AbstractUV/H2O2 is a commonly used advanced oxidation process for removing non-biodegradable organic compounds. However, additional efforts are needed to understand the relative significance of operational factors during the UV/H2O2. Here, we investigated the effect and specific contribution of crucial factors such as H2O2 concentration, UV intensity, and reaction time on the color and total organic carbon (TOC) removal efficiency in textile wastewater using the one-factor-at-a-time method and multi-layer perceptron (MLP) analysis. The results showed that color removal was enhanced with high H2O2 concentration, high UV intensity, and long reaction time. Overall, more than 99% removal of color was achieved by the UV/H2O2, utilizing the following parameters: H2O2 concentration of 5 mM, UV intensity of 26.6 W/m2, and reaction time of 180 min. On the other hand, the removal of TOC was increased by high H2O2 concentration and long reaction time, but not by high UV intensity. In the MLP analysis, the H2O2 concentration was identified as the primary factor affecting both color and TOC removal, accounting for 43% and 50% reduction of the color and TOC, respectively. Overall, this study helps to understand the relative importance of the most critical operating factors in treating actual textile wastewater by the UV/H2O2.
Graphical Abstract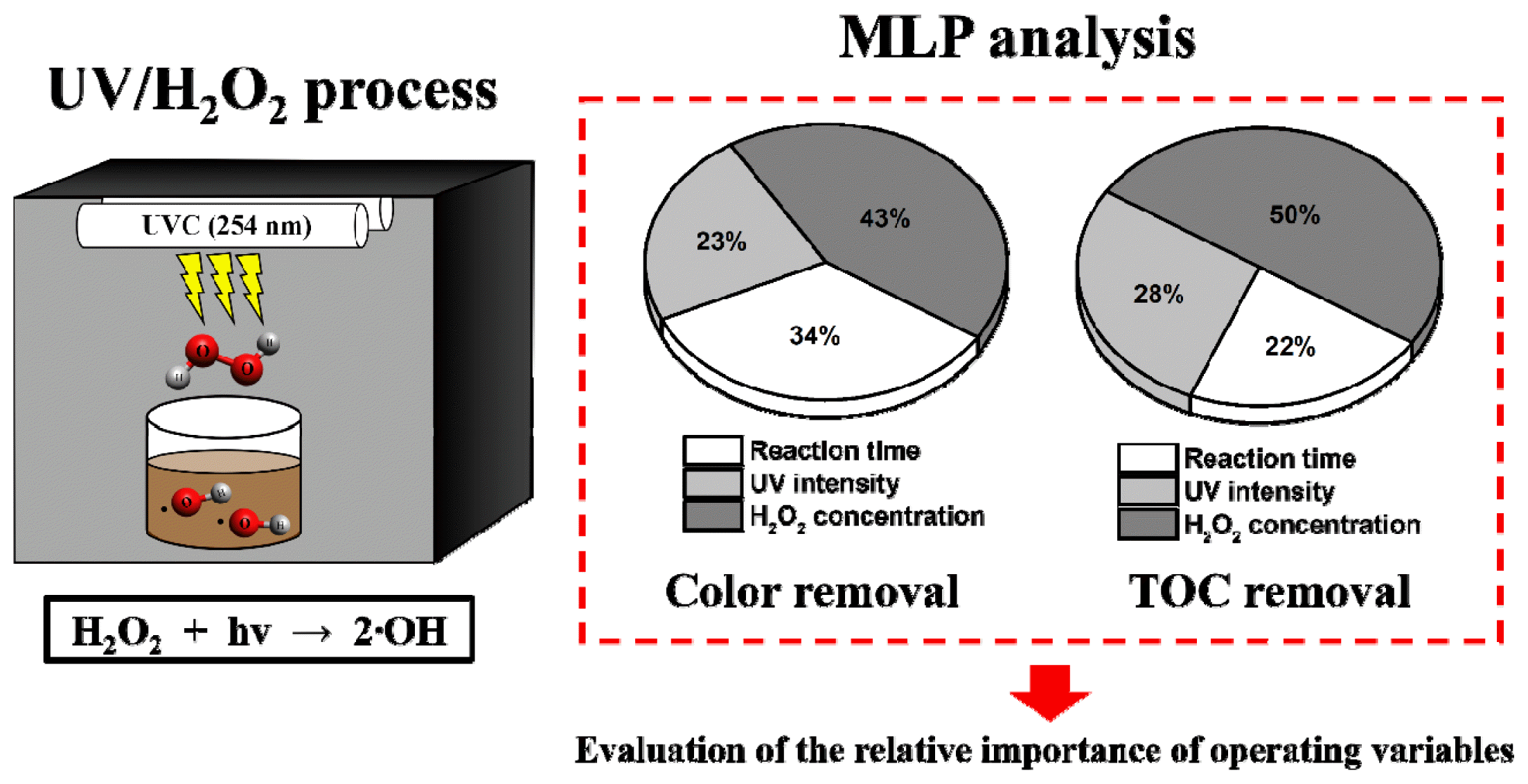
1. IntroductionDyes are widely used in the manufacturing and operation processes in numerous industries, such as the textile, leather, paper, pulp, prints, and paint industries [1, 2]. As a result, a significant volume of textile wastewater has been discharged into aquatic environments. For instance, the textile industry alone employs over 10,000 tons of dyes [3], with approximately 8%–20% of unused dyes and other chemicals being released into effluents [4]. It is estimated that tons of dye waste originating from these industries are discharged into aquatic environments every day [5]. Textile wastewater typically contains color-causing compounds that are resistant to biodegradation due to their high chemical stability [6, 7]. Moreover, most of these pollutants are toxic, carcinogenic, and mutagenic [8, 9]. Therefore, discharging these residual dyes without proper removal poses a serious threat to both aquatic ecosystems and human health [10]. The presence of dyes in water can lead to the absorption or reflection of sunlight [11], resulting in reduced photosynthesis by aquatic plants and depletion of oxygen in the aquatic ecosystem [12]. Consequently, a high concentration of dyes in water hinders the biological activity of aquatic organisms. In humans, exposure to dyes can cause allergic reactions, cancer, and eye irritation [13, 14]. Furthermore, even at low concentrations of less than 1 mg/L, dyes can cause aesthetic issues in water due to their distinctively unpleasant visibility [15]. As awareness of public health, aesthetic, and sanitary concerns in water increases, there is a growing demand for higher levels of color removal.
Conventional wastewater treatment methods, such as adsorption, coagulation, filtration, and biological treatment, have limitations when it comes to effectively removing non-biodegradable color-causing organic compounds [16]. To overcome these limitations, advanced oxidation processes (AOPs) such as Fenton [17], UV/H2O2 [18], and ozonation [19] have been introduced in textile wastewater treatment to enhance the removal of residual organic pollutants. AOPs employing strong oxidizing agents can generate various reactive oxygen species, including hydroxyl radicals (•OH), which react with non-biodegradable organic compounds [20–22].
