AbstractIn this study, 31 household spray-type cleaning products from 10 categories were examined to identify the substances contained in these products and evaluate their resulting toxicities. After substance detection via LC-QTOF-MS, suspect and non-target screenings were performed using UNIFI software. A total of 48 substances were identified during suspect screening. Among them, surfactant (freq. 22/31(71.0%)), plasticizer (freq. 23/31(74.2%)), antioxidant (freq. 20/31(64.5%)) were detected with high frequencies. Among the 48 compounds, tributyl citrate acetate was the most frequently detected (frequency 13/31, 41.9%). In addition, 51 substances were identified via non-target analysis. Among them, oleamide, a type of surfactant, showed a high frequency of detection (22/31(71.0%)). The toxicities of the 98 substances (suspect: 47 (except for confidence level 5) and non-target: 51) identified were evaluated using the European Chemicals Agency (ECHA) database and Toxtree. Among them, 20 substances were classified as a substance of caution. The risk assessment for 3 substances identified with references standards was also performed. The suspect and non-target screening technique proved to be a useful method that can be applied when there are many non-specific chemicals and their components in the consumer products are not well known.
Graphical Abstract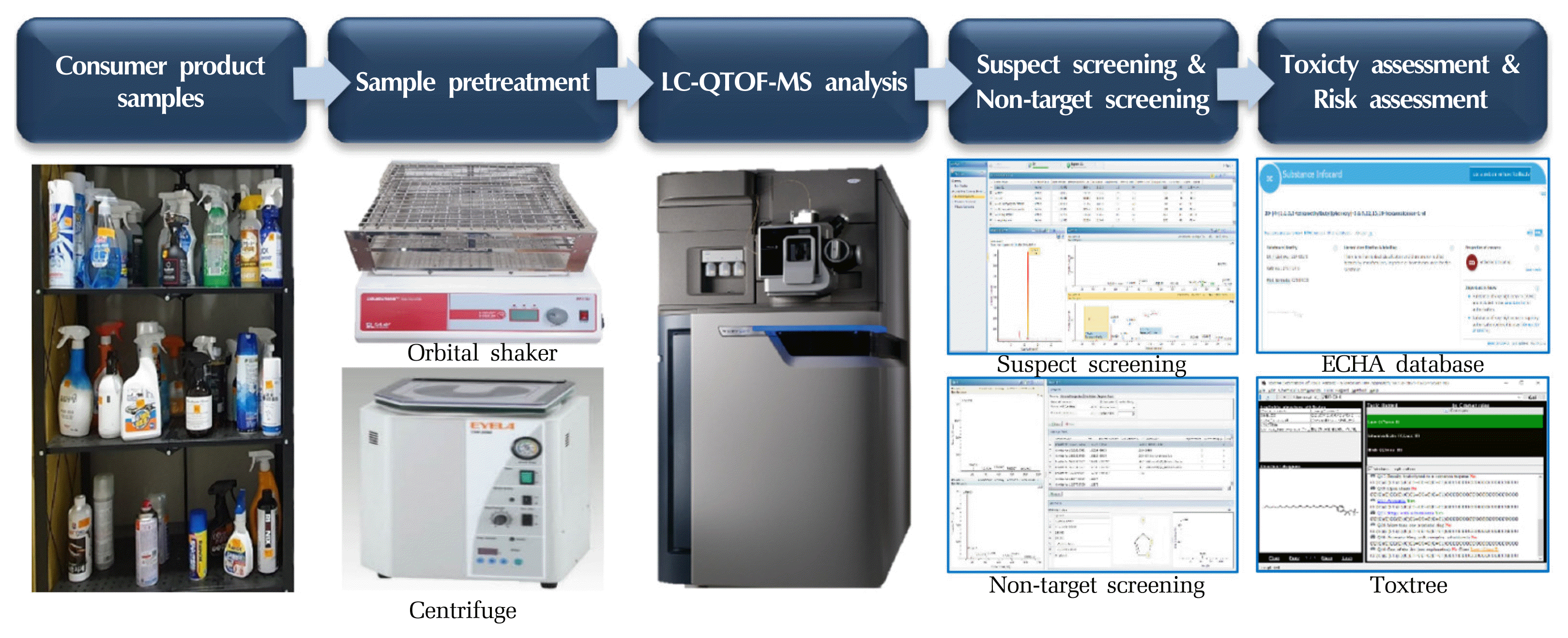
1. IntroductionNumerous chemicals are used daily, especially those found in consumer products. As the use of consumer products comprising numerous chemicals increases, the risk of human exposure to these chemicals increases as they can be transmitted to humans via various routes [1]. However, studies on the potential exposure of chemicals to humans from daily consumer products are lacking due to the following reasons. First, due to the ingredient disclosure requirement in many countries, consumer products may list numerous ingredients [2]. However, most consumer products only list general ingredients or do not list complete ingredients. Consequently, the information provided on product labels and material safety data sheets (MSDSs) is insufficient for fully identify product constituents and their potential health effects due to exposure [3]. Secondly, consumers may be simultaneously exposed to multiple substances in various products. For example, substances, such as benzophenone-3, bisphenol-A, phthalates, and polybrominated diphenyl ethers, can have synergetic effects on the human body, even when the chemicals are individually present at safe levels [4, 5], Thirdly, once commercialized, it is difficult to stop the use of chemicals, even if it is known to have adverse health effects. For example, polyhexamethylene guanidine (PHMG), a humidifier disinfectant associated with pulmonary fibrosis outbreaks in South Korea, was mainly used as a deterrent to clean carpets and swimming pools. PHMG included methylisothiazolinone (MIT) and benzisothiazolinone (BIT), which are used as disinfectants. Notably, the harmful effect of these chemicals on the human body was underestimated at the time of the outbreaks [4, 6].
To reduce the risk of exposure of substances contained in household consumer products and their adverse effects on humans, first, the substances in the products should be identified. Although most previous studies have analyzed chemicals using targeted analytical methods, the studies that analyze undisclosed or unintended components of consumer products and identify chemicals other than target compounds are lacking.
Owing to the recent development of advanced monitoring methods, the detection of known chemical classes and classes that were not previously targeted for detection has become a prominent topic in environmental research [7]. In particular, high-resolution mass spectrometry (HR-MS) has been used to identify substances in the products, screen by-products, and unintended chemicals, and assess the indicated substance information provided for the products [8]. Advances in high-resolution mass spectrometry can not only enable the detection of targeted compounds, but also identify expected (suspect) compounds using existing databases, libraries, or software matching algorithms. These advances have made it possible to closely examine HR-MS spectra to identify previously unknown compounds (off-targets) [7, 9, 10]. Gas chromatography high-resolution mass (GC-HRMS) has been used primarily for chemical analysis, especially semi-volatile organic compounds (SVOC), in products using suspect and non-suspect screening [8, 11, 12]. However, there are not many studies using liquid chromatography (LC-HRMS) [13].
Therefore, in this study, 31 household cleaning products from 10 categories (bathroom/toilet, kitchen, glass, metal, air conditioner, automobile, carpet, multipurpose, floor, and bowling ball) were examined to identify the chemicals in the products and evaluate their toxicities. To identify the substances in the products, suspect and non-target screening methods via liquid chromatography quadrupole time-of-flight mass spectrometry (LC-QTOF-MS) was adopted using the UNIFI platform (Waters, Milford, MA USA). To estimate the toxicity of the identified substances, the ToxTree open-source program tool (http://toxtree.sourceforge.net/index.html) and the European Chemicals Agency (ECHA) database [15] were used to estimate the toxicities of identified substances. Finally, the risk assessment was performed for substances identified with reference standards.
