AbstractThe separation of toxic boron from seawater using nanofiltration (NF) membrane technology is challenging due to the neutral charge associated with boric acid at seawater pH. In this study, the ethylenediamine functionalized Graphene oxide (EDA-GO) derivative is utilized as a nanofiller to coat the active top layer of the NF membrane over the polyethersulfone ultrafiltration support. The coating involved a self-assembly reaction of hydrolyzed trimesic acid (TMA) molecules facilitated by a triethylamine base. The unique hypothesis for coating enabled the -NH2 of EDA-GO to interact with the -COOH of TMA via hydrogen bonding interactions. The rejection shown by the M-0.05 membrane with 0.05wt% loading of EDA-GO towards MgSO4, Na2SO4, and NaCl was >98%, >97%, and 63%, respectively. The same membrane produced water flux in the range of 35–37 L/m2h with the modest significance of the salts on the membrane flux. The M-0.05 membrane showed significantly higher rejection towards boron (>47%) with a flux of 16.7 L/m2h for the beachwell seawater feed owing to the repulsive interaction between the borate ions and -NH2 and -OH groups. The rejection and flux offered by the M-0.05 membrane towards seawater's monovalent and divalent constituents were much higher than the commercial NF-90 and NF-270.
Graphical Abstract
1. IntroductionReverse osmosis (RO) is the most extensively used method for desalinating seawater at the moment. The RO water recovery is limited to an average of 35–40% due to severe fouling and scaling issues on the membrane surface [1]. As a result, RO requires more frequent membrane cleaning and replacement over a shorter period, reducing process efficiency and raising operational costs. The scaling issue is not limited to membrane-based RO technology also most frequently encountered in thermal-based desalination plants such as Multi-Stage Flash (MSF) [2]. Effective seawater pre-treatment method selection is crucial to avoid the aforementioned issues. Several pre-treatment systems and techniques have been developed over decades through significant research, resulting in many promising technologies, including nanofiltration (NF) membrane technology. Membrane-based NF is widely used in a variety of applications such as biotechnological, pharmaceutical, and wastewater treatment applications, NF is also employed in the food, dairy, and vegetable oil processing, fruit juice, plant extracts, and beverage sectors [3]. Seawater pre-treatment using NF before any desalination process offers various advantages. The scaling and fouling issues related to thermal or membrane-based desalination are mostly addressed by NF [4]. The typical pressure needed for the NF process is lower than that for reverse osmosis, ranging from 20 to 40 bar [5].
Thin-film nanocomposite (TFN) membranes are a recent advancement in NF technology, in which the inclusion of minute nanomaterial into the TFC membrane matrix improves membrane flow, rejection, and antifouling qualities significantly [6]. Karki et al. synthesized the TFC membrane via a novel vapor phase interfacial polymerization between the aqueous phase monomer and vapour phase of organic monomer (TMC) which is the greener method [7]. TiO2 nanoparticles were used as nanofillers to enhance the heavy metal separation property of the membranes. The results showed 87.0 ± 2% rejection of Na+, 86.8 ± 2% rejection of Cu2+, 77.0 ± 2% rejection of Hg2+, and 83.4 ± 1.5% rejection of Pb2+ ions by using TFN membranes with the improved antifouling and permeation properties. The integration of nanomaterial creates more nano-channels, which helps the nano-membrane’s water transport properties. As a result, the pressure required to operate nano-membranes will reduce by 50% to 10–25 bar, resulting in a more energy-efficient process. Li et al. used mesoporous silica SBA-15 as a nanofiller during the synthesis of NF membrane through coating using trimesoyl chloride and piperazine [8]. The membrane performance results revealed that the incorporation of SBA-15 improved the permeate flux from 32.4 to 45.6 L/m2h accompanied by a slight decrease of MgSO4 rejection from 97.88 to 85.23%. The SBA-15 contributed to excellent anti-fouling results due to the improved surface hydrophilicity, effective surface area, and pore size of the membrane top separation layer. Rahimi et al. developed NF membranes consisting of a highly porous and hydrophilic nanostructure of NH2-modified MCM-41 in the top active layer [9]. The resultant membrane exhibited the highest flux of 65.43 L/hm2 with improved antifouling properties with a flux recovery ratio (FRR) of around 97.0 %. Park et al synthesized the mesoporous silica containing zirconium (Zr-MCM-48) NF membranes using porous alumina support. The developed NF membranes showed narrow pore size distribution [10]. The study revealed that only 3% of Zr was necessary to significantly increase the alkaline resistance, as evidenced by the Zr-MCM-48 membranes' exceptional stability in an alkaline solution with a pH of 12. Ali et al studied an integrated system as tertiary treatment and the performance of a solar photocatalysis reactor as pretreatment for the removal of total organic carbon (TOC) and turbidity from municipal wastewater [11]. To avoid ultrafiltration (UF) membrane fouling, the procedure included UV sunlight, UV sunlight/H2O2, and UV sunlight/TiO2 nanocatalysts as pretreatment phases. The findings revealed that turbidity and TOC had the highest removal efficiencies, at 95% and 31%, respectively. With the use of UV, UV/H2O2, and UV/TiO2, the maximum removal efficiencies of the turbidity were 40%, 75%, and 95%, respectively. Kadhum et al. examined three methods to lower the chemical oxygen demand (COD) for wastewater that was contaminated with phenolic compounds such as batch adsorption, electrochemical processing, and adding a granular third electrode (GTE) to an electrochemical process [12]. As an effective adsorbent, adsorption was carried out using either nanoparticle zero-valent iron (nFe0) or silty clay-supported for nFe0 (SC-nFe0). Adsorption with nFe0 and SC-nFe0 resulted in maximal COD removal rates of 89.5% and 84.2%, respectively. The greatest removal rates were 79.8%, 93%, and 94%, respectively, when the electrochemical process and GTE were used in an electrochemical process with nFe0 or SC-nFe0.
TFN-NF membranes, on the other hand, have only been used in lab-scale research for seawater pre-treatment. To maximize the design of nanocomposite membranes for large-scale practical applications, the following difficulties must be addressed: i) the impacts of nanomaterials on membrane structures and how to correlate them with membrane performance. The specific contributions of surface hydrophilicity, porosity, charge, and membrane morphology on membrane performance; ii) nanomaterial aggregation is a common problem and approaches for better nanomaterial dispersion inside polymer matrices need to be further explored and, iii) it is critical to ensure nanoparticle compatibility with polymers. Both the optimal membrane performance and the stability of nanofillers within the membrane polymer matrix will be determined by compatibility. They are crucial for optimizing the loading concentration and durability of nanocomposite membranes, and iv) the task of developing an antifouling characteristic is highly desirable, since bacteria cells, extracellular biomaterials, and other foulants will be reduced on the membrane surface.
