1. Introduction
Several waves of the different mutations of the SARS-CoV-2 virus have demonstrated that face masks are an essential part of personal protective equipment (PPE), not only for frontline healthcare workers but for the general population as well. This has also resulted in severe and acute shortages of efficient and cost-effective masks. In the early days of the pandemic, healthcare facilities ran out of PPE and were forced to reuse single-use masks for days on end. Additionally, a positive correlation has been found between long-term exposure to air pollution and one’s susceptibility and vulnerability to coronavirus (e.g.: SARS and SARS-CoV-2 in 2003 and 2019, respectively) [1,2,3,4,5]. For example, if the amount of PM2.5, particulate matter with diameters under 2.5 μm, increases by a mere 1 μg/m3, the COVID-19 death rate was projected to increase by 11% in 2020 [2].
Air pollution can lead to severe health conditions, causing 7 million deaths every year [6]. This is exacerbated by the fact that 99% of the people in the world live in areas that exceed the air quality guidelines of the World Health Organization, 10 μg/m3, causing respiratory illnesses such as lung cancer, COPD, and respiratory infections, as well as cardiovascular diseases, and strokes [6]. These impacts are caused due to the size of the PM2.5 particles—small enough to pass through the nasal passage, penetrate the lungs, and enter the bloodstream yet large enough to avoid the effect of Brownian motion [7,8,9]. Access to effective and affordable filtration devices has remained a challenge because developing countries with limited resources (such as those in Asia and Africa) correlate with the areas of the world suffering from pollution most severely due to their reliance on fossil fuels, dense urbanization, and a lack of adequate measures of controlling pollution and its side-effects [6].
Respiratory viruses are amongst the most frequent causative agents of disease in humans, sometimes leading to endemic and pandemic proportions. Certain respiratory diseases (including those caused by excessive air pollution) can lead to the angiotensin-converting enzyme 2 (ACE2) receptors being overexpressed in human respiratory cells, leading to viral attachment. One such virus, SARS-CoV-2’s spike protein can attach to the overexpressed receptors in the epithelial cells of the respiratory tract. Therefore, the severity of the disease may increase due to the overexpressed receptors [8]. However, the spike protein in the SARS-CoV-2 virus can be oxidized by metal-based nanoparticles due to their generation of reactive oxygen species [10,11]. This researcher has previously identified a select combination of nanoparticles that are effective in filtering both particulate matter and virus particles [12,13,14].
Researchers worldwide have focused on the safety of nanoparticles for human use and their potential risks [15,16]. For example, silver nanoparticles have shown great promise towards disinfection capability and cancer treatment [11,16], but acute pulmonary effects from the inhalation of silver nanoparticles have also been found [16]. Hence a careful selection of nanoparticles with attention to their permissible exposure limits, as per OSHA standards [18] is critical, especially for application on face masks.
