AbstractCoal is used in most of the power and chemical plants to meet energy needs which produce various waste ashes. Reuse of these ashes as electroactive materials has great importance for sustainable development. In this study, it was detected that the main components of coal fly (CFA) and bottom ashes (CBA) were oxides of silica (SiO2), iron (Fe2O3), aluminium (Al2O3), and magnesium (MgO), besides carbon. These are well-known electrorheological (ER) active materials. The aim of this study is to reveal dielectric and electroactive vibration damping capabilities of CFA and CBA. According to the dielectric and ER flow tests carried out in insulating silicone oil (SO), the optimum concentration of particles was determined to be 35 wt.% for both ashes. Higher ER yield stress (τy = 135 Pa), higher ER efficiency (32.8), and better viscoelastic properties (τc = 128 Pa, G′ = 680 kPa) under 3.0 kV mm−1 applied electric field were obtained for 35CFA/SO suspension system compared to 35CBA/SO (τy = 125 Pa, EReff = 24.0, τc = 55 Pa, G′ = 260 kPa). Although it was concluded that both ashes can be upcycled to sustainable and smart vibration damping alternative materials, better performance was observed for CFA particles.
Graphical Abstract
1. IntroductionElectrorheological (ER) fluids are composed of polarizable dispersed particles in an insulating dispersing medium such as mineral and silicone oils [1, 2]. ER fluids are a type of viscoelastic material that exhibits dominant liquid-like viscous flow under off-electric field while they show dominant solid-like elastic behavior under on-electric field conditions, rapidly and reversibly. This phenomenon occurs via formation of fibril-like structures due to the electric field induced attractive forces between dispersed dielectric particles. Thus, ER fluids can be used in industrial applications as electrical-mechanical interfaces. The usage of ER fluids in vibration damping devices such as shock absorbers, car suspension systems, and automotive engine mounts provides the engineering of electroactive soft materials based on smart vibration damping systems. Generally, the components of a damper are a chamber, a piston, and a fluid inside the chamber. Electroactive vibration dampers can be fabricated by filling the chamber with a suitable smart ER fluid [3].
In recent years, sustainable materials have gained significant attention due to their potential as a sustainable alternative to readily available synthetic materials. Natural polymers such as alginate, cellulose, and chitosan, as well as clays like halloysite, diatomite, bentonite, and montmorillonite are some examples for the sustainable natural materials. While these materials may exhibit poor mechanical properties and limited functionalities, their utilizations as ER active materials provide incorporating them into advanced smart applications. For example, natural polymers such as alginate, cellulose, or chitosan suspensions have been reported to show ER activities [4–6]. It was conducted from the studies that the yield stresses of these suspensions were found to increase as follows: for alginate, from τy = 0.5 Pa to τy = 90 Pa under 3.0 kV mm−1; for cellulose, from τy = 1.0 Pa to τy = 1200 Pa under 3.0 kV mm−1; and for chitosan, from τy = 30 Pa to τy = 400 Pa under 7.0 kV mm−1. In addition, some clays such as halloysite, diatomite, bentonite, and montmorillonite were reported as ER active particles due to their dielectric and electrical properties [7–10]. The high aspect ratio and charged surfaces of clays enable them to form fibril-like structures under on-electric field due to rising stiffness and resulting in increased viscosities of suspensions. The reported the yield stresses of the suspensions of these clays were increased from τy = 0.1 Pa to τy = 11 Pa under 5.0 kV mm−1 for halloysite, τy = 12 Pa to τy = 98 Pa under 3.5 kV mm−1 for diatomite, τy = 0.15 Pa to τy = 0.30 Pa under 1.0 kV mm−1 for bentonite, and τy = 0 Pa to τy = 2500 Pa under 4.0 kV mm−1 for montmorillonite. Thus, it can be concluded that ER activities of clays strongly depend on their chemical structure, particle size, morphology, and ionic additives. While all these materials are derived from sustainable natural sources, there is only one study in the literature about ER activities of waste materials. This study was carried out on spent coffee grounds, and the results revealed a high ER activity of coffee grounds/SO system with the yield stress increasing from τy = 0 to τy = 700 Pa at 3.0 kV mm−1 condition. It can be attributed to the polarizability of coffee ground’s content of cellulose, hemicellulose, and lignin [11]. Thus, there is a gap about ER activity of various waste materials having polarizable content in the literature.
Coal is used as a fossil fuel in most of the power and chemical plants. The thermal process of coal generates fly and bottom ashes in high quantities, which is annually 600–800 million tons worldwide [12]. These waste ashes are collected and disposed as not only slurry into ponds or oceans but also dry solid particles into landfills [13–15]. The coal ash can appear as non-hazardous to the environment; however, anything that is more than the required amount has a toxic effect on nature. When high amounts of coal ash are disposed of as waste, heavy metals or synthetic particles will be absorbed by the soil or the sea life. For example, coal dust at the concentration of 40 mg L−1 was dangerous to the scallop nucleus [16]. To prevent this ecological threat, coal ashes have already been reused as filler in cement, concrete, structural fill, and agriculture [17–19]. Although the construction industry uses coal ashes as puzzolonic additive the most, they consume only 30% of the annually produced coal ashes [20]. For these reasons, it is vital to investigate new utilization areas for coal ash wastes such as dielectric and electroactive vibration damping material.
It was reported in the technical literature that coal ashes are composed of 61% silica (SiO2), 27% alumina (Al2O3), 4.4% iron oxide (Fe2O3), and 0.003% calcium oxide (CaO) [21, 22] beside small amount of carbonaceous residues and metal oxides such as 0.6% magnesium oxide (MgO), 0.4% potassium oxide (K2O), 0.1% sodium oxide (Na2O), and 0.5% titanium dioxide (TiO2) by mass [23]. Although the compositions of coal ashes differ depending on the region and the industry obtained, it is reasonable to say that the main contents of coal ashes are polarizable under externally applied electric field strengths and show chemical similarities with clays. Thus, coal ash particles can show electric field induced ER activity. The reusability of coal ashes as ER active material offers a cost-effective, innovative, and intelligent approach. Additionally, high thermal stability of coal ashes can be advantageous to prepare high performance ER fluids to perform at rush environmental conditions.
There are two types of coal ashes obtained from the upper and the bottom part of the furnaces: coal fly ash (CFA) and coal bottom ash (CBA). It is well known that CFA and CBA are complex residues that show electric field induced polarization. Chemical and physical compositions, moisture content, density, crystalline structure and processing temperature of CFA and CBA residues will affect their dielectric and electroactive responses [24, 25]. These properties collectively suggest that CFA and CBA can show different electroactive properties as sustainable dielectric and electroactive vibration damping materials.
The aim of this study is to reveal and to compare the possible reuse of CFA and CBA as electroactive smart materials in ER vibration damping systems by taking their structures and performances into account. To the best of our knowledge, there have not hitherto been any reported studies in the literature on this subject. Thus, this study will contribute to very limited ER activity studies of waste materials and will also bring about the detailed structural, morphological, thermal, electrical, dielectric and vibration damping properties of waste ashes to the literature. It is expected in this study that the suspensions of CFA/SO and CBA/SO systems will show vibration damping properties due to the inorganic mineral contents of coal ashes, allowing them to be reused in the future as value-added smart materials.
2. Experimental2.1. MaterialsCoal ashes were kindly supplied by Marmara Kimya Sanayi (MKS) Integrated Chemical Industry Inc. in Bursa, Türkiye. CFA and CBA were obtained from the upper and bottom parts of the steam boiler, respectively. CFA is a fine powder of light components that enters the flue gas, entrained by gas-phase by-products upward from the furnace. CBA settles down on the furnace floor due to the minerals left over from the combustion process of the coal used. CFA and CBA were used without any purification. To obtain fine-grade particles for further uses, coal ashes were grounded using a miller (Retsch, MM400, Germany). Silicone oils (polydimethylsiloxane with η = 1 Pa s, ρ = 0.970 g mL−1 and η = 4.57×10−3 Pa s, ρ = 0.967 g mL−1) and 2-[4-(2,4,4-trimethylpentan-2-yl)phenoxy]ethanol (Triton-X100, ρ = 1.065 g mL−1) were purchased from Sigma Aldrich (Germany) and used as received.
2.2. Characterizations of CFA and CBAThe structural, morphological, thermal and electrical characterizations were performed for CFA and CBA particles as follows.
The morphology and elemental compositions of CFA and CBA were investigated via SEM (Scanning Electron Microscopy, ZEISS, GeminiSEM 300, Germany) operated with an accelerating voltage of 10 kV and an Energy Dispersive X-Ray Analysis attachment (EDX, Bruker, XFlash 6-100, Germany). ATR-FTIR (Thermo Scientific, NICOLET, IS50, USA) working with a resolution of 2 cm−1 and scan of 64 was used to identify the dominant functional groups of CFA and CBA. Furthermore, the chemical compositions were determined by an X-ray fluorescence (XRF) analyzer (Rigaku, Supermini 200, Japan).
The sizes of CFA and CBA particles were calculated by taking 10 different points in SEM images mentioned above using ImageJ software. Additionally, the morphologies and the crystal structures were examined using the X-ray diffraction (XRD) technique (GNR, APD 2000 Pro, Italy). XRD patterns were obtained in the scanning angle range of 10° ≤ 2θ ≤ 75° with CoKα radiation (λ = 0.17903 nm). The patterns were converted to a CuKα radiation pattern with a simple equation of Eq. (1) derived from Bragg’s law to compare with the literature.
Thermal properties of CFA and CBA were examined via thermal gravimetric analysis (TGA, TA Instruments, SDT 650, USA) with a heating rate of 10 °C min−1 in a temperature range of 30–900 °C under N2(g) atmosphere. Electrical conductivity measurements were carried out at 25 °C by the four-probe technique (Entek Electronic, FPP 470-A, Turkiye) using compressed pellets with known dimensions (13 mm diameter and 0.2 mm height). The average electrical conductivity measurements were obtained from five different points on the pellets and the conductivities were calculated using the Van der Pauw equation of Eq. (2) [26].
where ρ is the resistivity of the material, I0 is current, ΔV is voltage difference, and c is the thickness of the pellet.
The pellets were also used to calculate the apparent densities of CFA and CBA using a simple equation of Eq. (3).
where ρ, m, and V are the apparent density, mass, and volume of the pellet, respectively.
