AbstractMost of previous studies proved bicarbonate (HCO3−) was free radical scavenger in advanced oxidation processes, however, in this study, the positive influence of HCO3− was confirmed in heterogeneous Fenton. The results showed that the degradation efficiency of Rhodamine B increased from 13.7% to 95.3% by adding appropriate concentration of HCO3−, and the zeolite supported Co and Cu oxides (Co/Cu/zeolite) could catalyze the HCO3− enhanced Fenton process. The response surface method was employed to optimize the concentration of HCO3− needed in the heterogeneous Fenton system. Radicals quenching results showed that 1O2 and O2•− rather than ·OH played important roles in the degradation of Rhodamine B. Mechanism studies indicated that carbonate radical (CO3•−) was the key intermediate in the formation of 1O2 and O2•−, and CO3•− could be generated through the reaction between HCO3− and ·OH. In addition, peroxymonocarbonate (HCO4−), which further produced CO3•−, was also generated in the bicarbonate enhancement system. The electron transfer between Cu and Co promoted the heterogeneous Fenton process in generating ·OH that reacted with HCO3−, or catalyzed HCO4− to generate CO3•−.
Graphical Abstract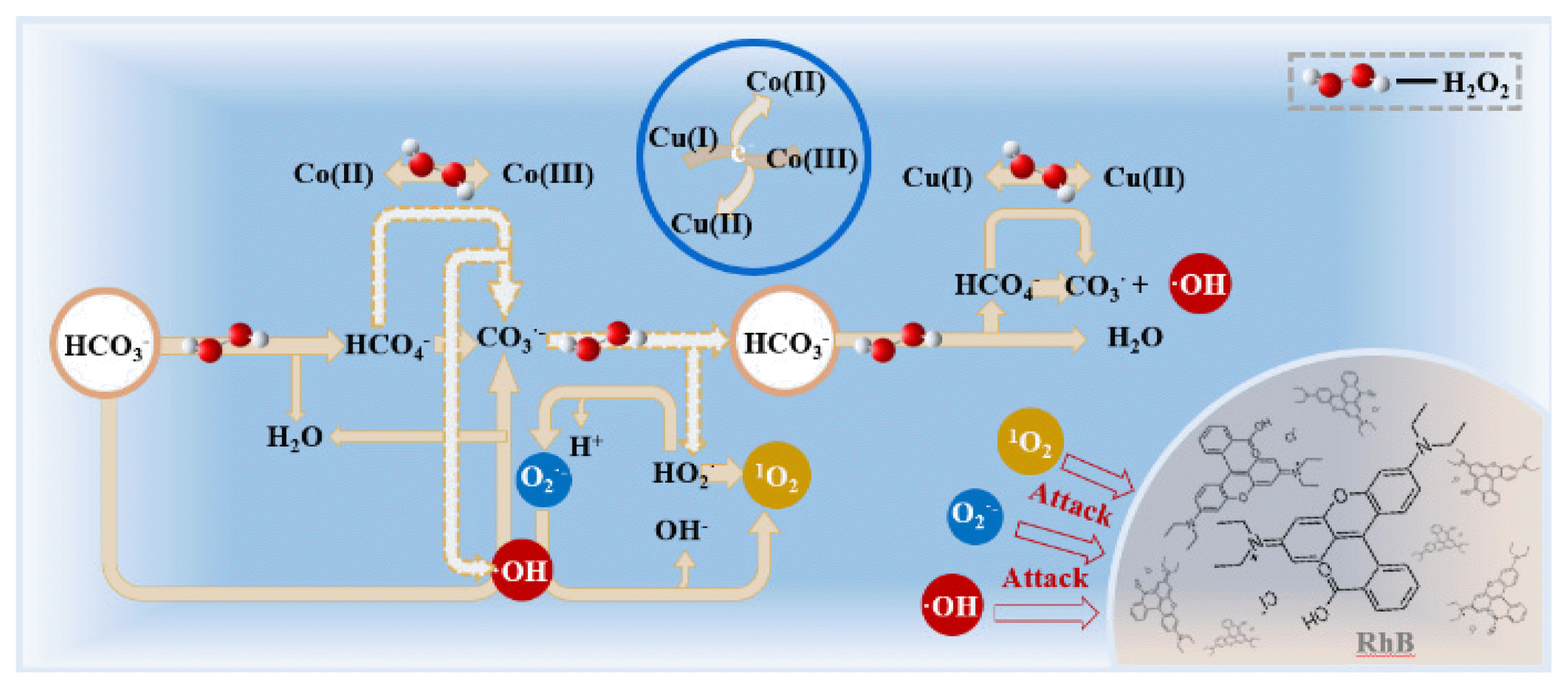
1. IntroductionA large number of refractory organic compounds, such as Rhodamine B, carbamazepine, tetracycline hydrochloride, were commonly contained in wastewater from dye and pharmaceuticals industries, and would cause potential risks to water ecosystems and human health if they were discharged without effective treatment [1–5]. Advanced oxidation processes (AOPs), including Fenton process, photocatalytic oxidation, and ozone oxidation, employed free radicals to oxidize refractory organics and were widely used to treat highly concentrated wastewater from industrial sources[6–8]. Since the efficiency of AOPs highly depended on whether free radicals could effectively attack and oxidize organics, coexisting ions, or organic compounds in wastewater, such as chloride ions (Cl−), nitrate ions (NO3−), sulfate ions (SO42−), and natural organic matters (NOM), would affect the efficiency via participating in free radical chain reactions. Chavalit et al. introduced Cl−, NO3− and H2PO4− into Fenton process and found that Cl− and H2PO4− had a significant inhibition on nitrobenzene degradation while nitrate had slight effect [9]. Previous studies showed that different anions may impose varying degrees of influence on AOPs in degrading organic pollutants [9–11].
Bicarbonate (HCO3−) was a common anion, and in same specific natural waters and industrial wastewaters, the concentration of HCO3− could range from 0.7mM to 10mM [12]. In most studies, HCO3− was believed to be a scavenger of free radicals and would reduce the oxidation efficiency of AOPs [13, 14]. However, it had been proved that HCO3− would effectively promote the production of free radicals under certain AOPs conditions. In 1980, it was found that peroxymonocarbonate (HCO4−) could be generated through the reaction between HCO3− and H2O2 in aqueous solution [15, 16]. The generated HCO4− further decomposed into CO3•− and ·OH, which subsequently generated HO2•, O2•−, and 1O2 [17]. This oxidation process was called bicarbonate activated hydrogen peroxide (BAP). Recently, BAP system for oxidation of organic pollutants in wastewater has been given more and more attentions, due to its convenient operation, easy material acquisition, no secondary pollution, and so on [18]. Therefore, it had been studied in degrading refractory organics compounds, such as azo dyes, aryl sulfide, aliphatic amines, chlorophenols, and so on [17, 19–24].
