AbstractSurface modification of zeolite is known to enhance its adsorption capacity for volatile organic compounds (VOCs). In this study, a hydrophobic adsorbent, NaY@LDHs, with layered double hydroxides (LDHs) as the shell and NaY zeolite as the core, was successfully synthesized. It was found that treating LDHs with ethanol can result in a larger specific surface area and pore volume, enhancing the adsorption performance of toluene under wet conditions. SEM imaging reveals that the LDHs shell grows vertically on the surface of the NaY zeolite. The dynamic adsorption experiment of toluene showed that under humid conditions, NaY@LDHs exhibited a saturated adsorption capacity of 32.42 mg/g, representing a significant 87% increase compared to the parent NaY zeolite. The kinetic results show that the adsorption of toluene on NaY@LDHs is primarily physical adsorption, and other factors influence the adsorption process in addition to intraparticle diffusion. Therefore, the air layer formed by the LDHs shell on the surface of NaY zeolite imparts a hydrophobic effect, making NaY@LDHs a promising material for adsorbing volatile organic compounds in a wet environment.
Graphical Abstract
1. IntroductionThe World Health Organization defines volatile organic compounds (VOCs) as a series of organic compounds with boiling temperatures at ambient temperatures ranging from 50°C to 260°C [1]. VOCs are important precursors of O3 and PM2.5, and to a certain extent are prone to photochemical pollution and haze, which seriously pollute the atmospheric environment and pose a health hazard to human beings [2, 3]. VOCs mainly originate from chemical plants, pharmaceutical plants, textile industries, fuel combustion, oil refining industry and so on [4–6]. Most volatile organic compounds include aliphatic hydrocarbons, many of which are chlorinated halogenated compounds, aromatics, alcohols, ethers, esters, aldehydes, amines, acids, and petroleum hydrocarbon compounds, are toxic [7, 8]. Some VOCs are also carcinogenic to humans [9, 10]. Various technologies for dealing with VOCs have been developed. Owing to low production cost, aptness, convenience and simple operation, adsorption has been widely used in the production process. Adsorption occurs when a fluid comes into contact with a porous solid and one or more components of the fluid collect on the solid surface [11]. This method can effectively separate and collect VOCs, and the adsorbent can be reused after desorption [4]. Adsorbents suitable for industrial use should have the following characteristics: high specific surface area, adsorption capacity and porosity, high mechanical strength, hydrophobicity, and thermal stability [12]. Activated carbon, metal organic frameworks and zeolites are all commonly used VOCs adsorption materials [4, 13–14].
Among various materials, activated carbon (AC) has excellent adsorption performance due to its high specific surface area and high porosity, and is currently the most widely used adsorbent [15–17]. Due to its extensive surface area, AC has a high adsorption affinity [18]. However, AC is a naturally non-polar adsorbent, which inevitably limits its adsorption capacity for hydrophilic VOCs [14]. Shin et al. [19] studied the effect of water vapor on the adsorption capacity of activated carbon and discovered that the adsorption performance plummet significantly as the water vapor concentration increases. For polar VOCs, polar functional groups need to be added to the surface of activated carbon to enhance its adsorption effect on polar VOCs [20]. Activated carbon may be unsuitable for regenerative adsorption processes because of easy clogging of its pores and reduction of its efficiency that arise due to excessive carbon loss, low thermal stability, and moisture as well as certain chemical interferences [21]. Metal organic frameworks (MOFs) are a type of crystalline porous material, having up to 90% free space and an interior surface area of approximately 6000 m2/g [22]. However, due to the inadequate stability of most MOFs, their tendency to self-decompose in the aqueous phase, and their high production cost limit their use for the removal of environmental contaminants [23].
Zeolite is a commonly used adsorbent for volatile organic compounds, possessing a variable pore structure, overall stability, and low preparation cost. The key factors determining its adsorption capacity are the physical structure and chemical modification of zeolites [24]. Due to its adjustable pore size, significant thermal stability, and ease of surface modification. It can be designed as a microporous material with uniform pores and appropriate wett-ability, which is conducive for the selective adsorption of VOCs [25]. Y-type zeolite is a faujasite (FAU) zeolite, whose structure consists of silicon-oxygen tetrahedron and aluminum-oxygen tetrahedron with a rich microporous structure [12]. Y zeolite is commonly utilized in catalytic and adsorptive processes due to its substantial specific surface area (exceeding 700 m2/g), ready availability, and high thermal stability [26]. Their ability to adsorb toluene diminishes in wet environments due to their inherently hydrophilic nature [26, 27]. The main reasons for this are the relatively low silica-aluminium ratio (Si/Al) of the NaY zeolite and the greater polarity of the zeolite framework, which has a strong affinity for highly polar water. Overall, improving the hydrophobicity of Y zeolites for toluene adsorption is required.
So far, researchers have conducted numerous studies on the hydrophobic modification of zeolite and put forward several modification schemes [11]. While dealumination may lead to the loss of elemental silicon and the disruption of the zeolite structure, silylation may impact the thermal stability of zeolite. In order to obtain hydrophobic properties, a shell structure was loaded on the surface of the zeolite without affecting the characteristics of the zeolite itself. Layered double hydroxides (LDHs) are a class of compounds with a layered structure, which have application value in the field of adsorption due to their unique structure and properties. It is an anion exchange two-dimensional layered compound composed of two different valence metal cations and anions, which are located on and between the layers, respectively. The chemical composition of LDH is [M2+ 1−xM3+ x(OH)2]x+(An−)x/n·mH2O, where M2+ and M3+ can be divalent and trivalent metal cations, respectively. The value of variable x equals M2+/(M2++M3+) and is in the range of 0.17–0.33; An− can be various anions [28]. In 2013, Wang and O 'Hare [29] reported an aqueous miscible organic solvent treatment (AMOST) method for synthesizing porous and highly dispersed LDHs powders, which is simple and efficient. In 2014, O'Hare and his team [28] found that aqueous miscible organic solvents (methanol and acetone) can effectively extract chemically adsorbed water from LDH surface, prevent piling and aggregating on the surface of LDH particles during drying and effectively increasing their specific surface area (up to 458.6 m2g−1) and pore volume. O'Hare's team used co-precipitation to coat the SiO2 surface with LDHs to form a core-shell composite structure with a tunable particle size, and morphology, and it showed that the vertically growing lamellar structure on the surface of the microporous zeolite can effectively improve its hydrophobicity [30]. In addition, they found that LDHs sheets without AMO treatment are prone to collapse and stack and form stone-like particles, which may clog the pores of the zeolite framework. The mesoporous and flexible chemical characteristics of the AMO-LDH coating should allow for effective pore structure and alkali/acid properties adjustment without compromising the zeolite's properties [31]. The research by O'Hare's team, which has not been applied to VOCs for the time being, offers new ideas for the application of LDHs in adsorption and catalysis. Li et al [32]. loaded microporous zeolites Y, ZSM-5, and TS-1 with uniformly and vertically organized LDH, which successfully improved the hydrophobicity of the three zeolites, but did not find the choice of AMO solvent and the proportion of bimetallic. Liu et al. [33] studied the adsorption performance of NaY@meso-SiO2 core-shell composites at low concentrations of VOCs under humid conditions and found that coating mesoporous materials on microporous surfaces can provide a transport channel for VOCs molecules to enter the lining and increase internal diffusion. Lu et al. [34] found in the large-scale study of hydrophobic organic polymers in core-shell structure that toluene adsorbed in porous organic polymers can further diffuse and migrate to the inside of Y zeolite. Vacant adsorption sites on the surface of organic polymers can continue to adsorb toluene molecules, resulting in higher toluene adsorption capacity.
In this work, LDHs were synthesized by co-precipitation. As a typical VOC substance, toluene is volatile and appears as a colorless liquid floating on water but insoluble in water. In many VOC studies, toluene was chosen as an adsorbent [35–38], and toluene was also chosen as an adsorbent in this experiment. To obtain higher hydrophobicity and toluene adsorption, three aqueous organic solvents and deionized water were chosen to treat the LDHs and the effect of different Mg/Al ratios on the pore capacity and adsorption properties was compared. On this basis, the optimal LDHs composition was combined with NaY zeolite to form a new core@shell structure, and its hydrophobicity was further investigated and mechanistically speculated.