In a typical NF membrane synthesis process, an interfacial polymerization process involving diamine or polyamines and polyacid monomers forms a polyamide (PA) layer over the ultrafiltration (UF) support. The most often utilized monomer for NF membrane top layer synthesis is the PA layer, which comprises meta-phenylene diamine as an amine monomer and trimesoyl chloride (TMC) as an acid monomer. The PA-based NF membranes are hydrophobic and prone to fouling [13]. Incorporating nanoparticles into hydrophobic PA layers may result in particle instability on the surface due to compatibility issues. Recently, we have developed a new approach for casting a self-assembled trimesic acid (TMA) active layer over the UF support membrane by performing the reaction of trimesoyl chloride with triethylamine [14]. The self-assembly reaction on the membrane surface provided dense, smooth, and hydrophilic structures on the NF membrane surface. Also, boron in seawater is a toxic element that exists as neutral boric acid species at seawater pH. Boron concentrations in seawater range from 0.5 to 9.6 mg/L with an average of 4.6 mg/L. Boron and its compounds are the second group of toxic substances and long-term consumption of water and food with higher boron content causes issues with the neurological, reproductive, cardiovascular, and endocrine systems [15]. The World Health Organization (WHO) included this component on a list of requirements for drinking water and set the allowable boron level at 0.5 mg/L. Most of the commercial NF membranes are charged and less selective towards the boron ion separation. Thus, the synthesis of boron selective NF membrane needs special attention and there are several laboratory-scale attempts to synthesize the NF membrane targeting the boron separation [16–20]. The NF membrane synthesized through self-assembly reaction showed a high propensity to reject the boron in seawater at seawater pH [15]. Therefore, the current study further assesses the effect of loading nanoparticles into TFN-NF membranes using a newly designed self-assembly process on the membrane performance parameters including the selectivity towards the boron separation.
Recently, graphene oxide (GO) having a high surface area and strong mechanical is proven as a promising nanofiller to achieve a high selectivity/separation factor, enhance permeability, and improve membrane desalination technology and wastewater treatment applications [21]. Due to its exceptional electric conductivity and extraordinarily high surface area to-mass ratio, graphene has drawn a lot of interest. Due to its distinct properties that improve the mass transit of reactants to catalysts, graphene has also been demonstrated to optimize the activity of surface-assembled metal nanoparticle catalysts [22]. The interaction property of the GO with the different transition metal oxides and iron-doped TiO2 finds application in textile waste treatment, sensors, and pesticide binding [23–25]. Graphene oxide (GO) is an attractive class of nanoparticles for improving the performance of NF membrane surfaces owing to its improved charged characteristics resulting from bulk concentrations of oxygen functional groups such as hydroxyl, epoxy, and carboxyl [26]. These oxygen functions can create a strong hydrogen network with water molecules, giving them a higher hydrophilic characteristic. The presence of abundant oxygen groups in the structure of GO enhances the adsorbate molecule's capacity for binding, therefore, GO is an efficient adsorbent for removing boron from wastewater. Additionally, the hydroxyl and carboxylic acid groups of GO are protonated in an acidic media, which results in a positive surface charge. The hydrogen bonding between oxygen and hydrogen molecules is the primary cause of the adsorption of boric acid in an acidic media on the protonated graphene oxide surface. The surface loses protons as the pH rises and changes to a negatively charged one and hence promotes the hydrogen bonding interactions [27]. Valizadeh et al. studied the influence of the number of oxygen atoms present on the functionalizing chemical groups of GO on the size, alignment, and separation performance of the resultant laminar GO membranes [28]. The protocol included a more-ordered deposition of GOs on the polyethersulfone membrane through the pressure-assisted self-assembly method and the formation of highly durable GO membranes possessing smoother surface morphology and higher antifouling properties. The double-oxidized GO-based membranes provided a significantly high-water flux of 230 Lm−2h−1 in 2.5 bar transmembrane pressure, with excellent rejection of 99.9% for methylene blue dye with superior antifouling properties. The incorporation of GO in the membrane matrix is more focused on improving water permeation, anti-fouling, and antimicrobial for the NF application [29]. GO is highly negatively charged (approximately −30 to −58 mV at pH 5–8) and for the NF membrane, the solute rejection is strongly dependent on both the steric hindrance (size exclusion) effect and the Donnan exclusion (charge repulsion) mechanism. Hence, the highly negatively charged GO nanosheets have great potential to be used to modify membrane surface charge for enhanced salt separation, in addition to improvement in water flux. Geng et al. synthesized imidazole-modified carboxylated graphene oxide (ICGO) to enhance the flux, biofouling resistance, and long-run stability of the TFC NF membranes [30]. The pure water flux of 0.5 wt% ICGO loaded NF membrane was 69.8 Lm−2h−1 at 0.6 MPa, which was 80.8% higher than the control NF membrane. Liu et al. prepared amino acid cross-linked GO-impregnated TFC NF membranes to enhance the water permeation and ionic rejection properties [31]. The resultant TFN membrane exhibited 191.0 Lm−2h−1bar−1 water permeance which is four times higher than that of pristine GO membrane with >98% rejection during FeCl3 filtration tests. Also, the NH2 groups aminated GO have a tendency to interact with the -COOH groups of the self-assembled NF active layer.
To investigate the possibility of primary-amine functionalization and its potential to further boost the cross-linking density and hence improve membrane separation performance, we chose ethylenediamine (EDA), a more reactive amine-containing primary amine group on both sides. Also, the tendency of EDA-modified GO to covalently link with the carboxylic acid may enhance the dispersion property of the nanoparticles in the self-assembled layer [32,33]. This material has high dispersion in water and negative zeta potential at neutral pH essential to improve the charge and morphological features of the TFN NF membranes in favor of seawater pre-treatment. In literature, the application of nano derivatives of ethylenediamine to construct thin films has been explored for the potential application in the enhanced removal of heavy metal ions from wastewater [34–36]. In the current study, EDA-GO will be employed as a nanofiller during the application of the TMA layer over the UF support. During the self-assembly process, the TEA molecules first penetrate the membrane support layer, enabling the hydrolysis of TMC to TMA molecules, and then involves in covalent cross-linking with the self-assembled TMA molecules. This study is unique in terms of studying the interaction of the self-assembled TMA molecules with the -NH2 functional groups EDA-GO and its effect on the membrane characteristics and performance parameters such as flux and rejection.
2. Experimental2.1. MaterialsPolyethylene glycol (PEG) with different molecular weights with purity >99% and all types of inorganic salts used in the study were procured from Merck (with purity >99%). Triethylamine (purity >99%) and trimesoylchloride (assay >97%) were procured from Sigma Aldrich Co. The polyethersulfone UF substrate membrane with molecular weight cut-off 5000 Da was procured from Sterlitech Corporation, USA. EDA-GO nanoparticles were procured from Ad-Nano technology, India.