Researchers also found that the introduction of HCO3− could improve the overall oxidation capacity of Fenton/Fenton-like systems. For example, the degradation efficiency of acid orange II increased about 40% by introducing 20mM NaHCO3 into a cobalt oxide catalyzed Fenton process [25, 26]. Despite the activation of HCO3−, catalysts still played crucial roles in both homogeneous and heterogeneous Fenton processes. Without catalysts, the oxidation ability of BAP system was relatively poor and needed high concentrations of HCO3− and hydrogen peroxide. Liu et al added 60mM H2O2 and 5mM HCO3− to wastewater containing chlorophenols, and the removal efficiency was less than 5% after 2 h. However, after adding 0.1 g/L Co-Mn oxides under the same experimental conditions, the removal efficiency rose to 70% [22]. Therefore, a series of catalysts, including Co-LDHs, CuO-MgO-Al2O3, CoxOy-N/GAC, had been developed to intensify the HCO3− activation of heterogeneous Fenton process [23, 25, 27]. The introduction of HCO3− ensured Fenton-like reaction be conducted under neutral or slightly alkaline condition, which could protect the heterogeneous catalysts from metal ions leaching out and guaranteed the reusability of catalysts [28, 29]. At present, fewer studies have been conducted on the HCO3− accelerating Fenton process, and new catalysts for HCO3− accelerating Fenton needed to be developed. Moreover, the activation of HCO3− on Fenton system should be investigated detailly from the point of reaction mechanism and oxidative radicals.
In this study, zeolite supported Cu and Co oxides were prepared using sol-gel method to be used to catalyze HCO3− advancement Fenton process for degradation of Rhodamine B (RhB). Response surface method (RSM) was used to investigate the interactions between various reaction factors to obtain optimal dosage of HCO3− for the enhancement of Fenton process. The reaction mechanism was studied by radical quenching, electron paramagnetic resonance (EPR) detecting, and X-ray photoelectron spectroscopy (XPS) characterization of the catalyst, in order to explain the positive role of HCO3− in the system.
2. Material and Methods2.1. ChemicalsCu(NO3)2·3H2O and powder zeolite were purchased from Shanghai Maclin Biochemical Technology Co., LTD. H2SO4, NaOH and H2O2 were purchased from Beijing Chemical Plant. Co(NO3)2·6H2O was purchased from Tianjin Guangfu Fine Chemical Research Institute. NaHCO3 and citric acid were purchased from Tianjin Fuchen Chemical Reagent Co., LTD. All the above chemicals were analytical reagents. The water used in the experiment was previously deionized and stored at room temperature.
2.2. Preparation of Co/Cu/zeoliteSol-gel method was employed to prepare composite heterogeneous Fenton catalyst and citric acid was used as the complexing agent. First of all, zeolite was alkali-activated at 500°C, and washed using deionized water to neutral pH; secondly, the right amount of Co(NO3)2·6H2O, Cu(NO3)2·3H2O, and zeolite were added into a beaker, according to Co: Cu=1:2 (molar ratio) and metal: zeolite =1:2 (mass ratio). After mixed thoroughly in a thermostat water bath (60°C), citric acid was added into the solution following the molar ratio of metal ions: citric acid =1:1. Then, the composite gel was obtained by evaporation, and was calcinated for 2 h in a muffle furnace at 600°C. When cooled, the powder catalyst was obtained by grinding and stored for further use.
2.3. CharacterizationThe morphologies of the catalyst were observed by the SEM test (HITACHI S-4700, Japan), the acceleration voltage and beam current were 0.02–30 kV and 3 pA-20 nA, respectively. Energy dispersive X-ray spectroscopy (EDS) was additionally conducted during SEM measurement. X-ray Powder Diffraction (XRD) patterns of the catalyst were achieved using an X-ray diffractometer (Rigaku D/Max2500VB2+/PC, Japan) at a diffraction angle range of 5° to 90° (2θ range). Fourier transform infrared spectroscopy (FTIR) spectra of the catalyst were collected using an FTIR spectrometer (Nicolet 6700, USA), and the spectra of 500–4000 cm−1 were recorded. The specific surface area and pore size distribution of the samples were obtained by BET analysis (ASAP2460, USA). X-ray photoelectron spectroscopy (XPS) (ESC ALAB-250, USA) was used to analyze the change of elemental valence state before and after catalyst reaction, and the binding energy was adjusted by carbon C1 s (284.60 eV). EPR (Bruker EMX plus, Germany) signals for TEMP were recorded at ambient temperature.
2.4. Degradation ExperimentSimulated wastewater was prepared by dissolving 10 mg RhB in 1.0 L deionized waster. The degradation experiments were conducted in 250mL conical flasks at room temperature of 25°C and mixed at 200 rpm. The initial pH was adjusted at 8.0–8.5 by 0.5 M H2SO4 or NaOH solution. The influences of HCO3− concentration, catalyst dosage, H2O2 dosage, and reaction time on the system were investigated. In this study, the center composite design (CCD) based RSM was used to design experimental groups. The NaHCO3 concentration, H2O2 dosage, catalyst dosage and reaction time were selected as independent parameters, and the degradation efficiency of RhB was used as the response variable. Table 1 showed the experimental factors and levels of the response surface method. At certain intervals, 3.0 mL water samples were collected and filtered through 0.22 μm Millipore membranes, and immediately measured using an UV-vis spectrophotometer to determine the residual RhB concentration at 554 nm according to a previously determined standard curve.
Through data fitting of the experimental system, it was found that the fitting effect of the pseudo-first-order reaction equation was best, and the calculation of the formula was shown in Eq. (1), where C0 was the initial concentration of the pollutant (mg/L), Ct was the instantaneous concentration of the pollutant (mg/L), t was reaction time (h) and k was the rate constant (h−1).
2.5. Oxidative Radical DeterminationThe activity and utilization of oxidative radical have great influence on the removal efficiency of pollutants [30–32]. In this study, isopropanol (IPA), p-benzoquinone (BQ) and diethylenetriamine (DABCO) [33] was used as trapping agents of ·OH, O2•− and 1O2, respectively, to evaluate the effects of the ROS in the HCO3− enhanced heterogeneous Fenton system [34]. EPR (EMXplus 10/12, Bruker, Germany) spectroscopy was employed to detect the 1O2 captured by 2,2,6,6-tetramethylpiperidine (TEMP).