2. Experimental2.1. MaterialsNaY zeolites were bought from Catalyst Plant of Nankai University. Toluene (99.8%), acetone (99.8%), anhydrous ethanol (99.8%), methanol (99.8%), Mg(NO3)2·6H2O (AR), Al(NO3)3·9H2O (AR), Na2CO3 (AR) were purchased from Sinopharm Chemical Reagent Co., Ltd. Deionized water (DI) was used throughout the experimental processes.
2.2. Preparation of LDHsLDHs were synthesized by co-precipitation, pH adjustment, and treated with aqueous miscible organic solvents to increase their surface area. Magnesium and aluminum were selected to form bimetallic carbonates ions. Mg(NO3)2·6H2O and Al(NO3)3·9H2O were dissolved in deionized water, Na2CO3 solution was added to them with continuous stirring.. The pH of the resulting solution was adjusted to 10 using 1 mol/L NaOH solution and solution was stirred for 4 hours. The solids were filtered out, and the solids were put into an aqueous organic solvent and stirred for 4 h. The remaining solids were filtered out and dried in an 80°C oven to obtain the LDHs.
Different proportions of bimetallic solutions were chosen then were adjusted the pH and stirred for 4 h. The samples were washed by respective solvents (distilled water, anhydrous enthanol, acetone, methanol) to obtain different samples represented as LDHs-W, LDHs-E, LDHs-A and LDHs-M, respectively.
2.3. Preparation of NaY@LDHs AdsorbentsNaY zeolite was placed in deionized water for sonicate treatment for 30 min, then a certain amount of Na2CO3 would then be added and sonicated for 6 min. The bimetallic solution was dropwise added into the NaY suspesion while stirring vigorously. The pH was adjusted to 10 with 1 mol/L NaoH and stirred for 1 h. Then the solid would be sifted out and placed in deionized water or aqueous miscible organic solvent and made 1 h stirring. The solid would be filtered again and put in the same solution for continuous stirring for 12 h. After the final filtration, the solid would be dried in an 80°C oven to obtain NaY@LDHs.
2.4. Characterization2.4.1. X-ray diffractionX-ray diffraction spectroscopy can be used to analyze crystal structure and grain size. The powder sample was flattened in a sample tank using a D8 Advance X-ray diffractometer (Germany, Bruker AXS) equipped with Cu-K radiation (40 kV, 100 mA) at 0.02° steps from 3° to 80°. The XRD spectrum was generated by irradiating the X-ray of the crystal powder.
2.4.2. N2 adsorption-desorption testThe texture characteristics were obtained by performing N2 adsorption / desorption isotherms at 77 K on the adsorption instrument JW-BK112 (Beijing JWGB Sci & Tech Co., Ltd.). Before each measurement, the sample was placed in a quartz tube at 523 K and 10−2 Pa for 3 hours to remove the adsorbed impurities and water, and then the adsorption and desorption experiments were performed. The Brunauer-Emmett-Teller (BET) method was utilized for calculating the total specific surface area (SBET), and the HK/SF method was used to ensure the micropore surface area (Smicro) and micropore volume (Vmicro). The isothermal curve was obtained by volume method, and the pore size distribution was obtained by non-local density functional theory (NLDFT) method.
2.4.3. Field emission scanning electron microscope (FE-SEM) analysisThe surface morphology of the sample can be observed and analyzed using a scanning electron microscope. The field emission scanning electron microscope (FE-SEM) Nova NanoSEM 450 (FEI, America) was applied. The surface of the sample was plated with platinum, and the SEM image was obtained in the secondary electron mode at 3 kV and 3 A beam energy.
2.4.4. Fourier transforms infrared spectroscopy (FTIR) analysisFourier transform infrared spectroscopy can identify functional groups in a sample based on the wavelength of absorption. FTIR spectra of samples were recorded using a Nicolet Magna IR-550 spectrometer (Thermo Fisher Scientific, China). The powder sample was mixed with potassium bromide (KBr) and pressed into pieces. FTIR spectra were recorded over a wide wavenumber range between 400 and 4000 cm−1.
2.4.5. Water contact angles analysesThe water contact angle (WCA) of the sample surface was measured at room temperature with a contact angle goniometer (Powereach JC2000A, China), and the hydrophobicity of the sample surface was determined by the change of WCA.
2.4.6. Adsorption measurement of VOCsToluene is a typical volatile organic compound, so toluene was used as an adsorbate, and the adsorption capacity of the adsorbent to volatile organic chemicals was evaluated by dynamic adsorption method. Toluene dynamic breakthrough curves were obtained under dry (RH=0%) and wet (RH=50%) conditions. The adsorption experiment was performed in a fixed-bed reactor, 0.2 g of adsorbent (20 mesh–40 mesh) is filled in a quartz tube, pretreat for 2 hours in a N2 flow at 150°C to remove water and other impurities from the adsorbent. The adsorption experiment was performed at 4°C. There was a toluene concentration of 550 ppm at the gas inlet, with 20% toluene vapor, and a total flow rate of 100 mL/min was maintained for experiments under dry and wet conditions, respectively. Toluene at the outlet was analyzed using a gas chromatograph (GC 7900, Techcomp Co., Ltd.) equipped with a flame ionization detector (FID).
The adsorption performance was evaluated by observing the change of the adsorption curve Ci/C0 (the ratio of toluene concentration at the inlet to the gas concentration at i minute). The calculation equations of toluene adsorption capacity as follows (Eq. (1)) [39]:
where Q (mg/g) is adsorption capacity of toluene; F (ml/min) is gas flow rate; C0 (mg/m3) is the inlet gas concentration; Ci (mg/m3) is the gas concentration at i minute; W (g) is adsorbent quantity; ts (min) is adsorption saturation time.
3. Results and Discussion3.1. Textural Properties of LDHs with Different SolventThe mechanism of using LDH to treat organic solvents is that solvent molecules replace water molecules between layers and on the surface of the LDH. Due to the low boiling point, the solvent molecules between layers can be lost easily, which can weaken the interlayer interactions and result in the collapse of the formation on porous materials [29]. The inorganic ions on LDHs are replaced by organic ions in the solvent, making the layers hydrophobic. According to the literature, pure LDHs including LDHs-A, LDHs-E, LDHs-W and LDHs-M were synthesized, and with the raw material ratio of Mg/Al=2:1 [40, 41]. The powder X-ray diffraction (XRD) patterns of pure LDHs are shown in Fig. 1. The XRD pattern of as-synthesized LDHs showed intense and symmetrical peaks at low angles, and some asymmetric peaks at high angles, showing similarity with the structure of highly crystalline LDHs. The XRD peaks at 2θ=11.39, 23.02, 34.88, 38.78, 46.03, and 61.34 can be attributed to the reflections of (003), (006), (009), (101), (0010), (108), and (110) planes of Mg2Al1–LDH (JCPDS No. 48–0601). The reflections belong to the typical features of LDHs structures, indicating the successful experimental synthesis of LDHs. It is generally considered that the higher and narrower characteristic peaks of XRD indicate the larger grain size. Comparison of the XRD spectra of the experimentally synthesized LDHs shows that LDHs-W has the lowest crystallinity, and LDHs-M is slightly better; it is worth paying attention that the characteristic peaks of LDHs-E and LDHs-A are more intense, which indicates they are more crystalline and that the LDHs crystal lamellae are larger in size. As shown in Fig. 1, compared to LDHs-W, the first three characteristic peaks of LDHs-A and LDHs-E shift towards high angles. It is considered to be caused by the shrinkage of LDHs crystal cells and a decrease in lattice constants after treatment with acetone and ethanol solvents. The mechanism of the AMOST process is not fully known, but it is thought that the XRD results are related to the polarity and viscosity of the AMO solvent. The viscosities (20°C) of methanol, ethanol, water, and acetone are known to be 0.6,1.2,1 and 0.32 Pa·s respectively; the polarities are 6.6, 4.3,10.2 and 5.4 respectively. It is considered that despite the low polarity of acetone, its lower viscosity prevents it from acting fully in the LDH lamellae, resulting in low crystallinity.