2.2. Membrane SynthesisThe methodology used to coat a self-assembled trimesic acid layer over a polyethersulfone (PES) support was comparable to a polyamide synthesis protocol using a diamine monomer (meta-Phenylene diamine) and a polyfunctional acid (Trimesoyl chloride) published in the literature [37]. First, the neat TFC-NF membrane was synthesized by soaking the UF support membrane in deionized (DI) water at 50 °C for 10 min. Then the UF support was clamped on the glass plate and a 50 mL aqueous solution of 1.0 wt % triethylamine was poured onto the membrane top surface at 25 °C. After 2 min, the glass plate was held vertically to remove the surplus amine solution. The amine-treated UF support was now reacted with the 0.1 wt% solution of trimesoyl chloride (TMC) in n-hexane for 2 min. The hydrolysis reaction of TMC to trimesic acid was enhanced by placing the glass plate inside a hot air oven at 90 °C for 10 min. The glass plate was placed into a DI water bath at 25 °C after the cured membrane was removed from the oven and allowed to cool to 25 °C. The washing process was repeated one more time, and the resulting TFC membrane was placed in a water bath to await further characterization and testing. The TFN membranes were prepared by incorporating the different compositions of EDA-GO in the range of 0.01 to 0.10 wt% into the aqueous triethylamine (TEA) solution. To attain the uniform dispersion of EDA-GO in TEA solution the mixture was exposed to ultrasonication for 15 min using a SONICS Vibra cell at an amplitude of 30% and pulse 25. The resultant amine mixture was poured over the surface of the UF support, and the ensuing coating protocol is quite similar to the above-described TFC membrane synthesis. The different compositions of the synthesized membranes with their codes are presented in Table 1.
2.3. Membrane Characterization InstrumentationThe surface and cross-sectional morphological images of the newly fabricated membranes were recorded using Zeiss Gemini SEM 360 scanning electron microscope. The 3-D AFM images of the membrane surfaces were taken using an atomic force microscope (AFM) Nano-Observer model from Nanosurf, CSI Instruments, France. The contact angle equipment from KINO (model: SL200KB), USA was used to determine the surface contact angles. The measurements were carried out three times for each sample, and the average value was then reported. Aqueous solutions of polyethylene glycol (PEG) with molecular weights Mw= 100, 200, 400, 500, and 1000 Da were used to evaluate the molecular weight cut-off (MWCO) of TFC-NF and TFN-0.05EDA-GO membranes. The PEG feed solution concentration was kept constant at 1g/L in each experiment, and the pressure applied during rejection testing was 10 bar at 25 °C. Gel Permeation Chromatography (GPC, Agilent Technologies, model: 1260 Infinity) with a RI detector was used to determine the amounts of PEG in permeate samples. The membrane surface charge was evaluated at pH 7 by measuring the streaming potential using the zeta potential instrument from France (model: ZetaCAD). A common approach involved measuring the sample in a NaCl solution (10−2 M, pH 5.5) and quickly equilibrating the electrolyte within the sample cell by circulating it. For a flat membrane sample of an area of 75 x 25 mm, a special cell model ZC1200 was employed for tangential measurement. The cell was made up of two rectangular pieces of the sample that are positioned face-to-face to form a narrow gap through which the electrolyte can travel.
2.4. Performance Tests for TFC and TFN MembranesThe newly fabricated NF membranes were subjected to flux and salt rejection study using the newly designed NF test unit in cross-flow mode at 25–26 °C and applied pressure of 0.6 MPa. At first, the membranes were pre-filtered with DI water at 1.0 MPa to reach a steady state before performing any tests. The feedstock solutions were prepared by dissolving Na2SO4, MgSO4, and NaCl individually in DI water while maintaining a 1g/L concentration for the rejection and flow measurements [38]. The salt rejection (R) and water flux (Jw) was calculated using Eq. (1) and Eq. (2) respectively and the average values of 3 different trials were reported.
where R is the salt rejection and Cp and Cf are the concentrations of the permeate and feed solutions respectively measured in terms of conductivity using the conductivity meter from Thermo Fischer Scientific. The water flux (Jw) during seawater pre-treatment was determined using Eq. (2).
where A (m2) is the effective membrane area and ΔV (L) is the volume of water collected over the time of Δt (h).
2.5. Boron Rejection TestsA boron rejection test was performed using a protocol described in the literature [20], where a solution containing 5 mg/L boron was generated by mixing boric acid in distilled water. Given the potential application of the synthesized NF membranes for actual saltwater pretreatment applications, where the boron content in seawater typically ranges in the range of 4–5 mg/L, the boron concentration in the current investigation was set at 5 mg/L for boron rejection testing. The boron standard (1000 mg/ml) is used for this. 0.1–1 M sodium bicarbonate solutions were used to modify the pH of the solution to 6, 8, or 10, and flux and rejection tests were made at 25–26 °C and 0.6 MPa. Eq. 1 was used to calculate the membranes' boron rejection efficiencies, with the boron concentration obtained using Agilent Technologies' Inductively coupled plasma–optical emission spectrometer (ICP-OES), model: 5900 ICP-OES.
2.6. Seawater Pre-treatment Performance Tests
Fig. S1 depicts the NF system and its flow-sheet diagram used in the research. Convergence Industries, the Netherlands designed and built the NF system for this study. The NF system can be used to test the pre-treatment performance of novel and commercially available flat-sheet NF membranes. A chemical cleaning system and backwash functionality are included in the system. To evaluate the seawater pretreatment performance of the newly fabricated NF membranes the NF system consisted of an acrylic membrane housing cell (Fig. S1b) with a surface area of 0.6 m2. The design is extremely adaptable, allowing it to operate at low pressures and/or low flows (e.g., 2 bar transmembrane pressure). The high-pressure side of the NF unit's pumps, piping, and necessary connections are all seawater resistant. Hastelloy C-grade materials were used. The NF system may be used to monitor characteristics like NF water flux and salt rejection by comparing conductivity differences.
The feed water sample for the NF tests was taken from the beachwell of the Doha Research Plant located at the Doha West Desalination plant of Kuwait. Table S1 shows the chemical composition of the beachwell seawater utilized as feed solution during the real seawater pre-treatment tests. The filtration trials were carried out at a temperature of 25–26 °C and a pressure of 10 bar across the transmembrane. The Inductively coupled plasma–optical emission spectrometer (ICP-OES) from Agilent Technologies was used to calculate the seawater boron rejection efficiency of the membranes depending on the boron concentration.
3. Results and Discussion3.1. Characterization of Aminated GOThe detailed physical characteristics of the EDA-GO provided by the manufacturer are presented in Table S2. The EDA modification was performed to achieve a higher dispersion in the aqueous medium. According to the manufacturer’s BET analysis data, the EDA-GO had a surface area in the range of 60–200 m2/g, with an amine ratio of 2–5%, and a bulk density of 0.1 g/cm3.
The typical 2D nanosheet morphologies of EDA-GO are presented in SEM images with wrinkled and folded textures (Fig. S2). The SEM images revealed thin sheets that had been randomly aggregated and had distinct edges, wrinkled surfaces, and with average thickness of 5–10 nm.
According to the manufacturer's information, the aminated GO was made using a two-step procedure: The first phase was COOH functionalization using a combination of sulfuric and nitric acids, while the second involved carboxylate GO reaction with ethylenediamine to attain -NH2 functional groups. The FTIR spectrum of aminated GO is shown in Fig. 1.