The zeta (ζ)-potentials of CFA and CBA in aqueous (water) and non-aqueous (SO: η = 4.57×10−3 Pa s) suspensions were measured at 25°C by Laser Doppler Electrophoresis technique using Malvern Zeta-sizer Nano ZS (England) instrument. The instrument was equipped with a 4-mW He–Ne laser operating at λ = 633 nm and a non-invasive backscatter system measuring the light scattered at a backscatter angle of 173°. The suspensions were prepared with 0.1 g L−1 and well dispersed via ultrasonication. Triton-X was added to the suspension in SO at 100 ppm concentration. After 30 minutes of rest, the supernatant liquids were taken and used for the measurements [27]. Additionally, the represented results of ζ-potentials were averages of 300 repeated measurements. Since Brownian motions are very weak in viscous medium such as SO used in the preparation of CFA/SO and CBA/SO fluids, the ζ-potential measurements were performed in SO having low viscosity (η(SO) = 4.57×10−3 Pa s). Since, the electrical double layer around the dispersed particles in SO is very thick. To diminish the electrical double layer, the measurements in SO were also performed in the presence of a non-ionic surfactant, Triton-X.
2.3. Dielectric Characterizations of CFA/SO and CBA/SO SuspensionsCFA, CBA, and SO were vacuum dried for 24 h at 50°C to remove any moisture present. To determine the optimum suspension concentration, the suspensions were prepared by dispersing CFA and CBA at 15–40% by weight with 5% increments in SO and designated as CFA/SO and CBA/SO by indicating the percentage of coal ash contents. The suspensions at 5 and 10 wt.% were prepared and analyzed, but they were ER inactive. All the suspensions were homogenized using a probe sonicator (Sonics, Vibracell, USA) and used for the rest of the measurements.
Dielectric spectra of the suspensions were analyzed using Agilent E4980A Precision Impedance Analyzer equipped with a 16452A model liquid test fixture (Japan). Capacitance (Cp) and resistance (Rp) were measured in the frequency range of 20 Hz-2 MHz at 25°C under 1 V constant AC voltage. The dielectric constant (ɛ′) and dielectric loss (ɛ″) values were calculated as a function of frequency by using the following equations of Eq. (4–10).
where ɛr is the relative dielectric constant, ɛ is the dielectric constant of the sample, and ɛ0 is the dielectric constant of the vacuum.
where ɛr′ and ɛr″ are real and imaginary parts of the dielectric constant, respectively.
where d is a gap between the electrodes, A is the electrode area, and ω is the angular frequency.
The dielectric characterization results are interpreted by fitting to the Cole-Cole model of Eq. (8) which is a simple method to define dielectric relaxation of the materials [28].
where ω is the angular frequency, λ the is relaxation time, α is a parameter for the broadness of relaxation time distribution and fmax is the peak of ɛ″ vs. f spectrum, and f is the frequency applied, ɛ0′ is dielectric constant as f → 0, and ɛ∞′ is dielectric constant as f.
2.4. Electroactive Vibration Damping Characteristics of CFA/SO and CBA/SO SuspensionsFirstly, to determine the best concentration of coal ash particles in the SO suspensions, the flow curves of all the suspensions were examined using a torque electrorheometer (Thermo-Haake, RS600 Rheometer, Germany) with a 1.0 mm gap between the 33 mm parallel plates at 25°C. The high DC electric field generator (FUG Electronics HCL 14, Germany) was connected to the rheometer for applications of external electrical field strengths (E) from 0 kV mm−1 to 3.0 kV mm−1 with 1.0 kV mm−1 increments. The higher electric field strengths are not used to avoid the risk of electrical breakdown. The flow curves showed the changes in shear stresses (τ) and viscosities (η) as a functions of shear rates (γ) in a range of 1–300 s−1. This shear rate range was suitable for calculation of the yield stress values under varying electric field strengths. The flow curves were fitted to the proper models using the OriginPro software. The well-known Bingham and Cho-Choi-John models could not fit and explain ER behavior of the suspensions [29]. The best models for the suspensions were decided to be the Herschel-Bulkey model of Eq. (11) under zero applied electric field and the Seo-Seo model of Eq. (12) under applied electric field strengths [30, 31].
where τy is the yield stress, k is the consistency index, and n is the flow index. The fluid exhibits shear-thinning if n<1, whereas it exhibits shear-thickening behaviour if n>1.
The yield stress (τy) values were calculated from the fitting equations. The optimum concentration of CFA and CBA in SO was determined by considering both dielectric and ER flow curves.
Secondly, the time dependent ER responses of the optimized CFA/SO and CBA/SO suspensions were investigated under the controlled shear rate mode with constant and unit shear rate (γ̇ = 1.0 s−1) under rising electric field strengths. The ER responses of optimized CFA/SO and CBA/SO suspensions were investigated under applied electric field strengths with 0.5 kV mm−1 increments and 30 s recovery intervals. This was aimed to understand whether the ER response is reversible, or not.
Thirdly, the dynamic rheological measurements were carried out to reveal the viscoelastic properties of optimized CFA/SO and CBA/SO suspension systems. Linear viscoelastic regions (LVR) for the suspensions under various electric fields from 0 kV mm−1 to 3.0 kV mm−1 with 1.0 kV mm−1 increments were determined regarding the following experiments. According to the result obtained from LVR measurements, the applicable shear stress value for frequency dependent, time dependent, and creep-recovery tests was determined to be 5 Pa for the both CFA/SO and CBA/SO suspensions under E = 0, 1.0, 2.0, and 3.0 kV mm−1 conditions. This stress value was in the LVR region under applied electric field, and the suspensions can undergo recoverable elastic deformation in this region. The frequency and time dependent viscoelastic properties were measured in the reasonable ranges of 1–100 Hz and 0–200 s, respectively. By this way, time dependent stability of electric field induced viscoelastic properties will be examined. The effect of gradual increase in temperature on elastic moduli of the suspensions were examined in the temperature range of 25–80°C with the application of shear stress of τ =5 Pa. The results have revealed the thermal stability of viscoelastic properties of CFA/SO and CBA/SO suspensions. The creep-recovery tests were performed by measuring the strains during the application of τ = 5 Pa for 100 s to deform and the release of the stress (τ = 0) for 200 s to recover in a sequence. This will indicate the magnitude of elastic character of suspensions under applied electric field.
2.5. Anti-sedimentation Characteristics of CFA/SO and CBA/SO SuspensionsThe anti-sedimentation stabilities (i.e., the resistance of dispersed particles against gravitational sedimentation) of optimized CFA/SO and CBA/SO suspensions were followed at room temperature for 20 days, after which the anti-sedimentation ratios were fixed. The heights of particle-rich and oil-rich phases of the suspensions were measured using a digital caliper. Then, the anti-sedimentation stabilities of the suspensions were calculated by taking the percentage ratios of the particle-rich phases to the total height of the suspensions.
3. Results and Discussion3.1. Characterizations of CFA and CBAThe compositions of CFA and CBA were revealed by the SEM-EDX measurements, which showed the present atoms in a particular area. The results of atomic percentage (Table S1) indicated that both CFA and CBA have similar contents with mainly Si, O, Fe, and Al atoms as well as small amounts of Mg and C atoms. These results are consistent with the previously reported literature studies on coal ashes [32–34]. Although the presence of C atoms in coal ashes might be due to the residual carbonaceous materials, the low amounts of C atoms might be attributed to the excess oxidation condition in the steam boiler [35]. Considering the high atomic percentage of O atoms, it might be suggested that both ashes are composed of SiO2, Al2O3, and Fe2O3, which are well-known electroactive materials [36, 37].
Oxides compositions of the coal ashes were revealed via XRF measurements (Table S1). The main components were determined to be SiO2, Al2O3, and Fe2O3 which are consistent with the literature on bottom ash samples [38]. Furthermore, it was determined that CFA contained more Fe2O3 than CBA whereas CBA contained more MgO than CFA. These findings were in accordance with the above-mentioned EDX result. Furthermore, similar results were reported previously, and suggested that oxides of alkaline earth metals and alkaline metals cause the fracture of bridging oxygen bonds [39]. Total oxides of earth alkaline and alkaline metals in CFA and CBA were calculated as 11.1% and 10.6% by mass, respectively. Thus, CFA is expected to be more amorphous than CBA. Additionally, the presence of small amounts of CaO indicated that CFA and CBA were F-type coal ashes, containing less than 7 wt.% CaO as reported for fly ash [40].
In order to comprehend the structural differences between CFA and CBA, their ATR-FTIR spectra (Fig. 1(a)) were examined. There were minor fluctuations in the 3000–3400 cm−1 range, especially for CBA. This might be attributed to the presence of small amount of -OH groups and water molecules in both CFA and CBA structures. In addition, the weak band at 1470 cm−1 was only observed for CBA that might be attributed to the presence of Mg-O vibrations [41]. ATR-FTIR indicated that the amount of Mg in the CBA structure is higher than CFA which is in accordance with the EDX and XRF results. The intense bands at 1025 cm−1 in both curves were related to the asymmetric stretching vibrations of Si-O-Si groups, according to the previously reported studies on coal ash [42, 43]. Additionally, the relatively intense bands at 770 cm−1 and 680–600 cm−1 in both curves might be attributed to the symmetric stretching vibrations of Si-O and Al-O groups, respectively.
The chemical contents of CFA and CBA were similar to the clays such as montmorillonite and bentonite, which are composed of oxides and hydroxides of Si, Al, Fe, and Mg atoms [44]. For example, SiO2 contents of CFA (53.5 wt.%) and CBA (56.1 wt.%) were closer to the Si contents in sepiolite (52 wt.%) [45], montmorillonite (53 wt.%) [46], laponite (60 wt.%) [47], and bentonite (60 wt.%) [48]. Since clays are generally considered as electroactive materials, CFA and CBA suspensions may also show ER activity [49].