The N2 adsorption desorption isotherms and pore distributions of LDHs are shown in Fig. 2. According to the IUPAC classification, due to the small specific surface area of LDHs-W, it exhibits a similar type I adsorption isotherm, a microporous material with a relatively small outer surface [42]. However, since LDHs-W also has hysteresis loops, all four materials belong to type IV adsorption isotherms, indicating that the properties of this material are in the mesoporous range with multilayer adsorption cycles. Capillary condensation and adsorption lead to the formation of hysteresis loops in the isothermal hysteresis of the mesoporous material, indicating that LDHs have a typical mesoporous structure [43]. It is clear that LDHs-E has the highest pore volume. Fig. 2 (b) shows the pore size distribution of samples obtained using the Barrett-Joyner-Halenda (BJH) method analysis according to the N2 adsorption-desorption isotherm. The pore size of LDHs is mainly concentrated around 3–50 nm, and they are mainly mesoporous. LDHs-E and LDHs-A even have macroporous structures, which are thought to be related to the stacking of their layered structures. Table S1 summarizes the detailed structural characteristics. LDHs-E has the largest total pore volume (1.530 cm3/g) and total specific surface area (409 m2/g), followed by LDHs-A (pore volume 0.055 cm3/g and specific surface area 196 m2/g) and LDHs-W has the smallest specific surface area and produces almost no pore volume. There is a network of hydrogen bonds formed by water molecules between the layers of LDHs, which are both bonded to hydroxyl hydrogen in the LDH layer and coordinated with anionic guests between layers [44]. It is believed that due to the entry of acetone and ethanol molecules, the interlayer water molecules have been replaced, disrupting the hydrogen bonding network between the layers, leading to delamination of LDH layers and the formation of pores in stacking, resulting in an increase in specific surface area and pore volume.
3.2. Adsorption Evaluation of LDHs with Different SolventThe breakthrough curves and times of LDHs under different relative humidity conditions are shown in Fig. 3(a) (b). The breakthrough time of LDHs-M, LDHs-W and LDHs-A was lower than 10 min when dynamic adsorption experiments were performed at RH=0%, and the breakthrough time of LDHs-E for toluene adsorption was longer about 14 min, indicating that the LDHs material treated with anhydrous ethanol has more adsorption sites and larger specific surface area (Fig. 3 (a)). The breakthrough time of dynamic adsorption experiments of LDHs-M, LDHs-W and LDHs-A under RH=50% water vapor was less than 5 min, and the breakthrough time of LDHs-E is about 10 min in the adsorption of toluene (Fig. 3 (b)). The other three LDHs have a very small specific surface area where both toluene and water are adsorbed, reaching saturation before replacement of toluene by water molecules occurs. LDHs-E has the largest specific surface area and pore volume, both toluene and water are adsorbed at the initial stage of adsorption. As the adsorption proceeds, the adsorption bed gradually saturates, and the continued entry of water molecules will replace the toluene in the adsorption site, and the substituted toluene enters the next dry unsaturated section. Adsorption cannot continue due to the eventual saturation of the bed's adsorption site, resulting in an increase in the concentration of toluene at the exit. When the replacement is completed and the toluene adsorption is saturated, the concentration of toluene at the outlet is the same as that at the inlet.
The results of toluene adsorption capacity of LDHs treated with different solvents calculated by Eq. (1) are shown in Fig. 3(c) (d). The toluene adsorption capacity of LDHs treated with different solvents under wet conditions was higher than that under dry conditions. LDHs-E has excellent toluene adsorption capacity under dry and wet conditions. The toluene adsorption capacity of LDHs-E under dry conditions is superior to that under humid conditions. Under wet conditions, the saturated adsorption capacity of LDHs-E is 16.1 mg/g, which is still the highest among the four samples. Combining the above data with the article by Wang and O'Hare [44], although anhydrous ethanol may be more favorable for the increase of specific surface area of LDHs, giving LDHs more adsorption sites. Still, the problem of competition for adsorption between the molecules of water and VOCs has not been settled yet. Therefore, it is believed that choosing a less polar and more viscous AMO solvent (e.g., anhydrous ethanol) treatment is more favorable for LDHs crystal synthesis and hydrophobization.
3.3. Adsorption Kinetics of LDHs with Different SolventThe properties of porous media, the flow rate of toluene trapped in the pores, and the volume residual saturation are different. When volatile organic compounds are present in porous media, they exhibit an inherent delay effect [45, 46]. It is not convenient to analyze the mass transfer, but the adsorption kinetics can be calculated. Vasudevan et al. [47] studied the combined effects of rate-limiting dissolution and adsorption of semi-volatile organic compounds in saturated porous systems and concluded that the adsorption of soils and sediments with different physicochemical properties generally follows a nonlinear behavior. At higher pore volumes, the molar fraction of lower solubility components increases, which can lead to higher effective solubility. At low residual saturation and low molar fraction, mass transfer limitations for more soluble compounds are due to low dissolution rate coefficients rather than reduced concentration gradients [48]. Using the adsorption model, the sequential process of mass transfer can be described, and the rate-limiting step of the adsorption process can be inferred [49].
In order to describe the adsorption process of toluene on the sample, the adsorption behavior of four LDHs was fitted by pseudo first-order kinetic model and pseudo second-order kinetic model. Pseudo first-order model often best describes adsorption processes that are dominated by physical adsorption [50]. The combination of physical adsorption and chemical adsorption, where chemical adsorption dominates, is described using a pseudo second-order model [51]. These two models are shown in Eq. (2) and (3), respectively:
The pseudo first-order model:
The pseudo second-order model:
where Qt and qe are the adsorption capacities (mmol/g) at time t and at equilibrium time; k1, k2 are the rate constant for pseudo first-order model and pseudo second-order model, respectively. As can be seen from Fig. S1, the fitting curves of both the pseudo first-order model and the pseudo second-order model are smooth curves, so the process of LDHs adsorbing toluene can be described by these two models. As shown in Table S2, among the fitting results of the two models, the R2 of the pseudo first-order model for the four types of LDHs is greater than that of the pseudo second-order model, and closer to 1, indicating that the quasi first order kinetic model can more accurately describe the adsorption behavior of LDHs for toluene, with physical adsorption being the dominant factor.
3.4. Textural Properties of LDHs with Different Mg/Al RatiosThe results of Chen et al. [30] showed that LDH thickness could be controlled by adjusting the Mg/Al ratio. In order to investigate the relationship among LDH thickness, the adsorption capacity and hydrophobicity of VOCs, samples with Mg/Al=3:1 (LDHs-3:1) were synthesized with the same other conditions and compared with Mg/Al=2:1 (LDHs-2:1). Its N2 adsorption-desorption isotherms and structural parameters are shown in Fig. 4(a) and Table S3. LDHs-3:1 has the same type IV adsorption isotherm as LDHs-2:1, proving that it has mesoporous material characteristics. When the molar ratio of Mg/Al increases, the specific surface area of LDHs-3:1 is 435 m2/g, which is slightly increased by 6.3% compared to LDHs-2:1, and the volume decreases by about 4.1%. Fig. 4(b) shows that both LDHs-3:1 and LDHs-2:1 have Bragg reflections at the corresponding positions. LDHs-3:1 has a lower peak, which is thought to be less crystalline than LDHs-2:1 and contains more lamellar structures that have not collapsed to form pore channels, so it has a higher specific surface area and a lower pore volume.