As the production and extensive usage of face masks has significantly increased due to the COVID-19 pandemic, it has generated more than eight million tons of pandemic-associated plastic waste globally, with more than 25,000 tons entering the global oceans affecting the land and marine environment in a short period, contributing to micro-plastic pollution [19,20].
The objective of this study is to develop an engineered face mask with optimized nanoparticle layering for the filtration of air pollutants like PM2.5 and viral pathogens like SARS-CoV-2. Furthermore, the goal of this work is to develop a simple and versatile application technique of the nanoparticles such that it can be applied in different parts of the world, thus providing an affordable and comparable alternative to expensive PPE. A rechargeable concept has been developed to increase the durability and reusability of face masks to find a sustainable solution to the adverse environmental effects caused by the increased usage of this critical protective equipment. This engineered face mask aims to provide an effective and sustainable solution to air pollution, airborne viral pathogens, and plastic pollution.
2. Materials and Methods
2.1. Nanoparticle Deposition Method
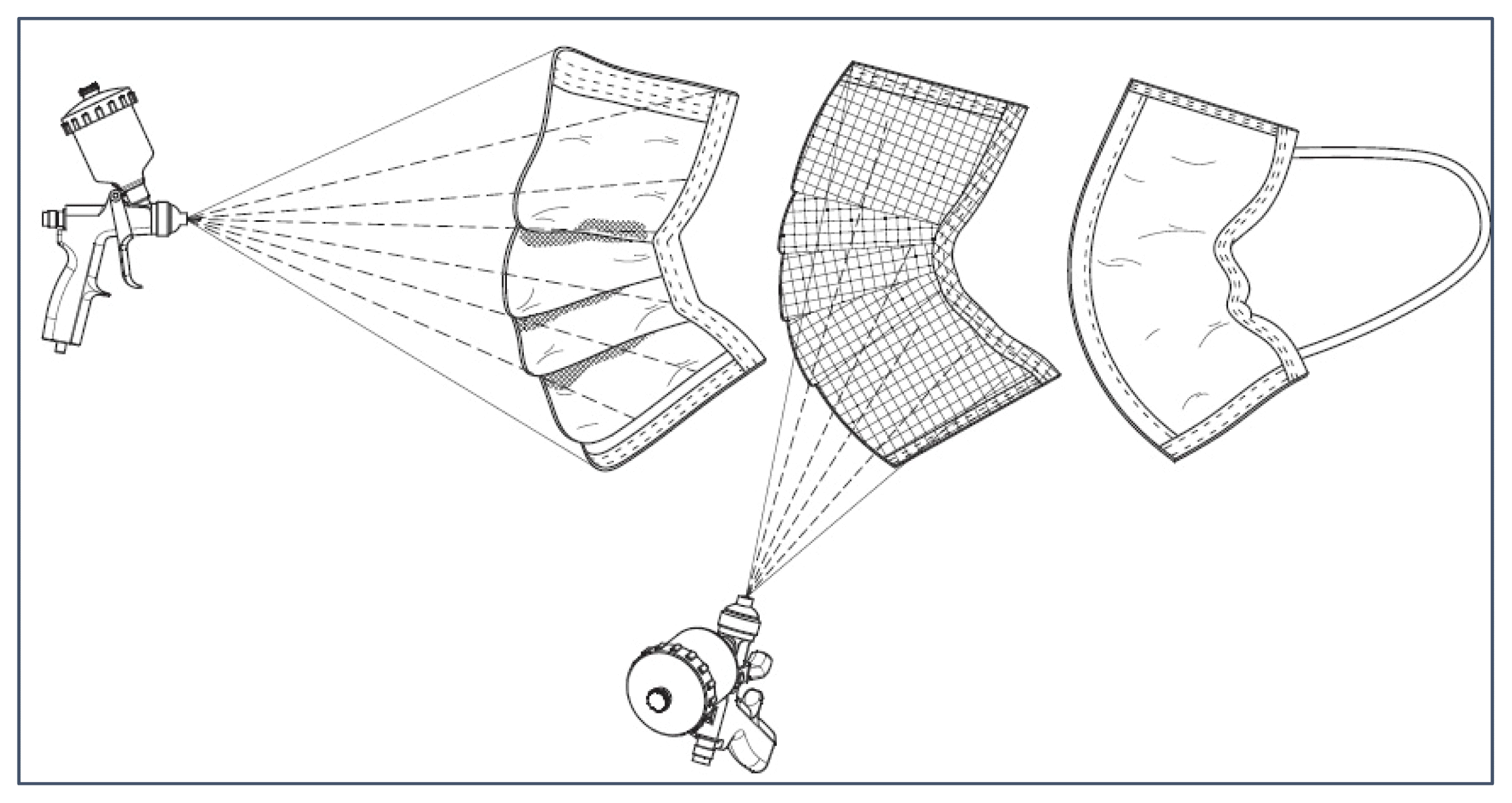
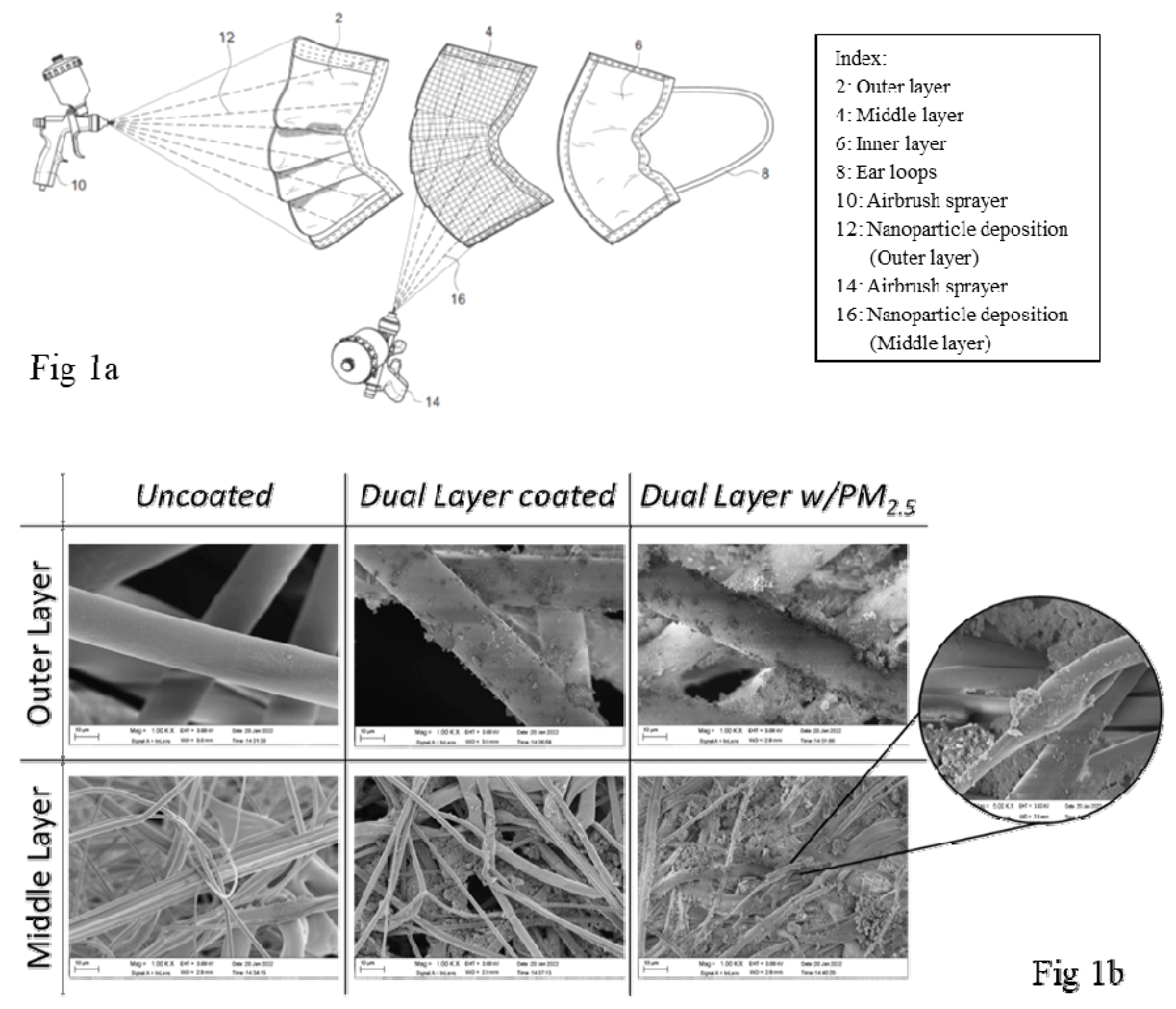
There were several nanoparticles used for this study: graphene (Alfa Aesar™ nanoplatelets aggregates, S.A. 500m2/g, Fisher-Scientific), titanium dioxide (TiO2 Anatase, 99.5% 40nm, US Research Nanomaterials, Inc. (USRNI)), zinc oxide (ZnO, 99+%, 35–45 nm, USRNI), copper oxide (CuO, 99%, 40nm, USRNI), and silicon dioxide (SiO2, 99.5+%, 15–20nm, P-type, Porous, USRNI). These nanoparticles were combined to create a suspension with ethanol (200 Proof (100%), USP/EP/ACS, Fisher-Scientific), appearing as a slurry [13]. A pressurized sprayer system (both the Gocheer airbrush sprayer, 25 psi, 10 LPM, and the Preval Airless Paint Sprayer, 70 psi, were tested) was used to aerosolize the nanoparticle suspension, spraying the masks at 15–18 cm (Fig 1a). The masks were left to air-dry for 8 hours (minimum), then used for testing. A layered deposition technique was applied by cutting the mask open from its periphery and applying selective nanoparticles in the outer and middle layers of the masks. SiO2 nanoparticles were deposited on the outer non-woven layer for its desiccant and adsorption properties [21] while the other nanoparticles (ZnO, TiO2, Graphene, CuO) were deposited onto the melt-blown middle layer to improve filtration efficiency and retention efficacy [22,23,24, 25,26]. The inner layer of the mask is typically made of adsorbent materials to trap mucus, saliva droplets, and moisture from exhaled air from the user. Hence, this layer was left uncoated; also reducing the risk of nanoparticle inhalation.