2. Materials and Methods2.1. Standards and ReagentsMethyl tertiary butyl ether (MTBE) was purchased from Sigma-Aldrich (St. Louis, MO, USA) for sample preparation. Herein, 0.1% formic acid (Sigma-Aldrich, ACS grade) in methanol (Fisher Chemicals, LC-MS grade, Loughborough, UK) and 0.1% formic acid in acetonitrile (Honeywell, HPLC grade, Morristown, USA) was used as the mobile phase for liquid chromatography (LC). Reference standards containing tributyl citrate acetate (C20H34O8), glyceryl monostearate (C21H42O4) and 2-(2-butoxyethoxy)ethyl acetate (C10H20O4) were purchased from Sigma-Aldrich. Stock solutions were prepared with water from a Milli-Q water system (R = 18.2 MΩ/cm, Millipore, St. Louis, USA).
2.2. Selected SamplesOf the products governed under the Chemical Product Safety Act of Korea [14], 31 cleaning products that can be purchased online were selected. The products were classified into 10 groups according to their intended use: bathroom/toilet (n=8), kitchen (n=6), glass (n=5), metal (n=2), automobile (n=2), air conditioner (n=2), carpet (n=2), multipurpose (n=2), floor (n=1), and bowling ball (n=1). Detailed information is provided in Table S1 (Supporting Information).
2.3. Sample PreparationAll samples were pretreated using the method described by Guo and Kannan [15] with minor modifications. Since it is difficult to detect all the substances in the consumer products, this study focused on hydrophobic substances with high bioaccumulation [16,17], to extract mainly hydrophobic substances, 0.2 g of each sample was added to 5 mL of MTBE and mixed evenly. The sample was then shaken in an orbital shaker for 30 min and centrifuged at 3500 rpm for 20 min to obtain 5 mL of the supernatant. After this process was repeated, 10 mL of the extract was obtained, purged with nitrogen (99% purity), then concentrated to 1 mL, and finally filtered with a 0.45 μm membrane filter for instrument analysis.
2.4. Sample AnalysisUltra-high performance liquid chromatography quadrupole time-of-flight mass spectrometry (UPLC-QTOF-MS; Acquity UPLC System coupled with a SYNAPT G2-Si mass spectrometer, Waters, Milford, MA, USA) with an Acquity UPLC BEH C18 column (100 mm × 2.1 mm, 1.7 μM, Waters) was used for suspect and non-target screening. Mobile phases A (0.1% formic acid in deionized water) and B (0.1% formic acid in acetonitrile) for positive mode, and (A) deionized water and (B) acetonitrile for negative mode were used according to the following gradient mode program at a flow rate of 0.3 mL/min: 0–16 min, 10–99% B; 16–21 min, 99% B; 21–24 min, 99–10% B. The auto-sampler was maintained at 15 °C and the injection volume was 2 μL. MS analysis was performed with electrospray ionization (ESI) in the m/z range of 50–1200; detailed information is provided in Table S2 (Supporting Information). A lock spray source (leucine enkephalin positive: 556.2771 Da, negative: 554.2615 Da) was used as the reference lock mass. All samples were run in LC-QTOF-MSe continuum mode. Triplicate injections were performed for all samples in positive and negative modes. To prevent contamination of the analytical instrument, LC and TOF-MS were cleaned before analysis.
2.5. Data Screening and IdentificationData obtained after instrument analysis were screened using the UNIFI platform (Waters, Milford, MA USA) (Fig. 1). If the same substance was detected both in the sample and a blank, any response below three times the blank response (instrument detection limit) was removed. In addition, all chromatograms were manually screened, and the peaks were classified by manually evaluating their fit. The substance identification workflow for the suspect and non-target screening is shown in Fig. 2, and the confidence level of the identified substances was determined using the method described by Schymanski et al. [18] (Table 1).
2.5.1. Suspect screening processAll samples were filtered using the UNIFI platform to compare the mass spectra, retention times, molecular formulas, and structures of a library (Waters, Extractables, and Leachables HRMS Library) containing 581 substances (Table S3). Compound identification was based on mass error (< ±10 ppm), chromatographic width ratio (< 1.5), response (> 50), theoretical fragments found (> 0), and isotope match intensity root-mean-square (RMS) percentage (< 20) (Fig. 2). The mass error is usually set to 5 ppm or less, but in this study, the range was set to ±10 ppm because even the peaks of which the mass error exceeding ±5 ppm have the isotope match root-mean-square (RMS) value of 20 or less (the recommended value by Waters), and the shapes of those chromatograms were sharp.
2.5.2. Non-target screening processThe substances that were not identified during suspect screening were subjected to non-target screening via the following four steps (Fig. 2) [19]; First, after instrument analysis, the number of features was appropriately reduced with the filtering process by the chromatographic width ratio (1.5) and response (1,000); Secondly, to determine the empirical formula for substances that were not identified after the filter step, i-FIT™, which is the UNIFI algorithm system, was used to obtain the i-FIT confidence percentage, which is a potential molecular formula; Thirdly, possible names and structures associated with the empirical formula or exact mass were searched using online libraries, such as ChemSpider Library, PubChem, and Thomson Pharma libraries. Finally, the UNIFI program was used to identify a possible structural candidate by comparing the experimental spectrum of an unidentified compound obtained at high energy with its theoretically predicted fragments [20–23]. The candidate structures were then ranked based on the relative intensity of the fragments, the number of matched fragment ions, and the number of citations in the literatures. In this step, the number of match fragmentations was set to > 5, the intensity was set to > 50%, and the number of citations was set to > 10.
2.6. Risk AssessmentAmong the substances identified with the standard product in this study, risk assessment was performed. The estimated daily intake (EDI) levels via dermal contact of cleaning products were determined using Eq. (1):
where C is a chemical's concentration in cleaning products (ng/ml, ppb), A is the amount of cleaning products consumed per time (g/time), F the frequency of use (time/day), and abs. the absorption rate [24]. Table S4 summarizes the values assigned for each parameter in the equation [25].
3. Results & Discussions3.1. Suspect ScreeningFrom suspect screening of 31 cleaning products, 48 chemicals were identified using LC-QTOF-MS (Table 2), of which 40 were from positive and 8 from negative modes. The sample information with identified chemicals is provided in Supporting Information (Table S6-1: positive and Table S6-2: negative). Based on their usage, 48 substances were classified as follows: surfactant (n=10) (frequency: 22/31(71.0%)), plasticizer (n=6) (freq. 22/31(74.2%)), antioxidant (n=4) (freq. 20/31(64.5%)), UV absorber (n=3) (freq. 4/31(12.9%)), fragrance (n=2) (freq. 2/31(6.5%)), foam boosting substance (n=2) (freq. 4/31(12.9%)), solvent (n=2) (freq. 10/31(32.3%)), viscosity controlling substance (n=2) (freq. 9/31(29.0%)), buffers (n=1) (freq. 1/31(3.2%)), other plastic additives (n=1) (freq. 1/31(3.2%)), and unknown (n=15). The detailed identified substances were discussed as follows.
3.1.1. Plasticizer categoryPlasticizers account for approximately one-third of the global plastic additives market in terms of consumption [30], and have been scrutinized for their contribution to environmental and health related problems [31, 32]. Many plasticizers are endocrine-disrupting chemicals (EDCs) that can adversely affect reproductive development [33]. In particular, di-(2-ethylhexyl) phthalate (DEHP) and other phthalates such as dibutyl phthalate (DBP), and benzyl butyl phthalate (BBP) have been registered as candidates of high concern by the Registration, Evaluation, Authorization, and Restriction of Chemicals (REACH). As a result, many countries have various regulations regarding plasticizers as ingredients in consumer products [34].