In Fig. 1, the FTIR spectrum of EDA-GO displayed a characteristic peak at 1649 cm−1 corresponding to the scissoring vibrations of the primary −NH2 group [39]. The peak at 1530 cm−1 could be ascribed to the N-C-C-N stretching mode of the EDA molecule [39]. The asymmetric and symmetric -C-H stretching appeared at 2340 cm−1. The band at 1215 cm−1 appeared due to the bending vibrations of the -C-H group, and the -C-N stretching of the primary amine was visible at 1046 cm−1 [39].
3.2. Membrane Characterization3.2.1. SEM analysis
Fig. 2 shows the surface and cross-sectional morphology of the TFC and TFN-0.05EDA-GO NF membranes. The surface of the M-0 membrane (Fig. 2a and 2b) did not show any porous nodular structures, after adding the EDA-GO nanoparticles the surface of the M-0.05 membrane having 0.5 wt% of EDA-GO showed lengthy nodular structures (Fig. 2c and 2d). In the cross-sectional views, the top TMA coating layer was visible for both the M-0 and M-0.05 membranes (Fig. 2e and Fig. 2f), followed by the long finger-like voids of the underlying PES UF membrane. The amino-functionalized GO nanoparticles could interact with trimesic acid to form amide-type linkages (-NH-CO-) providing the dense top layer [40]. The rough and nodular structures that appeared on the surfaces of the M-0.01 and M-0.05 membrane surfaces were similar to that of a traditional polyamide film [41], and the surface morphology of the membranes was changed compared to the nascent M-0 membrane by the addition of EDA-GO in the aqueous solution [42,43].
3.2.2. Contact angle analysisThe contact angle study (Fig. S3) demonstrated that the control M0 membrane has lower surface hydrophilicity with a contact angle (CA) of 56.94 ° compared to the EDA-GO-impregnated membranes. In general, the CA reveals the wettability property of the membrane surface indicating the affinity of the membrane surface toward the water molecules. The hydrophilic properties of the EDA-GO nanoparticles inserted into the active layer of the NF membrane led to a further fall in CA value to 53.1 ° for the M-0.05 membrane when 0.05 wt% EDA-GO was added [44].
3.2.3. AFM analysisThe surface AFM images of the M-0, M-0.01, and M-0.05 membranes in Fig. 3 indicated that the coating of the self-assembled TMA layer consisting of EDA-GO nanoparticles increased the average surface roughness of the TFN-NF membranes. The M-0.05 membrane with optimal EDA-GO loading composition of 0.05 wt% showed surface roughness of 635 nm while the neat M-0 membrane showed 476 nm. This suggested increased roughness owing to EDA-GO loading due to nanoparticle aggregation on its surface via the agglomeration effect [45]. The active -NH2 groups of EDA on the membrane surface resulted in an excessive increase in ionic interaction among the surface chains, causing some of them to wrinkle to enhance roughness [46]. Generally, the increased surface roughness increases the water contact angle of the membrane making the surface more hydrophobic. The roughness of the surface lowers the surface energy, which may reduce the wettability of water molecular and thus increase the contact angle and hydrophobicity. On the contrary, the decreased surface contact angle values with the higher loading of the EDA-GO are attributed to the accumulation of additional polar -NH2 groups on the membrane surface to enhance the affinity of the membrane surface toward the water molecules [47].
3.2.4. MWCO and zeta potential measurementsAs revealed in Fig. 4, the pH-dependent zeta potential investigation demonstrated that the M-0.05 membrane had the highest negative streaming potential at neutral pH. Thus, EDA-GO nanoparticles can form strongly negatively charged membrane surfaces. Though EDA-GO was slightly positively charged at pH 7, the increased negative zeta potential at the higher loading of the EDA-GO is attributed to the unreacted -COOH groups on the GO after modification using the EDA molecules [48]. The highest negative streaming potential for the M-0.05 membrane was observed at pH ~7 and was contributed by the carboxylic acid functionalities of EDA-GO and trimesic acid groups present on the membrane surface [49]. In principle, the increased surface charge of the TFN NF membranes retard the particle agglomeration due to the stronger electrostatic repulsive interactions [50]. The % rejection vs. molecular weight graph, as illustrated in Fig. 4 was used to calculate the membrane MWCO. According to the graph, the MWCOs of TFC-NF and TFN-EDA-GO membranes are 180 and 215 Da, respectively. TFC and TFN membranes demonstrated lower MWCO values than commercial NF-90 (MWCO=200–300 Da) and NF270 (MW=300–400 Da) membranes, despite the addition of EDA-GO providing denser structures to the membrane surface [51].
3.3. Boron Rejection by NF MembranesAt a neutral pH, the nascent TFC NF membrane M-0 offered a 13% boron rejection, as seen in Fig. 5. The M-0.05 membrane demonstrated the highest boron rejection of 47.8% at neutral pH when the loading of EDA-GO was increased to 0.05 wt%. At neutral pH, the M-0.05 membrane gave significantly higher rejection than the commercial NF-90 (18%) and NF-70 (22%), as shown in Fig. 5. NF-based seawater pre-treatment is usually inefficient for the boron rejection due to the neutral charge associated with the boron at the seawater pH [11]. Thus, the EDA-GO integrated membrane could be useful in seawater pre-treatment, particularly in RO-based desalination. Despite the fact that the MWCO of M-0.05 (195 Da) and NF-90 (190 Da) are comparable [51], the former membrane showed stronger boron rejection at pH 7. As illustrated in Fig. S4, this could be the effect of undissociated boric acid molecules in seawater behaving similarly to water due to their low molecular weight, the absence of electrostatic charges, and its tendency to form hydrogen bonding interactions with the abundant -COOH (of TMA) and -NH2 (of EDA-GO) groups present on the membrane surface [52]. Such interactions narrowed the pores on the membrane surface which decreased the passage of small solutes like salt and boron [53,54]. At pH 11, the M-0.05 membrane provided the highest boron rejection of 97% as at higher pH boric acid will be fully dissociated to borate form. The dissociated borate form will be fully hydrated, resulting in a larger size and an enhancement of the negative charge of the ion. This results in higher rejection both by exclusion and repulsion by the negatively charged membrane [55].