2.2. Surface Morphology of Nanoparticle Coatings
A scanning electron microscope (Zeiss Ultra-55 SEM with a Schottky field emission source and resolution of 1 nm at 15 KV, 1.7 nm at 1 KV) was used to characterize the surface morphology of the different masks tested. Figure 1b demonstrates images of three typical surgical masks; one without any coatings, one with nanoparticle coatings before and one after exposure to PM2.5, at 1000X magnification. These images illustrate the differences in fiber size and density of the inner and outer layers of the surgical masks. The middle layer has much thinner fibers (300 μm diameter) randomly oriented to enable the impaction and diffusion mechanisms of particle entrapment. Examining the images of the coated masks confirms the adhesion of the nanoparticles to fibers in the outer and middle layers of the mask. The images labeled ‘Dual Layer w/PM2.5’ confirm the entrapment of particulate matter onto the nanoparticle surfaces of the coated masks, as also seen in the enhanced view with 5000X magnification. This is due to the characteristic properties of the embedded nanoparticle, which enhance the preexisting filtration mechanisms of the masks. The nanoparticles’ high surface-to-volume ratios and photocatalytic activation properties amplify the diffusion and electrostatic attraction of the masks, increasing their efficacy.
2.3. Experimental Set-up
The particulate filtration efficiency (PFE) of the masks was tested using a designed wind tunnel (Fig 2a). The body of the tunnel was created using a 122x61x30.5 cm plexiglass enclosure, with a 10.2 cm x 244 cm aluminum exhaust tube (Imperial) connecting the PM2.5 source to the wind tunnel’s inlet. The source of PM2.5 was tested using either incense sticks, wood chips, or paraffin. A draft between the inlet and outlet was created using a small fan (Holmes HAOF 85, 23x23 cm) inside the tunnel. To simulate human breathing, vacuum pumps (HSH-Flow, 6W, 8 LPM, 120 KPa) were attached to two silicone mannequin heads (Yephets, 23x15x10 cm). PM2.5 levels were measured using laser particle detectors (Temtop, LKC-1000S) connected between the mannequins and vacuum pump by plastic tubing (Ø 0.64 cm) and funnels (Ø 7 cm). One mannequin was fitted with a nanoparticle-coated mask, while the other was tested without a mask as a control. The pressure drop of the filters was measured using a manometer (PerfectPrime, AR1890P2). The photocatalytic properties of the nanoparticles were measured using a UV lamp (Houlight, UV-A, 385–400 nm, 10W) and a 5000K lamp (Hyperikon, 15W) as a control, simulating daylight. Two different types of masks—surgical (Dr. Moxa, 3-layer disposable, 33% melt blown, 67% non-woven) and KN95 (WWDoll, 5-layer, 51% non-woven, 29% melt blown, 20% hot air cotton)—were tested with nanoparticles, and without any nanoparticles as controls. Measuring and comparing the flow rate (μg/m3) of PM2.5, of the masked (ϕ2) and control (ϕ1) mannequins placed side-by-side and exposed to the same environment was used to evaluate the PFE as shown in Eq. (1),
where ϕ1 is the PM2.5 without mask, and ϕ2 is the PM2.5 with mask.
The mannequin with the coated mask was connected to a vacuum pump (used to simulate human breathing) to test for nanoparticle retention efficacy (NRE) (Fig 2c). Two different vacuum pumps were used to simulate regular breathing (Lab Fish, Oilless Diaphragm, 8 LPM) and strenuous breathing (Zeny, Single-Stage 5 Pa Rotary Vane, 85 LPM). A fine-pore filter paper (Supertek, Grade 1, Ø 110 mm, 9 nm pore Ø) collected any dislodged nanoparticles. In the experimental set-up, it was enclosed between two funnels (creating a collection chamber) and a gasket for sealing the chamber (Plumb Pak, Ø 3.8cm). The two funnels were then secured with binder clips (Acco, 3.2 cm). The sealing capabilities of the gasket were verified using the soap-bubble leak test. A plexiglass enclosure (61x30.5x30.5 cm) encased this set-up to maintain similar environmental conditions throughout the length of the experiment.