3. Results and Discussion3.1. Catalyst Characterization
Fig. 1 showed the characterization of Co/Cu/zeolite. The XRD characteristic peaks were sharp and clear, indicating that the prepared catalyst was of high purity and crystallization degree. Fig. 1b showed the FTIR spectra of catalyst before and after the reaction, the vibration absorption peaks of Co-O bond and Cu-O bond at 690.5 cm−1 and 561.3 cm−1 corresponded to the active components of Co and Cu oxides, respectively, and the peak pattern was basically unchanged after the reaction, it showed that the structure of the catalyst had high stability and did not change significantly during the process. Fig. 1c showed the specific surface area of the catalyst was 17.87m2/g, the pore size was 204.9 nm, and the pore volume was 0.183 cm3/g. Macropores accounted for a large proportion, while mesopores and micropores accounted for a small proportion, and the catalyst was a kind of macroporous material. Therefore, a certain amount of pollutants could be adsorbed, but the smaller specific surface area limited the adsorption capacity of the material [35], so the catalyst was less effective for the adsorption of pollutants. This was confirmed by the result in Fig. 2b where the RhB removal rate was less than 30% with the addition of Co/Cu/zeolite catalyst only. According to IUPAC classification standard, the isothermal curves of the catalyst belonged to type IV isotherms, and the loop belonged to type H3 hysteretic ring, indicating that the pore structure in the catalyst was irregular. It could be observed from Fig. 1d that cobalt oxides and copper oxides were spherical, and uniformly attached to the surface of zeolite, providing multiple active sites for the reaction. The surface morphology of the catalysts did not change significantly before and after the reaction (Fig. S1) and provided a basis for the reusability of the catalysts, which was consistent with the above characterization. Fig. 1e showed the proportion of cobalt and copper elements of the catalyst was about 2%–3%, the proper metal proportion could ensure its catalytic oxidation capacity and reduce ions leaching.
3.2. Enhancement of HCO3−The influence of CH3COO−, SO42−, Cl−, CO32−, and HCO3− on the oxidation capacity of heterogeneous Fenton was investigated as showed in Fig 2a. The results showed that CH3COO− would inhibit but all other anions would promote the oxidation capacity of heterogeneous Fenton. Among them, HCO3− had the best promotion effect, which could degrade RhB, achieving 5 times increase in the degradation efficiency of RhB compared with traditional heterogeneous Fenton system (13.7% to 95.3%). In addition, CO32− could also effectively enhance the degradation since part of CO32− would be converted to HCO3− through hydrolysis. Fig 2b and Fig. S2 showed the degradation efficiency of RhB by HCO3− activated H2O2 system was much less than that by Co/Cu/zeolite catalyzed Fenton process. Moreover, the pseudo-first-order reaction rate constant (k1) was 0.0323 h−1 for HCO3− activated H2O2 system, indicating that the BAP process without catalyst had a much lower reaction rate, while k1 was 0.158 h−1 for only Co/Cu/zeolite catalyzed Fenton process. However, when Co/Cu/zeolite was added together with HCO3− into the Fenton system, the reaction rate constant k1 was 3.181 h−1, about 20 times higher than that of only Co/Cu/zeolite catalyzed process. The reason was that the introduction of HCO3− changed the heterogeneous Fenton oxidation environment and increased the variety of active species responsible for pollutants degradation.
3.3. Optimizing Conditions for HCO3− Enhanced Heterogeneous Fenton3.3.1. RSM model establishmentCCD combined with RSM could provide a superior alternative for investigation of such a complex system [36]. This study used the CCD based RSM to design the optimizing experiment (Table S1). The multivariate regression model between RhB degradation efficiency (%) and the concentration of HCO3−, H2O2, Co/Cu/zeolite dosage, and reaction time was established as showed in Eq. (2).
As shown in Table 2, the p-value of the RSM model was less than 0.0001, indicating that the actual experiment results were well fitted with the regression model. All of the p-values of factors A, B, C and D were less than 0.05, indicating that the concentration of HCO3−, H2O2, catalyst dosage, and reaction time had significant effects on the degradation efficiency of RhB. The p-value of the misfitting and the coefficient of variation CV also indicated that the model was of high confidence.
The contour plots were drawn to illustrate interactions between the concentration of HCO3−, H2O2, catalyst dosage and reaction time (Fig. S3). The contour plot in Fig. S3a suggested a significant interaction between HCO3− concentration and catalyst dosage on RhB degradation efficiency. It was worth noting that when the concentration of HCO3− was 2 g/L (about 24mM), the 90% contour line exhibited an obvious turning point. The results indicated that when the concentration of HCO3− was less than 24mM, increasing the concentration of HCO3− would promote the Fenton reaction, thus the dosage of catalyst needed would decrease. However, if the concentration of HCO3− was more than 24mM, the excessive HCO3− would quench free radicals, thus the dosage of catalyst needed would increase. Furthermore, the contour plots in Fig. S3b and Fig. 4 suggested that interactions between the concentration of HCO3− and H2O2 as well as reaction time were both significant, and therefore the concentration of HCO3− should be given properly attention when used as enhanced reagent for Fenton process.
3.3.2. Model verificationAccording to the regression model, the optimal conditions for the HCO3− enhanced heterogeneous Fenton oxidation of RhB were as follows: HCO3− 24mM, H2O2 was 30mM, catalyst dosage 0.3 g/L, and reaction time 43 min. The actual experiment was conducted under these conditions, and the degradation efficiency was 95.4%±1.12%, close to the predicted 94.3% by the model. In addition, a linear regression between the actual results and predicted values of the model was achieved, the measured value of RhB degradation efficiency was found to be very close to those estimated by the RSM model (Fig. S4).
3.4. Mechanism of HCO3− Enhanced Heterogeneous Fenton3.4.1. Composition of oxidative radicalsThe activity and utilization rate of oxidative radicals had great influence on the degradation efficiency of water pollutants [30, 32]. In order to reveal oxidative radicals that played dominant roles in pollutant degradation, oxidative radicals masking experiments were carried out, and isopropanol (IPA), p-benzoquinone (BQ) and diazabicyclooctane (DABCO) were selected to capture ·OH, O2•− and 1O2, respectively [34] (Fig. 3a and Fig. S5).
