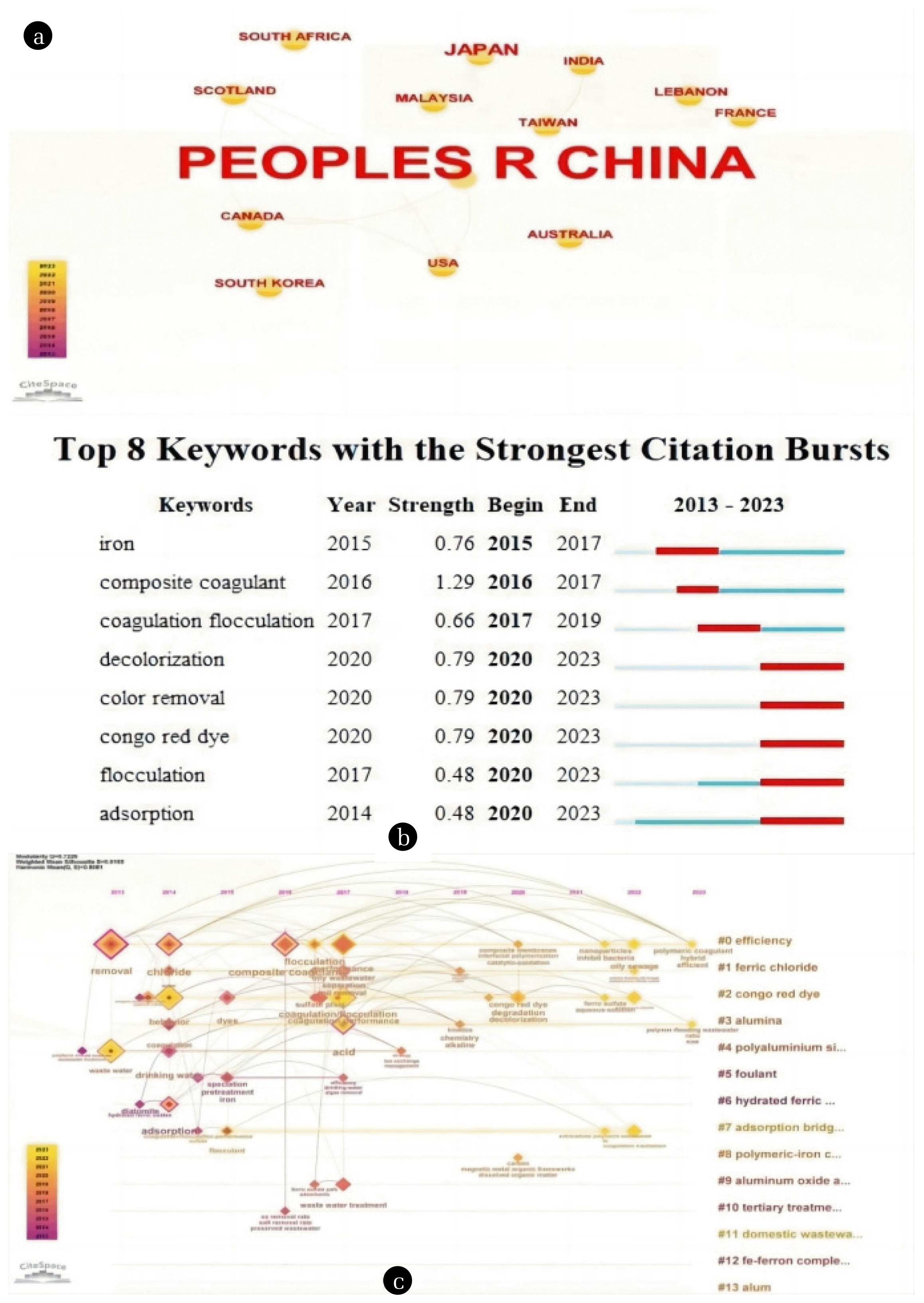
Table. 1Comparison between conventional aluminum and iron flocculants and PAS metal ions in content of hydrolyzed species
|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||