Among the different AOPs developed thus far, UV/H2O2 is particularly effective, as it generates •OH via the photodecomposition of H2O2 during UV irradiation. This enables the decomposition and mineralization of non-biodegradable organic compounds in industrial wastewater [23]. Moreover, the UV/H2O2 process offers several advantages: (1) a simple mechanism, (2) no sludge production, (3) relatively low oxidant cost, and (4) accidental effect for disinfecting water. Furthermore, non-biodegradable organic compounds can be completely decomposed into harmless substances such as CO2 and H2O after the UV/H2O2 process [24]. However, partially degraded intermediates, formed during incomplete mineralization, may exhibit greater toxicity than the original dyes [25]. Hence, to evaluate the suitability of the UV/H2O2 process for advanced textile wastewater treatment, it is crucial to assess the total organic carbon (TOC) removal efficacy.
The treatment of textile wastewater using UV/H2O2 is influenced by experimental conditions such as the H2O2 concentration [26], UV intensity [27], and reaction time [28]. However, distinguishing their specific impacts can be challenging, which highlights the need for the development and implementation of appropriate approaches. In the wake of rapid advancements in machine learning algorithms over recent years, numerous researchers have applied these technologies across diverse sectors, including scientific studies, industrial production, and energy [29]. Among the most commonly used strategies, the multi-layer perceptron (MLP), a class of artificial neural networks (ANN), has been used in many research fields that require complex analysis and prediction [30, 31]. MLP with multiple hidden layers, is capable of solving non-linear problems by utilizing the activation functions [32]. MLP generally excels in classification, prediction, recognition, and interpretation tasks [33]. For instance, Jaffari et al. utilized MLP analysis to predict the adsorption capacities of biochar-based materials for a range of heavy metal ions [34]. Also, Iftikhar et al. employed MLP to model and predict the adsorption capacities of carbon-based adsorbents for manufactured dyes [35]. However, very few studies have investigated the impact of various conditions on the UV/H2O2 process using MLP. Therefore, this study aims to identify the most important factors in textile wastewater treatment by UV/H2O2 using MLP.
In this study, we evaluated the removal efficiency of color resulting from dissolved non-biodegradable organic substances by pre-filtering the colloidal or suspended materials in actual textile wastewater. The effect of several factors such as H2O2 concentration, UV intensity, and reaction time in the UV/H2O2 process was investigated through the one-factor-at-a-time method. Additionally, the relative importance of these conditions in the removal of color and TOC was investigated via MLP analysis, which helps a comprehensive understanding of the interaction between H2O2 concentration, UV intensity, and reaction time. The treatment of actual textile wastewater using UV/H2O2 can provide insights into the impacts of experimental conditions, in addition to enabling the assessment of the applicability of the UV/H2O2 process in real-world textile wastewater treatment technologies.
2. Materials and Methods2.1. ChemicalThe dye wastewater used for analysis and characterization was obtained from a local textile wastewater treatment plant (Pocheon, South Korea). The main components of dye wastewater are summarized in Table 1. Mean values were obtained from at least two measurements. The hydrogen peroxide (≥ 34.5%, H2O2) and methanol (MeOH, ≥ 99.5%, CH3OH) were procured from Samchun Chemicals (Pyeongtaek, South Korea). Sodium sulfite anhydrous (≥ 96%, Na2SO3) was purchased from Daejung Chemicals (Siheung, South Korea).
2.2. Color and TOC Removal via the UV/H2O2 ProcessPrior to the experiments, the dye wastewater was subjected to pretreatment using a microfiber filter (1.2 μm, Whatman GF/C) to eliminate any suspended solid particles. The UV/H2O2 process was conducted in a black acrylic photoreactor [36, 37] and UVC lamp(s) were used as a light source (TUV 4W G4 T5, Philips, USA). The UVC intensities were measured as 15.12, 26.56, 50.78, 65.86 and 80.30 W/m2 using UV radiometers. The UV/H2O2 process was carried out for 120 min (UVC lamp number = 2, H2O2 concentration = 5 mM). Here, we focused on the effect of three crucial factors on the efficiency of color removal. UVC intensity (5.12, 26.56, 50.78, 65.86 and 80.30 W/m2), H2O2 concentration (0.2, 0.5, 2, 5, and 8 mM), and reaction time (10, 60, 120, 180, and 240 min) were adjusted in this study. For the color removal experiments, 50 mL of samples containing moderate amounts of H2O2 were placed under the UVC lamps in a quartz beaker with steady stirring using a magnetic bar. After the experiments, to quench the residual H2O2, 0.5 mL of Na2SO3 (1.6 M) solution was instantly spiked into the samples. Additionally, there was no obvious change in pH after the reaction (± 0.1). The color change was analyzed using a UV-VIS spectrophotometer (DR 3900, HACH), and the TOC concentration of the samples was measured using a Total Organic Carbon Analyzer (TOC-V CSN, SHIMADZU). The hydroxyl radical (•OH) scavenging test was performed by adding 20 mM MeOH in the solution. Moreover, the optical properties of the samples were analyzed to confirm the degradation of color using a UV-VIS spectrophotometer (DR 3900, HACH) at a wavelength of 720 nm. All the results of one-factor-at-a-time method were represented as the mean of duplicate experiments and the error bars represent the range of the values.
2.3. MLP AnalysisMLP is a type of ANN that consists of multiple hidden layers, including an input layer, hidden layers, and an output layer with full connectivity between adjacent perceptrons [38, 39]. Each perceptron in the MLP applies an activation function to the input from the previous layer to obtain an output. These activation functions enable the MLP to compute complex and non-linear relationships in the data, making it suitable for solving non-linear problems. During training, the backpropagation algorithm is employed to adjust the weights and minimize the error between the predicted and actual outputs. Therefore, the MLP model can provide more accurate predictions by capturing non-linear relationships compared to other approaches. The experimental conditions were determined using a central composite design (Fig. 1), and data were collected for different conditions. The input variables and their ranges were selected as follows: H2O2 concentration of 2, 5, and 8 mM; UV intensity of 15.12, 26.56, and 50.78 W/m2; and reaction times of 60, 120, and 180 min, respectively. Detailed information about the MLP application is presented in Table S1–S3. To avoid the weighted fitting of MLP to the specific input variable which have relative bigger absolute values, each input variables are normalized (Coded values, Table S1). Separate MLP models were trained for each target variable, including color removal rate (−) and TOC removal rate (−). The experimental conditions derived from the Central Composite Design (CCD) represent optimal conditions for investigating the impact of individual factors and their interactions. Consequently, when training the MLP using this data, there is a risk of underfitting if only a subset of the data is employed. Conversely, utilizing all available data introduces the potential for overfitting. To strike a balance between these concerns, our study adopts a strategy aimed at minimizing overfitting while maximizing the utilization of the entire dataset as Fig. S1. The optimal hyperparameters for the MLP models were determined through a grid search with 5-fold cross-validation using all available data and the MLP models with the optimal hyperparameters were trained using the entire dataset. The relative importance of the input variables was examined using Shapley Additive explanations (SHAP) based on the trained MLP models and the entire dataset. Additionally, to evaluate the performance of the MLP model, additional experiments (see section 3.5) were conducted under the following conditions: H2O2 concentration of 2.30, 2.60, 3.80, and 8.00 mM; UV intensity of 15.12 and 26.56 W/m2; and reaction times of 66, 90, 96, and 114 min, respectively. All these processes were conducted using the Python programming language using the ‘sklearn’ and the ‘shap’ packages.
