AbstractChemical waste salt is a hazardous waste generated during chemical production, and it is crucial to complete the recycling of the waste salt in a practical way. In the case of waste salt recycling by rotary kiln pyrolysis carbonization, ringing affects the pyrolysis efficiency and process running, thus a study on waste salt ringing was conducted. The results showed that the particle size is the key to the ring formation in rotary kiln, and pyrolysis temperature, pyrolysis time and temperature distribution in the rotary kiln also affect the material discharge rate during pyrolysis of waste salt in the rotary kiln. Thermogravimetric analysis and SEM images revealed that the volatilization of organic matter in the material due to pyrolysis caused a decrease in the abrasion resistance of the waste salt particle, which was the factor behind the ringing during pyrolysis in the rotary kiln. This study may provide a reference for the implementation of similar waste salt pyrolysis carbonization processes.
Graphical Abstract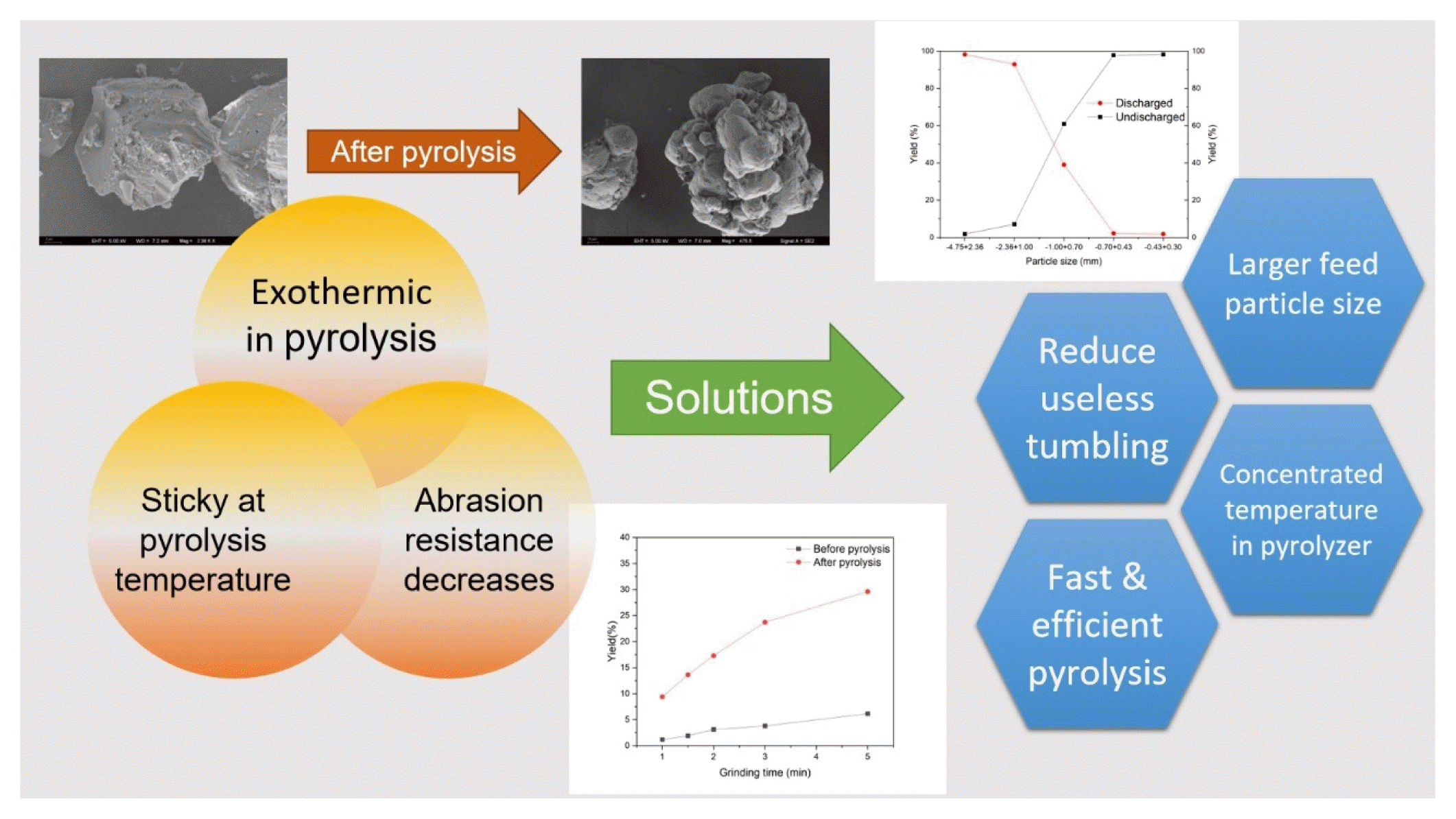
1. IntroductionDuring the production of organic and inorganic chemical products such as drug synthesis [1], pesticide intermediates [2], and printing and dyeing [3], a large amount of salt-containing wastewater is produced, and after evaporation and concentration, it forms solid waste that cannot be used as industrial raw material salt. Waste salt has a wide variety, large yield, complex composition, high toxicity, high treatment costs, environmental hazards [4], and if long-term storage will occupy a large number of sites [5], causing serious pollution and damage to the ecological environment [6]. Under the demand of clean production and sustainable development [7], it is very necessary to adopt practical methods for resource utilization of industrial waste salt and complete the separation of its target components [8].
At present, for the separation of target components in the resource utilization of industrial waste salts, the methods can be mainly divided into thermal treatment, advanced oxidation [9], adsorption precipitation, extraction and other technical routes [10]. Thermal treatment can be divided into two routes: pyrolysis carbonization and high-temperature melting. Pyrolysis carbonization [11] is the decomposition and carbonization of organic matter in waste salts at temperatures lower than the melting point of inorganic salts [12] and in a controlled oxygen atmosphere, which has greater advantages in reducing carbon emissions and energy consumption than high-temperature melting [13]; it is also a simpler process than advanced oxidation, adsorption and extraction, and reduces the introduction of additional chemicals in the treatment of waste salts, avoiding the generation of new waste and related treatment problems [14], which has a better prospect for promotion and application [15].
The core of the pyrolysis carbonization process is the pyrolysis reactor in the pyrolysis system [16]. The type of pyrolysis reactor has an important impact on the removal of organic impurities, heat and mass transfer, pyrolysis efficiency and process cost [17]. The reactor types under study include fixed beds, fluid bed, rotary kiln, etc. Fixed-bed reactors are easy to design [18], manage and maintain, but they often face problems of uneven carbonation and equipment sticking when treating waste salts [19]. Fluidized-bed reactors have better mass and heat transfer performance [20] but may increase the mechanical wear of materials due to the special fluidization requirements [21]. Rotary kiln is a kind of thermal reactor widely used in many industries such as building materials, metallurgy, chemical industry, environmental protection, etc [22]. In the process of pyrolysis [23], the cylinder of rotary kiln continuously rotates to mix the materials, which enhances the efficiency of mass and heat transfer of the reaction materials in the process of pyrolysis [24]. By adjusting the tilting angle and rotating speed of the rotary kiln, the residence time of the reactants in the cylinder can be controlled, and then the reaction process of the reactants in the rotary kiln can be effectively controlled [25]. The rotary kiln can be fed continuously to facilitate large batches of pyrolysis reactions and reduce the emission of pollutants in the reaction process. The rotary kiln is highly adaptable to materials of various particle sizes [26], but it is prone to the problem of ringing due to the uneven temperature of the material within and the nature of the feed material [27].