The results in Fig. S5a showed that when the concentration of IPA was 2.5mM, the decolorization efficiency decreased about 25% in comparison with no IPA added. In addition, the increase of IPA from 2.5mM to 10 mM did not pose an increased inhibition on degradation of RhB, indicating that all ·OH had been captured and ·OH contributed to about 25% of the total degradation of RhB. The decolorization efficiency decreased with the increase of the concentration of BQ, and the maximum inhibition ratio was about 50% when BQ was 10 mM, indicating that O2•− was one of the main oxidative radicals. However, when DABCO was added, a maximum decolorization inhibition of 80% was achieved when DABCO was 2.5 mM, indicating that 1O2 was the main oxidative radical for RhB degradation. The reaction rate of pseudo-first-order kinetics also revealed that the RhB degradation was extremely slow when 1O2 was captured. The results also illustrated that 1O2 and O2•− were the most important oxidative radicals in the HCO3− enhanced heterogeneous Fenton process, but interestingly, ·OH exhibited less significance role than expected in traditional Fenton process. Therefore, the addition of HCO3− in Fenton system promoted the formation of 1O2 and O2•−.
EPR experiment was conducted to detect the formation of 1O2 (Fig. 3b), where TEMP was employed as the spin trapping agent of 1O2. As the concentration of NaHCO3 increased, the signal of 1O2 became stronger, but when the concentration of NaHCO3 was 24 mM, the strongest signal of 1O2 was obtained, which was in consistent with the results of optimized concentration of HCO3− by RSM in Section 3.3.2. When the concentration of HCO3− increased to 36 mM, the inhibition effect of HCO3− itself could be observed from the weaker signal of 1O2 in Fig. 3b.
In addition, 1O2 mostly appeared as the dominant active substance on the activation site of catalyst surface in heterogeneous Fenton process [37, 38]. It had the characteristics of strong oxidizing property, long lifetime and long migration distance, and was more selective in pollutants oxidation [38–40]. From the point of view of ROS and RhB reaction, previous studies have revealed that RhB molecules contained bonds in alkene structures and conjugated C=C bonds in aromatic benzene rings, and 1O2 tended to react with them through [π2+π2] 1,2-cycloaddition and [π2+π 4] 1,4-cycloaddition [41]. Furthermore, Schenck ene reaction also probably occurred in RhB molecules, which transferred allylic hydrogens of carbon atoms to 1O2 [41, 42].
In the process of HCO3− enhanced Fenton reaction, two free radical reaction paths could be established to explain the formation of O2•− and 1O2. Firstly, HCO3− reacted with H2O2 to generate HCO4−, which had strong oxidation ability but short life time [22]. HCO4− decomposed to generate CO3•− and ·OH, which could be accelerated by catalyst Co/Cu/zeolite. Then, CO3•− reacted with H2O2 to generate HCO3− and HO2•, thus the recycling of HCO3− was achieved in this step. The generated HO2• decomposed to O2•−, which further reacted with ·OH to form 1O2, or two HO2• molecules reacted them-self to generate 1O2 (Eq (8–10)). Secondly, according to previous studies, HCO3− could react with ·OH, present in Fenton process, to generate CO3•−, and the generated CO3•− participated in the formation of HO2•, and then O2•− and 1O2 [17, 22, 43].
In traditional heterogeneous Fenton process without HCO3−, ·OH could react with H2O2 to generate HO2•, which decomposed or reacted with itself to generate O2•− and ended up generating 1O2. However, the second-order reaction rate constant of ·OH reacting with H2O2 to produce HO2• was 1.7–4.5×104 M−1 s−1 [44, 45], and the second-order reaction rate constant of CO3•− reacting with H2O2 to produce HO2• was 4.3×105 M−1 s−1 [13]. Therefore, the rate constant of CO3•− path was an order of magnitude faster than the ·OH path in generating O2•− and 1O2, which could explain the enhanced degradation efficiency of RhB by the addition of HCO3−.
Therefore, in the oxidative radicals quenching experiments, when the ·OH was captured by IPA, the reaction of HCO3− with H2O2 in generating HCO4− was not affected, and the HCO4− could be continually catalyzed by Co/Cu/zeolite to generate CO3•−, and then generated O2•− and 1O2. The moderate decrease of degradation efficiency of RhB (about 25%) was ascribed to the blocked reaction path of HCO3− with ·OH. In addition, if O2•− was captured, 1O2 could only be generated through the self-reaction of HO2•. The detailed reaction process was shown in Fig. 4 and Eq. (3–11).
3.4.2. XPS analysisThe XPS spectra (Fig. 5) of Co/Cu/zeolite before and after use exhibited C 1s, O 1s, and Co 2p and Cu 2p spin orbit peaks. The characteristic peaks at around 933.9 eV and 953.9 eV were ascribed to Cu 2p3/2 and 2p1/2 from Cu(I), and the peaks at 935.8 eV and 955.8 eV were corresponded to Cu 2p3/2 and 2p1/2 from Cu(II). According to the analysis of element proportion, Cu(II) accounted for 55.9% and 57.2% before and after the reaction, respectively, indicating that the ratio of Cu(I) and Cu(II) remained stable. Fig. 5b showed that the spin orbit peaks at around 781.2 eV and 796.7 eV were ascribed to Co 2p3/2 and 2p1/2 from Co(II), and the peaks at 780.0 eV and 795.5 eV were corresponded to Co 2p3/2 and 2p1/2 from Co(III). The proportion of Co(III) after the reaction was 67.4%, more than 50.5% before the reaction. Fig. 4 and Eq. (5) showed the electron transfer between Cu and Co that simultaneously catalyzed the heterogeneous Fenton process and the conversion of HCO4− to CO3•−. The relative stable valence state of Cu could ensure the recycling between Co(III) and Co(II), and thus the catalytic efficiency of Co/Cu/zeolite.
In addition, Cu and Co ions slightly leached out after 5 times reuse, which was less than 150 μg/L and 50 μg/L in the solution, respectively (Fig. S6a). Compared to the first time, the degradation efficiency of RhB only decreased about 5% when the catalyst was used 5 consecutive times (Fig. S6b). The catalyst prepared in this study had good stability and less metals leaching given that the leaching concentration of Co ions in other studies was about 200–300 μg/L [23, 25, 46].
