AbstractThe market for lithium-ion batteries (LiBs) is growing rapidly, the demand for lithium (Li) in the form of lithium carbonate (Li2CO3), which is the most common lithium mineralization form, is therefore also increasing significantly. Li is conventionally extracted as Li2CO3 using sodium carbonate (Na2CO3) to precipitate Li ions in an aqueous Li solution. However, Na2CO3 can also be replaced by CO2, which highlights the potential of using CO2 as a sustainable and economically viable alternative. This review focuses on technologies that utilize CO2 for Li2CO2 precipitation. First, the use of CO2 gas and Na2CO3 as carbonate sources are compared, and the need to consider important operating conditions with CO2 bubbling are then presented. Attempts made to increase the specific surface area of the reaction surface to enhance the utilization of CO2 gas and to produce micro-sized Li2CO3 powders are then reported, and the limitations associated with CO2 gas utilization are discussed. Although CO2 precipitation has limitations in terms of efficiency, scalability, and the fine-tuning of reaction conditions, this review shows that if CO2 precipitation technology is further developed, its use could be key to extracting and recycling next-generation Li.
Graphical Abstract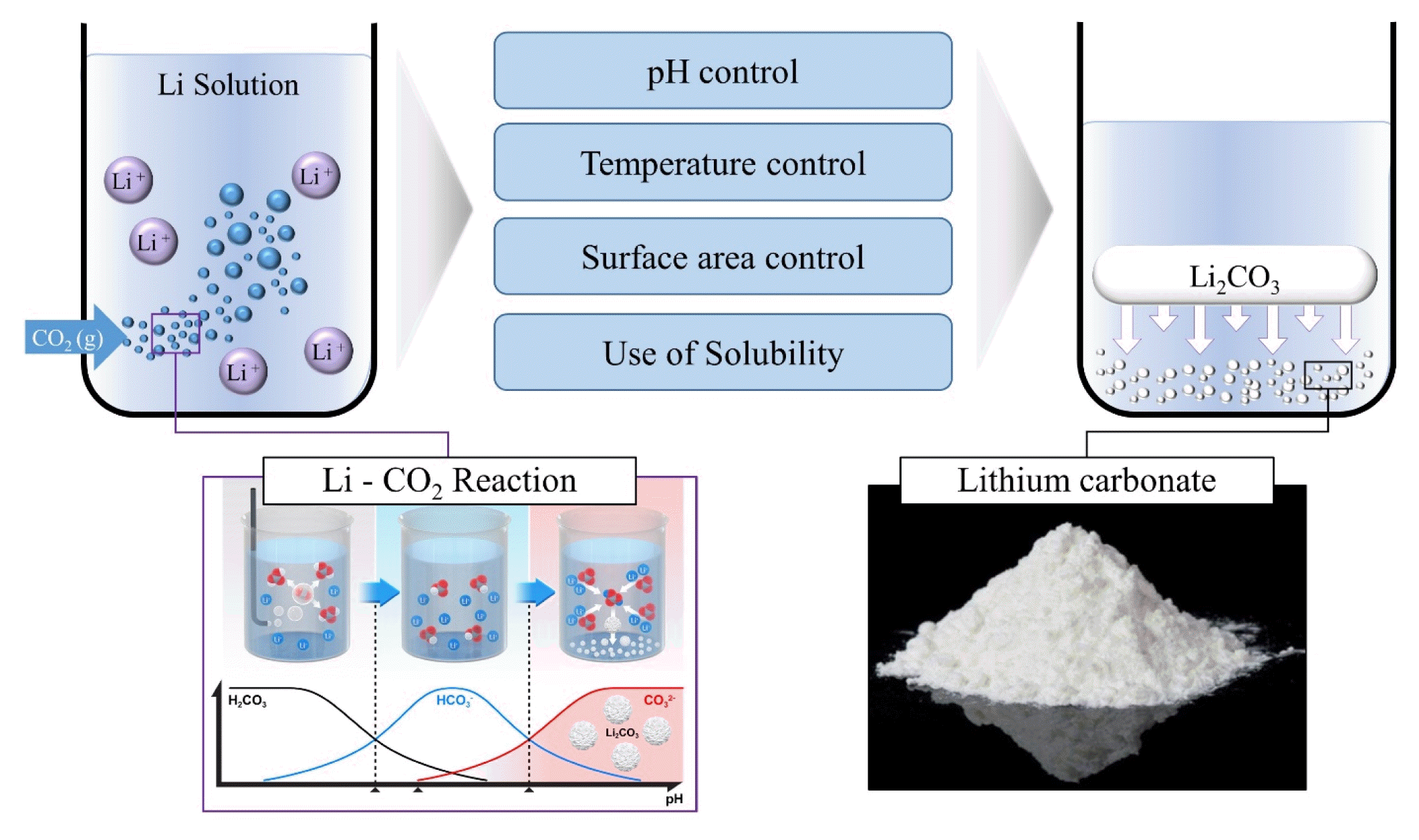
1. Introduction1.1. Development of the Lithium-ion Battery (LiB)The advent of lithium (Li)-ion technology in 1991 was marked by the commercialization of the first LiB, and this signified the beginning of a transformative epoch in technology [1,2]. The notable advantages of Li in batteries compared to other metals are its high electrochemical potential and energy density [3]. These compelling benefits have resulted in LiBs becoming indispensable constituents of portable electronic devices.
The introduction of LiB technology resulted in an unprecedented increase in its use, which was initially fueled by the adoption of portable electronics (such as mobile phones, computers, smartphones, and tablets) [4]; however, the scope of the technology broadened as it matured. By 2010, LiBs had permeated the power tool market and further expanded into the realm of electric vehicles. However, despite the diversity of their applications, portable electronics remained the dominant consumer of LiBs until the early-2010s [5], and the pattern of LiB consumption has since changed dramatically. Once predominantly consumed by portable electronics, the demand landscape has expanded and diversified. A significant amount of usage now relates to larger applications, such as electric vehicles and grid-scale energy storage systems [6]. This substantial shift indicates a transition towards large-scale energy storage solutions, and it underscores the influential role of LiBs in the evolving landscape of sustainable energy technologies.
From a mere few thousand units in 2010, the stock of electric vehicles (EVs) had increased dramatically to 11.3 million by 2020, and projections indicate a burgeoning fleet of 142 million EVs on roads by 2030 [7]. In parallel with this growth, the global production of Li tripled between 2010 and 2020 [8]. Based on these projections, the demand for Li is expected to reach 2 million tons per year by 2030 [9], and current projections estimate an 18- to 20-fold increase in the demand for Li by 2050, if existing extraction policies persist. However, the use of novel and more sustainable extraction strategies could potentially escalate this demand 40-fold by 2050 [10].
With the development of lithium secondary batteries, Li has become an essential resource for modern technology, especially in the form of Li carbonate (Li2CO3). This compound is the main source of Li, and it accounts for most of the world’s Li production (~60%) [11,12]. The demand for LiBs alone is expected to increase the production of Li2CO3 to a scale of 2 million metric tons annually by 2030 [13]. Li2CO3 is typically obtained from the hard rock mining of spodumene ore and from Li concentrated in the salt lake brine [14]. In hard rock mining, spodumene is chemically processed to liberate Li ions (Li+), which are then precipitated as Li2CO3, primarily through the addition of sodium carbonate (Na2CO3) [15–17]. Na2CO3 is also commonly employed as the carbonate source to recover Li from the salt lake brine via a series of steps involving the evaporation of brine, lithium concentration, and inducing Li2CO3 precipitation [18–20].
1.2. LiBs and Carbon Dioxide (CO2)It is acknowledged that the accumulation of atmospheric carbon dioxide (CO2) is a serious threat to the planet [21], and the Paris Agreement in 2015 established long-term temperature goals aimed to control average global warming at 2°C and to limit it to 1.5°C by the end of the 21st century [22]. In this respect, the rapidly growing use of the LiB is recognized as a carbon reduction technology that can ameliorate the problems associated with increases in atmospheric CO2 caused by decades of industrialization and urbanization.
