AbstractMolybdenum disulfide (MoS2) has become an attractive faradic material for capacitive deionization (CDI) process, but it still suffers from several drawbacks, such as low electrical conductivity, inferior hydrophilicity and easy restacking. Hence, we integrated MoS2 with high nitrogen content carbonized polyaniline (MoS2/CP) as intercalation cathode for CDI. The disordered entanglement between MoS2 and CP nanosheets enlarged interlayer spacing, improved pore structure and surface area which can provide multiple charge transfer routes and endow more embedding sites to storage Na+. The Mo-N-C bonds improve the electrical conductivity and wettability to facilitate ions diffusion process as well as ensure the cyclic stability of composites electrode. Moreover, the charge transfer between Na+ and N-containing functional groups is beneficial to forming pseudo-capacitance. Accordingly, the MoS2/CP electrode possess a large specific capacitance of 99.1 F g−1 at 5 mV s−1, which is 36% higher than MoS2. The hybrid cell AC//MC-2 delivers a remarkable desalination capacity (29.14 mg g−1), a rapid desalination rate (2.9 mg g−1 min−1) and favorable cyclic durability at 1.2 V in 500 mg L−1 NaCl solution. The superior desalination performance of MoS2/CP electrode was evaluated based on a capacitance-controlled contribution of 85.8% and a diffusion-controlled contribution of 14.2%.
Graphical Abstract
1. IntroductionInsufficient fresh water has become one of the serious problems and caught increasing attention [1]. Efficient desalination of saline or brackish water is a possible approach to deal with the crisis. However, orthodox desalination techniques include reverse osmosis [2], thermal evaporation [3], and electrodialysis [4], are suffering from potential environmental pollution and large energy consumption. Capacitive deionization (CDI), also known as electrosorption, is emerging as an alternative desalination method due to its economic cost, energy-efficiency (typically ≤ 2 V), and environmental friendliness [5–7].
The physicochemical properties of electrode materials play an essential role in determining CDI performance. Up to now, owing to the numerous advantages of huge specific surface area (SSA), outstanding electronic conductivity, and superior electrochemical stability, various carbon-based materials including activated carbon (AC) [8], carbon aerogel [9], carbon nanofibers [10], carbon nanotubes (CNT) [11], graphene [12], and their composites [13–15] have been widely used as CDI electrodes. Despite these merits above, disordered pore arrangement, the presence of micropores and overlapping effect of the electric double layers (EDLs) lead to low salt adsorption capacity in desalination process, which greatly restricted its development. In recent years, inspired by the research of sodium-ion batteries, intercalation electrode materials have appeared [16]. Na+ can be reversibly embedded in the layer or tunnel of the intercalation materials, accompanied by the faradaic charge transfer without changing the initial crystal structure. The co-ion expulsion effect can be effectively alleviated [17]. Moreover, the gap sites of the intercalated material increase the cyclic stability, yield greater ion storage capacity and rapid adsorption kinetics, resulting in pseudo-capacitive storage capacity and charge efficiency far more superior than the conventional carbon-based materials [18,19].
Among all of these intercalation materials, two-dimensional (2D) layered transition-metal molybdenum disulfide (MoS2) is composed of S-Mo-S cells stacked vertically via weak Van der Waals force between layers, which is quite attractive due to its flexible interlayer intercalation and high theoretical specific capacitance [20,21]. Also, previous studies have explored the desalination performance of MoS2 with different morphologies. The salt adsorption capacities (SACs) are in the range of 8.81–24.6mg g−1 and higher than carbon-based materials [22,23]. However, the inferior electrical conductivity and poor hydrophilicity of MoS2 greatly affect the charge transfer rate. Within this context, integrating MoS2 with carbon-based materials is a simple and effective approach to overcome the above disadvantages [24]. For instance, Gao et al. used expanded MoS2 nanosheets supported by reduced graphene oxide (MoS2/rGO) as electrode, which exhibits a saturated SAC of 34.20mg g−1 in 300mg L−1 NaCl solution at 1.4 V [25]. Cai et al. developed MoS2 connected with CNT and carbon spheres (MoS2@CNT-CS) electrode with an improved SAC of 25.35 mg g−1 at 1.2 V in 500 mg L−1 NaCl solution [26]. In particular, nitrogen doping of carbon materials has a significant effect on improving wettability, conductivity and contributing pseudo-capacitance. Recently, Tian et al. combined MoS2 with nitrogen-doped highly ordered mesoporous carbon (MoS2/NOMC) as the intercalated electrode, which achieved the remarkable SAC of 30.49mg g−1 at 1.6 V in 250mg L−1 NaCl solution [27]. Even worse, the serious restacking of MoS2 sheets makes a large number of adsorption sites difficult to use, which will reduce the desalination performance. The primary objective of the research is to develop novel MoS2 composites with the characteristics of expanded interlayer space, enhanced specific capacitance, fast charge transfer and ionic storage, and good cyclic stability.
Conducting polymers such as polyaniline (PANI) are quite attractive owing to their high theoretical specific capacitance, easy preparation, low production cost, and environmental friendliness [28]. Meanwhile, PANI has a large number of amine and imine functional groups, which is beneficial to enhance the CDI performance. Unfortunately, when PANI is used alone as an electrode material, the low practical capacitance caused by agglomeration and the severe performance fading due to the volume change restrict its application [29,30]. According to previous studies, PANI is commonly coupled with various carbonaceous materials to enhance the capacitance of carbon material, and the stability of PANI can be improved. For instance, Haq et al. synthesized ion-exchange (-NH2+ and-SOH3−) PANI-AC composites electrode, which achieved the SAC of 17.7 mg g−1 in 8.55 mM NaCl solution at 1 V [31]. Tian et al. fabricated an advanced PANI-decorated activated carbon fiber (ACF/PANI) electrode, which demonstrated a maximum SAC of 19.9 mg g−1 at 1.2 V (200 mg L−1 NaCl), a low charge transfer resistance (1.17 Ω) and a robust regeneration performance (2800 min for 28 circles) [32]. More importantly, as a low-cost nitrogen-containing precursors, PANI can facilitate the introduction of nitrogen-containing active sites inside the carbon matrix at high temperatures [33]. Until now, few studies have focused on combining MoS2 with PANI-derived carbon to exert synergetic advantages for CDI application, and the mechanism of nitrogen-doping into MoS2-carbon nanocomposites on their desalination performance has been reported scarcely.
Inspired by the above research, for the first time, this study integrates the high nitrogen-doped carbonized polyaniline with MoS2 to prepare electrode (MoS2/CP) for CDI process. We speculate that the disordered entanglement between MoS2 and CP nanosheets will be beneficial to reduce the agglomeration of MoS2 layers and endow the composites with convenient charge transfer pathway. The formation of strong Mo-N-C bonds between CP and MoS2 can be conductive to improving wettability and electrical conductivity as well as cyclic stability. Pyridinic and pyrrolic-N groups can introduce defects to provide more open channels, and the charge transfer between Na+ and N-rich functional groups induce pseudo-capacitance which will improve the total capacitance of the electrode.
Herein, the efficient removal of Na+ by hybrid CDI (HCDI) cell with MoS2/CP electrode from saline water was explored. The MoS2/CP composites were developed via a facile hydrothermal route, and the composites combined with different CP content were compared and investigated. The desalination capacity of MoS2/CP containing 0.56 g CP (MC-2) as the cathode was evaluated by adsorption isotherm and kinetic fitting method. In addition, the physical and chemical properties of MC-2 before and after the removal process of Na+ were analyzed by XPS. The mechanism of desalination during electrosorption process with MC-2 was proposed, as well.