4. ConclusionIn this study, the experimental results and mechanisms of HCO3− enhanced heterogeneous Fenton process were studied for degradation of aqueous pollutants RhB. Co/Cu/zeolite was prepared using sol-gel method as the catalyst for HCO3− enhanced heterogeneous Fenton process. The degradation results confirmed that HCO3− effectively promoted the oxidation capacity of heterogeneous Fenton process, and the optimal reaction conditions for HCO3− enhanced heterogeneous Fenton were obtained using response surface method. The radical quenching and EPR experiment indicated that 1O2 was the main oxidative radicals, followed by O2− and ·OH. In addition, it was illustrated that HCO4− and CO3•− formed in HCO3− enhanced heterogeneous Fenton were important radicals in the formation of 1O2. Finally, the XPS analysis indicated that the electron transfer between Cu and Co effectively catalyzed the heterogeneous Fenton process and the conversion of HCO4− to CO3•−. The above research results showed that HCO3− enhanced heterogeneous Fenton process was a promising wastewater treatment technique and provided insights for future research on Fenton advanced oxidation.
AcknowledgmentsThis research was supported by National Natural Science Foundation of China (52270147).
References1. Yue Y, Shen C, Ge Y. Biochar accelerates the removal of tetracyclines and their intermediates by altering soil properties. J. Hazard. Mater. 2019;380:2183–2188.
http://10.1016/j.jhazmat.2019.1208211
2. Zhao Q, Liu LJ, Li SZ, Liu R. Built-in electric field-assisted charge separation over carbon dots-modified Bi2WO6 nanoplates for photodegradation. Appl. Surf. Sci. 2019;465:164–171.
http://10.1016/j.apsusc.2018.09.168
3. Zhou HR, Wen ZP, Liu J, Ke J, Duan XG, Wang SB. Z-scheme plasmonic Ag decorated WO3/Bi2WO6 hybrids for enhanced photocatalytic abatement of chlorinated-VOCs under solar light irradiation. Appl. Catal. B: Environ. 2019;242:76–84.
http://10.1016/j.apcatb.2018.09.090
4. Liang YT, Pei M, Wang DD, Cao SN, Xiao X, Sun B. Improvement of Soil Ecosystem Multifunctionality by Dissipating Manure-Induced Antibiotics and Resistance Genes. Environ. Sci. Technol. 2017;51:4988–4998.
http://10.1021/acs.est.7b00693
5. Deshpande BD, Agrawal PS, Yenkie MKN. Nanoparticles aided AOP for degradation of p-nitro benzoic acid. Mater. Today. Proc. 2020;32:519–523.
http://10.1016/j.matpr.2020.02.924
6. Oller I, Malato S, Sanchez-Perez JA. Combination of Advanced Oxidation Processes and biological treatments for wastewater decontamination-a review. Sci. Total. Environ. 2011;409:4141–4166.
http://10.1016/j.scitotenv.2010.08.061
7. Li XL, Yang HY, Pan J, et al. Variation of the toxicity caused by key contaminants in industrial wastewater along the treatment train of Fenton-activated sludge-advanced oxidation processes. Sci. Total. Environ. 2023;858:159856.
http://10.1016/j.scitotenv.2022.159856
8. Saravanan A, Deivayanai VC, Kumar PS, et al. A detailed review on advanced oxidation process in treatment of wastewater: Mechanism, challenges and future outlook. Chemosphere. 2022;308:136524.
http://10.1016/j.chemosphere.2022.136524
9. Ratanatamskul C, Chintitanun S, Masomboon N, Lu MC. Inhibitory effect of inorganic ions on nitrobenzene oxidation by fluidized-bed Fenton process. J. Mol. Catal. A: Chem. 2010;331:101–105.
http://10.1016/j.molcata.2010.08.007
10. Pignatello JJ. Dark and photoassisted iron(3+)-catalyzed degradation of chlorophenoxy herbicides by hydrogen peroxide. Environ. Sci. Technol. 1992;26:944–951.
http://10.1021/es00029a012
11. Lu MC, Chen JN, Chang CP. Effect of inorganic ions on the oxidation of dichlorvos insecticide with Fenton’s reagent. Chemosphere. 1997;35:2285–2293.
http://https://doi.org/10.1016/S0045-6535(97)00307-X
12. Wilkin RT, Digiulio DC. Geochemical Impacts to Groundwater from Geologic Carbon Sequestration: Controls on pH and Inorganic Carbon Concentrations from Reaction Path and Kinetic Modeling. Environ. Sci. Technol. 2010;44:4821–4827.
http://10.1021/es100559j
13. Wu CL, Linden KG. Phototransformation of selected organophosphorus pesticides: roles of hydroxyl and carbonate radicals. Water Res. 2010;44:3585–3594.
http://10.1016/j.watres.2010.04.011
14. Yu DY, Wu MH, Lin JX, Zhu JT. Economical Low-Temperature Bleaching of Cotton Fabric Using an Activated Peroxide System Coupling Cupric Ions with Bicarbonate. Fibers. Polym. 2018;19:1898–1907.
http://10.1007/s12221-018-7963-z
15. Philip Jones D, Griffith WP. Alkali-metal peroxocarbonates, M2[CO3]·nH2O2, M2[C2O6], M[HCO4]·nH2O, and Li2[CO4]· H2O. J. Chem. Soc., Dalton Trans. 1980;2526–2532.
http://10.1039/DT9800002526
16. Flangan J, Philip Jones D, Griffith WP, Skapski AC, West AP. On the existence of peroxocarbonates in aqueous solution. J. Chem. Soc., Chem. Commun. 1986;20–21.
http://10.1039/C39860000020
17. Zhao SP, Xi HL, Zuo YJ, Wang Q, Wang ZC, Yan ZY. Bicarbonate-activated hydrogen peroxide and efficient decontamination of toxic sulfur mustard and nerve gas simulants. J. Hazard. Mater. 2018;344:136–145.
http://10.1016/j.jhazmat.2017.09.055
18. Fakhraian H, Valizadeh F. Activation of hydrogen peroxide via bicarbonate, sulfate, phosphate and urea in the oxidation of methyl phenyl sulfide. J. Mol. Catal. A: Chem. 2010;333:69–72.
http://10.1016/j.molcata.2010.09.017
19. Bennett DA, Yao H, Richardson DE. Mechanism of Sulfide Oxidations by Peroxymonocarbonate. Inorg. Chem. 2001;40:2996–3001.
http://10.1021/ic000910h
20. Yao HR, Richardson DE. Bicarbonate Surfoxidants: Micellar Oxidations of Aryl Sulfides with Bicarbonate-Activated Hydrogen Peroxide. J. Am. Chem. Soc. 2003;125:6211–6221.