3.4. Seawater Pre-treatment Test
Fig. 6 shows the rejection offered by the neat TFC and the EDA-GO-loaded membranes towards the various salts. The different membranes rejected the salts in the order of Na2SO4 > MgSO4>NaCl. The M-0.05 membrane with 0.05 wt% of EDA-GO loading attained the highest salt rejection and flux as shown in Fig. 6 and Fig. S5. In NF technology, the separation principle is based on sieving and electrical or Donnan effects [56]. According to Donnan’s effects, a charged membrane surface attracts oppositely charged ions, making it simpler for them to move through the membrane, while repelling ions with the same charge, helping to keep them in the solution. The TFN-EDA-GO membrane surface has both the positive and negatively charged groups on its surface contributed from -NH3 + and -COOH groups, respectively. However, the zeta potential study revealed a negatively charged surface due to the presence of abundant carboxyl groups on the membrane surface contributed by the unreacted GO and trimesic acid molecules. The negatively charged membrane surface would reject multivalent anion SO4 2− and attract multivalent cation Mg2+ more strongly than the monovalent anion Cl− and cation Na+ due to the greater valency ions should interact significantly with the charged membrane surface [57]. As a result, the membrane demonstrated a strong rejection of Na2SO4 with divalent anions and monovalent cations. Despite the fact that sodium ions are smaller than magnesium ions, the strong rejection of sodium salt (Na2SO4) indicates that the electrical effect dominates the steric hindrance effect between the ions and charged groups on the membrane surface [58,59]. MgSO4 has a higher rejection than NaCl due to the bigger size of both Mg2+ and SO4 2− than Cl− and Na+. Due to the identical concentrations of feed solutions and similar pressure and temperature settings used during filtration, the charge effect or steric hindrance had a lesser impact on the water flux of the membrane.
It was observed that EDA-GO nanoparticles successfully prevented their agglomeration in the coated layer and had a greater impact on the enhanced membrane's flux [60]. From Fig. S5, the water flux of the M-0.05 membrane altered in the range of 35–37 L/m2h with little significance of the salts on the membrane flux. The significantly increased flux is largely due to the relatively hydrophilic layer generated by the insertion of hydrophilic EDA-GO nanoparticles, which offered new pathways for water molecules [61]. Also, all of the EDA-GO incorporated membranes had a higher flux than the neat TFC membrane could be due to the functionalization with EDA increasing the interlayer distance functionalized GO and resulting in thinner stacked graphene sheets [62]. Irrespective of the increased MWCO, the flux value of the M-0.1 membrane was reduced could be the effect of excessive loading of the EDA-GO above the optimal loading composition resulting in the agglomeration of nanoparticles on the membrane surface [63].
The optimal performer membrane with 0.05 wt% loading of EDA-GO was used for the study. As revealed in Table 2, the M-0.05 membrane showed excellent selectivity towards boron rejection (>40.0%), with a flux of 16.7 L/m2h for the beachwell seawater sample taken from Kuwait's Doha East desalination facility. Table 2 includes the comparison of the flux and rejection offered by the newly synthesized NF membranes with the commercially available NF membranes tested towards the real seawater pre-treatment application. Accordingly, the performance results of the newly synthesized NF membranes were compared with the five commercially available NF membranes. A 0.05 wt% addition of EDA-GO nanoparticles increased hazardous boron rejection by three times as compared to commercial NF90. Also, the rejection offered by the membrane towards the other ionic constituents present in the seawater was much higher compared to the commercial NF-90 and NF-270 membranes. The increased flux and selectivity of the membrane were attributed to the entire effect of strong compatibility between EDA-GO and -COOH groups of the TMA on the membrane surface [15]. Thus, the incorporation of EDA-GO into the top selective layer of the NF membrane revealed an efficient method to modify the NF membranes for the potential application of membranes for seawater pre-treatment application. Most importantly, the scalant and hardness ions were rejected much higher than the commercial NF membranes as observed in Table 2.
3.5. Flux Stability TestThe long-term seawater filtration trails were performed to measure the flux stability of the EDA-GO loaded membrane over 24 h at 10 bar applied pressure and 24 °C. The results are in Fig. S6 and over a four-day running period, a consistent flow of permeate water was achieved with permeability in the range of 1.66 to 1.64 L/m2hbar. As a result, the flux stability test demonstrated the membrane's long-term stability over the test time by avoiding the risk of fouling from the foulants found in seawater.
4. ConclusionsOverall, this study explored the use of aminated EDA-GO nanoparticle-impregnated TFN membrane for real seawater pre-treatment application. The -NH2 groups of the EDA-GO assisted in overcoming the compatibility issue with the membrane surface by interacting with the -COOH groups. The membrane morphology was altered to lengthy nodular structures to enhance the water permeation characteristics. EDA-GO contributed to the improved surface negative charge and hydrophilicity of the membrane. During seawater pre-treatment, the 0.05 wt% EDA-GO incorporated TFN membrane could achieve boron rejection of > 40%, which is the greatest of any commercial NF membrane at seawater pH. The newly developed TFN NF membranes have the best chance of rejecting scaling ions especially Ca2+, SO4 2−, and Mg2+ from seawater at seawater pH. Thus, newly developed TFN membranes can potentially find seawater pre-treatment applications in RO or MSF plants to reduce the scaling and fouling issues.
AcknowledgementsThe authors express their gratitude to the Director-General of Kuwait Foundation for the Advancement of Sciences, Kuwait for financially supporting this research project (grant code: PN19-15EM-05).
Data Availability Statement
All data generated or analyzed during this study are included within the manuscript.
