AbstractAdsorption using solid adsorbents is a promising technique for capturing carbon dioxide (CO2) to reduce greenhouse gas emission. In the present work, palm shell-based activated carbon was functionalized with eco-friendly activating agents including potassium carbonate, potassium acetate, binary deep eutectic solvent (DES) composed of choline chloride and ethylene glycol, and ternary DES composed of choline chloride, urea and ethylene glycol by impregnation method. Post-combustion CO2 adsorption performance of the functionalized adsorbents was evaluated in a fixed-bed adsorption column under varying adsorption temperature (25–55°C) and inlet CO2 concentration (15–20%), followed by a cyclic CO2 adsorption study to determine the regeneration ability of the adsorbents. The results revealed that activated carbon modified with potassium acetate (ACPA) exhibited remarkably high CO2 adsorption capacity of 116.5 mg/g and breakthrough time of 54 min at 25°C and 15% inlet CO2 concentration. Furthermore, ACPA demonstrated good regeneration ability even after seven adsorption-desorption cycles. Interestingly, it was found that activated carbon modified with ternary DES (AC-DES 4) exhibited significantly higher adsorption capacity than activated carbon modified with binary DES (AC-DES 2). It is worth mentioning that the present work is the first study that uses ternary DES as activating agent for such purpose.
Graphical Abstract
1. IntroductionThe steady increase in greenhouse gas concentration in the atmosphere is one of the critical factors causing climate change and global warming since the Industrial Revolution. Carbon dioxide (CO2) accounts for the most significant fraction of greenhouse gas emission in the atmosphere. The major factor which contributes to increasing CO2 emission is anthropogenic activities through burning of fossil fuels to meet growing energy demand. Hence, reducing global CO2 emission is crucial to mitigate the impact of climate change in alignment with the United Nations’ Sustainability Development Goals (UN SDGs). CO2 accounts for 81% of the total greenhouse gas emission, reaching 5,425 million metric tons of CO2 equivalent in 2018 [1]. The global average concentration of atmospheric CO2 in December 2022 is 419 parts per million (ppm). This figure is significantly higher than that before the industrial revolution started in the mid-1700s where atmospheric CO2 concentration was 280 ppm [2]. SDG 13 and the Paris Agreement are among the most significant global responses to mitigate the threat of climate change. In 2015, the UN established SDG 13 which urges the public to take various climate-positive actions to combat the impact of climate change through regulating greenhouse gas emission [3]. As a signatory to the Paris Agreement, Malaysia aims to cut down CO2 emission at national level by 45% in 2030 relative to 2005 level [4]. One of the well-known technologies to reduce CO2 emission is through Carbon Capture, Storage and/or Utilization (CCSU) [5, 6]. There are many CO2 capture techniques available, including absorption [7], adsorption [8] and membrane separation [9]. Among all the CO2 capture techniques, adsorption using solid porous materials has attracted attention as the most promising technique for CO2 capture. It presents many potential advantages including low energy consumption for adsorbent regeneration, low cost of porous material, high selectivity and adsorption capacity, fast kinetics, ease of handling, and high thermal stability [6, 10, 11]. There are several types of adsorbents available for CO2 adsorption, for instance, activated carbon [12], zeolite [13], molecular sieve [13], metal oxides [14] and graphene [14–16]. Each type of adsorbent has its own limitation and shortcomings. Among all, activated carbon is a promising option for the application of CO2 capture due to low cost, high porosity and favorable surface chemistry which contributes to significant CO2 adsorption capacity [17].
Typically, activated carbon is prepared from fossil fuels such as petroleum coke which is a non-renewable resource [5, 8]. Due to sustainability considerations, there is a growing trend of using biomass materials and agricultural wastes as raw material to synthesize activated carbon [8]. For instance, palm shell is a suitable raw material for producing activated carbon in Malaysia due to the abundance of oil palm in the country. Activated carbon is usually synthesized by chemical activation whereby a chemical activating agent is impregnated onto the carbon precursor, and subsequently the impregnated solid undergoes thermal treatment followed by a washing step [18]. The selection of chemical activating agent is important in the synthesis and modification of activated carbon. Activating agents have great influence on pore development and the enhancement of surface characteristics of activated carbon. Surface area, porosity and surface chemistry between adsorbent and adsorbates are among the most significant factors which determine the high adsorption performance of adsorbent. The regeneration performance of the modified activated carbon allows us to assess the viability of using such adsorbent for large-scale application of CO2 adsorption in the industry. Conventional chemical activating agents such as potassium hydroxide (KOH), sodium hydroxide (NaOH), phosphoric acid (H3PO4) and zinc chloride (ZnCl2) are known to yield high CO2 adsorption capacity for activated carbon [19]. However, these conventional activating agents have intrinsic shortcomings. For example, KOH, NaOH and H3PO4 are corrosive whereas KOH and ZnCl2 are hazardous to the environment [19]. Improper disposal of KOH and ZnCl2 to the surroundings without treatment would cause environmental pollution. Due to the environmental hazards presented by the conventional activating agents, there is a need to conduct a more thorough study on eco-friendly chemical activating agents for the activation of porous carbon for CO2 adsorption.
Potassium carbonate (K2CO3) and potassium acetate (CH3COOK) are potential substitutes for KOH due to low toxicity and reduced environmental impact [20]. The intercalation of metallic potassium ions into carbon network during activation contributes to well-developed porosity in the activated carbon. K2CO3 and CH3COOK have been used in the activation of porous carbon for various adsorption purposes such as dye removal and metal ions removal. However, there are very few published works regarding the use of K2CO3 and CH3COOK activated carbon for CO2 capture [18, 20, 21]. Deep eutectic solvents (DESs) are other examples of environmentally friendly chemicals that show great potential as activating agents [20]. To the best of our knowledge, there are only few studies use green solvent (DESs) as activating agent to enhance the surface properties of porous carbon for CO2 adsorption. Therefore, the area of concern of this research would be the application of green solvent as activating agents in the modification of activated carbon for CO2 adsorption.
DES is a green solvent that has received much attention for its potential in various applications such as biodiesel treatment, metal electropolishing and synthesis application. As a sustainable alternative to the conventional ionic liquids, DESs presents many advantages such as high biodegradability, low toxicity, low cost, easy preparation, high recyclability and high thermal stability [21, 22]. The preparation of DESs involves mixing a hydrogen bond acceptor (HBA) and a hydrogen bond donor (HBD) to form liquid-phase eutectics at ambient condition [21]. Typical HBAs are quaternary salts such as cholinium, phosphonium or ammonium-based halide salts, whereas common examples of HBDs are carboxylic acid, urea, ethylene glycol, triethylene glycol, glycerol and carbohydrates of different types [21–24]. Similar to ionic liquids, DESs are designable by changing the combination of HBAs and HBDs. Different combinations of HBAs and HBDs would have different physical and chemical properties for different specific applications [24–25]. The conventional DESs consist of two single components, hence they are known as binary DESs. Introducing a third component (another HBD) results in the formation of ternary DESs which have enhanced properties [25]. DESs could be used as chemical activating agents in the modification of activated carbon. Based on the published articles, most of the DESs used for CO2 capture by adsorption on the activated carbon are choline chloride based or ammonium based binary DESs. There were only a few studies on the use of DES in the modification of activated carbon for CO2 adsorption. The DES combinations which have been studied are choline chloride-glycerol mixture, choline chloride-urea mixture, tetra-n-butyl ammonium bromide-glycerol mixture, choline hydroxide-urea mixture and choline hydroxide-glycerol mixture [25, 26]. More studies are therefore required to develop activated carbon using different combinations of binary and ternary DESs as novel activating agents, with special interest on ternary DESs.