http://10.1021/ja0274756
21. Balagam B, Richardson DE. The Mechanism of Carbon Dioxide Catalysis in the Hydrogen Peroxide N-Oxidation of Amines. Inorg. Chem. 2008;47:1173–1178.
http://10.1021/ic701402h
22. Pi L, Yang N, Han W, et al. Heterogeneous activation of peroxymonocarbonate by Co-Mn oxides for the efficient degradation of chlorophenols in the presence of a naturally occurring level of bicarbonate. Chem. Eng. J. 2018;334:1297–1308.
http://10.1016/j.cej.2017.11.006
23. Jawad A, Lu XY, Chen ZQ, Yin GC. Degradation of Chlorophenols by Supported Co–Mg–Al Layered Double Hydrotalcite with Bicarbonate Activated Hydrogen Peroxide. J. Phys. Chem. A. 2014;118:10028–10035.
http://10.1021/jp5085313
24. Xu AH, Li XX, Xiong H, Yin GC. Efficient degradation of organic pollutants in aqueous solution with bicarbonate-activated hydrogen peroxide. Chemosphere. 2011;82:1190–1195.
http://10.1016/j.chemosphere.2010.11.066
25. Duan L, Chen YL, Zhang KX, Luo HY, Huang JX, Xu AH. Catalytic degradation of Acid Orange 7 with hydrogen peroxide using CoxOy-N/GAC catalysts in a bicarbonate aqueous solution. Rsc. Adv. 2015;5:84303–84310.
http://10.1039/c5ra13603b
26. Duan L, Sun BZ, Wei MY, et al. Catalytic degradation of Acid Orange 7 by manganese oxide octahedral molecular sieves with peroxymonosulfate under visible light irradiation. J. Hazard. Mater. 2015;285:356–365.
http://10.1016/j.jhazmat.2014.12.015
27. Li YB, Guo LS, Huang DK, et al. Support-dependent active species formation for CuO catalysts: Leading to efficient pollutant degradation in alkaline conditions. J. Hazard. Mater. 2017;328:56–62.
http://10.1016/j.jhazmat.2016.12.063
28. Jawad A, Chen ZQ, Yin GC. Bicarbonate activation of hydrogen peroxide: A new emerging technology for wastewater treatment. Chinese. J. Catal. 2016;37:810–825.
http://10.1016/s1872-2067(15)61100-7
29. Zhou L, Song W, Chen ZQ, Yin GC. Degradation of Organic Pollutants in Wastewater by Bicarbonate-Activated Hydrogen Peroxide with a Supported Cobalt Catalyst. Environ. Sci. Technol. 2013;47:3833–3839.
http://10.1021/es400101f
30. Wang TC, Jia HZ, Guo XT, et al. Evaluation of the potential of dimethyl phthalate degradation in aqueous using sodium percarbonate activated by discharge plasma. Chem. Eng. J. 2018;346:65–76.
http://10.1016/j.cej.2018.04.024
31. Guo H, Jiang N, Wang HJ, et al. Enhanced catalytic performance of graphene-TiO2 nanocomposites for synergetic degradation of fluoroquinolone antibiotic in pulsed discharge plasma system. Appl. Catal. B: Environ. 2019;248:552–566.
http://10.1016/j.apcatb.2019.01.052
32. Tang SF, Li N, Yuan DL, et al. Comparative study of persulfate oxidants promoted photocatalytic fuel cell performance: Simultaneous dye removal and electricity generation. Chemosphere. 2019;234:658–667.
http://10.1016/j.chemosphere.2019.06.112
33. Grigalavicius M, Mastrangelopoulou M, Berg K, et al. Proton-dynamic therapy following photosensitiser activation by accelerated protons demonstrated through fluorescence and singlet oxygen production. Nat. Commun. 2019;10:3986.
http://10.1038/s41467-019-12042-7
34. Cao Y, Qian XC, Zhang YX, et al. Decomplexation of EDTA-chelated copper and removal of copper ions by non-thermal plasma oxidation/alkaline precipitation. Chem. Eng. J. 2019;362:487–496.
http://10.1016/j.cej.2019.01.061
35. Deshpande BD, Agrawal PS, Yenkie MKN, Dhoble SJ. Prospective of nanotechnology in degradation of waste water: A new challenges. Nano-Struct. Nano-Objects. 2020;22:100442.
http://10.1016/j.nanoso.2020.100442
36. Ji J, Liu Y, Yang XY, Xu J, Li XY. Multiple response optimization for high efficiency energy saving treatment of rhodamine B wastewater in a three-dimensional electrochemical reactor. J. Environ. Manage. 2018;218:300–308.
http://10.1016/j.jenvman.2018.04.071
37. Yan QY, Zhang JL, Xing MY. Cocatalytic Fenton Reaction for Pollutant Control. Cell. Rep. Phys. Sci. 2020. 1:
http://10.1016/j.xcrp.2020.100149
38. Yi QY, Ji JH, Shen B, et al. Singlet Oxygen Triggered by Superoxide Radicals in a Molybdenum Cocatalytic Fenton Reaction with Enhanced REDOX Activity in the Environment. Environ. Sci. Technol. 2019;53:9725–9733.
http://10.1021/acs.est.9b01676
39. Ogilby PR. Singlet oxygen: there is indeed something new under the sun. Chem. Soc. Rev. 2010;39:3181–3209.
http://10.1039/B926014P
40. Zhao QQ, Zhang R, Ye DX, Zhang S, Chen H, Kong JL. Ratiometric Fluorescent Silicon Quantum Dots–Ce6 Complex Probe for the Live Cell Imaging of Highly Reactive Oxygen Species. ACS. Appl. Mater. Interfaces. 2017;9:2052–2058.
http://10.1021/acsami.6b12047
41. Maranzana A, Canepa C, Ghigo G, Tonachini G. Theoretical Study on the Reactivity and Regioselectivity of the Ene Reaction of 1Δg O2 with α,β-Unsaturated Carbonyl Compounds. Eur. J. Org. Chem. 2005;2005:3643–3649.
http://https://doi.org/10.1002/ejoc.200500215
42. DeRosa MC, Crutchley RJ. Photosensitized singlet oxygen and its applications. Coord. Chem. Rev. 2002;233:351–371.
http://10.1016/s0010-8545(02)00034-6
43. Pan HP, Gao Y, Li N, Zhou Y, Lin QT, Jiang J. Recent advances in bicarbonate-activated hydrogen peroxide system for water treatment. Chem. Eng. J. 2021;408:127332.