NotesAuthor Contribution R.K.A (Research Scientist): Conceptualization, Supervision. M.A (Research Scientist): Data curation, Writing - original draft. G.B (Associate Research Scientist).: Visualization, Investigation. M.A.R (Senior Technician): Methodology. J.P.T (Research Associate): Writing - review & editing. All authors read and approved the final manuscript. References1. Jafari M, Vanoppen M, van Agtmaal JMC, et al. Cost of fouling in full-scale reverse osmosis and nanofiltration installations in the Netherlands. Desalination. 2021;500:114865–114876. https://doi.org/10.1016/j.desal.2020.114865
2. Dhakal N, Salinas-Rodriguez SG, Hamdani J, et al. Is Desalination a Solution to Freshwater Scarcity in Developing Countries? Membranes. 2022;12:381–396. https://doi.org/10.3390%2Fmembranes12040381
3. Yadav D, Hazarika S, Ingole PG. Recent development in nanofiltration (NF) membranes and their diversified applications. Emergent Mat. 2022;5:1311–1328. https://doi.org/10.1007/s42247-021-00302-6
4. Elazhar F, Elazhar M, Filali N, Belhamidi S, Elmidaoui A, Taky M. Potential of hybrid NF-RO system to enhance chloride removal and reduce membrane fouling during surface water desalination. Sep. Purif. Technol. 2021;261:118299–118306. https://doi.org/10.1016/j.seppur.2021.118299
5. Ray H, Perreault F, Boyer TH. Rejection of nitrogen species in real fresh and hydrolyzed human urine by reverse osmosis and nanofiltration. J. Environ. Chem. Eng. 2020;8:103993–104002. https://doi.org/10.1016/j.jece.2020.103993
6. Samsami S, Sarrafzadeh MH, Ahmadi A. Surface modification of thin-film nanocomposite forward osmosis membrane with super-hydrophilic MIL-53 (Al) for doxycycline removal as an emerging contaminant and membrane antifouling property enhancement. Chem. Eng. J. 2021;43:133469–133478. http://dx.doi.org/10.1016/j.cej.2021.133469
7. Karki S, Ingole PG. Development of polymer-based new high performance thin-film nanocomposite nanofiltration membranes by vapor phase interfacial polymerization for the removal of heavy metal ions. Chem. Eng. J. 2022;446:137303–137314. https://doi.org/10.1016/j.cej.2022.137303
8. Li Q, Li Z, Yu H, et al. Effects of ordered mesoporous silica on the performances of composite nanofiltration membrane. Desalination. 2013;327:24–31. https://doi.org/10.1016/j.desal.2013.08.002
9. Rahimi Z, Zinatizadeh AA, Zinadini S, van Loosdrecht M, Younesi H. A new anti-fouling polysulphone nanofiltration membrane blended by amine-functionalized MCM-41 for post treating waste stabilization pond's effluent. J. Environ. Manage. 2021;290:112649–112663. https://doi.org/10.1016/j.jenvman.2021.112649
10. Park PH, Saputra H, Nishiyama N, Egashira Y, Ueyama K. Synthesis of zirconium-containing mesoporous silica Zr-MCM-48 membranes with high alkaline resistance for nanofiltration. Stud. Surf. Sci. Catal. 2003;146:327–330. https://doi.org/10.1016/S0167-2991(03)80391-9
11. Ali NS, Kalash KR, Ahmed AN, Albayati TM. Performance of a solar photocatalysis reactor as pretreatment for wastewater via UV, UV/TiO2, and UV/H2O2 to control membrane fouling. Sci. Rep. 2022;121:16782–16792. 10.1038/s41598-022-20984-0
12. Kadhum ST, Alkindi GY, Albayati TM. Determination of chemical oxygen demand for phenolic compounds from oil refinery wastewater implementing different methods. Desal. Water Treat. 2021;231:44–53. 10.5004/dwt.2021.27443
13. Freger V, Gilron J, Belfer S. TFC polyamide membranes modified by grafting of hydrophilic polymers: An FT-IR/AFM/TEM study. J. Membr. Sci. 2002;209:283–292. https://doi.org/10.1016/S0376-7388(02)00356-3
14. Simsek A, Korkmaz D, Velioglu YS, Ataman OY. Determination of boron in hazelnut (Corylus avellana L.) varieties by inductively coupled plasma optical emission spectrometry and spectrophotometry. Food Chem. 2003;83:293–296. https://doi.org/10.1016/S0308-8146(03)00122-5
15. Kumar R, Ahmed M, Ok S, Garudachari B, Thomas J. Boron selective thin film composite nanofiltration membrane fabricated via a self-assembled trimesic acid layer at a liquid–liquid interface on an ultrafiltration support. New J. Chem. 2019;43:3874–3883. https://doi.org/10.1039/C8NJ05670F
16. Lan N, Wang KY, Weber M, Maletzko C, Chung TS. Investigation of novel molecularly tunable thin-film nanocomposite nanofiltration hollow fiber membranes for boron removal. J. Membr. Sci. 2021;620:118887–118895. https://doi.org/10.1016/j.memsci.2020.118887
17. Ghiasi S, Mohammadi T, Tofighy MA. Hybrid nanofiltration thin film hollow fiber membranes with adsorptive supports containing bentonite and LDH nanoclays for boron removal. J. Membr. Sci. 2022;655:120576–120587. https://doi.org/10.1016/j.memsci.2022.120576
18. Tu KL, Chivas AR, Nghiem LD. Effects of membrane fouling and scaling on boron rejection by nanofiltration and reverse osmosis membranes. Desalination. 2011;279:269–277. https://doi.org/10.1016/j.desal.2011.06.019
19. Liu L, Xie X, Qi S, et al. Thin film nanocomposite reverse osmosis membrane incorporated with UiO-66 nanoparticles for enhanced boron removal. J. Membr. Sci. 2019;580:101–109. https://doi.org/10.1016/j.memsci.2019.02.072
20. Ali Z, Sunbul Y, Pacheco F, et al. Defect-free highly selective polyamide thin-film composite membranes for desalination and boron removal. J. Membr. Sci. 2019;578:85–94. https://doi.org/10.1016/j.memsci.2019.02.032
21. Karki S, Ingole PG. Chapter Four - Graphene-based thin film nanocomposite membranes for separation and purification. Comprehensive Analytical Chemistry, Elsevier. 2020;73–97.
22. Al-Nayili A, Majdi HS, Albayati TM, Saady NMC. Formic acid dehydrogenation using noble-metal nanoheterogeneous catalysts: towards sustainable hydrogen-based energy. Catalysts. 2022;12:324–336. https://doi.org/10.3390/catal12030324
23. Khan MS, Riaz N, Shaikh AJ, et al. Graphene quantum dot and iron co-doped TiO2 photocatalysts: Synthesis, performance evaluation and phytotoxicity studies. Ecotoxicol. Environ. Saf. 2021;226:112855–3112864. https://doi.org/10.1016/j.ecoenv.2021.112855
24. Ahmad S, Ayoub MH, Khan AM, et al. Diverse comparative studies for preferential binding of graphene oxide and transition metal oxide nanoparticles. Colloids Surf. A: Physicochem. Eng. Asp. 2022;647:129057–129068. https://doi.org/10.1016/j.colsurfa.2022.129057
