AbstractAccurate and real-time detection of pesticides is needed to ensure food safety and ecosystem protection. A biosensor was developed to determine organophosphate pesticides using acetylcholinesterase (AChE) and silver nanoparticle (AgNP) immobilized on the alginate-chitosan film matrix. The sensing principle is based on organophosphate pesticides (OP) inhibiting AChE activity. AChE hydrolyzes acetylthiocoline (ATCh) to thiocholine (TCh) and acetic acid (AA). The −SH group on TCh induces AgNP aggregation, resulting in fading of the film’s brownish-yellow color. The presence of OP in the sample inhibited AChE activity, leading to a decrease in TCh and color fading. The biosensor response is determined by measuring the Red Green Blue (RGB) value of the film. The biosensor offers rapid detection directly in-situ and real time without the use of sophisticated instruments, with optimal measurement conditions of pH 7, incubation time of 1 minute, and concentrations of AgNP, ATCh, and tris-HCl buffer of 10 μg/mL, 50, and 7 mM, respectively. Biosensors have excellent sensitivity, reproducibility, and selectivity. Measurement of OP levels in nine vegetable samples revealed that the proposed colorimetric probe’s results agreed well with the GC result as the reference method. Therefore, this method can be applied in monitoring food and environment OP levels.
Graphical Abstract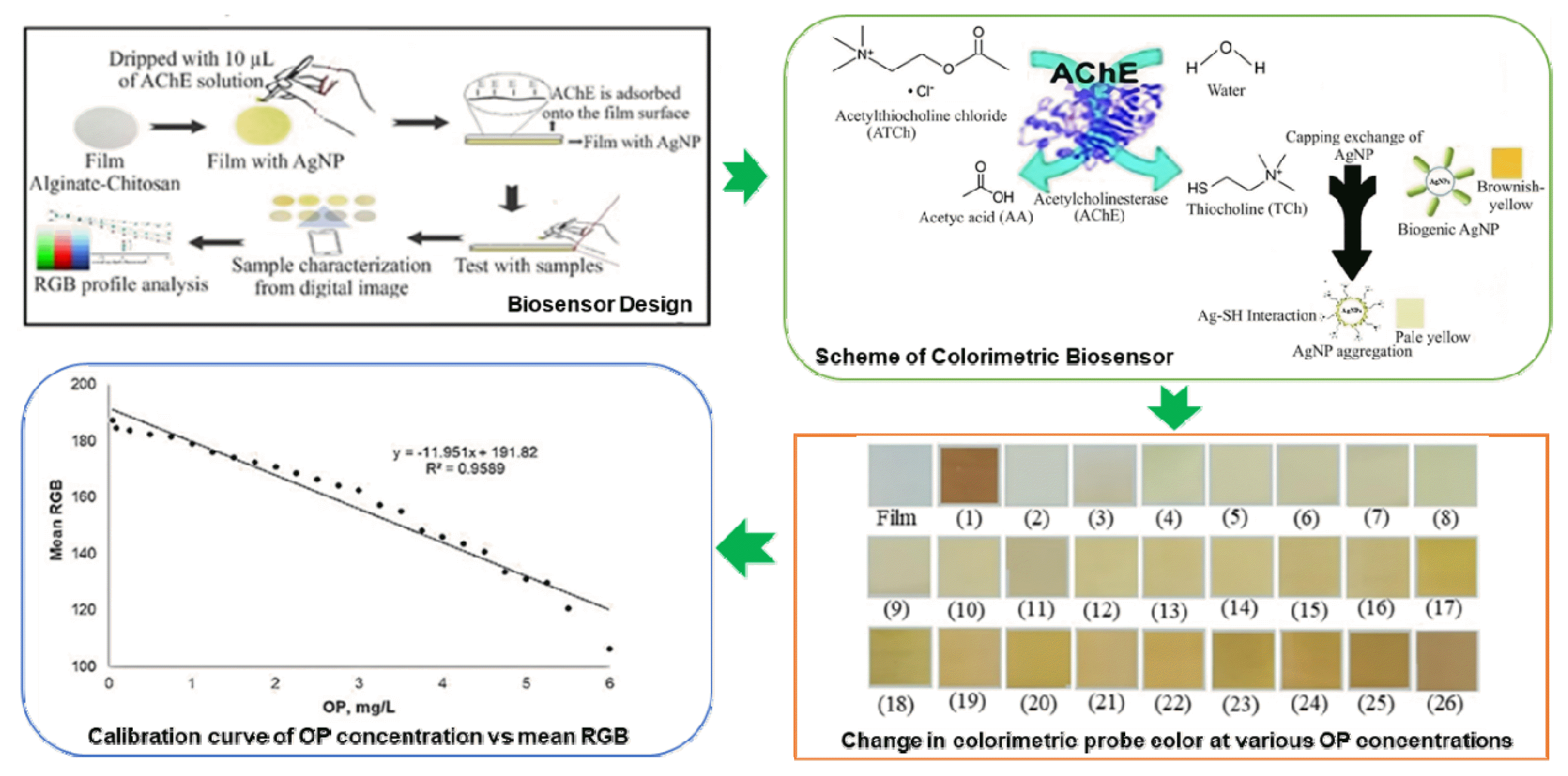
1. IntroductionApplying controlled pesticides is an approach of minimizing the harmful effects of pesticides. Pesticides are required to increase crop yield by effectively suppressing pests, but they can also be harmful to non-target organisms and the environment [1]. Pesticides’ persistent nature and mobility allow them to contaminate all human needs, including water, soil, air, and crops [2,3]. Therefore, pesticide detection is required to ensure consumer food safety and ecosystem protection.