2. Materials and Methods2.1. MaterialsAmmonium molybdate ((NH4)6Mo7O24˙4H2O), thiourea (CN2H4S), aniline, P-toluenesulfonic acid monohydrate (HPTS), ammonium persulfate (CH4N2S), sodium hydroxide (NaOH), sodium chloride (NaCl), anhydrous ethanol (CH3CH2OH), polyvinylidene fluoride (PVDF) and N-methyl-2-pyrrolidone (NMP) were analytical grade and supported by the Sinopharm Chemical Reagent Co., Ltd. (China). Conductive carbon black (Carbon ECP-600JD) was purchased from LION Specialty Chemicals Co., Ltd. (Japan). Pure aluminium plates used as current collectors were provided from Tianjin Bodi Chemical Co., Ltd. (China).
2.2. Preparation of Carbonized PANIThe precursor PANI was synthesized via the same method described in the literature [34]. Briefly, 10mL of 0.21M aniline was added into 500mL of 0.3 M HPTS solution at 3°C. Subsequently, 85.9 mL of 1 M CH4N2S solution was dropped slowly to polymerize the monomer. The reaction process was carried out under stirred continuously for 2 h. Then, the dark green precipitate was obtained by vacuum filtration, rinsed repeatedly with deionized (DI) water, and dried at 60°C overnight [35]. The product was carbonized in a tubular furnace at 500°C with the heating rate of 10°C min−1 for 2 h under N2. Finally, the resulting sample was grinded and referred to as CP.
2.3. Synthesis of MoS2/CP CompositesTypically, (NH4)6Mo7O24˙4H2O (1.24 g) and CN2H4S (2.28 g) were dissolved in DI water (60mL). Subsequently, 0.56 CP powder was added into the above solution with an ultrasound for 60 min. Then the mixture solution was sealed and subjected to hydrothermal route at 200°C for 24 h. The product was centrifuged and washed with DI water and anhydrous ethanol several times. Finally, the MoS2/CP (MC-2) composites were obtained after drying at 60°C overnight in a vacuum oven. The prepared samples were labeled as MC-1 and MC-3 according to the amount of CP (1.12 and 0.37 g), respectively. Pure MoS2 was also prepared in the same way except for the addition of CP.
2.4. Fabrication of ElectrodesThe electrode material was a mixture of active material (MoS2, MC-1, MC-2, MC-3 or AC), conductive carbon black, and PVDF with a mass ratio of 8:1:1. For electrochemical measurement, the active material (8 mg), conductive carbon black (1mg), and PVDF (1 mg) were dissolved in NMP (about 0.4 mL) with stirring for 3 h to ensure uniformity. Afterwards, the slurry mixture was coated onto the graphite paper (1×1 cm2) by using a pipette (1000 uL), and further dried in a vacuum oven at 60°C for 12 h to remove the residual organic solvent. The mass loading of the prepared electrode was measured to be 9.6 mg. For CDI test, the electrodes were prepared as the same method as that of electrochemical measurement except aluminum plate as current collector. The aluminum plate was etched using sodium hydroxide solution (0.5 mol L−1) for 3 min and electrochemical corrosion for 5 min at 1 A/dm2 current density and rinsed thoroughly with DI water. The obtained slurry coated onto a aluminum plate (5×5 cm2) with the effective working area of 8.87 cm2 and the mass of coated material was 44.7 mg [36].
2.5. Material CharacterizationsScanning electron microscopy (FE-SEM, S4800 Hitachi, Japan) was employed to characterize the material morphology. The elements of the samples were analyzed by energy dispersive X-ray spectroscopy (EDX) attached to the SEM instrument. The crystalline phases were determined by X-ray diffraction (XRD, Beijing Puxi General Instrument Co., Ltd.), and measurement condition was Cu Kα radiation (λ = 0.154 nm) at 2θ ranging from 10° to 90°. The functional groups were identified by Fourier transform infrared spectroscopy (FTIR, Nicolet iS50, Thermo Fisher Technology Co. Ltd.). The surface elemental species were measured by X-ray photoelectron spectroscopy (XPS, ESCALAB 250XI, Thermo Fisher Scientific) under Al Kα radiation. The specific surface area (SSA) and pore structure were measured by a Brunauer-Emmett-Teller (BET) N2 adsorption method (JWBK122W, Beijing Jingwei Gaobo Science and Technology Co., Ltd.) at 423.1 K. The hydrophilicity was determined by the contact angle measuring instrument (Krüss, DSA100).
2.6. Electrochemical MeasurementsThe electrochemical behaviors including cyclic voltammetry (CV), electrochemical impedance spectroscopy (EIS), and galvanostatic charge/discharge (GCD) were conducted on an electrochemical workstation (CHI 760E, Chen hua) in 1M NaCl electrolyte with a typical three-electrode system. The as-prepared electrode, platinum sheet (1×1 cm2), and Ag/AgCl (saturated KCl solution) were applied as working electrode, counter electrode, and reference electrodes, respectively.
The specific capacitance (C, F g−1) of working electrode was calculated by Eq. (1):
where I, m, v, and ΔV correspond to the current response (A), the mass (g) of active material, the scan rate (V s−1), and voltage window (V), respectively.
The discharge specific capacitance was evaluated by the GCD curve from Eq. (2):
where I, Δt, m, and ΔV stand for the discharge current (A), discharge time (s), the mass (g) of active material, and voltage window (V), respectively.
2.7. Batch Mode CDI TestThe electrosorption performance of the prepared electrodes at different operational parameters, such as external voltages (0.8–1.4 V) and the initial NaCl concentrations (100–500 mg L−1) were evaluated using a batch-mode CDI experiment device (Fig. 1). It consisted of an electrosorption cell (Fig. S1), a peristaltic pump, a direct current (DC) regulated power supply, and an online conductivity meter. Electrosorption cell was the key component, in which MoS2/CP cathode and AC anode were placed in parallel, separated by a silicone rubber spacer with a thickness of 1 mm to prevent a short circuit. A square water flow channel with a size of 3 cm×3 cm in the center. The cyclic electrosorption experiment was carried out in 100 mL NaCl solution with a constant flow rate of 20 mL min−1. The concentration of NaCl solution was positively correlated with its conductivity (Fig. S2) which was recorded every 30 s by an ion conductivity meter (DDS-308F). Real-time detection of the current was conducted by multimeter (VC 8265, China). The salt adsorption capacity (SAC, mg g−1), charge efficiency (Λ), and average adsorption rate (SAR, mg g−1 min−1) were obtained by Eqs. (3)–(5):
where C0, Ct, V, m, F, and M represent the initial salt concentration (mg L−1), steady-state salt concentration (mg L−1), the volume of electrolyte (L), the mass (g) of active material in both electrodes, Faraday’s constant (96485 C mol−1), and molecular mass of NaCl (58.5 g mol−1), respectively.