3. Results and Discussion3.1. Effects of H2O2 Concentration on the Removal of Color and TOCThe color removal efficiency of the UV/H2O2 process was investigated under various H2O2 concentrations (Fig. 2a). As illustrated in the figure, the color removal efficiency increases with higher H2O2 concentrations. The removal efficiencies of 0.2, 0.5, 2, 5, and 8 mM of H2O2 were 0.00 ± 1.23)%, 14.22 ± 3.24)%, 32.58 ± 0.00)%, 91.60 ± 0.01)%, and 95.08 (± 0.02)%, respectively. In the UV/H2O2 process, H2O2 molecules absorb photons, which results in the generation of hydroxyl radicals (•OH) [ɛ = 18.7 M−1 cm−1 at 254 nm [40]] (Eq. (1)).
In general, the generated hydroxyl radicals (•OH) (oxidation potential: 2.8 eV) [41] have high reactivity towards color-causing organic compounds [42]. Therefore, higher initial H2O2 concentrations (5 and 8 mM) resulted in higher color removal efficiency rates. To confirm the contribution of •OH in the removal of the color, MeOH was used as a •OH radical scavenging agent (k•OH/MeOH = 9.7 108 M−1s−1) [43]. As illustrated in Fig. 2c, the addition of MeOH exactly hindered color removal reducing the removal ratio from 91.60 ± 0.01)% to 15.14 ± 1.95)%. Additionally, photolysis alone did not remove the color of textile wastewater, suggesting that •OH played a major role in the color removal during the UV/H2O2 process. The optical properties of the textile wastewater samples before and after reaction were investigated by UV-Vis spectrum (Fig. 2d). In contrast to the before reaction, there was a substantial decrease in light absorption within the 400 to 700 nm range after the UV/H2O2 reaction. This observation suggests a complete removal of color through the UV/H2O2 process. An increase in H2O2 concentration also significantly enhanced the TOC removal efficiency (Fig. 2b). The TOC removal efficiencies were 0.00 ± 0.44)%, 1.03 ± 0.36)%, 0.00 ± 0.60)%, 2.92 ± 0.06)%, and 32.29 ± 0.01)% at H2O2 concentrations of 0.2, 0.5, 2, 5, and 8 mM, respectively. In contrast to the result of the color removal experiment, a noteworthy difference in TOC removal efficiency was observed between H2O2 concentrations of 5 and 8 mM. TOC concentrations were decreased only at 8 mM of H2O2 even though the color removal efficiencies of 5 and 8 mM H2O2 were similar. This indicates that the generation of •OH was not sufficient to further mineralize the organic compounds with 5 mM of H2O2 (2.92%). On the other hand, the higher H2O2 concentration (8 mM) generated more •OH for the mineralization of organic compounds, leading to higher TOC removal (32.29%).
3.2. Effects of UV Intensity on the Removal of Color and TOCThe impact of UV intensity on the color removal efficiency was investigated by varying the number of UVC lamps (1, 2, and 3) and distances between lamps and the sample. The measured light intensities of different conditions were 5.12, 26.56, 50.78, 65.86 and 80.30 W/m2, respectively. Light intensity directly affects color removal efficiency in the UV/H2O2 process. As shown in Fig. 3a, an increase in light intensity led to an enhancement in color removal efficacy, which was consistent with previous studies [44, 45]. The color removals for 5.12, 26.56, 50.78, 65.86 and 80.30 W/cm2 were 49.61 (± 0.00)%, 91.60 (± 0.01)%, 94.66 (± 0.00)%, 97.71 (± 0.65)%, and 98.62 (± 0.65)%, respectively. In contrast, TOC degradation was negligible in all experimental conditions (Fig. 3b). The TOC removals were 0.44 (± 0.00)%, 2.92 (± 0.77)%, 2.49 (± 0.01)%, 2.82 (± 0.36)%, and 3.81 (± 1.04)% for 5.12, 26.56, 50.78, 65.86 and 80.30 W/m2, respectively. This result suggests that the degradation products that were generated during the UV/H2O2 process contribute to the TOC concentration of the reacted samples (Eq. (2)).
Likewise, the increase in UV intensity in the UV/H2O2 treatment is effective for color removal in textile wastewater but there are still limitations regarding the mineralization of non-biodegradable organic compounds to CO2 and H2O.
3.3. Effects of Reaction Time on the Removal of Color and TOCThe color removal efficiency of the UV/H2O2 process was also investigated at various reaction times (Fig. 4a). The color removal efficiencies were 3.21 (± 1.95)%, 40.00 (± 0.70)%, 91.60 (± 1.82)%, 99.22 (± 0.01)%, and 100.00 (± 0.00)% at reaction times of 10, 60, 120, 180, and 240 min, respectively. Longer reaction times significantly improved the color removal efficiency, with almost all of the color being removed at 180 min. As the reaction time increased, the frequency of reactions between OH radicals and organic compounds also increased, resulting in higher removal efficiency. The TOC removal efficiencies were 0.00 (± 0.37)%, 0.00 (± 0.66)%, 2.92 (± 0.77)%, 26.17 (± 0.01)%, and 32.31 (± 0.48)% at reaction times of 10, 60, 120, 180, and 240 min, respectively (Fig. 4b). A noticeable difference in TOC removal efficiency was observed at 240 min, which was consistent with previous studies [46, 47]. Therefore, our findings confirmed that longer reaction times improve TOC removal efficiency. These results further suggest that an increase in H2O2 concentration and reaction time affects TOC removal efficiency, whereas an increase in UV intensity has no significant effect.
3.4. Comparison of Operational Variable Effects via MLP AnalysisThe relative importance of the experimental conditions was analyzed using MLP. Fig. 5 illustrates the ratio of the mean absolute SHAP value for the experimental conditions, including H2O2 concentration, UV intensity, and reaction time. In terms of color removal, H2O2 concentration (43%) was the most important factor, followed by reaction time (34%) and UV intensity (23%) (Fig. 5a). Similarly, for TOC removal in the UV/H2O2 process, H2O2 concentration (50%) was the most important factor, followed by UV intensity (28%) and reaction time (22%) (Fig. 5b). Overall, H2O2 concentration emerged as the most significant factor [48, 49], and different impacts of reaction time and UV intensity were observed between color and TOC removal in the UV/H2O2 process.