In this study, the most frequently identified substance from the suspect screening of the cleaning products is tributyl citrate acetate (CAS No. 77-90-7, freq. 13/31(41.9%)) (Fig. 3). Tributyl citrate acetate is known as a plasticizer and has a specific migration limit of 60 mg/kg by the European Food Safety Authority [35]. This chemical is mainly used in surface treatment, washing, and cleaning products [36]. Citrates-based plasticizers are reported to be relatively safer than conventional phthalate plasticizers in terms of reproductive, developmental, acute, and genotoxicity, but there is insufficient information on their endocrine disrupting properties and various toxic endpoints, so in vivo and in vitro studies are needed [37, 38]. Malnes et al. measured tributyl citrate acetate at a concentration of 6.3 ng/L (max. 29.0 ng/L) at 15 sewage treatment plants (STPs) in Sweden, with a detection frequency of 100% [39]. Lee et al. also reported that tributyl citrate acetate was detected in wastewater treatment plants (WWTPs) in South Korea at concentrations of 29–19000 ng/g and with frequencies of 73–100% [40]. The higher detection frequency (41.9%) of tributyl citrate acetate in this study can be explained by its universal use in cleaning products as a plasticizer.
Another identified chemical by suspect screening is luperox 101 (CAS No. 78-63-7, freq. 10/31(32.3%)) which was detected primarily in bathroom/toilet products (Fig. S1). Many household chemicals use polypropylene as packaging [41], and luperox 101 is used as a viscosity modifier for polypropylene [42]. The high detection frequency of Luperox 101 is most likely due to the frequent use of polypropylene product containers and the elution of Luperox 101 from the container. Luperox 101 has been shown to be cytotoxic and corrosive to the skin [43].
3.1.2. Surfactant categoryOwing to their physicochemical properties, surfactants have broad applications in household cleaning products, including laundry detergents, surface cleansers, and personal care products [44]. The interaction of surfactants with the skin can produce clinical manifestations, including skin dryness, changes in skin texture, modification of permeability properties, and inflammation [45].
Among the surfactants identified in the household cleaning product samples by suspect screening, n,n-bis(2-hydroxyethyl)dodecanamide (CAS No. 120-40-1, freq. 1/31(3.2%)) (Fig. S2) is classified as List 7 Substances for which some toxicological data exist, but for which an acceptable daily intake (ADI) or a Tolerable Daily Intakes (TDI) could not be established (EC, 1999) [46]. It is reported that n,n-bis(2-hydroxyethyl)dodecanamide is widely used in detergents for dishwashing, washing machine products for cleaning fabrics, and polish metal surface cleaners [47].
Another substance identified in the household cleaning product samples, tetraethylene glycol (CAS No. 112-60-7, freq. 1/31(3.2%)), is used as a surfactant in surface treatment, and washing or cleaning products [48]. Tetraethylene glycol is known as a mild skin irritant and can affect the central nervous system, liver, kidney, and reproductive function of humans after long periods of exposure [49].
Another surfactant, octoxynol 7 (CAS No. 2497-59-8, frequency 4/31 (12.9%)), has been reported to induce estrogen both in vivo and in vitro because it mimics the effect of estradiol [50, 51]. In addition, among the identified surfactants, hexadecanol (CAS No. 36653-82-4, freq. 15/31 (48.4%)) has little toxicity by Toxtree and ECHA, despite its high detection frequency.
3.1.3. Antioxidant categorySynthetic antioxidants are a group of critical anthropogenic chemicals broadly used in foodstuffs, personal care products, plastics, lubricants, and rubber products for protection from oxidative degradation [52–55]. According to recent studies, some antioxidants can be present in human urine, serum, plasma, breast milk, and the placenta, indicating human exposure [56–60].
Among these antioxidant categories, 2,6-di-tert-butyl-4-ethylphenol (CAS No. 4130-42-1, freq. 1/31(3.2%)) (Fig. S3), was identified in the samples examined herein. This substance is known as an antioxidant and stabilizer in polymers employed in food manufacturing [61], and is widely used to prevent free-radical-mediated oxidation in fluids (e.g., fuels and oils) and other materials [62]. It is reported that 2,6-di-tert-butyl-4-ethylphenol not only hinders the survival and reproduction of aquatic organisms, but also endangers human health [63, 64].
Bis(2,4-di-tert-butylphenyl) pentaerythritol diphosphate (CAS number 97994-11-1, freq. 18/31(58.1%)), one of the organophosphate esters (OPEs), is a family of anthropogenic additives with high production and application volumes, and widely used in many industrial and household products [65]. OPEs are often observed in human serum and urine, and there is growing interest in the identification of OPE in the environment due to its endocrine-disrupting potential toxicity [66–68].
3.1.4. Fragrance categoryStudies have shown that a variety of household cleaners include fragrances with unique scents, and fragrance products, including air fresheners, deodorants, cleaning products, laundry detergents, fabric softeners, essential oils, scented candles, soaps, personal care products, perfumes, and hand sanitizers [69, 70]. Owing to increased usage and exposure, clinical cases of health conditions that are caused, triggered, and exacerbated by fragrance products are increasing.
In the fragrance category, diethyl sebacate (CAS No. 110-40-7, freq. 1/31(3.2%)) was identified in kitchen cleaner samples (Sample No. 10) (Fig. S4). It is widely used as a food flavoring agent and is also used in perfumes [71]. It is reported that diethyl sebacate can cause allergic contact dermatitis [72].
3.1.5. UV absorberOne of the UV absorbers detected in this study, octabenzone (CAS No. 1843-05-6, freq. 1/31(3.2%)), is frequently used in cleaning products and household care [73]. Octabenzone is not readily biodegradable and is potentially persistent [74]. It is also reported that octabenzone causes a concentration- and time-dependent loss of cell viability accompanied by a depletion of intracellular adenosine triphosphate (ATP) in rat hepatocytes [74]. Another substance identified in this study was tinuvin 144 (CAS No. 63843-89-0, freq. 2/31(6.5%)) (Fig. S5). Tinuvin 144, a type of polyurethane as a UV absorber [75], was detected in the sample No. 12 and No. 26. This chemical is mainly used in adhesives, sealants, coatings and paints, and paint removers [74], can be detected as polyurethane foam in various cleaning agents [76–78]. Of note, tinuvin 144 is a hindered amine that is toxic to the immune system, liver, blood, and male reproductive system [79].
3.2. Non-target ScreeningSubstances that are not identified in the MS library after instrument analysis can be categorized as substances through a non-target screening process. Through the non-target screening process, a total of 51 substances were identified in the positive mode from the samples, and no substances were identified in the negative mode (Table 3). 51 substances were classified based on their usage categories as follows: surfactant (n=6) (frequency: 28/31(90.3%)), emulsifier (n=4) (freq. 2/31(6.5%)), fragrance (n=2) (freq. 2/31(6.5%)), PFAS (n=4) (freq. 3/31(9.7%)), %)), antistatic agent (n=4) (freq. 8/31(25.8%)),viscosity controlling substance (n=1) (freq. 1/31(3.2%)), and unknown (n=30). Sample information with substances identified is provided in Table S7.
Among 51 substances, oleamide (CAS No. 301-02-0, freq. 22/31(71.0%)) (Fig. S6) was the most frequently detected substance via non-target screening in this study. Oleamide, is one of the surfactant, has a variety of industrial uses including as a slip agent, a lubricant, and a corrosion inhibitor [80]. Oleamide is known to be leached out of polypropylene plastics [81]. It is reported that, in rats, the oral LD50 of undiluted oleamide DEA was 12.4 mL/kg [82]. Next, as one of the emulsifiers, myreth-3 (CAS No. 26826-30-2, freq. 1/31 (3.2%)) (Fig. S7), was detected in kitchen products. The emulsifier is a type of surfactant and is a substance that helps immiscible liquids, such as water and oil, mix well [83]. In addition, as one of the PFASs, 4,4,4-trifluorobutanal (CAS number 406-87-1, frequency 1/31 (3.2%)) (Fig. S8) was detected in bathroom/toilet products. According to Gluge et al., various PFAS are detected in cleaning products [84]. Also, palmitamidopropyl dimethylamine (CAS No. 39669-97-1, freq. 3/31 (9.7%)) (Fig. S9) detected in this study is one of the antistatic agents. Cleaning products are balanced blends of cleaning and surface care ingredients that create a “care film” that protects from chemical breakdown or abrasion to maintain and improve surfaces, and in some cases may have antistatic effects [85].