It was important to ensure that any dislodged nanoparticles would be within the Permissible Exposure Limit (PEL), per OSHA’s standards, if inhaled [18]. Therefore, the NRE of the masks was tested. The PEL Utilization of the masks was determined by measuring the weight of nanoparticles dislodged from the mask after eight hours of operation (ωi) in the experimental set-up to the PEL limit set by OSHA for that specific nanoparticle (ωiPEL). Calculating a weighted average of the nanoparticles was enabled by collecting the total weight of the nanoparticles using a micro-balance (0.1 mg accuracy) through a gravimetric method. This is a test procedure recommended by the Center for Disease Control (CDC) [27], allowing one to use the nanoparticles ratio of molecular weights to calculate their individual weights, Eq. (2).
where n is the number of NPs in mask, i is the NP type, ωi is the weight of NPi inhaled from mask (mg), ωiPEL is the permissible exposure limit for NPi (mg/m3), v̇j is the breathing flow rate for time j, (lpm), tj is the time at this breathing rate (hours), and PEL is the permissible exposure limit per OSHA standard.
To test for virus filtration efficiency (VFE, Fig 2b), nebulized NaCl particles (0.9% saline solution made from distilled water and table salt) were used as a surrogate for respiratory droplets charged with viruses, as advised by test protocol of the CDC [27,28]. The nebulizer (Mayluck, 0.25 ml/min atomization rate, 0.5–10 μm particle size) was connected to a mannequin that simulated the exhalation of NaCl particles that passed to another mannequin wearing the mask being tested that simulated the inhalation of the particles. The mannequins were separated at a distance of 25 cm, and the nebulizer ran for 20 minutes per mask tested. This set-up was designed to deposit quantifiable amounts of NaCl particles in the collection chamber so they could be accurately and precisely quantified by the microbalance. Similar to the previous set-up, these mannequins were placed within a plexiglass chamber (122x30.5x30.5 cm) during testing so the outside environment would not disturb the flow of NaCl particles, maintaining consistent results. As seen in figure 2c, comparing the weight of NaCl that passed through the mask onto the fine filter paper (γ2) to the control without the mask (γ1) was used to calculate VFE, Eq. (3),
where γ1 is the NaCl (virus surrogate) inhaled without mask, and γ2 is the NaCl inhaled with mask.
Virus filtration efficiency with viral pathogens, VFE2, was measured with viral pathogens using the experimental set-up as illustrated in Fig 2d. A nebulizer (Aeroneb, NEB-1000, >0.3 ml/min, VMD 4–6 μm) was operated for 30 seconds to aerosolize the virus particles through a piece of the mask being tested, into a sealed collection chamber (Kent Scientific, VetFlo 0530LG, 22.9 x 15.2 x 15.2 cm). A vacuum pump (Gast, 1531-320-G557X, 8 LPM) was operated for 5 minutes to create a negative pressure for the aerosolized viral particles to get collected at the impinger (Kontes Midget Impinger, TS 24/40). This solution was analyzed for plaque forming units (PFU2) and compared to the extracted stock solution (PFU1) using the plaque assay method [29] to calculate the VFE2 filtration efficiency, Eq. (4),
where PFU1 is the plaque forming units in the virus exposure aerosol, and PFU2 is the plaque forming units reconstituted from the filter.
2.4. Viral Load Calculation
Prior literature and studies focused on respiratory droplet sizes [29] indicated that using particle sizes between 0.8 and 5 μm was necessary for this experiment. The median mass aerodynamic diameter (MMAD) of the droplets produced by the nebulizer used in this study was 5.3 μm. This result correlates with the range of sizes for typical aerosolized respiratory droplets, as detailed in previous work published by this researcher [13]. The viral load in this experiment was calculated using the MMAD of the nebulized particles produced, the time of exposure of the masks to the particles, the volume of the enclosure, and the volume flow rate of the nebulizer [13].
3. Results
3.1. Filtration Efficiency
The nanoparticle coatings on regular surgical masks improved particulate and virus filtration efficiency. VFE improved by ≈140% for dual-layer masks as compared to uncoated ones (Fig 3a) while PFE also improved by ≈100% (Fig 3b). The improvement from single-layer to dual-layer coating is more significant in VFE than in PFE. It is hypothesized that this is due to the filtration mechanisms and the filtrate being tested. The PFE experiment specifically enhances the mask’s preexisting mechanisms of diffusion and electrostatic attraction through the nanoparticles that entrap PM2.5 particles due to their unique properties (their high surface-to-volume ratio, and photocatalytic capabilities). This interaction happens similarly in the outer and middle layers of the mask. In the VFE experiment, the aerosolized NaCl particles are adsorbed more in the outer layer due to the desiccant properties of the SiO2 nanoparticles. However, the virucidal properties of metal-based nanoparticles like CuO and ZnO are not activated with the NaCl surrogate. Ongoing research by this author as described in Fig 2d will be used to validate this effect.
Two types of pressurized spraying systems, the airbrush, and the pressurized sprayer were used in this study to evaluate the improvement in filtration efficiency of the masks. The airbrush spraying system was more effective in improving PFE as compared to the pressurized sprayer, as seen in Fig 3c. The low-pressure airbrush sprayer is more controllable and enhances the uniformity in deposition, which in turn improves the adherence of the nanoparticles onto the mask fibers thus increasing the particulate adsorption surfaces.
3.2. Versatility
The versatility of the nanoparticle-coated masks was evaluated by exposing them to different sources of PM2.5: incense, paraffin, and wood chips. Incense [31,32] and wood chips are used to simulate the pollution from wildfires and indoor cooking [33,34], the two major sources of PM2.5. Paraffin (candles) is used to simulate indoor pollution in communities with limited resources who do not have access to electricity. The PFE was found to be independent of the source of PM2.5, as seen in Fig 3d. The size of the PM particles is the primary driver for the diffusion and electrostatic attraction filtration mechanisms, which are enhanced by the presence of nanoparticles in the fibrous layers of the masks. The consistency in the size of the PM2.5 particles generated from these various sources explains the consistency in the filtration efficiency. A previous study by this author also investigated the different sources of air pollution and found this filtration mechanism independent of the sources and global levels of air pollution [14].