3.5. Verification of Multi-Layer Perceptron ApplicabilityPair plots of the observed data and MLP predictions are shown in Fig. 6. The MLP demonstrated high predictability for all data, achieving the highest R2 value of 0.9995 and the lowest mean square error (MSE) of 6.7×10−5 for color removal (Fig. 6a). In contrast, the R2 value for the additional data was relatively low at 0.4172, indicating that the MLP model was overfitted. This underscores the imperative to identify supplementary strategies for averting overfitting when training an MLP model with the entire set of experimental conditions derived from the CCD. Additionally, the MLP showed considerably low predictability for TOC (Fig. 6b). Since TOC does not vary much with experimental conditions (Table S2), additional research is necessary to assess the predictability of the MLP for TOC through an appropriate fitting. The results of the comparison between observed data and MLP predictions for actual dyeing wastewater treatment confirmed that MLP is an appropriate predictive model for color removal.
4. ConclusionThis study examined the efficiency of color and TOC removal under various conditions and determined the importance of each factor using MLP analysis during the treatment of actual textile wastewater via the UV/H2O2 process. The color removal efficiency improved with increasing H2O2 concentration, UV intensity, and reaction time. However, the TOC removal efficiency only partially improved with H2O2 concentration and reaction time, whereas the effect of UV intensity was negligible. Therefore, it is necessary to further control the experimental conditions to fully mineralize the non-biodegradable organic compounds. MLP analysis revealed that H2O2 concentration is the most significant factor influencing the efficiency of color and TOC removal in the UV/H2O2 process. Moreover, the high predictability of the MLP in color removal suggests that accurate prediction of color removal efficiency is feasible when the UV/H2O2 process is implemented in actual textile wastewater treatment technologies. The utilization of MLP analysis is a promising approach for accurately assessing the relative importance of various factors. This can offer insight into the interactions among H2O2 concentration, UV intensity, and reaction time, thereby facilitating the determination of optimal operating conditions.
Overall, this study contributes to understanding the impacts and specific contributions of different experimental conditions on color and TOC removal. In future research and applications, these results can provide valuable information, aiding in the decision-making process for experiments. Furthermore, the concept of integrating machine learning with the SHAP application holds profound importance for future advancement and implementation in enhancing the UV/H2O2 process efficiency. However, additional observed data is still required to achieve precise prediction for TOC removal using machine learning models such as MLP.
NotesAuthor Contributions C.Y.S. (M.Sc. student) conducted all experiments and wrote the manuscript. J.K.K. (Assistant Professor) helped in developing the conceptualization, methodology, and wrote the manuscript. Y.J.L. (Ph.D. student) helped in developing the methodology of the study and revised the manuscript. C.G.L. (Associate professor) made supervision, final checking, revised the manuscript and helped with publishing. J.K. (Assistant Professor), S.J.P. (Professor), Y.M.C. (Researcher Fellow) and G.A.S. (Professor) revised the manuscript. References1. Kang Y, Jang J, Kim S, et al. PIP/TMC interfacial polymerization with electrospray: novel loose nanofiltration membrane for dye wastewater treatment. ACS Appl. Mater. Interfaces. 2020;12:36148–36158.
https://doi.org/10.1021/acsami.0c09510
2. Ma J, Hou L, Li P, Zhang S, Zheng X. Modified fruit pericarp as an effective biosorbent for removing azo dye from aqueous solution: study of adsorption properties and mechanisms. Environ. Eng. Res. 2022;27:200634.
https://doi.org/10.4491/eer.2020.634
3. Al Tohamy R, Ali SS, Li F, et al. A critical review on the treatment of dye-containing wastewater: Ecotoxicological and health concerns of textile dyes and possible remediation approaches for environmental safety. Ecotoxicol. Environ. Saf. 2022;231:113160.
https://doi.org/10.1016/j.ecoenv.2021.113160
4. Uday USP, Bandyopadhyay TK, Bhunia B. Bioremediation and detoxification technology for treatment of dye (s) from textile effluent. InTech; London: 2016. p. 1–124.
5. Mogale R, Akpomie KG, Conradie J, Langner EHG. Isoreticular aluminium-based metal-organic frameworks with structurally similar organic linkers as highly efficient dye adsorbents. J. Mol. Struct. 2022;1268:133648. 10.1016/j.molstruc.2022.133648
6. Ismail GA, Sakai H. Review on effect of different type of dyes on advanced oxidation processes (AOPs) for textile color removal. Chemosphere. 2022;291:132906.
https://doi.org/10.1016/j.chemosphere.2021.132906
7. Shindhal T, Rakholiya P, Varjani S, et al. A critical review on advances in the practices and perspectives for the treatment of dye industry wastewater. Bioengineered. 2021;12:70–87.
https://doi.org/10.1080/21655979.2020.1863034
8. Alderete BL, da Silva J, Godoi R, et al. Evaluation of toxicity and mutagenicity of a synthetic effluent containing azo dye after advanced oxidation process treatment. Chemosphere. 2021;263:128291.
https://doi.org/10.1016/j.chemosphere.2020.128291
9. Karabulut K, Atav R. Dyeing of cotton fabric with natural dyes without mordant usage part I: determining the most suitable dye plants for dyeing and UV protective functionalization. Fiber. Polym. 2020;21:1773–1782. 10.1007/s12221-020-9365-2
10. Detjob A, Boonnorat J, Phattarapattamawong S. Synergistic decolorization and detoxication of reactive dye Navy Blue 250 (NB250) and dye wastewater by the UV/Chlorine process. Environ. Eng. Res. 2023;28:220221.
https://doi.org/10.4491/eer.2022.221
11. Gita S, Hussan A, Choudhury T. Impact of textile dyes waste on aquatic environments and its treatment. Environ. Ecol. 2017;35:2349–2353.
12. Hernández Zamora M, Martínez Jerónimo F. Congo red dye diversely affects organisms of different trophic levels: a comparative study with microalgae, cladocerans, and zebrafishembryos. Environ. Sci. Pollut. 2019;26:11743–11755.
https://doi.org/10.1007/s11356-019-04589-1
13. Dawood Affat SS. Classifications, advantages, disadvantages, toxicity effects of natural and synthetic dyes: a review. Iraqi J. Agric. Sci. 2021;8:130–135.
http://doi.org/10.32792/utq/utjsci/v8/1/21
14. Dawood S, Sen T. Review on dye removal from its aqueous solution into alternative cost effective and non-conventional adsorbents. J. Chem. Proc. Eng. 2014;1:1–11. 10.17303/jce.2014.105
15. Fito J, Abrham S, Angassa K. Adsorption of methylene blue from textile industrial wastewater onto activated carbon of Parthenium hysterophorus. Int. J. Environ. Res. 2020;14:501–511.