3. Results and discussion3.1. Morphology and StructuresThe micro-morphology and structure of CP, MoS2, and MC-2 were identified by SEM. CP shows a thin sheet structure (Fig. 2a). MoS2 presents a granular shape with uneven size and irregular shape (Fig. 2b). In contrast, the MC-2 composites display a 3D flower-like architecture which are composed of loosely stacked and interconnected nanosheets (Fig. 2c–d). Nano-flowers mixed with CP sheet are interconnected to form a well-opened porous framework, and the enrich porosity increases the accessible surface area for ion accumulation. Ultra-thin 3D flower-like MoS2 nanosheets give a large number of exposed edges, which provides multiple routes for Na+ fast interlayer insertion and accelerate the electron transfer rate during electrosorption process. The content and distribution of different elements in MC-2 were analyzed by EDS. The element mappings (Fig. 2e) show that Mo, S, C, O, and N are uniformly distributed throughout the sample, and the nitrogen content is 2.7 wt% (Fig. S3), revealing the successful preparation of MC-2 composites.
The XRD patterns of as-prepared samples were shown in Fig. 3a. The diffraction profiles of CP exhibit a (002) broad band centered at 20.7°, which is typically amorphous carbon [19,33]. MoS2 and MC-2 display similar crystal characteristics. The four characteristic diffraction peaks of MC-2 located at 13.80°, 32.85°, 39.59° and 57.60° are assigned to the (002), (100), (103), and (110) planes of hexagonal 2H-MoS2 (JCPDS no. 37-1492) [37,38], respectively. The (002) diffraction peak of MC-2 shifts slightly to lower diffraction angles. Based on the Bragg equation (2dsinθ=nλ) calculation, the interlayer distance increases from 0.825 nm for MoS2 to 0.841 nm for MC-2, which is favorable to absorb more ions. In addition, the intensity of the (002) diffraction peak is slightly reduced, indicating that MC-2 composites possess much lower crystallinity. A large number of MC-2 sheets have a relatively loose structure in agreement with the SEM analysis results. It shows that the disordered entanglement between MoS2 and CP nanosheets is beneficial to inhibit the accumulation of MoS2. The expanded interlayer spacing is helpful to accommodate more intercalated Na+ during cyclic charge/discharge process.
Fig. 3b shows the FTIR spectra of CP, MoS2, and MC-2. In the spectrum of MC-2 composites, the diffraction peaks at 1555 and 1471 cm−1 belong to C=C stretching of the quinoid ring and C=C stretching vibration of the benzenoid ring, respectively, which are the characteristic diffraction peaks of CP. The diffraction peaks at 1430 and 1336 cm−1 are assigned to C-N stretching of the benzenoid ring. The peaks at 1010 and 791 cm−1 are attributed to C-C stretching and N-H out-of-place bending vibration [39], respectively. These vibrational modes do not exist in the pure MoS2. Moreover, the Mo-S vibrational mode (543 cm−1) in both MoS2 and MC-2 composites remains unchanged [40,41].
The SSAs and pore structures of CP, MoS2, and MC-2 were investigated via N2 adsorption-desorption isotherm (Fig. 4a). At relative pressure (P/P0) between 0–1.0, the prepared samples possess a type IV isotherm curve with a type H3 hysteresis loop, implying the existence of mesopores [42]. According to BET method, the SSAs of CP, MoS2, and MC-2 were 32.40, 8.88, and 13.78 m2 g−1, respectively. The introduction of CP increases the SSA of MoS2, which may create more effective electroactive sites for Na+ storage. Therefore, the diffusion distance between electrons and Na+ is shortened, and the electrochemical kinetics is improved. The hydrophilicity of CP, MoS2 and MC-2 was evaluated by the contact angle tests, as demonstrated in Fig. 4b. Compared with CP (46.2°) and MoS2 (13.5°), the contact angle of MC-2 (11.3°) is slightly reduced, which indicates that the hydrophilicity of MC-2 sample is improved. It may be ascribed to the unpaired electrons generated by N atoms, which leads some structural defects and disorder of MC-2 composites, changes the charge distribution and improves the hydrophilicity. The enhanced hydrophilicity facilitate the penetration process of electrolyte and ions migration to electroactive sites, thus improving the desalination performance.
3.2. Electrochemical Performance Analysis
Fig. 5a shows the voltammograms of all the as-prepared electrodes at 10 mV s−1 in the working potential range of −0.4 to 0.4 V. The CV curve of CP electrode maintains a standard rectangle, demonstrating typical EDL capacitance characteristics. The voltammogram of MoS2 shows a pair of obvious redox peaks, which is mainly caused by multiple phase transitions accompanied by insertions and extractions of Na+ within the electrode [43,44]. Likewise, the redox peaks are also detected after MoS2 combined with CP of different mass, reflecting pseudo-capacitance behavior in MoS2/CP composites electrodes. Notably, with increasing the amount of CP, the specific capacitance of three composite electrodes increases at first and declines by comparing the enclosed area of the voltammograms. The capacitance of MC-2 is the highest, and the capacitance of MC-1 and MC-3 are less than that of original MoS2. The lager capacitance is more conductive to subsequent electrosorption performance [45].
Fig. 5b displays the voltammograms of MC-2 at various scan rates from 5 to 100 mV s−1. As the scan rate increased, the width of redox peaks gradually increases and becomes more obvious, which indicates that the composites electrode possesses good rate performance and pseudo-capacitance behavior during CV test. Fig. 5c depicts the specific capacitances of CP, MoS2, and MC-2 as a function of scan rates. The corresponding capacitances of the three electrodes gradually decrease with increasing scan rate, and the calculated capacitances follow the order of MC-2 > MoS2 > CP at all scan rates. The maximum capacitance of MC-2 is 99.1 F g−1, which is 36% higher than that of MoS2. It proves that the capacitance of pure MoS2 is increased after combined with CP, which is beneficial to salt adsorption in CDI.
Fig. 5d exhibits the electrical resistances of CP, MoS2, and MC-2 electrodes were measured by EIS. A circular arc and a sloped straight line are observed in the high-frequency area and low-frequency area, respectively. The radius of circular represents the the charge transfer resistance (Rct). The smallest semicircle and the increased inclined slope indicating that MC-2 exists the fastest charge transfer rate and enhanced ion diffusion behavior.
As shown in Fig. 5e, GCD tests were conducted on MoS2, CP, and MC-2 electrodes at a current density of 1 A g−1. In comparison with CP and MoS2, the discharge time of MC-2 electrode is obviously prolonged, demonstrating that it possesses the best charge release and storage capacity. Meanwhile, the MC-2 electrode delivers a discharge capacity of 60.9 F g−1 and a charge capacity of 64.1 F g−1 with a coulombic efficiency of 95%, which indicates the highly reversible charge transfer performance. Furthermore, the GCD test of MC-2 electrode shows a triangular curve at different current densities (Fig. 5f), which implies that the existence of pseudo-capacitance behavior.
Therefore, it can be anticipated that the best electrochemical property will endue MC-2 a favorable desalination performance.
3.3. CDI Performance EvaluationThe asymmetric HCDI cells were constructed to test the desalination capacity. Using the AC electrode as anode, and the as-prepared CP, MoS2, MC-1, MC-2, and MC-3 as cathode, respectively. The assembled cells were denoted as AC//CP cell, AC//MoS2 cell, AC//MC-1 cell, AC//MC-2 cell and AC//MC-3 cell. Fig. 6a illustrates the SAC of AC//MC-2 cell in different NaCl concentrations (100–500 mg L−1) at 1.2 V. It can be seen that the SAC values increase with the initial salt concentration. In Fig. 6b, the corresponding Ragone plots at different salt concentration present the trend of increased SAC and decreased SAR, which is attributed to the increase of electrical conductivity in NaCl solution with high concentration, resulting in the fast ion storage speed and high electrosorption capacity. Thus, in the 500 mg L−1 feed solution, the AC//MC-2 achieves the highest SAC and the fastest SAR.