3.5. Adsorption Evaluation of LDHs with Different Mg/Al RatiosAt RH=0% (Fig. 5(a)) the breakthrough time of LDHs-3:1 was slightly longer than that of LDHs-2:1; and at RH=50% (Fig. 5(b)), the breakthrough times of both were essentially the same, and both showed Ci/C0 over 1. However, the highest Ci/C0 point of LDHs-3:1 was higher, and the time required to reach adsorption saturation was also longer, indicating that under the Mg/Al=3:1 molar ratio synthesis condition, the effect of competing adsorption of water vapor on LDHs is greater. It can be observed that tiny difference in the breakthrough time of LDHs-2:1 under dry and wet conditions, indicating that the competition of water molecules can be effectively avoided by hydrophobic effect in the early stage of adsorption. Overall, it seems that the adsorption effect of LDHs synthesized by the two ratios does not differ much. Based on hydrophobicity, raw material consumption, and economic considerations for industrialization, it is considered that the most suitable LDHs is LDHs-E-2:1.
3.6. Textural Properties of NaY@LDHsNaY zeolite is a faujasite (FAU) zeolite with a high specific surface area, pore volume, and high temperature stability. Once water does not exist in this progress, NaY zeolite has a very large toluene adsorption capacity; but when water is present, while there is strong competition for adsorption, both the toluene adsorption capacity and the breakthrough time plummets. We loaded LDHs on NaY zeolites to make them both microporous and mesoporous, which facilitated the adsorption of VOCs while achieving the goal of improving their hydrophobicity. The morphology of NaY zeolite and the corresponding NaY@LDHs were examined by SEM and XRD analyses, as shown in Fig. S2 and Fig. 6(a). It shows that the NaY zeolite possesses smooth surface (Fig. S2(a)). The core-shell composite material maintains the original shape of the zeolite and forms a petal-like shell on the surface (Fig. S2(b)). As shown in Fig. 6(a), NaY@LDHs retains the original peak characteristics of zeolite compared with NaY, while the peak profile of LDHs appears. The expected (003), (006), (009) and (110) Bragg reflections were observed, indicating that LDH crystals grew on the surface of NaY zeolite. As could be seen in Fig. 6(b), according to the IUPA classification, NaY zeolite can be considered as type I and has mainly microporous properties. As shown in Table S4, the NaY zeolite has a microporous surface area that makes up approximately 98% of the total specific surface area and a microporous volume that makes up about 85% of the total volume. The isothermal curve of NaY @ LDHs has a hysteresis loop, which is a typical mesoporous material characteristic. It shows that after loading LDHs, the surface of NaY zeolite increases mesopores and provides more adsorption sites for toluene.
3.7. Adsorption Evaluation of NaY@LDHsThe results of toluene dynamic adsorption experiments of NaY zeolite and NaY@LDHs are shown in Fig. 7(a) (b). The breakthrough time of LDHs-2:1, NaY@LDHs and NaY zeolite under dry conditions was 12, 38 and 54 min, respectively. NaY@LDHs not much difference in breakthrough time compared to NaY zeolite. The breakthrough time of LDHs-2:1, NaY@LDHs and NaY zeolite under wet conditions is 8, 12 and 26 min, respectively. Compared with NaY zeolite, the breakthrough time of NaY@LDHs under wet conditions is greatly prolonged, and the value of Ci/C0 is significantly reduced, indicating that the loading of LDHs can effectively improve the adsorption capacity of NaY zeolite to toluene under wet conditions and reduce the competitive adsorption effect of water molecules. The toluene adsorption capacity of NaY zeolite and NaY@LDHs is shown in Fig. 7(c) (d). Loading LDHs on NaY zeolite can significantly improve the saturation adsorption capacity of toluene under humid conditions, which is about 87% higher than NaY zeolite. Kraus et al. [52] studied the effects of competitive adsorption and found that some boils were within the detection accuracy range, and toluene was almost completely replaced by water. Due to this strong selectivity, these zeolites can also be used in water flooding to recover valuable heat-sensitive chemicals. The strong effect of co-adsorbed water can often be explained by the strong affinity of most zeolites for this polar molecule. It is believed that this is related to the hydrophobic effect and mesoporous adsorption sites provided by LDHs, which will continue to be explored in subsequent studies.
3.8. Adsorption Kinetics of NaY@LDHsThe pseudo first-order model, pseudo second-order model, and Weber-Morris model [53] (i.e., the intraparticle diffusion model) were used to describe the toluene adsorption process of the NaY@LDHs (Fig. S3, and Table S5, Table S6).
Weber-Morris model:
The fitting results of the pseudo first-order model and pseudo second-order model to NaY, NaY@LDHs and LDHs-2:1 are smooth curves, indicating that these two models can be used to describe the toluene adsorption process of the above three samples. The R2 of the pseudo first-order model is greater than that of the pseudo second-order model, indicating that the toluene adsorption process of the three samples is dominated by physical adsorption.
The kinetic curve of toluene on the NaY@LDHs can be divided into three parts: outer surface diffusion, internal mesopora-macropore diffusion, and dissolved particles adsorbed from the active site to the inner surface of the pore (meso-macropore diffusion) [54]. As shown in Fig. S3 (d), since the plot of the second stage does not cross the origin, it shows that the intraparticle diffusion step is not the only factor in toluene adsorption, and there are other factors that affect the adsorption process. The meso-macropore adsorption with the lowest diffusion coefficient is the limiting factor in the adsorption process. The adsorption process of toluene on the NaY@LDHs is divided into two stages, the first stage of surface diffusion is mainly related to the specific surface area, and the second stage is the rapid diffusion of toluene inside the particles due to the porous and layered structure [55].
3.9. FTIR Spectra of NaY@LDHsThe FT-IR spectra is useful for identifying surface functional groups [56]. As the FT-IR spectrum of LDHs, NaY zeolite and NaY@LDHs shown in Fig. S4. Two distinguishable external and internal vibrations (TO4, T=Si or Al) exist within the tetrahedra of NaY zeolites [57]. Fig. S4 shows that NaY zeolites have distinct tetrahedral asymmetric stretching vibrations at 1057 and 1196 cm−1 and tetrahedral symmetric stretching vibrations at 668 cm−1. The peaks at about 3443 cm−1 and 1650 cm−1 are due to the stretching and bending vibration of the hydroxide layer and interlayer water [58].
The peak at 1384 cm−1 is the asymmetric stretching vibration of CO32−, and the peak at 664 cm−1 is the in-plane deformation vibration of CO32−. These two peaks indicate the presence of carbonate anions in LDHs nanosheets [59]. The non-polar bending mode of CO32− corresponds to the tiny shoulder at around 870 cm−1 [60]. The absorption peak for the C-O group is 1062 cm−1 [11]. In the low frequency region, the bands in the range of 400 to 700 cm−1 are attributed to metal-oxygen-metal and oxygen-metal-oxygen stretching [61]. The metallic characteristic peaks in the low frequency region are significantly reduced for NaY@LDHs compared to NaY, while there are distinctive characteristic peaks at 664 cm−1 and 1384 cm−1 compared to LDHs. Compared to NaY, the −OH peak at 1650 cm−1 of the NaY@LDHs became significantly smoother, indicating improved hydrophobicity.
3.10. Water Contact Angles TestThe water contact angle can directly reveal the outer surface's hydrophilic or hydrophobic qualities [26]. The images of the water drop on NaY and NaY@LDHs, surfaces are shown in Fig. S5. NaY zeolite exhibits superhydrophilicity and the contact angle is 0°. Therefore, the adsorption capacity of organic matter is weak when toluene and water coexist. After coated by LDHs layers, the NaY@LDHs shows a contact angle of 29°. The NaY@LDHs exhibited the coalescence of water droplets compared to NaY zeolites and showed significant hydrophobicity despite the small angle of water contact angle.