3.3. Characterization of Identified Compounds from the Suspect and Non-target ScreeningAll substances identified in the suspect and non-target screening are summarized in Table 2 (suspect screening) and Table 3 (non-target screening), along with their assigned confidence levels. Among the identified 99 substances, Confidence level 1 (confirmed structure) was assigned if the substance was identifiable based on a comparison with the reference standard among the screened substances. Information on the confidence level 1 substances compared to the reference standard is shown in Fig. 3, Fig. S10, and S11. 3 substances (tributyl citrate acetate, glyceryl monostearate and 2-(2-butoxyethoxy)ethyl acetate) were identified at a confidence level 1. Tributyl citrate acetate (retention time: 12.94 min) (Fig. 3), which is used as a plasticizer, was also detected in 13 of the 31 samples. Glyceryl monostearate (retention time: 15.43 min) (Fig. S10) is typically used as a surfactant and was detected in 5 of the 31 samples. 2-(2-Butoxyethoxy)ethyl acetate (RT: 6.75 min) (Fig. S11) is typically used as a solvent and was detected in 9 of the 31 samples.
Next, 45 substances, where no reference standards were available, were assigned to Confidence level 2 (probable structure) by available library spectra and the characteristic fragments during suspect screening (Table 2). In addition, 45 substances identified by non-target screening, although not conclusive MS fragments were identified, were assigned to Confidence level 3 (tentative candidates) (Table 3). Also, the chemical structures of 5 substances were confirmed based on the fragment information, but Confidence level 4 (unequivocal molecular formula) was assigned because insufficient evidence exists to propose possible structures (Table 3). Finally, one substance, which was identified as hexadecylamine (CAS No. 143-27-1, RT: 9.84 min) through library matching in the suspect-screening process, was assigned confidence level 5 (exact mass (m/z)). However, it was confirmed as a false positive because the retention time was different from the standard (RT: 10.83 min) (Fig. 4).
To provide a higher confidence level of data, it is believed that more standards should be purchased and compared to the identified substances.
3.4. Toxicity AssessmentECHA database can provide not only toxicity information but also substance regulatory information. The ECHA database was used to evaluate the toxicity of 98 substances identified in this study (suspect: 47 (except for confidence level 5) and non-target: 51). Among them, 26 identified substances were not included in the ECHA database, 35 substances were included in the ECHA database, but with no toxicity information. 31 substances were classified as “substances predicted as likely to meet the criteria for carcinogenicity, mutagenicity, or reproductive toxicity” in the ECHA database. Finally, 6 substances were included in the ECHA database, and have toxicities (Table S8), but do not meet the criteria for carcinogenicity, mutagenicity, or reproductive toxicity. Among 6 substances, octoxynol 7 (CAS No. 2497-59-8, freq. 4/31(12.9%)) is classified as an EDC, and tributyl citrate (CAS No. 77-94-1, freq. 3/31(9.7%)) has been listed under evaluation for EDCs.
For the assessment of the potential toxicity of the substances identified in the samples, Toxtree, a free QSAR tool, was used to determine the Cramer class of a chemical and estimate its relative toxic hazard [86]. In Toxtree, risk assessment for most human health effects is based on the threshold of a toxicological effect, usually derived from animal experiments. Toxtree was found to be a useful tool in facilitating the systematic evaluation of compounds through the Cramer scheme [87].
The Cramer classification scheme (decision tree) is the best-known approach for estimating the threshold of toxicity concern (TTC) of a chemical based on its chemical structure [88]. TTC concept can provide a means of waiving testing based on knowledge of exposure limits. This scheme was encoded into a software program called Toxtree, specifically commissioned by the European Chemicals Bureau (ECB). There are three Cramer classes, with Class 3 representing the most severe toxicity risk, and Class 3 compounds assigned the lowest TTC values. The classification criteria are listed in Table S9. The rule classified 58 substances as “highly toxic (Class 3)” and 27 substances as “low toxicity (Class 1)” based on their chemical structure. If the substances were found to be toxic in both evaluations (Toxtree: Cramer rules Class 3, ECHA: “substances predicted as likely to meet the criteria for carcinogenicity, mutagenicity, or reproductive toxicity”), they were classified as cautious substances.
Based on ECHA database and Toxtree tool, 20 substances (bisphenol A (2,3-dihydroxypropyl) glycidyl ether (BADGE-glycidyl), bis(oxiran-2-ylmethyl) cyclohex-4-ene-1,2-dicarboxylate, methyl 1,2,2,6,6-pentamethyl-4-piperidinyl sebacate, myristamide, myricetin, pentaerythritol triallyl ether, linalool oxide, (.+−.)-limonene, (Z)-N-Isopropyl-9-octadecenamide, (9Z)-N-methyl-9-octadecenamide, diethyl caprylamide, diisopropyl methylphosphonate, elaidamide, isostearamidopropyl dimethylamine, lauramide, methyl(trifluoromethyl)dioxirane, oleamide, palmitamidopropyl dimethylamine, Rionox MD-697(Naugard® XL-1), Succinylsulfathiazole) were classified as a substance of caution. These substances were classified as cautionary substances in both toxicity assessment tools (ECHA database and Toxtree) with a high level of toxicity (Table S8). Among 20 substances, myricetin [89], pentaerythritol triallyl ether [90], linalool oxide [91], (.+−.)-limonene [92], and diisopropyl methylphosphonate [92] are related to the consumer products based on literature reviews.
3.5. Risk AssessmentTo assess the potential risk by using cleaning products, we selected three substances (tributyl citrate acetate, glyceryl monostearate and 2-(2-butoxyethoxy)ethyl acetate; confidence level 1) which were identified with analytical standards, and the concentration of each compound was summarized in Table S10. The concentration of each substance was compared by comparing the response of each sample with the response of the standard calculated from LC-QTOF-MS analysis. Exposure risk evaluation was conducted for four product groups (bathroom/toilet, kitchen, glass, and multipurpose) for which dermal exposure coefficients could be obtained.
When the EDIs were evaluated by product group, they ranged from 1.71 × 10−4 to 1.08 × 10−3 (μg/kg bw/day) (Table 4). This is because the products are of the same type (spray), the amount of spray per application is similar, and the difference in concentration levels of each substance detected in the products is not significant. The HQ values for the substances within each product group were less than 1 (from 9.40 × 10−8 to 2.28 × 10−6 (μg/kg bw/day)), indicating that the potential hazards of these substances in each product group are low (Table 4).
However, since we only conducted the risk assessment using three substances having reference standards, and we did not perform the same approach on 20 substances classified as a substance of caution by the ECHA database and Toxtree tool. This is the limitation of this study. Future study is needed for the accurate risk assessment by comparing these 20 substances with reference standards to estimate the actual risk by exposing the substances in household cleaning products.