The morphologies and crystal structures of CFA and CBA were also investigated by XRD diffractograms (Fig. 1(b)) to determine the percentage crystallinity, to reveal the particle orientations and to identify the surface functional groups. The XRD patterns showed highly amorphous structures, and the percentage crystallinities from the peak integrations were calculated to be 15% and 17% for CFA and CBA, respectively, which may be attributed to the not well-defined layer structures of coal ashes. The relatively higher amorphous structure for CFA was attributed to the higher amount of the earth alkaline and alkaline metals (11.1 wt.%). It was reported in the literature that some quartz (SiO2), mullite (Al2SiO3), and hematite (Fe2O3) structures were observed through the crystallization of molten coal [50–52]. The relative amounts of quartz, mullite, and hematite crystals in CFA were calculated to be 4%, 10%, and 1%, respectively. On the other hand, the amounts of quartz, mullite, and hematite in CBA were determined to be 6%, 10%, and 1%, respectively. CFA and CBA were composed of similar percentages of mullite and hematite, which have surface charges due to the presence of Si-OH and Al-OH groups in mullite and Fe-OH groups in hematite. On the other hand, CBA had a higher percentage of quartz, having Si-OH groups on the surface. Thus, it can be suggested that the presence of quartz, mullite, and especially hematite might be one of the driving forces behind the ER activities of CFA and CBA particles.
TGA thermograms exhibited mass losses of CFA and CBA particles as a function of temperature (Fig. 1(c,d)). For both ashes, the first mass losses below 100°C were attributed to the removal of moisture. The second mass loss between 100–520°C for CFA and 100–410°C for CBA might be originated from the removal of adsorbed structural water molecules [53]. In addition, the total amounts of water were calculated to be 0.49% for CFA and 0.78% for CBA. The higher amount of structural water in CBA curve could be a reason for more prominent fluctuations in the 3000–3400 cm−1 range of ATR-FTIR spectrum. The weight losses in the third region might be attributed to the evaporation of residual carbonaceous materials in CFA and CBA [54]. The last regions with weight loss of 1.18% above 730°C for CFA and 0.40% above 625°C for CBA might be attributed to the dehydroxylation of metallic hydroxides [55]. Overall, the total mass loss of CFA (1.99%) was higher than CBA (1.48%) because of the presence of metallic hydroxides in CFA. In addition, the high thermal resistance of both ashes in a wide range of temperature might improve the industrial applicability of coal ash-based ER systems.
The SEM images in Fig. 2(a,b) demonstrated that CFA and CBA were similar sized irregular particles with some agglomerations. This might be explained by the fragmentation of large particles during the ground milling process to obtain fine particles [56]. The average sizes of CFA and CBA particles were calculated to be 2.0 ± 0.8 μm and 2.2 ± 0.7 μm, respectively. Similar results were reported in a previous study indicating that CFA composes of particles with irregular shapes and various sizes ranging from 2 to 10 μm [57]. The particles with various sizes might provide a high packing efficiency under an externally applied electric field, which will be an advantage for electroactive vibration damping applications of CFA and CBA due to the formation of stronger electric field induced fibril-like structures. A similar suggestion was previously reported for spent coffee grounds/SO suspension system [11].
One of the critical parameters for electroactive smart materials is their electrical conductivity values. The conductivity of ER active particles should be in the semiconducting range of 10−8–10−4 S cm−1 to form an excellent response to the externally applied electric field and to prevent electrical break-down. The conductivities (σ) of CFA and CBA were determined to be 1.6 ± 0.3 10−6 S cm−1 and 5.5 ± 0.6 10−7 S cm−1, respectively. Indeed, these results are in the desired range of a material to show a good electroactive performance. It is explained that high conductivity can enhance the ER effect by promoting the dielelctric polarization [58]. The higher conductivity of CFA might indicate a better ability to response to the applied electric field, and it might be attributed to a previously mentioned higher amount of Fe2O3 content in CFA.
The apparent densities and ζ-potentials of CFA and CBA particles were investigated to reveal the colloidal stabilities of the dispersed particles in CFA/SO and CBA/SO suspensions. The apparent densities of CFA and CBA particles were calculated to be ρ = 0.605 g mL−1 and ρ = 1.712 g mL−1, respectively. The density of CFA was lower than CBA which was attributed to the fracture of bringing oxygen bonds and the highly amorphous structure of CFA (85%). Moreover, there was a high-density mismatch between CBA (ρ = 1.712 g mL−1) and SO (ρ =0.970 g mL−1) resulting in a low anti-sedimentation stability [59]. Furthermore, the particles with high negative (ζ<−30 mV) or positive (ζ>+30 mV) ζ-potentials in suspensions are colloidally stable owing to the significant electrostatic repulsive forces acting between the dispersed particles [60]. The ζ-potentials of CFA and CBA particles in aqueous medium were determined to be −23.0 ± 0.6 mV and −32.1 ± 6.0 mV, respectively. The similar negative ζ-potentials for ashes in aqueous media were also reported in the literature for blast-furnace slag and fly ash [61]. On the other hand, the ζ-potentials of CFA and CBA particles in non-aqueous media (SO) were measured to be and −0.20 ± 0.10 mV and −0.40 ± 0.07 mV, respectively. Negative ζ-potential values of CFA and CBA in aqueous and non-aqueous media might be attributed to their similar compositions. On the other hand, the anti-sedimentation stabilities of both ashes were expected to be moderately lower in non-aqueous medium due to their low ζ-potentials.
3.2. Dielectric Characteristics of CFA/SO and CBA/SO SuspensionsElectrons of a dielectric material slightly shift throughout an electric field and cause dielectric polarization when an external electric field is applied. Therefore, it is essential to reveal dielectric properties for detailed explanation of ER behavior. Dielectric constant (ɛ′) and dielectric loss (ɛ″) of a material are described as the abilities of storage and dissipation of applied electrical energy, respectively. The difference in ɛ′ values at zero and infinite frequencies (Δɛ′ = ɛ0′-ɛ∞′) is a measure of a magnitude of dielectric polarizability in a material. In addition, dielectric relaxation is defined as 63% of the material returning to its initial state [62]. Therefore, the dielectric relaxation time (λ) is the response time of a material to applied electric field. Slow dielectric relaxations due to the orientational and interfacial polarizations are responsible for the expected electroactivity since they can result in a formation of strong electric field induced fibril-like structures [63].
In order to determine the optimum amounts of CFA and CBA to be dispersed in SO for ER measurements, the suspension with the strongest polarizability (Δɛ′ = ɛ0′-ɛ∞′) and the fastest response time (smaller λ) were investigated by dielectric measurements. The dielectric spectra of CFA/SO and CBA/SO suspensions within the concentration range of 15–40 wt.% with 5% increments (Fig. 3, S1, and S2) were fitted to the Cole-Cole equation of Eq. (8).
The fitting curves provided complete spectra of the suspensions at low and high frequencies so that the dielectric parameters could be calculated (Table S2). All the CFA/SO and CBA/SO suspensions showed the interfacial (Maxwell-Wagner) polarization under externally applied electric fields, arising from dielectric constant and conductivity differences between the dispersed particles and dispersing medium [64]. According to the simple mixture rule defined by Eq. (13), gradually increasing concentrations of dielectric CFA and CBA particles led to rising in Δɛ′ values, which indicate an increase in the polarizability of the suspensions due to the greater electric field induced dipoles.
where ɛ is the dielectric property, φ is the volume fraction, m is the mixture, 1 is the dispersing medium, and 2 is the dispersed phase.
The shortest response times (λ) were observed for 35CFA/SO and 30CBA/SO suspensions. These mean that the suspensions demonstrated dielectric saturation at concentration of 40 wt.%, beyond which the diffusion of particles might be restricted due to the crowded particles [65]. Compared to the dielectric properties of 40CFA/SO suspension (Δɛ′ = 13.0, λ = 1.4×101 s), 40CBA/SO showed lower polarizability (Δɛ′ = 6.6) and faster response time (λ = 2.0×10−2 s). The higher concentration of Fe2O3 in CFA might be responsible for the superior dielectric polarizability, since Fe2O3 has dielectric constant value greater than ɛ =5 [66]. On contrary, CBA contains higher proportion of SiO2, which has a relatively low dielectric constant within the range of ɛ =3.9–4.5 [67]. Dielectric properties of CFA and CBA films were reported previously in the literature indicating that CFA film follows the conduction mechanism [68] whereas, in our study, CFA/SO and CBA/SO suspension systems followed the interfacial polarization mechanism. Additionally, ɛ∞′ values of CFA and CBA films reported in the literature were 5.0 and 2.3, determined from the graphs, indicating high dielectric constant value of CFA [69,70]. This is in accordance with the dielectric results obtained in our study which revealed high dielectric constant for CFA/SO system (ɛ∞′ = 2.8) than CBA/SO system (ɛ∞′ = 2.4). It is also important to mention that CFA/SO and CBA/SO suspensions have higher polarizability (Δɛ′ = ɛ0′-ɛ∞′) than some clay suspension systems such as diatomite/SO [9], sepiolite/SO [71], and montmorillonite/SO [72]. This may be related to the higher content of Fe2O3, which is an excellent dielectric material [73], in CFA and CBA than in diatomite (0.48%) [9], sepiolite (0.05%) [74], and montmorillonite (3.6%) [75].
3.3. Electroactive Vibration Damping Properties of CFA/SO and CBA/SO Suspensions3.3.1. Effect of concentration on flow behaviorsFlow curves of all the CFA/SO and CBA/SO suspensions were investigated under various applied electric field strengths (Fig. 4(a,b), Fig. S3, S4). Shear thinning behaviors, a decrease in the viscosity with applied shear rate, were observed for all the suspensions under off and on-electric field conditions. The yield stress (τy) values observed to increase with rising concentration and applied electric field strengths; thus, the highest yield stress values were determined to be τy = 225 Pa for 40CFA/SO and τy = 130 Pa for 40CBA/SO at E = 3.0 kV mm−1 (Fig. S3 and Fig. S4). The polarization and viscous forces between CFA and CBA particles were stronger under the externally applied electric field than the hydrodynamic forces generated by the applied shear stresses. Hence, it was deduced that the electric field induced interfacial polarization of CFA and CBA particles resulted in an attraction between the dispersed particles and driven the systems to the formation of strong columnar structures. Moreover, 40CFA/SO suspension system showed higher τy values than 40CBA/SO suspension. Considering dielectric properties given in Table S2, the higher polarizability (Δɛ′ = 13) of 40CFA/SO lead to the formation of dense fibril-like structures under externally applied electric fields. However, 40CFA/SO suspension showed the longest dielectric relaxation times.