25. Ul Hassan Z, Abbas Z, Bakht K, et al. Dynamic light scattering and zeta-potential as a tool for understanding the mechanism of pesticides binding toward individual components of transition metal nanoparticles and graphene oxide hybrids. J. Environ. Sci. Health B. 2022;57:932–947. 10.1080/03601234.2022.2147348
26. Li H, Shi W, Du Q, Zhou R, Zhang H, Qin X. Improved separation and antifouling properties of thin-film composite nanofiltration membrane by the incorporation of cGO. App. Surf. Sci. 2017;407:260–275. https://doi.org/10.1016/j.apsusc.2017.02.204
27. Li Y, Du Q, Liu T, et al. Equilibrium, kinetic and thermodynamic studies on the adsorption of phenol onto graphene. Mater. Res. Bull. 2012;47:1898–1904. https://doi.org/10.1016/j.materresbull.2012.04.021
28. Valizadeh S, Naji L, Karimi M, Tafreshi SS, Heijman B, de Leeuw NH. Experimental and density functional theory studies of laminar double-oxidized graphene oxide nanofiltration membranes. Chem. Eng. Res. Des. 2022;188:590–606. https://doi.org/10.1016/j.cherd.2022.10.006
29. Wang J, Gao X, Yu H, et al. Accessing of graphene oxide (GO) nanofiltration membranes for microbial and fouling resistance. Sep. Purif. Technol. 2019;215:91–101. https://doi.org/10.1016/j.seppur.2019.01.018
30. Geng C, Zhao F, Niu H, et al. Enhancing the permeability, anti-biofouling performance and long-term stability of TFC nanofiltration membrane by imidazole-modified carboxylated graphene oxide/polyethersulfone substrate. J. Membr. Sci. 2022;664:121099–121111. https://doi.org/10.1016/j.memsci.2022.121099
31. Liu J, Wang S, Yang R, Li L, Liang S, Chen L. Bio-inspired graphene oxide-amino acid cross-linked framework membrane trigger high water permeance and high metal ions rejection. J. Membr. Sci. 2022;659:120745–120755. https://doi.org/10.1016/j.memsci.2022.120745
32. Li Z, He C, Wang Z, et al. Ethylenediamine-modified graphene oxide covalently functionalized with a tetracarboxylic Zn(ii) phthalocyanine hybrid for enhanced nonlinear optical properties. Photochem. Photobiol. Sci. 2016;15:910–919. https://doi.org/10.1039/C6PP00063K
33. Khatoon H, Iqbal S, Ahmad S. Covalently functionalized ethylene diamine modified graphene oxide poly-paraphenylene diamine dispersed polyurethane anticorrosive nanocomposite coatings. Prog. Org. Coat. 2021;150:105966–105977. https://doi.org/10.1016/j.porgcoat.2020.105966
34. Zawisza B, Baranik A, Malicka E, Talik E, Sitko R. Preconcentration of Fe(III), Co(II), Ni(II), Cu(II), Zn(II) and Pb(II) with ethylenediamine-modified graphene oxide. Microchim. Acta. 2016;183:231–240. https://doi.org/10.1007/s00604-015-1629-y
35. Velickovic ZS, Marinkovic AD, Bajic ZJ, et al. Oxidized and ethylenediamine-functionalized multi-walled carbon nanotubes for the separation of low concentration arsenate from water. Sep. Sci. Technol. 2013;48:2047–2058. https://doi.org/10.1080/01496395.2013.790446
36. Soleimani M, Afshar MG, Sedghi A. Amino-functionalization of multiwall carbon nanotubes and its use for solid phase extraction of mercury ions from fish sample. ISRN Nanotechnol. 2013;8:674289–674298. https://doi.org/10.1155/2013/674289
37. Zhang C. Graphene oxide quantum dots incorporated into a thin film nanocomposite membrane with high flux and antifouling properties for low-pressure nanofiltration. ACS Appl. Mat. Interf. 2017;9:11082–11094. https://doi.org/10.1021/acsami.6b12826
38. Atiyah NA, Albayati TM, Atiya MA. Functionalization of mesoporous MCM-41 for the delivery of curcumin as an anti-inflammatory therapy. Adv. Powder Technol. 2022;33:103417. https://doi.org/10.1016/j.apt.2021.103417
39. Segal L, Eggerton FV. Infrared Spectra of Ethylenediamine and the Dimethylethylenediamines. Appl. Spectrosc. 1961;15:116–117. https://opg.optica.org/as/abstract.cfm?uri=as-15-4-116
40. Wei S, Chen Y, Hu X, et al. Monovalent/divalent salts separation via thin film nanocomposite nanofiltration membrane containing aminated TiO2 nanoparticles. J. Taiwan Inst. Chem. Eng. 2020;112:169–179. https://doi.org/10.1016/j.jtice.2020.06.014
41. Safarpour M, Vatanpour V, Khataee A, Esmaeili M. Development of a novel high flux and fouling-resistant thin film composite nanofiltration membrane by embedding reduced graphene oxide/TiO2. Sep. Purif. Technol. 2015;154:96–107. https://doi.org/10.1016/j.seppur.2015.09.039
42. Peyravi M, Jahanshahi M, Rahimpour A, Javadi A, Hajavi S. Novel thin film nanocomposite membranes incorporated with functionalized TiO2 nanoparticles for organic solvent nanofiltration. Chem. Eng. J. 2014;241:155–66. https://doi.org/10.1016/j.cej.2013.12.024
43. Rajaeian B, Rahimpour A, Tade MO, Liu S. Fabrication and characterization of polyamide thin film nanocomposite (TFN) nanofiltration membrane impregnated with TiO2 nanoparticles. Desalination. 2013;313:176–88. https://doi.org/10.1016/j.desal.2012.12.012
44. Wang J, Sun Y, Yu M, Lu X, Komarneni S, Yang C. Emulsions stabilized by highly hydrophilic EDA-GO nanoparticles via van der Waals attraction. J. Colloid Interface Sci. 2021;589:378–387. https://doi.org/10.1016/j.jcis.2021.01.011
45. Davari S, Omidkhah M, Salari S. Role of polydopamine in the enhancement of binding stability of TiO2 nanoparticles on polyethersulfone ultrafiltration membrane. Colloids Surf. A: Physicochem. Eng. Asp. 2021;622:126694. https://doi.org/10.1016/j.colsurfa.2021.126694
46. Nasrollahi N, Aber S, Vatanpour V, Mahmoodi NM. The effect of amine functionalization of CuO and ZnO nanoparticles used as additives on the morphology and the permeation properties of polyethersulfone ultrafiltration nanocomposite membranes. Compos. B. Eng. 2018;154:388–409. https://doi.org/10.1016/j.compositesb.2018.09.027
47. Goh PS, Wong KC, Yogarathinam LT, Ismail AF, Abdullah MS, Ng BC. Surface modifications of nanofillers for carbon dioxide separation nanocomposite membrane. Symmetry. 2020;12:1102–1135. https://doi.org/10.3390/sym12071102
48. Schneible JD, Shi K, Young AT, et al. Modified gaphene oxide (GO) particles in peptide hydrogels: a hybrid system enabling scheduled delivery of synergistic combinations of chemotherapeutics. J. Mater. Chem. B. 2020;8:3852–3868. https://doi.org/10.1039/D0TB00064G
49. Artug G, Hapke J. Characterization of nanofiltration membranes by their morphology, charge and filtration performance parameters. Desalination. 2006;200:178–180. https://doi.org/10.1016/j.desal.2006.03.287