Fig. 6c shows the isotherm adsorption process of the cell AC//MC-2 in various initial NaCl concentrations, which was fitted by the Langmuir (Eq. (S1)) and Freundlich (Eq. (S2)) models. The comparative parameters are displayed in Table S1. The greater value of correlation coefficient (R2=0.9867) indicates Freundlich model can better fit the experimental data than that of Langmuir model (R2=0.9818). It corroborates that the electrosorption process of MC-2 is dominated by multilayer adsorption, which can be related to ion intercalation in MoS2 nano-flowers.
Fig. 6d exhibits the effects of various external voltages on the SACs of AC//CP, AC//MoS2, and AC//MC-2 cells. It can be found that under different voltages, the SAC of AC//CP is quite low, and the SAC of AC//MC-2 is significantly superior to that of AC//MoS2. As the voltage increased from 0.8 to 1.2 V, the SACs of AC//MC-2 and AC//MoS2 cells improve from 9.02 and 14.02 mg g−1 to 23.53 and 29.14 mg g−1, respectively. A greater electrical potential can produce stronger electric field force for the electrodes to capture more anion and cation from the saline water [25,46,47]. It is noticeable that the SAC values of AC//MC-2 are always the highest at any voltage. Additionally, the SAC values of the three cells decline as the voltage reaches 1.4 V. The reduced SAC can be attributed to the fact that higher voltage causes water splitting and some side reactions. Therefore, the optimal applied voltage is determined to be 1.2 V.
Fig. 6e displays the CDI performance of the as-prepared electrodes were measured by recording the SAC vs desalination time at 1.2 V in 500 mg L−1 NaCl solution. The AC//MoS2, AC//MC-1, AC//MC-2, and AC//MC-3 cells reach adsorption equilibrium at approximate 30, 31, 28, and 35 min, respectively. The equilibrium SAC decreases in the order of AC//MC-2 > AC//MoS2 > AC//MC-3 > AC//MC-1, which is correspond to the result of CV measurement. In addition, kinetic models were employed to analyze the experimental data, and the relevant adsorption kinetic parameters are listed in Table S2. The calculated values of equilibrium electro-sorptive capacity qe of pseudo-first-order kinetic (Eq. (S3)) model are good consistency with the experimental data, and the regression correlation coefficient (R2) is larger than that of pseudo-second-order kinetic (Eq. (S4)) model. The results indicate that pseudo-first-order kinetic can better describe the electrosorption process of the four cells, and the electrostatic interaction between Na+ in the solution and the electrode plays an important role in the electrosorption. And the maximum electrosorption rate constant k1 (0.1395) is achieved by AC//MC-2, demonstrating the enhanced desalination kinetic process for MC-2 electrode.
The relationship between SAR vs. SAC is reflected by Kim-Yoon Ragone plots. As shown in Fig. 6f, the AC//MoS2 and AC//MC-2 cells exhibit a decreased SAR and an increased SAC with the increase of the time. In addition, the Ragone plot of cell AC//MC-2 dominates the upper-right region with a higher SAC (29.14 mg g−1) and a more rapid SAR (2.9 mg g−1 min−1), indicating that AC//MC-2 presents a much more excellent desalination kinetics than AC//MoS2. Based on the calculation of Eq. (4), the charge efficiency of AC//MC-2 is 66.8%.
3.4. Desalination Mechanism of MC-2 Composites ElectrodeThe reaction kinetic and Na+ storage behavior in the MC-2 electrode can be investigated by quantitatively determined the contribution ratio of capacitance and diffusion control to the total capacitance via Eq. (6):
where k1v and k2v1/2 refer to capacitance-controlled and diffusion-controlled current, respectively. From Fig. 7a, the contribution of pseudo-capacitive process occurs on the surface of MC-2 electrode is 85.8%, and the diffusion control process by Na+ intercalation into the bulk material is 14.2% (100 mV s−1). The results indicate that Na+ intercalation reaction mainly by forming the pseudo-capacitance. Meanwhile, the pseudo-capacitive process indicates rapid Na+ storage dynamics [48].
More chemical information before and after desalination of MC-2 was further analyzed by XPS. In Fig. 7b, the survey spectra elucidate the presence of C, Mo, S, O as well as N elements, in line with the characterization results of EDS mappings. Compared with the XPS spectra before desalination, F peak is observed which is mainly originated from PVDF binder used in the preparation of MC-2 electrode. It is notable that a small Na peak appears at 1077 eV, indicating that MC-2 electrode does absorb Na+. The C 1s spectrum of MC-2 composites (Fig. 7c) displays four characteristic peaks which are associated with sp2-hybridized C=C (284.2 eV), sp3-hybridized C-C (284.7 eV), C-N (285.2 eV), and C-O (285.9 eV) bonds, respectively [49,50].
As exhibited in N 1s spectra (Fig. 7d), the peak at 394.9 ev is derived from chemical bond Mo-N-C (394.9 ev), which was constructed after N (CP) in-situ partially substituted S in MoS2 to form N-doped MoS2 (N-MoS2) during the hydrothermal route. The existence of Mo-N-C bond can effectively improve the electrical conductivity and wettability of composites [48,51]. The peaks at 399.3 and 401.8 eV belong to pyridinic and pyrrolic-N, respectively. After electrosorption, the peak positions of pyridinic and pyrrolic-N shift to the right and left, respectively. The two peaks overlap to form a notably enhanced peak near 399.8 eV corresponds to the charge transfer between Na+ and N-rich functional groups during electrosorption. The charge transfer facilitates the formation of pseudo-capacitance, thus increasing the specific capacitance of MC-2 electrode.
In Fig. 7e, the Mo 3d high-resolution spectra at 229.1 (Mo 3d5/2) and 232.0 eV (Mo 3d3/2) correspond to Mo4+ [52]. Peaks at 228.3 eV and 235.9 eV are consistent with Mo2+ and Mo6+, respectively. It can be found that Mo has multiple valence states, which is beneficial to improve the SAC through the pseudo-capacitance reactions. In addition, the S 2p spectrum can be deconvoluted into S 2p3/2 (161.5 eV) and S 2p1/2 (162.8 eV) orbitals of S2− (Fig. 7f), respectively. After electrosorption, the peak intensities of Mo 3d (236.3 eV) and Mo 3d5/2 (228.9 eV) increase notably. It can be attributed to the electrochemical reaction during the electrosorption process, which results in partial Mo4+ transforming into Mo6+ and partial Mo6+ is reduced to Mo2+ [53]. The relatively low peak intensities of Mo2+ and Mo6+ clearly indicate that Mo mainly exists in the form of MoS2. The peak locations of S 2p shift from 161.5 and 162.8 eV to 161.9 and 163.0 eV [54], respectively. The shift in peak positions of Mo 3d3/2 and Mo 3d5/2 of Mo4+, S 2p3/2 and S 2p1/2 of S2− can be neglected. Most MoS2 crystals keep their original crystallographic structure after adsorption, which confirms that Na+ intercalated MoS2 dominates the electrosorption process.