To determine the optimum contents of CFA and CBA in SO, electric field induced yield stresses and ER efficiencies defined by Eq. (14) of suspensions were compared. All the CFA/SO and CBA/SO suspensions examined showed non-Newtonian flow behaviors. The best fitting model for the flow curves under off and on-electric fields are decided to obey the Herschel–Bulkley and the Seo-Seo models, respectively. The Herschel-Bulkey model of Eq. (11) describes the non-linear flow curves of non-Newtonian fluids. The Seo-Seo model of Eq. (12) explains the deformation and reformation of the fibril-like structures with the shear rate under applied electric fields [30, 31]. Seo-Seo model allows to calculate the static yield stress (τsy) values by taking the structural realignments at low shear rates and Papanastasiou model into account which describes the entire flow curve of non-Newtonian fluids after yielding. Additionally, Seo-Seo model provides better predictions for ER fluids at high and low shear rate regions than commonly used Cho-Choi-John (CCJ) model [76]. If the yield stresses of ER fluids are proportional to E2 at low electric field strengths and E3/2 at high field strengths, the Seo-Seo model is expected to be applicable [77].
where τy(E≠0) and τy(E=0) are the shear stresses under on- and off-electric fields, respectively.
According to the results obtained from the fitting curves, ER efficiencies were increased up to 32.8 and 24.0 for 35 wt.% concentration of CFA/SO and CBA/SO suspensions, respectively. The lower ER efficiency values of 15.1 and 14.0 for 40 wt.% CFA/SO and CBA/SO suspensions, respectively, which might be attributed to the increasing off-field viscosity of the suspensions. A similar thickening effect resulting in low EReff was also observed in the literature for concentrated suspensions of various inorganic materials such as clay [49], sepiolite [71] and iron-II oxalate micro-rods [78]. Thus, the optimum content of CFA and CBA in SO was determined to be 35 wt.%. Further electroactive studies in this work were performed only for 35CFA/SO and 35CBA/SO suspension systems.
When 35CFA/SO and 35CBA/SO suspensions were compared, the best ER properties were obtained for 35CFA/SO system with τy = 135 Pa at E = 3.0 kV mm−1 and EReff = 32.8. This might be attributed to the better dielectric polarizability of 35CFA/SO suspension. Therefore, it might be concluded that dielectric polarizability is the major driving force on ER properties of coal as suspensions.
The ER results of CFA and CBA suspensions have been scrutinized with the previously reported clays suspensions due to their similar content of SiO2 and Al2O3. The 35CFA/SO and 35CBA/SO suspensions showed higher electric field induced yield stress values than montmorillonite, sepiolite and laponite dispersions [49,72]. On the other hand, diatomite and kaolin dispersions have higher electric field induced yield stress values than 35CFA/SO and 35CBA/SO. This may be attributed to the higher conductivities of diatomite (10−3 S cm−1) [9] and kaolin (10−4 S cm−1) [79] when compared to CFA (10−6 S cm−1), CBA (10−7 S cm−1), sepiolite (10−12 S cm−1) [80], montmorillonite (10−10 S cm−1) [81], and laponite (10−5 S cm−1) [82]. The 35CFA/SO suspension have had higher ER activities than Na-fluorohectorite clay (τy = 40 Pa at E = 2.0 kV mm−1), which is a synthetic and charged silica-based clay material [83]. 35CFA/SO suspension is also better ER fluid than the previously reported green ER fluid so-called cellulose/SO suspension (τy = 53 Pa at E = 3.0 kV mm−1) [84]. Unfortunately, when compared to the suspension of spent coffee grounds (τy = 700 Pa at E = 3.0 kV mm−1), 35CFA/SO suspension has had lower yield stress and ER activity [11]. The driving force behind the stronger yield stress of spent coffee grounds was reported to be the hemicellulose content which has stronger dielectric and conductivity properties than the main silica content of CFA [85].
Time dependent ER responses graphs depicted in Fig. 4(c) showed that both 35CFA/SO and 35CBA/SO suspensions were not only quickly responded to the applied electric fields but also completely returned to the initial conditions under the off-electric fields. The fast and reversible ER response could be related to the millisecond dielectric relaxation time of 35CFA/SO (λ= 230 ms) and 35CBA/SO (λ=23 ms). It can be concluded that both suspension systems were suitable for long-term use in potential smart electroactive vibration damping applications [86].
3.3.2. Effect of shear stress on the elastic moduliER fluids are viscoelastic materials that can show both viscous and elastic characteristics under various deformation conditions. Linear viscoelastic region (LVR) is an important parameter for viscoelasticity because it defines the maximum applicable stress range in which the recoverable deformation exists. The limit of LVR is called as the critical stress (τc). When a stress above the τc is applied to a system, the fibril-like structures break-down, the elastic modulus (G′) sharply decreases, and a non-recoverable deformation is perceived. The τc values at E=3.0 kV mm−1 for 35CFA/SO and 35CBA/SO were determined to be 128 Pa and 55 Pa, respectively (Fig. 5). Notably, the tc raised and the LVR region broadened for both suspensions as the applied electric field strength increased due to the enhanced interfacial polarization of the dispersed coal ash particles. The better elastic modulus (G′ = 680 kPa) and critical stress (τc = 128 Pa) values of 35CFA/SO than 35CBA/SO were attributed to the better electroactive properties such as electrical conductivity (σ=1.6×10−6 S/cm), dielectric polarizability (Δɛ′ =6.7), EReff (32.8) and Fe2O3 content (11.2%) than 35CBA/SO. Compared to the reported elastic moduli of clays such as montmorillonite (G′ =10 kPa) and diatomite (G′ = 30 kPa), 35CFA/SO system has also shown better overall viscoelastic properties [9,72].
3.3.3. Effect of frequency and time on elastic and viscous moduliIt is essential to obtain continuous and constant elastic behavior for vibration damping applications. Therefore, the effect of frequency and time on the viscous (G″) and elastic (G′) moduli were investigated for the 35CFA/SO and 35CBA/SO suspension systems. Time dependent curves depicted in Fig. 6(a,b) showed continuous and constant ER behaviors for both suspension systems. The highest elastic modulus (insert in Fig. 6(a,b)) was observed for 35CFA/SO at E =3.0 kV mm−1 as G′ = 680 kPa. The suspensions of clays like montmorillonite (G′ = 70 kPa) and bentonite (G′= 8 kPa) could not reach the values of G′ of 35CFA/SO and 35CBA/SO systems under on-electric field [7, 49]. This is related to the higher friction forces for coal ash suspensions under applied electric field providing better electroactive vibration damping capability. Additionally, the frequency dependent curves shown in Fig. 6(c,d) elucidated that both G″ and G′ moduli for 35CFA/SO and 35CBA/SO systems raised with increasing frequencies under the off-electric fields.
The cross-over points on the frequency curve under the off-electric field indicated gelation behaviors with gelation times of 4.0 ×10−2 s and 1.7×10−2 s for 35CFA/SO and 35CBA/SO, respectively. 35CBA/SO suspension has better gelation behavior with a shorter gelation time and higher gel strength (τc = 0.5 Pa) than 35CFA/SO under off-electric field. On the other hand, G″ and G′ moduli for both suspensions were nearly constant with changes in frequencies under the on-electric fields. The elastic moduli were dominant for both suspensions in the on-electric field curves due to the formations of electric field induced fibril-like columnar structures. This implied that both CFA/SO and CBA/SO suspensions had electroactive vibration damping capability due to the viscous and elastic behaviors under off and on-electric fields, respectively. Applying E = 3.0 kV mm−1 led to a higher increase in the elastic modulus of 35CFA/SO than 35CBA/SO indicating best electroactive vibration damping capability. This could be attributed to the higher dielectric polarizability of 35CFA/SO than 35CBA/SO. Stronger fibril-like structures formed in 35CFA/SO under the applied electric field, and this increased the amount of viscous friction forces to store the applied energy until eventual dissipation. Additionally, the constant and dominant elastic modulus under applied electric field improved the potential use of 35CFA/SO suspension for electroactive vibration damping applications.
The electroactive vibration damping properties were also investigated by calculating the damping factor (tanδ = G″/G′) values represented in Fig. 6(e,f). The tanδ values were higher than unity (tanδ>1) under off-electric field due to dominant viscous behavior. However, this value dropped below unity (tanδ<1) thanks to the dominating elastic character of the suspensions under on-electric field. The presence of viscous friction forces in the fluids provides resistance against the applied forces so the system has vibration damping capability due to the enhanced stiffness. Thus, electroactive vibration damping can be achieved by using both coal ash suspensions by switching the predominant viscoelastic property with the application of external electric field.
The effect of temperature on electroactive viscoelastic properties is competition of Brownian motions with the formation of fibril-like structures at higher temperatures and under applied electric field [87]. As seen in Fig. 6, 35CFA/SO and 35CBA/SO suspension systems showed high temperature resistance under applied electric field. This can be attributed to the better thermal properties of CFA and CBA which is in accordance with the TGA results. Under off-electric field conditions, elevated temperatures slightly decreased the elastic moduli of both 35CFA/SO and 35CBA/SO suspensions because the Brownian motions became dominant and so the rheological properties weaken. When the electric field is turned on, the fibril-like structure can resist to motion.
3.3.4. Creep and creep-recovery behaviorsCreep and creep-recovery behaviors identify the recovered and unrecovered portions of the viscoelastic materials when a deformation force is applied. Dissipation of the stored energy after removing the applied force is called the recovery, and the deformation is called elastic. The magnitude of recovery percentage defined in Eq. (15) shows the material parts having an elastic character.
where γi is the strain at the end of the creep phase, and γf is the average steady-state strain at the end of the recovery phase.
The percentage recoveries (inserted in Fig. 7) under the off-electric field were calculated to be 0.4% and 0.8% for 35CFA/SO and 35CBA/SO suspension systems, respectively. This indicates that both suspensions showed almost viscous deformation without application of electric field. On the contrary, the recoveries reached 92% and 49% under E=3.0 kV mm−1 for 35CFA/SO and 35CBA/SO suspensions, respectively. Indeed, 35CFA/SO suspension underwent stronger elastic deformation under on-electric field, indicating better electroactive vibration damping capability as mentioned above. This approved that CFA particles form more durable electric field induced fibril-like structures than CBA which is consistent with the dielectric properties and ER flow curves discussed above. Compared to the previously reported diatomite/SO suspension system (25% under E=3.5 kV mm−1), 35CFA/SO and 35CBA/SO showed excellent recoveries [9]. Thus, coal ash suspensions have better energy storage capacity than diatomite/SO system under applied electric field.
