AbstractFor efficient lithium recovery, the electrochemical lithium recovery (ELR) process that uses LiMn2O4 (LMO) electrodes with selectivity for lithium ions, has been introduced. The electrochemical system is environmentally friendly and allows for the recovery of lithium at a high yield, but the issue of manganese dissolution in LMO electrodes, decreasing their stability, remains to be solved. Herein, we suggest a solution to the existing problem through a rapid lithium recovery method that also enhances the stability of LMO electrodes through the state-of-charge (SoC) control approach. The retained discharge capacity of the system with a high current density (0.4 A/g) remains at 99.2% at 60% SoC after 300 cycles. Compared to the results under full charge/discharge operation (44.2% after 300 cycles), the proposed method demonstrates the state-of-charge (SoC) control adjustments at high current density levels to enhance the recovery rate and stability of the electrode. Additionally, high lithium-ion selectivity with a similar recovery rate is maintained at a high current density under 60% SoC operation compared to 100% SoC in lithium recovery tests. These results indicate that the SoC control strategy can increase the efficiency of ELR by improving the stability of the electrode under high-rate operational conditions.
Graphical Abstract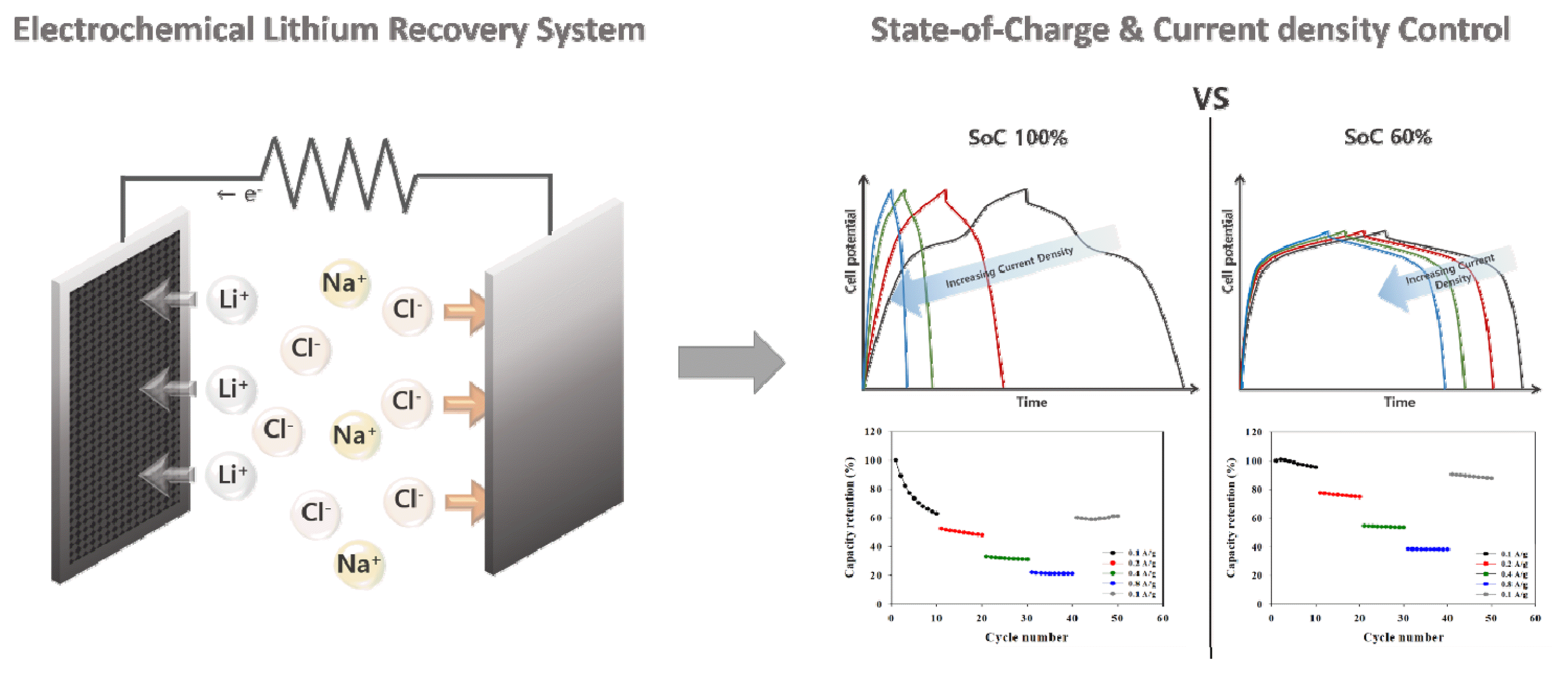
1. IntroductionAccording to a market survey on the demand for lithium, lithium usage in the battery industry has been increasing gradually [1–3]. At present, a sufficient supply of lithium is considered to be a major challenge due to the growth of the electric vehicle industry; hence, the economic value of lithium is increasing rapidly [4–8]. Currently, salt-lake brine, especially from South America, is considered a major source of lithium [9,10]. However, not only is lithium contained in such sources, but various other ions exist as well, such as sodium, potassium, calcium, and magnesium, typically at high concentrations, thus further complicating the extraction process of lithium ions and causing supply limitations [11].
