AbstractConstructed wetlands (CWs) are a promising ecological wastewater treatment technology. The method of biochar (BC) filler to enhance CWs has been widely researched in the past decade. This article summarizes the applications of BC-added CWs technology and consolidates the various impacts of BC filler on the operation of CWs, which can be divided into two aspects. One is to enhance the wastewater purification performance of CWs ecosystems, and the other is to improve the abilities of CWs to sequester carbon and reduce greenhouse gas (GHG) emissions. These two aspects are precisely among the environmental challenges facing humanity globally. The BC prepared from CWs plants used as CWs filler can solve excess biomass in CWs. Moreover, the large specific surface area of BC, excellent adsorption performance, and good biocompatibility improve the sewage treatment effect of CWs. The BC has a high potential for stimulating the growth of CWs plants, biomass carbon sequestration, inhibition of organic carbon mineralization in CWs substrates, and reducing GHG emissions, which mitigates global climate change. In short, the BC-added CWs is a technology that combines wastewater purification and carbon sequestration/GHG reduction. This study provides solid references for the engineering promotion of CWs based on BC filler.
Graphical Abstract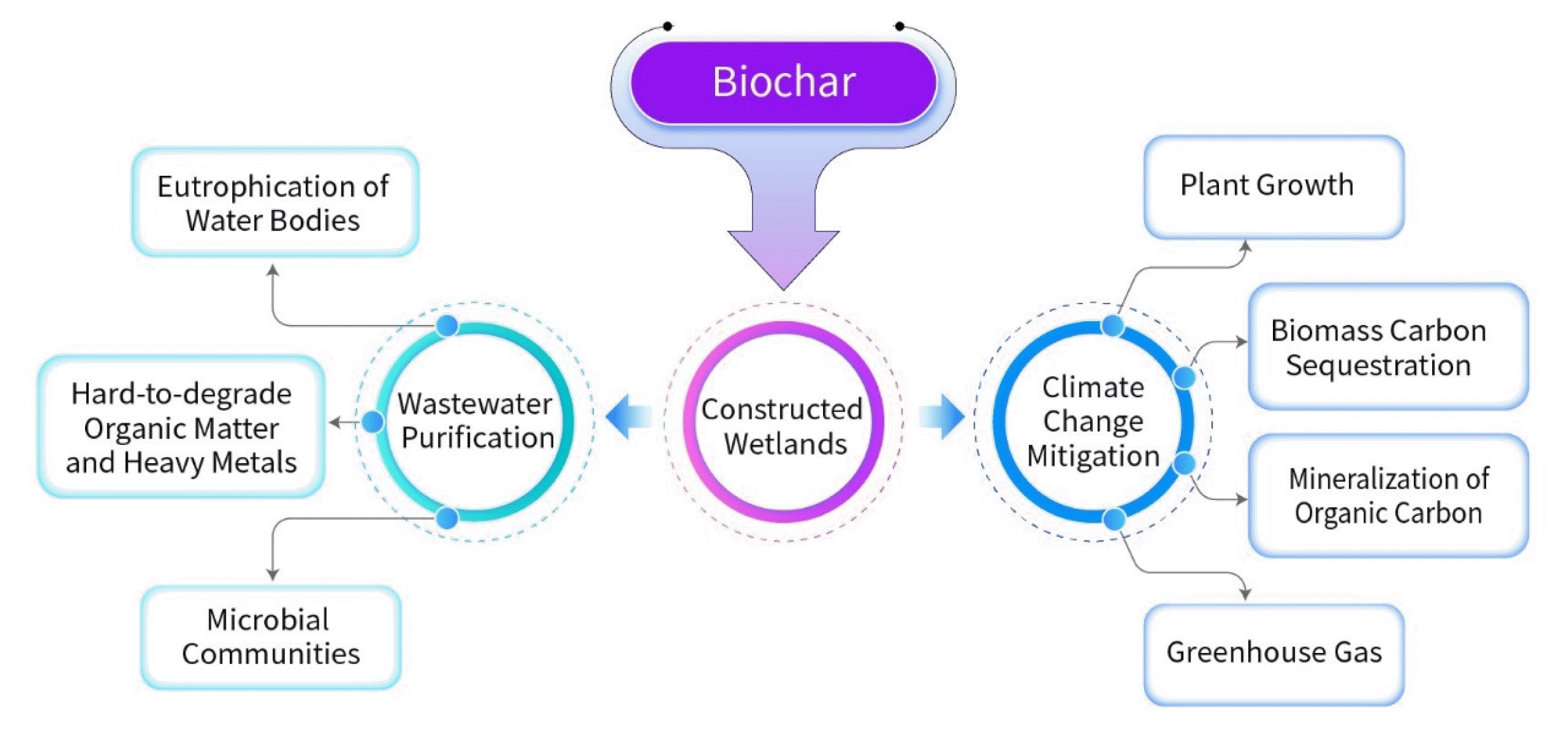
1. IntroductionClimate change and water resource issues have become important factors hindering global development [1]. Although significant technological advances have been made in the field of wastewater treatment, a large number of greenhouse gases (GHGs), such as carbon dioxide (CO2), methane (CH4), and nitrous oxide (N2O), are commonly emitted by conventional wastewater treatment technologies [2]. As a new emerging technology, constructed wetlands (CWs) technology has the potential to simultaneously address both wastewater purification and climate change mitigation.
The CWs are engineered wetlands systems designed and built by humans to mimic and utilize the functions of natural wetlands. They typically consist of water bodies, aquatic plants, media, microorganisms, etc [3]. The various pollutants from wastewater through physical, chemical, and biological processes such as plants absorption, media adsorption, and microbial degradation can be effectively removed by CWs [4, 5]. The CWs have high removal efficiency for pollutants, are easy to operate and maintain, are low-cost, and have good potential for wastewater and nutrient reuse [6]. The CWs can significantly contribute to sustainable wastewater management as ecological engineering systems [7]. However, there may be issues with CWs-treated wastewater not meeting discharge standards [8].
Meanwhile, compared to conventional wastewater treatment technologies, the CWs have relatively lower emissions of GHG during the sewage treatment process [9, 10]. CWs are carbon sinks over long-term operating conditions [11]. However, if wetlands plants are not harvested regularly, it will not only affect the efficiency of water purification but also release organic carbon into the environment as dead branches and leaves decompose, violently exacerbating the greenhouse effect [12]. As a result, a technology that can resourcefully utilize wetlands plants is urgently needed to improve the wastewater purification effect of wetlands and achieve carbon sequestration/GHG reduction functions.
Biochar (BC) is a type of carbon-rich product produced by high-temperature treatment of various biomasses (wood, straw, manure, leaves, etc.) under anaerobic or less aerobic conditions [13]. The BC has high physical stability, resistance to decomposition, a large specific surface area, and a porous structure. Many research results have shown that BC has positive effects on soil improvement, carbon sequestration, environmental remediation, and energy production [14].