Rotary kiln ring problem is mainly due to low-temperature eutectic clumps attached to the equipment solidified to form ring [28], the source of low-temperature eutectic can be divided into the composition of raw material components and fuel composition of the introduction of two aspects [29]. When the raw material composition contains more low melting point substances [30], at the working temperature of the process, these low-temperature substances first melt to form a liquid phase, the raw material in the unmelted particles wrapped together, after passing through the high-temperature section or by the cooling effect of the lower temperature induced air solidification and solidification attached to the rotary kiln wall. When the temperature of the rotary kiln requires an external heat source, if a fuel such as pulverized coal is selected to provide heat, the fuel quality will affect the process [31], and if the ash content in the fuel is high, the ash content in the fuel may provide the liquid phase which is used for the ringing and accelerate the process of ringing. Particles wrapped by liquid phase are more likely to be wrapped at smaller particle sizes, while particles at smaller particle sizes also tend to agglomerate and agglomerate without the need for liquid phase, and agglomeration will occur more easily when the liquid phase fills the gap and then cools [32]. As in sintered pellets in the chain grate rotary kiln process in the rotary kiln ring formation problem [33], the ring is formed under the joint action of the particle powder of the raw material and coal dust, coal dust provides a reducing atmosphere to increase the amount of liquid phase, and the ash in the component further provides a source of liquid phase and particles. The quality of the green pellets, the composition of coal ash, the combustion efficiency of pulverized coal, roasting temperature, FeO content and alkali metal input and other raw material properties and process factors, in the case of larger feed size of pellet raw materials, result in a large amount of powder being produced during the process, which starts to form agglomerates on the surface of the corroded kiln lining and gradually develops into rings. To some extent, the fine particle size and liquid phase produced for various reasons are the cause of rotary kiln rings, however, the finer particle size of the raw material facilitates the heat and mass transfer of the raw material [34], how to balance the two relationships is the key to the successful application of rotary kiln-related proces.
The pyrolysis carbonization method can remove organic matter-and separate industrial salt at a smaller cost. In the specific process implementation, problems such as softening of waste salt, bonding and caking in the pyrolysis equipment are often encountered to hinder the process implementation and operation [35]. A chemical plant in Hunan, China, used pyrolysis carbonization technology for waste salt, and the waste salt objects treated were easy to bond and dusty, and finally the rotary kiln equipment was selected as the pyrolysis reactor, but during the actual operation of the process, the problem of serious ringing in the rotary kiln of the pyrolysis equipment was encountered.
This study investigates the effect of different process conditions of rotary kiln pyrolysis on the change of discharge rate of pyrolysis waste salt, and systematically studies the mechanism of ringing problem of rotary kiln pyrolysis waste salt, including the effect of pyrolysis on the abrasion resistance of waste salt and the change of surface morphology, providing research ideas and theoretical methods to support the ringing problem encountered in the implementation of the resource utilization of waste salt pyrolysis carbonization process.
2. Materials and Methods2.1. MaterialsThe waste salt used in this study is a by-product of the production process of a chemical plant in Hunan Province, China. The X Ray Fluorescence (XRF), X-ray diffraction (XRD) shows that the waste salt contains mainly Na (28.27%), Cl (61.32%), O (9.87%) and trace amounts of Si (0.16%), S (0.15%), Al (0.01%), K (0.01%), some of the sodium and chloride formed sodium chloride, and the 9.87% oxygen element content indicates that it contains a large amount of organic matter, gas chromatography mass spectrometry (GCMS) showed that it included o-isopropoxyphenol, p-propoxyphenol, o-dipropoxybenzene and n-octanol, isooctanol, dibutyl ketone and others. The original particle size of the waste salt ranged from 0.1 m to 1 m. The materials used in the experiment were crushed and sieved to control particle sizes in the range of 0.30–0.43mm, 0.43–0.73mm, 0.70–1.00mm, 1.00–2.36mm, and 2.36–4.75mm.
2.2. Experimental MethodThe waste salt was subjected to pyrolysis experiments in rotary kiln and muffle furnace, respectively, where the structure of the rotary kiln pyrolysis equipment is shown in Fig. 1. In the rotary kiln pyrolysis experiment, waste salt particles with a certain particle size range are placed in the rotary kiln head which in front of the heating section, and then turn on the rotary switch that rotates the rotary kiln at the specified speed. The waste salt particles are forced to move toward the end of the rotary kiln driven by the inner spiral blade inside the cylinder, so that the residence time of the waste salt in the heating section of the rotary kiln can be controlled by controlling the rotational speed of the rotary kiln. The waste salt particles are finally driven by the inner spiral blade and discharged from the end of the kiln, the toxic and harmful organic waste gas generated during the pyrolysis process is carried away by the induced draft device located at the end of the kiln, which will be adsorbed by the multi-stage spraying tower to meet the environmental requirements for gas emissions, finally the organic waste gas adsorbed in liquid will be harmlessly treated by microbial degradation. Air is selected for the pyrolysis atmosphere and the waste salt fines which are undischarged in the rotary kiln cylinder are collected after the pyrolysis is completed.
In the muffle furnace pyrolysis experiment, the waste salt with the determined particle size was placed in a crucible, and it was ensured that there was only one layer of particles in the crucible and no contact between the particles, so that there was no adhesion or caking between the particles after pyrolysis. After that, the pyrolyzed waste salt particles were placed in an autogenous mill for a period of time, and finally the waste salt after self-grinding is sieved using a set of sieves [36].
2.3. Analysis TechniquesThe proportion of each product distribution of waste salt pyrolysis is measured by measuring the sodium content in the pyrolysis product [37]. Scanning electron microscopy (SEM) was used to observe the microscopic morphology of waste salt particles before and after pyrolysis. Fourier transform infrared spectroscopy (FTIR, CCR-1) was used to determine the organic composition of the waste salt particles. A thermogravimetric analyzer (TG-DTA6300) was used to study the weight loss and exotherm of the waste salt pyrolysis process.
3. Results and Discussion3.1. Characteristics of Raw Materials3.1.1. Elemental content and morphological analysis of raw materialsThe distribution state of the organic matter in the waste salt was analyzed by scanning electron microscopy (SEM) and EDS elemental mapping. As shown in Fig. 2(a1, a2, a3), the surface of the waste salt contaminated with organic matter is rougher and has more defects than the usual crystal particles [38], and the relatively flat surface also has more bumps attached to it, the angles are more disordered and appear irregular in comparison. Fig. 3 EDS elemental mapping of carbon (C) and oxygen (O) basically matches with the attached small particles, it can be assumed that the bumps are the small organic contaminants on the surface of the particles, and can be inferred that the small organic contaminants are closely embedded with the salt particles, the organic matter are not completely removed by gasification during the whole pyrolysis process, but are finally pyrolyzed into solid insoluble organic carbon [39].