Pesticide residues are commonly detected using gas chromatography (GC) or high-pressure liquid chromatography (HPLC) [4,5]. The weakness of the two analytical methods is the laboratory’s extraction and purification treatment, which requires solvents and a longer analysis time, increasing the risk of error. As an alternative technique, enzyme-based biosensors can be employed [6]. Biosensors use selective enzymes for substrates to analyze a pollutant quickly, reliably in small quantities, and efficiently. Although this method produces satisfactory results, it still relies on a high level of instrumentation automation as its transduction system; it requires laborious sample preparation steps, making it only suitable for laboratory-scale research.
Recently, a direct method for monitoring pesticide residues was developed using immobilized chromophores in a paper matrix. The previous research used 5,5′-dithiobis-(2-nitrobenzoic acid) as an indicator or sensor to quantify pesticides [7]. Metal-ligand complex compounds, such as cobalt hydroxide [CO(OH)2], have been utilized as a colorimetric agent for detecting glyphosate herbicide in aqueous solutions [8]. Most indicator or colorimetric sensors for pesticide quantitation use chemical reagent dyes or heavy metals. The release of these two reagents into the environment as waste creates new environmental and health issues. It is well recognized that heavy metals and synthetic dyes have a similar harmful effect on human health as pesticide residues. As an alternative colorimetric indicator, silver nanoparticles (AgNPs) can be used [9]. AgNPs are sensitive and selective as biosensing agents and are non-toxic and environmentally friendly [10].
AgNPs have unique optical properties, such as the SPR phenomenon, that provide color response and are sensitive to changes in the surrounding medium. This optical property is used to develop a visual biosensor for pesticide detection. The sensing principle of biosensors is based on organophosphate pesticides (OP) inhibiting acetylcholinesterase (AChE) activity [12]. AChE is an enzyme that hydrolyzes acetylcholine (ACh) substrates to yield choline (Ch) and acetic acid (AA). Using AgNPs as indicators in AChE inhibition-based biosensors is insufficient to rely solely on AChE activity. A second enzyme, choline oxidase (ChO), is required to catalyze the oxidation of Ch to betaine and hydrogen peroxide (H2O2). Finally, H2O2 spontaneously undergoes redox with AgNP, resulting in a color change due to Ag0 in AgNP converting to Ag+ ions [13,14]; which directly indicate the presence of OP [15]. However, in this study, only a single ChE enzyme was required for biosensing. In this biosensor, acetylthiocoline (ATCh) was used as a substrate while a film was used to support ChE enzyme immobilization in combination with AgNPs [16]. Single-film fabrication was used to visually detect the presence of OPs, and RGB value was used as the colorimetric biosensor. This biosensor allows OP monitoring to be performed directly in situ and in real time without requiring sophisticated laboratory instruments. Method validation was achieved by comparing the biosensor and GC results. The biosensor’s performance in determining OP in real samples was also tested.
2. Experimental Section2.1 ChemicalsThe biosensor was made using AChE from Electrophorus electricus (EC 3.1.1.7, VI-S type, 200 units/mL). The single film was prepared using sodium alginate from brown algae (300–400 centipoise, cP) and chitosan from crab shells (95% deacetylated). In addition, ATCh chloride (≥99%, TLC) was used as the substrate, and the profenofos (OP) was the analyte. All chemicals were also obtained from Sigma Aldrich (St. Louis, Missouri, USA) with analytical grade. Double distilled water was used to prepare the solutions.
2.2 Preparation of Biogenic AgNPThe biogenic AgNP was synthesized using electrolysis of green tea extract solution, as described by Hermanto et al. [17,18]. The anode and cathode are made of silver metal rods, and their polarity is switched every 1 minute by a controller. Both electrodes were immersed in green tea extract and connected to a 10 V DC voltage source. The formation of AgNPs was defined by a change in the color of the solution from greenish yellow to brownish yellow. The biogenic AgNP used as biosensor indicators in this study was pure, obtained by centrifugation at 12,000 rpm (MDX 310 Tomy Centrifuge, Japan) and freeze-drying (freeze-dryer Alpha 1–2LDplus with vacuum pump RZ 2.5, Germany). Separated biogenic AgNP is taken in 10 mg and redispersed in 1 mL of double distilled water; colloidal AgNP is used for further measurement.