However, it is essential to consider the lifecycle of these batteries to fully understand their environmental impact. Notably, the production and recycling of Li, a crucial component of LiBs, can be a significant source of CO2 emissions [23]. For example, an EV battery with an energy density of approximately 78 kWh/kg produces an estimated 172–196 kg CO2 eq/kWh through raw material extraction, cell manufacturing, and assembly [24,25]. Therefore, although LiBs are currently promoted as tools to achieve carbon neutrality, their production processes indirectly contribute to CO2 emissions.
To realize a circular economy and a sustainable Li production and recycling cycle, it is necessary to explore methods to mitigate these CO2 emissions. One promising approach involves the use of CO2 for Li precipitation. CO2 is an abundant greenhouse gas, and utilizing it for Li precipitation could provide dual benefits where CO2 emissions associated with Li production are mitigated and turned into a valuable asset [18]. This method has shown promise in several studies, and it represents a significant step toward a more sustainable and eco-friendly Li production process. CO2 is typically regarded as a waste product, but it could be transformed into a valuable asset for Li production, thereby contributing to a sustainable and circular economy [26–27]. In this context, it has become increasingly vital to explore and understand Li2CO3 precipitation methods while also investigating innovative techniques that could potentially revolutionize the industry and result in a more sustainable and low-carbon society [28]. In addition to the CO2 reduction benefits, the production of Li2CO3 using CO2 is expected to be beneficial in terms of Li2CO3 purity [29].
1.3. Precipitation of Li2CO3 using CO2In this review, we investigate and report the precipitation of Li2CO3 using CO2 as the carbonate source. Until recently, Na2CO3 has been used as the carbonate source for Li precipitation; however, CO2 could be a viable substitute, and carbonate mineralization is a promising carbon capture utilization technology that can be applied to the production of Li2CO3.
This study aims to overcome the existing limitations of Li precipitation methodologies by investigating the use of CO2 gas for Li precipitation. Leveraging CO2 for this purpose could potentially create a greener and more efficient approach for extracting Li, which aligns with the broader goals of advancing towards a low-carbon and sustainable future (Fig. 1). Despite its importance, there have been limited systematic reviews of Li2CO3 production using CO2. To the best of my knowledge, there has yet to be a comprehensive review paper addressing this specific topic. By analyzing the mechanics, methods, and environmental implications of using CO2 gas for Li precipitation, this review aims to determine its potential as a viable alternative to the traditional use of Na2CO3.
2. Li Extraction and Recycling Methods2.1. Use of Na2CO3 as a Carbonate SourceThis section focuses on the Li2CO3 precipitation processes used to extract and recycle Li and the prevailing reliance on Na2CO3 as the primary agent.
An Li-rich solution and a carbonate source are essential for all Li2CO3 production processes [11]. The Li-rich solution is prepared using the following methods in these three processes:
First, in hard rock mining, extracted spodumene ore undergoes a series of processing stages. It is first crushed and heated in a kiln to produce beta-spodumene, which is more reactive and amenable to Li extraction. The beta-spodumene is then subjected to acid roast using sulfuric acid to generate an Li sulfate (Li2SO4) solution [15,16].
Second, to recover Li from brine, an Li-rich solution is first prepared by concentrating the brine and separating the Li. Li salt-rich brines are prepared by extracting Li from underground reservoirs and concentrating the Li content through solar evaporation in large ponds. Concentrated brine can be pretreated with an Li-selective precipitant, such as phosphate, to produce a primary precipitate, which is re-dissolved to prepare an Li-rich solution [18,19].
Lastly, when recycling Li from spent LiBs, an Li-rich solution is prepared by acid leaching after shredding the spent battery, and this is considered to be a hydrometallurgical process [30]. Pyrometallurgy is also used as a recycling process for spent batteries; however, this method has now been limited for use in Li extraction due to its high energy consumption [31] and the potential loss of Li during the process [32].
A carbonate source is then prepared using Na2CO3. In Li extraction and recycling methodologies, Na2CO3 plays a pivotal role in the precipitation of Li from Li-enriched solutions [11], and the chemical process is described by the following equation:
Through this reaction, Li+ in the solution combine with sodium carbonate (Na2CO3) to produce Li2CO3. The Li2CO3 precipitates owing to its low solubility in water, which enables its separation from the rest of the solution [33].
However, the precipitation of Li2CO3 using Na2CO3 has notable drawbacks. One significant disadvantage is the production of sodium- based by-products, which require further processing or disposal [34]. This can lead to additional refining steps that add to the process costs. Furthermore, the use of Na2CO3 does not contribute to carbon capture or reduction strategies; therefore, exploring alternative methods, such as the use of CO2 as a carbonate source for Li precipitation, would provide both economic and environmental advantages [26, 27].
2.2. Li Precipitation with CO2The precipitation of Li2CO3 using CO2 gas is gaining attention from the Li production and recycling industry. The process essentially involves the reaction between the Li+ present in an aqueous solution and CO2, which results in Li2CO3.
As shown in Fig. 2, the precipitation of Li2CO3 is completed in three steps, and these are outlined as follows:
First, CO2 gas is dissolved in water to become carbonic acid (H2CO3), which lowers the pH of the solution:
However, if the pH is below 6.3, H2CO3 is the dominant carbonate species, which is theoretically unfavorable for Li2CO3 precipitation. When the pH exceeds 6.3, bicarbonate is the dominant carbonate species. When it reacts with Li, it exists as Li bicarbonate (LiHCO3), which is highly soluble and ionizable.
Therefore, in most cases Li and bicarbonate ions exist in water systems.
Finally, when the pH exceeds 10.3, carbonates are the dominant carbonate species:
2.3. Factors Influencing Li2CO3 PrecipitationAlthough a chemical understanding of the reaction is relatively straightforward, applying the process requires optimization of these reaction parameters. Various factors (such as temperature, pH, and the Li+ concentration of the solution) significantly influence the yield and efficiency of the reaction [36]; these factors are described in detail below:
First, precipitation is affected by temperature. the solubility of Li2CO3 shifts with increasing temperature and decreases with increasing temperature. Quantitatively, at room temperature (20°C), Li2CO3 has a solubility of approximately 0.18 mol/L, but this decreases to approximately 0.1 mol/L at higher temperatures of around 90°C. This decrease in solubility shows a linear correlation with temperature [37], which highlights the significance of temperature for the effective precipitation of Li2CO3 [38].
Second, pH strongly influences the carbonation process. For instance, the carbonate species in the solution may be bicarbonate or carbonate depending on the pH (refer to Eq. (5)). Considering that LiHCO3 is approximately seven times more soluble in water than Li2CO3 [39], it can be appeared that the Li2CO3 precipitation is affected by the pH. On the other hand, the pH of a solution affects the solubility of CO2, and the precipitation reaction occurs only when CO2 dissolved in the liquid phase actively participates in the carbonation reaction. The reaction sequence includes the following steps:
A critical aspect of this process is maintaining a high pH, which is typically achieved by introducing sufficient amounts of hydroxide ion. Despite the theoretical considerations favoring a pH above 10.33 and 8.95 for the formation of CO32− in freshwater and seawater, respectively [40], practical observations show that a pH above 8 is required for effective carbonate ion formation. This discrepancy may arise from various factors under real experimental conditions, including temperature and the presence of other ions.
Third, the process is influenced by the concentrations of the Li+ in the solution. Assuming that the supply of CO2 is sufficiently large, the concentration of Li+ determines whether supersaturation occurs and affects the precipitation reaction. The supersaturation ratio, calculated as the solubility-to-concentration ratio, must exceed 1 to induce the formation of Li2CO3 precipitates [41]. The supersaturation ratio affects both the nucleation and growth rates of the precipitates. A high supersaturation ratio is preferred when a small particle size is desired because it leads to a higher nucleation rate relative to the growth rate, and consequently to a smaller particle size [42]. The purity of the solution plays an important role in the success of this process, and certain ions in the reaction mixture can interfere with the formation of Li2CO3. For example, the presence of sodium or potassium ions can lead to the formation of their bicarbonates, which may compete with Li for CO2 and reduce the overall yield of Li2CO3. In addition, the presence of divalent ions, such as calcium or magnesium, may lead to the formation of insoluble carbonates, which can potentially cause scaling issues in industrial setups [43].