In recent years, BC is considered a promising CWs filler, enhancing the water purification capacity and reducing the GHG emissions of CWs [15]. Moreover, BC can sequester a large number of CO2 in solid form [16, 17]. The BC filler in CWs can absorb organic matter from water bodies, partially preventing the complete decomposition of organic matter and further enhancing the overall carbon sequestration effect of the CWs [18]. Chen et al. [19] concluded that adding BC filler to CWs can strengthen the adsorption performance of various pollutants within the systems and reduce GHG emissions in CWs. Di et al. [20] reported that biomass-derived BC has porous features that can reduce the density of the absorbing material. The BC of wood material having a large surface area and tiny pores was reported by Nirmaladevi et al. [21]. Li et al. [22] used corncob xylose residue to prepare the BC, which presented an extremely large specific surface area of 3043 m2/g, a total pore volume of 1.67 cm3/g, and an average pore diameter of 2.23 nm. The large specific surface area of BC can provide a good living space for various functional microorganisms [23], which may contain the functional genes required for CWs operation [24].
Overall, BC can be made from wetlands plants biomass and used back as CWs filler, which can not only improve the water purification efficiency of CWs but also solve the excess biomass in CWs and enhance the climate change mitigation function of the CWs [25, 26]. Currently, many connected individuals have designed many forms of CWs for various wastewaters utilizing various kinds of BC, wetlands plants, filler, and other raw materials (Table 1.).
The purpose of this review is to: (i) clarify the significance of BC prepared from excess CWs plants biomass, (ii) illuminate the role of BC filler in enhancing wastewater purification in CWs ecosystems, and (iii) elaborate on how BC filler enhances the carbon sequestration and GHG reduction functions of the CWs ecosystems.
2. Current Status of BC Application CWs Based on Bibliometric AnalysisThe subject term “Biochar” used on the “Web of Science” shows that the number of articles about BC has almost exponentially increased in the past decade, reaching 7494 in 2022, an 11-fold increase compared to 2013 (Fig. 1(b).). A search is conducted for the term “Biochar AND Constructed Wetlands” to compile information on the application of BC in CWs (Fig. 1(a).). A total of 287 articles were published from 2013 to 2022, of which 75 articles were published in 2022, accounting for one-quarter of the total. This result indicates that adding BC filler to CWs has received increasing attention worldwide in recent years. The application of BC is a popular research topic on CWs, and a systematic summary of the role played by BC in this context is needed.
3. Feasibility of BC Preparation from CWs Plants BiomassBC is a black solid material rich in carbon formed by the pyrolysis of biomass materials such as coconut shells, reeds, sawdust, agricultural and forestry waste, and sludge under limited oxygen conditions [27]. According to surveys, there are 6.75 million hectares of wetlands in China alone, which can produce about 107 Mt of wetlands plants biomass annually [28]. The surplus biomass is mostly not being utilized properly, and wetlands plants residues are generally abandoned, piled up, or burned, resulting in a waste of resources and potential environmental pollution [29]. Pyrolysis is the oldest, most common, and most mature technology for preparing BC from wetlands plants biomass [30], with a typical pyrolysis temperature between 300 ~ 900°C, ranging from a few minutes to several hours, and heating rates varying from 5°C to 30°C per minute [31]. Due to different preparation methods, BC prepared has slight differences in physical and chemical properties but has a certain potential for CWs filler [32]. In the current situation of increasingly prominent environmental issues, BC prepared from excess CWs plants biomass can avoid environmental pollution caused by burning, and can also be used as CWs filler to backfill the systems. The CWs filled with BC have the potential to enhance the function of CWs, and can greatly promote the industrial development of CWs technology.
4. The Impact of BC Filler on the Effectiveness of CWs in Purifying Wastewater4.1. Controlling Eutrophication of Water BodiesThe main removal mechanism of nitrogen in CWs is through the microbial nitrification and denitrification process, which converts nitrogen to N2O or nitrogen (N2). The plant uptake, adsorption, filtration, and ion exchange by filler can also play a role [33]. In recent years, Gupta et al. [34] found that using BC as CWs filler could effectively adsorb and remove organic matter, total nitrogen (TN), nitrate nitrogen (NO3−-N), and ammonia nitrogen (NH4+-N) from wastewater compared to gravel filler, with removal rates of 91%, 58.3%, and 92%. Similarly, Rozari et al. [35] investigated the pollutants removal efficiency of CWs with and without BC added to the gravel filler, and the results showed that the removal rates of TN, NH4+-N, and NO3−-N were significantly higher in CWs with BC addition. Cheng et al. [36] found that the efficient removal of nitrogen was basically completed within 12 hours of adding BC to CWs. On the one hand, the BC has a larger specific surface area, total pore volume, and micropore volume compared to gravel, which enhances its ability to fix and transfer oxygen. The dissolved oxygen (DO) level is a key factor in the smooth progress of nitrification reactions. In addition, the surface of BC is conducive to biofilm formation, promoting the enrichment of different microorganisms, and thereby improving nitrogen removal [37]. The CWs supplemented with BC can release additional dissolved organic matter (DOM) during operation to promote the denitrification process within the systems and enhance nitrogen removal [38]. Moreover, the plant-source carbon from BC-addition CWs is considered more important for denitrification in CWs than the direct release of carbon from BC [39]. On the other hand, the adsorption performance of gravel is limited and significantly lower than that of BC, and it is more likely to reach saturation under the same concentration conditions [40].
Zhou et al. [38] found that combining BC with aeration vertical flow CWs had excellent intermittent aeration treatment effects on low carbon/nitrogen ratio wastewater. Abedi et al. [41] found that the chemical oxygen demand (COD) and TN removal of CWs with BC addition reached 99.9%, which was much higher than that of CWs without BC addition (64.6%, 71.1%). It has shown that aerobic degradation of COD in CWs systems generally occurs before nitrification reactions, and DO in CWs is prioritized for COD degradation. The BC filler not only enables an efficient transfer of DO but also absorbs organic matter, improving the water purification effect of CWs [18]. In contrast, too much BC filler may slightly reduce the COD removal performance of the CWs, because the release of DOM increases the COD concentration in the CWs with BC addition [42]. The DOM released from BC is influenced by the pyrolysis temperature during preparation. BC with pyrolysis temperature above 650°C shows a substantial reduction in DOM concentration [42, 43]. In addition, factors such as the pH of the water bodies, raw material of biochar, and microbial community all have different degrees of influence on DOM and should be further explored [44].
The BC also enhances phosphorus uptake in CWs. The removal rates of nitrogen and phosphorus by plants were 15.4% and 14.2% in CWs with BC addition, respectively, compared to 8.00% and 8.30% in CWs without BC addition [45]. Guo et al. [32] found that removal rates of COD, TN, NH4+-N, and total phosphorus (TP) reached 96.34%, 100%, 80.02%, and 81.19%, a significant increase compared to the control group (96.13%, 69.67%, 62.27%, and 67.51%). The adsorption and precipitation of phosphorus by CWs filler are the main pathways for removing phosphorus in CWs, the rational selection of CWs filler is of great significance for phosphorus removal [46, 47]. The TP removal efficiency is continuously improved with the increase of the BC addition ratio [48, 49]. Although most studies indicate that the BC addition has a positive impact on phosphorus removal, several reports have shown BC addition in gravel-filled CWs does not promote phosphorus removal [50], and that mixed media of sand and BC are even less effective than pure sand in phosphorus removal [50, 51]. More research and applications related to phosphorus removal using BC filler are still needed. Table 2. records in detail the removal of pollutants in different types of CWs.