To the best of our knowledge, the influence of various environmentally-friendly activating agents to evaluate breakthrough adsorption performance has not been fully studied. In addition, to date, no research works have reported the application of ternary DES in the activation of porous carbon derived from palm shell waste for CO2 adsorption. Therefore, there is a need to conduct a more thorough study on eco-friendly activating agents for the modification of activated carbon derived from palm shell waste for capturing CO2.
In the present study, K2CO3, CH3COOK, binary DES (composed of choline chloride and ethylene glycol), and ternary DES (composed of choline chloride, urea and ethylene glycol) were used as activating agents for the modification of palm shell activated carbon through impregnation method. Sample characterization was conducted to examine the surface morphology, elemental composition and textural properties of the functionalized adsorbents. The adsorption performance of each adsorbent was evaluated in terms of CO2 adsorption capacity and breakthrough time under varying adsorption temperature (25–55°C) and inlet CO2 concentration (15–20%) using a fixed-bed continuous adsorption unit. Furthermore, cyclic CO2 adsorption study was conducted to gauge the regeneration ability of the adsorbents.
2. Materials and Methods2.1. Preparation of Raw MaterialsPotassium carbonate (≥99%), potassium acetate (≥99%), choline chloride (≥98%), ethylene glycol (≥98%) and urea (≥99 %) are purchased from Sigma Aldrich. Purified nitrogen (99.99%) and carbon dioxide (99.8%) are purchased from Alpha Gas Solution Sdn. Bhd., Malaysia. Palm shell activated carbon produced by steam activation is purchased from Biotek Abadi Sdn. Bhd., Malaysia. The particle size of activated carbon is 8 to 20 mesh.
2.2. Preparation of AdsorbentsThe procedure description in this section consists of two parts. The first part is about impregnation of activated carbon with K2CO3 and CH3COOK, whereas the second part is about impregnation of activated carbon with binary and ternary DESs. To begin with, stock solution of 1 M K2CO3 and 1 M CH3COOK are prepared in volumetric flasks by dilution method. Raw activated carbon is washed and dried overnight in the oven. The activated carbon is impregnated with 1 M K2CO3 and 1M CH3COOK solution in two separate beakers at impregnation ratio of 1:2 (wt.%) for 24 hours at room temperature. The functionalized adsorbents are filtered and washed with deionized water to remove leftover chemicals. The adsorbents are then dried in the oven at 150°C for 8 hours. The products are labelled as ACPC and ACPA (activated carbon modified with K2CO3 and CH3COOK) respectively.
On the other hand, prior to the impregnation of raw activated carbon with binary and ternary DES, it is required to synthesize the DES. To begin with, choline chloride (ChCl) and urea (U) are pre-dried overnight at 45°C in the vacuum oven to remove moisture. Each HBA is mixed with respective HBD according to the molar ratio listed in Table S1. The mixtures of HBA and HBD are heated at 80°C for 2 hours while being stirred at 350 rpm using magnetic stirrers until homogeneous solution is observed without any precipitates. The DESs formed are dried overnight in the vacuum oven to remove moisture and kept in well-sealed cups in a desiccator for later use. Only DESs that remain liquid phase are chosen for impregnation. Using a similar procedure as written earlier, raw activated carbon is impregnated with the chosen DESs in separate beakers at impregnation ratio of 1:2 (wt.%) for 24 hours at room temperature. The functionalized adsorbents are filtered and washed with deionized water to remove leftover chemicals. The adsorbents are then dried in an oven at 150°C for 5 hours. The products are labelled as AC-x, where x denotes the abbreviation listed in Table S1. Besides, the viscosity of DES samples is measured using a viscometer (Model: IKA ROTAVISC lo-vi).
2.3. Evaluation of CO2 Adsorption Performance
Fig. 1 shows a laboratory-scale fixed bed CO2 adsorption unit for the evaluation of adsorption performance of solid adsorbents. There are six major components. Label 1 is digital mass flow controllers whereas label 2 is data logger. Label 3 is an adsorption column whereas label 4 is rotameter. Label 5 is the CO2 analyzer whereas label 6 is temperature controllers.
CO2 adsorption study is conducted using a fixed-bed adsorption column with 15 mm inner diameter and 350 mm effective length, surrounded with heating jackets used to maintain column temperature. The column temperature is adjusted using temperature controllers. Feed stream is a mixture of CO2 and nitrogen. The feed flow rate and inlet CO2 concentration are adjusted using digital mass flow controllers. The parameters of this adsorption study are adsorption temperature and inlet CO2 concentration whereas the variables of interest are CO2 adsorption capacity and breakthrough time. To achieve this, the CO2 adsorption performance for each adsorbent is evaluated at different adsorption temperature (25 to 55°C) and different inlet CO2 concentration (15 to 20%) while maintaining the other parameters including pressure (atmospheric pressure, 1 atm) and inlet flow rate (200 mL/min). The mass of adsorbent used for breakthrough adsorption study was 25g. The outlet CO2 concentration is continuously measured and recorded using a CO2 analyzer (Model: Alpha Omega Instruments Series 9610) equipped with a data logger (Model: Graphtec GL820). The adsorption process is continued until it reaches the saturation point where the outlet concentration of CO2 (Ct) equals the inlet concentration of CO2 (C0). Breakthrough curves are plotted for each adsorbent at each operating condition using the outlet CO2 concentration data recorded by the data logger. A breakthrough curve is a plot of Ct/C0 against time. The duration for which Ct/C0 remain zero is taken as breakthrough time whereas the time at which Ct/C0 = 1 is taken as saturation time. The CO2 adsorption capacity and breakthrough time of each adsorbent at different operating conditions are tabulated for data analysis.