The above analysis suggests that charge transfer between Na+ and doped N from CP, as well as the various oxidation states (from +2 to +6) of Mo atoms in MoS2 greatly facilitate the formation of the pseudo-capacitance. The increased capacitance due to nitrogen-doping is the predominant contribution for the better CDI performance of MC-2.
3.5. Stability of MC-2 ElectrodeThe GCD measurement was performed to obtain the electrochemical stability of electrode. After 20 consecutive GCD cycles, the specific capacitance remains almost constant (Fig. 8a), revealing that MC-2 electrode possesses an excellent capacitance retention rate. The regeneration performance was evaluated by six adsorption/desorption cycles (one adsorption/desorption cycle lasts about 100 min) at 1.2 V. As shown in Fig. 8b, once the voltage was imposed, the conductivity decreased sharply until reaching equilibrium. Ions in the saline solution were adsorbed on electrode materials, thus reducing the conductivity of the solution. The electrodes were regenerated by applying a reversed voltage. With the captured ions released, the conductivity increased again and returned to the initial value. The SAC of AC//MC-2 cell remains stable during six adsorption/desorption cycles, which confirms that the MC-2 can be well regenerated and reused. It is ascribed to the stable Mo-N-C bonds between N-MoS2 and CP guarantee good structural strength for MC-2 electrode.
4. ConclusionsIn summary, through elaborate control of polymerization ratio, the 3D flower-like N-containing MoS2/CP nanocomposites were successfully synthesized by facile hydrothermal method. The electrosorption process of Na+ fits well with the Freundlich and pseudo-first-order kinetic model, signifying that the adsorption of Na+ on MoS2/CP is dominated by multilayer adsorption and electrostatic interaction. Moreover, 85.8% of Na+ storage is ascribed to the capacitance-controlled contribution including the pseudo-capacitance while the remaining 14.2% accounted for diffusion-controlled process.
The asymmetric HCDI cell with as-fabricated MoS2/CP as cathode, manifesting a higher SAC (29.14 mg g−1) and higher SAR (2.9 mg g−1 min−1) than AC//MoS2. It also presents considerable advantages over most electrode materials recently reported as listed in Table S3. The enhanced CDI behavior can be mainly ascribed to the following reasons: (i) The introduction of CP effectively improves the flaky disordered stacking of the pure MoS2 and the SSA of MoS2/CP, which provide abundant intercalation sites to adsorb Na+ and endow multiple paths to accelerate the electron transfer rate. (ii) The charge transfer between Na+ and N-containing functional groups forms extra pseudo-capacitance. The specific capacitance of MoS2/CP exhibits 99.1 F g−1 which is 36% higher than MoS2 (at scan rate 5 mV s−1). (iii) Mo-N-C bonds not only improve the electrical conductivity and hydrophilicity to accelerate ions diffusion process, but also guarantee structural stability of hybrid electrode. Thus, the MoS2/CP can keep favorable capacitance retention rate and regeneration performance after several cycles.
For the first time, the composites of MoS2 and PANI-derived carbon were used as the Na+ capturing electrode of HCDI, and the mechanism of nitrogen doping enhanced the deionization performance for MoS2/CP electrode was also studied. However, the influences of PANI-derived carbon fabricated under different carbonization temperatures on desalination performance of MoS2-based composite electrodes were not discussed, since more in-depth research is needed. The MoS2/CP composites prepared in this study possess a great potential as a cost-effective and eco-friendly electrode material for CDI application.
AcknowledgmentsThis research is financially supported by the Science and Technology Planning Project of Shaanxi Provincial Water Resources Department (2022slkj-5). We also gratefully acknowledge the support provided by Key R&D plan of Shaanxi province (2022SF-578).
References1. Mi M, Liu X, Kong W, Ge Y, Dang W, Hu J. Hierarchical composite of N-doped carbon sphere and holey graphene hydrogel for high-performance capacitive deionization. Desalination. 2019;464:18–24.
https://doi.org/10.1016/j.desal.2019.04.014
2. Qin M, Deshmukh A, Epsztein R, Patel SK, Owoseni OM, Walker WS, Elimelech M. Comparison of energy consumption in desalination by capacitive deionization and reverse osmosis. Desalination. 2019;455:100–114.
https://doi.org/10.1016/j.desal.2019.03.016
3. Xue Y, Du X, Ge Z, Yang L. Study on multi-effect distillation of seawater with low-grade heat utilization of thermal power generating unit. Appl. Therm. Eng. 2018;141:589–599.
https://doi.org/10.1016/j.applthermaleng.2018.05.129
4. Al-Amshawee S, Yunus MYBM, Azoddein AAM, Hassell DG, Dakhil IH, Hasan HA. Electrodialysis desalination for water and wastewater: A review. Chem. Eng. J. 2020;380:122231.
https://doi.org/10.1016/j.cej.2019.122231
5. Tikhonov NA, Tokmachev MG, Bakhia T, Khamizov RK. Modeling the process of capacitive deionization of solutions at supposing complex structure of the pores of the electrodes. J. Math. Chem. 2021;59:1054–1067.
https://doi.org/10.1007/s10910-021-01226-6
6. Liu Y, Du X, Wang Z, Zhang L, Chen Q, Wang L, Liu Z, Dou X, Zhu H, Yuan X. MoS2 nanoflakes-coated electrospun carbon nanofibers for “rocking-chair” capacitive deionization. Desalination. 2021;520:115376.
https://doi.org/10.1016/j.desal.2021.115376
7. Tang K, Kim Y-h, Chang J, Mayes RT, Gabitto J, Yiacoumi S, Tsouris C. Seawater desalination by over-potential membrane capacitive deionization: Opportunities and hurdles. Chem. Eng. J. 2019;357:103–111.
https://doi.org/10.1016/j.cej.2018.09.121
8. Ahn D, Kim D, Park JH, Kim N, Lim E, Kim C. Enhanced desalination performance of nitrogen-doped porous carbon electrode in redox-mediated deionization. Desalination. 2021;520:115333.
https://doi.org/10.1016/j.desal.2021.115333
9. Liu X, Liu H, Mi M, Kong W, Ge Y, Hu J. Nitrogen-doped hierarchical porous carbonaerogel for high-performance capacitive deionization. Sep. Purif. Technol. 2019;224:44–50.
https://doi.org/10.1016/j.seppur.2019.05.010
10. Men L, Chen C, Liu A, Zhou J, Yu S, Wei Z. Activated carbon fiber–loaded single layer graphene oxide–based capacitive deionization electrode materials for water desalination applications. Ionics. 2022;28:1903–1913.
https://doi.org/10.1007/s11581-022-04473-y
11. Bao S, Duan J, Zhang Y. Characteristics of Nitric Acid-Modified Carbon Nanotubes and Desalination Performance in Capacitive Deionization. Chem. Eng. Technol. 2018;41:1793–1799.
https://doi.org/10.1002/ceat.201700448
12. Zhang G, Li W, Chen Z, Long J, Xu C. Freestanding N-doped graphene membrane electrode with interconnected porous architecture for efficient capacitive deionization. Carbon. 2022;187:86–96.
https://doi.org/10.1016/j.carbon.2021.10.081
13. Wang H, Sun W, Liu Y, Ma H, Li T, Lin KA, Yin K, Luo S. Large-scale chemical vapor deposition synthesis of graphene nanoribbions/carbon nanotubes composite for enhanced membrane capacitive deionization. Electroanal. Chem. 2022;904:115907.