In addition, the reaction between Li and CO2 can be promoted by increasing CO2 partial pressure [44], and it can also be influenced by factors such as the stirring rpm and CO2 feed rate. Therefore, it is important to optimize the reaction conditions to achieve the desired Li2CO3 precipitation. The following sections present information from various studies focusing on the production of Li2CO3 using CO2.
3. Techniques Used in CO2-based Li2CO3 PrecipitationThis section describes the precipitation of Li2CO3 using CO2. The schematic conditions presented in studies based on CO2 utilization in each stage are presented in Table 1. The table summarizes the CO2 supply flow rate, stirring, carbonation time, recovery rate, purity, particle size, pH control, and temperature, which are most commonly considered in experiments. Each category in Table 1 are aligned with each subsection of this section.
3.1. Comparison between CO2 and Na2CO3 MethodsIn this section, previous studies comparing the precipitation of Li2CO3 with CO2 and that of Li2CO3 with Na2CO3 are presented. Establishing this comparison provides a foundation for analyzing and assessing the innovations and potentials of using CO2-based processes. The analysis aims not to resolve or identify the challenges with each method but to offer a comparative perspective of Li2CO3 precipitation methodologies.
Han et al. conducted experiments on the semi-batch precipitation of Li2CO3 using two distinct methods: precipitation using CO2 (heterogeneous) and precipitation using an aqueous Na2CO3 solution (homogeneous) [45]. For both methods, the shape of the primary crystals varied slightly depending on the temperature: ellipsoid shapes were observed at 50°C, while elongated sheets were formed at 25°C. In precipitation using CO2, the final pH value played an important role, and it was ascertained that a level of 8 was required to halt the CO2 input. A higher rotation speed resulted in smaller particle sizes because the smaller bubbles accelerated the chemical reaction. Particles were larger at a higher temperature, and a doubled crystal yield was observed at 50°C. However, the gas feed rate did not significantly influence the particle size.
In the case of precipitation using an aqueous Na2CO3 solution, the particle size was also smaller at higher rotational speeds, but the pumping rate of the Na2CO3 solution made no notable impact. The crystal yield was higher than that obtained by precipitation using CO2, because a higher concentration of CO32− was provided by the Na2CO3 saturated solution. A high alkaline concentration was required to precipitate Li2CO3 from the Li2SO4 solution. The use of solid Na2CO3 improved the recovery of Li without increasing the overall solution volume. It was considered that the residues from both processes could potentially be used to precipitate other salts, such as Li3PO4. The study concluded that both methods were viable for recovering Li as Li2CO3 salt from a Li2SO4 solution; however, it was acknowledged that additional research is needed to enhance the yield and crystal purity.
In a comprehensive analysis conducted by Battaglia et al., the precipitation performance of Li2CO3 in terms of recovery and purity was examined using both CO2 and Na2CO3, as indicated in Table 2 [34]. In their approach, an aqueous Li solution derived from dilute Li-rich brine was used for CO2 precipitation. The pH of this solution was meticulously regulated at 8.5 or higher through the addition of NaOH. From their findings, the CO2 precipitation method was found to perform well in terms of recovery and purity in all comparisons, although a lower recovery rate was observed in the reference case. In particular, the authors found that CO2 precipitation was very effective in removing Mg, as shown in Table 2. Furthermore, by incorporating an ethanol washing step, they successfully obtained Li2CO3 with a near purity of 100%. These results underscore the potential of using CO2 as a precipitant to obtain high-purity Li2CO3.
Although the CO2 precipitation method for Li2CO3 extraction has not been as extensively studied as the Na2CO3 approach, it shows promise for optimization. Although many studies have reported higher recovery rates with Na2CO3, there is a potential to enhance the novel CO2 method through better temperature and pH management, which suggests its potential to match, or even surpass the efficiency of the conventional method. The following sections focus on research efforts that have sought to enhance the performance of the CO2 method by integrating various novel methods.
3.2. Case Studies of Direct CO2 BubblingThis section introduces the technique used to feed CO2 gas by bubbling it directly into an aqueous Li solution to precipitate Li. This method provides a substantial advantage by preventing the introduction of Na-based impurities that are typically associated with traditional Na2CO3 precipitation, where implementing pure CO2 gas circumvents extraneous contamination. However, this approach presents a significant challenge: the persistent decrease in pH inherent to the use of CO2 gas necessitates consistent and meticulous adjustments to preserve optimal pH levels.
One study introduced the use of CO2 as a substitute for Na2CO3 for the carbonation of Li hydroxide in a concentrated KOH solution to produce Li2CO3 [46]. This study revealed that the process can be divided into four distinct stages: Stage I, rapid consumption of free hydroxide ions; Stage II, burst nucleation; Stage III, gradual precipitation; and Stage IV, the formation of bicarbonate, as shown in Fig. 3a. In Stage I, the carbonation rate gradually increased as the concentration of hydroxide ions decreased rapidly, indicating that reaction in Eq. (8) was predominant. This stage prepares for the supersaturation necessary for the nucleation of Li2CO3. In Stage II, a direct correlation between the carbonation rate and time was observed, indicating the burst nucleation of Li2CO3 and the predominance of reaction in Eq. (9). In stage III, the carbonation rate began to level off, indicating that the precipitation of Li2CO3 was near equilibrium. Reactions in Eq. (8) and Eq. (9) occurred predominantly during this stage. Finally, in stage IV, the presence of bicarbonate indicated an excess injection of CO2, and the reverse reaction of Eq. (9) was dominant during this period. As shown in Fig. 3b–c, the effect of temperature was dominant during the early stages (stages I and II). Temperature significantly affected the maximum carbonation rate and the particle size distribution (PSD) of Li2CO3. As the temperature increased from 30°C to 90°C, the maximum carbonation rate also rose from ~67% to ~83%, primarily due to the decrease in solubility of Li2CO3. Interestingly, the maximum volume average diameter of Li2CO3 particles was achieved at 70°C, illustrating the intricate influence of temperature on the particle size of Li2CO3. Furthermore, the study found a correlation between the absorption rate of CO2 and the crystallization rate of Li2CO3, suggesting that crystallization notably enhances the CO2 absorption efficiency, especially at its onset. Li2CO3 produced during the carbonation process was of high purity and crystallinity, validating the carbonation process as a promising approach for CO2 utilization and Li recovery from potent alkaline solutions.
Menghua Tian’s research group [47] made several noteworthy observations and suggested that the presence of ammonium ions ([NH4+]) significantly influences the precipitation of Li2CO3. Specifically, these ions can impede the recarbonation of Li2CO3 crystals (i.e., dissolution of Li2CO3) and prevent over-carbonation, thereby simplifying the gas–liquid reactive crystallization process. In the research, it was found that by increasing the NH3 · H2O concentration from 200 g/L to 400 g/L, the supersolubility of the solution increased from 0.38 mol/L to 0.55 mol/L, the reactive crystallization induction period was extended from 38 min to 50 min, [Li+] conversion improved from 43.0% to 49.6% within 120 min, and the particle size decreased, from around 52 μm to 28 μm. This enhancement resulted in a longer induction period and a more stable metastable solution. They also found that the absorption process of CO2 in an NH3·H2O solution was more temperature-sensitive than that in an LiOH–CO2 system. This characteristic can be effectively utilized to regulate the quantity of absorbed CO2, thereby providing a mechanism for controlling the crystallization process.
In their further research [48], Tian et al. demonstrated that the reaction temperature significantly affects the Li2CO3 particle size and distribution. Increasing the temperature from 25°C to 60°C doubled the mean particle size. They also found that higher temperatures decreased the secondary nucleation rate, while increasing the growth rate. These findings provide crucial insights into particle size regulation during Li2CO3 production. In their innovative approach, they introduced microbubbles into a weakly basic LiCl-NH3·H2O–CO2 system to enhance gas–liquid mass transfer and CO2 absorption, which resulted in improved local supersaturation and the creation of reactive micro-zones [35]. Using CO2 as a precipitant for the production of Li2CO3 helped to avoid metal ion impurities and provided a pathway for beneficial CO2 conversion. In addition, the introduction of microbubbles significantly reduced the particle size of Li2CO3 from a D90 of 32.5 μm to 14.6 μm. This reduction was attributed to increased local supersaturation, which promoted nucleation and the formation of reactive micro-zones that inhibited crystal growth. This method yielded a Li2CO3 product that met the battery-grade requirements in terms of both purity and particle size.