3.3. Rechargeability
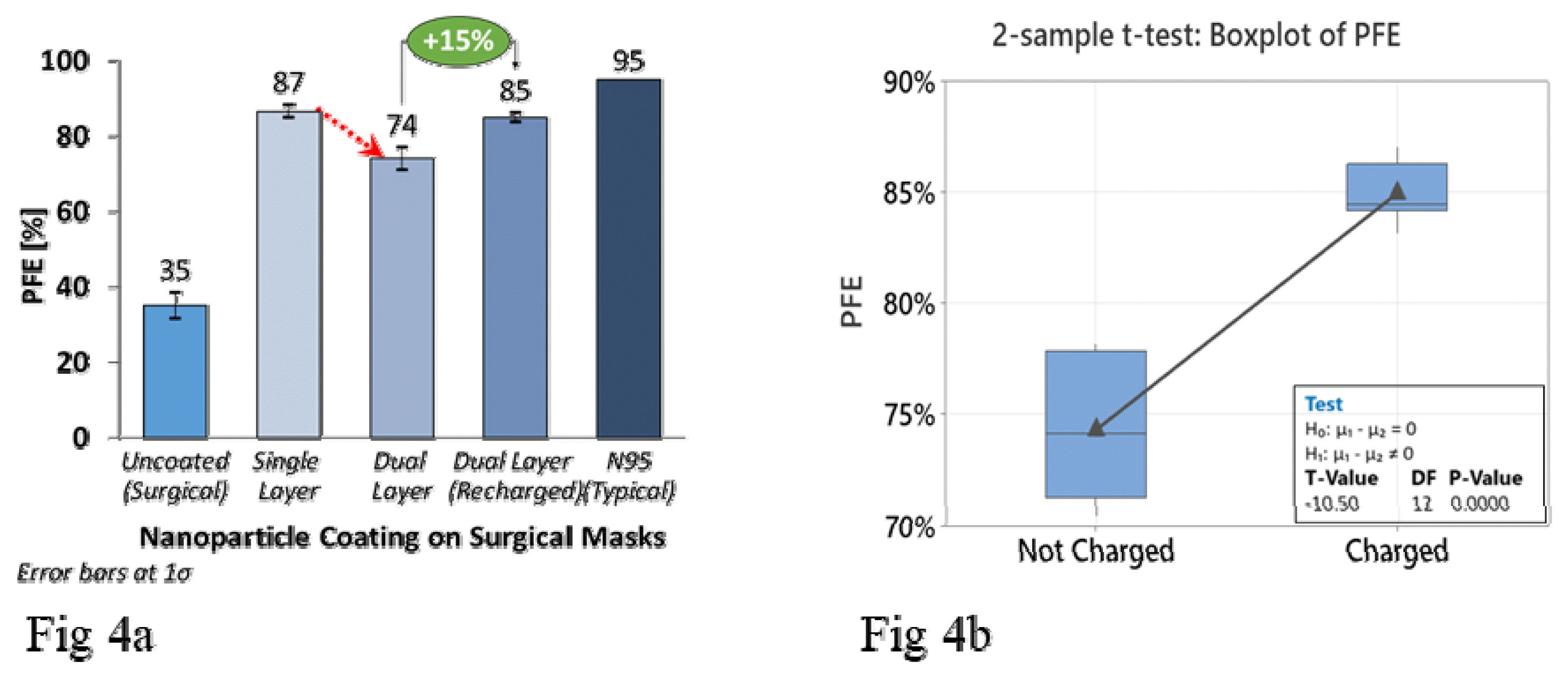
A degradation in PFE of the dual-layer coated masks was initially observed, as seen in Fig 4a. This decline can be attributed to the degeneration of the electrostatic field in the middle electret layer of the mask in presence of moisture/ethanol that was used as a solvent for the nanoparticles during the deposition process, which has been also observed by other researchers [35,36,37]. The masks were then recharged and the PFE was restored by ≈15%, to the levels of a single-layer coated mask (Fig. 4a). A 2-sample t-test confirmed the statistical significance of charging over multiple samples (Fig. 4b). Typically, the melt-blown layers are electrostatically charged by corona discharge or triboelectric methods [36]. However, a 3V D-cell battery pack was able to restore the electrostatic field with a charging time of 8–10 hours.
3.4. Durability
Durability testing was performed on the coated and uncoated masks with an accelerated testing method by increasing the amount of PM2.5 particles (increasing the number of incense sticks) to perform the tests within a reasonable amount of measurement time window, as is typical in the industry. The assumption behind this method of testing is that the efficiency reduction is primarily dependent on the number of particles cumulatively flowing through the mask. This is because the primary methods of particle capture enhanced by nanoparticles are via adsorption and electrostatic attraction mechanisms.
Each incense stick generates about 2 times the PM2.5 particles (300 ppm) as compared to a typical polluted city (150 ppm). Twenty incense sticks were used simultaneously to generate 40 times the regular exposure in a polluted city. Datapoints were collected every 12 minutes, thus simulating 8 hours (a workday) of mask wear, as expressed in Eq. (5),
where taccel is the equivalent accelerated time of one workday (8 hours), ttest is the tested time (12 mins), pcity is the PM2.5 of a typical polluted city (150 μg/m3), pincense is the PM2.5 per incense stick (300 μg/m3), and nincense is the number of incense sticks used (20) for the accelerated testing.