https://doi.org/10.1016/j.jelechem.2021.115907
14. Martinez J, Colan M, Catillon R, Huaman J, Paria R, Sanchez L, Rodriguez JM. Desalination Using the Capacitive Deionization Technology with Graphite/AC Electrodes: Effectof the Flow Rate and Electrode Thickness. Membranes (Basel). 2022;12:1–12.
https://doi.org/10.3390/membranes12070717
15. Lee B, Park N, Kang KS, Ryu HJ, Hong SH. Enhanced Capacitive Deionization by Dispersion of CNTs in Activated Carbon Electrode. Acs Sustainable Chem. Eng. 2017;6:1572–1579.
https://doi.org/10.1021/acssuschemeng.7b01750
16. Zhang X, Liu K, Zhang S, Miao F, Xiao W, Shen Y, Zhang P, Wang Z, Shao G. Enabling remarkable cycling performance of high-loading MoS2@Graphene anode for sodium ion batteries with tunable cut-off voltage. J. Power Sources. 2020;458:228040.
https://doi.org/10.1016/j.jpowsour.2020.228040
17. Ding Z, Xu X, Li J, Li Y, Wang K, Lu T, Hossain MSA, Amin MA, Zhang S, Pan L, Yamauchi Y. Nanoarchitectonics from 2D to 3D: MXenes-derived nitrogen-doped 3D nanofibrous architecture for extraordinarily-fast capacitive deionization. Chem. Eng. J. 2022;430:133161.
https://doi.org/10.1016/j.cej.2021.133161
18. Srimuk P, Su X, Yoon J, Aurbach D, Presser V. Charge-transfer materials for electrochemical water desalination, ion separation and the recovery of elements. Nat. Rev. Mater. 2020;5:517–538.
https://doi.org/10.1038/s41578-020-0193-1
19. Allah AE, Wang J, Kaneti YV, Li T, Farghali AA, Khedr MH, Nanjundan AK, Ding B, Dou H, Zhang X, Yoshio B, Yamauchi Y. Auto-programmed heteroarchitecturing: Self-assembling ordered mesoporous carbon between two-dimensional Ti3C2Tx MXene layers. Nano Energy. 2019;65:103991.
https://doi.org/10.1016/j.nanoen.2019.103991
20. Yin T, Zhang Y, Dong D, Wang T, Wang J. Highly efficient capacitive removal of Cd2+ over MoS2-Carbon framework composite material in desulphurisation wastewater from coal-fired power plants. J. Clean. Prod. 2022;355:131814.
https://doi.org/10.1016/j.jclepro.2022.131814
21. Pan B. Preparation and Electrosorption Desalination Performance of Peanut Shell-BasedActivated Carbon and MoS2
. Int. J. Electrochem. Sc. 2020;1861–1880.
https://doi.org/10.20964/2019.12.74
22. Xing F, Li T, Li J, Zhu H, Wang N, Cao X. Chemically exfoliated MoS2 for capacitive deionization of saline water. Nano Energy. 2017;31:590–595.
https://doi.org/10.1016/j.nanoen.2016.12.012
23. Jia F, Sun K, Yang B, Zhang X, Wang Q, Song S. Defect-rich molybdenum disulfide as electrode for enhanced capacitive deionization from water. Desalination. 2018;446:21–30.
https://doi.org/10.1016/j.desal.2018.08.024
24. Huang K-J, Wang L, Liu Y-J, Liu Y-M, Wang H-B, Gan T, Wang L-L. Layered MoS2–graphene composites for supercapacitor applications with enhanced capacitive performance. Int. J. Hydrogen Energ. 2013;38:14027–14034.
https://doi.org/10.1016/j.ijhydene.2013.08.112
25. Gao L, Dong Q, Bai S, Liang S, Hu C, Qiu J. Graphene Oxide-Tuned MoS2 with an Expanded Interlayer for Efficient Hybrid Capacitive Deionization. Acs Sustainable Chem. Eng. 2020;8:9690–9697.
https://dx.doi.org/10.1021/acssuschemeng.0c01453
26. Cai Y, Zhang W, Fang R, Zhao D, Wang Y, Wang J. Well-dispersed few-layered MoS2connected with robust 3D conductive architecture for rapid capacitive deionization process and its specific ion selectivity. Desalination. 2021:520.
https://doi.org/10.1016/j.desal.2021.115325
27. Tian S, Zhang X, Zhang Z. Novel MoS2/NOMC electrodes with enhanced capacitive deionization performances. Chem. Eng. J. 2021:409.
https://doi.org/10.1016/j.cej.2020.128200
28. Nie P, Yan J, Zhu G, Liu J. Inverted hybrid-capacitive deionization with polyaniline nanotubes doped activated carbon as an anode. Electrochim. Acta. 2020:339.
https://doi.org/10.1016/j.electacta.2020.135920
29. Wu J, Zhang Qe, Wang J, Huang X, Bai H. A self-assembly route to porous polyaniline/reduced graphene oxide composite materials with molecular-level uniformity for high-performance supercapacitors. Energ. Environ. Sci. 2018;11:1280–1286.
https://doi.org/10.1039/C8EE00078F
30. Pattananuwat P, Thammasaroj P, Nuanwat W, Qin J, Potiyaraj P. One-pot method to synthesis polyaniline wrapped graphene aerogel/silver nanoparticle composites for solid-state super-capacitor devices. Mater. Lett. 2018;217:104–108.
https://doi.org/10.1016/j.matlet.2018.01.058
31. Haq Ou, Choi J-H, Lee Y-S. Synthesis of ion-exchange polyaniline-carbon composite electrodes for capacitive deionization. Desalination. 2020:479.
https://doi.org/10.1016/j.desal.2019.114308
32. Tian S, Zhang Z, Zhang X, Ken Ostrikov K. Capacitative deionization using commercial activated carbon fiber decorated with polyaniline. J. Colloid Interf. Sci. 2019;537:247–255.
https://doi.org/10.1016/j.jcis.2018.11.025
33. Zornitta RL, Barcelos KM, Nogueira FGE, Ruotolo LAM. Understanding the mechanism of carbonization and KOH activation of polyaniline leading to enhanced electrosorption performance. Carbon. 2020;156:346–358.
https://doi.org/10.1016/j.carbon.2019.09.058
34. Jelmy EJ, Ramakrishnan S, Devanathan S, Rangarajan M, Kothurkar NK. Optimization of the conductivity and yield of chemically synthesized polyaniline using a design of experiments. J. Appl. Polym. Sci. 2013;130:1047–1057.
https://doi.org/10.1002/app.39268
35. Zhang Z, Zhou Z, Peng H, Qin Y, Li G. Nitrogen- and oxygen- containing hierarchical porous carbon frameworks for high-performance supercapacitors. Electrochim. Acta. 2014;134:471–477.
http://dx.doi.org/10.1016/j.electacta.2014.04.107
36. Liu Y, Zhang X, Gu X, Wu N, Zhang R, Shen Y, Zheng B, Wu J, Zhang W, Li S, Huo F. One-step turning leather wastes into heteroatom doped carbon aerogel for performance enhanced capacitive deionization. Micropor. Mesopor. Mat. 2020;303.