A coupled reaction and solvent extraction process for the production of Li2CO3 from LiCl and CO2 was introduced in Zhou et al. [49] Considering that the reaction is not spontaneous because the pH is lowered by carbonation, this study emphasized the need to remove hydrogen chloride to allow the reaction to proceed uninterrupted. The resultant Li2CO3 crystals from the room temperature open reactor process were observed to have a dual PSD: micro-sized particles (0.1–1 μm) and larger ones (10–100 μm). Interestingly, the bulk crystals fell within a smaller size range. This study provided insights into the crystal growth conditions. Radial growth occurred in the free aqueous phase resulting in large flakes or bulk crystals, whereas growth within water-in-oil structures yielded smaller ellipsoidal crystals. This study also revealed that factors such as a large phase ratio, surfactant addition, low temperature, and a short reaction time can help increase the formation of small-particle crystals while curtailing the growth of larger-particle crystals.
Similar to the application of microbubbles and the formation of water-in-oil structures, which significantly improved the precipitation of Li2CO3 in the aforementioned research, similar systematic adjustments can also lead to enhanced sedimentation characteristics. In the next section, we introduce studies that have employed a range of systems to exploit large specific surface area and enhance the properties and production of Li2CO3.
3.3. Use of Improved CO2 Gas-based Technology for Large Specific Surface AreaIn this section, our focus moves towards improving the CO2 gas reaction rate by utilizing large specific surface areas. A fundamental principle of chemistry is that an increased reaction surface area accelerates the reaction rate. In the context of Li2CO3 production, systems that artificially create structures with large specific surface areas, such as films or small droplet shapes, can increase the interaction area between Li+ and CO3 2−, thereby enhancing the reaction efficiency. Innovative studies have expanded the reactive interface by incorporating techniques such as spraying the liquid onto CO2 gas or passing the liquid through a microchannel, including microfilm. In this section, we explore such systems, how they operate, and their impact on the production of Li2CO3, thereby shedding light on their potential to improve traditional production methods.
An effective strategy used to increase the specific surface area involves the reaction being conducted in the form of a thin film, as shown in Fig. 4a [50]. In this study, a system for constructing a falling film was developed. In an associated study, the gas–liquid reactive crystallization of CO2 gas and LiOH solution was systematically investigated using a falling-film column to produce Li2CO3 crystals. This study identified three critical fluid dynamic parameters of the falling-film column: the Reynolds number, falling-film thickness, and exposure time. The reaction time was 26 min at pH 9 in a falling-film column, whereas that in a conventional stirred reactor was 60 min. During crystallization, the mean particle size changed from 5.72 μm at t = 7.5 min to 41.62 μm at t = 10 min, and then to 74.78 μm at t = 20 min. When the CO2 flow rate increased from 0.5 L/min to 1.0 L/min, the carbonation time decreased from 20 min to 15 min. At varying temperatures from 10°C to 40°C, there was no difference in the absorbed CO2, but the output of Li2CO3 at 40°C was 0.71 mol/L, which was more than triple the amount of 0.22 mol/L at 10°C. In addition, as the temperature increased from 10°C to 40°C, the volume mean particle size increased from 49.60 μm to 96.29 μm. It was determined that to obtain high Li2CO3 yields, the final pH of the carbonation reaction should be maintained within the range of 9.0–9.5. Interestingly, crystallization expedited the transport of CO3 2− from the interfacial liquid film to the bulk solution, thereby promoting the overall absorption rate. The resulting products exhibited high purity (99.8%), with the particles displaying a distinctive flower-like composition comprising numerous plate-like primary crystals.
Sun et al. [51] used a spinning disk reactor (SDR) to synthesize Li2CO3 through the liquid reactive crystallization of LiOH and CO2 (Fig. 4b). The study showed that the particle size and yield rate of Li2CO3 were influenced by multiple factors. In this respect, ultrasound was found to be a primary determinant of particle size, as it prevented agglomeration and resulted in smaller particle sizes. Other factors, such as the reaction temperature, CO2 gas flow rate, and the spinning disk rotation speed were also found to influence the particle size. For example, when the concentration of LiOH solution was 1.5 mol/L at a temperature at 20°C, the yielding rate was less than 30%, whereas with an LiOH solution with a concentration of 2.0 mol/L at a temperature at 40°C, the yielding rate rocketed to 73%. The authors also found that high temperature increased the average volume size of the Li2CO3 particles and increasing the temperature from 20°C to 40°C resulted in an average particle size of 37–90 μm. Several operational parameters were also investigated, including the rotational speed of the rotating disc, circulation speed of the LiOH slurry, CO2 flow rate, and the influence of the ultrasonic field. The ultrasonic field, temperature, and CO2 flow rate were considered to be the main factors affecting the particle size. In contrast, the yield was significantly affected by the temperature, LiOH solution concentration, and CO2 flow rate, while the ultrasonic field and rotational speed of the rotating disc had limited effects. The scanning electron microscopy images revealed flower-shaped particles composed of plate-like primary crystals.
In another study by Sun et al. [52], spray pyrolysis of LiHCO3 was employed to produce Li2CO3 hollow spheres (Fig. 4c). The X-ray diffraction pattern of the product closely matched the standard pattern, suggesting a pure Li2CO3 crystalline phase in the synthesized product. Scanning electron microscopy revealed that the self-assembled hollow spheres were composed of primary particles approximately 200 nm in size. Furthermore, a crystal size distribution analysis indicated that the macro-volume means crystal size ranged from 4 μm to 9 μm, and this varied based on experimental conditions. Notably, the Brunauer–Emmett–Teller (BET) surface area reached 7.24 m2/g, surpassing the highest value previously reported and suggesting its potential application in the Li-battery industry.
One method used to broaden the specific surface area within confined spaces involves the formation of microbubbles within small tubes (Fig. 4d) [42]. A study adopting this approach utilized a microfiltration membrane dispersion microreactor to execute the precipitation reaction between LiOH and CO2, using a water–ethanol mixture as the solvent. This research underlines the crucial roles played by the interphase mass-transfer rate of CO2 and the solubility of Li2CO3 in achieving successful nanoparticle synthesis. The solubility ratio of LiOH to Li2CO3 surged to nearly two orders of magnitude higher than that in water when the mass fraction of ethanol (ϕ) was approximately 0.9. Nanoparticles with a narrow size distribution (coefficient of variation (CV) < 0.3) were reliably obtained with a ϕ value of 0.870 or above. The particle size demonstrated sensitivity to ϕ and the flux of the liquid/slurry feed, with increases in either parameter causing a reduction in the particle size from 150 nm to 30 nm. The use of LiOH slurry up to a concentration of 10 wt% slightly increased the particle size, but it did not influence the uniformity of the nanoparticle morphology. Given the potential for solvent recycling through partial water removal, this method of Li2CO3 nanoparticle preparation is a green process in which water is the only byproduct.
The studies discussed in this section have advanced the technology to precipitate Li2CO3 by utilizing the large specific surface area of the gas–liquid interface. In the next section, we will explore the precipitation of Li2CO3 using the unique properties of solubility and temperature.
3.4. Exploiting Solubility Differences between LiHCO3 and Li2CO3Extracting Li from various sources is a complex process due to the presence of multiple impurities, including Mg2+, Na+, Ca2+, K+, B, and SO42−. As over 99.9% pure Li2CO3 is required for LiB production [53], it is essential to remove these impurities. Of these impurities, the removal of Mg2+ (3.4–113.7 g/L) is of particular significance, due to their high prevalence in Li-bearing (0.06–1.21 g/L) sources [54].