4.2. Removal of Hard-to-degrade Organic Matter and Heavy MetalsIn recent years, due to its characteristics such as large specific surface area, high degree of aromatization, good stability, and rich microporous structure, BC has been proven a green adsorbent for the removal of hard-to-degrade pollutants in water, such as herbicides, pesticides, and heavy metals, in many studies [52]. Chang et al. found [53] that CWs filled with walnut shell BC pyrolyzed at 450°C were more effective in removing Hg than the gravel CWs, which was mainly attributed to Hg adsorption and precipitation on the CWs filler. Cheng et al. [36] found that adding 20% BC to CWs significantly improved the removal efficiency of Microcystins (MC-LR) and reduced the potential risk of MC-LR over three days. Uchimiya et al. [54] found that chicken manure BC prepared at high temperatures had good adsorption efficiency for atrazine. A study by Ahmad et al. [55] also noted that BC prepared with tofu residue and peanut shells at 700°C was much more effective in the adsorption of trichloroethylene than BC prepared at 300°C. In contrast, a study by Sun et al. [56] showed that BC prepared at low temperatures (400°C) showed better adsorption of Norflurazon and Nluridone. Abedi et al. [41] found that the addition of BC approximately doubled the removal rates of Phenols, Pb, and Mn, reaching 99%. Guo et al. [32] confirmed that the addition of BC increased the removal of Zn from 8.15% to 22.38%, and As from 35.58% to 74.09%. Tang et al. [57] had reached similar conclusions in degradation experiments with Chlorpyrifos, Endosulfan, Fenvalerate, and Diuron.
Based on the analysis of various pollutants using BC-based CWs, hard-to-degrade organic matter, and heavy metals are mainly removed by BC adsorption through mechanisms such as electrostatic attraction, hydrophobic effects, hydrogen bonding, pore filling, ion/ligand exchange, complexation, precipitation, and π-binding interactions [58]. On the other hand, the growth and reproduction of microorganisms related to the degradation of these pollutants may be stimulated by BC filler. Mittal et al. [59], conducting antibacterial testing on BC, observed substantial microbial growth without any obvious inhibition zones, and found bacterial colonies inhibiting ciprofloxacin. The BC, which is added to CWs, has a dual role of adsorbing pollutants and enhancing biodegradation. The above expressions were summarized in Table 3.
However, CWs dealing with heavy metal contaminants (As, Zi, Ni, Cr, Cu) and organic pollutants (polycyclic aromatic hydrocarbons, chlorinated hydrocarbons) may accumulate these contaminants in CWs plants [60]. The use of BC prepared from contaminated plant residues may pose some environmental risks due to the fact that heavy metals and polycyclic aromatic hydrocarbons are generally difficult to volatilize during high-temperature carbonization and remain in biochar [61]. Moreover, BC does not always play a positive role in the treatment of complex contamination. Zheng et al [62]. found that the addition of BC to rice soils contaminated with As-Pb-Cr resulted in a 98% and 752% reduction in Pb and Cr content in rice stems, respectively, but As content in stems and soil pore water increased by 3.3- and 14.2-fold, respectively.
4.3. Enrichment of Microbial Communities in CWsThe CWs rely mainly on the action of microbial communities for the purification of sewage. Due to its large surface area and porous structure, BC is considered a carrier for microbial growth in bioreactors [63]. Antibacterial testing of BC shows [59] significant microbial growth around the BC, indicating that BC can serve as a supporting substrate for microbial adhesion and growth. Kizito et al. [64] found that adding BC to vertical flow CWs can enhance sustainable wastewater treatment performance and increase microbial diversity. Deng et al. [65] studied the microbial community structure and metabolic characteristics of CWs systems with different proportions of BC, the results showed that the addition of BC enhanced the diversity of wetland microbial communities, promoted the biodegradation of extracellular polymeric substance (EPS), and improved the removal efficiency of nitrogen and organic matter in the CWs. Cheng et al. [36] found that the addition of BC improved the microbial community and increased the relative abundance of a variety of functional degrading bacteria. In addition, a lot of studies have shown that microorganisms can be stably attached to BC and are less likely to be lost due to sudden changes in hydraulic conditions or by predation, which maintains microbial diversity to a certain extent. It has also been suggested that BC can promote the structural diversity of nitrifying bacterial communities, promote the abundance and activity of ammonia-oxidizing bacteria, etc., and increase the nitrification rate [66, 67]. BC also has a rich source of carbon and a porous structure, providing a good habitat for microbial growth [68].
5. The Effectiveness of BC Filler in Increasing Carbon Sequestration and Reducing GHG Emissions in CWs Ecosystems5.1. Improving Plants Biomass of CWs by BCThe plants biomass in CWs is an important component of the carbon sinks role played by CWs [69]. The addition of BC affects the physical and chemical conditions of CWs, such as conductivity, DO, and pH, improving nutrient uptake by plant roots [39]. To some extent, the ability of plant roots can be assisted by BC [70]. BC can improve the CWs substrates environment and make the soil fertile [71]. The BC filler can provide rich nutrients to surface plants and increase plants biomass production [68]. BC also released substances that directly affect plants growth, increasing the biomass of above-ground and below-ground parts by 1.9 and 1.5 times, respectively, compared to control systems without BC [45]. In the study by Li et al. [72], it was found to be 1.73 times and 1.37 times. These benefits are mainly attributed to the various nutrients released by BC (such as K, P, N, Mg, and Ca) and the increased porosity of the cultivation medium due to the oxygen conditions created by BC, which is extremely beneficial for plants growth [73]. The addition of BC filler can promote nutrient-using efficiency in CWs plants by enhancing plant growth through nutrient uptake, oxygen release, organic matter secretion, and providing more attachment sites for root-associated microbes [57]. In addition, studies have shown that the addition of BC increases nitrogen content in plant biomass, which improves the ability to remove nitrogen from plants [72]. The addition of woody BC significantly increased the nitrogen content of reed from 1.40 mg/g to 1.95 mg/g [39]. A study by Kasak et al. found that the addition of BC increased plants nitrogen uptake from 40.7 g/m2 to 78.1 g/m2 [45]. Plants can thrive in the CWs of BC filler.
5.2. The Biomass Carbon Sequestration Potential of BCWith the increasing use of fossil fuels worldwide, reducing GHG emissions has become a global challenge [74]. As an important component of the global carbon cycle, plants can fix and convert atmospheric CO2 into organic compounds through photosynthesis [75]. Hence, plants are highly regarded for sequestering carbon. However, once the plants life cycle is complete, burning or decay of the plants material releases the previously fixed CO2 back into the atmosphere, contradicting the goal of carbon sequestration.
Kong et al. discovered [76] that charring plants into BC can be stable without rapid decomposition, thus promising to development of an effective carbon sequestration technology. At present, it is generally believed that BC has excellent thermal stability, chemical stability, and microbial stability, which derives from the tight structure of aromatic rings and alkyl groups in BC. This results in higher resistance to biological and chemical degradation than other organic carbon. On the other hand, the organic components on the surface of BC can form new agglomerate structures with minerals and organic matter in the CWs, reducing the risk of microbial degradation through physical protection [77]. Guo et al. [78] further pointed out that the stability of BC not only depends on the degree of aromatic carbon aggregation but also on the integrity of the aggregated aromatic carbon and the silica-carbon complex, which is an important protective mechanism. A study extrapolated experimentally and through modeling that the amount of carbon lost from BC due to mineralization and degradation (including DOM) is between 3 ~ 26% at the annual scale, and further extrapolated that the half-life of BC may be between 102 ~ 107 years, confirming the high stability of BC [79].