3.11. Analysis of Hydrophobic MechanismThe hydrophobic LDHs material can be hydrophobized by the AMOST method [40, 62–63]. Based on the above findings, a schematic diagram of the hydrophobic mechanism of LDHs loaded on the surface of zeolite was constructed in Fig. 8. LDHs are treated with AMO solvent, and the solvent molecules replace the bound water on the surface of the lamellae, providing them with hydrophobic properties and weakening the interactions between the lamellae, resulting in the stacking of the lamellae to form a pore structure. It is considered that LDHs rely on stable chemical bonding connections on zeolite, vertical structural growth, formation of air layers, and sheet stacking to produce mesoporous volume and increase the roughness of the molecular sieve surface to achieve effective improvement of toluene adsorption capacity and hydrophobicity.
According to the above experimental results and the article of Li et al. [40], after the vertical loading of LDHs on the surface of NaY zeolite, the composites are similar to lotus leaves with many regular dense villi on the surface, which can effectively improve the hydrophobic properties of the composites [64]. Therefore, pure zeolite is considered to have a smoother surface and a large contact area with water molecules, so it exhibits hydrophilicity [11]. Although LDHs are also hydrophilic [65], when vertically loaded on the surface of zeolites, an air layer will be formed on the surface, reducing the contact area between the composite surface and water molecules. Therefore, the NaY@LDHs composites have excellent adsorption and hydrophobic properties.
4. ConclusionIn this study, NaY@LDHs was synthesized by co-precipitation. When the molar ratio of Mg/Al is 2:1, and LDHs are treated with solvents with high viscosity and low polarity, a large specific surface area (up to 409 m2/g) and pore volume can be obtained, and the hydrophobicity and adsorption capacity can be effectively improved. It was found that high Mg/Al ratio and solvents with higher polarity reduced crystallinity and pore volume. Coating LDHs on NaY zeolite to form an air layer on its surface and reduce the contact area between water molecules and zeolite, which can effectively extend their penetration time under wet conditions. The kinetic simulation results show that the adsorption process of toluene on the adsorbent conforms to the pseudo first-order model, which is mainly physical adsorption. The adsorption process is mainly divided into two processes, and it is affected by a variety of factors. LDHs coating has the potential and effectiveness in improving the adsorption performance of NaY zeolite wet humid conditions.
AcknowledgementsThis study received no specific financing from funding agencies in the public, commercial, or non-profit sectors.
NotesAuthor Contributions Y. Y. L. (Master student) conducted experiments, wrote the final manuscript, and revised it. T. Y. (PhD student) proposed the experimental idea and collected the relevant literature. J. W. X. (Master student) conducted the drawing. N. W. L. (Associate Professor) supervised the research and co-wrote the manuscript. L. S. (Professor) supervised the research and co-wrote the manuscript. X. M. (Associate Professor) supervised experiments and co-authored and revised manuscripts. References1. Sarigiannis DA, Karakitsios SP, Gotti A, Liakos IL, Katsoyiannis A. Exposure to major volatile organic compounds and carbonyls in European indoor environments and associated health risk. Environ Int. 2011;37(4)743–765.
https://doi.org/10.1016/j.envint.2011.01.005
2. Yu G, Wei Y, Cheng J, Jiang T, Ling C, Xu B. Health risk assessment and personal exposure to Volatile Organic Compounds (VOCs) in metro carriages — A case study in Shanghai, China. Sci. Total Environ. 2017;574:1432–1438.
https://doi.org/10.1016/j.scitotenv.2016.08.072
3. Liang X, Chen X, Zhang J, et al. Reactivity-based industrial volatile organic compounds emission inventory and its implications for ozone control strategies in China. Atmos. Environ. 2017;162:115–126.
https://doi.org/10.1016/j.atmosenv.2017.04.036
4. Li X, Zhang L, Yang Z, Wang P, Yan Y, Ran J. Adsorption materials for volatile organic compounds (VOCs) and the key factors for VOCs adsorption process: A review. Sep. Purif. Technol. 2020;235:116213.
https://doi.org/10.1016/j.seppur.2019.116213
5. Tang J, Zhuang JB, Aljerf L, Xia H, Wang TZ, Gao BY. Numerical simulation modelling on whole municipal solid waste incineration process by coupling multiple software for the analysis of grate speed and air volume ratio. Process Saf. Environ. Protect. 2023;176:506–527.
https://doi.org/10.1016/j.psep.2023.05.101
6. Liang YQ, Tang J, Xia H, Aljerf L, Gao BY, Akele ML. Three-Dimensional Numerical Modeling and Analysis for the Municipal Solid-Waste Incineration of the Grate Furnace for Particulate-Matter Generation. Sustainability. 2023. 1516:22.
https://doi.org/10.3390/su151612337
7. Bari MA, Kindzierski WB. Ambient volatile organic compounds (VOCs) in Calgary, Alberta: Sources and screening health risk assessment. Sci Total Environ. 2018;631–632:627–640.
https://doi.org/10.1016/j.scitotenv.2018.03.023
8. Huang B, Lei C, Wei C, Zeng G. Chlorinated volatile organic compounds (Cl-VOCs) in environment — sources, potential human health impacts, and current remediation technologies. Environ. Int. 2014;71:118–138.
https://doi.org/10.1016/j.envint.2014.06.013
9. Klett C, Duten X, Tieng S, Touchard S, Jestin P, Hassouni K, et al. Acetaldehyde removal using an atmospheric non-thermal plasma combined with a packed bed: Role of the adsorption process. J. Hazard. Mater. 2014;279:356–364.
https://doi.org/10.1016/j.jhazmat.2014.07.014
10. Gałęzowska G, Chraniuk M, Wolska L. In vitro assays as a tool for determination of VOCs toxic effect on respiratory system: A critical review. Trac-Trends Anal. Chem. 2016;77:14–22.
https://doi.org/10.1016/j.trac.2015.10.012
11. Aljerf L. High-efficiency extraction of bromocresol purple dye and heavy metals as chromium from industrial effluent by adsorption onto a modified surface of zeolite: Kinetics and equilibrium study. J. Environ. Manag. 2018;225:120–132.
https://doi.org/10.1016/j.jenvman.2018.07.048
12. Deng H, Pan T, Zhang Yan, Wang L, He H. Adsorptive removal of toluene and dichloromethane from humid exhaust on MFI, BEA and FAU zeolites: An experimental and theoretical study. Chem. Eng. J. 2020;394:124986.
https://doi.org/10.1016/j.cej.2020.124986
13. Zhang X, Gao B, Creamer AE, Cao C, Li Y. Adsorption of VOCs onto engineered carbon materials: A review. J. Hazard. Mater. 2017;338:102–123.
https://doi.org/10.1016/j.jhazmat.2017.05.013
14. Zhu L, Shen D, Luo KH. A critical review on VOCs adsorption by different porous materials: Species, mechanisms and modification methods. J. Hazard. Mater. 2020;389:122102.
https://doi.org/10.1016/j.jhazmat.2020.122102
15. Zhang Z, Xu M, Wang H, Li Z. Enhancement of CO2 adsorption on high surface area activated carbon modified by N2, H2 and ammonia. Chem Eng J. 2010;160(2)571–577.
https://doi.org/10.1016/j.cej.2010.03.070
16. Sun W, Lipka SM, Swartz C, Williams D, Yang F. Hemp-derived activated carbons for supercapacitors. Carbon. 2016;103:181–192.
https://doi.org/10.1016/j.carbon.2016.02.090
17. Heidarinejad Z, Dehghani MH, Heidari M, Javedan G, Ali I, Sillanpää M. Methods for preparation and activation of activated carbon: a review. Environ Chem Lett. 2020;18(2)393–415.
https://doi.org/10.1007/s10311-019-00955-0
18. Kang Y-J, Jo H-K, Jang M-H, et al. A Brief Review of Formaldehyde Removal through Activated Carbon Adsorption. Appl. Sci. 2022. 1210:5025.
https://doi.org/10.3390/app12105025
19. Shin H-C, Park J-W, Park K, Song HC. Removal characteristics of trace compounds of landfill gas by activated carbon adsorption. Environ Pollut. 2002;119(2)227–236.