2.3 Fabrication of Visual BiosensorThe film functioned as a support material for immobilizing AgNP and AChE. Single films were made by mixing alginate hydrosol and chitosan hydrosol, as done by Hermanto et al. [19]. In a petri dish, 1 μL of Tris-HCl buffer solution (pH 6.5) was added, and the mixture was stirred with a magnetic stirrer until homogeneous. The colloidal AgNP 10 μg/mL (10 μL) was added. The mixture was stirred for 10 seconds with a magnetic stirrer at 300 rpm, then transferred to a mold with a 1×1 cm2 dimension and stored at 4°C for 72 hours to dry. Single AgNP-immobilized films were then dripped with 10 μL of AChE solution (prepared with 200.0 units/mL AChE in 40 μL buffer solution pH 7.0) and aged overnight at 4°C. The entrapment technique for AgNPs and the adsorption technique for AChE were utilized in their specific immobilization processes to prevent AChE-AgNP reactions resulting from metal-induced enzyme inhibition. The effective immobilization of AChE and AgNP on the film was confirmed by Energy Dispersive Spectroscopy (EDS) analysis (JEOL-JEM, Japan). The single film produced by this fabrication is used for additional measurements.
2.4 Measurement ProcedureA series of OP standard solutions were prepared using 50 mM ATCh solution in Tris-HCl buffer solution pH 7.0 as a solvent, and measurements were performed on a single film produced by the previous method. The amount of OP present is proportional to AChE’s inhibition of the hydrolysis reaction. It changes the color of the AgNPs, and the biosensor response can be observed visually and displayed as a calibration curve between concentration vs. mean RGB. Color intensity changes are measured using RGB and ImageJ software version 1.49. Nine vegetables were obtained as real samples from local markets in Mataram, West Nusa Tenggara, Indonesia (onion, tomato, cayenne pepper, potato, carrot, eggplant, long beans, water spinach, and Chinese cabbage).
2.5 Determination by GC-MS MethodTo validate the method in the quantitation of OP, the GC method was used as a comparison [15,20]. The GCMS (QP210 Ultra, Shimadzu) used an RTX®-5MS column as the stationary phase and helium gas as the mobile phase, with 1 μL of sample injected using an autosampler at a temperature of 250°C. The peak chromatogram retention time was used to identify the OP. A series of standard solutions were injected, and the retention time was observed; then, a calibration curve was made by plotting the concentration vs the peak area of the chromatogram. The same parameters were used to inject samples, and the chromatogram area was observed, and concentration were calculated.
3. Results and Discussion3.1 Biosensor SchemeThe present study employs a colorimetric biosensor based on AChE inhibition and AgNPs as probes (Fig. 1), as described in the literature [21–23]. Immobilized AgNPs dispersed well in the film, resulting in a yellow-brown film. As shown in Fig. 1, elemental analysis was performed to confirm the presence of AgNPs and AChE in the films. There is a significant amount of new elements, such as chlorine and sodium, which are components of the AChE skeleton that are adsorbed on the film surface. Furthermore, the decrease in Ag percentage and the increase in silicon and oxygen weight percentages (due to AChE’s free hydroxyl groups) provide additional evidence of the presence of enzymes on the film surface [24].
The substrate for this enzymatic reaction is ATCh, which is positively charged and tends to adsorb onto the surface of the negatively charged AgNPs. In this case, it can be prevented by trapping AgNPs in the film and positioning AChE on its surface, allowing ATCh to interact with AChE first. ATCh undergoes hydrolysis due to the enzymatic activity of AChE, producing TCh and AA [25]. The released TCh is positively charged and has an additional group (thiol group, −SH) [16]. TCh with such characteristics is more likely to replace the biogenic role of capping agent in AgNPs. The powerful Ag-SH interaction, which occurs readily on the surface of AgNP, plays a role in the capping exchange of AgNP. As a result, AgNP aggregation occurred; it was accompanied by a change of AgNP plasmonics as well as its color from brownish yellow to pale yellow. The OP reduces TCh formation by inhibiting the enzymatic activity of AChE. Increasing the OP concentration decreased AgNP aggregation and, as a result, the discoloration of AgNPs in the film.
Based on the above scheme (Fig. 2), it will be easier to fabricate an analytical device that is easy, simple, fast, and low cost while still having high sensitivity in quantifying OP based on AChE inhibition and plasmonic AgNP. More research on the colorimetry of biosensors for the quantitation of OPs in real samples is required. As a result, determining the performance of these biosensors is both an exciting challenge and a great opportunity for advancement.
3.2 Optimization of Colorimetric BiosensorBefore determining the colorimetric biosensor’s character, several parameters that affect the biosensor’s performance must be optimized, as shown in Table 1. Based on several colloidal AgNPs concentrations tested, a concentration of 10 μg/mL could cover the entire detection area (Table 1). It is done to achieve an even distribution of color in each section of a single film, resulting in a uniform response in the detection area.