Global agricultural waste can produce 373 Mt of BC per year and have the potential to sequester 0.55 Pg CO2 [80]. The potential for carbon sequestration by BC is enormous. Compared to biomass entering the soil directly, sustaining complete degradation to CO2 and returning to the atmosphere, the stabilization of carbon in the form of BC is considered a “carbon-negative” pathway, which can effectively achieve GHG emissions reduction and biomass carbon sequestration [81, 82].
5.3. Reducing the Mineralization of Organic Carbon in CWs Substrates by BCThe addition of BC can reduce the mineralization of CWs substrates organic carbon. The soil carbon pool is the largest carbon pool in terrestrial ecosystems and an important part of the global carbon cycle, dominating the global carbon balance. A small change in the soil carbon pool can affect the global carbon balance. Studies have shown that CWs substrates organic carbon mineralization is the main source of CO2 release from CWs substrates, and controlling the mineralization rate of CWs substrates organic carbon plays an important role in reducing atmospheric CO2 [83]. The BC can achieve high carbon sinks to the soil carbon pool through a mechanism of sorption of CWs substrates organic carbon and aggregates. Researchers conducted a 532-day incubation experiment using soils collected from central Amazonia (Brazil) and showed that the total mineralization rate of soil organic carbon containing BC was 25.5% lower than that of the without BC control, demonstrating the inhibitory effect of BC on organic carbon mineralization [84].
Although the addition of BC to CWs may increase CH4 emissions, CWs are still net carbon sinks in the context of the CWs ecosystems as a whole [11]. Overall, Landry et al. [85] reported that the carbon sequestered by CWs ecosystems may be 2 to 15 times greater than the carbon they emit. Luan et al. and Meng et al. [86, 87] suggested that the majority of wetlands are carbon sinks from an ecological perspective when they are not destroyed. Estimates of GHG fluxes between CWs and natural wetlands indicates that CWs have higher carbon sequestration potential than natural wetlands [88].
5.4. Reducing GHG of CWs by BCGHG is a significant factor in global climate change, and GHG reduction is crucial to mitigating the greenhouse effect [89]. This adverse environmental impact could undermine the benefits and sustainability of CWs [90]. GHGs, such as N2O, CO2, and CH4, may be generated through the conversion of carbon and nitrogen by microorganisms and plants, released into the air [91]. Therein, CH4 and N2O have 28 and 298 times more global warming potential (GWP) than CO2 [92]. GHG produced in CWs is correlated with water bodies composition, microbial species, filler, etc. The DO, pH, microbial community, plants growth, GHG emissions can be regulated by BC in CWs [93]. The BC applied to the CWs not only changes the native physicochemical properties of CWs substrates in several aspects such as bulk weight, porosity, pH, cation exchange, soil mineral elements, and organic matter but also has some effects on CWs microbial community, changing the emission of GHG [94]. GHG emissions strategies in CWs have received increasing attention in recent years [95].
Some studies have found that the N2O emissions from BC-added CWs are lower than those from other CWs [96]. The N2O emissions were reported to be reduced from 418.75 μg·m−2·h−1 to 60.54 μg·m−2·h−1 [38]. Abedi et al. [41] found that N2O emissions were reduced to 1/6 of the original level after adding BC to CWs. The average N2O fluxes in the BC-added and none-BC CWs were 253.8, 219.4, 183.9, 167.8 μg·m−2·h−1 and 351.2, 300.3, 259.8, 238.9 μg·m−2·h−1 at influent C/N ratios of 3, 6, 9, 12, respectively [97]. The addition of BC significantly reduces N2O fluxes, and N2O emissions decrease with increasing C/N ratio and oxygen supplementation in CWs [97]. BC effectively facilitates nitrification and denitrification processes in CWs, reducing the byproducts of nitrogen intermediate [98]. Spokas et al. [99] and Yanai et al. [100] reported that BC could be used as a means to reduce or suppress N2O. The BC-rich CW can increase the activity of nitrifying and denitrifying bacteria, reducing N2O emissions. There may be another explanation: BC as a potential carbon source can promote complete denitrification to reduce N2O emissions and enhance the bio-transformation of N2O to N2 [101, 102]. The lime treatment of alkaline BC can promote the activity of N2O reductase in denitrification and enhance the biological conversion of N2O to N2 [101]. In addition, BC can adsorb the generated N2O for subsequent biological consumption [103] or abiotic reduction catalyzed by metal oxides on the biochar surface [102].
However, the BC filler may increase the emission of CH4 compared to gravel-based CWs [104]. The BC has a high redox-active property and charging and discharging capacities that facilitates the direct interspecies electron transfer between methanogens and anaerobic bacteria, as well as providing labile organic carbon for CH4 [105]. Overall, when considering multiple GHGs, BC-added CWs generally exhibit low GWP [106]. For instance, an 18 ~ 24% lower GWP in BC-added CWs compared to gravel-based CWs was reported by Guo et al [97].
6. ConclusionsThis review provides an overview of the application of BC in CWs, detailing its effects on wastewater purification and carbon sequestration/GHG reduction, with the aim of enhancing the sustainability of CWs. Excess biomass from CWs can be prepared into BC which is used as CWs filler. This approach can help to solve the problem of excess biomass and prevent secondary pollution while promoting the engineering development of CWs. The CWs with BC filler can improve the removal of various water quality indicators including COD, TN, NO3−-N, NH4+-N, TP, hard-to-degrade organic matter, and heavy metals. These benefits are primarily due to the large specific surface area of BC providing adsorption sites for contaminants, supporting microbial colonization and growth, and increasing microbial diversity in the CWs. However, the addition of BC filler can have a negative impact sometimes. This is related to a variety of factors (the raw material of BC, the amount of BC dosed, pollutant type, the characteristics of the wetland substrate, etc.) and needs to be further explored. On the other hand, BC filler can increase plant biomass and promote plant growth from the perspective of climate change mitigation. The BC has high stability, and as a solid form of carbon sequestration represents a “carbon-negative” pathway. The inhibition of organic carbon mineralization by BC in CWs substrates can improve carbon sinks efficiency. The BC-added CWs typically exhibit low GWP and release few GHG, such as N2O. However, future research is recommended to evaluate the feasibility of wider applications and long-term operation of BC-added CWs.
AcknowledgmentsThis research was supported by the National Natural Science Foundation of China (51708087).