https://doi.org/10.1016/S0269-7491(01)00331-1
20. Zhao H, Tang Z, He M, et al. Effect of oxygen functional groups on competitive adsorption of benzene and water on carbon materials: Density functional theory study. Sci. Total Environ. 2023;863:160772.
https://doi.org/10.1016/j.scitotenv.2022.160772
21. Khan FI, KrGhoshal A. Removal of Volatile Organic Compounds from polluted air. J Loss Prev Process Ind. 2000;13(6)527–545.
https://doi.org/10.1016/S0950-4230(00)00007-3
22. Kalmutzki MJ, Diercks CS, Yaghi OM. Metal-Organic Frameworks for Water Harvesting from Air. Adv. Mater. 2018. 3037:e1704304.
https://doi.org/10.1002/adma.201704304
23. Liu D, Gu W, Zhou L, et al. Recent advances in MOF-derived carbon-based nanomaterials for environmental applications in adsorption and catalytic degradation. Chem. Eng. J. 2022;427:131503.
https://doi.org/10.1016/j.cej.2021.131503
24. Shen X, Du X, Yang D, Ran J, Yang Z, Chen Y. Influence of physical structures and chemical modification on VOCs adsorption characteristics of molecular sieves. J. Environ. Chem. Eng. 2021. 96106729.
https://doi.org/10.1016/j.jece.2021.106729
25. Zhang L, Peng Y, Zhang J, Chen L, Meng X, Xiao FS. Adsorptive and catalytic properties in the removal of volatile organic compounds over zeolite-based materials. Chin J Catal. 2016;37(6)800–809.
https://doi.org/10.1016/S1872-2067(15)61073-7
26. Yin T, Meng X, Jin L, Yang C, Liu N, Shi L. Prepared hydrophobic Y zeolite for adsorbing toluene in humid environment. Microporous Mesoporous Mat. 2020;305:110327.
https://doi.org/10.1016/j.micromeso.2020.110327
27. Lu SC, Liu QL, Han R, et al. Core-shell structured Y zeolite/hydrophobic organic polymer with improved toluene adsorption capacity under dry and wet conditions. Chem. Eng. J. 2021;409:11.
https://doi.org/10.1016/j.cej.2020.128194
28. Chen C, Yang M, Wang Q, Buffet J-C, O'Hare D. Synthesis and characterisation of aqueous miscible organic-layered double hydroxides. J Mater Chem A. 2014;2(36)15102–15110.
https://doi.org/10.1039/C4TA02277G
29. Wang Q, O'Hare D. Large-scale synthesis of highly dispersed layered double hydroxide powders containing delaminated single layer nanosheets. Chem Commun. 2013;49(56)6301–6303.
https://doi.org/10.1039/C3CC42918K
30. Chen C, Felton R, Buffet JC, O'Hare D. Core-shell SiO2@LDHs with tuneable size, composition and morphology. Chem Commun. 2015;51(16)3462–3465.
https://doi.org/10.1039/C4CC10008E
31. Chen C, Byles CFH, Buffet JC, Rees NH, Wu Y, O'Hare D. Core-shell zeolite@aqueous miscible organic-layered double hydroxides. Chem Sci. 2016;7(2)1457–1461.
https://doi.org/10.1039/C5SC03208C
32. Li R, Chong S, Altaf N, Gao Y, Louis B, Wang Q. Synthesis of ZSM-5/Siliceous Zeolite Composites for Improvement of Hydrophobic Adsorption of Volatile Organic Compounds. Front. Chem. 2019;7:505.
https://doi.org/10.3389/fchem.2019.00505
33. Liu H, Wei K, Long C. Enhancing adsorption capacities of low-concentration VOCs under humid conditions using NaY@ meso-SiO2 core–shell composite. Chem. Eng. J. 2022;442:136108.
https://doi.org/10.1016/j.cej.2022.136108
34. Lu S, Liu Q, Han R, et al. Core-shell structured Y zeolite/hydrophobic organic polymer with improved toluene adsorption capacity under dry and wet conditions. Chem. Eng. J. 2021;409:128194.
https://doi.org/10.1016/j.cej.2020.128194
35. Zhang X, Gao B, Zheng Y, Hu X, Creamer AE, Annable MD, et al. Biochar for volatile organic compound (VOC) removal: Sorption performance and governing mechanisms. Bioresour. Technol. 2017;245:606–614.
https://doi.org/10.1016/j.biortech.2017.09.025
36. Kante K, Florent M, Temirgaliyeva A, Lesbayev B, Bandosz TJ. Exploring resistance changes of porous carbon upon physical adsorption of VOCs. Carbon. 2019;146:568–571.
https://doi.org/10.1016/j.carbon.2019.02.039
37. Kutluay S, Temel F. Silica gel based new adsorbent having enhanced VOC dynamic adsorption/desorption performance. Colloid Surf. A-Physicochem. Eng. Asp. 2021;609:125848.
https://doi.org/10.1016/j.colsurfa.2020.125848
38. Jeong YK, Lee S-B, Kweon S, et al. Synergistic Inorganic/Inorganic Hybrid Approach for Fabricating a BTX Gas Adsorbent with High Performance and Thermal Stability. ACS Sustain Chem Eng. 2023;11(12)4652–4661.
https://doi.org/10.1021/acssuschemeng.2c06591
39. Yin T, Meng X, Wang S, Yao X, Liu N, Shi L. Study on the adsorption of low-concentration VOCs on zeolite composites based on chemisorption of metal-oxides under dry and wet conditions. Sep. Purif. Technol. 2022;280:119634.
https://doi.org/10.1016/j.seppur.2021.119634
40. Li R, Xue T, Bingre R, Gao Y, Louis B, Wang Q. Microporous Zeolite@Vertically Aligned Mg-Al Layered Double Hydroxide Core@Shell Structures with Improved Hydrophobicity and Toluene Adsorption Capacity under Wet Conditions. ACS Appl Mater Interfaces. 2018;10(41)34834–34839.
https://doi.org/10.1021/acsami.8b15118
41. Huang M, Lu G, Pu J, Qiang Y. Superhydrophobic and smart MgAl-LDH anti-corrosion coating on AZ31 Mg surface. J. Ind. Eng. Chem. 2021;103:154–164.
https://doi.org/10.1016/j.jiec.2021.07.031
42. Sing KS. Reporting physisorption data for gas/solid systems with special reference to the determination of surface area and porosity (Recommendations 1984). Pure Appl Chem. 1985;57(4)603–619.
https://doi.org/10.1351/pac198557040603
43. Thommes M, Kaneko K, Neimark AV, Olivier JP, Rodriguez-Reinoso F, Rouquerol J, et al. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl Chem. 2015;87(9–10)1051–1069.
https://doi.org/10.1515/pac-2014-1117
44. Wang Q, O'Hare D. Recent advances in the synthesis and application of layered double hydroxide (LDH) nanosheets. Chem Rev. 2012;112(7)4124–4155.
https://doi.org/10.1021/cr200434v
45. Vasudevan M, Suresh Kumar G, Nambi IM. Numerical study on kinetic/equilibrium behaviour of dissolution of toluene under variable subsurface conditions. Eur J Environ Civ Eng. 2014;18(9)1070–1093.
https://doi.org/10.1080/19648189.2014.922902
46. Vasudevan M, Nambi IM, Suresh Kumar G. Scenario-based modelling of mass transfer mechanisms at a petroleum contaminated field site-numerical implications. J. Environ. Manage. 2016;175:9–19.
https://doi.org/10.1016/j.jenvman.2016.03.009
47. Vasudevan M, Kumar GS, Nambi IM. Numerical studies on kinetics of sorption and dissolution and their interactions for estimating mass removal of toluene from entrapped soil pores. Arab J Geosci. 2015;8(9)6895–6910. https://doi.org/10.1007/s12517-014-1681-7
48. Vasudevan M, Suresh Kumar G, Nambi IM. Numerical modelling of multicomponent LNAPL dissolution kinetics at residual saturation in a saturated subsurface system. Sadhana-Acad. Proc. Eng. Sci. 2014. 3961387–1408.