3.4. Anti-sedimentation PropertiesSedimentation of suspensions is related to the density, size, and intermolecular interactions of dispersed and dispersing phases [88]. It is important to show high anti-sedimentation stability of suspensions for long-term use in industrial applications. The calculated anti-sedimentation ratio for 35CFA/SO showed no sedimentation or phase separation within the first few days, then showed gradual decrease for a period of 6 days and eventually levelled off at the end of 20 days. On the other hand, 35CBA/SO suspension system showed gradual phase separation starting from the first day which continued for a period of 8 days and then almost levelled off at the end of 20 days. (Fig. 8). Ultimately 35CFA/SO and 35CBA/SO systems showed 85% and 62% anti-sedimentation stabilities, respectively within 20 days of measurements. The low anti-sedimentation stability of CBA suspension might be attributed to the density mismatch with SO (ρCBA = 1.712 g mL−1, ρSO = 0.967 g mL−1) as discussed previously in the text.
4. ConclusionsTo investigate the possible upcycling of coal ash as a smart material, CFA and CBA samples were obtained from a steam boiler of a chemical company. CFA and CBA were mainly composed of SiO2, Fe2O3, and Al2O3, which are well-known electroactive materials. According to the characterization results, CFA contained relatively more Fe2O3 (11.2 wt.%) than CBA (10 wt.%). Thus, the conductivity of CFA (1.6×10−6 S cm−1) was higher than CBA (5.5×10−7 S cm−1). TGA thermograms also indicated that CFA had a higher amount of metal hydroxides, which are highly polarizable compounds, than CBA. All of these could be the reason of the better dielectric polarizability of 35CFA/SO suspension (Δɛ = 6.7, λ = 2.3×10−1 s) than 35CBA/SO (Δɛ = 5.9, λ = 2.3×10−2 s). Although the best dielectric properties were obtained for 40wt.% suspensions, ER flow tests showed that 35wt.% suspensions exhibited the highest ER efficiency. Thus, the optimum concentration of suspensions was determined to be 35wt.%. When two coal ash suspensions were compared, 35CFA/SO had higher yield stress (τy = 135 Pa under E = 3.0 kV mm−1) and ER efficiency (EReff = 32.8) than 35CBA/SO (τy = 125 Pa under E = 3.0 kV mm−1, EReff = 24.0).
Additionally, the viscoelastic tests indicated that the both suspensions had electroactive vibration damping capability with a dominant viscous character (G″) under the off-electric field and an elastic character (G′) under the on-electric field. The electric field-induced critical yield stress and elastic modulus were higher for 35CFA/SO (τc = 128 Pa, G′ = 680 kPa) than 35CBA/SO (τc = 55 Pa, G′ = 260 kPa). All the results concluded that both suspensions have electroactive vibration damping capability, and 35CFA/SO suspension was stronger ER fluid than 35CBA/SO owing to higher electrical, thermal, dielectric, τy, EReff, electroactive viscoelastic properties and anti-sedimentation stability data. Moreover, not only the submicron size (≤10 μm) and reasonable electrical conductivity (10−8<σ<10−4 S cm−1) of CFA but also the fast response time to the applied electric field (≤millisecond) and the high anti-sedimentation stability (85%) of 35CFA/SO suspension offer a potential for futher electroactive vibration damping applications of waste coal ashes as a sustainable raw material.
NotesReferences1. Plachy T, Sedlacik M, Pavlinek V, Stejskal J, Graca MP, Costa LC. Temperature-dependent electrorheological effect and its description with respect to dielectric spectra. J. Intel. Mat. Syst. Str. 2016;27(7)880–886.
https://doi.org/10.1177/1045389X15600801
2. Santos J, Goswami S, Calero N, Cidade MT. Electrorheological behaviour of suspensions in silicone oil of doped polyaniline nanostructures containing carbon nanoparticles. J. Intel. Mat. Syst. Str. 2019;30(5)755–763.
https://doi.org/10.1177/1045389X18818776
3. Hao T. Applications of electrorheological fluids. Electrorheological Fluids: The Non-Aqueous Suspensions. Elsevier: Studies in Interface Science; 2005. p. 518–551.
https://doi.org/10.1016/S1383-7303(05)80025-0
4. Ko YG, Lee HJ, Chun YJ, Choi US, Yoo KP. Positive and Negative Electrorheological Response of Alginate Salts Dispersed Suspensions under Electric Field. ACS Appl. Mater. Interfaces. 2013;5(3)1122–1130.
https://doi.org/10.1021/am302891w
5. Zhang S, Winter WT, Stipanovic AJ. Water-activated cellulose-based electrorheological fluids. Cellulose. 2005;12(2)135–144.
https://doi.org/10.1007/s10570-004-0345-2
6. Kuznetsov NM, Zagoskin YD, Vdovichenko AY, et al. Enhanced electrorheological activity of porous chitosan particles. Carbohydr. Polym. 2021;256:117530.
https://doi.org/10.1016/j.carbpol.2020.117530
7. Cabuk M, Yavuz M, Unal HI, Erol O. Synthesis, characterization and electrorheological properties of biodegradable chitosan/bentonite composites. Clay Miner. 2013;48(1)129–141.
https://doi.org/10.1180/claymin.2013.048.4.09
8. Xiang L, Zhao X. Preparation of montmorillonite/titania nanocomposite and enhanced electrorheological activity. J. Colloid Interface Sci. 2006;296(1)131–140.
https://doi.org/10.1016/j.jcis.2005.08.059
9. Ozdemir C, Gumus OY, Calis-Ismetoglu G, Unal HI. Electroactively smart vibration damping suspensions of diatomite/silicone oil. Rheol. Acta. 2022;61(7)459–472.
https://doi.org/10.1007/s00397-022-01342-3
10. Kuznetsov NM, Stolyarova DY, Belousov SI, et al. Halloysite nanotubes: Prospects in electrorheology. Express Polym. Lett. 2018;12(11)958–965.
https://doi.org/10.3144/expresspolymlett.2018.82
11. Chun Y, Ko YG, Do T, Jung Y, Kim SW, Su Choi U. Spent coffee grounds: Massively supplied carbohydrate polymer applicable to electrorheology. Colloids Surf. A Physicochem. Eng. 2019;562:392–401.
https://doi.org/10.1016/j.colsurfa.2018.11.005
12. Wang SB, Boyjoo Y, Choueib A, Zhu ZH. Removal of dyes from aqueous solution using fly ash and red mud. Water Res. 2005;39(1)129–138.
https://doi.org/10.1016/j.watres.2004.09.011
13. Horiuchi S, Kawaguchi M, Yasuhara K. Effective use of fly ash slurry as fill material. J. Hazard. Mater. 2000;76(2–3)301–337.
https://doi.org/10.1016/S0304-3894(00)00205-3
14. Kim B, Prezzi M. Compaction characteristics and corrosivity of Indiana class-F fly and bottom ash mixtures. Constr. Build. Mater. 2008;22(4)694–702.
https://doi.org/10.1016/j.conbuildmat.2006.09.007
15. Sathonsalowaphak A, Chindaprasirt P, Pimraksa K. Workability and strength of lignite bottom ash geopolymer mortar. J. Hazard. Mater. 2009;168(1)44–50.
https://doi.org/10.1016/j.jhazmat.2009.01.120
16. Benitez-Polo Z, Velasco LA. Effects of suspended mineral coal dust on the energetic physiology of the Caribbean scallop. Environ. Pollut. 2020;260:114000.
https://doi.org/10.1016/j.envpol.2020.114000
17. Chindaprasirt P, Jaturapitakkul C, Chalee W, Rattanasak U. Comparative study on the characteristics of fly ash and bottom ash geopolymers. Waste Manag. 2009;29(2)539–543.
https://doi.org/10.1016/j.wasman.2008.06.023
18. Yunusa IAM, Loganathan P, Nissanka SP, et al. Application of Coal Fly Ash in Agriculture: A Strategic Perspective. Crit. Rev. Env. Sci. Tec. 2012;42(6)559–600.
https://doi.org/10.1080/10643389.2010.520236
19. Monika F, Prayuda H, Cahyati MD, Augustin EN, Rahman HA, Prasintasari AD. Engineering Properties of Concrete Made with Coal Bottom Ash as Sustainable Construction Materials. Civ. Eng. J. (Tehran). 2022;8(1)181–194.
https://doi.org/10.28991/Cej-2022-08-01-014
20. Mehta PK. Role of pozzolanic and cementious material in sustainable development of the concrete industry. In : Fly Ash, Silica Fume, Slag and Natural Pozzolans in Concrete Conference; 1998; Bangkok. p. 1–20.
https://doi.org/10.14359/5969
21. Ahmaruzzaman M. A review on the utilization of fly ash. Prog. Energy Combust. 2010;36(3)327–363.
https://doi.org/10.1016/j.pecs.2009.11.003
22. Ali AA, Al-Attar TS, Abbas WA. A Statistical Model to Predict the Strength Development of Geopolymer Concrete Based on SiO2/Al2O3 Ratio Variation. Civ. Eng. J. (Tehran). 2022;8(3)454–471.
https://doi.org/10.28991/Cej-2022-08-03-04
23. Blissett RS, Rowson NA. A review of the multi-component utilisation of coal fly ash. Fuel. 2012;97:1–23.
https://doi.org/10.1016/j.fuel.2012.03.024
24. Motasemi F, Afzal MT, Salema AA, Mouris J, Hutcheon RM. Microwave dielectric characterization of switchgrass for bioenergy and biofuel. Fuel. 2014;124:151–157.
https://doi.org/10.1016/j.fuel.2014.01.085
25. Salema AA, Yeow YK, Ishaque K, Ani FN, Afzal MT, Hassan A. Dielectric properties and microwave heating of oil palm biomass and biochar. Ind. Crops Prod. 2013;50:366–374.