NotesReferences1. Gupta S, Srivastava P, Patil SA, Yadav AK. A comprehensive review on emerging constructed wetland coupled microbial fuel cell technology: Potential applications and challenges. Bioresour. Technol. 2021;320:124376.
https://doi.org/10.1016/j.biortech.2020.124376
2. Tansel B, Jolis D, Ho CFH. Gaseous emissions from wastewater facilities. Water. Environ. Res. 2004;76:1343–1374.
https://doi.org/10.2175/106143004x142086
3. Faulwetter JL, Gagnon V, Sundberg C, et al. Microbial processes influencing performance of treatment wetlands: A review. Ecol Eng. 2009;35:987–1004.
https://doi.org/10.1016/j.ecoleng.2008.12.030
4. Fang YK, Sun Q, Fang PH, et al. Integrated constructed wetland and bioelectrochemistry system approach for simultaneous enhancment of p-chloronitrobenzene and nitrogen transformations performance. Water. Res. 2022;217:118433.
https://doi.org/10.1016/j.watres.2022.118433
5. Vymazal J. The use of hybrid constructed wetlands for wastewater treatment with special attention to nitrogen removal: A review of a recent development. Water. Res. 2013;47:4795–4811.
https://doi.org/10.1016/j.watres.2013.05.029
6. Zhao M, Wang S, Wang HS, et al. Application of sodium titanate nanofibers as constructed wetland fillers for efficient removal of heavy metal ions from wastewater. Environ. Pollut. 2019;248:938–946.
https://doi.org/10.1016/j.envpol.2019.02.040
7. Li D, Zheng B, Chu Z, Liu Y, Huang M. Seasonal variations of performance and operation in field-scale storing multipond constructed wetlands for nonpoint source pollution mitigation in a plateau lake basin. Bioresour. Technol. 2019;280:295–302.
https://doi.org/10.1016/j.biortech.2019.01.116
8. Vymazal J. The use constructed wetlands with horizontal sub-surface flow for various types of wastewater. Ecol. Eng. 2009;35:1–17.
https://doi.org/10.1016/j.ecoleng.2008.08.016
9. Koutsou OP, Fountoulakis MS, Matsoukas C, Fyllas NM, Stasinakis AS. Estimation of N2O emissions from wastewater characteristics in constructed wetlands. J. Environ. Chem. Eng. 2021;9:106632.
https://doi.org/10.1016/j.jece.2021.106632
10. Pan T, Zhu XD, Ye YP. Estimate of life-cycle greenhouse gas emissions from a vertical subsurface flow constructed wetland and conventional wastewater treatment plants: A case study in China. Ecol. Eng. 2011;37:248–254.
https://doi.org/10.1016/j.ecoleng.2010.11.014
11. Wang F, Tang J, Ye S, Liu J. Blue Carbon Sink Function of Chinese Coastal Wetlands and Carbon Neutrality Strategy. Bull Chinese. Aca. Sci. 2021;36:241–251.
https://doi.org/10.16418/j.issn.1000-3045.20210215101
12. Janssen MA, Walker KF. Processing of riparian and wetland plant litter in the River Murray, South Australia. Hydrobiologia. 1999;411:53–64.
https://doi.org/10.1023/a:1003891720922
13. Wang Z, Guo H, Shen F, et al. Biochar produced from oak sawdust by Lanthanum (La)-involved pyrolysis for adsorption of ammonium (NH4
+), nitrate (NO3
−), and phosphate (PO4
3−). Chemosphere. 2015;119:646–653.
https://doi.org/10.1016/j.chemosphere.2014.07.084
14. Cui X, Wang J, Wang X, et al. Biochar from constructed wetland biomass waste: A review of its potential and challenges. Chemosphere. 2022;287:132259.
https://doi.org/10.1016/j.chemosphere.2021.132259
15. Zhou X, Liang CL, Jia LX, Feng LK, Wang RG, Wu HM. An innovative biochar-amended substrate vertical flow constructed wetland for low C/N wastewater treatment: Impact of influent strengths. Bioresour. Technol. 2018;247:844–850.
https://doi.org/10.1016/j.biortech.2017.09.044
16. Smith P. Soil carbon sequestration and biochar as negative emission technologies. Global. Change. Biol. 2016;22:1315–1324.
https://doi.org/10.1111/gcb.13178
17. Sheng YQ, Zhan Y, Zhu LZ. Reduced carbon sequestration potential of biochar in acidic soil. Sci. Total. Environ. 2016;572:129–137.
https://doi.org/10.1016/j.scitotenv.2016.07.140
18. Zhang SP, Wang L, Wei W, et al. Enhanced roles of biochar and organic fertilizer in microalgae for soil carbon sink. Biodegradation. 2018;29:313–321.
https://doi.org/10.1007/s10532-017-9790-0
19. Chen X, Zhu H, Yan B, et al. Greenhouse gas emissions and wastewater treatment performance by three plant species in subsurface flow constructed wetland mesocosms. Chemosphere. 2020;239:124795.
https://doi.org/10.1016/j.chemosphere.2019.124795
20. Di XC, Wang Y, Fu YQ, Wu XM, Wang P. Wheat flour-derived nanoporous carbon@ZnFe2O4 hierarchical composite as an outstanding microwave absorber. Carbon. 2021;173:174–184.
https://doi.org/10.1016/j.carbon.2020.11.006
21. Nirmaladevi S, Boopathiraja R, Kandasamy SK, Sathishkumar S, Parthibavarman M. Wood based biochar supported MnO2 nanorods for high energy asymmetric supercapacitor applications. Surf. Interfaces. 2021;27:27.
https://doi.org/10.1016/j.surfin.2021.101548
22. Li YX, Shang HR, Cao YN, Yang CH, Feng YJ, Yu YL. High performance removal of sulfamethoxazole using large specific area of biochar derived from corncob xylose residue. Biochar. 2022;4:004.
https://doi.org/10.1007/s42773-021-00128-9
23. Palansooriya KN, Wong JTF, Hashimoto Y, et al. Response of microbial communities to biochar-amended soils: a critical review. Biochar. 2019;1:3–22.
https://doi.org/10.1007/s42773-019-00009-2
24. Rajan RJ, Sudarsan JS, Nithiyanantham S. Microbial population dynamics in constructed wetlands: Review of recent advancements for wastewater treatment. Environ. Eng. Res. 2019;24:181–190.
https://doi.org/10.4491/eer.2018.127
25. Harvey OR, Kuo L-J, Zimmerman AR, Louchouarn P, Amonette JE, Herbert BE. An Index-Based Approach to Assessing Recalcitrance and Soil Carbon Sequestration Potential of Engineered Black Carbons (Biochars). Environ. Sci. Technol. 2012;46:1415–1421.
https://doi.org/10.1021/es2040398
26. Nair RR, Mondal MM, Weichgrebe D. Biochar from co-pyrolysis of urban organic wastes-investigation of carbon sink potential using ATR-FTIR and TGA. Biomass. Convers. Biorefin. 2022;12:4729–4743.
https://doi.org/10.1007/s13399-020-01000-9
27. Sohi SP. Carbon Storage with Benefits. Science. 2012;338:1034–1035.
https://doi.org/10.1126/science.1225987
28. Cui X, Hao H, He Z, Stoffella PJ, Yang X. Pyrolysis of wetland biomass waste: Potential for carbon sequestration and water remediation. J. Environ. Manage. 2016;173:95–104.
https://doi.org/10.1016/j.jenvman.2016.02.049
29. Jazinaninejad M, Nematollahi M, Zamenjani AS, Tajbakhsh A. Sustainable operations, managerial decisions, and quantitative analytics of biomass supply chains: A systematic literature review. J. Cleaner Prod. 2022;374:133889.
https://doi.org/10.1016/j.jclepro.2022.133889
30. Xiong J, Liang L, Shi W, et al. Application of biochar in modification of fillers in bioretention cells: A review. Ecol. Eng. 2022;181:106689.