http://10.1016/j.cej.2020.127332
44. Ghatak HR. Advanced Oxidation Processes for the Treatment of Biorecalcitrant Organics in Wastewater. Crit. Rev. Environ. Sci. Technol. 2014;44:1167–1219.
http://10.1080/10643389.2013.763581
45. Bianco B, Ide DM, Veglio F. Fenton treatment of complex industrial waste-water: optimization of process conditions by surface response method. J. Hazard. Mater. 2011;186:1733–1738.
http://10.1016/j.jhazmat.2010.12.054
46. de Lima LB, Pereira LO, de Moura SG, Magalhaes F. Degradation of organic contaminants in effluents-synthetic and from the textile industry-by Fenton, photocatalysis, and H2O2 photolysis. Environ. Sci. Pollut. Res. Int. 2017;24:6299–6306.
http://10.1007/s11356-016-6973-x
Fig. 2Influence of different anions on the degradation of RhB (a): anions concentration 20mM, H2O2 dosage 30mM, Co/Cu/zeolite dosage 0.3 g/L, reaction time 1 h; The effect of NaHCO3 and catalyst on the degradation of RhB (b): NaHCO3 concentration 30mM, H2O2 dosage 20mM, Co/Cu/zeolite dosage 0.2 g/L, reaction time 2 h. 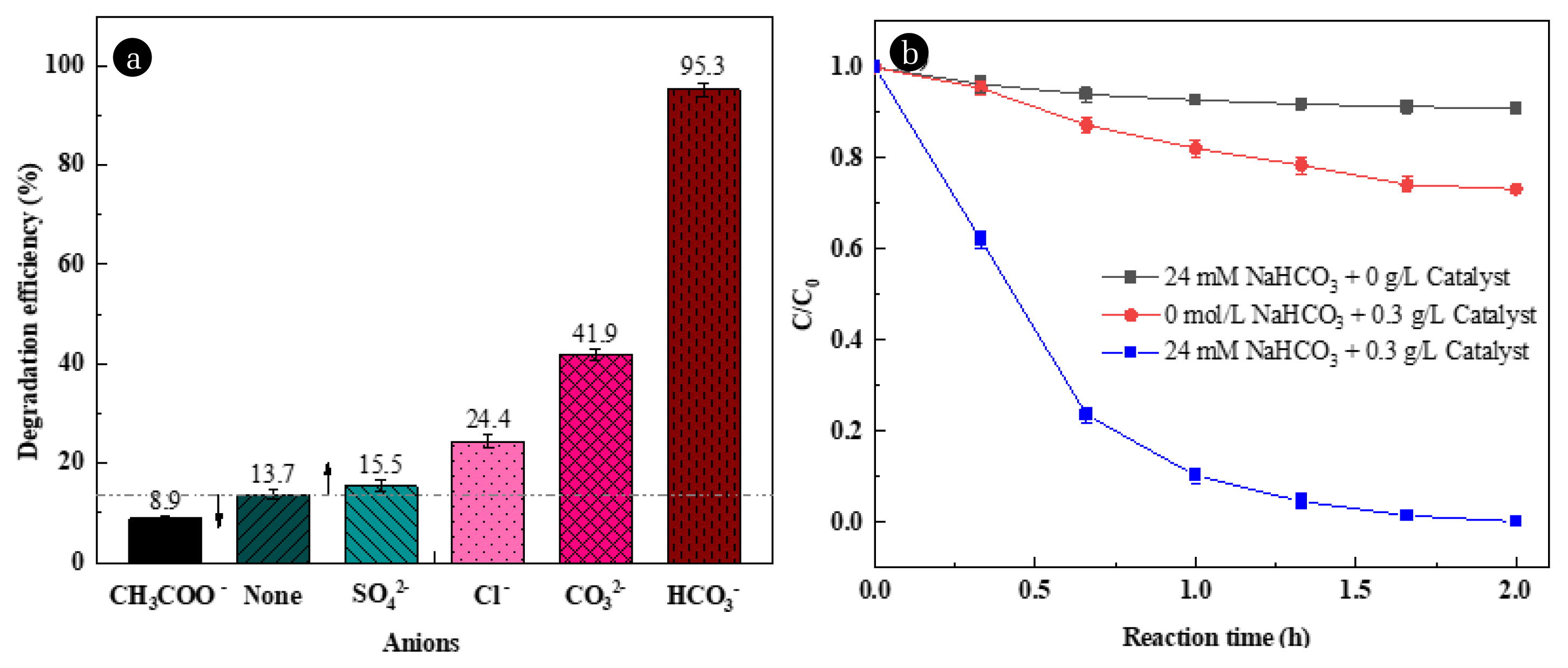
Fig. 3Oxidative radicals quenching experiment (a): NaHCO3 concentration 24mM, H2O2 dosage 30mM, Co/Cu/zeolite dosage 0.3 g/L, and EPR detection signal of 1O2 at different NaHCO3 concentrations (b). 