2.4. Regeneration StudyBased on the CO2 adsorption and breakthrough performance, the adsorbent which yields the highest CO2 adsorption capacity and longest breakthrough time is selected for regeneration study. The adsorption procedure is conducted as mentioned in the earlier section. After completion of each adsorption process, the adsorbent is purged under inert nitrogen gas flow. The introduction of nitrogen gas reduces the partial pressure of CO2 in the adsorption column, thus favoring the CO2 desorption process. Regeneration was performed under flowing N2 at a temperature of 90°C and 180°C for 10 min. The adsorption-desorption procedure is repeated over seven cycles. For each cycle of regeneration study, the outlet CO2 concentration data from the data logger is used to determine the CO2 adsorption capacity and breakthrough time. The result of regeneration study is tabulated to analyze the regeneration potential of the adsorbent.
2.5. Sample CharacterizationThe surface morphology and elemental composition of the adsorbents were analyzed using Scanning Electron Microscopy (SEM) and Energy Dispersive X-ray Spectroscopy (EDX) equipment (Model: Tescan VEGA-3). The textural properties of the adsorbents such as surface area, micropore volume and pore width are analyzed through nitrogen adsorption-desorption studies at 77 K employing the Micromeritics ASAP 2020 instrument. Surface area and average pore width of the adsorbents are determined by applying Brunauer-Emmett-Teller (BET) method whereas micropore volume is obtained using t-plot method.
3. Results and Discussion3.1. Selection of DES for Adsorption Study
Table S2 shows the physical state of each DES mixture at ambient condition. It is observed that DES 1 and DES 3 exist in heterogeneous phase containing liquid and crystalline solid, whereas DES 2 and DES 4 exist in homogeneous liquid phase. Theoretically, DES with good HBA:HBD molar ratio should be clear, transparent liquid without any crystalline solids at room temperature [27]. Only DES 2 and DES 4 fulfil this criterion, which justified that they are successfully synthesized. Since DES 1 and DES 3 were poorly formed, they would not be further investigated in this study. Another criterion of a good DES is to have low viscosity [27]. Table S3 shows the viscosity of DES 2 and DES 4 at various temperatures. The viscosities of binary DES (DES 2) and ternary DES (DES 4) were 28.4 mPa.s and 34.7 mPa.s. It is observed that DES 2 and DES 4 exhibit lower viscosity, suggesting that they are better solvents in comparison to DES composed of choline chloride and urea (527.1 mPa.s) at 30°C [28]. This justifies our selection of DES 2 and DES 4 for subsequent adsorption study.
3.2. Surface MorphologyThe surface morphology of the adsorbents was examined through FESEM analysis. Figs. 2 (a–e) shows the surface morphology images of all samples obtained at a magnification of 4.7 kX. As shown in Fig. 2(a), the surface of raw activated carbon is relatively smooth and flat with few porous structures. Figs. 2(b) until (e) show that modification of raw activated carbon through chemical impregnation significantly increases the porosity of adsorbents. The functionalized activated carbons exhibit noticeable porous structures with relatively more cavities and irregular pores on the surfaces, resulting in rough appearance of the adsorbent surfaces. This could be due to the dehydration and elimination reaction between activating agents and carbon materials which results in the release of volatile products during activation, giving rise to pore development that favors CO2 adsorption [29]. The improvement in surface morphology of the adsorbents showed that these chemicals are effective activating agents which enhance the porosity of adsorbents. The degree of porosity in ACPA and ACPC is relatively higher when compared to AC-DES 2 and AC-DES 4. Possible reason is due to the intercalation of atomic potassium into carbon structure, leading to high porosity development in ACPA and ACPC [18]. Interestingly, it is observed that ternary DES activation results in higher degree of porosity in AC-DES 4 when compared to binary DES activation in AC-DES 2, suggesting that ternary DES is more effective than binary DES in the pore development of activated carbon.
3.3. Elemental CompositionThe elemental composition of the adsorbents was examined through EDX analysis. Table S4 shows the elemental composition of each adsorbent. Overall, all adsorbents have a high carbon content of more than 77.43 wt.%. High carbon content is favorable for physical adsorption due to van der Waals interactions between adsorbent and adsorbates [30, 31]. In comparison to raw activated carbon, the carbon loss in ACPA is relatively smaller than that in ACPC, suggesting that CH3COOK activation results in milder sample burn-off than K2CO3 activation. On the other hand, the presence of nitrogen element in AC-DES 2 (4.11 wt.%) and AC-DES 4 (7.89 wt.%) is attributed to the deposit of nitrogen-containing compounds such as choline chloride and urea upon impregnation. The presence of nitrogen element in these two functionalized samples justifies that the impregnation process is successful. The nitrogen content in AC-DES 4 is relatively higher than that in AC-DES 2 because DES 4 is composed of two nitrogen-containing components, i.e., choline chloride and urea, whereas DES 2 is composed of only one nitrogen-containing component, i.e., choline chloride.
3.4. Surface Area and PorosityThe textural parameters of the adsorbents were examined through BET surface area and porosity analysis. Fig. 3 shows the nitrogen adsorption-desorption isotherm of each adsorbent obtained at 77 K. The isotherms of the adsorbents are a combination of Type I and Type IV isotherms according to the standard International Union of Pure and Applied Chemistry (IUPAC) classification. At low relative pressure region (0 < P/P0 <0.05), a steep gradient is observed indicating a significant increase in the volume of nitrogen adsorbed. This corresponds to Type I isotherm which indicates the presence of significant amount of micropores in the adsorbents. At 0.45<P/P0 <0.90, a slim and long hysteresis loop with the adsorption and desorption isotherms parallel to each other is observed. This corresponds to Type H4 hysteresis loop which indicates the presence of limited amount of mesopores [32]. This result was associated with the results of pore size distribution for all carbon both samples (see Fig. 4).
Based on Fig. 4, the pore size distribution for all adsorbents is within the range of 5 to 15 Å, which shows that most of the pores in the adsorbents are microporous. The BET surface area and micropore volume of all the functionalized adsorbents (except ACPA) was reduced after impregnation with activating agents, following the order of Raw AC > AC-DES 2 > AC-DES 4 > ACPC. According to Aliasa et al. [33], the deposit of activating agents on the adsorbent surfaces during impregnation blocks the pores resulting in reduced surface area and micropore volume. Meanwhile, it creates more active sites for enhancing CO2 adsorption, leading to larger CO2 uptake. On the contrary, the increase in BET surface area and micropore volume after impregnation with CH3COOK is in line with the study conducted by Singh et al. [18] which states that both high surface area and high micropore volume collectively contributes to high CO2 adsorption capacity in ACPA (Table S5).