4. ConclusionsAmong the cleaning products frequently used in daily life, 31 spray type products were subjected to suspect and non-target screening by LC-QTOF-MS to identify the substances in the products. Based on the suspect screening process, plasticizers (freq. 23/31(74.2%)), surfactants (freq. 22/31(71.0%)), and antioxidants (freq. 20/31(64.5%)) were detected with high frequencies. From the non-target analysis, oleamide, a type of surfactant, was identified with the highest frequency of 22/31(71%). From toxicity evaluation using the ECHA database and Toxtree, among the 98 identified substances, 31 substances were classified as “substances predicted as likely to meet criteria for carcinogenicity, mutagenicity, or reproductive toxicity,” and 58 substances were classified as “highly toxic (Class 3).” 20 substances were classified as a substance of caution. Finally, a risk assessment was conducted for three substances identified with reference standards.
Our results indicate that household cleaning products contain various substances which can be toxic, in addition to listed general ingredients on the labels. Therefore, continuous monitoring of products containing these substances in consumer products is needed as future studies. A more complete risk assessment associated with exposure from the use of household cleaning products containing these substances is also needed by examining concentrations with more reference standards.
AcknowledgmentsThis work was supported by Korea Environment Industry & Technology Institute (KEITI) through Technology Development Project for Safety Management of Household Chemical Products Program (or Project), funded by Korea Ministry of Environment (MOE) (2021002970003, 1485019148(NTIS))
NotesConflict-of-Interest statement The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper. Author Contributions J.H.P (Ph.D. candidate) conducted the experiments and data processing and wrote the manuscript. H.J.Y. (Master candidate) conducted the experiments. C.Y. (Professor) concepted, received the funding, and supervised the research. K.L. (Professor) concepted and supervised the research. K.D.Z (Professor) conceived, supervised the research, wrote and revised the manuscript. References1. Kim JH, Kim TS, Yoon HJ, et al. Health risk assessment of dermal and inhalation exposure to deodorants in Korea. Sci. Total Environ. 2018;625:1369–1379.
https://doi.org/10.1016/j.scitotenv.2017.12.282
2. Lunny S, Nelson R, Steinemann A. Something in the air but not on the label: a call for increased regulatory ingredient disclosure for fragranced consumer products. Univ. NSW Law J. 2017;40:1366.
https://doi.org/10.53637/FZXH4269
3. Steinemann AC, Macgregor IC, Gordon SM, et al. Fragranced consumer products: Chemicals emitted, ingredients unlisted. Environ. Impact Assess. Rev. 2011;31(3)328–333.
https://doi.org/10.1016/j.eiar.2010.08.002
4. Li D, Suh S. Health risks of chemicals in consumer products: A review. Environ. Int. 2019;123:580–587.
https://doi.org/10.1016/j.envint.2018.12.033
5. Kortenkamp A, Faust M. Regulate to reduce chemical mixture risk. Science. 2018;361(6399)224–226.
https://doi.org/10.1126/science.aat9219
6. Park DU, Yang KW, Kim J, et al. Characteristics of the Molecular Weight of Polyhexamethylene Guanidine (PHMG) Used as a Household Humidifier Disinfectant. Molecules. 2021;26(15)4490.
https://doi.org/10.3390/molecules26154490
7. Shin HM, Moschet C, Young TM, Bennett DH. Measured concentrations of consumer product chemicals in California house dust: Implications for sources, exposure, and toxicity potential. Indoor Air. 2020;30(1)60–75.
https://doi.org/10.1111/ina.12607
8. Phillips KA, Yau A, Favela KA, et al. Suspect Screening Analysis of Chemicals in Consumer Products. Environ. Sci. Technol. 2018;52(5)3125–3135.
https://doi.org/10.1021/acs.est.7b04781
9. Moschet C, Anumol T, Lew BM, Bennett DH, Young TM. Household Dust as a Repository of Chemical Accumulation: New Insights from a Comprehensive High-Resolution Mass Spectrometric Study. Environ. Sci. Technol. 2018;52(5)2878–2887.
https://doi.org/10.1021/acs.est.7b05767
10. Krauss M, Singer H, Hollender J. LC–high resolution MS in environmental analysis: from target screening to the identification of unknowns. Anal. Bioanal. Chem. 2010;397(3)943–951.
https://doi.org/10.1007/s00216-010-3608-9
11. Wang CM. Development of Novel Methods of Analysis for Indoor Air Pollutants [dissertation]. NewYork: Univ. of NewYork; 2017.
12. Kim K. Variability and Temporal Trends of Semivolatile Organic Compounds in Biological and Environmental Media [dissertation]. Ann Arbor: Univ. of Texas; 2020.
13. Sardar SW, Choi Y, Park N, Jeon J. Occurrence and Concentration of Chemical Additives in Consumer Products in Korea. Int. J. Environ. Res. Public Health. 2019;16(24)5075.
https://doi.org/10.3390/ijerph16245075
14. Koreaministry of Environment. Living environment & safety information system [Internet]. Korea: 2022. [cited 01 November 2022]. Available from: http://ecolife.me.go.kr/ecolife/
15. Guo Y, Kannan K. A Survey of Phthalates and Parabens in Personal Care Products from the United States and Its Implications for Human Exposure. Environ. Sci. Technol. 2013;47(24)14442–14449.
https://doi.org/10.1021/es4042034
16. Targowski T. Out of concern for diligence in science--commentary to the paper of T. Gólczewski “Spirometry--comparison of Lubinski’s prediction equations for Polish population with ECSC/ERS and Falaschetti’s equations”. Adv. Respir. Med. 2012;80(2)186–8.
https://doi.org/10.5603/ARM.27604
17. Akamatsu M. HYDROPHOBICITY OF CHEMICAL COMPOUNDS INCLUDING PESTICIDES IN THE ENVIRONMENT. J. Environ. Sci. Sustainable Society. 2021;10:27–30.
https://doi.org/10.3107/jesss.10.MR07
18. Schymanski EL, Jeon J, Gulde R, et al. Identifying Small Molecules via High Resolution Mass Spectrometry: Communicating Confidence. Environ. Sci. Technol. 2014;48(4)2097–2098.
https://doi.org/10.1021/es5002105
19. Ccanccapa-Cartagena A, Pico Y, Ortiz X, Reiner EJ. Suspect, non-target and target screening of emerging pollutants using data independent acquisition: Assessment of a Mediterranean River basin. Sci. Total Environ. 2019;687:355–368.
https://doi.org/10.1016/j.scitotenv.2019.06.057
20. Gago-Ferrero P, Schymanski EL, Bletsou AA, Aalizadeh R, Hollender J, Thomaidis NS. Extended Suspect and Non-Target Strategies to Characterize Emerging Polar Organic Contaminants in Raw Wastewater with LC-HRMS/MS. Environ. Sci. Technol. 2015;49(20)12333–12341.
https://doi.org/10.1021/acs.est.5b03454
21. Hollender J, Schymanski EL, Singer HP, Ferguson PL. Nontarget Screening with High Resolution Mass Spectrometry in the Environment: Ready to Go? Environ. Sci. Technol. 2017;51(20)11505–11512.
https://doi.org/10.1021/acs.est.7b02184
22. Kaufmann A, Butcher P, Maden K, Walker S, Widmer M. Using In Silico Fragmentation to Improve Routine Residue Screening in Complex Matrices. J. Am. Soc. Mass Spectrom. 2017;28(12)2705–2715.
https://doi.org/10.1007/s13361-017-1800-2
23. Zedda M, Zwiener C. Is nontarget screening of emerging contaminants by LC-HRMS successful? A plea for compound libraries and computer tools. Anal. Bioanal. Chem. 2012;403(9)2493–2502.
https://doi.org/10.1007/s00216-012-5893-y
24. Koo HJ, Lee BM. Estimated exposure to phthalates in cosmetics and risk assessment. J. TOXICOL. ENV. HEALTH PT A. 2004;67(23–24)1901–1914.