From the results of infrared spectral analysis, as shown in Fig. 4(a), its comparison with the spectrum of analytically pure sodium chloride shows that the organic matter contained in the waste salt contains mainly -CH, -C6H5, -N=O in terms of functional groups, which make it easy to break and generate intramolecular bonds during the pyrolysis process, resulting in exothermic and volume changes [40].
Pyrolysis of waste salt with different particle sizes was performed at a heating rate of 20°C/min, holding temperature of 600°C and holding time of 40 min to investigate the effect of particle size on the mass loss rate of waste salt pyrolysis, and the results are shown in Fig. 5. The highest weight loss rate of waste salt pyrolysis reached 16.05% when the particle size was 1.00–2.36 mm, and the weight loss rate of waste salt was 15.61% when the particle size was 0.30–0.43mm, which was slightly less than the highest value, and the three particle sizes of 2.36–4.75mm, 0.70–1.00mm, and 0.43–0.70mm had similar mass loss rates, which were 14.85%, 14.88%, and 14.83%.
The weight change of the waste salt after pyrolysis is mainly caused by the volatile organic matter volatilization during the pyrolysis process. The difference in the pyrolysis mass loss rate of different particle sizes of waste salt is caused by the fact that the particle size affects the thermal process of the waste salt on the one hand, and the different volatile organic matter content of waste salt particles of different particle sizes on the other hand [41]. From the results of Fig. 3, the fluorescence of Na and Cl partially overlap with the concentrated area of C and O fluorescence, but mainly located in different parts, which indicates that the components in the waste salt are not homogeneously mixed, and therefore will make the difference on the components of different particle size after crushing [42], and finally reflected in the properties such as the mass loss rate of different waste salt particle size.
3.1.2. Thermogravimetric analysisThe waste salt particles of 2.36–4.75mm particle size were selected for thermogravimetric testing as shown in Fig. 4(b), and the heating rate of TG-DTG was 10°C/min. It can be seen through the TG-DTG curve that the weight loss rate of the whole heating process is relatively smooth until it approaches 800°C, the TG curve showed an overall uniform downward trend. The DTG curve as a whole fluctuates above and below −0.25%, and there are several relatively obvious peaks at 300–350°C and 500–550°C. Although the DTG curve was not smooth, it was always maintained at the level of −0.25%, indicating that the organic matter in the waste salt was in a state of random distribution, which made the weight loss rate fluctuate around a certain level as the pyrolysis proceeded. When the pyrolysis temperature reached 750°C, the weight loss rate began to rise rapidly, and when the temperature exceeded 800°C, the TG curve maintained a smooth and steady decline, and the DTG curve, compared with that before 750°C, no longer fluctuated violently around −0.25% but began to increase rapidly and smoothly downward in absolute value, which indicated that the waste salt particles had already started to melt at 750°C during the pyrolysis process [43]. The melting point of NaCl, which constitutes the majority of the waste salt particles, is 801°C in pure crystalline form, and there are usually stages of exothermic processes when pyrolysis of organic matter is carried out under aerobic atmospheric pressure conditions, therefore, the melting of the waste salt starts at 750°C may due to the incorporation of organic matter on the one hand, and the exothermic state of waste salt pyrolysis process on the other hand.
3.2. Factors Influencing the Discharge Rate of Pyrolysis Waste Salt in Rotary KilnThrough experiments with the pyrolysis of waste salt in rotary kiln, the effects of three main factors on the discharge rate of pyrolysis waste salt in rotary kiln, which are waste salt particle size, pyrolysis temperature and pyrolysis time, were investigated. The results showed that the most influential factor in the discharge rate was waste salt particle size. As shown in Fig. 6(a), the discharge rate of waste salt decreased with decreasing particle size, and decreases by 5.27% when the particle size is reduced from 2.36–4.75mm to 1.00–2.36mm. When the particle size continues to decrease to 0.70–1.00mm and 0.43–0.70mm, the discharge rate decreases significantly and rapidly to 39.03% and 2.19%, as the particle size range continues to decrease to 0.30–0.43mm, the discharge rate is 1.80%, which means that in the pyrolysis time of 40 mins and pyrolysis temperature of 600°C, and the particle size is less than 0.70mm, more than 95% of the waste salt remains in the rotary kiln which cannot be discharged.
When the particle size is 0.70–1.00mm, more than 60% of the waste salt still remains in the rotary kiln that cannot be discharged. Waste salt with organic matter often has the problem of increased viscosity and causes poor movement at high temperatures [44]. When the particle size is so small that gravity cannot overcome the resistance caused by increased viscosity, which is manifested in the pyrolysis of waste salt in the rotary kiln as the fines of waste salt cannot be discharged, and after a period of accumulation, the phenomenon of ringing is formed. It is worth noting that the variation of discharge rate is not significant correlated with the mass loss rate, 1.00–2.36mm has the highest mass loss rate, but still has a discharge rate of 92.93% which is only second to the highest value and is not significantly affected by the high mass loss rate.
The effect of pyrolysis time and pyrolysis temperature on the discharge rate is different from the effect of particle size on the discharge rate of pyrolysis waste salt in the rotary kiln. In the experiments of pyrolysis temperature 600°C and particle size 2.36–4.75mm, as shown in Fig. 6(b), the overall discharge rate was in a decreasing trend with the increase of pyrolysis time, although it was 0.50% higher at 50 mins than at 40 mins, but the discharge rate at 50 mins was still different from 98.91% at the highest discharge rate of 20 mins which is 0.21% lower. When the pyrolysis time increased from 20 mins to 30 mins, the pyrolysis discharge rate only decreased by 0.01%, and when the pyrolysis time continued to increase to 40 mins and 60 mins, the pyrolysis discharge rate decreased to 98.20% and 97.78%.
The effect of pyrolysis temperature on the discharge rate is similar to the effect of pyrolysis time on the discharge rate in the pyrolysis of waste salt in rotary kiln, Fig. 6(c) shows that the overall trend of pyrolysis discharge rate decreases with the increase of pyrolysis temperature, but at the pyrolysis temperature of 625°C, the discharge rate reaches the highest 99.12%, which is higher than the second highest discharge rate of 99.10% at the pyrolysis temperature of 550°C. However, the discharge rate dropped to 98.20% and 98.12% at 600°C and 650°C, which was 1% different from the pyrolysis discharge rate of 99.10% and 99.09% at 550°C and 575°C, showing a relatively obvious difference.