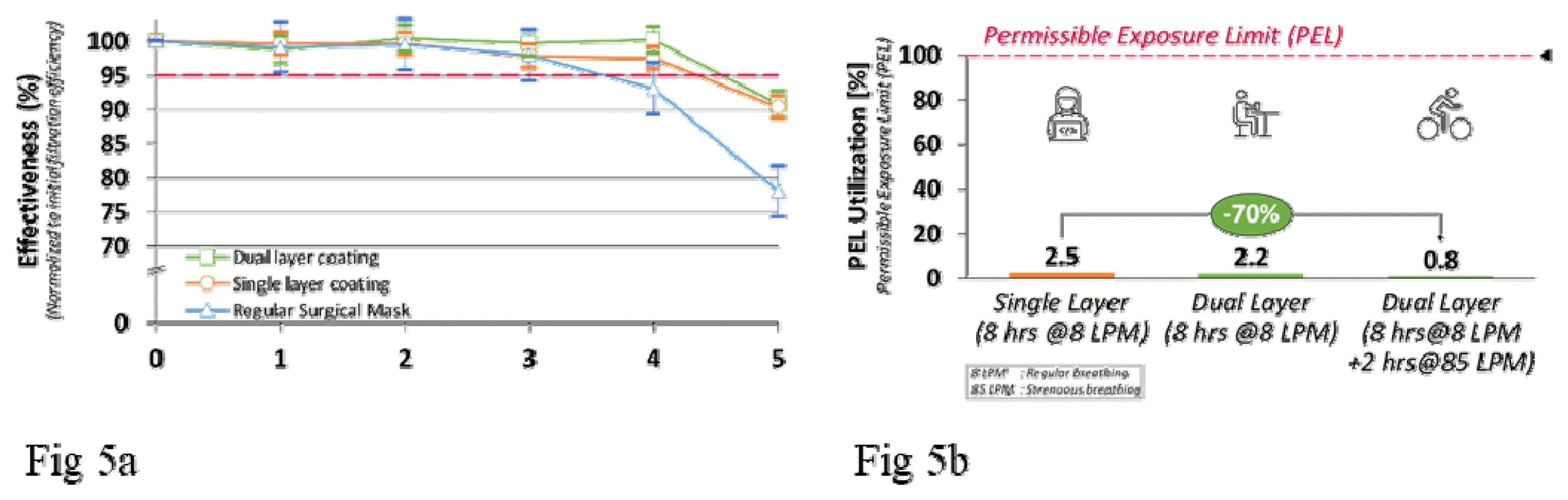
It was observed (Fig 5a) that the coated masks were able to retain ≈95% of their initial effectiveness over 4 equivalent days of exposure.
3.5. Safety
Nanoparticle safety was one of the most critical aspects of this study. The chosen nanoparticles are known for their clinical safety and are hence used in pharmaceutical applications such as pill coatings and sunscreens. However, the dislodged nanoparticles calculated by the PEL utilization expression in Eq. (2) were evaluated for single-layer and dual-layer coated masks. The dual-layer masks were also tested using different breathing patterns–regular breathing at 8 LPM and strenuous breathing (e.g., during exercising, biking, etc.) at 85 LPM. All coated masks were well within (≈2%) of the PEL limits as specified by OSHA standards (Fig 5b). Dual layer coating technique reduced PEL utilization further by ≈70% as compared to single-layer coatings.
3.6. Breathability
The pressure drop, calculated by the Bernoulli equation Eq. (6), significantly impacts the mask’s breathability and the mask wearer’s comfort.
In this equation, P is the pressure of the fluid (air), ρ is its density, υ is the velocity of the fluid, g is the gravitational acceleration, h is the height, subscript1 denotes upstream conditions and subscript2 is for downstream conditions. This equation can be simplified, considering that the height of both sides is constant and there is frictionless and incompressible flow at a constant rate, from Eq. (6) to Eq. (7):
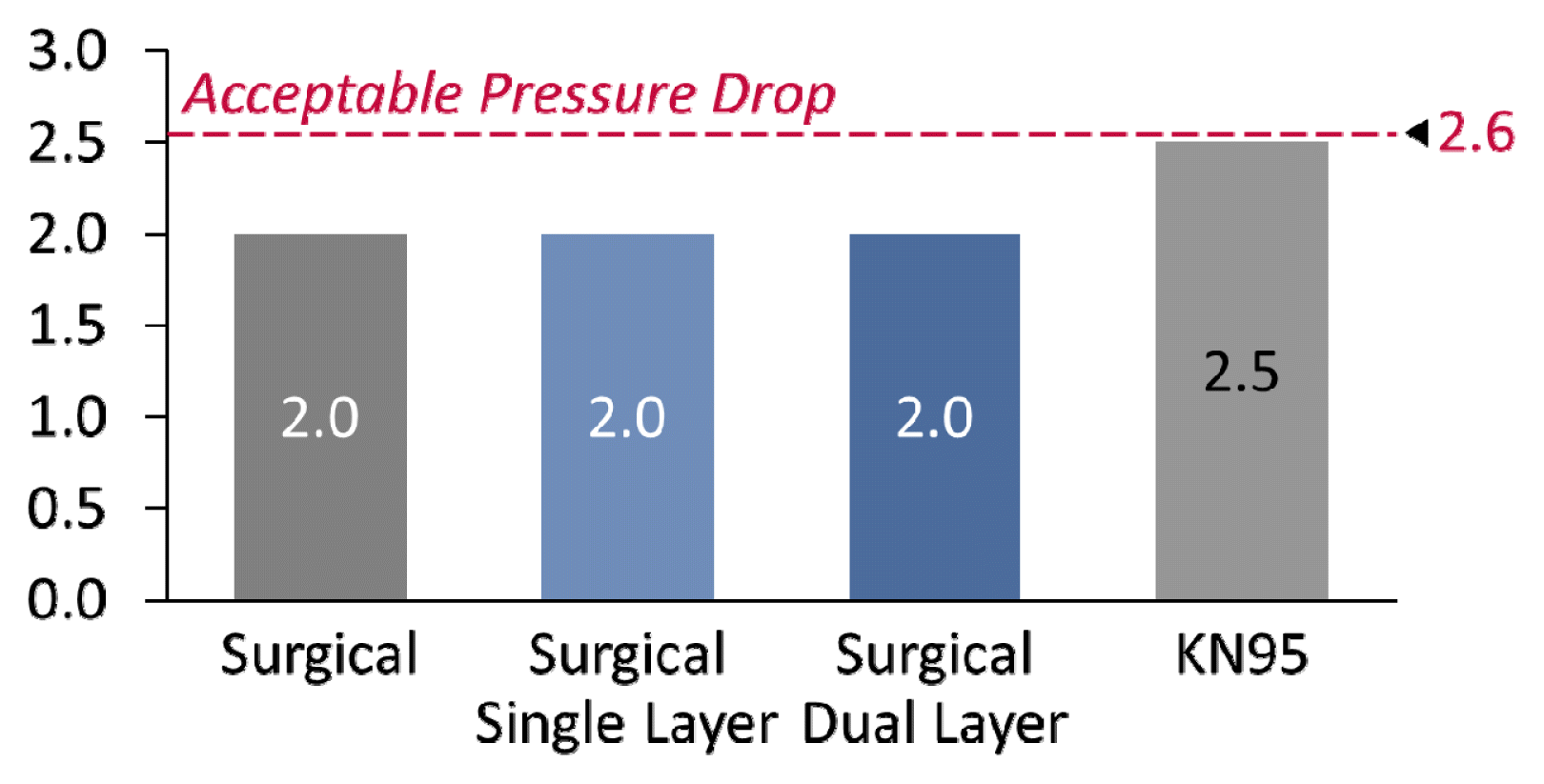
A manometer was used to measure pressure drop; its tubes were on either side of the masked mannequin to collect data on upstream and downstream conditions. All of the masks measured were well within the CDC’s pressure drop requirements [38], according to the manometer’s results (Fig 6). As can be expected, the 5-layer KN95 masks have a higher pressure drop as compared to the 3-layer surgical masks.