In the IUPAC classification of pore size, the pores of activated carbon can be classified into three major categories: (i) micropore (diameters less than 2 nm or 20 Å), (ii) mesopore (diameters between 2–50 nm or 20–500 Å) and (iii) macropore (diameters greater than 50 nm) [34, 35]. Pore size distribution is important to determine pore structure and is related to the accessibility of pore space of CO2 molecules [36]. Burnett, Jeyner, Halenda (BJH) method is widely used to analyze pore size distribution for porous carbon. However, the non-local or nonlinear density functional theory (NLDFT) method is suitable to be used to study micropore and BJH method to determine mesopores characteristics [37]. Fig. 4 shows that the micropore size distribution of raw AC, ACPC, ACPA and AC-DES 2 samples were mostly distributed in a range of 10–20 Å (1–2 nm). The micropore size distribution fluctuates in the range of 5–15 Å for raw AC, ACPC, ACPA and AC-DES 2 samples indicating that they are dominant in the micropore area. This wide range of pore width and micropore characteristics are important for CO2 adsorption [35]. Therefore, it can be concluded that narrow micropores are perfect sites for CO2 adsorption to occur. It can be observed from Fig. 4 that the AC-DES 4 sample had both micropores and mesopores characteristics. Several intense peaks were found with diameters of 10–20 Å, 20–25 Å and 25–30 Å.
3.5. CO2 Adsorption Study3.5.1. Effect of adsorption temperature
Fig. 5 shows the effect of adsorption temperature ranging from 25°C to 55°C on CO2 adsorption by various adsorbents. It is observed that when adsorption temperature increases, breakthrough time decreases. For instance, the maximum breakthrough time for the ACPA is 54 min at 25°C (low temperature). Meanwhile, breakthrough time decreased to 31 min with an increase in the adsorption temperature at 55°C (high temperature). The adsorption capacity of adsorbents becomes smaller as shown in Table S6. The trend of shorter breakthrough time and reduced adsorption capacity at elevated temperature suggests that the CO2 adsorption process that occurs in the adsorbents is physisorption with exothermic nature [39]. According to Le Chatelier’s principle, reduced temperature is favorable for exothermic reaction [31]. The adsorbate molecules under physisorption are attracted to the adsorbent by weak van der Waals forces which would be overcome at high temperature [40]. Consequently, less CO2 molecules are being adsorbed at high temperature. The adsorbents reached the equilibrium saturation stage faster and breakthrough occurs in shorter time. As shown in Table S6, the reduction in adsorption capacity of all adsorbents is at least two-fold as the temperature increases from 25°C to 55°C. This shows that CO2 adsorption exhibits significant dependence on the adsorption temperature whereby lower temperature at 25°C favors substantial adsorption. Among the five adsorbents, it is observed that ACPA has the highest adsorption capacity (116.5 mg/g) and longest breakthrough time (54 min) at 25°C, signifying the great potential of CH3COOK activated carbon for CO2 adsorption.
3.5.2. Effect of inlet CO2 concentration
Figs. 6 (a–e) shows the effect of inlet CO2 concentration ranging from 15 % to 20 % on CO2 adsorption by various adsorbents. It is observed that when the inlet CO2 concentration increases, breakthrough time becomes shorter. The increase in the inlet CO2 concentration corresponds to the increase in the number of CO2 molecules available per unit volume of feed. As a result, a steeper concentration gradient builds up between gaseous and solid phase, which facilitates the mass transfer of CO2 molecules from the feed to the adsorbent porous sites, leading to faster saturation of the adsorbent bed. Consequently, breakthroughs occur in shorter time. Moreover, the increase in the inlet CO2 concentration results in larger uptake of CO2 molecules as indicated by the increase in adsorption capacity of adsorbents that is shown in Table S7. This could be due to the increased concentration gradient that provides greater driving force to overcome the mass transfer resistance of CO2 molecules, leading to larger CO2 uptake [41, 42]. The trend of shorter breakthrough time and larger adsorption capacity at increased inlet CO2 concentration is in agreement with the results published by Auta and Hameed [42] and Tan et al. [39]. Interestingly, the reduction in breakthrough time due to the increase in inlet CO2 concentration is relatively smaller when compared to the reduction in breakthrough time due to the increase in adsorption temperature. This suggests that the influence of inlet CO2 concentration is relatively less significant when compared to that of adsorption temperature. Among the five adsorbents, it is observed that ACPA has the highest adsorption capacity and longest breakthrough time despite changes in inlet CO2 concentration, signifying the great potential of CH3COOK activated carbon for CO2 adsorption.
3.5.3. Effect of different activating agents
Fig. 7 shows the effect of different activating agents on CO2 adsorption by various adsorbents. It is observed that adsorption capacities increase in the order of Raw AC (10.8 mg/g) < AC-DES 2 (32.4 mg/g) < AC-DES 4 (66.9 mg/g) < ACPC (90.6 mg/g) < ACPA (116.5 mg/g). Based on Table S8, adsorption capacity of the adsorbents increases in the same order. Overall, all the functionalized adsorbents exhibit improvement in terms of breakthrough time and adsorption capacity when compared to raw activated carbon, showing that the chemicals used in this study are effective activating agents for modification of activated carbon. Among the potassium salt-based activated carbons, ACPA yields higher adsorption capacity and longer breakthrough time than ACPC. This could be attributed to the fact that ACPA has more porous structures than ACPC that favors CO2 adsorption as confirmed by SEM analysis. The adsorption capacity of ACPA obtained in this study is comparable with the literature result published Singh et al. [18]. Among the DES-based activated carbons, AC-DES 4 yields higher adsorption capacity and longer breakthrough time than AC-DES 2. In addition to SEM analysis, this could be explained by EDX analysis which shows that AC-DES 4 has higher nitrogen content than AC-DES 2, thus enhancing the interaction between acidic CO2 molecules and basic sites on adsorbent surface, leading to larger CO2 uptake. Interestingly, the adsorption capacity of AC-DES 4 is two times larger than that of AC-DES 2, indicating that ternary DES is significantly more effective than binary DES in the modification of activated carbon. It is worth mentioning that the present work is the first study that uses ternary DES as activating agent in the modification of activated carbon for capturing CO2. Furthermore, it is found that the CO2 adsorption capacity of AC-DES 4 is significantly higher when compared to published results by Zulkurnain et al. [43, 44] studied the modification of sea mango activated carbon using DES composed of choline chloride and glycerol as the activating agent. A larger CO2 uptake of up to 9.851 mg/g was observed in the DES-activated carbon in comparison to non-functionalized activated carbon. Therefore, activated carbon modified with DES was used in this study, signifying the great potential of ternary DES in the modification of activated carbon for CO2 adsorption.
3.6. Regeneration StudyRegeneration is an important indicator of the adsorption performance for an adsorbent. A good adsorbent should be thermally stable, reusable, and able to offer consistent adsorption performance after multiple adsorption-desorption cycles. For chemical adsorption (amine modified adsorbent), regeneration could be achieved completely at temperature up to 200°C [44]. For physical adsorption, successful regeneration occurred at 150°C and only 0.1 mmol capacity reduced for 10 cycles. The best regeneration temperature for activated carbon is in the range of 150°C and 230°C [45]. For vacuum-temperature swing adsorption, complete regeneration occurred at 55°C for 10 min [45].