https://doi.org/10.1016/j.micromeso.2020.110303
37. Sun K, Yao X, Yang B, Jia F, Song S. Oxygen-incorporated molybdenum disulfide nanosheets as electrode for enhanced capacitive deionization. Desalination. 2020;496:114758.
https://doi.org/10.1016/j.desal.2020.114758
38. Wang Q, Jia F, Huang A, Qin Y, Song S, Li Y, Arroyo MAC. MoS2@sponge with double layer structure for high-efficiency solar desalination. Desalination. 2020;481:114359.
https://doi.org/10.1016/j.desal.2020.114359
39. Zornitta RL, García-Mateos FJ, Lado JJ, Rodríguez-Mirasol J, Cordero T, Hammer P, Ruotolo LAM. High-performance activated carbon from polyaniline for capacitive deionization. Carbon. 2017;123:318–333.
http://dx.doi.org/10.1016/j.carbon.2017.07.071
40. Zhao Y, Kang S, Qin L, Wang W, Zhang T, Song S, Komarneni S. Self-assembled gels of Fe-chitosan/montmorillonite nanosheets: Dye degradation by the synergistic effect of adsorption and photo-Fenton reaction. Chem. Eng. J. 2020;379:122322.
https://doi.org/10.1016/j.cej.2019.122322
41. Aditya T, Nayak AK, Pradhan D, Pal A, Pal T. Fabrication of MoS2 decorated reduced graphene oxide sheets from solid Mo-precursor for electrocatalytic hydrogen evolution reaction. Electrochim. Acta. 2019;313:341–351.
https://doi.org/10.1016/j.electacta.2019.05.034
42. Wang L, Liu Y, Hao J, Ma Z, Lu Y, Zhang M, Hou C. Construction of an S-schemeTiOF2/HTiOF3 heterostructures with abundant OVs and OH groups: Performance, kinetics and mechanism insight. J. Colloid Interf. Sci. 2023;640:15–30.
https://doi.org/10.1016/j.jcis.2023.02.097
43. Zhao M, Zhao Z, Ma X, Zhao J, Ye M, Wen X. Carbon-embedded hierarchical and dual-anion C@MoSP heterostructure for efficient capacitive deionization of saline water. Electrochim Acta. 2021;387:138494.
https://doi.org/10.1016/j.electacta.2021.138494
44. Wen X, Zhao M, Zhao Z, Ma X, Ye M. Hierarchical and Self-Supported Vanadium Disulfide Microstructures@ Graphite Paper: An Advanced Electrode for Efficient and DurableAsymmetric Capacitive Deionization. Acs Sustainable Chem. Eng. 2020;8:7335–7342.
https://doi.org/10.1021/acssuschemeng.0c00712
45. Ai K, Ruan C, Shen M, Lu L. MoS2 Nanosheets with Widened Interlayer Spacing for High-Efficiency Removal of Mercury in Aquatic Systems. Adv. Funct. Mater. 2016;26:5542–5549.
https://doi.org/10.1002/adfm.201601338
46. Wang Z, Yan T, Fang J, Shi L, Zhang D. Nitrogen-doped porous carbon derived from a bimetallic metal–organic framework as highly efficient electrodes for flow-through deionization capacitors. J. Mater. Chem. A. 2016;4:10858–10868.
https://doi.org/10.1039/C6TA02420C
47. Ding M, Shi W, Guo L, Leong ZY, Baji A, Yang HY. Bimetallic metal–organic framework derived porous carbon nanostructures for high performance membrane capacitive desalination. J. Mater. Chem. A. 2017;5:6113–6121.
https://doi.org/10.1039/C7TA00339K
48. Li J, He T, Zhao Y, Zhang X, Zhong W, Zhang X, Ren J, Chen Y. In-situ N-doped ultrathin MoS2 anchored on N-doped carbon nanotubes skeleton by Mo-N bonds for fast pseudocapacitive sodium storage. J. Alloy. Compd. 2022;897:163170.
https://doi.org/10.1016/j.jallcom.2021.163170
49. Wang L, Niu M, Liu Y, Xie Y, Ma Z, Zhang M, Hou C. The Ovs surface defecting of an S-scheme g-C3N4/H2Ti3O7 nanoheterostructures with accelerated spatial charge transfer. J. Colloid Interf. Sci. 2023;645:639–653.
https://doi.org/10.1016/j.jcis.2023.04.178
50. Zheng P, Wang L, Wang Q, Zhang J. Enhanced capacitive deionization by rGO@PEI/MoS2 nanocomposites with rich heterostructures. Sep. Purif. Technol. 2022;295:121156.
https://doi.org/10.1016/j.seppur.2022.121156
51. Tian S, Zhang X, Zhang Z. Capacitive deionization with MoS2/g-C3N4 electrodes. Desalination. 2020;479:114348.
https://doi.org/10.1016/j.desal.2020.114348
52. Yan Y, Xia B, Li N, Xu Z, Fisher A, Wang X. Vertically oriented MoS2 and WS2 nanosheets directly grown on carbon cloth as efficient and stable 3-dimensional hydrogen-evolving cathodes. J. Mater. Chem. A. 2015;3:131–135.
https://doi.org/10.1039/C4TA04858J
53. Peng W, Wang W, Qi M, Miao Y, Huang Y, Yu F. Enhanced capacitive deionization of defect-containing MoS2/graphene composites through introducing appropriate MoS2 defect. Electrochim. Acta. 2021;383:138363.
https://doi.org/10.1016/j.electacta.2021.138363
54. Nguyen TKA, Wang T-H, Doong R-a. Architectures of flower- like MoS2 nanosheet coated N-doped carbon sphere electrode materials for enhanced capacitive deionization. Desalination. 2022;540:115979.
https://doi.org/10.1016/j.desal.2022.115979
Fig. 2SEM images of (a) CP, (b) MoS2, and (c, d) MC-2. (e) The EDS elemental mapping images of Mo, S, C, O, and N. 