The next optimization step is to determine the optimal substrate concentration (ATCh). At the optimum concentration, the amount of substrate that occupies the active site of the enzyme is saturated, so that adding substrate does not increase the reaction rate (steady state). Sensor response increased at ATCh concentrations ranging from 10 to 50 mM, indicating an increase in the rate of enzymatic reactions. However, the sensor response was nearly the same at concentrations of 50 and 100 mM. Since there was saturation of ATCh to AChE at this concentration, the ATCh concentration of 50 mM was chosen for this study (Table 1) and used for further measurements.
The effects of pH and buffer concentration on enzymatic reactions have also been studied in this study. As in the literature, phosphate-containing solutions are strictly avoided in this biosensor to prevent competition between solutions with analyte targets that both contain phosphate groups. As a result, this biosensor employs a tris HCl buffer solution. A series of pH and buffer concentration investigations produced the optimum at pH 7 and concentration at 7 mM of tris HCl buffer solution, which was used for further measurements. As soon as ATCh hydrolyzes, a TCh product is formed, which can change the color of a single film. The film functions as a matrix for immobilizing AgNPs (entrapment) and AChE (adsorption). Then, by diffusion and penetration to the film, TCh interacts with the AgNPs as a colorimetric biosensor probe. Plasmonic AgNPs changed the film’s color, and the biosensor response was determined as the RGB value of the film. Incubation time is needed to convince that the AChE catalytic activity and the plasmonic AgNP have been completed, inducing a change in the color of the AgNPs in the film from brownish yellow to pale yellow, about 1 minute in this study.
3.3 The Response of The Colorimetric BiosensorThe biosensor was developed by immobilizing 10 μL of colloidal AgNP and 10 μL of AChE solution on a single film surface. The test solution was prepared by mixing 2.5 μL of the Tris HCl buffer pH 7.0 and 2.5 μL of 50 mM ATCh. The test solution (5 μL) was dripped onto the surface of a single film with a response time of 1 minute. Reduced color fading indicates the biosensor’s response to AChE catalytic activity inhibitors. Due to AgNP aggregation by TCh, the enzyme-substrate reaction produced a pale-yellow color. In contrast, after OP inhibition, the film’s color changed to a brownish-yellow color that faded in comparison to the color of the initial AgNP-immobilized film. Fig. 3 depicts the colorimetric biosensor used in OP quantitation. The color change of the colorimetric probe at various OP concentrations can be observed with the naked eye, allowing for quick and reliable analysis of pollutants in small quantities and with simple operation.
The calibration curve is plotting by evaluating the colorimetric probe against the OP concentration. A linear curve was obtained within the OP concentration interval of 0.05 to 6.0 mg/L, as shown in Fig. 3. A linear correlation was obtained by plotting the colorimetric response using the mean RGB vs OP concentration, resulting in a correlation coefficient (r) of 0.98. The colorimetric probe has an OP detection range of 0.05 to 6.0 mg/L. At higher OP concentrations, browning of the film on the colorimetric probe was observed, indicating that AgNP aggregation was reduced. The limit of detection (LOD, 3σ) is 0.04 mg/L OP. Table 2 compares the linear range and LOD of various sensors and colorimetric biosensors for the determination of profenofos. The LOD of the proposed colorimetric biosensor is lower than that of previous methods. Even the commonly used HPLC reference method in laboratories for determining OP has a detection limit of 0.104 mg/L [31]. In small quantities, the colorimetric probe is better and more reliable because it detects the maximum OP level (0.05 mg/kg) permitted by the Ministers of Health and Agriculture of the Republic of Indonesia [32] and SNI [33]. The LOD value is sensitive enough to detect profenofos exposure at human-harmful concentrations. Another advantage of the proposed colorimetric probe is that it can analyze OP quickly and easily, allowing for in-situ and real-time analysis. It is also relatively inexpensive, with production costs of less than $5.
The proposed colorimetric probes in this study provide better biosensor performance with a lower detection limit of 0.04 mg/L of OP. The developed colorimetric probe only uses AChE but still exceeds previous studies that used AChE/ChO in terms of working range. Using a single enzyme also has the advantage of lowering production costs. Another cost advantage due to the easy operation of the biosensor, the colorimetric probe can be observed directly with the naked eye.
The use of highly sensitive indicators, such as AgNPs, contributes to the proposed biosensor’s excellence. The slope of the curve indicates sensor sensitivity, or the sensor’s ability to detect changes in response per unit concentration of analyte; the larger the indicated value, the higher the sensor’s sensitivity. The colorimetric probe has a slope of 11.951 a.u L/mg, a greater value than previous studies of 0.0331 and 0.0085 a.u L/mg, which used bromothymol blue and poly(sulfobetaine methacrylate) as indicators [12,25]. Moreover, AgNP offers good accuracy and long-term stability [17].