Fig. 4Illustration of the proposed reaction mechanisms of HCO3− enhanced Co/Cu/zeolite catalyzed heterogeneous Fenton process. 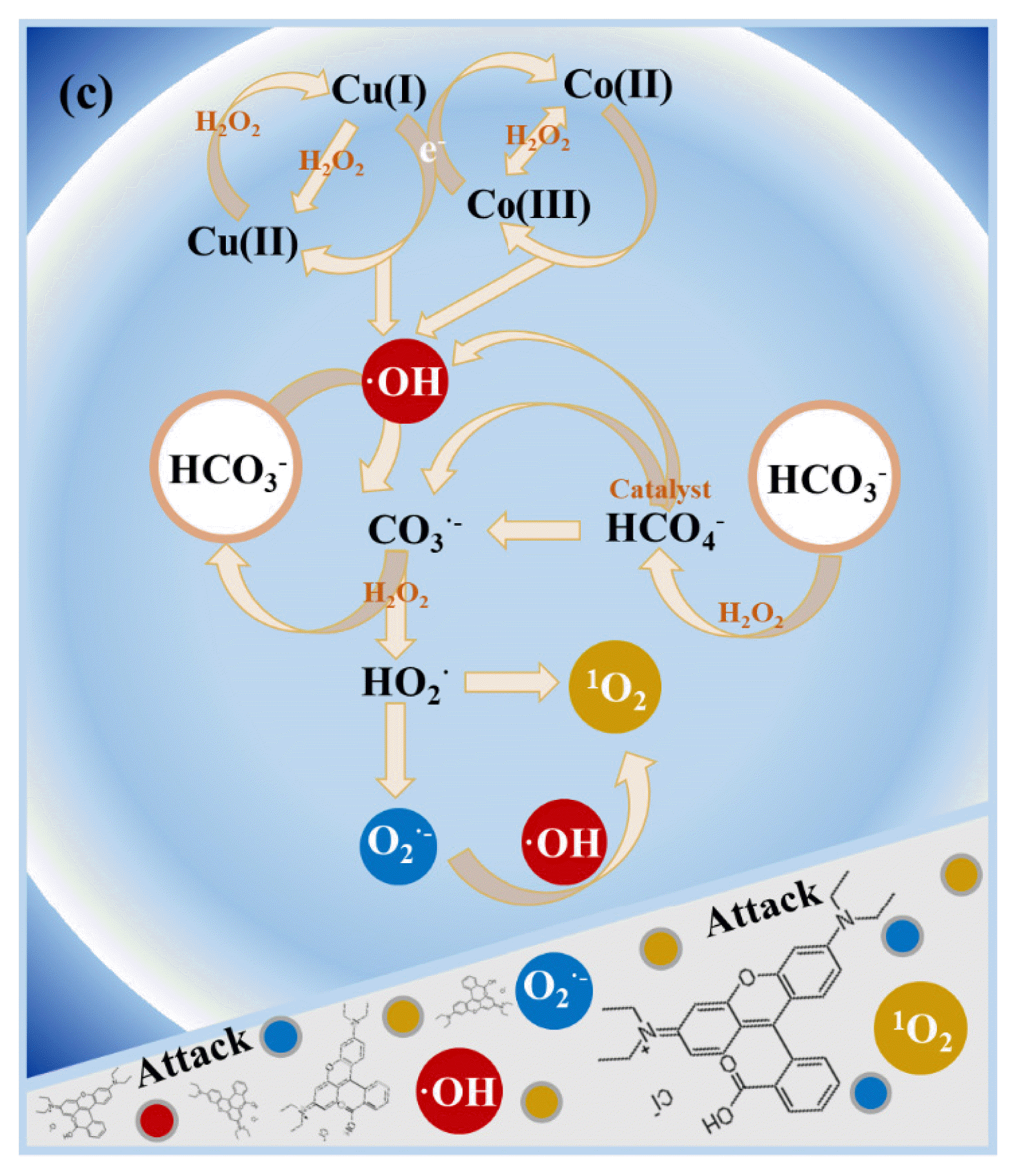
Fig. 5XPS diagram of the survey spectra (a), Co 2P (b) and Cu 2P (c) of Co/Cu/zeolite before and after use. 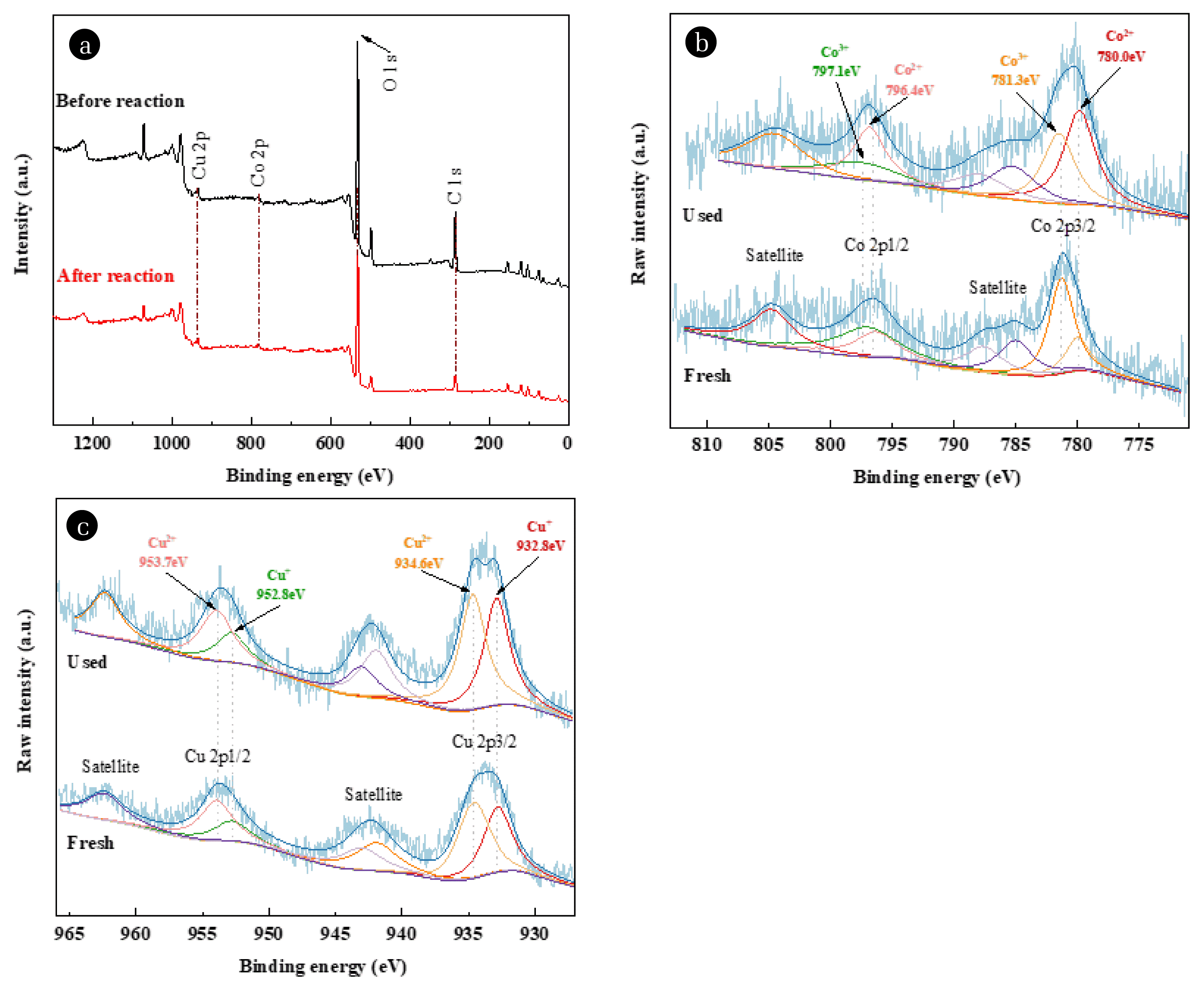
Table 1The experimental factors and limits of the RSM design
Table 2ANOVA results for the degradation efficiency |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||