Fig. 5(a) CV curves of CP, MoS2, MC-1, MC-2, and MC-3 electrodes (10 mV s−1). (b) MC-2 electrode CV curves (5–100 mV s−1). (c) Specific capacitances of CP, MoS2, and MC-2 electrodes (5–100 mV s−1). (d) EIS spectra of CP, MoS2, and MC-2 electrodes. (e) GCD curves of CP, MoS2, and MC-2 electrodes (1 A g−1). (f) GCD curves of MC-2 electrode (1–10 A g−1). 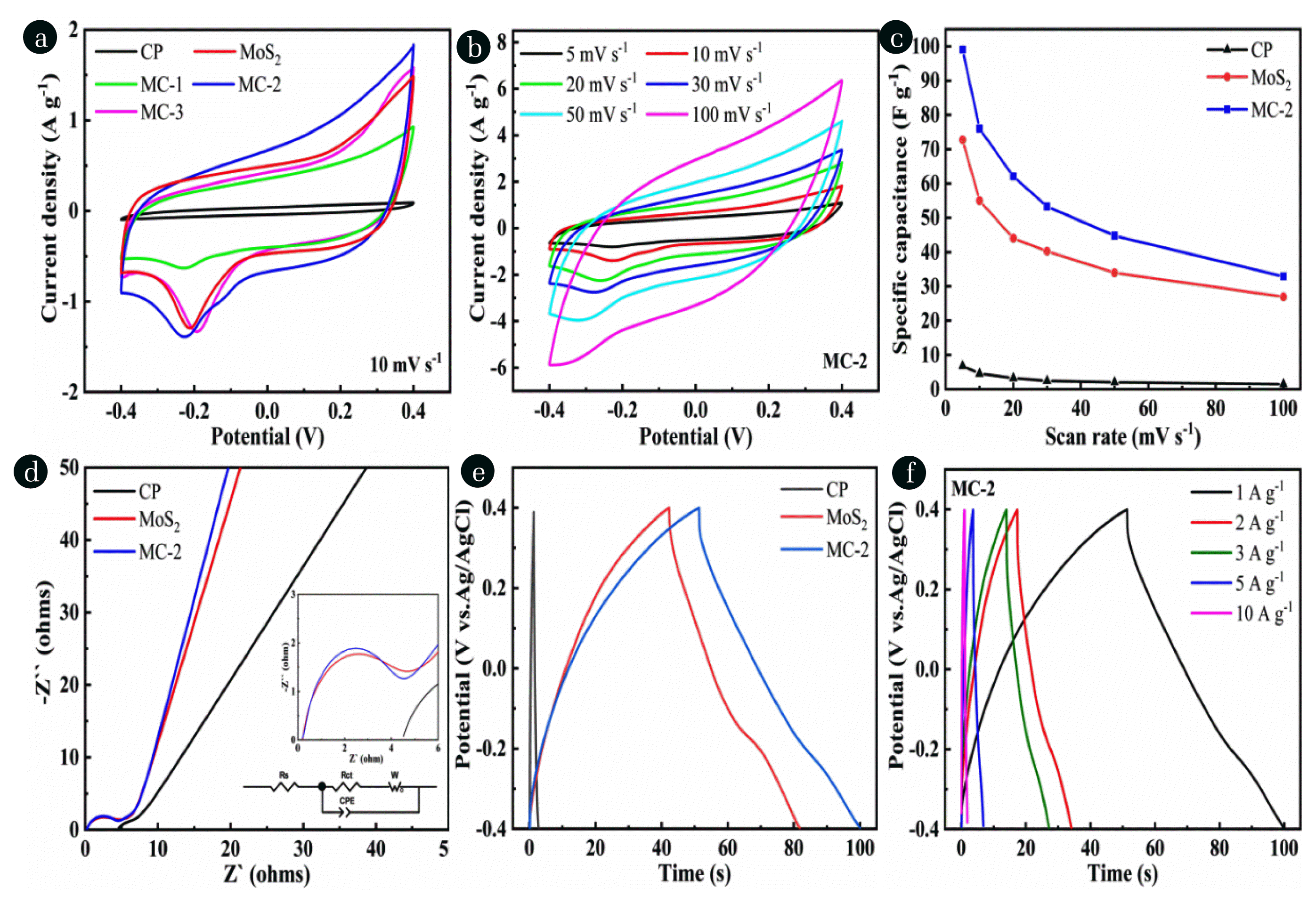
Fig. 6(a) The SAC, (b) Ragone plots, and (c) isotherm adsorption curves of the cell AC//MC-2 in different NaCl concentrations (1.2 V). (d) The SACs of AC//CP, AC//MoS2, and AC//MC-2 cells at different voltages (500 mg L−1). (e) The SACs and desalination kinetics of the AC//MoS2, AC//MC-1, AC//MC-2, and AC//MC-3 cells, and (f) Ragone plots of the AC//MoS2 and AC//MC-2 cells (1.2 V and 500 mg L−1). 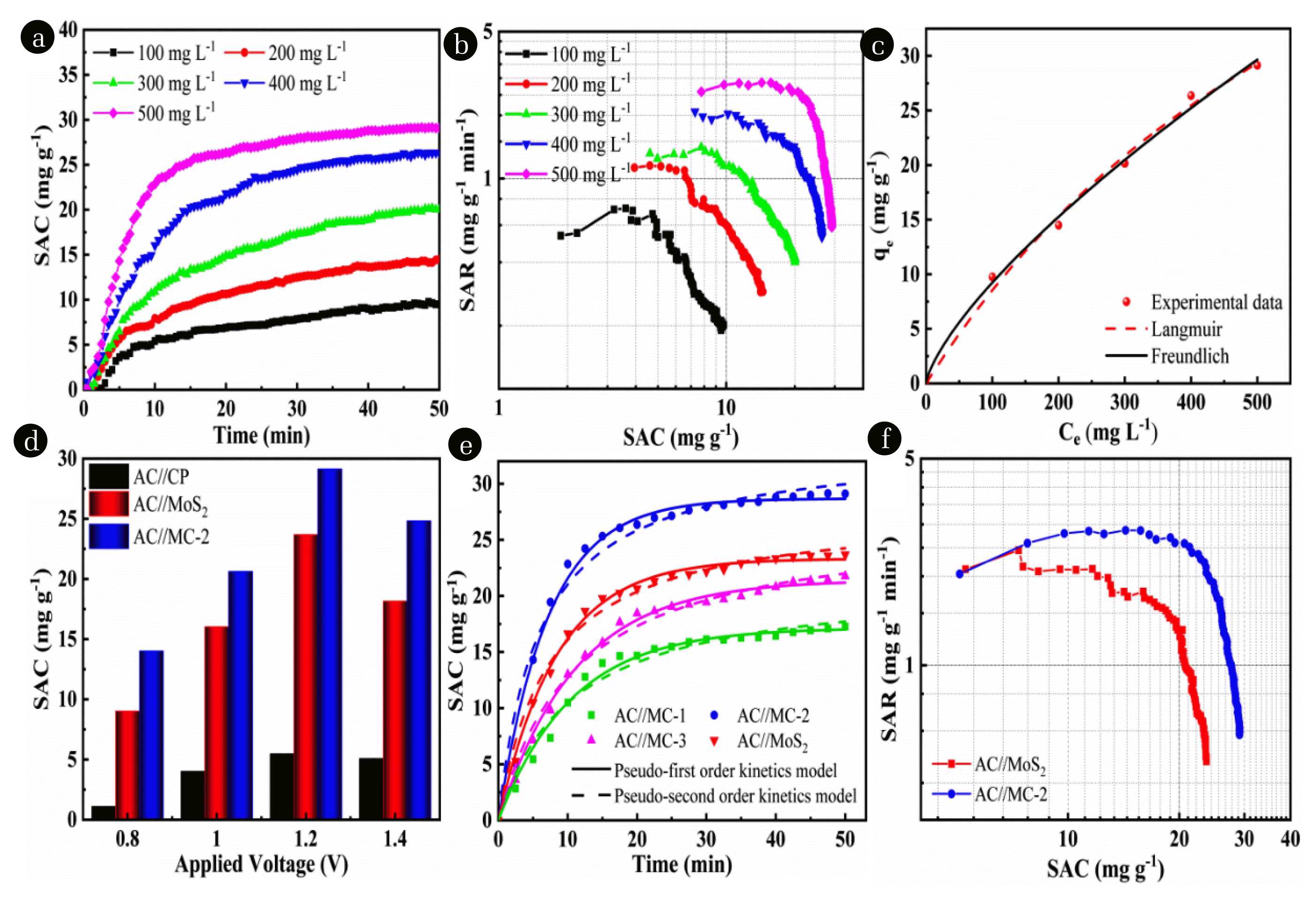
|
|
||||||||||||||||||||||||||||||||||||