In the present work, ACPA was chosen for regeneration study because it yielded the best adsorption performance in terms of adsorption capacity and breakthrough time despite changes in adsorption temperature and inlet CO2 concentration. ACPA was subjected to seven adsorption-desorption cycles at 25°C and 55°C using 90°C and 180°C regeneration temperatures. As shown in Fig. 8, when using 90°C regeneration temperature, the adsorption capacity of ACPA at 25°C was 116.5 mg/g at the first cycle. It was slightly reduced to 107.9 mg/g at the second cycle and became stable at 103.6 mg/g from the third to seventh cycle. The loss in the adsorption capacity is attributed to the filling of porous sites by CO2 molecules that are chemically bonded to the leftover activating agents deposited on the adsorbent surface [46]. Moreover, another possible reason is because the temperature is not high enough to break the chemical bonds. At 55°C, the adsorption capacity of ACPA remained constant at 60.8 mg/g for all the seven cycles. The results show that ACPA exhibits good regeneration ability, with only a slight (11 %) reduction in the adsorption capacity at 25°C. As observed in Fig. 8, regeneration could be attained completely (complete regenerability) at regeneration temperature of 180°C due to the adsorption capacity remaining constant up to seven cycles.
4. ConclusionsThe present work has achieved its main objective to develop eco-friendly chemical activating agents for the modification of activated carbon for CO2 adsorption. The results revealed that activated carbon modified with potassium acetate (ACPA) exhibited remarkably high CO2 adsorption capacity of 116.5 mg/g at 25°C and 15% inlet CO2 concentration. Furthermore, ACPA demonstrated good regeneration ability even after seven adsorption-desorption cycles, which showed its potential for large-scale applications. Interestingly, it was found that activated carbon modified with ternary DES composed of choline chloride, urea, and ethylene glycol (AC-DES 4) exhibited high adsorption capacity of 66.9 mg/g of CO2 at 25°C and 15% inlet CO2 concentration. This figure is significantly higher when compared to all the existing published results of activated carbon modified with binary DES, signifying the great potential of ternary DES in the modification of activated carbon for CO2 adsorption. It is worth mentioning that the present work is the first study that uses ternary DES as activating agent for such purpose. In future work, more research efforts are recommended to investigate the use of ternary DES composed of other combinations of HBAs and HBDs in the modification of activated carbon for capturing CO2.
AcknowledgementsThis study was supported by Sunway University under Internal research grant (GRTIN-IGS-CCDCU[S]-07-2022) and Research Reward Output grant (GRTIN-RRO-18-2022).
NotesAuthor Contribution Statement F.H. (Senior Research Fellow) contributed to conception and design of the study, performed the analysis, wrote the final draft of the manuscript, revised the manuscript and supervision. L.B.K. (Undergraduate student, research project) conducted all the experiments and wrote the manuscript. R.Y. (Professor) advised on the design of experiment, reviewed the manuscript and supervision. M.K.A (Professor) provided resources for the experiments and reviewed the final draft of the manuscript. All authors contributed to manuscript revision and approved the submitted version. References1. EPA. Inventory of U.S. Greenhouse Gas Emissions and Sinks Fast Facts and Data Highlights. 2018. [cited 4 April 2022]. Available from: https://www.epa.gov/sites/production/files/2020-04/documents/fastfacts-1990-2018.pdf
3. Climate Change. 2020. [cited 19 November 2022]. Available from: https://www.un.org/sustainabledevelopment/climate-change/
4. Susskind L, Chun J, Goldberg S, Gordon JA, Smith G, Zaerpoor Y. Breaking out of carbon lock-in: Malaysia’s path to decarbonization. Front. Built. Environ. 2020;6:21.
https://doi.org/10.3389/fbuil.2020.00021
5. Chirone R, Paulillo A, Coppola A, Scala F. Carbon capture and utilization via calcium looping, sorption enhanced methanation and green hydrogen: A techno-economic analysis and life cycle assessment study. Fuel. 2022;328:125255.
https://doi.org/10.1016/j.fuel.2022.125255
6. Li L, Zhao N, Wei W, Sun Y. A review of research progress on CO2 capture, storage, and utilization in Chinese academy of sciences. Fuel. 2013;108:112–130.
https://doi.org/10.1016/j.fuel.2011.08.022
7. Cheng J, Wu C, Gao W, Li H, Ma Y, Liu S, Yang D. CO2 absorption mechanism by the deep eutectic solvents formed by mono-ethanolamine-based protic ionic liquid and ethylene glycol. Int. J. Mol. Sci. 2022;23. 10.3390/ijms23031893.
8. Hong SH, Chung K, Bang G, Kim KM, Lee CH. Adsorption equilibria and kinetics of CO2, CH4, CO, N2, and H2 on KOH-treated activated carbon pellets up to 1000 kPa. Chem. Eng. J. 2022;431:133396.
https://doi.org/10.1016/j.cej.2021.133396
9. Odunlami OA, Vershima DA, Oladimeji TE, Nkongho S, Ogunlade SK, Fakinle BS. Advanced techniques for the capturing and separation of CO2 – A review. Results. Eng. 2022;15:100512.
https://doi.org/10.1016/j.rineng.2022.100512
10. Abuelnoor N, AlHajaj A, Khaleel M, Vega LF, Abu-Zahra MRM. Activated carbons from biomass-based sources for CO2 capture applications. Chemosphere. 2021;282:131111.
https://doi.org/10.1016/j.chemosphere.2021.131111
11. Yang J, Yue L, Hu X, Wang L, Zhao Y, Lin Y, et al. Efficient CO2 capture by porous carbons derived from coconut shell. Energ. Fuel. 2017;31(4)4287–4293.
https://doi.org/10.1021/acs.energyfuels.7b00633
12. Singh J, Bhunia H, Basu S. Adsorption of CO2 on KOH activated carbon adsorbents: Effect of different mass ratios. J. Environ. Manage. 2019;250:109457.
https://doi.org/10.1016/j.jenvman.2019.109457
13. Ahsan S, Ayub A, Meeroff D, Jahandar Lashaki M. A comprehensive Comparison of Zeolite-5A molecular sieves and Amine-Grafted SBA-15 silica for cyclic adsorption-desorption of carbon dioxide in enclosed environments. Chem. Eng. J. 2022;135139.
https://doi.org/10.1016/j.cej.2022.135139
14. Firdaus RM, Desforges A, Rahman Mohamed A, Vigolo B. Progress in adsorption capacity of nanomaterials for carbon dioxide capture: A comparative study. J. Clean. Prod. 2021;328:129553.
https://doi.org/10.1016/j.jclepro.2021.129553
15. dos Santos TC, Mancera RC, Rocha MVJ, da Silva AFM, Furtado IO, Barreto J, Stavale F, Archanjo BS, de M, Carneiro JW, Costa LT, et al. CO2 and H2 adsorption on 3D nitrogen-doped porous graphene: Experimental and theoretical studies. J. CO2 Util. 2021;48:101517.