https://doi.org/10.1080/15287390490513300
25. Envbigdata. Household Chemical Product Exposure Information [Internet]. Korea: 2022. [cited 20 Octber 2022]. Available from: https://www.bigdata-environment.kr/user/data_market/detail.do?id=72ae64b0-11d5-11eb-bc79-3b11eb915d6d
26. EPA. Risk Characterization Handbook [Internet]. USA: 2022. [cited 15 Octber 2022]. Available from: https://www.epa.gov/risk/risk-characterization-handbook
27. European Commission. SCIENTIFIC COMMITTEE ON TOXICITY, ECOTOXICITY AND THE ENVIRONMENT (CSTEE) [Internet]. European Union; 2022. [cited 21 Octber 2022]. Available from: https://ec.europa.eu/health/ph_risk/committees/sct/documents/out45_en.pdf
28. ECHA. 2-(2-butoxyethoxy)ethyl acetate [Internet]. European Union; 2022. [cited 21 Octber 2022]. Available from: https://echa.europa.eu/registration-dossier/-/registered-dossier/14826
29. Tang S, Chen Y, Song G, Liu X, Shi Y, Xie Q, Chen D. A cocktail of industrial chemicals in lipstick and nail polish: Profiles and health implications. Environ. Sci. Technol. Lett. 2021;8(9)760–765.
https://doi.org/10.1021/acs.estlett.1c00512
30. Lerner I. Plasticizers to reach $8 billion by 2004. 1stLook Smart: ProQuest; 2003. p. 26.
31. Kim DY, Chun SH, Jung Y, et al. Phthalate Plasticizers in Children’s Products and Estimation of Exposure: Importance of Migration Rate. Int. J. Environ. Res. Public Health. 2020;17(22)8582.
https://doi.org/10.3390/ijerph17228582
32. Rahman MBrazel CS. The plasticizer market: an assessment of traditional plasticizers and research trends to meet new challenges. Prog. Polym. Sci. 2004;29(12)1223–1248.
https://doi.org/10.1016/j.progpolymsci.2004.10.001
33. Matsumoto M, Hirata Koizumi M, Ema M. Potential adverse effects of phthalic acid esters on human health: a review of recent studies on reproduction. Regul Toxicol Pharmacol. 2008;50(1)37–49.
https://doi.org/10.1016/j.yrtph.2007.09.004
34. Kawakami T, Isama K, Jinno H. Skin transferability of phthalic acid ester plasticizers and other plasticizers using model polyvinyl chloride sheets. J. Environ. Sci. Health Part A-Toxic/Hazard. Subst. Environ. Eng. 2020;55(10)1163–1172.
https://doi.org/10.1080/10934529.2020.1795503
35. He PX, Ling Y, Yong W, et al. Determination of 22 alternative plasticizers in wrap film by solid phase extraction and ultra-high performance supercritical fluid chromatography-tandem mass spectrometry. J. Chromatogr. A. 2022;1669:462916.
https://doi.org/10.1016/j.chroma.2022.462916
36. ECHA. Tributyl O-acetylcitrate [Internet]. European Union; 2022. [cited 10 October 2022]. Available from: https://echa.europa.eu/da/substance-information/-/substanceinfo/100.000.971
37. Woong K, Gye MC. Maleficent Effects of Phthalates and Current States of Their Alternatives: A Review. Korean Soc. Environ. Biol. 2017;35(1)21–36.
https://doi.org/10.11626/KJEB.2017.35.1.021
38. Chiellini F, Ferri M, Morelli A, Dipaola L, Latini G. Perspectives on alternatives to phthalate plasticized poly(vinyl chloride) in medical devices applications. Prog. Polym. Sci. 2013;38(7)1067–1088.
https://doi.org/10.1016/j.progpolymsci.2013.03.001
39. Malnes D, Ahrens L, Köhler S, Forsberg M, Golovko O. Occurrence and mass flows of contaminants of emerging concern (CECs) in Sweden's three largest lakes and associated rivers. Chemosphere. 2022;294:133825.
https://doi.org/10.1016/j.chemosphere.2022.133825
40. Lee YS, Lee S, Lim JE, Moon HB. Occurrence and emission of phthalates and non-phthalate plasticizers in sludge from wastewater treatment plants in Korea. Sci. Total Environ. 2019;692:354–360.
https://doi.org/10.1016/j.scitotenv.2019.07.301
41. Deshwal GK, Panjagari NR. Review on metal packaging: materials, forms, food applications, safety and recyclability. J. Food Sci. Technol.-Mysore. 2020;57(7)2377–2392.
https://doi.org/10.1007/s13197-019-04172-z
42. Sadik T, Massardier V, Becquart F, Taha M. Radical grafting of polar monomers onto polypropylene by reactive extrusion. J. Appl. Polym. Sci. 2013;129(4)2177–2188.
https://doi.org/10.1002/app.38942
43. Laura A, Cecilia HS, Mark S. Greener Solutions U. STEELCASE FINAL REPORT. UC Berkeley. 2016;
https://bcgc.berkeley.edu/sites/default/files/steelcase_report_final-2016.pdf
44. Polefka TG. Surfactant interactions with skin. 1stTaylor & Francis Group: CRC Press; 1999. p. 433–468.
45. Clint JH. Surfactant aggregation. 1stSpringer Science & Business Media; 2021. p. 1–283.
46. Efsa Panel on Food Contact Materials E, FlavouringsAids P. Scientific Opinion on the safety evaluation of the substance N,N-bis(2-ydroxyethyl)dodecanamide, CAS No. 120-40-1, for use in food contact materials. EFSA Journal. 2010;8(10)1837.
https://doi.org/10.2903/j.efsa.2010.1837
47. PubChem. N, N-Bis(2-hydroxyethyl)dodecanamide [Internet]. 2022. [cited 21 October 2022]. Available from: https://pubchem.ncbi.nlm.nih.gov/compound/N_N-Bis_2-hydroxyethyl_dodecanamide
48. ECHA. 3,6,9-trioxaundecane-1,11-diol [Internet]. European Union; 2022. [cited 11 October 2022]. Available from: https://echa.europa.eu/da/substance-information/-/substanceinfo/100.003.627
49. Schladt L, Ivens I, Karbe E, Rühl Fehlert C, Bomhard E. Subacute oral toxicity of tetraethylene glycol and ethylene glycol-administered to Wistar rats. Exp. Toxicol. Pathol. 1998;50(3)257–265.
https://doi.org/10.1016/S0940-2993(98)80096-1
50. Nimrod AC, Benson WH. Environmental estrogenic effects of alkylphenol ethoxylates. Crit. Rev. Toxicol. 1996;26(3)335–364.
https://doi.org/10.3109/10408449609012527
51. Johnson JW. Final report on the safety assessment of octoxynol-1, octoxynol-3, octoxynol-5, octoxynol-6, octoxynol-7, octoxynol-8, octoxynol-9, octoxynol-10, octoxynol-11, octoxynol-12, octoxynol-13, octoxynol-16, octoxynol-20, octoxynol-25, octoxynol-30, octoxynol-33, octoxynol-40, octoxynol-70, octoxynol-9 carboxylic acid, octoxynol-20 carboxylic acid, potassium octoxynol-12 phosphate, sodium octoxynol-2 ethane sulfonate, sodium octoxynol-2 sulfate, sodium octoxynol-6 sulfate, and sodium octoxynol-9 sulfate. Int. J. Toxicol. 2004;23:59–111.
https://doi.org/10.1080/10915810490274306
52. Liu R, Mabury SA. Synthetic Phenolic Antioxidants in Personal Care Products in Toronto, Canada: Occurrence, Human Exposure, and Discharge via Greywater. Environ. Sci. Technol. 2019;53(22)13440–13448.