The coefficient of variation (CV) in measuring the biosensor response at an OP concentration of 1.00 mg/L with three repetitions on different films is used to express biosensor reproducibility. The reproducibility of the colorimetric probe calculated from the CV was 0.17% (Fig. 4a), indicating good reproducibility. The colorimetry probe was kept at 4°C when not in use to keep its activity. Testing of colorimetric probes for 1 mg/L of OP quantitation during storage can be observed in Fig. 4b. The results show that the colorimetric probe can still be used for up to 10 days of storage. At 10 days of storage, the mean RGB colorimetric probe showed an intensity equal to 1.25 mg/L. AgNP aggregation may be caused by oxidation by the surrounding air or interaction with moisture during storage. As a result, this colorimetric probe can be stored at 4°C for 10 days and still produce good results.
The selectivity of the colorimetric probe was determined by adding some of the possible interfering substances found in vegetable samples at a concentration of 1 mg/L [34], such as starch, quercetin, phosphate, and Hg(II). The selectivity of colorimetric probes for this purpose was investigated using a separate solution method, in which the interference solution and OP were investigated for sensor response using a colorimetric probe at concentrations of 1 mg/L each. Fig. 5a shows that the interference values are Hg(II)> phosphate> quercetin> starch. Phosphate, quercetin, and starch did not interfere significantly. Hg(II) ions, on the other hand, cause relatively large interference due to their strong inhibitory effect on AChE activity. Furthermore, AgNP undergoes an oxidation reaction to become Ag+ in the presence of Hg(II) ions since the standard reduction potential for Ag+ is lower than that of Hg(II) [35]. As shown in Fig. 5b, measurements of the localized surface plasmon resonances (LSPRs) intensity from AgNPs before and after the addition of Hg(II) ions at varied concentrations of 0 to 1.5 mg/L provide evidence to support this explanation. The addition of Hg(II) decreased the intensity of the AgNP LSPR, indicating a decrease in AgNP concentration due to Ag oxidation to Ag+.
For this reason, the presence of Hg(II) in the sample as an interfering agent should be reduced. First, masking Hg(II) with ethylenediaminetetraacetic acid (EDTA) can be used for this case. EDTA is a chelating agent capable of binding metal ions via covalent coordinates to form multidentate ligands [36,37]. Thus, the interference effect of Hg(II) on the colorimetric probe in the OP quantization can be reduced, as shown in Fig. 5a.
Analytical method validation is the process of testing the performance characteristics of the analytical method through a series of laboratory tests to ensure accurate and reliable measurement results so that it can be trusted. The validation of colorimetric probes using GC as the standard method for determining OP in sample. Then, the measurement results of the GC and biosensor methods are compared. Here, the sample was spiked with OP at a 1 mg/L concentration. The results of OP quantitation in vegetable samples are summarized in Table 3. Table 3 shows the measured OP sample concentration values corrected by adding a standard OP solution. The samples tested were vegetables obtained from local markets in Mataram, Indonesia, and the results obtained showed that some were detected to contain OP. However, the level is below the threshold set by the authorities of the Republic of Indonesia, making it safe for consumption. The colorimetric probe developed produced OP quantitation results in vegetable samples that were not significantly different from the GC reference method. GC is a widely used and official AOAC method for quantifying real sample OPs. Statistical analysis (t-test) revealed no statistically significant difference between the two methods. The results of the biosensor measurements agreed with the results from the GC. Therefore, a colorimetric performance biosensor based on plasmonic AChE and AgNP inhibition can be widely utilized for measuring OP levels in real samples.
4. ConclusionsA visual biosensor for directly detecting pesticides was successfully designed using AChE and AgNPs as colorimetric probes. Its sensing principle is based on the inhibition of AChE activity by OP. As biosensor indicators, AgNPs with a distinct color that changes due to environmental influences are immobilized on alginate-chitosan films. AChE was also adsorbed on the film surface, allowing the single film to serve as a probe for colorimetry. As a sensing agent, AChE catalyzes the hydrolysis of ATCh substrates to TCh, which replaces the AgNP capping, causing AgNP aggregation and film fading. AChE activity was inhibited by OP analytes, which reduced AgNP aggregation and film color fading. The RGB values were plotted against the OP concentrations to create a calibration curve. The biosensor performed best at pH 7, incubation time of 1 minute, and concentrations of AgNP, TCh, and buffer of 10 μg/mL, 50, and 7 mM, respectively. The biosensor’s analytical properties are excellent, with a linear range of 0.05–6.00 mg/L, LOD of 0.04 mg/L, reproducibility (CV) of 0.17%, and probe stability of up to 10 days. Using AgNPs and AChE as calorimetric probes improves the biosensor’s sensitivity and selectivity. Method validation on several vegetable samples using the standard GC method revealed that both measurement results were in line. As a result, this biosensor is appropriate for on-site and real-time OP measurements.
AcknowledgmentsThis study was supported by the National Competitive Basic Research Program funded by the Kementerian Pendidikan, Kebudayaan, Riset dan Teknologi Republik Indonesia (Kemendikbudristek RI) [decree number: 2349/UN18.L1/PP/2023].