In order to overcome the limitations and disadvantages of current lithium recovery methods, such as the limited reserves and interference by other ions, it is desirable to develop an efficient system for better lithium recovery from brine [12,13]. The conventional method for lithium recovery is the lime soda evaporation process, which involves concentrating raw brine through solar irradiation and wind and then obtaining lithium metal or a lithium compound through an additional process [14]. Although this method is relatively inexpensive, it is time-consuming and cannot be applied to all salt lakes due to environmental issues [15]. Moreover, the efficiency of lithium recovery depends greatly on the chemical composition of the brine [16]. Additionally, water shortages have been observed in Chile due to the high-water consumption that takes place during the evaporation process [11,17,18]. As a result, alternative lithium recovery processes such as precipitation, ion-exchange, adsorption, solvent extraction, membrane processes, and electrochemical methods have been considered [19–28]. Among these, electrochemical methods have been developed to supplement existing lithium recovery processes [29–33].
An electrochemical lithium recovery (ELR) system can produce electrodes with selectivity for lithium, allowing for the extraction of solutions in which lithium ions are captured selectively from source water [34]. This method utilizes the electrochemical ion exchange process, which is environmentally friendly and efficient, and enables the recovery of lithium even in cases of low lithium concentrations in salt lakes [12]. Recent studies have reported the recovery of lithium at high selectivity levels using λ-MnO2 cathodes and Ag/AgCl anodes in aqueous electrolyte systems [35]. LiMn2O4 (lithium manganese oxide, LMO) is an inexpensive material with high lithium selectivity [36]. However, LMO electrodes have low stability, leading to rapid damage to the electrode due to manganese dissolution during repeated charging and discharging cycles [12,37]. These characteristics are particularly problematic when the lithium supply rate is crucial, and they reduce the efficiency of LMO electrodes [14,38–40]. To overcome these limitations, it is necessary to focus on improving the stability of LMO electrodes while maintaining their efficient lithium recovery rates.
To enhance the stability of LMO electrodes, our group reported a novel method to increase the stability of LMO electrodes by utilizing some of the capacity for lithium recovery, as opposed to using 100% of the capacity [37]. However, the stability of the system was demonstrated only under low-current-density operation (±0.1 A/g), and additional investigations are required to enhance the efficiency of the ELR system under rapid operation. Herein, we propose a method to ensure high selectivity, a high recovery rate, and good electrode stability by adjusting the state-of-charge (SoC) control during rapid operation of the ELR system in an effort to enhance the recovery rate of lithium (Fig. 1). This study investigates the lithium-ion and sodium-ion composition of Salar de Atacama, a salt lake in Chile [9,10]. The selectivity of lithium and the recovered concentration of lithium were calculated using a method that involves the setting of a high current density with SoC control. In addition, capacity changes were monitored through an electrochemical analysis.
2. Materials and Methods2.1. ReagentsTo produce the LMO positive electrode in this study, a slurry of 4 g was prepared by mixing LiMn2O4 powder, Super P as a conducting agent, and PTFE (wt%) as a binder at a weight ratio of 80:10:10. The slurry was spread thinly to a thickness of 200 μm using a heating roll press at a surface temperature of 60°C. The remaining solvent was removed from the electrode by placing it in a vacuum oven at 60°C for 12 hours. The resulting electrode was attached to a carbon sheet using carbon paste. The negative or counter electrode used in this study was an Ag electrode. It was prepared by mixing Ag, Super P as a conducting material, and PTFE as a binder at a ratio of 80:10:10, after which it underwent heating roll pressing process and a vacuum oven treatment, like the LMO electrode. Subsequently, the electrode was charged at 0.5 V for 15 minutes to realize a silver/silver chloride electrode.
2.2. Electrode AnalysisAn electrochemical assessment was carried out using a battery cycler (WBCS3000, WonAtech, Republic of Korea), with the fabricated electrode analyzed using cyclic voltammetry (CV) and galvanostatic charge-discharge (GCD) techniques. To assemble the electrochemical cell, a circular filter with a diameter of 19 mm was placed between a circular LMO electrode (9 mm) and an Ag electrode (18 mm). Electrode stability tests involved repeated charging and discharging cycles. An X-ray diffractometer (D-MAX2500, Rigaku, Japan) was used to measure the X-ray diffraction patterns of the electrodes.