Any technique employed for the separation of similar elements must exploit the minor differences between them. Mg2+ has a high charge density that is double that of Li+, but it has roughly the same ionic radius and is readily hydrated [55]. Therefore, the extraction efficiency of Li depends heavily on the Mg-to-Li mass ratio, and effective separation is crucial. This challenge was addressed by utilizing a strategy that capitalizes on the solubility differences between LiHCO3 and Li2CO3. When CO2 is dissolved in water at lower pH levels, it dissociates into HCO3−. In the pH range where HCO3− dominates, there is potential for the formation of LiHCO3. However, LiHCO3 is considered to be metastable; therefore, it is generally believed that LiHCO3 exists in an ionic state as separate Li+ and bicarbonate (HCO3−) ions (Fig. 2).
The subsequent application of heat leads to the precipitation of Li2CO3, during which CO2 is introduced into the preliminarily precipitated Li2CO3, converting it back into LiHCO3. This solution is then dissolved in water to facilitate the removal of impurities including Mg2+. The final round of heat application precipitates the solution, yielding high-purity Li2CO3. Leveraging the differences in solubility and meticulous purification processes effectively eliminates Mg2+ and other impurities and significantly contributes towards the manufacture of high-quality LiBs.
Focusing on the influence of various factors that enhance precipitation via solubility, the research conducted by Tao on refining crude Li2CO3 procured from Qinghai’s salt lake brines in China is instructive [56]. This study comprehensively investigated the direct carbonation of Li2CO3 slurries using CO2–water solutions, wherein Li2CO3 was converted into the more soluble LiHCO3. Experiments were conducted in a three-phase mechanically agitated slurry-bed reactor under various conditions. The parameters analyzed included CO2 pressure, temperature, the CO2 flow rate, Li2CO3 particle size, slurry filling degree, and the agitation speed. The findings demonstrated that an increase in the CO2 pressure, agitation speed, CO2 flow rate, or a decrease in the Li2CO3 particle size, slurry filling degree, reaction temperature, or solid concentration resulted in an increased Li2CO3 dissolution rate. Interestingly, the immersion time before carbonation had a negligible effect on the carbonation ratio of Li2CO3. The process kinetics are well represented by the equation 1 – (1 – X)1/3 = kt, where X represents the conversion of Li2CO3, k is the reaction rate constant, and t is the reaction time up to 50 min. This equation describes the dynamic nature of Li2CO3 carbonation. Using multiple carbonation cycles, it was possible to convert all insoluble Li2CO3 into LiHCO3. This study highlights the importance of maintaining room temperature, considering high CO2 partial pressures (>0.6 MPa), performing multiple carbonation cycles, and ensuring a high agitation speed for optimal Li2CO3 carbonation. Moreover, this process enables potential CO2 recovery by heating the LiHCO3 solution under vacuum.
Xu developed a process for creating battery-grade Li2CO3 from Damxungcuo saline lake [14] that involved a two-stage Li2CO3 precipitation process in a hydrometallurgical system to remove impurities. Initially, industrial-grade Li2CO3 was obtained by removing Fe3+, Mg2+, and Ca2+ from Li-containing liquor. Subsequently, the industrial-grade Li2CO3 was treated with CO2 to yield more soluble bicarbonates, while EDTA-Li was used to chelate Ca2+ and Mg2+. The decomposition of Li2CO3 at 85°C yielded insoluble Li2CO3, resulting in a high purity (99.6%) and homogeneous Li2CO3 in the final precipitate. The process effectiveness was influenced by certain factors; for example, an L/S mass ratio of 30:1 favored Li2CO3 slurry formation and hot water washing promoted ion removal. Furthermore, the cyclic use of the filtrate assisted in improving Li recovery, thereby paving the way for large-scale production.
Linneen focused on the transformation of industrial-grade Li chloride into high-purity battery-grade Li2CO3 and achieved an impressive purity of >99.95% [38] through a four-step precipitation process. The treatment solution was concentrated in the salt lake, and it contained contaminants such as K, Na, Mg, Ca, Cu, Ni, and iron chloride at concentrations ranging from 2.5 M to 10 M. In the first step, the heavy metals and the majority of Mg were eradicated by increasing the pH of the solution, and the remaining Mg and Ca were then removed through the addition of sodium oxalate, which successfully reduced the calcium levels to 5–6 ppm in the 10 M solution. A higher molarity and ionic strength of the LiCl solution facilitated more effective impurity removal. Finally, high-purity Li2CO3 was obtained through two stages of precipitation: initial precipitation from the brine solution, followed by a second purification step using pressurized CO2. This final step allowed for the removal of entrapped Na and K from the first precipitate, ultimately yielding >99.95 wt% purity Li2CO3.
Zhou et al. successfully synthesized battery-grade Li2CO3 via a carbonation–decomposition method using crude Li2CO3 that contained various impurities [57]. The starting material was impure, defective, crude Li2CO3 with a purity of 98.56 wt%, and contained 5,873 ppm of SO42−, along with other impurities such as Na, K, Ca, Fe, and Mg. This study underlines the significance of using filter operations to enhance product quality. After filtration, the purity of Li2CO3 increased significantly to 99.65 wt%, and the amounts of SO42− and Fe dropped to 222.0 ppm and 6.6 ppm, respectively. Interestingly, despite the use of the qualitative filter paper, the Ca content increased from 14.9 ppm in crude Li2CO3 to 249.6 ppm. The study also employed ethylenediaminetetraacetic acid disodium salt (EDTA-2Na) for further purification, and it was highly efficient at removing metal cations, particularly Ca and Fe ions. Post-EDTA-2Na treatment, the Ca and Fe contents plummeted to 2.9 ppm and 0.7 ppm, respectively, improving the whiteness of the sample to 97.8 Wb. The removal efficiencies obtained for Ca, Fe, and SO42− were calculated as 80.5%, 99.4%, and 97.5%, respectively.
The research conducted by Meng Shi focused on a unique electrodialysis (ED) method for recovering Li and manganese from spent LiBs [58]. Unlike the hydrometallurgical routes traditionally used for Li recycling, this study used electrodialysis (ED), employing a CO2-capture agent, N-methyldiethanolamine, as a regenerable catholyte. Both surrogate solutions and Li-rich leachates were tested to recover Li and Mn as Li2CO3 and MnCO3, respectively. The process achieved battery-grade purity levels (99.6% purity) of recovered Li2CO3 from both the surrogate and leachate solutions. Notably, the energy consumption of the ED process was low, at 0.5 kW h/g Li, and Li2CO3 yields as high as 48.4% were reported. Despite the Li2CO3 yield being lower than what is typically needed for industrial applications, this study highlights several factors that can be optimized to increase the yield, such as the anolyte pH, Li2CO3 precipitation temperatures, and the choice of catholyte tertiary amine solutions. The ED method also suggests the possibility of a carbon-negative process with CO2 as the only chemical consumed. This study presents a promising, environmentally friendly, and energy-efficient method for recovering Li2CO3 during LiB recycling.
3.5. CO2 Utilization as Leachate and PrecipitantThe method proposed here leverages CO2 as a leaching agent to recycle spent Li iron phosphate (LiFePO4) at room temperature [29]. This strategy relies on the weak acidity of H2CO3, which restricts the leaching of Fe and P elements, thereby ensuring high extraction selectivity for Li. Notably, the role of CO2 is two-fold; in addition to facilitating the leaching process, it also aids in the chemical precipitation of leached Li+ to yield value-added Li2CO3.
The CO2-leaching strategy exhibits clear environmental and economic advantages. A life cycle analysis revealed that it consumes low amounts of energy and would reduce greenhouse gas emissions and provide substantial economic benefits. With an impressive recovery yield of high-purity Li2CO3 products and the ability to fix approximately 120 kg of CO2 per ton of spent LiFePO4, the use of this strategy demonstrates effective carbon reduction.