https://doi.org/10.1007/s12046-014-0282-1
49. Vasudevan M, Ajithkumar PS, Singh RP, Natarajan N. Mass transfer kinetics using two-site interface model for removal of Cr(VI) from aqueous solution with cassava peel and rubber tree bark as adsorbents. Environ Eng Res. 2016;21(2)152–163.
https://doi.org/10.4491/eer.2015.152
50. Yu S, Wang X, Liu F, Xiao K, Kang C. Adsorption of acetone, ethyl acetate and toluene by beta zeolite/diatomite composites: preparation, characterization and adsorbability. Environ Sci Pollut Res. 2022;29(53)80646–80656.
https://doi.org/10.1007/s11356-022-21308-5
51. Guo M, Liu Q, Lu S, et al. Synthesis of Silanol-Rich MCM-48 with Mixed Surfactants and Their Application in Acetone Adsorption: Equilibrium, Kinetic, and Thermodynamic Studies. Langmuir. 2020;36(39)11528–11537.
https://doi.org/10.1021/acs.langmuir.0c01933
52. Kraus M, Trommler U, Holzer F, Kopinke F-D, Roland U. Competing adsorption of toluene and water on various zeolites. Chem. Eng. J. 2018;351:356–363.
https://doi.org/10.1016/j.cej.2018.06.128
53. Nayak AK, Pal A. Development and validation of an adsorption kinetic model at solid-liquid interface using normalized Gudermannian function. J. Mol. Liq. 2019;276:67–77.
https://doi.org/10.1016/j.molliq.2018.11.089
54. Bagheri M, Masoomi MY, Morsali A. High organic sulfur removal performance of a cobalt based metal-organic framework. J. Hazard. Mater. 2017;331:142–149.
https://doi.org/10.1016/j.jhazmat.2017.02.037
55. Ma X, Wang W, Sun C, Li H, Sun J, Liu X. Adsorption performance and kinetic study of hierarchical porous Fe-based MOFs for toluene removal. Sci. Total Environ. 2021;793:148622.
https://doi.org/10.1016/j.scitotenv.2021.148622
56. Wei G, He Q, Zhang T, et al. Tunable infrared radiation properties of hybrid films co-assembled with semiconductor quantum chips and exfoliated ultra-thin LDH nanosheets. J. Alloys Compd. 2018;751:215–223.
https://doi.org/10.1016/j.jallcom.2018.04.073
57. Karge HG. Characterization by infrared spectroscopy. Microporous Mesoporous Mat. 1998;22(4)547–549.
https://doi.org/10.1016/S1387-1811(98)80021-8
58. Zhao X, Mao L, Cheng Q, Liao F, Yang G, Chen L. Dual-cation preintercalated and amorphous carbon confined vanadium oxides as a superior cathode for aqueous zinc-ion batteries. Carbon. 2022;186:160–170.
https://doi.org/10.1016/j.carbon.2021.10.013
59. Zhang G, Wu L, Tang A, et al. Active corrosion protection by a smart coating based on a MgAl-layered double hydroxide on a cerium-modified plasma electrolytic oxidation coating on Mg alloy AZ31. Corrosion Sci. 2018;139:370–382.
https://doi.org/10.1016/j.corsci.2018.05.010
60. Khanal S, Lu Y, Jin D, Xu S. Effects of layered double hydroxides on the thermal and flame retardant properties of intumescent flame retardant high density polyethylene composites. Fire Mater. 2022;46(1)107–116.
https://doi.org/10.1002/fam.2951
61. Chen H, Zhang F, Fu S, Duan X. In Situ Microstructure Control of Oriented Layered Double Hydroxide Monolayer Films with Curved Hexagonal Crystals as Superhydrophobic Materials. Adv Mater. 2006;18(23)3089–3093.
https://doi.org/10.1002/adma.200600615
62. Li Q, Chen Q, Jiang K, Lei S, Deng Y, Bao J. Boosting high-current water electrolysis: Superhydrophilic/superaerophobic nanosheet arrays of NiFe LDH with oxygen vacancies in situ grown on iron foam. Int J Hydrog Energy. 2023;48(46)17501–17511.
https://doi.org/10.1016/j.ijhydene.2023.01.184
63. Hadj-Abdelkader NEH, Beltrao-Nunes A-P, Belkhadem F, Benselka N, Roy R, Azzouz A. New insights in MgAl and MgFe-LDH affinity towards carbon dioxide – role of the hydrophilic character on CO2 retention strength. Appl. Clay. Sci. 2020;198:105829.
https://doi.org/10.1016/j.clay.2020.105829
64. Bhushan B, Jung YC, Koch K. Micro-, nano- and hierarchical structures for superhydrophobicity, self-cleaning and low adhesion. Philos. Trans. R. Soc. A-Math. Phys. Eng. Sci. 2009. 36718941631–1672.
https://doi.org/10.1098/rsta.2009.0014
65. Zümreoglu-Karan B, Ay AN. Layered double hydroxides — multifunctional nanomaterials. Chem Pap. 2012;66(1)1–10.
https://doi.org/10.2478/s11696-011-0100-8
Fig. 3Adsorption breakthrough curves of toluene under (a) dry and (b) wet conditions; Toluene adsorption capacity of LDHs in (c) RH=0% and (d) RH=50%. 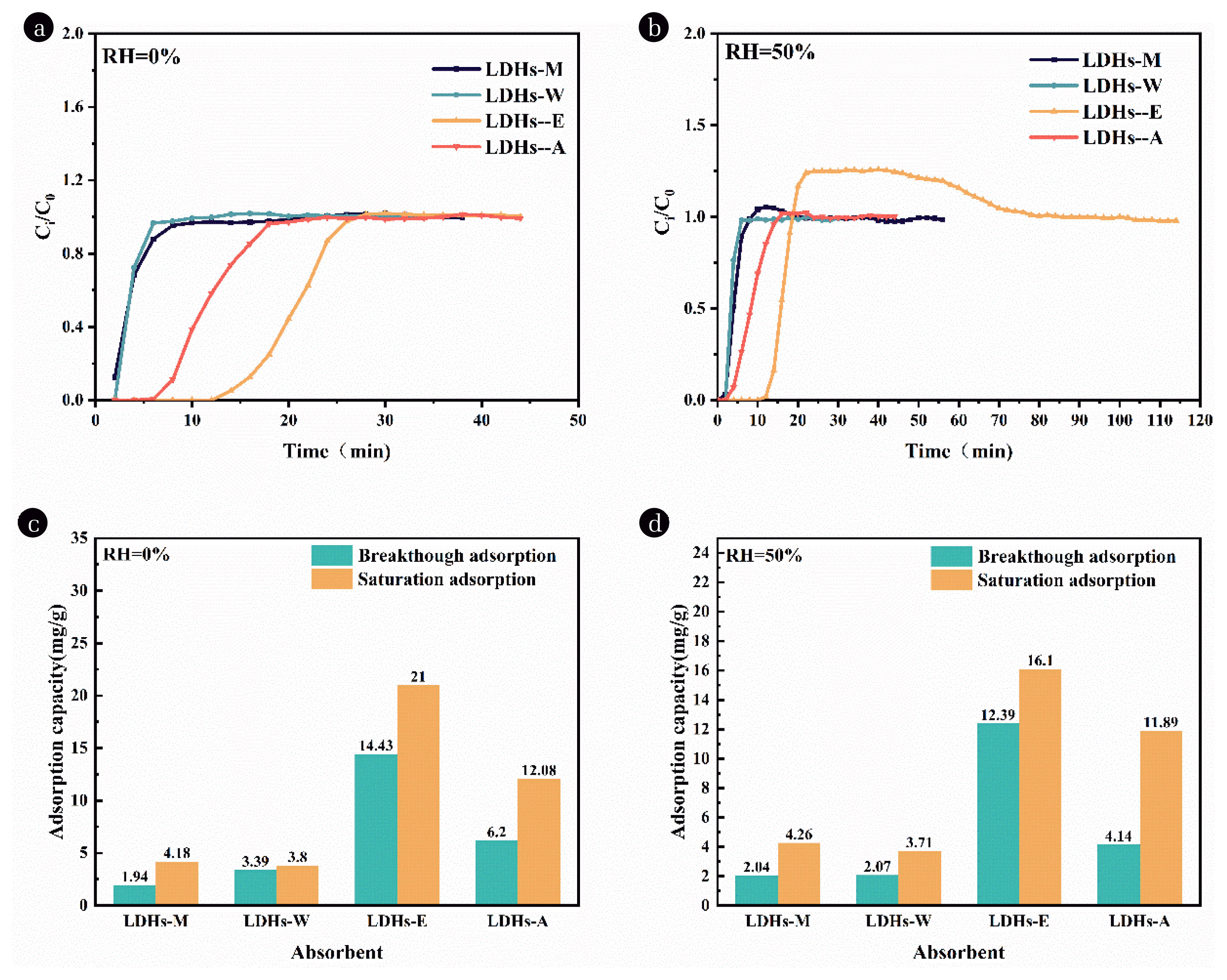
Fig. 4(a) N2 adsorption/desorption isotherms of LDHs-2:1 and LDHs-3:1; (b) XRD patterns of LDHs-2:1 and LDHs-3:1. 