https://doi.org/10.1016/j.jcou.2021.101517
16. Tian Y, Lin Y, Hagio T, Hu YH. Surface-microporous graphene for CO2 adsorption. Catal. Today. 2020;356:514–518.
https://doi.org/10.1016/j.cattod.2020.06.002
17. De Gisi S, Lofrano G, Grassi M, Notarnicola M. Characteristics and adsorption capacities of low-cost sorbents for wastewater treatment: A review. Sustain. Mater. Technol. 2016;9:10–40.
https://doi.org/10.1016/j.susmat.2016.06.002
18. Singh J, Basu S, Bhunia H. CO2 capture by modified porous carbon adsorbents: Effect of various activating agents. J. Taiwan. Inst. Chem. Eng. 2019;102:438–447.
https://doi.org/10.1016/j.jtice.2019.06.011
19. Chiang YC, Juang RS. Surface modifications of carbonaceous materials for carbon dioxide adsorption: A review. J. Taiwan. Inst. Chem. Eng. 2017;71:214–234.
https://doi.org/10.1016/j.jtice.2016.12.014
20. Shi M, Xiong W, Tu Z, Zhang X, Hu X, Wu Y. Task-specific deep eutectic solvents for the highly efficient and selective separation of H2S. Sep. Purif. Technol. 2021;276:119357.
https://doi.org/10.1016/j.seppur.2021.119357
21. Yue L, Xia Q, Wang L, Wang L, DaCosta H, Yang J, Hu X. CO2 adsorption at nitrogen-doped carbons prepared by K2CO3 activation of urea-modified coconut shell. J. Colloid. Interface. Sci. 2018;511:259–267.
https://doi.org/10.1016/j.jcis.2017.09.040
22. Aboshatta M, Magueijo V. A comprehensive study of CO2 absorption and desorption by Choline-Chloride/Levulinic-Acid-Based deep eutectic solvents. Molecules. 2021;26. 10.3390/molecules26185595.
23. Ma C, Liu C, Lu X, Ji X. Techno-economic analysis and performance comparison of aqueous deep eutectic solvent and other physical absorbents for biogas upgrading. Appl. Energy. 2018;225:437–447.
https://doi.org/10.1016/j.apenergy.2018.04.112
24. Poole CF. Chapter 2 - Solvent selection for liquid-phase extraction. Handbooks in Separation Science. Elsevier. 2020. 45–89.
https://doi.org/10.1016/B978-0-12-816911-7.00002-5
25. Wang J, Baker SN. Pyrrolidinium salt based binary and ternary deep eutectic solvents: green preparations and physiochemical property characterizations. Green. Process. Synth. 2017;7(4)353–359.
https://doi.org/10.1515/gps-2017-0060
26. Cui G, Lv M, Yang D. Efficient CO2 absorption by azolide-based deep eutectic solvents. Chem. Commun. 2019;55(10)1426–1429.
https://doi.org/10.1039/C8CC10085C
27. Pam A. Innovative activated carbon based on deep eutectic solvents (DES) and H3PO4. C. 2019;5:
https://doi.org/10.3390/c5030043
28. Shekaari H, Zafarani-Moattar MT, Mohammadi B. Thermophysical characterization of aqueous deep eutectic solvent (choline chloride/urea) solutions in full ranges of concentration at T =(293.15–323.15) K. J. Mol. Liq. 2017;243:451–461.
https://doi.org/10.1016/j.molliq.2017.08.051
29. Adinata D, Wan Daud WMA, Aroua MK. Preparation and characterization of activated carbon from palm shell by chemical activation with K2CO3
. Bioresour. Technol. 2007;98(1)145–149.
https://doi.org/10.1016/j.biortech.2005.11.006
30. Rashidi NA, Yusup S. A review on recent technological advancement in the activated carbon production from oil palm wastes. Chem. Eng. J. 2017;314:277–290.
https://doi.org/10.1016/j.cej.2016.11.059
31. Rashidi NA, Yusup S, Borhan A. Isotherm and thermodynamic analysis of carbon dioxide on activated carbon. Procedia. Eng. 2016;148:630–637.
https://doi.org/10.1016/j.proeng.2016.06.527
32. Chang SS, Clair B, Ruelle J, Beauchêne J, Di Renzo F, Quignard F, Gril J. Mesoporosity as a new parameter for understanding tension stress generation in trees. J. Exp. Bot. 2009;60(11)3023–3030.
https://doi.org/10.1093/jxb/erp133
33. Aliasa N, Ahmad Zaini MA, Kamaruddin M. Roles of impregnation ratio of K2CO3 and NaOH in chemical activation of palm kernel shell. J. Appl. Sci. Process. Eng. 2017;4:195–204.
https://doi.org/10.33736/jaspe.436.2017
34. Li Z, Ren T, Li X, Qiao M, Yang X, Tan L, Nie B. Multi-scale pore fractal characteristics of differently ranked coal and its impact on gas adsorption. Int. J. Min. Sci. Technol. 2023;
https://doi.org/10.1016/j.ijmst.2022.12.006
35. Wang Z, Fu X, Pan J, Deng Z. Effect of N2/CO2 injection and alternate injection on volume swelling/shrinkage strain of coal. Energy. 2023;275:127377.
https://doi.org/10.1016/j.energy.2023.127377
36. Ma X, Xu W, Su R, Shao L, Zeng Z, Li L, Wang H. Insights into CO2 capture in porous carbons from machine learning, experiments and molecular simulation. Sep. Purif. Technol. 2023;306:122521.
https://doi.org/10.1016/j.seppur.2022.122521
37. Maarof HI, Ajeel MA, Daud WMAW, Aroua MK. Electrochemical properties and electrode reversibility studies of palm shell activated carbon for heavy metal removal. Electrochim. Acta. 2017;249:96–103.
https://doi.org/10.1016/j.electacta.2017.07.171
38. Thubsuang U, Manmuanpom N, Chokaksornsan N, Sommut C, Singhawat K, Payaka A, Wongkasemjit S, Chaisuwan T. Efficient CO2 adsorption on porous carbon with nitrogen functionalities based on polybenzoxazine: High-pressure adsorption characteristics. Appl. Surf. Sci. 2023;607:155120.
https://doi.org/10.1016/j.apsusc.2022.155120
39. Tan YL, Islam MA, Asif M, Hameed BH. Adsorption of carbon dioxide by sodium hydroxide-modified granular coconut shell activated carbon in a fixed bed. Energy. 2014;77:926–931.
https://doi.org/10.1016/j.energy.2014.09.079
40. Borhan A, Yusuf S. Activation of rubber-seed shell waste by malic acid as potential CO2 removal: Isotherm and kinetics studies. Materials. 2020;13(21)4970.
https://doi.org/10.3390/ma13214970
41. Auta M, Hameed BH. Preparation of waste tea activated carbon using potassium acetate as an activating agent for adsorption of Acid Blue 25 dye. Chem. Eng. J. 2011;171(2)502–509.