2.3. Lithium Recovery TestsFor the lithium recovery tests, the LMO and Ag electrodes are attached to a titanium plate (3.0 cm × 3.0 cm) using carbon paste with a gap (1.0 cm) between the two electrodes. The lithium recovery cell has a structure identical to that reported in our previous study, and the recovery process consists of two steps. First, the cell is immersed in the target solution (25 mL), which contains 30 mM each of LiCl, NaCl, MgCl2, KCl, and CaCl2, and is discharged by current density: 0.5 mA/cm2 (0.011 A/g), 0.9 mA/cm2 (0.020 A/g), and 1.3 mA/cm2 (0.029 A/g) at different SoC levels, respectively. As a pre-treatment, the LMO electrode was charged in 30 mM KCl solution at a voltage range of 0 ~ 1.0 V for 100% SoC condition. In the case of the SoC 60%, the operation voltage range (0 ~ 0.802 V) was set that approximately 60% of the capacity was used compared with the capacity of 100% SoC data, as performed in our previous study [37]. After this discharge process, the cell is immersed in a reservoir solution (30 mM KCl) and the system is then charged to delithiate the lithium from the LMO electrode. The concentrations of each of the ions were analyzed by means of ion chromatography (IC).
2.4. Calculations and Analytical MethodsThe stability performance of the electrodes during charging and discharging are determined through changes in the specific capacity (Q, mAh/g). The specific capacity was calculated as follows, using the test time, current density, and the active material weight value.
In this equation, I is the current (A), t is the test time (sec), m is the active material weight (g).
The capacity retention (%) can be determined according to the change in the capacity of the electrode calculated through the ratio of the capacity (Qn, mAh/g) of the corresponding cycle to the initial capacity (Qi, mAh/g), as shown below.
3. Results and DiscussionIn order to confirm the selective chemical reaction of lithium ions in the LMO electrodes, a cyclic voltammetry analysis (Fig. 2) was carried out with different solutions, which in this case were a NaCl-LiCl mixed solution (3.3 M NaCl, 0.21 M LiCl) with a composition similar to that of the "Salar de Atacama" source, as well as solutions containing NaCl (1.0 M) and LiCl (1.0 M). As shown in Fig. 2, the LMO electrode has high selectivity for lithium ions, as a chemical reaction is observed with lithium ions in both the mixed solution and the 1.0 M LiCl solution conditions, whereas no reaction with sodium ions occurs in the 1.0 M NaCl solution condition [41–45]. The peaks observed in the 1.0 M LiCl solution condition indicate the intercalation and de-intercalation of lithium ions due to a chemical reaction with the LMO electrode [39,40,46]. These results suggest that the LMO electrode has strong affinity towards lithium ions and that it can effectively intercalate and de-intercalate lithium ions, as shown in our previous studies [30,31,37].
Figs. 3(a) and (b) show the results of GCD curves under different current conditions with state-of-charge (SoC) conditions, using a mixed solution of 3.3 M NaCl and 0.21 M LiCl. The current conditions were changed to 0.1, 0.2, 0.4, 0.8, and 0.1 A/g every 10 cycles, and the SoC level (100% and 60%) was set by a voltage cut operation. As shown in the GCD data, the capacity at the 100% SoC level operation (using the full capacity of the LMO electrode) decreased dramatically during ten cycles, whereas the capacity remained relatively constant with less of a capacity reduction under a higher current density at the 60% SoC level.
Fig. 3(c) and Fig. 3(d) show the capacity retention rate at the time of discharge for each cycling condition. The capacity retention rates after ten cycles of charge and discharge at the first 0.1 A g−1 in the 100% SoC and 60% SoC cases were 62.9% and 95.4%, respectively. At a relatively high current density (0.8 A/g), the capacity retention rates after 40 cycles compared to 31 cycles under 100% SoC and 60% SoC were correspondingly 95.9% and 99.2%. Overall, the cyclic performance at various current densities under 60% SoC control exhibits better rate behavior with excellent stability compared to 100% SoC operation.
To verify the long-term stability of LMO at a high current density, 300 charge/discharge cycles were conducted under a rapid operation condition (0.4 A/g). Fig. 4 shows the charge/discharge curves and capacity retention rates during operation at 100% and 60% SoC. As shown in Figs. 4(a) and (c), the capacity decreases dramatically during 30 cycles, decreasing to 44.2% after 300 cycles while under the 100% charge and discharge condition. In contrast, the capacity at 60% SoC exhibits a similar charge/discharge profile when cycling (Fig. 4(b)), and the discharge capacity remains at 99.2% after 300 cycles. These results suggest that the stability of the electrode under a high current density increases significantly when only a part of the capacity (60% SoC) is used compared to the total capacity (100% SoC) of the electrode.