NotesAuthor Contribution D.H. (Dr.) performed the study conceptualization, methodology and writing-original draft; N.I. (M.Sc.) and S.H (Ph.D.) perfomed the validation, data curation and the study supervision; S.R.K. (Ph.D.) has done the study investigation, methodology and visualization; R.W. (Dr.) worked on the study software, resources and methodology; H.M. (M.Sc.) investigated the formal analysis and project administration. All authors contributed to the study writing, review and editing. References1. Pathak VM, Verma VK, Rawat BS, et al. Current status of pesticide effects on environment, human health and it’s eco-friendly management as bioremediation: A comprehensive review. Front. Microbiol. 2022;13:1–29.
https://doi.org/10.3389/fmicb.2022.962619
2. Rajmohan KS, Chandrasekaran R, Varjani S. A review on occurrence of pesticides in environment and current technologies for their remediation and management. Indian J. Microbiol. 2020;60(2)125–138.
https://doi.org/10.1007/s12088-019-00841-x
3. Chuluun B, Iamchaturapatr J, Rhee JS. Phytoremediation of organophosphorus and organochlorine pesticides by acorus gramineus. Environ. Eng. Res. 2009;14(4)226–236.
https://doi.org/10.4491/eer.2009.14.4.226
4. Kang SM, Won JH, Han JE, Kim JH, Kim KH, Sung SH. Chromatographic method for monitoring of pesticide residues and risk assessment for herbal decoctions used in traditional Korean medicine clinics. Molecules. 2023. 28(8)(3343)1–9.
https://doi.org/10.3390/molecules28083343
5. Liang HC, Bilon N, Hay MT. Analytical methods for pesticide residues. Water Environ. Res. 2013;85(10)2114–2138.
https://doi.org/10.2175/106143013x13698672323227
6. Kuswandi B, Mascini M. Enzyme inhibition based biosensors for environmental monitoring. Curr. Enzym. Inhib. 2005;1(3)207–221.
https://doi.org/10.2174/157340805774580484
7. Kavruk M, Özalp VC, Öktem HA. Portable bioactive paper-based sensor for quantification of pesticides. J. Anal. Methods Chem. 2013;2013(932946)1–8.
https://doi.org/10.1155/2013/932946
8. Da Silva CR, Neri LCM, De Souza CTA, De Oliveira BJR, Bianchi RF, Santos IJB. Colorimetric nanosensor for the determination of glyphosate herbicide in aqueous solutions. Biointerface Res Appl. Chem. 2023;13(1)1–17.
https://doi.org/10.33263/BRIAC131.073
9. Prosposito P, Burratti L, Venditti I. Silver nanoparticles as colorimetric sensors for water pollutants. Chemosensors. 2020;8(2)1–29.
https://doi.org/10.3390/CHEMOSENSORS8020026
10. Loiseau A, Asila V, Boitel-Aullen G, Lam M, Salmain M, Boujday S. Silver-based plasmonic nanoparticles for and their use in biosensing. Biosensors. 2019;9(2)1–39.
https://doi.org/10.3390/bios9020078
11. Kaur J, Singh PK. Enzyme-based optical biosensors for organophosphate class of pesticide detection. Phys. Chem. Chem Phys. 2020;22(27)15105–15119.
https://doi.org/10.1039/d0cp01647k
12. Kuswandi B, Fikriyah CI, Gani AA. An optical fiber biosensor for chlorpyrifos using a single sol-gel film containing acetylcholinesterase and bromothymol blue. Talanta. 2008;74(4)613–618.
https://doi.org/10.1016/j.talanta.2007.06.042
13. Sequeira CA. Silver nanoparticles for hydrogen peroxide sensors. Biomed. J. Sci. Tech. Res. 2021;40(4)32438–32443.
https://doi.org/10.26717/bjstr.2021.40.006481
14. Tagad CK, Kim HU, Aiyer RC, et al. A sensitive hydrogen peroxide optical sensor based on polysaccharide stabilized silver nanoparticles. RSC Adv. 2013;45(3)22940–22949.
https://doi.org/10.1039/C3RA44547J
15. Hermanto D, Ismillayli N, Muliasari H, Wirawan R, Kamali SR. Silver-based plasmonic nanoparticles for biosensor organophosphate pesticides using a single film containing acetylcholinesterase/choline oxidase. Glob. J. Environ. Sci. Manag. 2024;10(1)1–12.
https://doi.org/10.22035/gjesm.2024.01
16. Garai-Ibabe G, Saa L, Pavlov V. Thiocholine mediated stabilization of in situ produced CdS quantum dots: Application for the detection of acetylcholinesterase activity and inhibitors. Analyst. 2014;139(1)280–284.
https://doi.org/10.1039/c3an01662e
17. Hermanto D, Ismillayli N, Irmawanti I, et al. Electrochemically synthesized biogenic silver nanoparticles using green tea extract as a promising antioxidant. Karbala Int. J. Mod. Sci. 2023;9(1)80–88.