50. Verwey EJW, Overbeek JTG. Theory of the stability of lyophobic colloids. Elsevier; Amsterdam: 1948. p. 413–414.
51. Fujioka T, Khan SJ, McDonald LJA, Nghiem LD. Nanofiltration of trace organic chemicals: a comparison between ceramic and polymeric membranes. Sep. Purif. Technol. 2014;36:258–264. https://doi.org/10.1016/j.seppur.2014.08.039
52. Ezechi EH, Isa MH, Kutty SRBM. Boron in produced water: challenges and improvements: A comprehensive review. J. Appl. Sci. 2012;12:402–415. 10.3923/jas.2012.402.415
53. Liu L, Xie X, Qi S, et al. Thin film nanocomposite reverse osmosis membrane incorporated with UiO-66 nanoparticles for enhanced boron removal. J. Membr. Sci. 2019;580:101–109. https://doi.org/10.1016/j.memsci.2019.02.072
54. Mehanathan S, Jaafar J, Nasir AM, et al. Adsorptive membrane for boron removal: challenges and future prospects. Membranes. 2022;12:798–811. https://doi.org/10.3390/membranes12080798
55. Redondo J, Busch M, De Witte JP. Boron removal from seawater using FILMTECTM high rejection SWRO membranes. Desalination. 2003;156:229–238. https://doi.org/10.1016/S0011-9164(03)00345-X
56. Joseph N, Ahmadiannamini P, Hoogenboom R, Vankelecom IFJ. Layer-by layer preparation of polyelectrolyte multilayer membranes for separation. Polym. Chem. 2014;5:1817–1831. https://doi.org/10.1039/C3PY01262J
57. Wu C, Liu S, Wang Z, et al. Nanofiltration membranes with dually charged composite layer exhibiting super-high multivalent-salt rejection. J. Membr. Sci. 2016;517:64–72. https://doi.org/10.1016/j.memsci.2016.05.033
58. Zhao FY, An QF, Ji YL, Gao CJ. A novel type of polyelectrolyte complex/MWCNT hybrid nanofiltration membranes for water softening. J. Membr. Sci. 2015;492:412–421. https://doi.org/10.1016/j.memsci.2015.05.041
59. Ji Y, An Q, Zhao Q, Chen H, Qian J, Gao C. Fabrication and performance of a new type of charged nanofiltration membrane based on polyelectrolyte complex. J. Membr. Sci. 2010;357:80–89. https://doi.org/10.1016/j.memsci.2010.04.003
60. Kim NH, Kuil T, Lee JH. Simultaneous reduction, functionalization and stitching of graphene oxide with ethylenediamine for composites application. J. Mat. Chem. A. 2013;1:1349–1358. https://doi.org/10.1039/C2TA00853J
61. Ju H, Duan J, Lu H, Xu W. Cross-linking with diamine monomers to prepare graphene oxide composite membranes with varying d-spacing for enhanced desalination properties. Front. Chem. 2021;9:779304–779315. https://doi.org/10.3389/fchem.2021.779304
62. Pruna A, Carcel AC, Benedito A, Gimenez E. Effect of synthesis conditions on CO2 capture of ethylenediamine-modified graphene aerogels. App. Surf. Sci. 2019;487:228–235. https://doi.org/10.1016/j.apsusc.2019.05.098
63. Razmjou A, Resosudarmo A, Holmes RL, Li H, Mansouri J, Chen V. The effect of modified TiO2 nanoparticles on the polyethersulfone ultrafiltration hollow fiber membranes. Desalination. 2012;287:271–280. https://doi.org/10.1016/j.desal.2011.11.025
64. Llenas L, Ribera G, Martinez-Llado X, Rovira M, de Pablo J. Selection of nanofiltration membranes as pretreatment for scaling prevention in SWRO using real seawater. Desal. Water Treat. 2013;51:930–935. https://doi.org/10.1080/19443994.2012.714578
Fig. 2SEM images of the NF membranes: a and b) surface of the M-0 at two different magnifications, c and d) surface images of the M-0.05 at two different magnifications, e) and f) cross-sectional images of M-0 and M-0.05 NF respectively. 
Fig. 6The rejection offered by the membranes during MgSO4, Na2SO4, and NaCl filtration experiments. 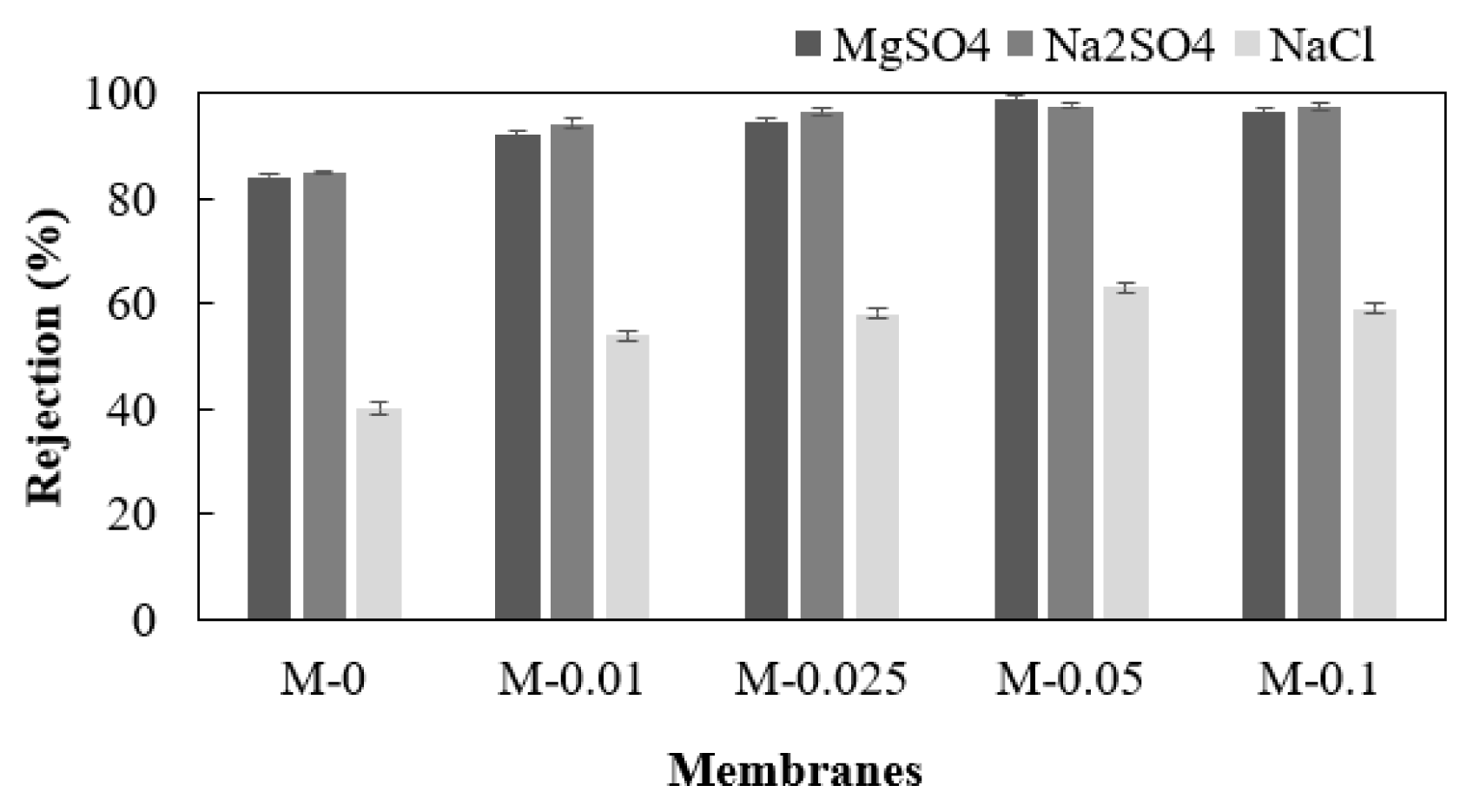
Table 1The chemical compositions of the top active layers of the synthesized NF membranes.
Table 2Comparison of salt rejection/flux data between TFC-NF, TFN-0.05EDA-GO, and the commercial NF membranes during the seawater pre-treatment.
|
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||