https://doi.org/10.1016/j.ecoleng.2022.106689
31. Tomczyk A, Sokolowska Z, Boguta P. Biochar physicochemical properties: pyrolysis temperature and feedstock kind effects. Rev. Environ. Sci. Bio. 2020;19:191–215.
https://doi.org/10.1007/s11157-020-09523-3
32. Guo X, Cui X, Li H. Effects of fillers combined with biosorbents on nutrient and heavy metal removal from biogas slurry in constructed wetlands. Sci. Total. Environ. 2020;703:134788.
https://doi.org/10.1016/j.scitotenv.2019.134788
33. Xu CL, Feng YL, Li HR, Yang Y, Wu RF. Research progress of phosphorus adsorption by attapulgite and its prospect as a filler of constructed wetlands to enhance phosphorus removal from mariculture wastewater. J. Environ. Chem. Eng. 2022;10:108748.
https://doi.org/10.1016/j.jece.2022.108748
34. Gupta P, Ann T-w, Lee S-M. Use of biochar to enhance constructed wetland performance in wastewater reclamation. Environ. Eng. Res. 2016;21:36–44.
https://doi.org/10.4491/eer.2015.067
35. de Rozari P, Greenway M, El Hanandeh A. Nitrogen removal from sewage and septage in constructed wetland mesocosms using sand media amended with biochar. Ecol. Eng. 2018;111:1–10.
https://doi.org/10.1016/j.ecoleng.2017.11.002
36. Cheng R, Hou S, Wang J, Zhu H, Shutes B, Yan B. Biochar-amended constructed wetlands for eutrophication control and microcystin (MC-LR) removal. Chemosphere. 2022;295:133830.
https://doi.org/10.1016/j.chemosphere.2022.133830
37. Verhamme DT, Prosser JI, Nicol GW. Ammonia concentration determines differential growth of ammonia-oxidising archaea and bacteria in soil microcosms. Isme. J. 2011;5:1067–1071.
https://doi.org/10.1038/ismej.2010.191
38. Zhou X, Wang X, Zhang H, Wu H. Enhanced nitrogen removal of low C/N domestic wastewater using a biochar-amended aerated vertical flow constructed wetland. Bioresour. Technol. 2017;241:269–275.
https://doi.org/10.1016/j.biortech.2017.05.072
39. Du L, Zhao Y, Wang C, et al. Removal performance of antibiotics and antibiotic resistance genes in swine wastewater by integrated vertical-flow constructed wetlands with zeolite substrate. Sci. Total. Environ. 2020;721:137765.
https://doi.org/10.1016/j.scitotenv.2020.137765
40. Li JH, Lv GH, Bai WB, Liu Q, Zhang YC, Song JQ. Modification and use of biochar from wheat straw (Triticum aestivum L.) for nitrate and phosphate removal from water. Desalin. Water Treat. 2016;57:4681–4693.
https://doi.org/10.1080/19443994.2014.994104
41. Abedi T, Mojiri A. Constructed wetland modified by biochar/zeolite addition for enhanced wastewater treatment. Environ. Technol. Innov. 2019;16:100472.
https://doi.org/10.1016/j.eti.2019.100472
42. Li J, Fan JL, Zhang J, Hu Z, Liang S. Preparation and evaluation of wetland plant-based biochar for nitrogen removal enhancement in surface flow constructed wetlands. Environ. Sci. Pollut Res. 2018;25:13929–13937.
https://doi.org/10.1007/s11356-018-1597-y
43. Zimmerman AR, Ouyang L. Priming of pyrogenic C (biochar) mineralization by dissolved organic matter and vice versa. Soil Biol. Biochem. 2019;130:105–112.
https://doi.org/10.1016/j.soilbio.2018.12.011
44. Wu HM, Dong XY, Liu H. Evaluating fluorescent dissolved organic matter released from wetland-plant derived biochar: Effects of extracting solutions. Chemosphere. 2018;212:638–644.
https://doi.org/10.1016/j.chemosphere.2018.08.110
45. Kasak K, Truu J, Ostonen I, et al. Biochar enhances plant growth and nutrient removal in horizontal subsurface flow constructed wetlands. Sci. Total. Environ. 2018;639:67–74.
https://doi.org/10.1016/j.scitotenv.2018.05.146
46. Lan W, Zhang J, Hu Z, et al. Phosphorus removal enhancement of magnesium modified constructed wetland microcosm and its mechanism study. Chem. Eng. J. 2018;335:209–214.
https://doi.org/10.1016/j.cej.2017.10.150
47. Liu Y, Feng L, Liu Y, Zhang L. A novel constructed wetland based on iron carbon substrates: performance optimization and mechanisms of simultaneous removal of nitrogen and phosphorus. Environ. Sci. Pollut. Res. 2023;30:23035–23046.
https://doi.org/10.1007/s11356-022-23754-7
48. Chand N, Kumar K, Suthar S. “Cattle dung biochar-packed vertical flow constructed wetland for nutrient removal”: Effect of intermittent aeration and wastewater COD/N loads on the removal process. J. Water. Process. Eng. 2021;43:102215.
https://doi.org/10.1016/j.jwpe.2021.102215
49. Guo XF, Cui XY, Li HS, Xiong BH. Purifying effect of biochar-zeolite constructed wetlands on arsenic-containing biogas slurry in large-scale pig farms. J. Cleaner Prod. 2021;279:123579.
https://doi.org/10.1016/j.jclepro.2020.123579
50. Zhou X, Wang RG, Liu H, Wu SB, Wu HM. Nitrogen removal responses to biochar addition in intermittent-aerated subsurface flow constructed wetland microcosms: Enhancing role and mechanism. Ecol. Eng. 2019;128:57–65.
https://doi.org/10.1016/j.ecoleng.2018.12.028
51. de Rozari P, Greenway M, El Hanandeh A. Phosphorus removal from secondary sewage and septage using sand media amended with biochar in constructed wetland mesocosms. Sci. Total Environ. 2016;569:123–133.
https://doi.org/10.1016/j.scitotenv.2016.06.096
52. Ahmad M, Rajapaksha AU, Lim JE, et al. Biochar as a sorbent for contaminant management in soil and water: A review. Chemosphere. 2014;99:19–33.
https://doi.org/10.1016/j.chemosphere.2013.10.071
53. Chang J, Peng D, Deng S, Chen J, Duan C. Efficient treatment of mercury(II)-containing wastewater in aerated constructed wetland microcosms packed with biochar. Chemosphere. 2022;290:133302.
https://doi.org/10.1016/j.chemosphere.2021.133302
54. Uchimiya M, Wartelle LH, Lima IM, Klasson KT. Sorption of Deisopropylatrazine on Broiler Litter Biochars. J. Agri. Food Chem. 2010;58:12350–12356.
https://doi.org/10.1021/jf102152q
55. Ahmad M, Lee SS, Dou X, et al. Effects of pyrolysis temperature on soybean stover- and peanut shell-derived biochar properties and TCE adsorption in water. Bioresour. Technol. 2012;118:536–544.
https://doi.org/10.1016/j.biortech.2012.05.042
56. Sun K, Keiluweit M, Kleber M, Pan Z, Xing B. Sorption of fluorinated herbicides to plant biomass-derived biochars as a function of molecular structure. Bioresour. Technol. 2011;102:9897–9903.