Additionally, the pressure drop of the masks (their ‘breathability’) was not measurably impacted by the deposition of nanoparticles. This was expected because the nanoparticles are unable to significantly block the pores of the mask. The pores, typically 10–90 μm [39], are significantly larger than the size of the nanoparticles (15–45 nm).
3.7. Measurement Uncertainty
Gage R&R was used to test the repeatability and reproducibility of this study, using the analytical software, Minitab. Its results were used to determine the reliability of the experimental setups. Four different mask types were tested on three different days with ten repetitions per mask, for the PFE set-up. 97% of the variation was due to ‘part-to-part variation’ – variation due to the natural differences between the different masks and coatings. Merely 3% of the variation in the results was attributed to the repeatability and reproducibility of the masks (Fig 7a). The total Gage R&R being 3% is deemed to be an acceptable measurement uncertainty [40]. Interaction analysis of the masks over different days of operation, illustrated in Fig 7b, verifies the reproducibility of the experiment over multiple days.
4. Discussion and Conclusions
4.1. Dual-layer Nanoparticle-Coated Masks
Typical surgical face masks are made of an outer non-woven layer for moisture absorption, a middle melt-blown electret layer for adsorption and filtration, and an inner soft non-woven layer for vapor/mist absorption. Particle entrapment in the middle layer is typically dominated by four mechanisms–inertial impaction, interception, diffusion, and electrostatic attraction [38]. Diffusion and interception mechanisms are typically effective for particle sizes between 1 and 3 μm like PM2.5. Viral particles encased in moisture droplets, typically in the range of 3–5 μm [30,41], can also be captured by these mechanisms. Electrostatic attraction is facilitated by coulombic forces for oppositely charged particles and by electrostatic induction for charge-neutral particles [9].
The dual-layer coated masks used in this study are deposited with SiO2 nanoparticles on the outer layer to enhance the adsorption of moisture-encased virions with their desiccant properties and their large specific area. The middle layer of these masks is deposited with graphene, TiO2, CuO, and ZnO nanoparticles. Graphene, with its hexagonal lattice structure, promotes adsorption and acts as a platform for other nanoparticles [23]. TiO2 generates the superoxide anion and OH radicals in presence of light which oxidizes the particulate matter into CO2 and H2O [24]. CuO increases the antiviral capability of TiO2 by enhancing its photoactivity by lowering the bandgap of Cu-TiO2, making the electron jump between bands quicker, especially in the visible light spectrum, since TiO2 works better in the UV light spectrum. ZnO and CuO, as metal-based nanoparticles, have the capability to denature the spike protein in the SARS-CoV-2 virus due to their generation of reactive oxygen species (ROS) that oxidize nucleic acids and viral proteins [26].
4.2. Rechargeable Masks
Many multi-layered masks, such as surgical, KN95, and N95 masks, contain a middle layer made of polypropylene fibers arranged as a melt-blown mesh. This layer is electrostatically charged by corona discharge, photoionization, tribo-electrification, or a liquid contact charging method [9]. These methods cannot be deployed in a hospital or household setting for regular usage. The electrostatic field is also known to degrade in presence of moisture and/or alcohol treatment cycles [35,36]. Hence, a simpler method of rechargeability would be beneficial for public use to enable the reusability of these much-needed masks, as well as reduce the significant plastic pollution that is now generated by billions of people using masks. The method demonstrated here entails recharging the mask overnight with a 3V battery cell, which can restore the degradation in efficiency. For this deposition technique, the electrostatic charging process may need to be re-sequenced post the deposition process step, to preserve the electrostatic charge. Furthermore, it can be envisioned that masks can be recharged after a day’s wear by plugging them into an outlet, much like the recharging of mobile phones. Continuous recharging techniques with solar cells embedded in the masks can also be envisioned for sustainability.
4.3. Nanoparticle Safety and Future Applications
While many nanoparticles are known to have many beneficial properties, ongoing research has indicated several nanoparticles to have harmful effects on humans [15,16,17]. Therefore, the nanoparticles used in this study were selected carefully based on their clinical safety and non-toxicity. Some are used in biomedical applications (such as pill coatings) and cosmetics (such as sunscreens) [42,43]. Tests were conducted to determine the safety of the masks, based on their risk of being dislodged from the mask layers and inhaled at unsafe amounts. The amount of nanoparticle dislodgment was 3% of the permissible exposure limit per OSHA regulations [18]. The dual-layer coating improves this further to less than 1% of the PEL, even for regular and strenuous breathing cycles (Fig 5b).