https://doi.org/10.33640/2405-609X.3276
18. Hermanto D, Ismillayli N, Fatwa DH, et al. Bio-mediated electrochemically synthesis of silver nanoparticles using green tea (Camellia sinensis) leaves extract and their antibacterial activity. South African J. Chem. Eng. 2024;47:136–141.
https://doi.org/10.1016/j.sajce.2023.11.004
19. Hermanto D, Mudasir M, Siswanta D, Kuswandi B, Ismillayli N. Optical fiber mercury biosensor based on immobilized urease and bromothymol blue onto the alginate-chitosan membrane in the flow-system. Kuwait J. Sci. 2022;49(1)1–13.
https://doi.org/10.48129/kjs.v49i1.9400
20. Alen Y, Adriyani F, Suharti N, Nakajima S, Djamaan A. Determination of profenofos pesticide residue in tomato (Solanum lycopersicum L.) using gas chromatography technique. Der Pharm. Lett. 2016. 88137–141.
https://api.semanticscholar.org/CorpusID:49578038
21. Li H, Guo J, Ping H, et al. Visual detection of organophosphorus pesticides represented by mathamidophos using Au nanoparticles as colorimetric probe. Talanta. 2011;87(1)93–99.
https://doi.org/10.1016/j.talanta.2011.09.046
22. Xia N, Wang Q, Liu L. Nanomaterials-based optical techniques for the detection of acetylcholinesterase and pesticides. Sensors. 2015;15(1)499–514.
https://doi.org/10.3390/s150100499
23. Mane PC, Shinde MD, Varma S, et al. Highly sensitive label-free bio-interfacial colorimetric sensor based on silk fibroin-gold nanocomposite for facile detection of chlorpyrifos pesticide. Sci. Reports Nat. Res. 2020;10(1)1–14.
https://doi.org/10.1038/s41598-020-61130-y
24. Saleem M, Rafiq M, Seo SY, Lee KH. Acetylcholinesterase immobilization and characterization, and comparison of the activity of the porous silicon-immobilized enzyme with its free counterpart. Biosci. Rep. 2016;36(2)1–11.
https://doi.org/10.1042/BSR20150154
25. Kumar DN, Rajeshwari A, Alex SA, Sahu M, Raichur AM, Chandrasekaran N, Mukherjee A. Developing acetylcholinesterase-based inhibition assay by modulated synthesis of silver nanoparticles: Applications for sensing of organophosphorus pesticides. RSC Adv. 2015;5(76)61998–62006.
https://doi.org/10.1039/c5ra10146h
26. Zhu J, Yin X, Zhang W, et al. Simultaneous and sensitive detection of three pesticides using a functional poly(sulfobetaine methacrylate)-coated paper-based colorimetric sensor. Biosensors. 2023;13(3)1–16.
https://doi.org/10.3390/bios13030309
27. Badawy MEI, El-Aswad AF. Bioactive paper sensor based on the acetylcholinesterase for the rapid detection of organophosphate and carbamate pesticides. Int. J. Anal. Chem. 2014;2014(536823)1–8.
https://doi.org/10.1155/2014/536823
28. Selvolini G, Băjan I, Hosu O, Cristea C, Săndulescu R, Marrazza G. DNA-based sensor for the detection of an organophosphorus pesticide: Profenofos. Sensors. 2018;18(7)1–12.
https://doi.org/10.3390/s18072035
29. Rana K, Bhamore JR, Rohit JV, Park TJ, Kailasa SK. Ligand exchange reactions on citrate-gold nanoparticles for a parallel colorimetric assay of six pesticides. New J. Chem. 2018;42(11)9080–9090.
https://doi.org/10.1039/c8nj01294f
30. Chen MJ, Yang HL, Si YM, Tang Q, Chow CF, Gong CB. Photoresponsive surface molecularly imprinted polymers for the detection of profenofos in tomato and mangosteen. Front Chem. 2020;8:1–11.
https://doi.org/10.3389/fchem.2020.583036
31. Mahajan R, Chatterjee S. A simple HPLC-DAD method for simultaneous detection of two organophosphates, profenofos and fenthion, and validation by soil microcosm experiment. Environ. Monit. Assess. 2018;190(327)1–8.
https://doi.org/10.1007/s10661-018-6710-7
32. Menkes Mentan. Join decision by the ministers of health and agriculture Republic Indonesia, No 881/Menkes/SKB VIII/ 1996, No 771/Kpts/Tp. 270/8/1996 about maximum limit of pesticide residues available on the agricultural. c1996. [cited August 17, 2009] [Online]. Available from: http://www.deptan.go.id
33. SNI:7313, Batas Maksimum Residu Pestisida pada Hasil Pertanian. c2008. [cited August 17, 2009] [Online]. Available from: http://www.chilealimentosinodata.cl/uploads/rules/indonesia-batas-maksimum-pestisida.pdf?v1.6
34. Gothwal A, Beniwal P, Dhull V, Hooda V. Preparation of electrochemical biosensor for detection of organophosphorus pesticides. Int. J. Anal. Chem. 2014;2014(303641)1–9.