https://doi.org/10.1016/j.biortech.2011.08.036
57. Tang X, Yang Y, Tao R, et al. Fate of mixed pesticides in an integrated recirculating constructed wetland (IRCW). Sci Total. Environ. 2016;571:935–942.
https://doi.org/10.1016/j.scitotenv.2016.07.079
58. Deng SJ, Chen JQ, Chang JN. Application of biochar as an innovative substrate in constructed wetlands/biofilters for wastewater treatment: Performance and ecological benefits. J. Cleaner Prod. 2021;293:126156.
https://doi.org/10.1016/j.jclepro.2021.126156
59. Mittal Y, Srivastava P, Kumar N, et al. Ultra-fast and low-cost electroactive biochar production for electroactive-constructed wetland applications: A circular concept for plant biomass utilization. Chem. Eng. J. 2023;452:138587.
https://doi.org/10.1016/j.cej.2022.138587
60. Zhang YF, Wang JM, Feng Y. The effects of biochar addition on soil physicochemical properties: A review. Catena. 2021;202:105284.
https://doi.org/10.1016/j.catena.2021.105284
61. Schievano A, Sciarria TP, Gao YC, et al. Dark fermentation, anaerobic digestion and microbial fuel cells: An integrated system to valorize swine manure and rice bran. Waste. Manage. 2016;56:519–529.
https://doi.org/10.1016/j.wasman.2016.07.001
62. Zheng RL, Cai C, Liang JH, et al. The effects of biochars from rice residue on the formation of iron plaque and the accumulation of Cd, Zn, Pb, As in rice (Oryza sativa L.) seedlings. Chemosphere. 2012;89:856–862.
https://doi.org/10.1016/j.chemosphere.2012.05.008
63. Chakraborty I, Sathe SM, Dubey BK, Ghangrekar MM. Waste-derived biochar: Applications and future perspective in microbial fuel cells. Bioresour. Technol. 2020;312:123587.
https://doi.org/10.1016/j.biortech.2020.123587
64. Kizito S, Lv T, Wu S, Ajmal Z, Luo H, Dong R. Treatment of anaerobic digested effluent in biochar-packed vertical flow constructed wetland columns: Role of media and tidal operation. Sci. Total. Environ. 2017;592:197–205.
https://doi.org/10.1016/j.scitotenv.2017.03.125
65. Deng C, Huang L, Liang Y, et al. Response of microbes to biochar strengthen nitrogen removal in subsurface flow constructed wetlands: Microbial community structure and metabolite characteristics. Sci. Total. Environ. 2019;694:133687.
https://doi.org/10.1016/j.scitotenv.2019.133687
66. DeLuca TH, MacKenzie MD, Gundale MJ, Holben WE. Wildfire-produced charcoal directly influences nitrogen cycling in ponderosa pine forests. Soil. Sci. Soc. Am. J. 2006;70:448–453.
https://doi.org/10.2136/sssaj2005.0096
67. Song Y, Zhang X, Ma B, Chang SX, Gong J. Biochar addition affected the dynamics of ammonia oxidizers and nitrification in microcosms of a coastal alkaline soil. Biol. Fert. Soils. 2014;50:321–332.
https://doi.org/10.1007/s00374-013-0857-8
68. Lehmann J, Rillig MC, Thies J, Masiello CA, Hockaday WC, Crowley D. Biochar effects on soil biota - A review. Soil. Boil Biochem. 2011;43:1812–1836.
https://doi.org/10.1016/j.soilbio.2011.04.022
69. Liu YS, Feng L, Liu YZ, Zhang LQ. A novel constructed wetland based on iron carbon substrates: performance optimization and mechanisms of simultaneous removal of nitrogen and phosphorus. Environ. Sci. Pollut. Res. 2023;30:23035–23046.
https://doi.org/10.1007/s11356-022-23754-7
70. He K, He G, Wang C, et al. Biochar amendment ameliorates soil properties and promotes Miscanthus growth in a coastal saline-alkali soil. Appl. Soil. Ecol. 2020;155:103674.
https://doi.org/10.1016/j.apsoil.2020.103674
71. Du ZL, Zhao JK, Wang YD, Zhang QZ. Biochar addition drives soil aggregation and carbon sequestration in aggregate fractions from an intensive agricultural system. J. Soils. Sediments. 2017;17:581–589.
https://doi.org/10.1007/s11368-015-1349-2
72. Li J, Fan J, Liu D, Hu Z, Zhang J. Enhanced nitrogen removal in biochar-added surface flow constructed wetlands: dealing with seasonal variation in the north China. Environ. SoiI. Pollut. 2019;26:3675–3684.
https://doi.org/10.1007/s11356-018-3895-9
73. Werner S, Kaetzl K, Wichern M, Buerkert A, Steiner C, Marschner B. Agronomic benefits of biochar as a soil amendment after its use as waste water filtration medium. Environ Pollut. 2018;233:561–568.
https://doi.org/10.1016/j.envpol.2017.10.048
74. Aguirre-Villegas HA, Benson CH. Expectations for Coal Demand in Response to Evolving Carbon Policy and Climate Change Awareness. Energies. 2022;15:3739.
https://doi.org/10.3390/en15103739
75. Araujo OQF, Gobbi CN, Chaloub RM, Coelho MAZ. Assessment of the Impact of Salinity and Irradiance on the Combined Carbon Dioxide Sequestration and Carotenoids Production by Dunaliella salina: A Mathematical Model. Biotechnol. Bioeng. 2009;102:425–435.
https://doi.org/10.1002/bit.22079
76. Kong SH, Loh SK, Bachmann RT, Zainal H, Cheong KV. PALM KERNEL SHELL BIOCHAR PRODUCTION, CHARACTERISTICS AND CARBON SEQUESTRATION POTENTIAL. J. Oil. Palm Res. 2019;31:508–520.
https://doi.org/10.21894/jopr.2019.0041
77. Du Z-L, Zhao J-K, Wang Y-D, Zhang Q-Z. Biochar addition drives soil aggregation and carbon sequestration in aggregate fractions from an intensive agricultural system. J. Soil Sediment. 2017;17:581–589.
https://doi.org/10.1007/s11368-015-1349-2
78. Guo J, Chen B. Insights on the Molecular Mechanism for the Recalcitrance of Biochars: Interactive Effects of Carbon and Silicon Components. Environ. Sci. Technol. 2014;48:9103–9112.
https://doi.org/10.1021/es405647e
79. Zimmerman AR. Abiotic and Microbial Oxidation of Laboratory-Produced Black Carbon (Biochar). Environ. Sci Technol. 2010;44:1295–1301.
https://doi.org/10.1021/es903140c
80. Windeatt JH, Ross AB, Williams PT, Forster PM, Nahil MA, Singh S. Characteristics of biochars from crop residues: Potential for carbon sequestration and soil amendment. J Environ. Manage. 2014;146:189–197.
https://doi.org/10.1016/j.jenvman.2014.08.003
81. Tsai WT. Carbon-Negative Policies by Reusing Waste Wood as Material and Energy Resources for Mitigating Greenhouse Gas Emissions in Taiwan. Atmosphere. 2021;12:1220.