An XRD analysis was conducted to confirm the changes in the crystal structure of the electrode when cycled under the 60% and 100% SoC conditions. The LiMn2O4 XRD diffraction showed characteristic spinel structure peaks of [111], [311], [222], [400], [331], [511], [440], and [531], which were indexed to the Fd-3m (227) space group (JCPS file #35-0782). The XRD patterns of the pristine electrode and the electrode cycled under 60% SoC did not show significant changes in the peak positions or intensities, indicating that the crystal structure remained stable after cycling. However, the XRD patterns of the electrode cycled under 100% SoC showed peak shifts compared to the pristine electrode, indicating a change in the crystal structure. Specifically, the changes of the 440 and 511 peaks are evidence of a structural change of the LMO electrode, as reported in previous studies [37,47], and the peak shifts were more significant in the electrode cycled under 100% SoC, indicating that 60% SoC operation improved the structural stability of the electrode. Overall, the XRD analysis confirmed that cycling under a 60% SoC condition results in better structural stability of the electrode.
Figs. 6(a) and (b) show the concentration changes of the source water when the SoC level is adjusted at a high current density of 1.3 mA/cm2 (0.029 A/g). As shown in the figure, with regard to the concentration changes of the cations, both results exhibit high lithium-ion selectivity, proving that the SoC level does not affect the selectivity of lithium. Despite the fact that the lithium recovery rate at 60% SoC is lower (15.6%) than that in the 100% SoC case (23.1%), it is clear that the 60% SoC control scheme significantly enhances the stability of the ELR process, taking into account the results of the cycling tests (Figs. 3 and 4). To confirm the relationship between the lithium recovery rate and the current density, additional lithium recovery tests were conducted at different current densities (Fig. 3(c)). At a low current density of 0.5 mA/cm2 (0.011 A/g), the recovery rates averaged 60.4% and 37.1% at SoC levels of 100% and 60%, respectively. However, the difference in the recovery rate is reduced at a higher current density, indicating that the gap in the lithium recovery rates between the 100% and 60% SoC cases is narrowing. Therefore, the 60% SoC control scheme is shown to be an efficient method for ELR with a high current density.
4. ConclusionThis study reports a method to ensure efficient lithium recovery and good electrode stability when using a LiMn2O4 positive electrode and an Ag/AgCl negative electrode under various current density conditions in an ELR system and proposes a technique to increase the rate of the lithium supply while maintaining high recovery efficiency by means of a SoC control scheme. The results of cycling performance tests indicate that the ELR process exhibits better rate performance and stability under 60% SoC compared to 100% SoC operation. Additionally, the capacity retention rate improves as the current density increases. Furthermore, the study demonstrates that a high recovery rate with excellent lithium-ion selectivity is maintained even at a high current density upon control of the SoC level. In conclusion, the proposed technique can improve the ELR system by increasing the lithium recovery rate and enhancing the electrode stability.
AcknowledgmentsThis work was supported by was supported by Samsung Electronics Co., Ltd (IO221220-04278-01), Republic of Korea; 2023 Hongik University Innovation Support Program Fund, Republic of Korea; 2023 Hongik University Research Fund, Republic of Korea.
NotesReferences1. Martin G, Rentsch L, Höck M, Bertau M. Lithium market research – global supply, future demand and price development. Energy Storage Mater. 2017;6:171–179.
https://doi.org/10.1016/j.ensm.2016.11.004
2. Olivetti EA, Ceder G, Gaustad GG, Fu X. Lithium-ion battery supply chain considerations: Analysis of potential bottlenecks in critical metals. Joule. 2017;1(2)229–243.
https://doi.org/10.1016/j.joule.2017.08.019
3. Wanger TC. The lithium future-resources, recycling, and the environment. Conserv. Lett. 2011;4(3)202–206.
https://doi.org/10.1111/j.1755-263X.2011.00166.x
4. Cui J, Tan Q, Liu L, Li J. Environmental benefit assessment of second-life use of electric vehicle lithium-ion batteries in multiple scenarios considering performance degradation and economic value. Environ. Sci. Technol. 2023;57(23)8559–8567.
https://doi.org/10.1021/acs.est.3c00506
5. Brückner L, Frank J, Elwert T. Industrial recycling of lithium-ion Batteries—A critical review of metallurgical process routes. Metals. 2020;10(8)1107.
https://doi.org/10.3390/met10081107
6. Wrålsen B, Prieto-Sandoval V, Mejia-Villa A, O'Born R, Hellström M, Faessler B. Circular business models for lithium-ion batteries - stakeholders, barriers, and drivers. J. Clean. Prod. 2021;317:128393.
https://doi.org/10.1016/j.jclepro.2021.128393
7. Liu Z, Wei B, Wang L. Polyaniline-derived nitrogen-doped carbon/MoS2 nanocomposites as cathode for efficient hybrid capacitive deionization. Environ. Eng. Res. 2024;29(2)230204.
https://doi.org/10.4491/eer.2023.204
8. Kim S, Yoon H, Min T, Han B, Lim S, Park J. Carbon dioxide utilization in lithium carbonate precipitation: A short review. Environ. Eng. Res. 2024;29(3)230553.