From the experiments of the three sets of conditions factors for waste salt pyrolysis in the rotary kiln, it can be seen that the most influential factor on the discharge rate is the particle size of the waste salt particles, in the particle size of 2.36–4.75 mm, more than 98% of the waste salt particles can be discharged, once the particle size decreases, even if the waste salt pyrolysis mass loss rate increases, the discharge rate still decreases. Therefore, in the process of waste salt pyrolysis in rotary kiln, a larger particle size is more conducive to the continuous operation of pyrolysis under the premise of guaranteeing the pyrolysis effect. For the factor of pyrolysis time, although an extreme value occurred at 50 mins, the maximum value still happened at 20 mins, so the fast-in-fast-out pyrolysis strategy should be given priority when the waste salt is pyrolyzed in the rotary kiln. For the pyrolysis temperature, the maximum value occurred at 625°C, but the discharge rate decreases rapidly at 600°C and 650°C on both sides of it. Therefore, under the premise of fast-in and fast-out, if the pyrolysis carbonization effect at 550°C and 575°C meets the requirements, then there is not necessary to pursue the high temperature as much, and if the pyrolysis carbonization of organic matter does not reach the requirements, then the appropriate high temperature should be chosen, and the pyrolysis time should be increased appropriately.
3.3. Pyrolysis Effect on Abrasion Resistance of Waste Salt ParticlesThe effect of pyrolysis on the abrasion resistance of waste salt was investigated by simulating the pyrolysis of waste salt in rotary kiln through pyrolysis in muffle furnace and self-grinding of the pyrolyzed product in an autogenous mill, and the reasons for the effect of different pyrolysis conditions on the discharge rate when using rotary kiln for pyrolysis of waste salt particles. In this part of the experiment, waste salt particles of 2.36–4.75mm particle size were selected. The particle size distribution of the waste salt particles after self-grinding before and after pyrolysis is shown in Fig. 7(a) and Fig. 7(b), where the pyrolysis temperature of 600°C, the pyrolysis time of 40 mins, and the heating rate of 20°C/min are choice. With the increase of the self-grinding time, the fine particles gradually increased, and the increased fine particles before and after pyrolysis were mainly concentrated in 0.15–0.3mm and less than 0.15mm. This indicates that the particle size produced by the rotational movement of the waste salt particles during the whole process of pyrolysis in rotary kiln is basically less than 0.43mm particle size [45]. In the pyrolysis of waste salt particles in rotary kiln, when the pyrolysis particle size range is chosen to be 2.36–4.75mm, 1%–2% of the waste salt particles that are undischarged are also due to the rotational movement during the pyrolysis in the rotary kiln, which produces difficult to discharge particles with particle size less than 0.43mm.
The abrasion resistance of the waste salt after pyrolysis decreased significantly [46], as shown in Fig. 7(c), with the yield of particle size less than 0.43 mm, the same conditions as above, the yield before and after pyrolysis was 1.17% and 9.40% for 1 min of self-milling time, and 6.16% and 29.61% for 5 mins of self-milling, the difference of the yield of hard-to-discharge particles before and after pyrolysis increases with the increase of the self-milling time. As longer self-milling time means more self-milling tumbling movement of the salt particles, the rotary kiln rotation speed should not be selected too fast for pyrolysis, since high-speed rotation may result in an additional tumbling movement of the salt particles that are not used for forward movement, producing more fine salt particles that cannot be discharged.
It can be seen from the GCMS and infrared spectrum Fig. 4(a) analysis of the waste salt that the organic compounds in the waste salt can be mainly classified as long-chain hydrocarbons, aldehydes, benzene rings, heterocyclic compounds and aromatic nitro compounds, the specific gravity of these organic compounds is usually between 0.8–1.2 [47], and the specific gravity of NaCl is about 2.2. Taking the pyrolysis under the condition of temperature at 600°C and time at 40 mins as an example, the weight loss of the product is 14.85%. Assuming that the average specific gravity of the organic matter removed by pyrolysis in the waste salt is 0.95 and the average specific gravity of raw waste salt is 1.65, the volume percentage of voids left in the particles due to the removal of organic matter by pyrolysis is: (14.85/0.95)/(100/1.65)*100% =25.80%.
About 25% void makes the waste salt particles with high abrasion resistance drop dramatically on the process of pyrolysis [48], so that the rotary kiln pyrolysis, despite the selection of waste salt particles with a larger particle size of 2.36–4.75mm for pyrolysis, it still produces fine size waste salt particles that remain in the rotary kiln which is undischarged, and the yield of discharged waste salt will change with the change of pyrolysis conditions. When the pyrolysis mass loss rate is 16.05% and the porosity is 27.88%, it only increased by 2.08%, so the particle size is the dominant factor of discharge rate change in the rotary kiln experiment.
By changing the three factors of holding time, holding temperature and heating rate on the process of muffle pyrolysis, the self-grinding time was fixed at 2 mins, which investigated the effects of different factors on the distribution of fine particle size produced by self-grinding. With the increase of holding time, as shown in Fig. 8(a1, a2), taking the waste salt particles with particle size less than 0.15 mm produced after self-grinding as example, the yield of particle size less than 0.15mm after self-grinding gradually increased, and when the holding time reached 50 mins, the yield of the particles smaller than 0.15mm particle size after self-grinding rapidly dropped to 9.56%, which was only higher than 8.50% at 20 mins. When the holding time of muffle pyrolysis reached 60 mins, the yield of less than 0.15mm particle size after self-grinding increased back to 16.11%, while it was still less than the maximum value of 17.21% at the holding time of 40 mins.
Comparison with the results of the pyrolysis time discharge rate in the rotary kiln shows that both have an overall increase in the yield of fine waste salt particles with increasing holding time. At 60 mins, the yield of less than 0.43mm particle size was 18.69% compared with the result of pyrolysis in rotary kiln, which did not exceed the yield of particle size with less than 0.43 mm of 19.49% at the time of 40 mins, but the results of rotary kiln pyrolysis discharge rate showed that 97.98% discharge rate at 60 mins was the lowest value, i.e., at 60 mins of rotary kiln pyrolysis time, the most amount of less than 0.43 mm particle size of waste salt was produced.
As shown in Fig. 8(b1, b2), the increase in holding temperature first caused the yield of waste salt particles with particle size less than 0.43 mm to increase, and then showed a slight decrease in the cumulative yield under the sieve at 600°C, and it decreased rapidly to 4.79% and 1.31% at 625°C and 650°C. With the increase of the holding temperature, the organic matter was pyrolyzed in a more rapid way, and at the same time the heat release process becomes more intense, which make the abrasion resistance of the waste salt, which should be decreased due to the organic matter being volatilized by pyrolysis and voids generated, appear to melt to a certain extent due to the rapid accumulation of heat in the waste salt particles, so that the abrasion resistance is increased instead. It finally resulted in the yield of 1.31% of pyrolyzed waste salt particles with particle size less than 0.43 mm at a holding temperature of 650°C, which is less than 4.13% of waste salt particles without pyrolysis.