https://doi.org/10.1016/j.indcrop.2013.08.007
26. Weiss JD, Kaplar RJ, Kambour KE. A derivation of the van der Pauw formula from electrostatics. Solid State Electron. 2008;52(1)91–98.
https://doi.org/10.1016/j.sse.2007.07.029
27. Cabuk M. Colloidal Behaviors of Conducting Polymer/Chitosan Composite Particles. Advances in Colloid Science. 2016. 177–188.
https://doi.org/10.5772/65125
28. Dong YZ, Choi HJ. Electrorheological Characteristics of Poly(diphenylamine)/magnetite Composite-Based Suspension. Materials. 2019;121(18)2911.
https://doi.org/10.3390/ma12182911
29. Cho MS, Choi HJ, Jhon MS. Shear stress analysis of a semiconducting polymer based electrorheological fluid system. Polym. 2005;46(25)11484–11488.
https://doi.org/10.1016/j.polymer.2005.10.029
30. Seo YP, Choi HJ, Lee JR, Seo Y. Modeling and analysis of an electrorheological flow behavior containing semiconducting graphene oxide/polyaniline composite particles. Colloids Surf. A: Physicochem. Eng. Asp. 2014;457:363–367.
https://doi.org/10.1016/j.colsurfa.2014.06.011
31. Seo YP, Seo Y. Modeling and Analysis of Electrorheological Suspensions in Shear Flow. Langmuir. 2012;28(6)3077–3084.
https://doi.org/10.1021/la204515q
32. Koukouzas N, Vasilatos C, Itskos G, Mitsis I, Moutsatsou A. Removal of heavy metals from wastewater using CFB-coal fly ash zeolitic materials. J. Hazard. Mater. 2010;173(1–3)581–588.
https://doi.org/10.1016/j.jhazmat.2009.08.126
33. Langauer D, Cablik V, Hredzak S, Zubrik A, Matik M, Dankova Z. Preparation of Synthetic Zeolites from Coal Fly Ash by Hydrothermal Synthesis. Materials (Basel). 2021;14(5)1267.
https://doi.org/10.3390/ma14051267
34. Sandstrom K, Brostrom M, Eriksson M. Coal ash and limestone interactions in quicklime production. Fuel. 2021;300:120989.
https://doi.org/10.1016/j.fuel.2021.120989
35. Liu K, Jiang J, Takasu K, Wan J, Gao W. Effect of Prestirring Time on Carbon Removal from Coal Fly Ash in the Flotation Technology. ACS Omega. 2023;8(30)27794–27801.
https://doi.org/10.1021/acsomega.3c04121
36. Choi HJ, Jhon MS. Electrorheology of polymers and nanocomposites. Soft Matter. 2009;5(8)1562–1567.
https://doi.org/10.1039/b818368f
37. Yin JB, Zhao XP. Electrorheology of nanofiber suspensions. Nanoscale Res. Lett. 2011;6:256.
https://doi.org/10.1186/1556-276X-6-256
38. Tuan LQ, Thenepalli T, Chilakala R, Vu HHT, Ahn JW, Kim J. Leaching Characteristics of Low Concentration Rare Earth Elements in Korean (Samcheok) CFBC Bottom Ash Samples. Sustainability (Basel). 2019;11(9)2562.
https://doi.org/10.3390/su11092562
39. Zhang J, Zhang X, Liu B, Ekberg C, Zhao S, Zhang S. Phase evolution and properties of glass ceramic foams prepared by bottom ash, fly ash and pickling sludge. Int. J. Miner. Metall. Mater. 2022;29(3)563–573.
https://doi.org/10.1007/s12613-020-2219-5
40. Wang SB, Wu HW. Environmental-benign utilisation of fly ash as low-cost adsorbents. J. Hazard. Mater. 2006;136(3)482–501.
https://doi.org/10.1016/j.jhazmat.2006.01.067
41. Selvam NCS, Kumar RT, Kennedy LJ, Vijaya JJ. Comparative study of microwave and conventional methods for the preparation and optical properties of novel MgO-micro and nanostructures. J. Alloy. Compd. 2011;509(41)9809–9815.
https://doi.org/10.1016/j.jallcom.2011.08.032
42. Jutarosaga T, Jeoung JS, Seraphin S. Infrared spectroscopy of Si-O bonding in low-dose low-energy separation by implanted oxygen materials. Thin Solid Films. 2005;476(2)303–311.
https://doi.org/10.1016/j.tsf.2004.10.006
43. Mozgawa W, Krol M, Dyczek J, Deja J. Investigation of the coal fly ashes using IR spectroscopy. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2014;132:889–894.
https://doi.org/10.1016/j.saa.2014.05.052
44. Murray HH. Structure and Composition of the Clay Minerals and their Physical and Chemical Properties. Developments in Clay Science. Elsevier; 2006. p. 7–31.
https://doi.org/10.1016/S1572-4352(06)02002-2
45. Ozdemir O, Cinar M, Sabah E, Arslan F, Cefik MS. Adsorption of anionic surfactants onto sepiolite. J. Hazard. Mater. 2007;147(1–2)625–632.
https://doi.org/10.1016/j.jhazmat.2007.01.059
46. Ralla K, Sohling U, Riechers D, Kasper C, Ruf F, Scheper T. Adsorption and separation of proteins by a smectitic clay mineral. Bioproc. Biosyst. Eng. 2010;33(7)847–861.
https://doi.org/10.1007/s00449-010-0408-8
47. Marandi GB, Baharloui M, Kurdtabar M, Sharabian LM, Mojarrad MA. Hydrogel with high laponite content as nanoclay: swelling and cationic dye adsorption properties. Res. Chem. Intermediat. 2015;41(10)7043–7058.
https://doi.org/10.1007/s11164-014-1797-0
48. Raposo F, Borja R, Sanchez E, Martin A. A kinetic evaluation of the anaerobic digestion of two-phase olive mill effluent in batch reactors. J. Chem. Technol. Biotechnol. 2005;80(3)241–250.
https://doi.org/10.1002/jctb.1186
49. Ramos-Tejada M, Rodriguez JM, Delgado AV. Electrorheology of clay particle suspensions. Effects of shape and surface treatment. Rheol. Acta. 2018;57(5)405–413.
https://doi.org/10.1007/s00397-018-1086-8
50. Ahmad S, Kothari R, Singh HM, Tyagi VV, Singh B, Sari A. Experimental investigation of microalgal harvesting with low cost bottom ash: Influence of temperature and pH with zeta potential and thermodynamic function. Environ. Technol. Innov. 2021;22:101376.
https://doi.org/10.1016/j.eti.2021.101376
51. Sathiya Prabhakaran SP, Swaminathan G, Viraj VJ. Energy conservation - A novel approach of co-combustion of paint sludge and Australian lignite by principal component analysis, response surface methodology and artificial neural network modeling. Environ. Technol. Innov. 2020;20:101061.
https://doi.org/10.1016/j.eti.2020.101061
52. Khadyair A, Hmeed A, Yousif F. Effect of Applying Cold Plasma on Structural, Antibacterial and Self Cleaning Properties of α-Fe2O3 (HEMATITE) Thin Film. Emerg. Sci. J. 2022;6:75–85.
https://doi.org10.28991/ESJ-2022-06-01-06
53. Mushtaq S, Aslam Z, Ali R, et al. Surfactant modified waste ash for the removal of chloro and nitro group substituted benzene from wastewater. Water Sci. Technol. 2022;86(8)1969–1980.
https://doi.org/10.2166/wst.2022.324
54. Kaur R, Goyal D. Mineralogical Studies of Coal Fly Ash for Soil Application in Agriculture. Part. Sci. Technol. 2015;33(1)76–80.
https://doi.org/10.1080/02726351.2014.938378
55. Musyoka NM, Petrik LF, Hums E, Kuhnt A, Schwieger W. Thermal stability studies of zeolites A and X synthesized from South African coal fly ash. Res. Chem. Intermed. 2015;41(2)575–582.
https://doi.org/10.1007/s11164-013-1211-3
56. Ikumapayi OM, Akinlabi ET. Comparative Study Of The Variability In The Compositions And The Effect Of Milling Time On Coal Fly Ash And Wood Fly Ash Nanoparticles. Mater. Today: Proc. 2019;18:5556–5564.
https://doi.org/10.1016/j.matpr.2019.07.005
57. Ghassemi M, Andersen PK, Ghassemi A, Chianelli RR. Hazardous Waste from Fossil Fuels. Encyclopedia of Energy. Elsevier; 2004. p. 119–131.
https://doi.org/10.1016/B0-12-176480-X/00395-8
58. Yuan JH, Wang YD, Xiang LQ, Zhao XP, Yin JB. Understanding the enhanced electrorheological effect of reduced graphene oxide-supported polyaniline dielectric nanoplates by a comparative study with graphene oxide as the support core. IET Nanodielectr. 2021;4(3)143–154.
https://doi.org10.1049/nde2.12021
59. Choi SB. Sedimentation Stability of Magnetorheological Fluids: The State of the Art and Challenging Issues. Micromachines. 2022;13(11)1904.
https://doi.org/10.3390/mi13111904
60. Vogel R, Pal AK, Jambhrunkar S, et al. High-Resolution Single Particle Zeta Potential Characterisation of Biological Nanoparticles using Tunable Resistive Pulse Sensing. Sci. Rep. 2017;7(1)17479.
https://doi.org/10.1038/s41598-017-14981-x
61. Nagele E, Schneider U. The Zeta-Potential of Blast-Furnace Slag and Fly-Ash. Cem. Concr. Res. 1989;19(5)811–820.
https://doi.org/10.1016/0008-8846(89)90052-5
62. Moldoveanu S, David V. Solvent Extraction. Modern Sample Preparation for Chromatography. Elsevier; 2015. p. 131–189.
https://doi.org/10.1016/B978-0-444-54319-6.00006-2
63. Hao T. Dielectric properties of ER suspensions. Electrorheological Fluids: The Non-Aqueous Suspensions. Elsevier: Studies in Interface Science; 2005. p. 341–423.
https://doi.org/10.1016/S1383-7303(05)80022-5
64. Karthik R, Tummala V. Voltage dependent Maxwell-Wagner polarization in dielectric heterostructures. Mater. Today: Proc. 2017;4(8)8751–8757.