These engineered masks can be used as PPE for medical personnel. They have uses in areas of the world suffering from severe air pollution (such as parts of Asia) and in areas prone to forest fires (such as Australia and California). Additionally, with their viral protection capability, these masks can be used as a low-cost preventive measure against COVID and other airborne viruses. With their durability and rechargeability, these masks can help people in need as well as reduce the burden of plastic pollution.
4.4. Cost Effectiveness
Cost-effectiveness is critical for these coated masks as an alternative to KN95 or N95 masks. It is envisioned that the nanoparticle coating will be incorporated within the mask manufacturing process to optimize cost. In a typical mask manufacturing process, the polypropylene material is extruded and formed through a melt-blown process. The middle electret layer is charged typically by corona discharge or triboelectric means. The nanoparticle deposition would be introduced prior to the electrostatic charging process to prevent the reduction in charge from the nanoparticle solvent.
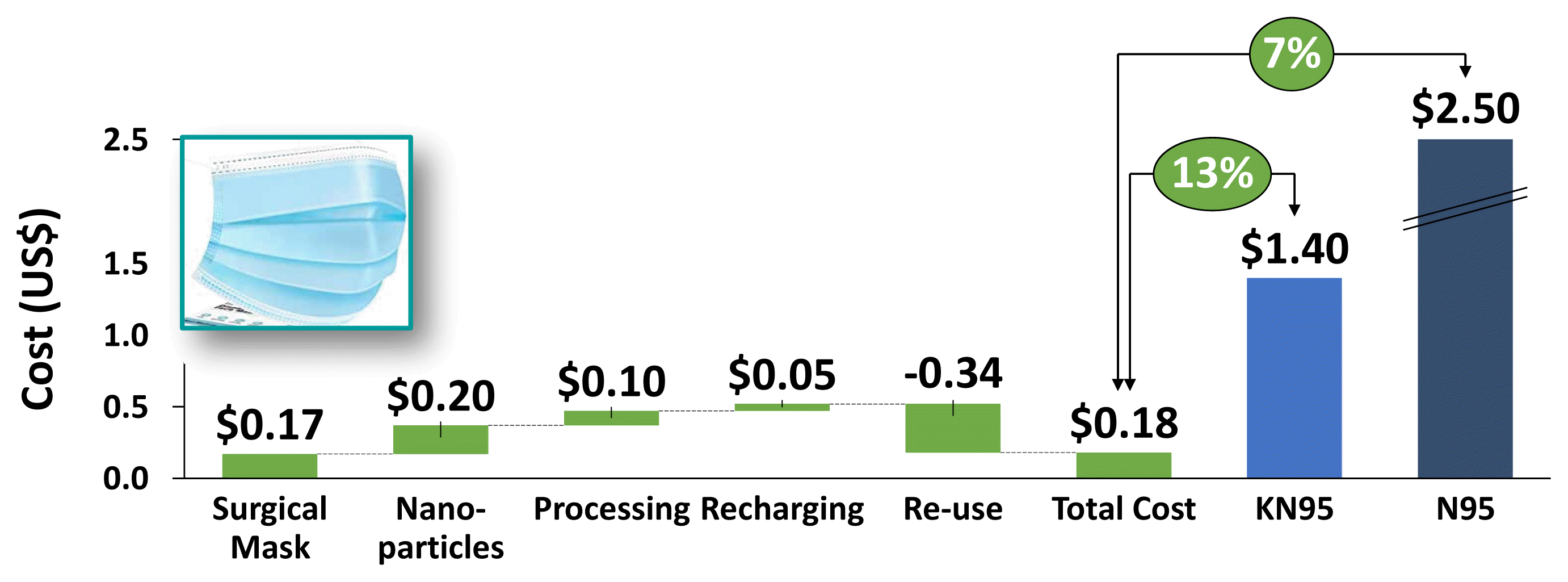
As seen in Fig 8, the additional cost of nanoparticles, processing, and recharging is offset by the re-useability of the mask making it as cost-effective as a regular surgical mask, thus being ≈7% of the cost of an N95 mask and ≈13% of the cost of a KN95 mask. Therefore, these nanoparticle-coated masks can be used as a cost-effective option when compared to costlier filtration systems that nevertheless have similar levels of filtration efficiency.
4.5. Limitations of this Study
This study is limited by the experimental design and the availability of equipment at the time of the experiments. The simulation of PM2.5 particles with incense sticks or wood chips introduces some uncertainty due to the additional volatile organic compounds (VOCs) and polycyclic aromatic hydrocarbons (PAHs) that are present. This can be avoided by using neutral challenge particles like NaCl as recommended by NIOSH [27]. The tests were done at different times of the day and on different days to explore the variation in ambient conditions. The uncertainty was captured in the gage R&R uncertainty analysis (Section 3.7). However, this testing was limited to the ambient temperature and humidity levels available in Florida, USA, and does not capture the entire range of variability in ambient conditions around the world. A controlled environment with humidifiers, dehumidifiers, heaters, and coolers can possibly simulate the entire range of ambient conditions. VFE was simulated using NaCl particles as surrogates for mucus-encased virus particles. Future studies with viral particles (Fig. 2d) will be used to validate the virucidal properties of the nanoparticles.
4.6. Conclusions
This study has demonstrated the feasibility of an engineered mask coated with selected nanoparticles with their unique physico-chemical properties which aid in particulate matter and viral pathogen filtration. The safety concerns associated with nanoparticles as documented by previous researchers have been considered. The nanoparticles used in this study are commonly used in pharmaceutical and cosmetic applications. The dual-layered deposition technique has improved filtration efficiency and retention efficacy thus being well within permissible exposure limits. These masks can be used worldwide for various problems due to their versatility in different applications. The rechargeability and durability make a significant impact on the cost-effectiveness and offer a sustainable alternative to a critical need. These nanoparticle-coated masks address the global challenges of air pollution, airborne viral diseases, and the growing impacts of plastic pollution.