The inconsistency trend between the results of muffle pyrolysis of waste salt particles and the rotary kiln pyrolysis of waste salt particles when the two factors of time and temperature are varied is probably due to the influence of the two different pyrolyzer structures of the rotary kiln and the muffle. When the pyrolysis process is carried out in the muffle furnace, it can be considered that the pyrolysis temperature which the waste salt particles are subjected is strictly according to the setting, whereas when the pyrolysis is carried out in the rotary kiln, the pyrolysis temperature which is subjected to is not uniformly distributed in time and space [49], and the waste salt particles always enter through the rotary kiln inlet first, and move forward under the propulsion of the spiral blade inside the rotary kiln, before entering the expected pyrolysis section, the waste salt particles already start to be subjected to a certain temperature that enables pyrolysis to take place. As the waste salt particles move forward to the intended heating section, the temperature distribution in the heating section is not uniform as well, the temperature distribution in the rotary kiln is at a point where there is a section below the set temperature and a section above the set temperature due to the induced air of the induced draft device. The temperature of the heating section, which enters at the very beginning, is below the set temperature, and as it moves forward, the actual pyrolysis temperature of the waste salt particles is then higher than the set temperature, and after leaving the heating section, due to the induced draft, the waste salt particles are still at a certain temperature that the pyrolysis reaction can take place during the discharge stage. The different distribution of the pyrolysis temperature to which the waste salt particles are subjected in the rotary kiln compared to the muffle furnace leads to an inconsistent correspondence of the pyrolysis results in the two pyrolyzers.
The heating rate of waste salt particles in the muffle furnace pyrolysis corresponds to a comprehensive indicator of the pyrolysis time and spiral blade pitch of the rotary kiln pyrolysis of the waste salt particles. The shorter the pyrolysis time, the faster the rotary kiln speed, the faster the waste salt particles are pushed forward, the pyrolysis temperature will rise faster, which means there is a higher heating rate, and the corresponding spiral blade pitch is larger, the waste salt particles are pushed forward faster, the heating rate is faster, when the pitch is smaller, more turns are required to push forward the distance of the large pitch, so the heating rate is slower [50]. In the heating rate experiment, as shown in Fig. 8(c1, c2), the yield of particle size less than 0.43 mm decreased overall with increasing heating rate, and had a second largest value of 14.82% at 12°C/min, but at a heating rate of 15°C/min, the yield decreased rapidly to a minimum value of 3.95%, then at 20°C/min, the yield of particle size less than 0.43 mm reached a maximum of 19.49%, and then with increasing pyrolysis rate, the yield decreases. The rise in heating rate means that when the waste salt particles are pyrolyzed, the pyrolysis reaction proceeds faster, the heat released per unit time increases, and the partial melting of the waste salt during the pyrolysis process is stronger, so the abrasion resistance decreases with the increase in heating rate. However, when the heating rate increases to a certain degree, the abrasion resistance increases rapidly and then decreases rapidly. This may be due to the fact that under this heating rate, the heating process of the waste salt is accelerated and the exotherm of pyrolysis under aerobic atmospheric pressure becomes more intense, thus strengthening the partial melting of the waste salt particles and increasing the abrasion resistance, while the volatile organic compounds are volatilized by rapid pyrolysis, resulting in voids and pores in the microstructure of the waste salt, causing the abrasion resistance to decrease in turn. This relationship of opposite trends and dynamic changes is the reason behind the phenomenon.
3.4. Alteration of Waste Salt Microstructure by Pyrolysis ProcessThe waste salt particles of 2.36–4.75 mm were subjected to pyrolysis in the rotary kiln at a pyrolysis temperature of 600°C and a pyrolysis time of 40 mins, the SEM observation of the discharged waste salt particles is shown in Fig. 2(b1, b2, b3). It can be seen that the surface morphology of the waste salt particles before (Fig. 2(a1, a2, a3)) and after pyrolysis has changed significantly. Before pyrolysis, the waste salt particles were more angular, and the small organic matters on the surface were attached to the large waste salt particles. After pyrolysis, the SEM images showed that the shape was mainly formed by oval-shaped particles which are relatively smooth in appearance rather than being dominated by angularity. As can be seen in Fig. 2(b2) and Fig. 2(b3), the surface of the waste salt particles after pyrolysis is observed to be rounded and smooth at smaller magnifications, but further increasing the magnification to observe the surface of the waste salt particles more clearly, the void structure of the residual angles before pyrolysis and the honeycomb-like densely distributed pores left by the volatilization of organic matter after pyrolysis can be observed [51].
The particles size less than 0.30mm produced by the self-grinding of waste salt particles are the major source of fines that is undischarged during pyrolysis of waste salt particles in the rotary kiln. In the process of pyrolysis, the organic matter in waste salt particles is continuously volatilized through pyrolysis, so that the strength of waste salt particles decreases, and their edges fall off in the form of fines under the abrasive action of self-grinding motion during the rotation and pushed forward by the spiral blade inside the rotary kiln. The particle size of the fines falling off from the waste salt particles is usually less than 0.30 mm, and when the waste salt particles of this particle size level are pyrolyzed in the rotary kiln, fines eventually remain in the rotary kiln and undischarged due to the dominant effect of the viscosity exhibited by the waste salt particles at high temperature than the effect of gravity.
When the rotary kiln pyrolyzes the waste salt, the continuous pyrolysis process causes the accumulation of undischarged fines which fall off from the waste salt particles [52], and the undischarged fines are gradually compacted in the subsequent continuous feeding, the organic matter in the undischarged waste salt fines at the pyrolysis temperature continues to be pyrolyzed and exothermic. As time continues, the undischarged waste salt particles gradually become denser and more finely porous, as shown in Fig. 2(c1, c2, c3), the overall morphological structure is no longer rounded but closer to the unpyrolyzed waste salt particles while lack of angularity compared to the discharged waste salt particles, the finer pores on the surface indicate the combined effect of the compaction and the pyrolysis process.
4. ConclusionsThis study shows that the conditions and causes for the ring formation and occurrence in the pyrolysis of waste salt particles in the rotary kiln are investigated: fine size waste salt particles are the key to the ringing problem in the rotary kiln pyrolysis section of the waste salt pyrolysis carbonization process. The fine-size waste salt particles are either not strictly controlled at the time of feeding or produced by self-grinding of waste salt particles that have decreased in abrasion resistance after pyrolysis, driven by the rotating motion of the rotary kiln.
For the waste salt pyrolysis carbonization process, in the rotary kiln pyrolysis section, it is important that the particle size is not too small: on the one hand, waste salt particles with a small particle size will directly lead to the existence of more fine particles that are unable to be discharged, on the other hand, by the self-grinding effect, small particle size means a larger specific surface area, resulting in waste salt particles in the process of being rotated forward will produce more fine particles. For pyrolysis conditions, shorter pyrolysis time and appropriately high temperature are essential to decrease the amount of fines produced. For the rotary kiln pyrolyzer, the pitch of the internal spiral blades that push the waste salt particles forward should not be selected too small, the appropriate large pitch can allow the waste salt particles to move forward more distance in each rotation, reducing the generation of fine waste salt particles that are hard to discharge due to useless rotational self-grinding, Thus, controlling parameters including temperature distribution in the heating section and pitch of the spiral blade in the rotary kiln also helps to improve the operation of pyrolysis section.