https://doi.org/10.3390/atmos12091220
82. Ganguly A, Brown RC, Wright MM. Techno-economic and greenhouse gas emission assessment of carbon negative pyrolysis technology. Green. Chem. 2022;24:9290–9302.
https://doi.org/10.1039/d2gc03172h
83. Bruun S, Clauson-Kaas S, Bobulska L, Thomsen IK. Carbon dioxide emissions from biochar in soil: role of clay, microorganisms and carbonates. Eur. J. Soil. Sci. 2014;65:52–59.
https://doi.org/10.1111/ejss.12073
84. Liang B, Lehmann J, Sohi SP, et al. Black carbon affects the cycling of non-black carbon in soil. Org. Gochem. 2010;41:206–213.
https://doi.org/10.1016/j.orggeochem.2009.09.007
85. Maltais-Landry G, Maranger R, Brisson J, Chazarenc F. Greenhouse gas production and efficiency of planted and artificially aerated constructed wetlands. Environ. Pollut. 2009;157:748–754.
https://doi.org/10.1016/j.envpol.2008.11.019
86. Luan J, Cui L, Song H, Wang Y. Foreign Research Progress on Carbon Cycle in Wetland Ecosystems. Wetland. Sci. 2012;10:235–242.
https://doi.org/1672-5948(2012)02-235-08
87. Weiqing M, Zhanlei WU, Zhongliang W. Control factors and critical conditions between carbon sinking and sourcing of wetland ecosystem. Ecol. Environ. Sci. 2011;20:1359–1366.
https://doi.org/10.1016/S1671-2927(11)60313-1
88. Kayranli B, Scholz M, Mustafa A, Hedmark A. Carbon Storage and Fluxes within Freshwater Wetlands: a Critical Review. Wetlands. 2010;30:111–124.
https://doi.org/10.1007/s13157-009-0003-4
89. Pattanayak A, Kumar KSK. Accounting for impacts due to climate change in GHG mitigation burden sharing. Clim. Policy. 2015;15:724–742.
https://doi.org/10.1080/14693062.2014.962468
90. Maucieri C, Barbera AC, Vymazal J, Borin M. A review on the main affecting factors of greenhouse gases emission in constructed wetlands. Agric. For. Meteorol. 2017;236:175–193.
https://doi.org/10.1016/j.agrformet.2017.01.006
91. Ji B, Chen J, Mei J, et al. Roles of biochar media and oxygen supply strategies in treatment performance, greenhouse gas emissions, and bacterial community features of subsurface-flow constructed wetlands. Bioresour. Technol. 2020;302:122890.
https://doi.org/10.1016/j.biortech.2020.122890
92. Tai PD, Li PJ, Sun TH, et al. Greenhouse gas emissions from a constructed wetland for municipal sewage treatment. J Environ. Sci. 2002;14:27–33.
https://doi.org/10.3321/j.issn:1001-0742.2002.01.005
93. Zhou X, Jia L, Liang C, Feng L, Wang R, Wu H. Simultaneous enhancement of nitrogen removal and nitrous oxide reduction by a saturated biochar-based intermittent aeration vertical flow constructed wetland: Effects of influent strength. Chem. Eng J. 2018;334:1842–1850.
https://doi.org/10.1016/j.cej.2017.11.066
94. Angst TE, Patterson CJ, Reay DS, Anderson P, Peshkur TA, Sohi SP. Biochar Diminishes Nitrous Oxide and Nitrate Leaching from Diverse Nutrient Sources. J. Environ. Qual. 2013;42:672–682.
https://doi.org/10.2134/jeq2012.0341
95. Wang F, Harindintwali JD, Yuan ZZ, et al. Technologies and perspectives for achieving carbon neutrality. Innovation. 2021;2:100180.
https://doi.org/10.1016/j.xinn.2021.100180
96. El-Mahrouky M, El-Naggar AH, Usman AR, Al-Wabel M. Dynamics of CO2 Emission and Biochemical Properties of a Sandy Calcareous Soil Amended with Conocarpus Waste and Biochar. Pedosphere. 2015;25:46–56.
https://doi.org/10.1016/s1002-0160(14)60075-8
97. Guo F, Zhang J, Yang X, He Q, Ao L, Chen Y. Impact of biochar on greenhouse gas emissions from constructed wetlands under various influent chemical oxygen demand to nitrogen ratios. Bioresour. Technol. 2020;303:122908.
https://doi.org/10.1016/j.biortech.2020.122908
98. Sun Y, Qi S, Zheng F, et al. Organics removal, nitrogen removal and N2O emission in subsurface wastewater infiltration systems amended with/without biochar and sludge. Bioresour Technol. 2018;249:57–61.
https://doi.org/10.1016/j.biortech.2017.10.004
99. Spokas KA, Novak JM, Venterea RT. Biochar’s role as an alternative N-fertilizer: ammonia capture. Plant. Soil. 2012;350:35–42.
https://doi.org/10.1007/s11104-011-0930-8
100. Yanai Y, Toyota K, Okazaki M. Effects of charcoal addition on N2O emissions from soil resulting from rewetting air-dried soil in short-term laboratory experiments. Soil. Sci. Plant Nutr. 2007;53:181–188.
https://doi.org/10.1111/j.1747-0765.2007.00123.x
101. Liu X, Mao P, Li L, Ma J. Impact of biochar application on yield-scaled greenhouse gas intensity: A meta-analysis. Sci Total. Environ. 2019;656:969–976.
https://doi.org/10.1016/j.scitotenv.2018.11.396
102. Singh BP, Hatton BJ, Singh B, Cowie AL, Kathuria A. Influence of Biochars on Nitrous Oxide Emission and Nitrogen Leaching from Two Contrasting Soils. J. Environ. Qual. 2010;39:1224–1235.
https://doi.org/10.2134/jeq2009.0138
103. Cornelissen G, Rutherford DW, Arp HPH, Dorsch P, Kelly CN, Rostad CE. Sorption of Pure N2O to Biochars and Other Organic and Inorganic Materials under Anhydrous Conditions. Environ. Sci. Technol. 2013;47:7704–7712.
https://doi.org/10.1021/es400676q
104. Hu S, Zhu H, Bañuelos G, et al. Factors Influencing Gaseous Emissions in Constructed Wetlands: A Meta-Analysis and Systematic Review. Int. J. Environ. Res. Public. Health. 2023;20:3876.
https://doi.org/10.3390/ijerph20053876
105. Yuan HY, Ding LJ, Zama EF, Liu PP, Hozzein WN, Zhu YG. Biochar Modulates Methanogenesis through Electron Syntrophy of Microorganisms with Ethanol as a Substrate. Environ. Sci. Technol. 2018;52:12198–12207.
https://doi.org/10.1021/acs.est.8b04121
106. Chen X, Zhu H, Banuelos G, Shutes B, Yan BX, Cheng R. Biochar reduces nitrous oxide but increases methane emissions in batch wetland mesocosms. Chem. Eng. J. 2020;392:124842.
https://doi.org/10.1016/j.cej.2020.124842
Table 1Comparison of different CWs types
Table 2Comparison of COD, NH4+-N, TN, NO3−-N, TP removal efficiency and N2O emissions in BC-added CWs
Table 3Effectiveness on the removal of hard-to-degrade pollutants in BC-added CWs
|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||