https://doi.org/10.4491/eer.2023.553
9. Hamzaoui AH, M'nif A, Hammi H, Rokbani R. Contribution to the lithium recovery from brine. Desalination. 2003;158(1)221–224.
https://doi.org/10.1016/S0011-9164(03)00455-7
10. Zolfani SH, Bazrafshan R, Ecer F, Karamaşa Ç. The suitability-feasibility-acceptability strategy integrated with bayesian BWM-MARCOS methods to determine the optimal lithium battery plant located in south america. Mathematics. 2022;10(14)2401.
https://doi.org/10.3390/math10142401
11. Tahil W. The trouble with lithium Implications of Future PHEV Production for Lithium Demand. Meridian Int. Res. 2007;
12. Lee J, Yu S, Kim C, Sung Y, Yoon J. Highly selective lithium recovery from brine using a λ-MnO2–Ag battery. Phys. Chem. Chem. Phys. 2013;15(20)7690–7695.
https://doi.org/10.1039/c3cp50919b
13. Wang H, Chen S, Fu C, et al. Recent advances in conversion-type electrode materials for post lithium-ion batteries. ACS Mater. Lett. 2021;3(7)956–977.
https://doi.org/10.1021/acsmaterialslett.1c00043
14. Xu X, Chen Y, Wan P, et al. Extraction of lithium with functionalized lithium ion-sieves. Prog. Mater. Sci. 2016;84:276–313.
https://doi.org/10.1016/j.pmatsci.2016.09.004
15. Kim N, Su X, Kim C. Electrochemical lithium recovery system through the simultaneous lithium enrichment via sustainable redox reaction. Chem. Eng. J. 2021;420(P2)127715.
https://doi.org/10.1016/j.cej.2020.127715
16. Wu L, Zhang C, Kim S, Hatton TA, Mo H, Waite TD. Lithium recovery using electrochemical technologies: Advances and challenges. Water Res. 2022;221:118822.
https://doi.org/10.1016/j.watres.2022.118822
17. Xu S, Song J, Bi Q, et al. Extraction of lithium from chinese salt-lake brines by membranes: Design and practice. J. Memb. Sci. 2021;635:119441.
https://doi.org/10.1016/j.memsci.2021.119441
18. Trócoli R, Erinmwingbovo C, Mantia FL. Optimized lithium recovery from brines by using an electrochemical Ion-Pumping process based on λ-MnO2 and nickel hexacyanoferrate. Chem. Electro. Chem. 2017;4(1)143–149.
https://doi.org/10.1002/celc.201600509
19. Yoon H, Lee J, Kim S, Yoon J. Review of concepts and applications of electrochemical ion separation (EIONS) process. Sep. Purif. Technol. 2019;215:190–207.
https://doi.org/10.1016/j.seppur.2018.12.071
20. Battistel A, Palagonia MS, Brogioli D, Mantia FL, Trócoli R. Electrochemical methods for lithium recovery: A comprehensive and critical review. Adv. Mater. 2020;32(23)1905440.
https://doi.org/10.1002/adma.201905440
21. Srimuk P, Su X, Yoon J, Aurbach D, Presser V. Charge-transfer materials for electrochemical water desalination, ion separation and the recovery of elements. Nat. Rev. Mater. 2020;5(7)517–538.
https://doi.org/10.1038/s41578-020-0193-1
22. Gamaethiralalage JG, Singh K, Sahin S, et al. Recent advances in ion selectivity with capacitive deionization. Energy. Environ. Sci. 2021;14(3)195–112.
https://doi.org/10.1039/d0ee03145c
23. Li X, Mo Y, Qing W, Shao S, Tang CY, Li J. Membrane-based technologies for lithium recovery from water lithium resources: A review. J. Memb. Sci. 2019;591:117317.
https://doi.org/10.1016/j.memsci.2019.117317
24. Zhang L, Li L, Rui H, et al. Lithium recovery from effluent of spent lithium battery recycling process using solvent extraction. J. Hazard. Mater. 2020;398:122840.
https://doi.org/10.1016/j.jhazmat.2020.122840
25. Cuong DV, Hou C. Nickel hexacyanoferrate incorporated with reduced graphene oxide for highly efficient intercalation desalination. Sep. Purif. Technol. 2022;295:121351.
https://doi.org/10.1016/j.seppur.2022.121351
26. Cuong DV, Wu P, Chen L, Hou C. Active MnO2/biochar composite for efficient as(III) removal: Insight into the mechanisms of redox transformation and adsorption. Water Res. 2021;188:116495.
https://doi.org/10.1016/j.watres.2020.116495
27. Zhao X, Yang S, Hou Y, et al. Recent progress on key materials and technical approaches for electrochemical lithium extraction processes. Desalination. 2023;546:116189.