https://doi.org/10.1016/j.matpr.2017.07.224
65. Agarwal P, Kim SA, Archer LA. Crowded, Confined, and Frustrated: Dynamics of Molecules Tethered to Nanoparticles. Phys. Rev. Lett. 2012;109(25)258301.
https://doi.org/10.1103/PhysRevLett.109.258301
66. Lunt RA, Jackson AJ, Walsh A. Dielectric response of Fe2O3 crystals and thin films. Chem. Phys. Lett. 2013;586:67–69.
https://doi.org/10.1016/j.cplett.2013.09.023
67. Kumar B, Kaushik BK, Negi YS. Perspectives and challenges for organic thin film transistors: materials, devices, processes and applications. J. Mater. Sci. Mater. Electron. 2014;25(1)1–30.
https://doi.org/10.1007/s10854-013-1550-2
68. Canımkurbey B. Investigation dielectric and morphological properties of fly ash collected from thermal power plant. Asia-Pac. J. Chem. Eng. 2020;15(4)e2437.
https://doi.org/10.1002/apj.2437
69. Raghavendra SC, Raibagkar RL, Kulkarni AB. Dielectric properties of fly ash. Bull. Mater. Sci. 2002;25(1)37–39.
https://doi.org/10.1007/BF02704592
70. Flesoura G, Garcia-Banos B, Catala-Civera JM, Vleugels J, Pontikes Y. In-situ measurements of high-temperature dielectric properties of municipal solid waste incinerator bottom ash. Ceram. Int. 2019;45(15)18751–18759.
https://doi.org/10.1016/j.ceramint.2019.06.101
71. Kutalkova E, Plachy T, Sedlacik M. On the enhanced sedimentation stability and electrorheological performance of intelligent fluids based on sepiolite particles. J. Mol. Liq. 2020;309:113120.
https://doi.org/10.1016/j.molliq.2020.113120
72. Liu Y, Zhao X, Yin J. Enhanced electro-responsive electrorheological efficiency of polyethylene oxide-intercalated montmorillonite nanocomposite suspension. Colloids Surf. A: Physicochem. Eng. Asp. 2023;666:131239.
https://doi.org/10.1016/j.colsurfa.2023.131239
73. Hayashida K. Highly improved dielectric properties of polymer/α-Fe2O3 composites at elevated temperatures. RSC Adv. 2016;6(69)64871–64878.
https://doi.org/10.1039/C6RA12752E
74. Ugurlu M, Kula I. The removal of colour, carotene and acidity from crude olive oil by using sepiolite. Int. J. Food Sci. Tech. 2007;42(3)359–365.
https://doi.org/10.1111/j.1365-2621.2006.01323.x
75. Komatsu LGH, Oliani WL, Lugao AB, Parra DF. Nanocomposites of irradiated polypropylene with clay are degradable? Radiat. Phys. Chem. 2016;118:11–15.
https://doi.org/10.1016/j.radphyschem.2015.06.004
76. Lu Q, Han WJ, Choi HJ. Smart and Functional Conducting Polymers: Application to Electrorheological Fluids. Molecules. 2018;23(11)2854.
https://doi.org/10.3390/molecules23112854
77. Seo Y. A new yield stress scaling function for electrorheological fluids. J. Non-Newton. Fluid Mech. 2011;166(3)241–243.
https://doi.org/10.1016/j.jnnfm.2010.11.010
78. Kutalkova E, Plachy T, Osicka J, Cvek M, Mrlik M, Sedlacik M. Electrorheological behavior of iron (II) oxalate micro-rods. RSC Adv. 2018;8(44)24773–24779.
https://doi.org/10.1039/C8RA03409E
79. Liu SX, Xue C, Yang H, et al. Intercalated hybrid of kaolinite with KH2PO4 showing high ionic conductivity (~10–4 S cm–1) at room temperature. Solid State Sci. 2017;74:95–100.