Fig. 5Adsorption breakthrough curves of toluene under (a) dry and (b) wet conditions of LDHs-2:1 and LDHs-3:1. 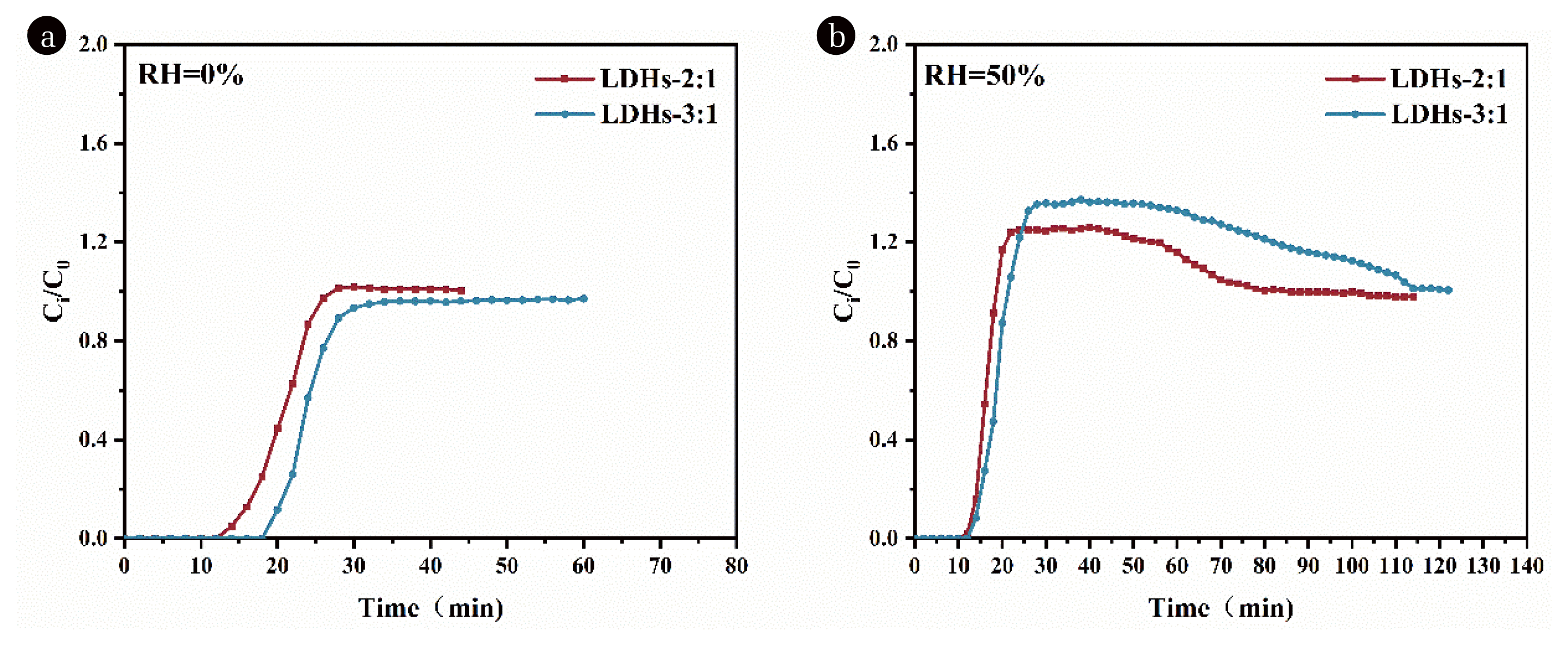
Fig. 6(a) XRD patterns of LDHs, NaY@LDHs and NaY; (b) N2 adsorption/desorption isotherms of NaY zeolite and NaY@LDHs. 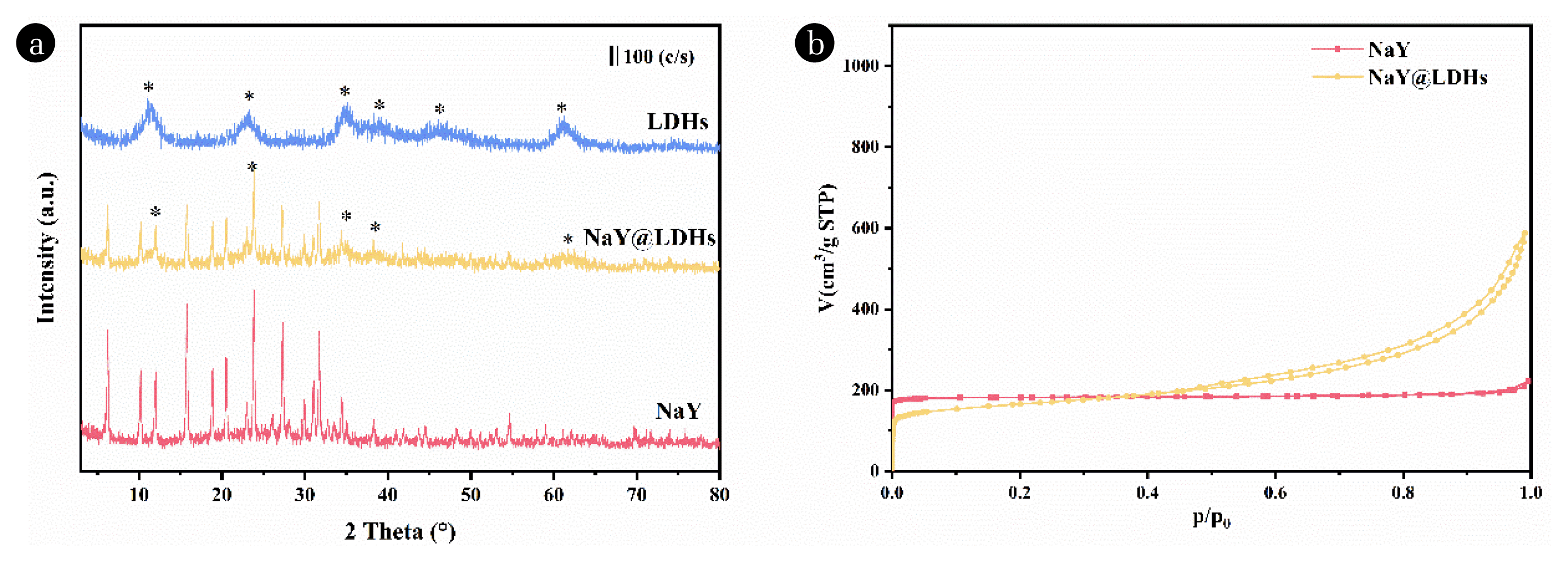
|
|
||||||||||||||||||||||||||||||||||||