In addition, the elimination of strong acids/bases and additional precipitants hindered the creation of high-salt wastewater, high-lighting the environmental compatibility of this method. These processes also caused the lowest energy consumption, greatest reduction in greenhouse gas emissions, and sizable economic profits per LiFePO4 cell unit. Specifically, the CO2 leaching strategy, particularly the CO2-O2 recycling route, exhibited the lowest energy consumption at 2.29 MJ per kg of LiFePO4 cells, the greatest reduction in greenhouse gas emissions at 194 g/kg of LiFePO4 cells, and substantial economic profits at $4.04 per kg of LiFePO4 cells.
4. Limitations Associated with Precipitating Li2CO3 with CO2The technologies reported in this review offer ways of mitigating CO2 emissions associated with Li production and harnessing, and they enable the beneficial use of CO2; however, they also have certain limitations that need to be addressed. For example, the process used to create Li2CO3 from Li and CO2 is comparatively slow. Unlike precipitation with Na2CO3, this procedure involves two stages: the dissolution of CO2 and the subsequent precipitation of Li. The optimal conditions for each step often diverge, potentially leading to procedural delays. These temporal constraints can impede the efficiency and scalability of Li2CO3 production, particularly in industries requiring large-scale production.
Another consideration is that the ideal reaction conditions need to be effectively managed. Both pH and temperature significantly influence the reaction rate and efficiency. The transition from CO2 to Li2CO3 typically occurs in alkaline conditions [26]; however, sustaining these conditions can be difficult and may require the use of additional chemicals. Moreover, the reaction is temperature- sensitive. CO2 dissolves effectively in water at lower temperatures, while Li precipitation necessitates higher temperatures.
Additionally, reactions utilizing lithium aqueous solution and CO2 gas specific surface area require additional systematic elements to increase the specific surface area of the reaction surface. To augment the reaction-specific surface area within the reaction vessel, the total reaction volume needs to be curtailed and the reacted area per hour amplified, which may result in a considerably smaller throughput per hour compared with those in other processes. Overcoming such challenges may require the use of specialized equipment, such as a stack-type reactor.
Finally, the storage and handling of CO2 poses safety risks. CO2 has a pivotal role in the reaction, but its management, particularly in a gaseous state, requires expertise and specific storage and handling equipment [28]. In addition, a controllable CO2 supply system is essential, which can increase the overall process cost.
To overcome these hurdles, ongoing research and development are required to optimize the CO2-facilitated Li2CO3 precipitation conditions and to increase efficiency and render the process more sustainable and cost-effective.
5. ConclusionsThis review highlights various approaches that use CO2 to recover Li and convert it into Li2CO3. The review first compares the use of CO2 gas and Na2CO3 as carbonate sources. While Na2CO3 is more commonly used as the carbonate source, CO2 can be used with a comparable performance and higher purity and recovery can be achieved under certain operating conditions. The important operating conditions that require consideration during CO2 bubbling are also reported. For instance, higher carbonate/ bicarbonate ratios, pH, and temperature are beneficial for Li2CO3 production. However, continuous CO2 bubbling results in a decrease in the pH of the solution; therefore, careful pH monitoring is required.
To speed up the reaction, systems have been designed to increase the reaction area between CO2 gas and the aqueous Li solution. Studies have reported exposing aqueous Li solution to CO2 gas over a large area to dissolve CO2 and precipitate Li2CO3 using a falling film or SDR. Precipitation techniques using large specific surface areas have also been introduced, such as those involving spraying aqueous Li solution in the form of droplets into a tank of CO2 under high pressure or spraying microbubbles into the microchannels through which aqueous Li solution flows.
Research exploiting the solubility difference between LiHCO3 and Li2CO3 for Li extraction has also been published, and this technique is useful for obtaining high-purity Li2CO3. In addition, technology used to precipitate Li2CO3 by utilizing CO2 gas as both a leachant and a precipitant has been introduced.
However, there are certain limitations associated with CO2-based Li2CO3 precipitation technology. One of the main limitations is the relatively slow processes of CO2 dissolution and Li precipitation. It is also difficult to manage ideal reaction conditions, particularly pH and temperature, which are required to increase the efficiency of the reaction. Other challenges include managing the large specific surface area of the Li aqueous solution and CO2 gas, which may require specialized equipment to improve reaction efficiency. Finally, storing and managing CO2 involves safety and cost risks.
If these limitations are addressed, this technology could become a key method for extracting next-generation Li. It is crucial to develop processes that convert CO2 to achieve carbon neutrality [59]. The production of Li2CO3 can be derived from three primary sources: hard rock mining, brine extraction, and recycling. Given that the CO2 precipitation method can be applied to all three of these processes, further development of this technique holds significant potential. Moreover, with a conscious shift towards more sustainable methodologies, the role of CO2 in gas–liquid crystallization processes have become a research focus, and its future role in high-quality carbonate production is promising [60,61]. With continued progress in this area, the possibility of achieving sustainable Li production and recycling processes may be achieved.
AcknowledgmentThis research was supported by project for Industry-University-Research Institute platform cooperation R&D funded Korea Ministry of SMEs and Startups in 2022 (S3312316), and by the New and renewable energy core technology development project of the Korea Institute of Energy Technology Evaluation and Planning (KETEP) granted financial resource from the Ministry of Trade, Industry & Energy, Republic of Korea(No. 20213030040590), and by Korea Institute of Machinery and Materials (NK245I), and by Development of key technologies for safety management of hydrogen charging infrastructure (1415180603).
NotesAuthor contributions S.K. (Post Doctor) Wrote all manuscript content. H.Y. (Senior Researcher) Organized and revised this manuscript. T.M. (Principal Researcher) and B.H. (Principal Researcher) Collected data used in manuscript. S.L. (Senior Researcher) and J.P. (Research Director) revised the manuscript. References1. Yoshino A. The birth of the lithium-ion battery. Angew. Chem. Int. Ed. Engl. 2012;51:5798–5800.
https://doi.org/10.1002/anie.201105006
2. Shionuma K, Yokokawa M, Nagaura T. Characteristics of lithium ion rechargeable battery. In : Proceeding of Abstracts 32nd Battery Symposium; 9 September 1991; p. 9–11.
3. Gruber PW, Medina PA, Keoleian GA, Kesler SE, Everson MP, Wallington TJ. Global lithium availability: A constraint for electric vehicles? J. Ind. Ecol. 2011;15:760–775.
https://doi.org/10.1111/j.1530-9290.2011.00359.x
4. Nishi Y. Lithium ion secondary batteries; past 10 years and the future. J. Power Sources. 2001;100:101–106.
https://doi.org/10.1016/S0378-7753(01)00887-4
5. Antipov EV, Abakumov AM, Drozhzhin OA, Pogozhev DV. Lithium-ion electrochemical energy storage: the current state, problems, and development trends in Russia. Therm. Eng. 2019;66:219–224.
https://doi.org/10.1134/S0040601519040013
6. Lebedeva N, Di Persio F, Boon-Brett L. Lithium ion battery value chain and related opportunities for Europe. European Commission; Petten: 2016.
7. Bibra EM, Connelly E, Gorner M, et al. Global EV Outlook 2021: Accelerating ambitions despite the pandemic.
8. Tabelin CB, Dallas J, Casanova S, et al. Towards a low-carbon society: A review of lithium resource availability, challenges and innovations in mining, extraction and recycling, and future perspectives. Miner. Eng. 2021;163:106743.
https://doi.org/10.1016/j.mineng.2020.106743
10. Xu C, Dai Q, Gaines L, Hu M, Tukker A, Steubing B. Future material demand for automotive lithium-based batteries. Commun. Mater. 2020;1:99.
https://doi.org/10.1038/s43246-020-00095-x
11. Chagnes A, Swiatowska J. Lithium process chemistry: Resources, extraction, batteries, and recycling. Elsevier; 2015.
12. Meng F, McNeice J, Zadeh SS, Ghahreman A. Review of lithium production and recovery from minerals, brines, and lithium-ion batteries. Miner. Process. Extr. Metall. Rev. 2021;42:123–141.
https://doi.org/10.1080/08827508.2019.1668387
13. Azevedo M, Campagnol N, Hagenbruch T, et al. Lithium and Cobalt. A Tale of Two Commodities. 2018;
14. Xu Z, Zhang H, Wang R, Gui W, Liu G, Yang Y. Systemic and direct production of battery-grade lithium carbonate from a saline lake. Ind. Eng. Chem. Res. 2014;53:16502–16507.