AcknowledgementThis research was supported by China Ocean Mineral Resources R&D Association under Grant No. JS-KTHT-2019-01 and No. DY135-B2-15.
NotesAuthor Contributions W.W. (Master student) conducted conceptualization, methodology, wrote the original draft and finalized the manuscript. Y.F. (Professor) participated in the coordination of the study and reviewed the manuscript. H.L. (Professor) participated in the coordination of the study and analyze the results. S.L. (Ph.D. student) and J.J. (Ph.D. student) helped analyze the results and assisted in the experiments. Y.Y. (Master student) and B.W. (Master student) assisted in the experiments. References1. Hoyos P, Pace V, Alcántara AR. Biocatalyzed synthesis of statins: A sustainable strategy for the preparation of valuable drugs. Catalysts. 2019;9:260.
https://doi.org/10.3390/catal9030260
2. Zhou HL, Jiang JC, Huang AC, et al. Calorimetric evaluation of thermal stability and runaway hazard based on thermokinetic parameters of O, O–dimethyl phosphoramidothioate. J. Loss Prev. Process Ind. 2022;75:104697.
https://doi.org/10.1016/j.jlp.2021.104697
3. Natarajan S, Bajaj HC, Tayade RJ. Recent advances based on the synergetic effect of adsorption for removal of dyes from waste water using photocatalytic process. J. Environ. Sci. 2018;65:201–222.
https://doi.org/10.1016/j.jes.2017.03.011
4. Yuvaraj A, Karmegam N, Ravindran B, et al. Recycling of leather industrial sludge through vermitechnology for a cleaner environment— A review. Ind. Crops Prod. 2020;155:112791.
https://doi.org/10.1016/j.indcrop.2020.112791
5. Popovych V, Stepova K, Prydatko O. Environmental hazard of Novoyavorivsk municipal landfill. InMATEC Web Conf. 2018;247:00025.
https://doi.org/10.1051/matecconf/201824700025
6. Yaashikaa PR, Kumar PS, Nhung TC, et al. A review on landfill system for municipal solid wastes: Insight into leachate, gas emissions, environmental and economic analysis. Chemosphere. 2022;8:136627.
https://doi.org/10.1016/j.chemosphere.2022.136627
7. Das S, Lee SH, Kumar P, Kim KH, Lee SS, Bhattacharya SS. Solid waste management: Scope and the challenge of sustainability. J. Cleaner Prod. 2019;228:658–678.
https://doi.org/10.1016/j.jclepro.2019.04.323
8. Zhao Z, Xu W, Wang Z, et al. Investigation of organic impurity and its occurrence in industrial waste salt produced by physicochemical process. Plos one. 2021;16:e0256101.
https://doi.org/10.1371/journal.pone.0256101
9. Pandis PK, Kalogirou C, Kanellou E, et al. Key points of advanced oxidation processes (AOPs) for wastewater, organic pollutants and pharmaceutical waste treatment: A mini review. Chem Engineering. 2022;6:8.
https://doi.org/10.3390/chemengineering6010008
10. Shindhal T, Rakholiya P, Varjani S, et al. A critical review on advances in the practices and perspectives for the treatment of dye industry wastewater. Bioengineered. 2021;12:70–87.
https://doi.org/10.1080/21655979.2020.1863034
11. Zheng X, Chen W, Ying Z, et al. Structure–reactivity correlations in pyrolysis and gasification of sewage sludge derived hydrochar: effect of hydrothermal carbonization. Energy Fuels. 2020;34:1965–1976.
https://doi.org/10.1021/acs.energyfuels.9b04275
12. Tang H, Xu M, Hu H, et al. In-situ removal of sulfur from high sulfur solid waste during molten salt pyrolysis. Fuel. 2018;231:489–494.
https://doi.org/10.1016/j.fuel.2018.05.123
13. Lin C, Chi Y, Jin Y, et al. Molten salt oxidation of organic hazardous waste with high salt content. Waste Manage. Res. 2018;36:140–148.
https://doi.org/10.1177/0734242X17748364
14. Tao R, Xing P, Li H, Sun Z, Wu Y. Recovery of spent LiCoO2 lithium-ion battery via environmentally friendly pyrolysis and hydrometallurgical leaching. Resour. Conserv. Recycl. 2022;176:105921.
https://doi.org/10.1016/j.resconrec.2021.105 921
15. Yang C, Wu H, Cai M, et al. Valorization of Biomass-Derived Polymers to Functional Biochar Materials for Supercapacitor Applications via Pyrolysis: Advances and Perspectives. Polym. 2023;15:2741.
https://doi.org/10.3390/polym15122741
16. Haghighat M, Majidian N, Hallajisani A. Production of bio-oil from sewage sludge: A review on the thermal and catalytic conversion by pyrolysis. Sustainable Energy Technol. Assess. 2020;42:100870.
https://doi.org/10.1016/j.seta.2020.100870
17. Kartik S, Balsora HK, Sharma M, et al. Valorization of plastic wastes for production of fuels and value-added chemicals through pyrolysis–A review. Therm. Sci. Eng. Prog. 2022;32:101316.
https://doi.org/10.1016/j.tsep.2022.101316
18. Taka AL, Klink MJ, Mbianda XY, Naidoo EB. Chitosan nanocomposites for water treatment by fixed-bed continuous flow column adsorption: a review. Carbohydr. Polym. 2021;255:117398.
https://doi.org/10.1016/j.carbpol.2020.117398
19. Cai J, Zeng R, Zheng W, et al. Synergistic effects of co-gasification of municipal solid waste and biomass in fixed-bed gasifier. Process Saf. Environ. Prot. 2021;148:1–2.
https://doi.org/10.1016/j.psep.2020.09.063
20. Hanchate N, Ramani S, Mathpati CS, Dalvi VH. Biomass gasification using dual fluidized bed gasification systems: A review. J. Cleaner Prod. 2021;280:123148.
https://doi.org/10.1016/j.jclepro.2020.123148
21. Wu Q, Wang S, Zhang K, Zhao Y, He Y. Numerical studies of gas-solid flow behaviors and wall wear in a swirling fluidized bed. Powder Technol. 2021;388:233–40.
https://doi.org/10.1016/j.powtec.2021.04.083
22. Jiang X, Li Y, Yan J. Hazardous waste incineration in a rotary kiln: a review. Waste Disposal Sustainable Energy. 2019;1:3–7.
https://doi.org/10.1007/s42768-019-00001-3
23. Liang Z, Yi L, Huang Z, Huang B, Han H. A novel and green metallurgical technique of highly efficient iron recovery from refractory low-grade iron ores. ACS Sustainable Chem. Eng. 2019;7:18726–18737.
https://doi.org/10.1021/acssuschemeng.9b05423
24. Zhang Z, Wu Y, Li H, Li X, Gao X. A simple step-change method to determine mean residence time in rotary kiln and a predictive model at low inclination. Powder Technol. 2018;333:30–37.
https://doi.org/10.1016/j.powtec.2018.04.002
25. Mungyeko Bisulandu BJ, Marias F. Modeling of the thermochemical conversion of biomass in cement rotary kiln. Waste Biomass Valorization. 2021;12:1005–1024.