https://doi.org/10.1016/j.desal.2022.116189
28. Biesheuvel PM, van der Wal A. Membrane capacitive deionization. J. Memb. Sci. 2010;346(2)256–262.
https://doi.org/10.1016/j.memsci.2009.09.043
29. Pasta M, Battistel A, Mantia FL. Batteries for lithium recovery from brines. Energy. Environ. Sci. 2012;5:9487–9491.
https://doi.org/10.1039/c2ee22977c
30. Kim S, Lee J, Kang JS, et al. Lithium recovery from brine using a λ-MnO2/activated carbon hybrid supercapacitor system. Chemosphere. 2015;125:50–56.
https://doi.org/10.1016/j.chemosphere.2015.01.024
31. Kim S, Kim J, Kim S, Lee J, Yoon J. Electrochemical lithium recovery and organic pollutant removal from industrial wastewater of a battery recycling plant. Environ. Sci. Water Res. Technol. 2018;4(2)175–182.
https://doi.org/10.1039/C7EW00454K
32. Kim S, Lee J, Kim S, Kim S, Yoon J. Electrochemical lithium recovery with a LiMn2O4–Zinc battery system using zinc as a negative electrode. Energy Technol. 2018;6(2)340–344.
https://doi.org/10.1002/ente.201700488
33. Yoon H, Min T, Lee J, Lee G, Jeon M, Kim A. Lithium-selective hybrid capacitive deionization system with a ag-coated carbon electrode and stop-flow operation. Environ. Sci. Water Res. Technol. 2023;9(2)5–57.
https://doi.org/10.1039/d2ew00791f
34. Zhao M, Ji Z, Zhang Y, et al. Study on lithium extraction from brines based on LiMn2O4/Li1-xMn2O4 by electrochemical method. Electrochim. Acta. 2017;252:350–361.
https://doi.org/10.1016/j.electacta.2017.08.178
35. Joo H, Lee J, Yoon J. Short review: Timeline of the electrochemical lithium recovery system using the spinel LiMn2O4 as a positive electrode. Energies. 2020;13(23)6235.
https://doi.org/10.3390/en13236235
36. Gao A, Sun Z, Li S, et al. Self-assembled layered lithium manganese oxide shows ultra-large adsorption capacity and high selectivity for lithium. Chem. Eng. J. 2023;471:144287.
https://doi.org/10.1016/j.cej.2023.144287
37. Kim B, Joo H, Lee J, Yoon J, Lee J. Enhancement of the cycling stability of an electrochemical lithium recovery system via state-of-charge (SoC) control. Desalination. 2023;553:116486.
https://doi.org/10.1016/j.desal.2023.116486
38. Xu P, Hong J, Qian X, et al. Materials for lithium recovery from salt lake brine. J. Mater. Sci. 2021;56(1)16–63.
https://doi.org/10.1007/s10853-020-05019-1
39. Ooi K, Miyai Y, Sakakihara J. Mechanism of lithium(1+) insertion in spinel-type manganese oxide. redox and ion-exchange reactions. Langmuir. 1991;7(6)1167–1171.
https://doi.org/10.1021/la00054a025
40. Feng Q, Miyai Y, Kanoh H, Ooi K. Lithium(1+) extraction/insertion with spinel-type lithium manganese oxides. characterization of redox-type and ion-exchange-type sites. Langmuir. 1992;8(7)1861–1867.
https://doi.org/10.1021/la00043a029
41. Feng Q, Miyai Y, Kanoh H, Ooi K. Lithium(1+) and magnesium( 2+) extraction and lithium(1+) insertion reactions with lithium magnesium manganese oxide (LiMg0.5Mn1.5O4) spinel in the aqueous phase. Chem. Mater. 1993;5(3)311–316.
https://doi.org/10.1021/cm00027a013
42. Kanoh H, Ooi K, Miyai Y, Katoh S. Selective electroinsertion of lithium ions into a platinum/.lambda.-manganese dioxide electrode in the aqueous phase. Langmuir. 1991;7(9)1841–1842.
https://doi.org/10.1021/la00057a002
43. Ammundsen B, Burns GR, Islam MS, Kanoh H, Rozière J. Lattice dynamics and vibrational spectra of lithium manganese oxides: A computer simulation and spectroscopic study. J. Phys. Chem. B. 1999;103(25)5175–5180.
https://doi.org/10.1021/jp984398l
44. Chitrakar R, Kanoh H, Makita Y, Miyai Y, Ooi K. Synthesis of spinel-type lithium antimony manganese oxides and their li+ extraction/ion insertion reactions. J. Mater. Chem. 2000;10(10)2325–2329.
https://doi.org/10.1039/b002465l
45. Zhang Q, Li S, Sun S, Yin X, Yu J. LiMn2O4 spinel direct synthesis and lithium ion selective adsorption. Chem. Eng. Sci. 2010;65(1)169–173.
https://doi.org/10.1016/j.ces.2009.06.045
46. Liu W, Kowal K, Farrington GC. Mechanism of the electrochemical insertion of lithium into LiMn2O4 spinels. J. Electrochem. Soc. 1998;145(2)459–465.
https://doi.org/10.1149/1.1838285
47. Qu Q, Fu L, Zhan X, et al. Porous LiMn2O4 as cathode material with high power and excellent cycling for aqueous rechargeable lithium batteries. Energy. Environ. Sci. 2011;4(10)3985–3990.
https://doi.org/10.1039/C0EE00673D
Fig. 1Schematic of the ELR system with state-of-charge (SoC) control adjustments under an increasing current density. 