https://doi.org/10.1021/ie502749n
15. Evans RK. An abundance of lithium: Part two. Lithium Abundance. Available online: http://www.evworld.com/library/KEvans_Lithium
accessed on 12 March 20202008. Jul
16. Meshram P, Pandey BD, Mankhand TR. Extraction of lithium from primary and secondary sources by pre-treatment, leaching and separation: A comprehensive review. Hydrometallurgy. 2014;150:192–208.
https://doi.org/10.1016/j.hydromet.2014.10.012
17. Chen Y, Tian Q, Chen B, Shi X, Liao T. Preparation of lithium carbonate from spodumene by a sodium carbonate autoclave process. Hydrometallurgy. 2011;109:43–46.
https://doi.org/10.1016/j.hydromet.2011.05.006
18. Kesler SE, Gruber PW, Medina PA, et al. Global lithium resources: Relative importance of pegmatite, brine and other deposits. Ore Geol. Rev. 2012;48:55–69.
https://doi.org/10.1016/j.oregeorev.2012.05.006
19. Liu G, Zhao Z, Ghahreman A. Novel approaches for lithium extraction from salt-lake brines: A review. Hydrometallurgy. 2019;187:81–100.
https://doi.org/10.1016/j.hydromet.2019.05.005
20. Swain B. Recovery and recycling of lithium: A review. Sep. Purif. Technol. 2017;172:388–403.
https://doi.org/10.1016/j.seppur.2016.08.031
21. Fan L, Liu CY, Zhu P, et al. Proton sponge promotion of electrochemical CO2 reduction to multi-carbon products. Joule. 2022;6:205–220.
https://doi.org/10.1016/j.joule.2021.12.002
22. Sun LL, Cui HJ, Ge QS. Will China achieve its 2060 carbon neutral commitment from the provincial perspective? Adv. Clim. Chang. Res. 2022;13:169–178.
https://doi.org/10.1016/j.accre.2022.02.002
23. Majeau-Bettez G, Hawkins TR, Strømman AH. Life cycle environmental assessment of lithium-ion and nickel metal hydride batteries for plug-in hybrid and battery electric vehicles. Environ. Sci. Technol. 2011;45:4548–4554.
https://doi.org/10.1021/es103607c
24. Velázquez-Martínez O, Valio J, Santasalo-Aarnio A, Reuter M, Serna-Guerrero R. A critical review of lithium-ion battery recycling processes from a circular economy perspective. Batteries. 2019;5:68.
https://doi.org/10.3390/batteries5040068
25. Kim HC, Wallington TJ, Arsenault R, Bae C, Ahn S, Lee J. Cradle-to-gate emissions from a commercial electric vehicle Li-ion battery: a comparative analysis. Environ. Sci. Technol. 2016;50:7715–7722.
https://doi.org/10.1021/acs.est.6b00830
26. Magzymov D, Dindoruk B, Johns RT. Carbon capture, utilization, and storage in the context of petroleum industry: a state-of-the-art review. In : SPE Improved Oil Recovery Conference; 18 Apr 2022; p. D031S031R001.
https://doi.org/10.2118/209368-MS
27. Breunig HM, Rosner F, Lim TH, Peng P. Emerging concepts in intermediate carbon dioxide emplacement to support carbon dioxide removal. Energy Environ. Sci. 2023;16:1821–1837.
https://doi.org/10.1039/D2EE03623A
28. Gaines L. The future of automotive lithium-ion battery recycling: Charting a sustainable course. SM&T. 2014;1:2–7.
https://doi.org/10.1016/j.susmat.2014.10.001
29. Xu C, Hu X, Yang Y, Jian Z, et al. Integrated process of CO2 sequestration and recycling spent LiFePO4 batteries. Energy Stor. Mater. 2023;60:102819.
https://doi.org/10.1016/j.ensm.2023.102819
30. Lv W, Wang Z, Cao H, et al. A critical review and analysis on the recycling of spent lithium-ion batteries. ACS Sustain. Chem. Eng. 2018;6:1504–1521.
https://doi.org/10.1021/acssuschemeng.7b03811
31. Yazami R, Touzain P. A reversible graphite-lithium negative electrode for electrochemical generators. J. Power Sources. 1983;9:365–371.
https://doi.org/10.1016/0378-7753(83)87040-2
32. Georgi-Maschler T, Friedrich B, Weyhe R, Heegn H, Rutz M. Development of a recycling process for Li-ion batteries. J. Power Sources. 2012;207:173–182.
https://doi.org/10.1016/j.jpowsour.2012.01.152
33. Mamontov MN, Gorbachev AV. The thermodynamic properties of lithium carbonate aqueous solution studied by the potentiometric method. J. Chem. Thermodyn. 2020;148:106146.
https://doi.org/10.1016/j.jct.2020.106146
34. Battaglia G, Berkemeyer L, Cipollina A, et al. Recovery of lithium carbonate from dilute Li-rich brine via homogenous and heterogeneous precipitation. Ind. Eng. Chem. Res. 2022;61:13589–13602.
https://doi.org/10.1021/acs.iecr.2c01397
35. Lu J, Tian M, Cao J, et al. Preparation of battery-grade lithium carbonate by microbubble enhanced CO 2 gas–liquid reactive crystallization. Green Chem. 2022;24:9084–9093.
https://doi.org/10.1039/D2GC03375E
36. Dong H, Koenig GM. A review on synthesis and engineering of crystal precursors produced via coprecipitation for multicomponent lithium-ion battery cathode materials. CrystEngComm. 2020;22:1514–1530.
https://doi.org/10.1039/C9CE00679F
37. Cheng W, Li Z, Cheng F. Solubility of Li2CO3 in Na–K–Li–Cl brines from 20 to 90° C. J. Chem. Thermodyn. 2013;67:74–82.
https://doi.org/10.1016/j.jct.2013.07.024
38. Linneen N, Bhave R, Woerner D. Purification of industrial grade lithium chloride for the recovery of high purity battery grade lithium carbonate. Sep. Purif. Technol. 2019;214:168–173.
https://doi.org/10.1016/j.seppur.2018.05.020
39. Qiao Z, Zhao L, Li N, et al. Highly efficient and environmental-friendly separation and purification of carbon nanotubes from molten salt via ultrasound-assisted carbonation. Sep. Purif. Technol. 2023;306:122630.
https://doi.org/10.1016/j.seppur.2022.122630
40. Lower SK. Carbonate equilibria in natural waters. Simon Fraser University; 1999. p. 544.
41. Sun Y, Song X, Wang J, Luo Y, Yu J. Unseeded supersolubility of lithium carbonate: Experimental measurement and simulation with mathematical models. J. Cryst. Growth. 2009;311:4714–4719.
https://doi.org/10.1016/j.jcrysgro.2009.09.013
42. Lu Y, Liu Y, Zhou C, Luo G. Preparation of Li2CO3 nanoparticles by carbonation reaction using a microfiltration membrane dispersion microreactor. Ind. Eng. Chem. Res. 2014;53:11015–11020.
https://doi.org/10.1021/ie5019832
43. Yang Y, Meng X, Cao H, Lin X, Liu C, Sun Y, Zhang Y, Sun Z. Selective recovery of lithium from spent lithium iron phosphate batteries: a sustainable process. Green Chem. 2018;20:3121–3133.
https://doi.org/10.1039/C7GC03376A
44. Abu-Eishah SI, Anabtawi MJ, Isaac SL. Upgrading of carbonaceous phosphate rocks by direct carbonation with CO2–water solutions. Chem. Eng. Process.: Process Intensif. 2004;43:1085–1094.
https://doi.org/10.1016/j.cep.2003.11.001
45. Han B, Haq RA, Louhi-Kultanen M. Lithium carbonate precipitation by homogeneous and heterogeneous reactive crystallization. Hydrometallurgy. 2020;195:105386.