https://doi.org/10.1016/j.cej.2011.04.017
42. Auta M, Hameed BH. Adsorption of carbon dioxide by diethanolamine activated alumina beads in a fixed bed. Chem. Eng. J. 2014;253:350–355.
https://doi.org/10.1016/j.cej.2014.05.018
43. Zulkurnai N, Md Ali U, Ibrahim N, Manan N. Carbon dioxide (CO2) adsorption by activated carbon functionalized with deep eutectic solvent (DES). IOP. Conf. Ser. Mater. Sci. Eng. 2017;206:12001.
https://doi.org/10.1088/1757-899X/206/1/012001
44. Petrovic B, Gorbounov M, Masoudi Soltani S. Influence of surface modification on selective CO2 adsorption: A technical review on mechanisms and methods. Micropor. Mesopor. Mater. 2021;312:110751.
https://doi.org/10.1016/j.micromeso.2020.110751
45. Papa E, Landi E, Natali Murri A, Miccio F, Vaccari A, Medri V. CO2 adsorption at intermediate and low temperature by geopolymer-hydrotalcite composites. Open. Ceram. 2021;5:100048.
https://doi.org/10.1016/j.oceram.2020.100048
46. Zulkurnai NZ, Md Ali U, Ibrahim N, Manan N. Carbon dioxide capture by deep eutectic solvent impregnated sea mango activated carbon. E3S. Web. Conf. 2018;34:2030.
https://doi.org/10.1051/e3sconf/20183402030
Fig. 3Nitrogen adsorption desorption isotherms of raw AC, ACPC, ACPA, AC-DES 2 and AC-DES 4 at 77 K 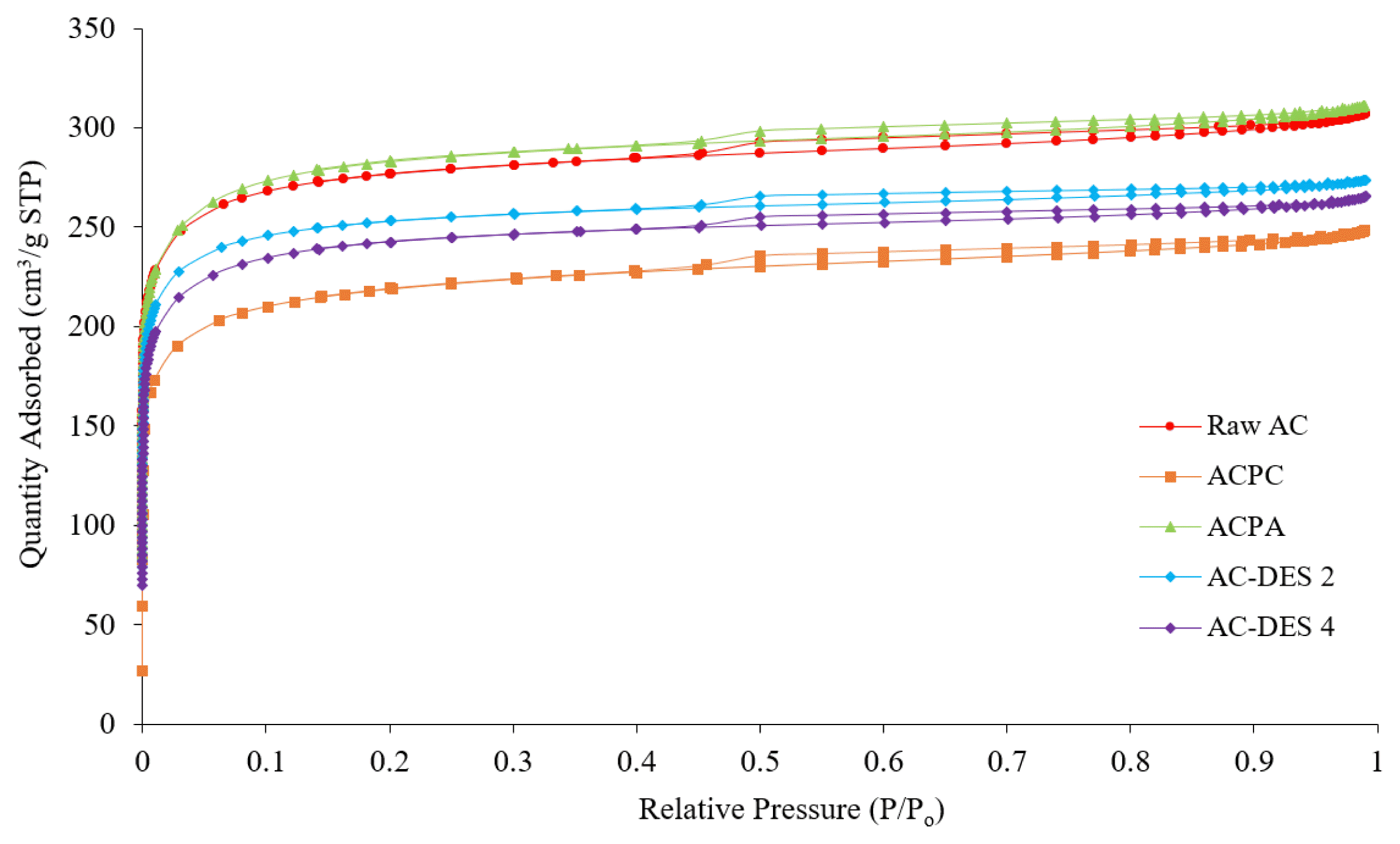
Fig. 5CO2 breakthrough curves of raw AC, ACPC, ACPA, AC-DES 2 and AC-DES 4. At temperature at 25–55°C, fixed inlet CO2 concentration at 15%, and inlet flow rate at 200 mL/min 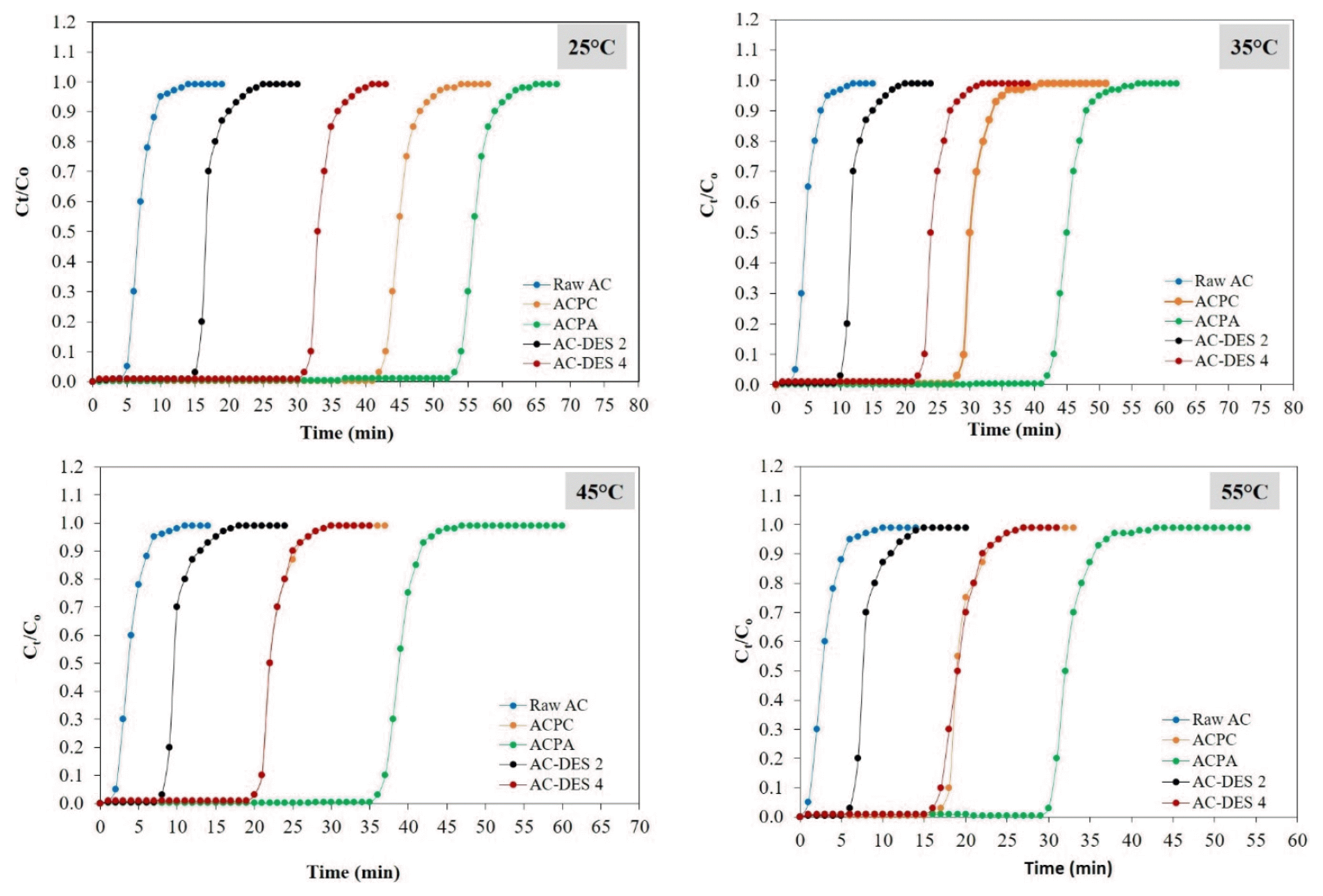
Fig. 6CO2 breakthrough curves of raw AC, ACPC, ACPA, AC-DES 2 and AC-DES 4. (Operating conditions: temperature at 25°C, inlet CO2 concentration at 15–20%, and inlet flow rate at 200 mL/min.) 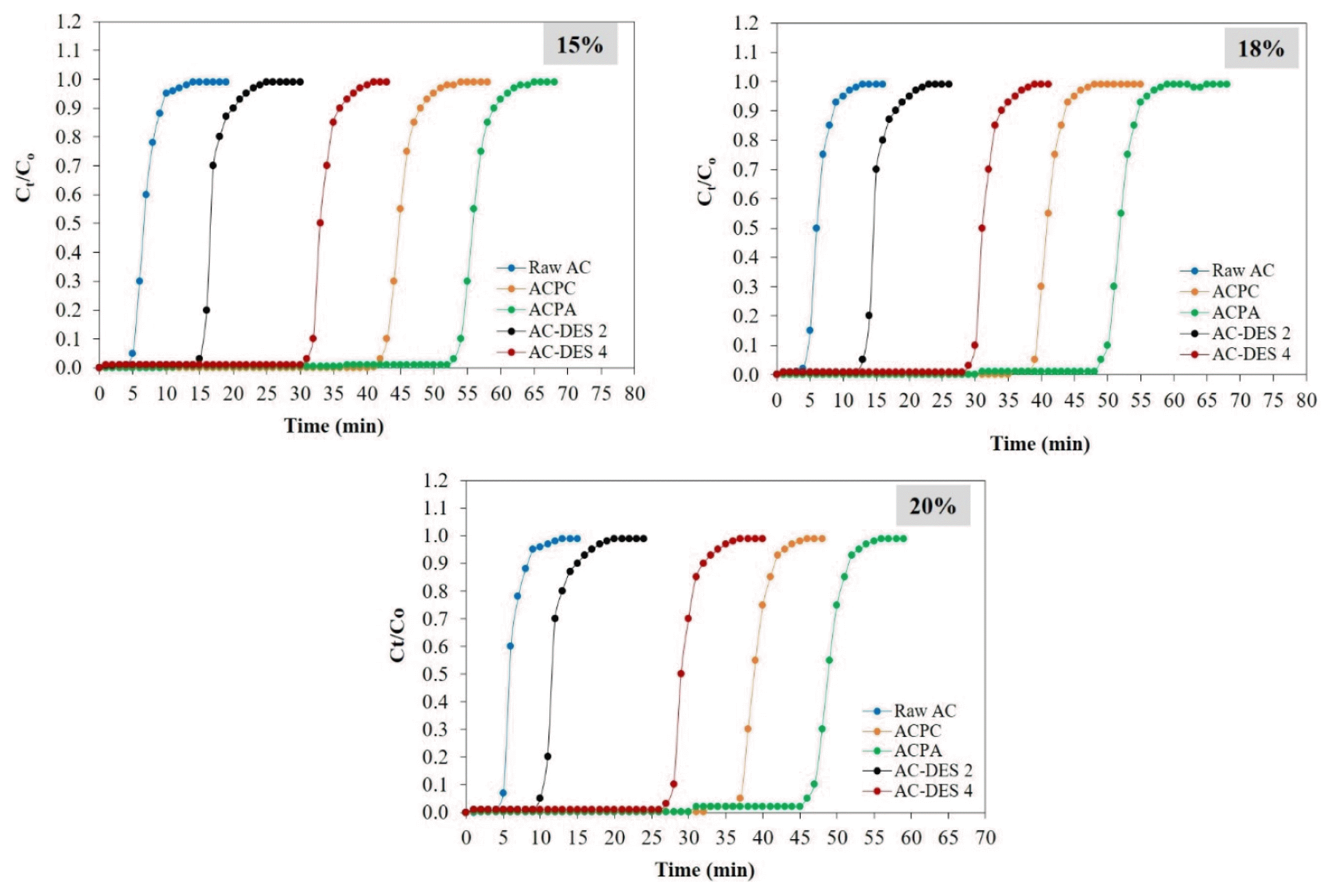
|
|
|||||||||||||||||||||||||||||||||||||