Fig. 2Lithium selectivity of LMO electrodes by a solution method using cyclic voltammetry (scan rate: 0.5 mV/s) with an aqueous solution containing 1.0 M LiCl (blue dashed line), 3.3 M NaCl + 0.21 M LiCl (black solid line), and 1.0 M NaCl (grey dashed line). 
Fig. 3Galvanostatic charge/discharge curves recorded at different current densities at the (a) 100% SoC level and the (b) 60% SoC level, and the discharge capacity retention outcomes at different current densities at the (c) 100% SoC level and the (d) 60% SoC level. 
Fig. 4Electrochemical performance outcomes of two electrodes under galvanostatic charge/discharge according to different SoC levels with 0.4 A/g in a mixed solution of NaCl 3.3 M, and 0.21 M LiCl; charge/discharge curve at 300 cycles at the (a) 100% SoC level and the (b) 100% SoC level; and capacity retention during 300 cycles at the (c) 100% SoC level and the (d) 60% SoC level. 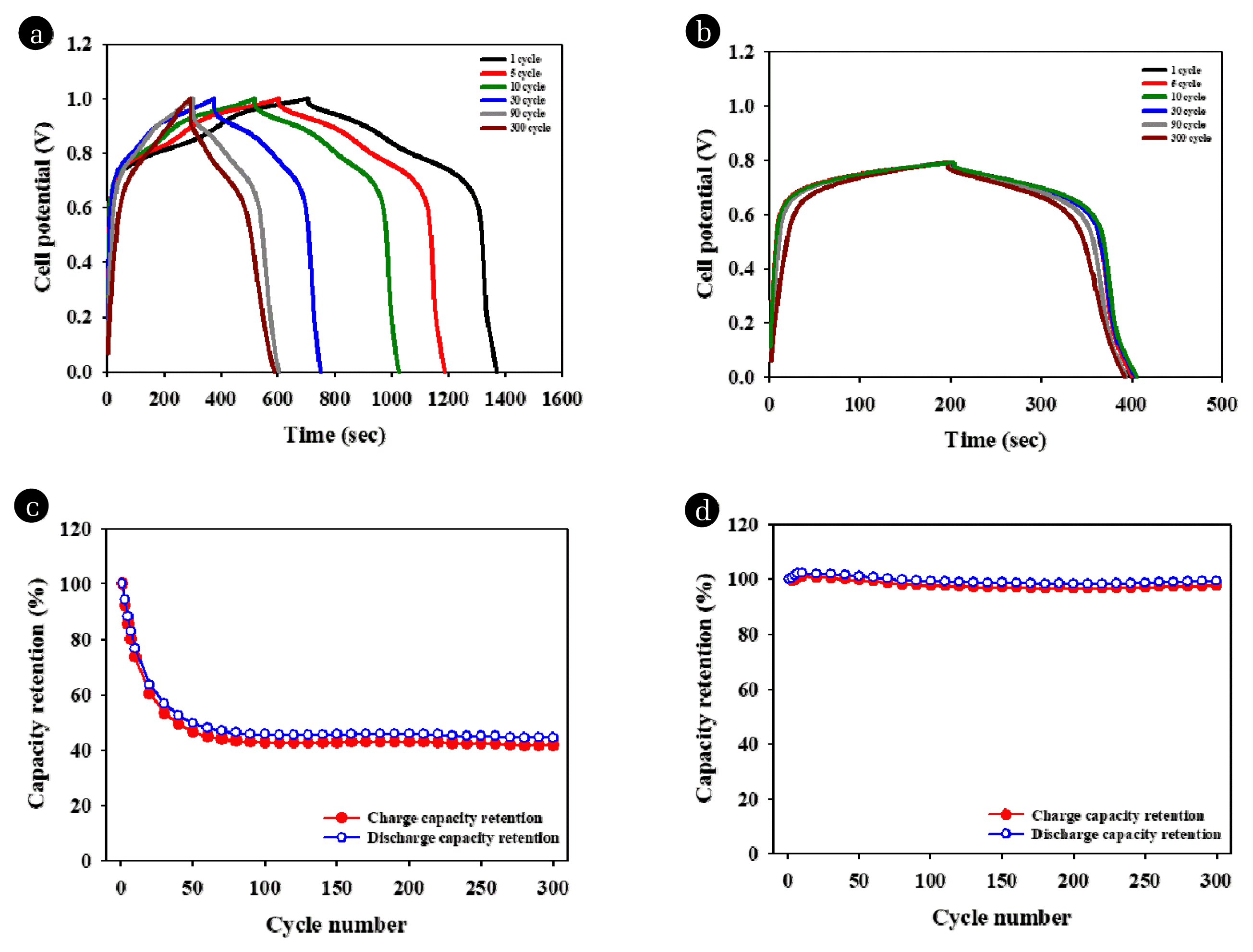
Fig. 5X-ray diffraction patterns of LiMn2O4 electrodes before and after 300 cycles under 60% and 100% SoC recorded using an X-ray diffractometer with a scan range (2θ) of 100 to 800 (cycling conditions referred to from Fig. 4). 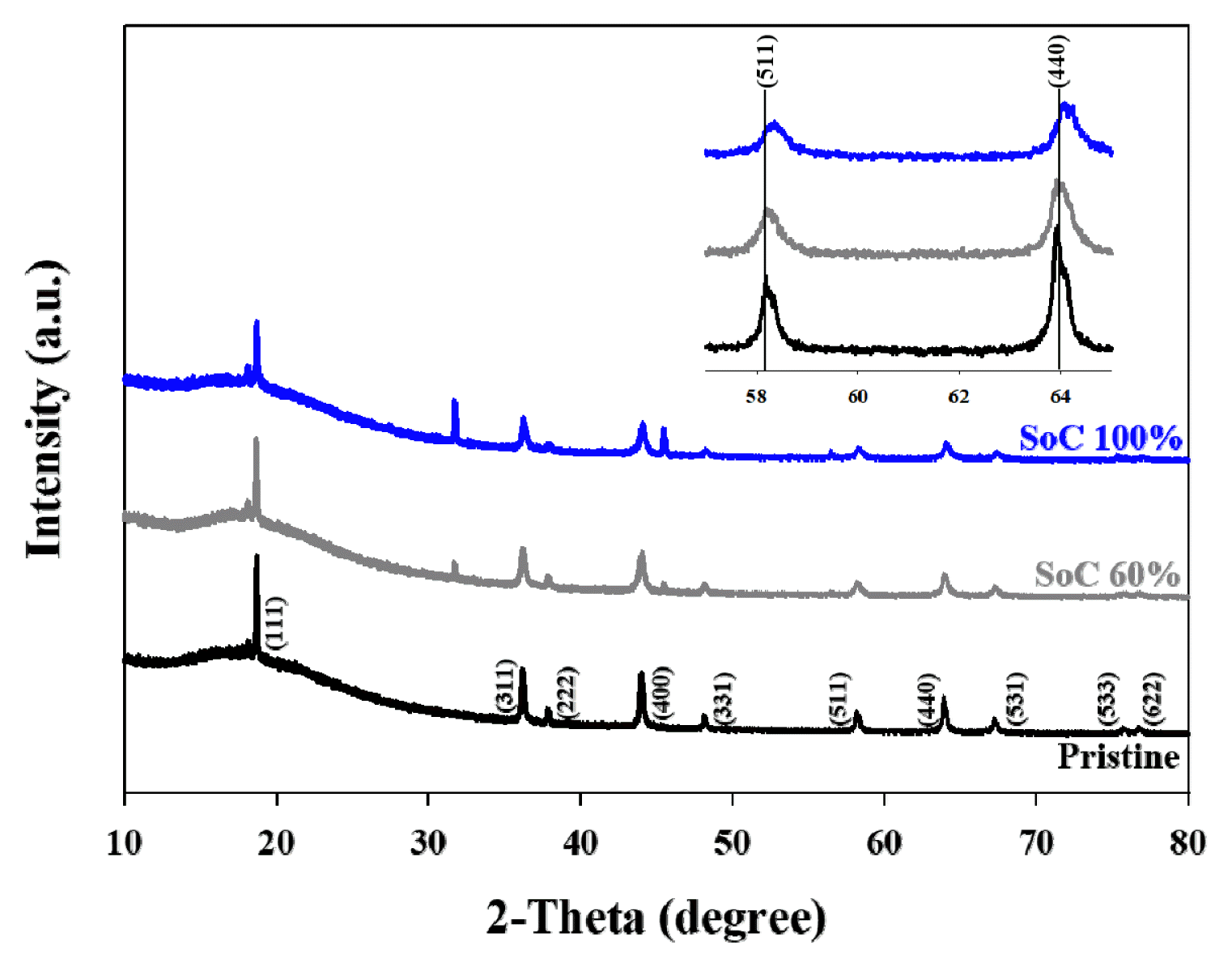
|
|
||||||||||||||||||||||||||||||||||