https://doi.org/10.1016/j.solidstatesciences.2017.10.007
80. Lokanatha S, Bhattacherjee S. Variation of electrical properties in sepiolite during dehydration. Bull. Mater. Sci. 1985;7(2)111–115.
https://doi.org/10.1007/BF02744418
81. Guseinov AA. Electrical properties of montmorillonite studied together with the processes occurring under thermal activation. Izv. Phys. Solid Earth. 2017;53(6)845–854.
https://doi.org/10.1134/S1069351317060015
82. Izci E. Dielectric properties of some cationic forms of laponite clay. Key Eng. Mater. 2004;264–268:1357–1360.
https://doi.org/10.4028/www.scientific.net/KEM.264-268.1357
83. Meheust Y, Parmar KPS, Schjelderupsen B, Fossum JO. The electrorheology of suspensions of Na-fluorohectorite clay in silicone oil. J. Rheol. 2011;55(4)809–833.
https://doi.org/10.1122/1.3579189
84. Liu Z, Zhao ZJ, Jin X, Wang LM, Liu YD. Preparation of Cellulose/Laponite Composite Particles and Their Enhanced Electrorheological Responses. Molecules. 2021;26(5)1482.
https://doi.org/10.3390/molecules26051482
85. Hao BN, Guo YX, Liu YD, Wang LM, Choi HJ. Highly transparent electrorheological fluids of silica nanoparticles: the effect of urea modification. J. Mater. Chem. C. 2016;4(33)7875–7882.
https://doi.org/10.1039/C6TC02154A
86. Liang Y, Huang D, Zhou X, et al. Efficient Electrorheological Technology for Materials, Energy, and Mechanical Engineering: From Mechanisms to Applications. Engineering. 2023;24:151–171.
https://doi.org/10.1016/j.eng.2022.01.014
87. Omambala JR, McIntyre EC, Gallo AA. Electrorheological Effects of Synthesized Octa-cyanopropylsilsesquioxane Cage Structure. ACS Omega. 2019;4(25)20955.
https://doi.org/20963.10.1021/acsomega.9b02105
88. Silfhout AM, Engelkamp H, Erne BH. Magnetic Sedimentation Velocities and Equilibria in Dilute Aqueous Ferrofluids. J. Phys. Chem. B. 2020;124(36)7989–7998.
https://doi.org/10.1021/acs.jpcb.0c06795
Fig. 3Dielectric spectra of (a) 35CFA/SO and (b) 35CBA/SO suspensions: dielectric constant (ɛ′) and dielectric loss factor (ɛ″) versus frequency (solid lines for Cole-Cole fitting). 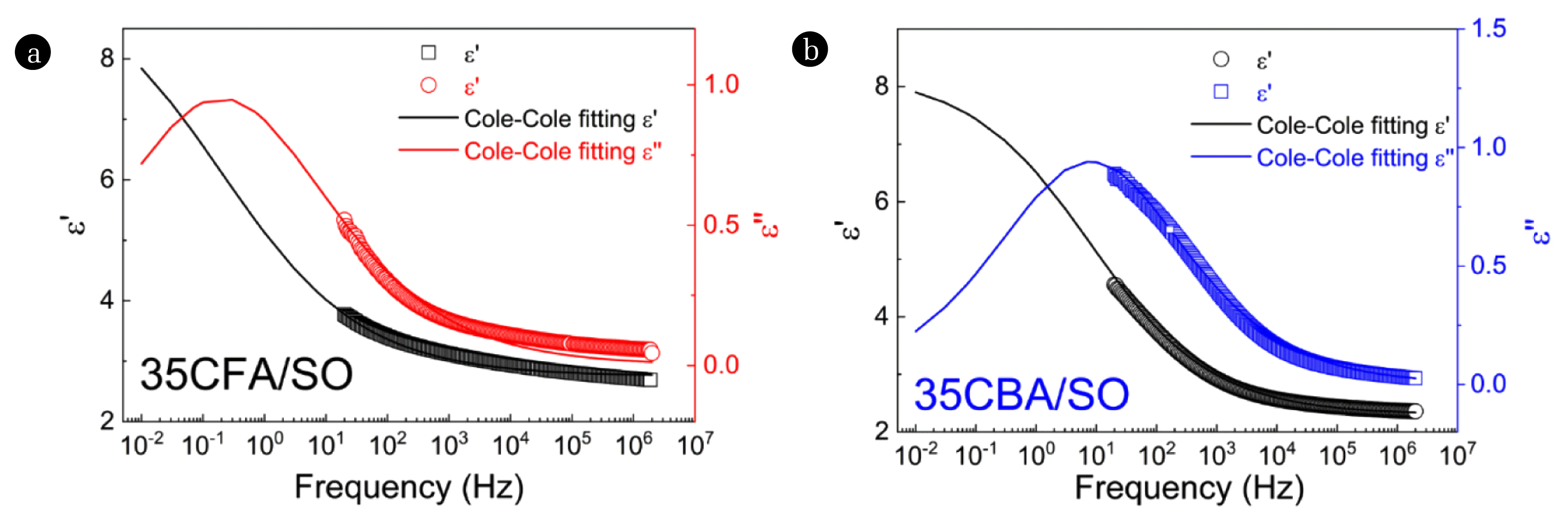
Fig. 4(a,b) The flow curves under various applied electric fields (solid blacklines, E = 0 kV mm−1, for the Hershel-Bulkley and colored lines, E ≠ 0 kV mm−1, for the Seo-Seo models fitting) and (c) time dependent ER responses of 35CFA/SO and 35CBA/SO suspensions. 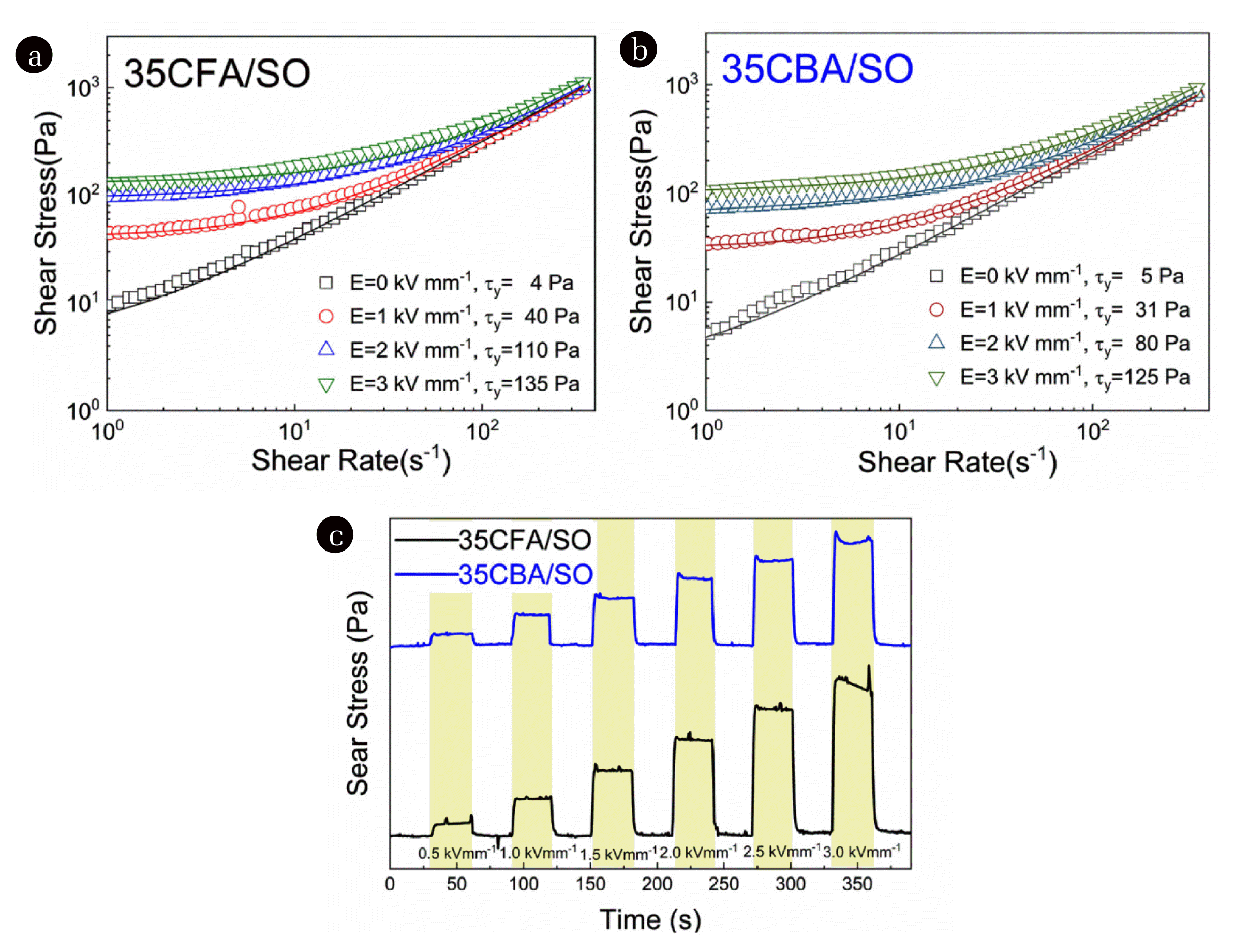
Fig. 5The changes of elastic moduli (G′) with shear stress under various electric field strengths for (a) 35CFA/SO and (b) 35CBA/SO suspensions. 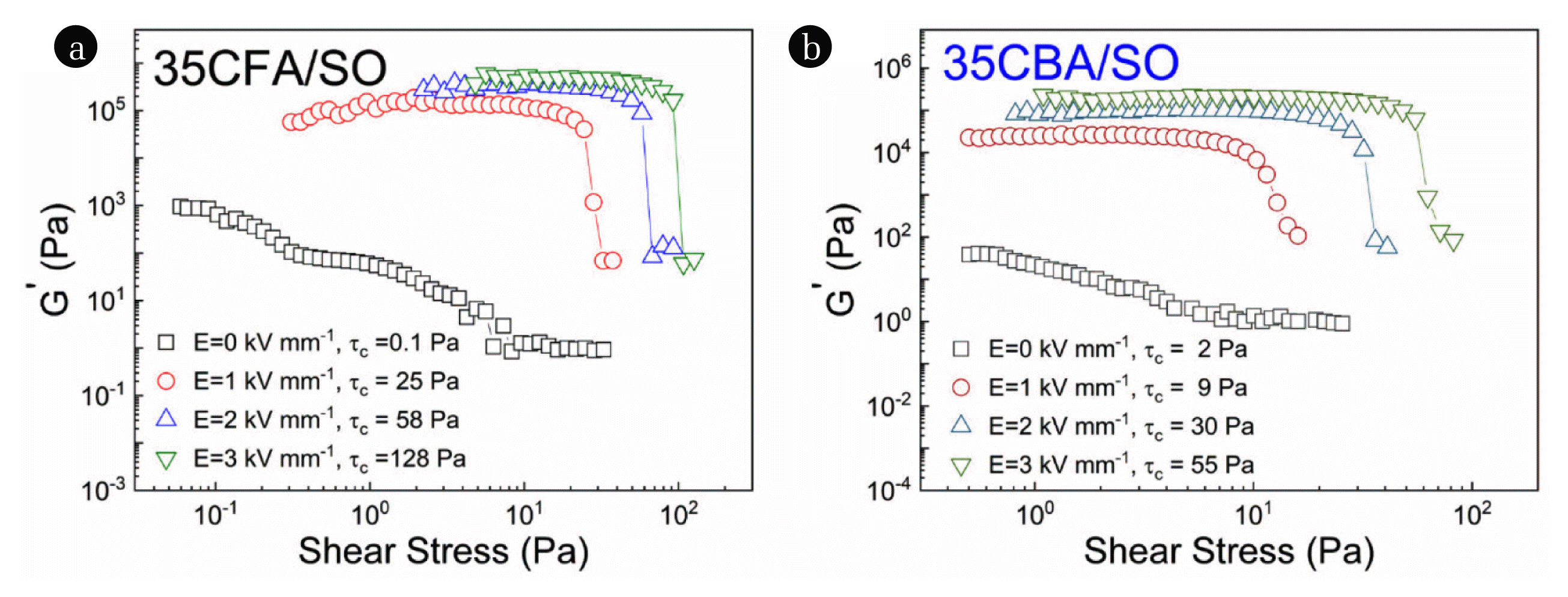
Fig. 6The changes in (a,b) the elastic (G′) moduli with time (f = 1 Hz), (c d) the elastic (G′) and viscous (G″) moduli with frequency, (e,f) damping factor (tanδ) with frequency, and (g,h) the elastic moduli (G′) with temperature in the range of 25–80°C by application of τ = 5 Pa for 35CFA/SO and 35CBA/SO suspensions. 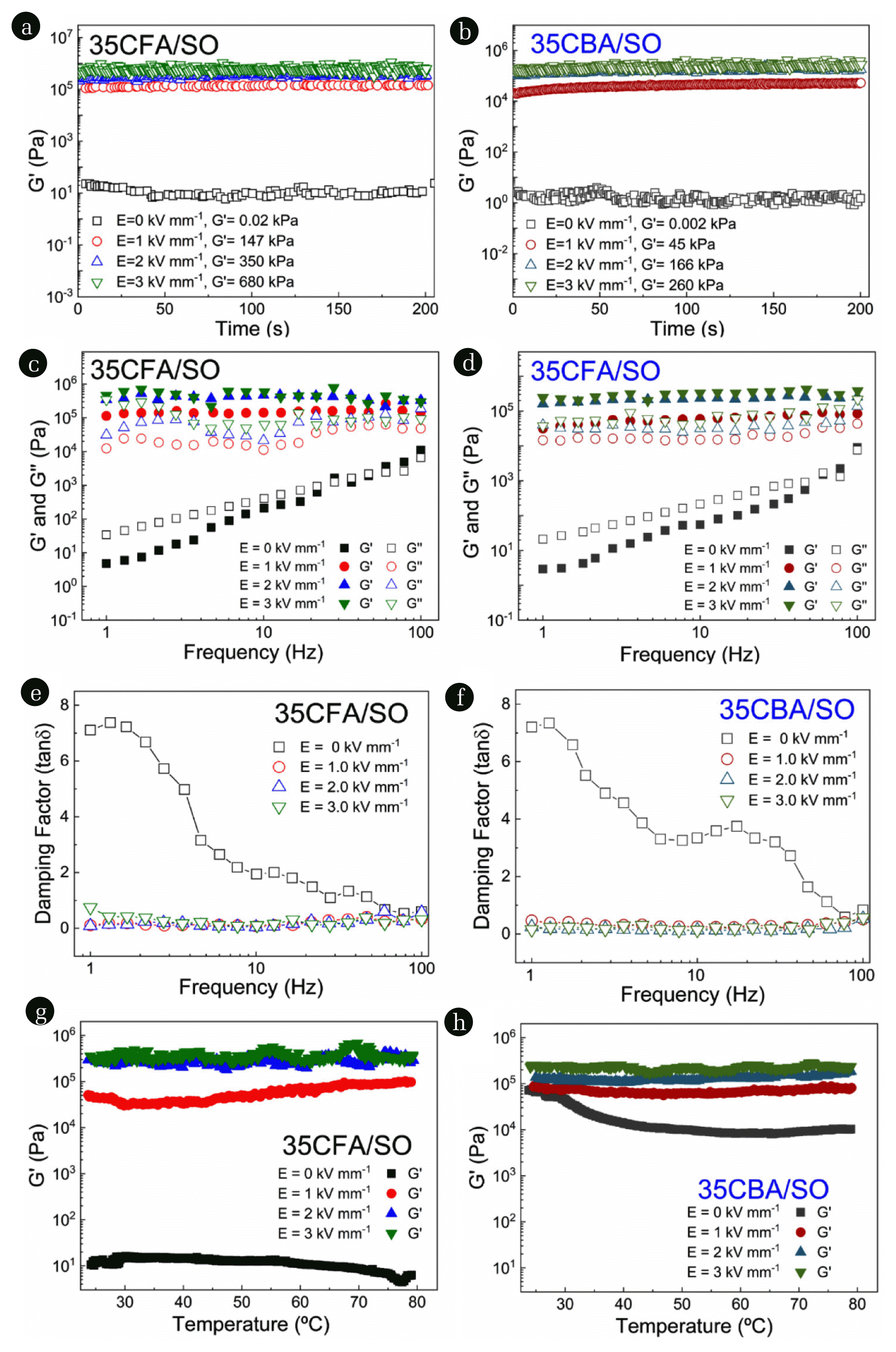
|
|
||||||||||||||||||||||||||||||||||||