https://doi.org/10.1021/acs.est.9b04120
53. Wang W, Asimakopoulos AG, Abualnaja KO, et al. Synthetic Phenolic Antioxidants and Their Metabolites in Indoor Dust from Homes and Microenvironments. Environ. Sci. Technol. 2016;50(1)428–434.
https://doi.org/10.1021/acs.est.5b04826
54. Zhang ZF, Zhang X, Sverko E, et al. Determination of Diphenylamine Antioxidants in Wastewater/Biosolids and Sediment. Environ. Sci. Technol. Lett. 2020;7(2)102–110.
https://doi.org/10.1021/acs.estlett.9b00796
55. Liu R, Mabury SA. Synthetic Phenolic Antioxidants: A Review of Environmental Occurrence, Fate, Human Exposure, and Toxicity. Environ. Sci. Technol. 2020;54(19)11706–11719.
https://doi.org/10.1021/acs.est.7b05493
56. Liu R, Mabury SA. Synthetic Phenolic Antioxidants and Transformation Products in Human Sera from United States Donors. Environ. Sci. Technol. Lett. 2018;5(7)419–423.
https://doi.org/10.1021/acs.estlett.8b00223
57. Du B, Zhang Y, Lam JCW, et al. Prevalence, Biotransformation, and Maternal Transfer of Synthetic Phenolic Antioxidants in Pregnant Women from South China. Environ. Sci. Technol. 2019;53(23)13959–13969.
https://doi.org/10.1021/acs.est.9b04709
58. Wang W, Kannan K. Quantitative identification of and exposure to synthetic phenolic antioxidants, including butylated hydroxytoluene, in urine. Environ. Int. 2019;128:24–29.
https://doi.org/10.1016/j.envint.2019.04.028
59. Liu R, Mabury SA. Unexpectedly high concentrations of 2,4-di-tert-butylphenol in human urine. Environ. Pollut. 2019;252:1423–1428.
https://doi.org/10.1016/j.envpol.2019.06.077
60. Murawski A, Schmied Tobies MIH, Rucic E, et al. Metabolites of 4-methylbenzylidene camphor (4-MBC), butylated hydroxytoluene (BHT), and tris(2-ethylhexyl) trimellitate (TOTM) in urine of children and adolescents in Germany – human bio-monitoring results of the German Environmental Survey GerES V (2014–2017). Environ. Res. 2021;192:110345.
https://doi.org/10.1016/j.envres.2020.110345
61. Blanco Zubiaguirre L, Zabaleta I, et al. Target and suspect screening of substances liable to migrate from food contact paper and cardboard materials using liquid chromatography-high resolution tandem mass spectrometry. Talanta. 2020;208:120394.
https://doi.org/10.1016/j.talanta.2019.120394
62. ChEBI. 2,6-di-tert-butyl-4-methylphenol [Internet]. 2022. [cited 15 October 2022]. Available from: https://www.ebi.ac.uk/chebi/searchId.do?chebiId=CHEBI:34247
63. Wolff MS, Teitelbaum SL, Mcgovern K, et al. Environmental phenols and pubertal development in girls. Environ. Int. 2015;84:174–180.
https://doi.org/10.1016/j.envint.2015.08.008
64. Li B, Liu R, Gao H, Tan R, Zeng P, Song Y. Spatial distribution and ecological risk assessment of phthalic acid esters and phenols in surface sediment from urban rivers in Northeast China. Environ. Pollut. 2016;219:409–415.
https://doi.org/10.1016/j.envpol.2016.05.022
65. Stapleton HM, Allen JG, Kelly SM, et al. Alternate and New Brominated Flame Retardants Detected in U.S. House Dust. Environ. Sci. Technol. 2008;42(18)6910–6916.
https://doi.org/10.1021/es801070p
66. Liu X, Ji K, Choi K. Endocrine disruption potentials of organophosphate flame retardants and related mechanisms in H295R and MVLN cell lines and in zebrafish. Aquat. Toxicol. 2012;114–115:173–181.
https://doi.org/10.1016/j.aquatox.2012.02.019
67. Carignan CC, Mínguez Alarcón L, Butt CM, et al. Urinary concentrations of organophosphate flame retardant metabolites and pregnancy outcomes among women undergoing in vitro fertilization. Environ. Health Perspect. 2017;125(8)087018.
https://doi.org/10.1289/EHP1021
68. Butt CM, Congleton J, Hoffman K, Fang M, Stapleton HM. Metabolites of organophosphate flame retardants and 2-ethylhexyl tetrabromobenzoate in urine from paired mothers and toddlers. Environ. Sci. Technol. 2014;48(17)10432–10438.
https://doi.org/10.1021/es5025299
69. Steinemann A. International prevalence of fragrance sensitivity. Air Qual. Atmos. Health. 2019;12(8)891–897.
https://doi.org/10.1007/s11869-019-00699-4?fbclid=IwAR04XzXzRI3n96axeqpl3S9rQULhth1iuZpAyUqwyFQHjWWg3Xl0zWm8AjE
70. Strugnell C, Jones L. Consumer perceptions and opinions of fragrances in household products. Nutr. Food Sci. 1999;99(4)
https://doi.org/10.1108/nfs.1999.01799daf.002
71. Moss HV. Allergic contact dermatitis due to Halotex solution. Arch. Dermatol. 1974;109(4)572–572.
https://doi.org/10.1001/archderm.1974.01630040076027
72. Hirao A, Oiso N, Hama M, Higashimori N, Tatsumi YKawada A. Allergic Contact Dermatitis from Diethyl Sebacate in a Topical Antimycotic Medicament. dermatitis. 2012;1:6.
http://dx.doi.org/10.4236/jcdsa.2012.23040
73. EPA. Cleaning products and household care [Internet]. USA: 2022. [cited 01 November 2022]. Available from: https://comptox.epa.gov/dashboard/search-results?input_type=puc&inputs=Cleaning%20products%20and%20household%20care.
74. Ministry of Environment and Food. Survey and health assessment of UV filters. [Internet]. 2015. [cited 02 November 2022]. Available from: https://www2.mst.dk/udgiv/publications/2015/10/978-87-93352-82-7.pdf
75. Scridb company. Ciba specialty chemicals. Additives for Polyurethane [Internet]. 2022. [cited 13 October 2022]. Available from: https://www.slideshare.net/sprados50/additivespolyurethane
76. Yaras A, Nodehi M, Ustaoglu A, et al. Cleaner production of polyurethane (PU) foams through use of hydrodesulfurization (HDS) spent catalyst. Environ. Sci. Pollut. Res. 2022;
https://doi.org/10.1007/s11356-022-21837-z
77. Fang H, Zhao P, Zhang C, et al. A cleaner polyurethane elastomer grouting material with high hardening strain for the fundamental rehabilitation: The comprehensive mechanical properties study. Constr. Build. Mater. 2022;318:125951.
https://doi.org/10.1016/j.conbuildmat.2021.125951
78. Lu J, Liao C, Cheng L, Jia P, Yin Z, Song L, Wang B, Hu Y. Cleaner production to a multifunctional polyurethane sponge with high fire safety and low toxicity release. J. Clean Prod. 2022;333:130172.
https://doi.org/10.1016/j.jclepro.2021.130172
79. EPA. TSCA New Chemicals Program (NCP) Chemical Categories [Internet]. USA: 2022. [cited 20 October 2022]. Available from: https://www.epa.gov/sites/default/files/2014-10/documents/ncp_chemical_categories_august_2010_version_0.pdf
80. WayBack Machine. Surfactants : Westco Oleamide a Slip Agent. Polyethylene Films [Internet]. 2022. [cited 31 October 2022]. Available from: https://web.archive.org/web/20070127054121/http://www.wrchem.com/Products/OLEAMIDE.htm
81. Peng J, Zhao Y, Hong Y, et al. Chemical Identity and Mechanism of Action and Formation of a Cell Growth Inhibitory Compound from Polycarbonate Flasks. Anal. Chem. 2018;90(7)4603–4610.