https://doi.org/10.1007/s41742-020-00273-2
16. Navas Cárdenas C, Rajendran S, Ramírez T, Muñoz F. Combined sedimentation, electrocoagulation and ozone processes for the wastewater treatment in an Ecuadorian MDF industry. Int. J. Environ. Sci. Technol. 2022;19:11699–11710. 10.1007/s13762-022-03961-y
17. Nidheesh PV, Gandhimathi R, Ramesh ST. Degradation of dyes from aqueous solution by Fenton processes: a review. Environ. Sci. Pollut. 2013;20:2099–2132. 10.1007/s11356-012-1385-z
18. Cha D, Lim G, Joo H, Yoon J, Lee C. Oxidative degradation of micropollutants by a pilot-scale UV/H2O2 process: Translating experimental results into multiphysics simulations. Environ. Eng. Res. 2023;28:220658.
https://doi.org/10.4491/eer.2022.658
19. Ulucan Altuntas K, Ilhan F. Enhancing biodegradability of textile wastewater by ozonation processes: optimization with response surface methodology. Ozone Sci. Eng. 2018;40:465–472. 10.1080/01919512.2018.1474339
20. Kilic MY, Abdelraheem WH, He X, Kestioglu K, Dionysiou DD. Photochemical treatment of tyrosol, a model phenolic compound present in olive mill wastewater, by hydroxyl and sulfate radical-based advanced oxidation processes (AOPs). J. Hazard. Mater. 2019;367:734–742. 10.1016/j.jhazmat.2018.06.062
21. Lee YJ, Jae Jeong Y, Sun Cho I, et al. The inhibitory mechanism of humic acids on photocatalytic generation of reactive oxygen species by TiO2 depends on the crystalline phase. Chem. Eng. J. 2023;476:146785. 10.1016/j.cej.2023.146785
22. Zhang X, Ma D, Zhu X. Insights into bicarbonate enhanced heterogeneous Fenton catalyzed by Co/Cu/zeolite for degradation of rhodamine B. Environ. Eng. Res. 2023;29:230095–230090. 10.4491/eer.2023.095
23. Lee YJ, Lee CG, Park SJ, Moon JK, Alvarez PJ. pH-dependent contribution of chlorine monoxide radicals and byproducts formation during UV/chlorine treatment on clothianidin. Chem. Eng. J. 2022;428:132444.
https://doi.org/10.1016/j.cej.2021.132444
24. Costantino F, Armirotti A, Carzino R, et al. In situ formation of SnO2 nanoparticles on cellulose acetate fibrous membranes for the photocatalytic degradation of organic dyes. J. Photochem. Photobiol. A. 2020;398:112599.
https://doi.org/10.1016/j.jphotochem.2020.112599
25. Sahoo M, Marbaniang M, Sinha B, Sharan R. Fenton and Fenton-like processes for the mineralization of Ponceau S in aqueous solution: Assessment of eco-toxicological effect of post treated solutions. Sep. Purif. Technol. 2014;124:155–162.
https://doi.org/10.1016/j.seppur.2014.01.021
26. Adeel M, Maniakova G, Rizzo L. Tertiary/quaternary treatment of urban wastewater by UV/H2O2 or ozonation: Microplastics may affect removal of E. coli and contaminants of emerging concern. Sci. Total Environ. 2024;907:167940. 10.1016/j.scitotenv.2023.167940
27. Hollman J, Dominic JA, Achari G, Langford CH, Tay JH. Effect of UV dose on degradation of venlafaxine using UV/H2O2: perspective of augmenting UV units in wastewater treatment. Environ. Technol. 2020;41:1107–1116. 10.1080/09593330.2018.1521475
28. Tuncer N, Sönmez G. Removal of COD and color from textile wastewater by the Fenton and UV/H2O2 oxidation processes and optimization. Water Air Soil Pollut. 2023;234:70. 10.1007/s11.270-023-06095-0
29. An H, Liu Z, Sun K, Peng B. A machine learning framework for intelligent prediction of ash fusion temperature characteristics. Fuel. 2024;362:130799. 10.1016/j.fuel.2023.130799
30. Chu JH, Kang JK, Park SJ, Lee CG. Ultrasound-activated peroxydisulfate process with copper film to remove bisphenol A: Operational parameter impact and back propagation-artificial neural network modeling. J. Water Process. Eng. 2021;44:102326. 10.1016/j.jwpe.2021.102326
31. Aghel B, Rezaei A, Mohadesi M. Modeling and prediction of water quality parameters using a hybrid particle swarm optimization–neural fuzzy approach. Int. J. Environ. Sci. Technol. 2018;16:4823–4832. 10.1007/s13762-018-1896-3
32. Yang H, Kang JK, Jeong S, Park SJ, Lee CG. Removal of perfluorooctanoic acid from water using peroxydisulfate/layered double hydroxide system: Optimization using response surface methodology and artificial neural network. Process. Saf. Environ. Prot. 2022;167:368–377.
https://doi.org/10.1016/j.psep.2022.09.032
33. Erdem E, Bozkurt F. A comparison of various supervised machine learning techniques for prostate cancer prediction. Avrupa Bilim ve Teknoloji Dergisi. 2021;610–620. 10.31590/ejosat.802810
34. Jaffari ZH, Abbas A, Kim CM, et al. Transformer-based deep learning models for adsorption capacity prediction of heavy metal ions toward biochar-based adsorbents. J. Hazard. Mater. 2024;462:132773. 10.1016/j.jhazmat.2023.132773
35. Iftikhar S, Zahra N, Rubab F, et al. Artificial neural networks for insights into adsorption capacity of industrial dyes using carbon-based materials. Sep. Purif. Technol. 2023;326:124891. 10.1016/j.seppur.2023.124891
36. Lee YJ, Son CY, Lee CG, et al. Enhancement of photocatalytic Cr(VI) reduction using mandarin peel extract as natural sacrificing agent. Alex. Eng. J. 2023;75:151–163. 10.1016/j.aej.2023.05.057
37. Lee YJ, Lee CG, Kang JK, Park SJ, Alvarez PJJ. Simple preparation method for Styrofoam–TiO2 composites and their photocatalytic application for dye oxidation and Cr(vi) reduction in industrial wastewater. Environ. Sci.: Water Res. Technol. 2021;7:222–230. 10.1039/d0ew00787k
38. Yahya SI, Rezaei A, Aghel B. Forecasting of water thermal conductivity enhancement by adding nano-sized alumina particles. J. Therm. Anal. Calorim. 2021;145:1791–1800. 10.1007/s10973-020-10452-0
39. Mohadesi M, Aghel B. Use of ANFIS/genetic algorithm and neural network to predict inorganic indicators of water quality. J. Chem. Pet. Eng. 2020;54:155–164. 10.22059/JCHPE.2020.264471.1244
40. Wang L, Chen Y, Chen B, Yang J. Generation of hydroxyl radicals during photodegradation of chloroacetic acids by 254 nm ultraviolet: A special degradation process revealed by a holistic radical determination methodology. J. Hazard. Mater. 2021;404:124040.