https://doi.org/10.1007/s12649-020-01001-9
26. Sanito RC, Bernuy-Zumaeta M, You SJ, Wang YF. A review on vitrification technologies of hazardous waste. J. Environ. Manage. 2022;316:115243.
https://doi.org/10.1016/j.jenvman.2022.115243
27. Wang Y, Zhang J, Liu Z. Rings growth behavior within a pre-reduction rotary kiln: The layered structure and formation mechanism. Powder Technol. 2019;356:73–82.
https://doi.org/10.1016/j.powtec.2019.08.015
28. Li H, Xie B, Zhu X, Li Q, Yang J. Erosion behaviour of rotary kiln refractory and its effects on ringing during steel-rolling oily sludge incineration. Waste Manage. 2023;164:162–70.
https://doi.org/10.1016/j.wasman.2023.04.005
29. Wang S, Guo Y, Fan J, et al. Characterization and comparison of deposits in a coal-fired rotary kiln for iron ore fluxed pellets and acidic pellets productions. Powder Technol. 2022;404:117454.
https://doi.org/10.1016/j.powtec.2022.117454
30. Liang Z, Yi L, Huang Z, et al. Insight of iron ore-coal composite reduction in a pilot scale rotary kiln: A post-mortem study. Powder Technol. 2019;356:691–701. https://doi.org/10.1016/j.powtec.2019.08.086
31. Bai K, Liu L, Pan Y, Zuo H, Wang J, Xue Q. A review: Research progress of flux pellets and their application in China. Ironmaking Steelmaking. 2021. 48:1048–63.
https://doi.org/10.1080/03019233.2021.1911770
32. Xiao X, Zhang S, Sher F, et al. A review on recycling and reutilization of blast furnace dust as a secondary resource. J. Sustainable Metall. 2021;7:340–57.
https://doi.org/10.1007/s40831-021-00377-9
33. Wang S, Guo Y, Liu K, et al. The deposit formation mechanism in coal-fired rotary kiln for iron ore pellet production: A review. Crystals. 2021;11:974–982.
https://doi.org/10.3390/cryst11080974
34. Saruwatari M, Nakamura H. Coarse-grained discrete element method of particle behavior and heat transfer in a rotary kiln. Chem. Eng. J. 2022;428:130969.
https://doi.org/10.1016/j.cej.2021.130969
35. Yang J, Wang S, Li Y, Zhang Y, Xu D. Novel design concept for a commercial-scale plant for supercritical water oxidation of industrial and sewage sludge. J. Environ. Manage. 2019;233:131–40.
https://doi.org/10.1016/j.jenvman.2018.11.142
36. Xu G, Li M, Lu P. Experimental investigation on flow properties of different biomass and torrefied biomass powders. Biomass Bioenergy. 2019;122:63–75.
https://doi.org/10.1016/j.biombioe.2019.01.016
37. Piana G, Ricciardi M, Bella F, Cucciniello R, Proto A, Gerbaldi C. Poly (glycidyl ether) s recycling from industrial waste and feasibility study of reuse as electrolytes in sodium-based batteries. Chem. Eng. J. 2020;15382:122934.
https://doi.org/10.1016/j.cej.2019.122934
38. Roy R, Weibel JA, Garimella SV. Modeling the formation of efflorescence and subflorescence caused by salt solution evaporation from porous media. Int. J. Heat Mass Transfer. 2022;189:122645.
https://doi.org/10.1016/j.ijheatmasstransfer.2022.122645
39. Liu T, Liu Z, Zheng Q, et al. Effect of hydrothermal carbonization on migration and environmental risk of heavy metals in sewage sludge during pyrolysis. Bioresour. Technol. 2018;247:282–290.
https://doi.org/10.1016/j.biortech.2017.09.090
40. Liu CH, Chu W, Li H, et al. Quantification and characterization of dissolved organic carbon from biochars. Geoderma. 2019;335:161–9.
https://doi.org/10.1016/j.geoderma.2018.08.019
41. Wang X, Gong Y, Qin J, Cheng J, Gong C, Jiang D. Deep removal of organic matter in glyphosate contained industrial waste salt by dielectric barrier discharge plasma. J. Environ. Chem. Eng. 2021;9:106295.
https://doi.org/10.1016/j.jece.2021.106295
42. Yan G, Zhang Z, Zhang B, et al. Preferential sequence crushing of copper ore based upon high-voltage pulse technology. Miner. Eng. 2019;131:398–406.
https://doi.org/10.1016/j.mineng.2018.11.035
43. Tan X, Su X, Yan Y, Uher C, Zhang Q, Tang X. New criteria for the applicability of combustion synthesis: The investigation of thermodynamic and kinetic processes for binary Chemical Reactions. J. Alloys Compd. 2021;860:158465.
https://doi.org/10.1016/j.jallcom.2020.158465
44. Defoort F, Grangier B, Chataing T, Ravel S, Ratel G, Valin S. Entrained flow gasification of hardwood bark: experimental characterization of inorganic matter versus equilibrium and viscosity predictions. Energy Fuels. 2021;35:12151–64.
https://doi.org/10.1021/acs.energyfuels.1c00993
45. Chen Z, Wang Y, Liao S, Huang Y. Grinding kinetics of waste glass powder and its composite effect as pozzolanic admixture in cement concrete. Constr. Build. Mater. 2020;239:117876.
https://doi.org/10.1016/j.conbuildmat.2019.117876
46. Li YH, Chang FM, Huang B, Song YP, Zhao HY, Wang KJ. Activated carbon preparation from pyrolysis char of sewage sludge and its adsorption performance for organic compounds in sewage. Fuel. 2020;266:117053.
https://doi.org/10.1016/j.fuel.2020.117053
47. Lee JE, Shim SB, Park JH, Chung I. Interfacial Properties and Melt Processability of Cellulose Acetate Propionate Composites by Melt Blending of Biofillers. Polym. 2022;14:4286.
https://doi.org/10.3390/polym14204286
48. Li Z, Shen A, Chen Z, Guo Y, Yang X. Research progress on properties of basalt fiber-reinforced cement concrete. Mater. Today Commun. 2022;104824.
https://doi.org/10.1016/j.mtcomm.2022.104824
49. Gao N, Jia X, Gao G, Ma Z, Quan C, Naqvi SR. Modeling and simulation of coupled pyrolysis and gasification of oily sludge in a rotary kiln. Fuel. 2020;279:118152.
https://doi.org/10.1016/j.fuel.2020.118152
50. Fu Y, Wang B, Zheng H, Zeng D, Xiao R. Simulation of heat transfer characteristics of tire particles in rotary kiln reactor. Int. J. Chem. React. Eng. 2021;19:1337–1349.
https://doi.org/10.1515/ijcre-2021-0176
51. Raymundo LM, Espindola JS, Borges FC, Lazzari E, Trierweiler JO, Trierweiler LF. Continuous fast pyrolysis of rice husk in a fluidized bed reactor with high feed rates. Chem. Eng. Commun. 2021;208:1553–63.