https://doi.org/10.1016/j.hydromet.2020.105386
46. Jiang Y, Liu C, Zhou X, Li P, Song X, Yu J. Toward CO2 utilization: Gas–liquid reactive crystallization of lithium carbonate in concentrated KOH solution. Energy Sources A: Recovery Util. Environ. 2021;43:3332–3344.
https://doi.org/10.1080/15567036.2019.1587068
47. Tian M, Wang Z, Cao J, Guo J, Gong X. Insight into lithium carbonate crystallization in the mild reaction system LiCl-NH3· H2O-CO2 by stabilizing the solution with NH3· H2O. J. Cryst. Growth. 2019;520:46–55.
https://doi.org/10.1016/j.jcrysgro.2019.05.020
48. Tian M, Guo J, Wang Z, Cao J, Gong X. Synergetic effect of secondary nucleation and growth on the lithium carbonate particle size in the gas–liquid reactive crystallization of LiCl–NH3· H2O–CO2. Particuology. 2020;51:10–17.
https://doi.org/10.1016/j.partic.2019.10.006
49. Zhou Z, Liang F, Qin W, Fei W. Coupled reaction and solvent extraction process to form Li2CO3: Mechanism and product characterization. AIChE J. 2014;60:282–288.
https://doi.org/10.1002/aic.14243
50. Sun YZ, Song XF, Jin MM, Jin W, Yu JG. Gas–liquid reactive crystallization of lithium carbonate by a falling film column. Ind. Eng. Chem. Res. 2013;52:17598–17606.
https://doi.org/10.1021/ie402698v
51. Sun Y, Song X, Wang J, Yu J. Preparation of Li2CO3 by gas-liquid reactive crystallization of LiOH and CO2. Cryst. Res. Technol. 2012;47:437–442.
https://doi.org/10.1002/crat.201100571
52. Sun Y, Song X, Wang J, Yu J. Preparation of lithium carbonate hollow spheres by spray pyrolysis. Cryst. Res. Technol. 2011;46:173–177.
https://doi.org/10.1002/crat.201000532
53. Virolainen S, Fini MF, Miettinen V, et al. Removal of calcium and magnesium from lithium brine concentrate via continuous counter-current solvent extraction. Hydrometallurgy. 2016;162:19–15.
https://doi.org/10.1016/j.hydromet.2016.02.010
54. Pramanik BK, Nghiem LD, Hai FI. Extraction of strategically important elements from brines: Constraints and opportunities. Water Res. 2020;168:115149.
https://doi.org/10.1016/j.watres.2019.115149
55. Le Poul N, Baudrin E, Morcrette M, Gwizdala S, Masquelier C, Tarascon JM. Development of potentiometric ion sensors based on insertion materials as sensitive element. Solid State Ion. 2003;159:149–158.
https://doi.org/10.1016/S0167-2738(02)00921-9
56. tao Yi W, yan Yan C, hua Ma P, qiang Li F, ming Wen X. Refining of crude Li2CO3 via slurry phase dissolution using CO2. Sep. Purif. Technol. 2007;56:241–248.
https://doi.org/10.1016/j.seppur.2007.01.015
57. Zhou M. Preparation of Battery Grade Li2CO3 from Defective Product by the Carbonation-Decomposition Method. Cryst. Res. Technol. 2023;58:2200112.
https://doi.org/10.1002/crat.202200112
58. Shi M, Diaz LA, Klaehn JR, Wilson AD, Lister TE. Li2CO3 recovery through a carbon-negative electrodialysis of lithium-ion battery leachates. ACS Sustain. Chem. Eng. 2022;10:11773–11781.
https://doi.org/10.1021/acssuschemeng.2c02106
59. Sullivan I, Goryachev A, Digdaya IA, et al. Coupling electrochemical CO2 conversion with CO2 capture. Nat. Catal. 2021;4:952–958.
https://doi.org/10.1038/s41929-022-00734-1
60. Chen P, Tang S, Yue H, Liu C, Li C, Liang B. Lithium enrichment of high Mg/Li ratio brine by precipitation of magnesium via combined CO2 mineralization and solvent extraction. Ind. Eng. Chem. Res. 2017;56:5668–5678.
https://doi.org/10.1021/acs.iecr.6b04892
61. Jung WM, Kang SH, Kim KS, Kim WS, Choi CK. Precipitation of calcium carbonate particles by gas–liquid reaction: Morphology and size distribution of particles in Couette-Taylor and stirred tank reactors. J. Cryst. Growth. 2010;312(22)3331–9.
https://doi.org/10.1016/j.jcrysgro.2010.08.026
Fig. 2Typical Li2CO3 precipitation process: a) Carbonic acid (H2CO3) is formed by supplying CO2 in an aqueous Li solution; b) carbonic acid ionizes to bicarbonate and exists as highly soluble LiHCO3 (Li+ and HCO3−). c) HCO3− is converted to CO32− at a high pH or high temperature, which binds to Li+ and precipitates as Li2CO3. 
Fig. 3Variations in the carbonation process with time: (a) changes in ion concentration during carbonation process of the LiOH–KOH solution: η indicates the carbonation rate (%); (b) variation in carbonation rate versus time at various temperatures; (c) variation in the concentration of CO32− versus time at various temperatures. 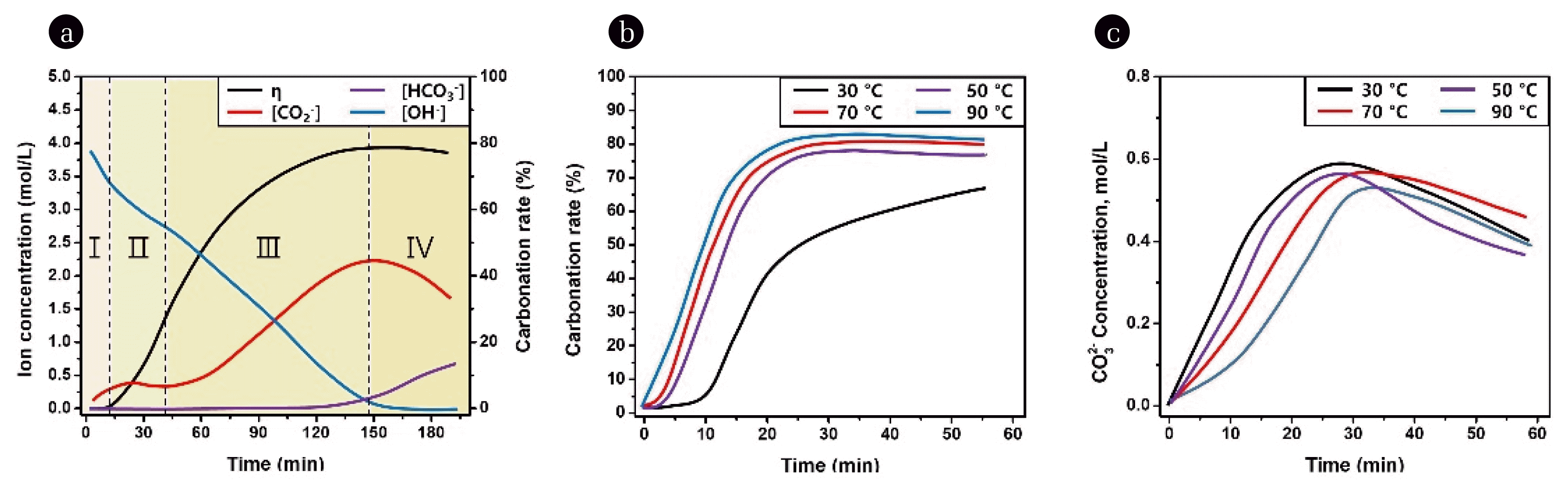
Fig. 4Different precipitation methods using a large specific surface area: a) reaction system where an Li+ solution descends and reacts with CO2 gas on a thin film; b) system utilizing a rotating plate to form a thin film; c) system that increases the specific surface area by spraying small droplets into the CO2 gas space; d) system that sprays bubbles from a microchannel to react with a large specific surface area in a small space. 
Table 1Summary of various conditions required for CO2 precipitation reported in literature.
Table 2Comparison of Li2CO3 precipitation recovery and purity when using CO2 and Na2CO3, respectively. |
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||