https://doi.org/10.1016/j.jhazmat.2020.124040
41. Dong C, Wang Z, Ye Z, et al. Superoxide radicals dominated visible light driven peroxymonosulfate activation using molybdenum selenide (MoSe2) for boosting catalytic degradation of pharmaceuticals and personal care products. Appl. Catal. B-Environ. 2021;296:120223.
https://doi.org/10.1016/j.apcatb.2021.120223
42. Lee YJ, Jeong YJ, Cho IS, et al. Facile synthesis of N vacancy g-C3N4 using Mg-induced defect on the amine groups for enhanced photocatalytic •OH generation. J. Hazard. Mater. 2023;449:131046. 10.1016/j.jhazmat.2023.131046
43. Xie P, Ma J, Liu W, et al. Removal of 2-MIB and geosmin using UV/persulfate: contributions of hydroxyl and sulfate radicals. Water Res. 2015;69:223–233. 10.1016/j.watres.2014.11.029
44. Jia Z, Zhang WC, Wang WM, Habibi D, Zhang LC. Amorphous Fe78Si9B13 alloy: An efficient and reusable photo-enhanced Fenton-like catalyst in degradation of cibacron brilliant red 3B-A dye under UV–vis light. Appl. Catal. B-Environ. 2016;192:46–56. 10.1016/j.apcatb.2016.03.048
45. Kucukcongar S, Alwindawi AGJ, Turkyilmaz M, Ozaytekin I. Reactive dye removal by photocatalysis and sonophotocatalysis processes using Ag/TiO2/Fe3O4 nanocomposite. Water Air Soil Pollut. 2023;234:103. 10.1007/s11.270-023-0613.6-8