https://doi.org/10.1080/00986445.2020.1798937
52. Yi L, Zhang N, Liang Z, Wang L, Xiao H, Huang Z. Coal ash induced ring formation in a pilot scale rotary kiln for low-grade iron ore direct reduction process: Characterization and mechanism. Fuel. 2022;310:122342.
https://doi.org/10.1016/j.fuel.2021.122342
Fig. 2SEM images of waste salt particles before pyrolysis (a1, a2, a3) and after pyrolysis in rotary kiln (discharged b1, b2, b3; undischarged c1, c2, c3). 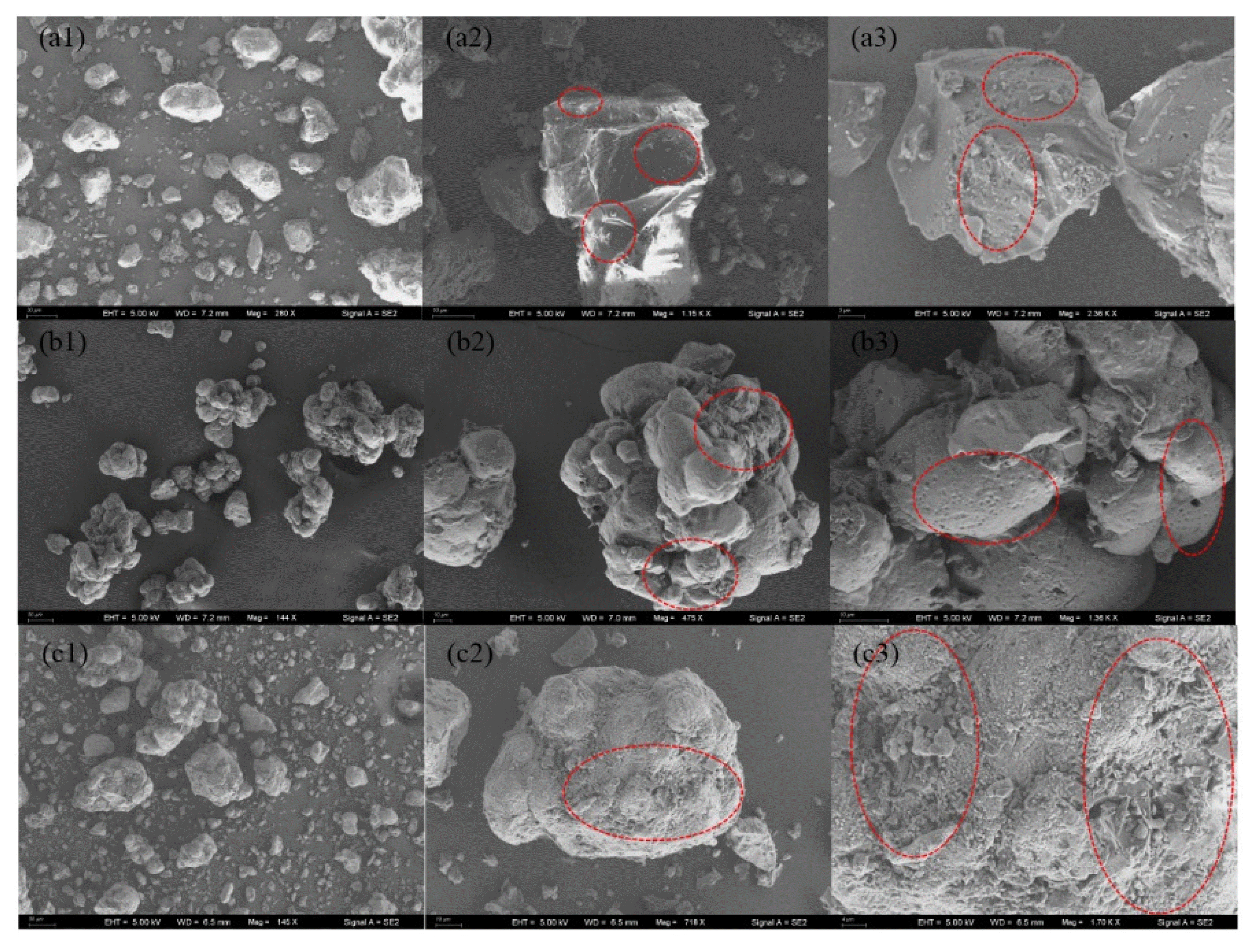
Fig. 6Effect of (a) particle size, (b) pyrolysis temperature, (c) pyrolysis time on the discharge rate of pyrolysis waste salt in rotary kiln. 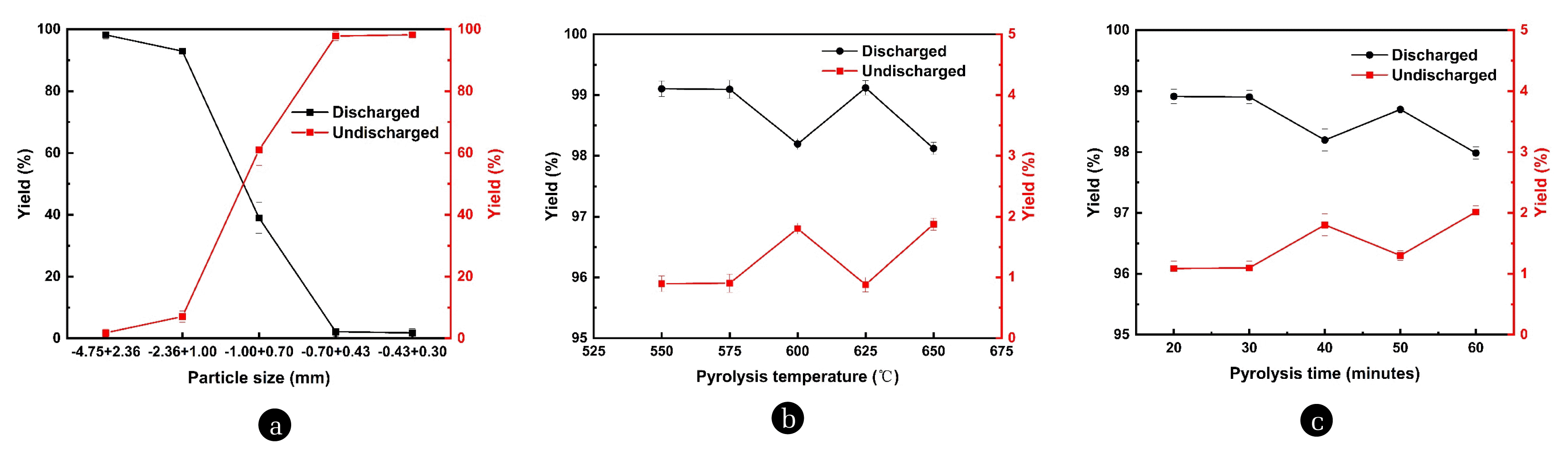
|
|
||||||||||||||||||||||||||||||||||