https://doi.org/10.1021/acs.analchem.7b05102
82. Cocamide D. Final report on the safety assessment of cocamide DEA, lauramide DEA, linoleamide DEA, and oleamide DEA. J. Am. Coll. Toxicol. 1986;5(5)
http://www.cir-safety.org/sites/default/files/118_draft_dea_suppl1.pdf
84. Glüge J, Scheringer M, Cousins IT, et al. An overview of the uses of per-and polyfluoroalkyl substances (PFAS). Environ. Sci.: Processes Impacts. 2020;22(12)2345–2373.
https://doi.org/10.1039/D0EM00291G
85. Wolkoff P, Schneider T, Kildesø J, Degerth R, Jaroszewski M, Schunk H. Risk in cleaning: chemical and physical exposure. Sci. Total Environ. 1998;215(1)135–156.
https://doi.org/10.1016/S0048-9697(98)00110-7
86. Tisler S, Tüchsen PL, Christensen JH. Non-target screening of micropollutants and transformation products for assessing AOP-BAC treatment in groundwater. Environ. Pollut. 2022;309:119758.
https://doi.org/10.1016/j.envpol.2022.119758
87. Patlewicz G, Jeliazkova N, Safford RJ, Worth AP, Aleksiev B. An evaluation of the implementation of the Cramer classification scheme in the Toxtree software. SAR QSAR Environ. Res. 2008;19(5–6)495–524.
https://doi.org/10.1080/10629360802083871
88. ChemSafetyPro. Introduction to Threshold of Toxicological Concern (TTC) Approach in Chemical Risk Assessment [Internet]. 2022. [cited 05 October 2022]. Available from: http://www.chemsafetypro.com/Topics/CRA/Introduction_to_Threshold_of_Toxicological_Concern_(TTC)_Approach_in_Chemical_Risk_Assessment.html
89. Hamdi H, Abid ES, Eyer J. Neuroprotective effects of Myricetin on Epoxiconazole-induced toxicity in F98 cells. Free Radic. Biol. Med. 2021;164:154–163.
https://doi.org/10.1016/j.freeradbiomed.2020.12.451
90. Patil A, Ferritto MS. Polymers for personal care and cosmetics: Overview. Polymers for personal care and cosmetics. 2013;3–11.
https://doi.org/10.1021/bk-2013-1148.ch001
91. Kern S, Dkhil H, Hendarsa P, Ellis G, Natsch A. Detection of potentially skin sensitizing hydroperoxides of linalool in fragranced products. Anal. Bioanal. Chem. 2014;406(25)6165–6178.
https://doi.org/10.1007/s00216-014-8066-3
92. Tomkins BA, Griest WH, Hearle DR. Determination of small dialkyl organophosphonates at microgram/L concentrations in contaminated groundwaters using multiple extraction membrane disks. Anal. Lett. 1997;30(9)1697–1717.
https://doi.org/10.1080/00032719708001688
Fig. 1UNIFI platform interface used in this study (ex. Luperox 101: CAS No. 78-63-7) (upper left: Total Ion Chromatogram (TIC) and lower left: extracted Ion Chromatogram (IC) for the selected component) 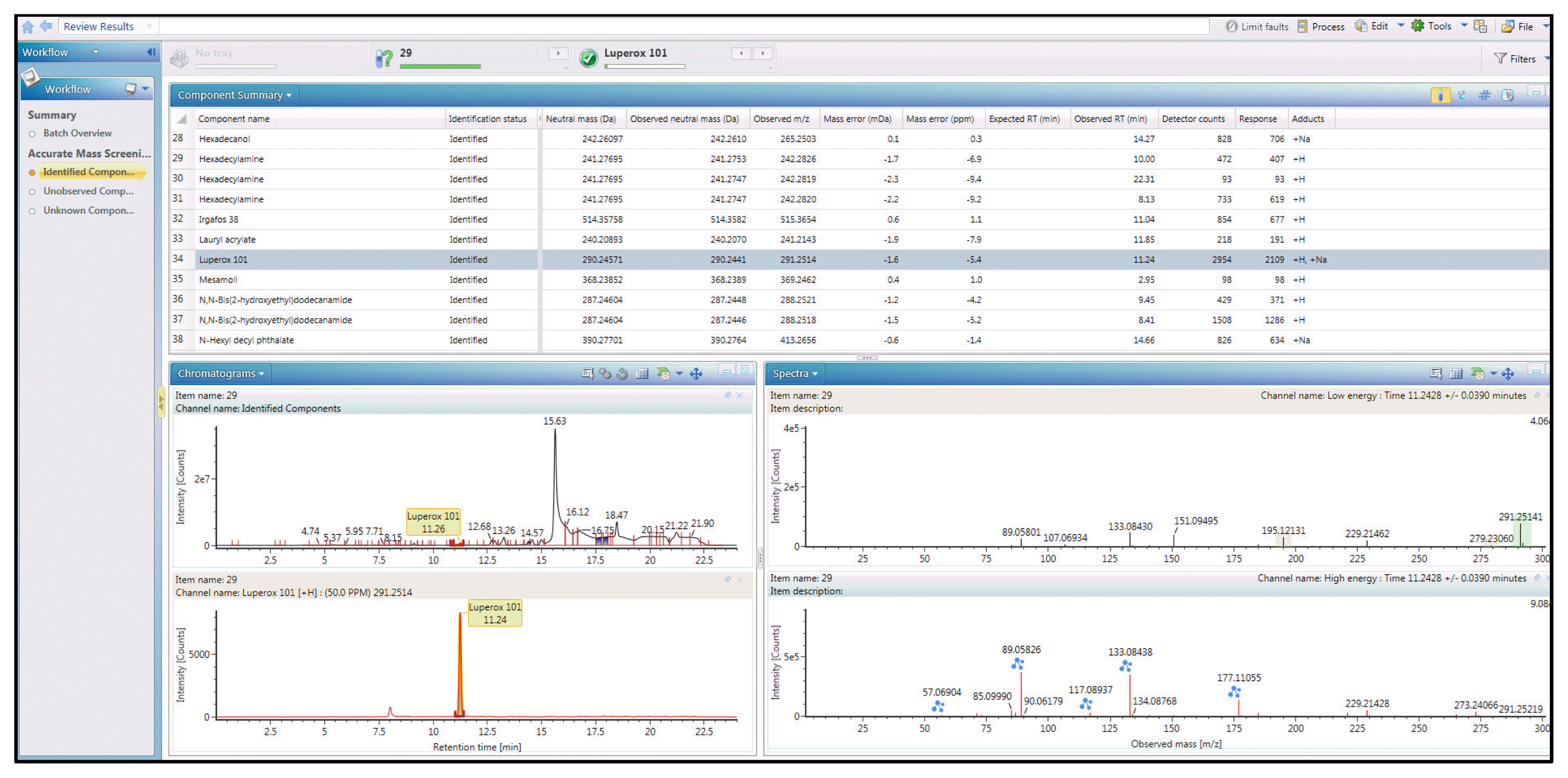
Fig. 3Comparison of the spectrum (bottom) between the identified substance (sample No. 20) and the reference standard (top) (Tributyl citrate acetate, CAS No. 77-90-7) 
Fig. 4False positive peak identified as hexadecylamine in the suspected screening by the Waters library (Reference standard (top, RT: 10.83) vs. No. 31 Sample (bottom, RT: 9.84 min)) 
Table 1Proposed identification confidence levels [18] Table 2Substances identified in the suspect screening
Table 3Substances identified in non-target screening Table 4Estimated daily intake (EDI), hazard quotient (HQ) of three substances identified by reference standards
|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||