https://doi.org/10.1155/2014/303641
35. Ghosh S, Maji S, Mondal A. Study of selective sensing of Hg2+ ions by green synthesized silver nanoparticles suppressing the effect of Fe3+ ions. Colloids Surfaces A Physicochem. Eng Asp. 2018;555:324–331.
https://doi.org/10.1016/j.colsurfa.2018.07.012
36. Shvoeva OP, Dedkova VP, Savvin SB. Influence of masking agents on the color reactions of dithizone with Pd(II), Hg(II), and Ag(I) ions after the sorption of their chloride complexes on the fibrous filled anion exchanger AV-17. J. Anal. Chem. 2004;59(7)623–627.
https://doi.org/10.1023/B:JANC.0000035271.85568.e0
37. Mohammadi S, Pourakbar L, Siavash-Moghaddam S, Popović-Djordjević J. The effect of EDTA and citric acid on biochemical processes and changes in phenolic compounds profile of okra (Abelmoschus esculentus L.) under mercury stress. Ecotoxicol Environ. Saf. 2021;208(111607)1–10.
https://doi.org/10.1016/j.ecoenv.2020.111607
Fig. 3(a) Calibration curve of OP concentration vs mean RGB, (b) the appearance of the colorimetric probe. The change in colorimetric probe color at various OP concentrations including (1) colorimetric probe color before reaction with the substrate, (2) 0.05, (3) 0.10, (4) 0.25, (5) 0.50, (6) 0.75, (7) 1.00, (8) 1.25, (9) 1.50, (10) 1.75, (11) 2.00, (12) 2.25, (13) 2.50, (14) 2.75, (15) 3.00, (16) 3.25, (17) 3.50, (18) 3.75, (19) 4.00, (20) 4.25, (21) 4.50, (22) 4.75, (23) 5.00, (24) 5.25, (25) 5.50, (26) 6.00 mg/L of profenofos. 
Fig. 4Reproducibility of the colorimetric probe for quantifying 1 mg/L of OP (a) at the same time, (b) with storage of colorimetric probes at 4°C 
Fig. 5(a) Selectivity of the colorimetric probe, (b) the effect of Hg(II) addition on LSPR intensity of AgNPs. 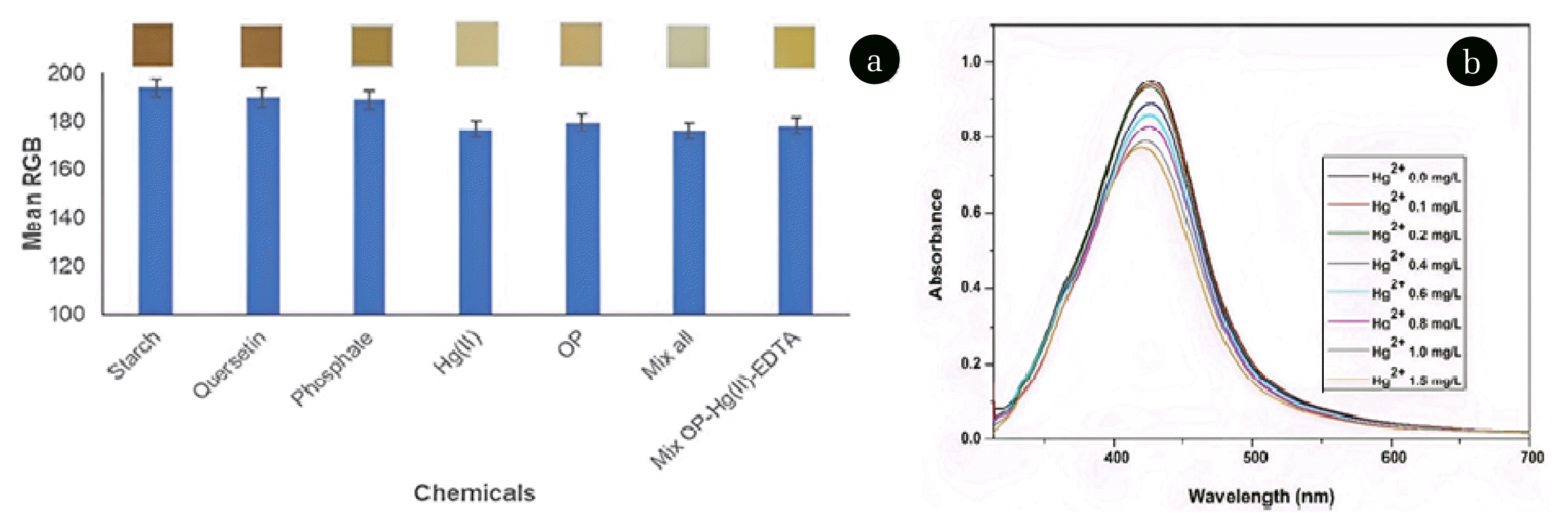
Table 1Optimization of colorimetric biosensor Table 2Sensor and biosensor for profenofos determination
Table 3Quantitation of OP concentrations in vegetable samples |
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||