46. Bustillo Lecompte CF, Knight M, Mehrvar M. Assessing the performance of UV/H2O2 as a pretreatment process in TOC removal of an actual petroleum refinery wastewater and its inhibitory effects on activated sludge. Can. J. Chem. Eng. 2015;93:798–807.
https://doi.org/10.1002/cjce.22180
47. Ji Q, He H, Gao Z, et al. UV/H2O2 oxidation of tri (2-chloroethyl) phosphate: Intermediate products, degradation pathway and toxicity evaluation. J. Environ. Sci. 2020;98:55–61.
https://doi.org/10.1016/j.jes.2020.05.015
48. Córdova RN, Nagel-Hassemer ME, Matias WG, Muller JM, de Castilhos AB. Removal of organic matter and ammoniacal nitrogen from landfill leachate using the UV/H2O2 photochemical process. Environ. Technol. 2019;40:793–806.
https://doi.org/10.1080/09593330.2017.1408692
49. Zhang A, Li Y. Removal of phenolic endocrine disrupting compounds from waste activated sludge using UV, H2O2, and UV/H2O2 oxidation processes: effects of reaction conditions and sludge matrix. Sci. Total. Environ. 2014;493:307–323.
https://https://doi.org/10.1016/j.scitotenv.2014.05.149
Fig. 2Effect of H2O2 concentration on the removal efficiency of the UV/H2O2 process. (a) removal of color and (b) removal of TOC (Initial color intensity = 124.50±2.12 ADMI, [TOC]0 = 14.87±1.34 mg C/L, UV intensity = 26.56 W/m2, reaction time = 120 min). (c) The removal efficiency of color by photolysis and UV/H2O2 in the presence of MeOH. (d) The UV-Vis spectrum of the textile wastewater samples before and after the UV/H2O2. 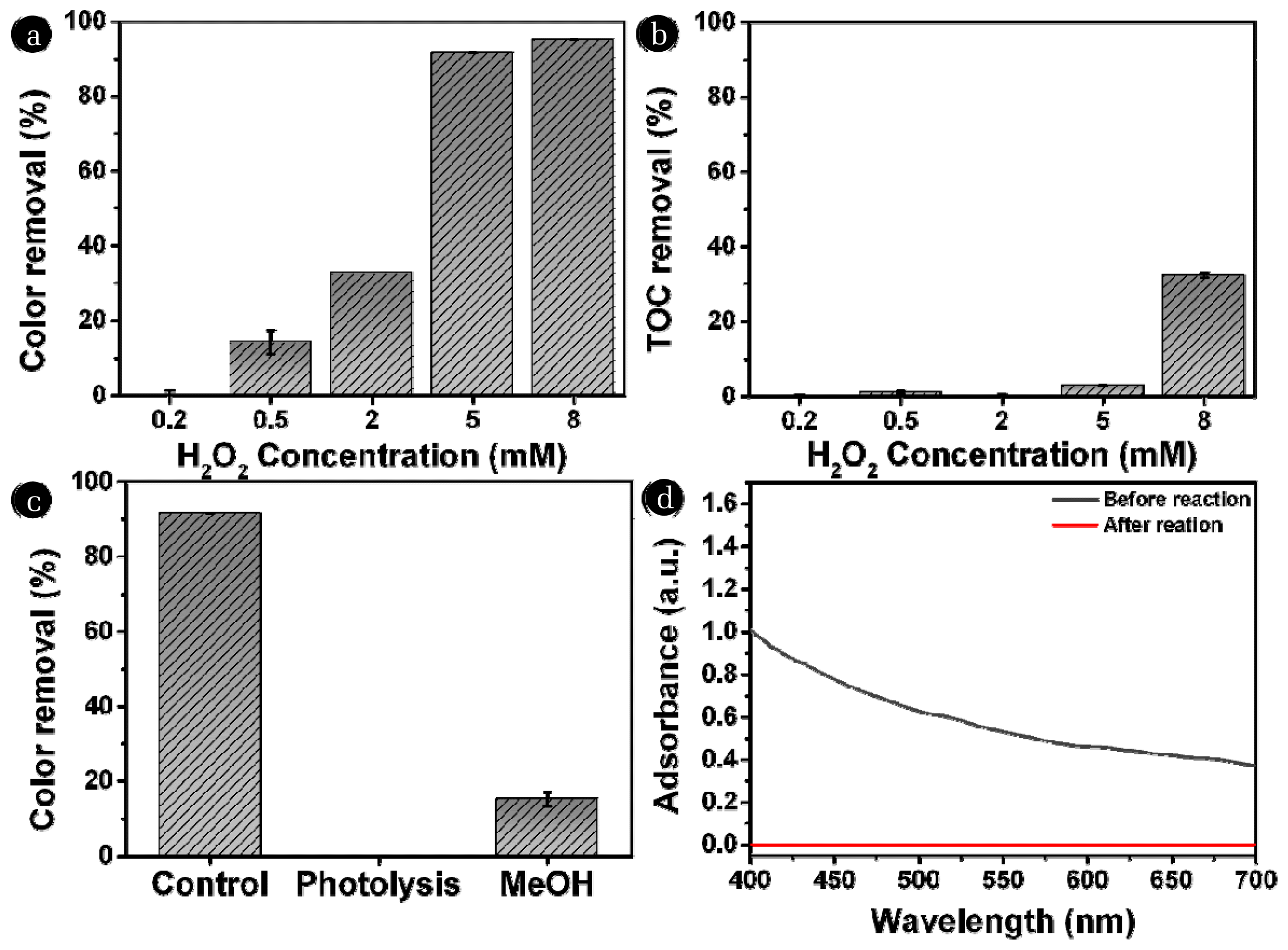
Fig. 3Effect of the UV intensity on the removal efficiency of UV/H2O2 process. (a) removal of color and (b) removal of TOC (Initial color intensity = 124.50±2.12 ADMI, [TOC]0 = 14.87±1.34 mg C/L, [H2O2]0 = 5 mM, reaction time = 120 min). 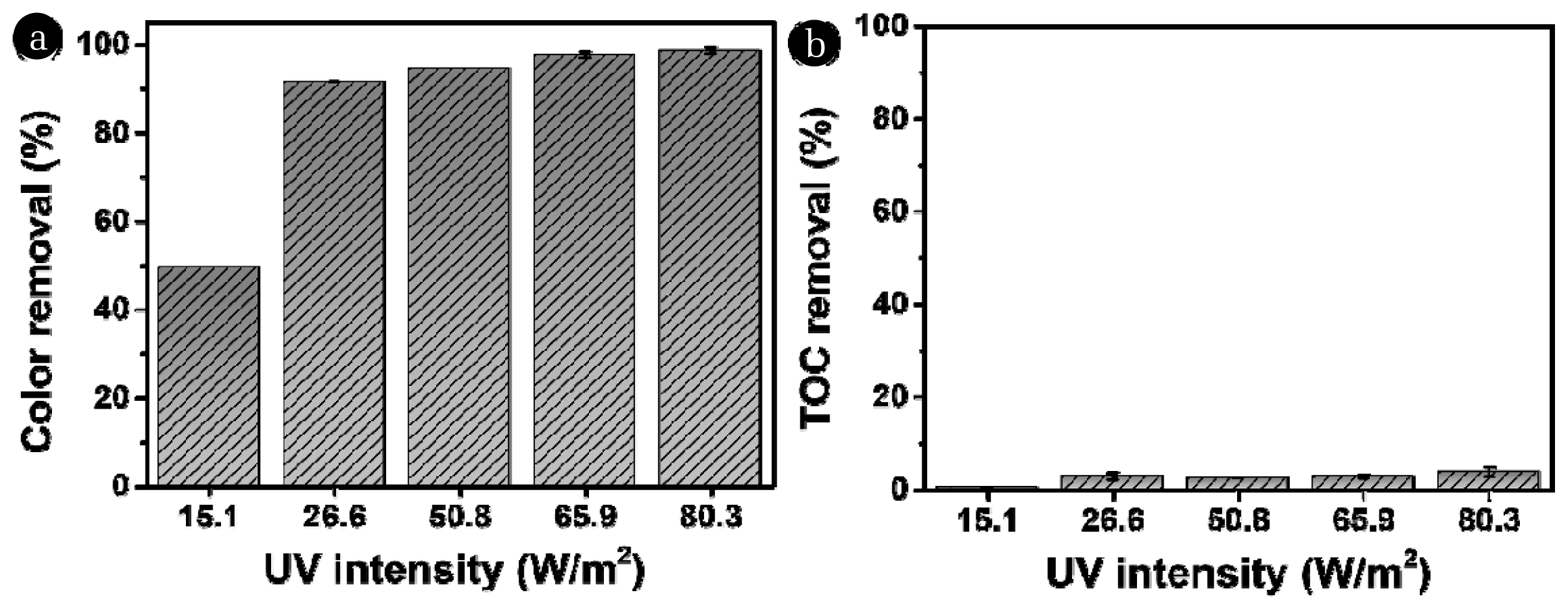
Fig. 4Effect of reaction time on the removal efficiency of the UV/H2O2 process. (a) removal of color and (b) removal of TOC (Initial color intensity = 124.50±2.12 ADMI, [TOC]0 = 14.87±1.34 mg C/L, [H2O2]0 = 5 mM, UV intensity = 26.56 W/m2). 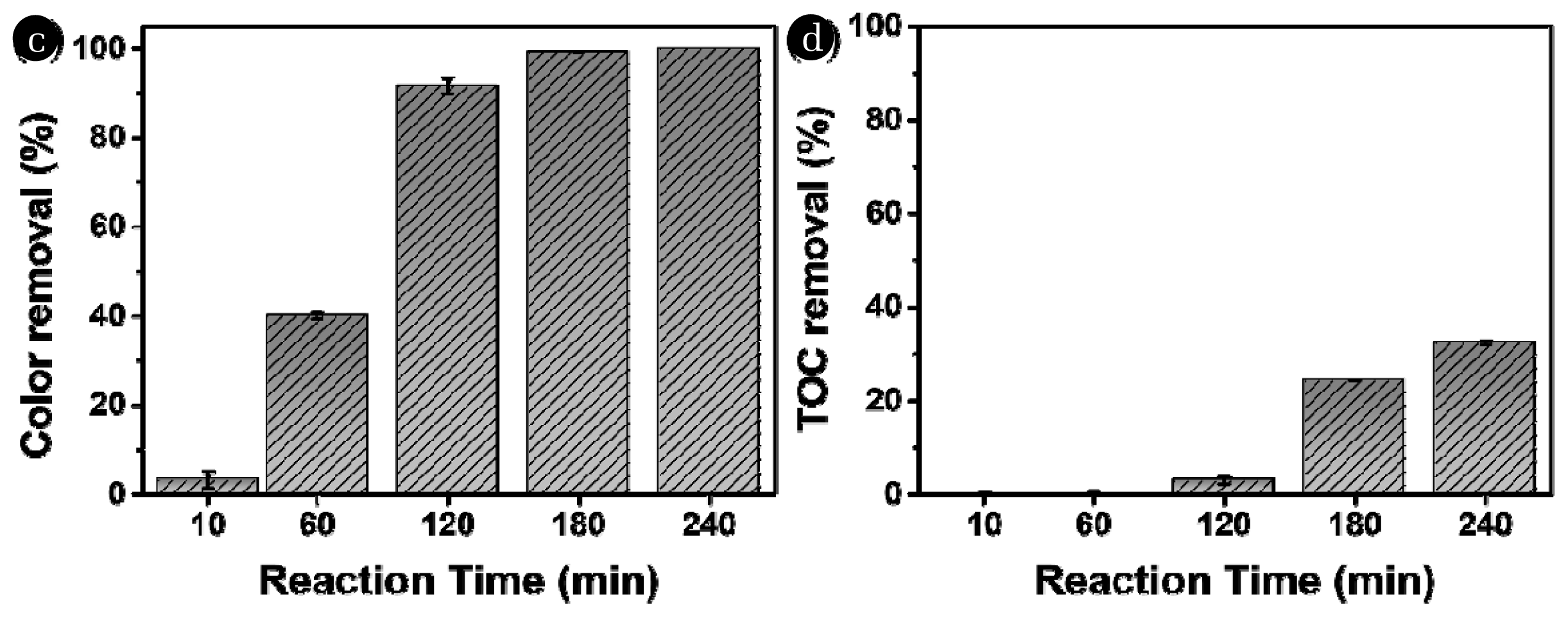
Fig. 5The ratio of mean absolute SHAP value for all data by MLP. (a) removal of color and (b) removal of TOC. 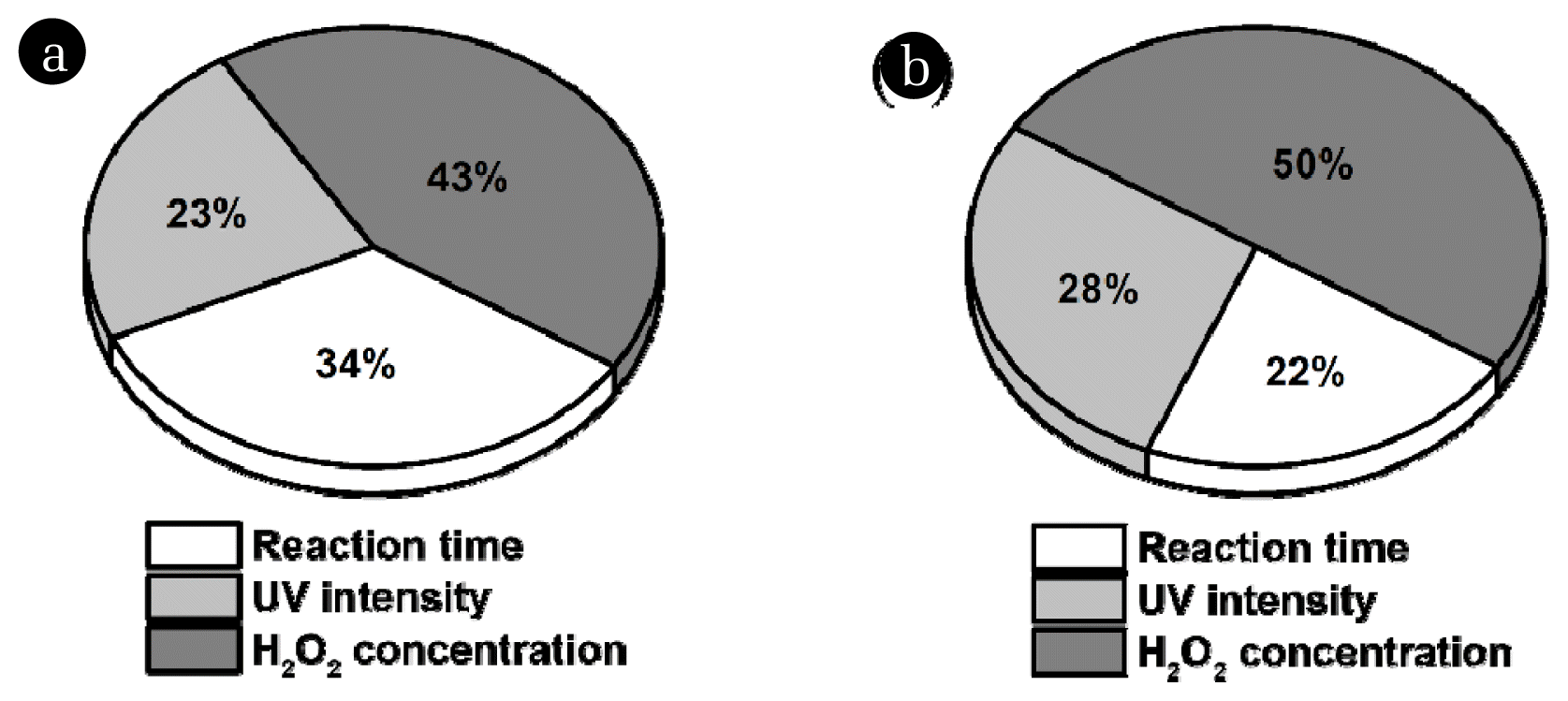
Fig. 6Observed data and MLP prediction comparison for all data and additional data. (a) removal of color and (b) removal of TOC. 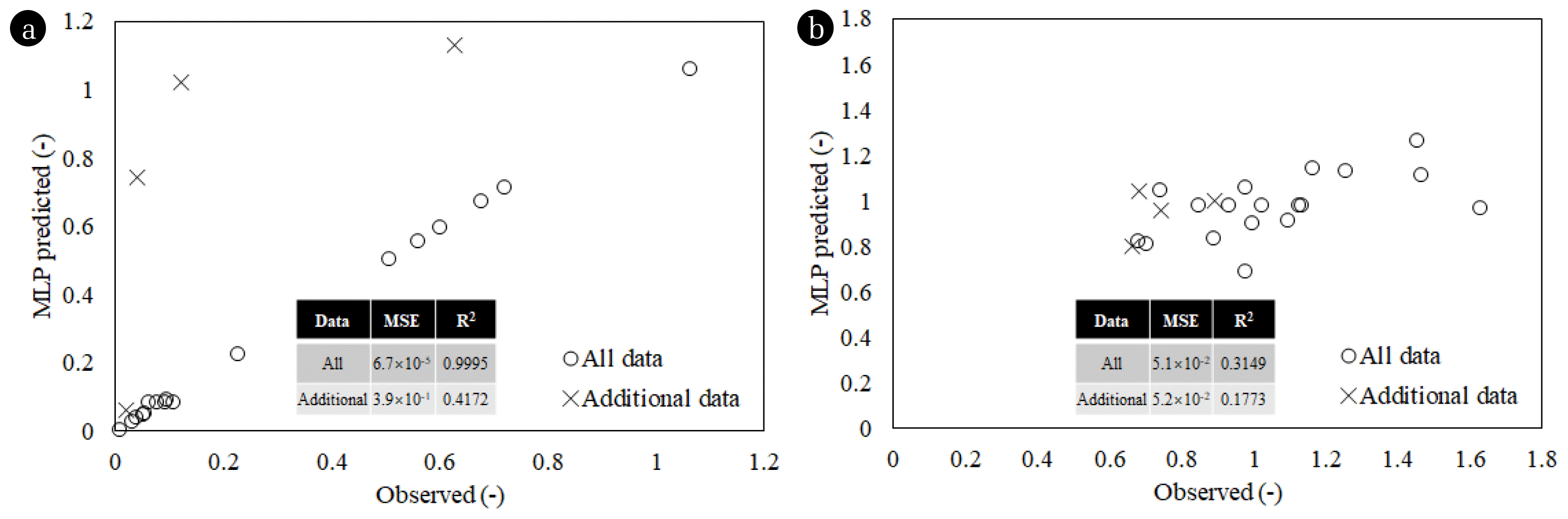
Table 1Wastewater composition |
|
||||||||||||||||||||||||||||||||||||