AbstractNitrous oxide (N2O) is an important greenhouse gas (GHG) and an ozone-depleting substance that can be emitted from landfills. Understanding the dynamics of N2O and its contribution to total emissions is critical to effective mitigation measures. This study outlines the dynamics of N2O in solid waste landfills and N2O emissions from them. N2O generation in anaerobic landfills is primarily due to denitrification and, to a lesser extent, nitrification, which occurs in the oxygenated cover soil layer and the working face. However, nitrification and denitrification are very limited within landfills. The landfill leachate contains high concentrations of ammonia (NH3). Thus, a significant amount of N2O is generated from aerobic processes during leachate treatment. Bioreactor landfills emit more N2O than traditional anaerobic landfills. The majority of N2O emitted from bioreactor landfills is generated through different pathways, such as hydroxylamine (NH2OH) oxidation, nitrifier denitrification, and heterotrophic denitrification. These processes are affected by several factors, including the carbon-to-nitrogen (C/N) ratio, NH3 oxidation rate NH3 oxidation rate, redox conditions, and temperature. Addressing N2O emissions from landfills will be necessary to achieve an integrated nitrogen management strategy that helps minimize N2O emissions.
Graphical Abstract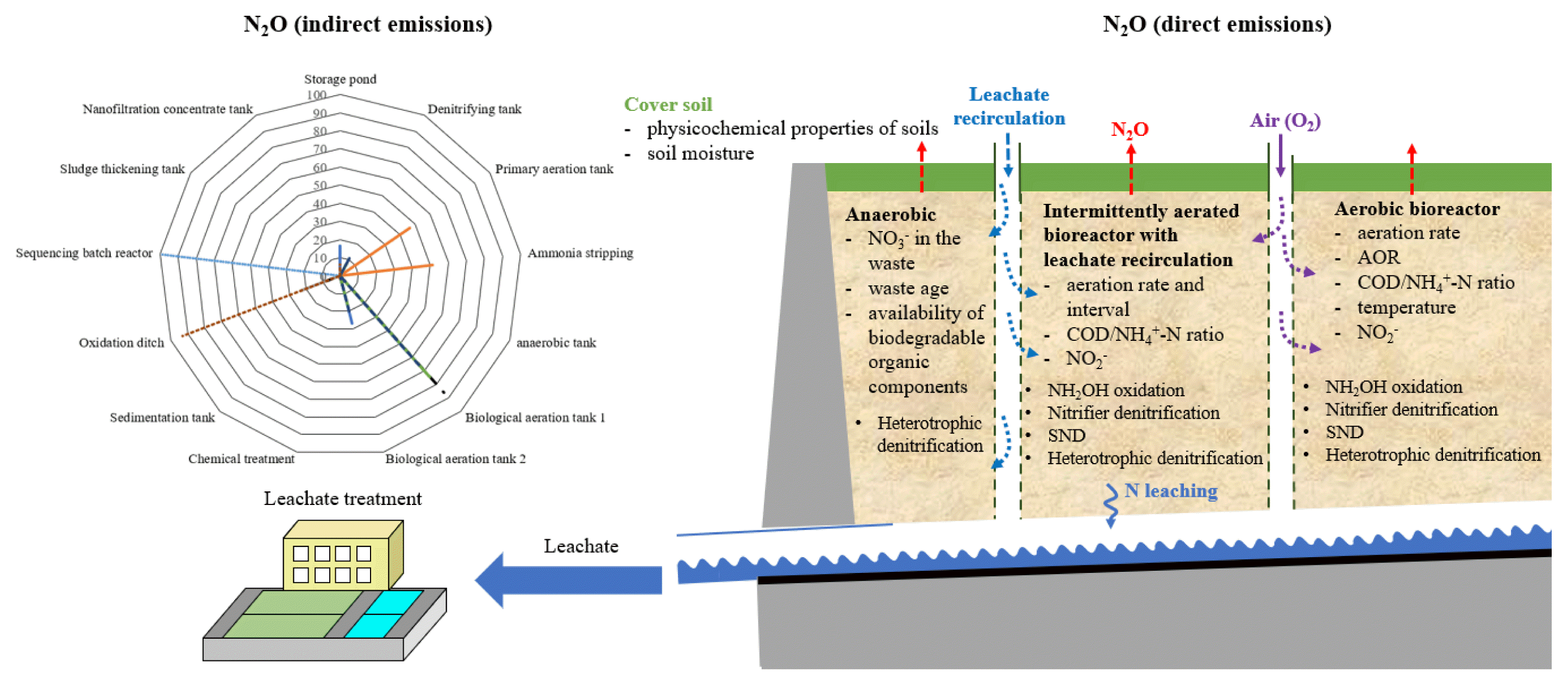
1. IntroductionAccording to the Intergovernmental Panel on Climate Change (IPCC), methane (CH4) and nitrous oxide (N2O) have global warming potentials that are 29.8 and 273 times higher, respectively, than carbon dioxide (CO2) over 100 years [1]. Human activities increase the concentration of N2O in the atmosphere. Since the pre-industrial era, the atmospheric N2O concentration has risen from 270 to 324 ppb, approximately 19% [2]. About 40% of the N2O emitted into the atmosphere originates from anthropogenic sources [3]. Human waste accounts for about 3% of the global anthropogenic N2O emissions, while agriculture accounts for about 60%. However, due to growing urbanization, human waste may become an important source of N2O emissions in the future [2].
Landfills are anthropogenic sources of greenhouse gases (GHGs), including CH4 and N2O. The environmental conditions in the landfills promote microbial production of N2O due to the high nitrogen (N) content in the organic waste contained in the landfills [4]. The contribution of N2O from anaerobic landfills is less than 20% of the contribution from CH4 as a CO2 equivalent contributing to the greenhouse effect [5].
N2O emissions from landfills can be divided into two categories. One is direct emissions of N2O generated in landfills to the atmosphere. The other is N2O emitted indirectly from the nitrogen in leachate from various wastes. The impact of N2O emissions from anaerobic landfills is relatively less than other systems, including agricultural and forest soils [6]. However, increasing attention has been given to bioreactor landfills, including leachate recirculation, aerobic, and hybrid landfills. Bioreactor landfills can produce large amounts of N2O, increasing the atmospheric N2O concentration [7–9]. Moreover, a significant amount of nitrogen is lost to leachate during the leaching process in anaerobic landfills. Consequently, a substantial amount of N2O is emitted from leachate treatment facilities [10].
Researchers have investigated the dynamics of N2O in various landfill operations [11–13] and quantified the emissions of N2O from landfill and leachate treatment facilities [14, 15]. N2O is a byproduct of nitrification and an intermediate in denitrification [16]. However, as shown by Nag et al. [4], the mechanisms of nitrification and denitrification in municipal solid waste (MSW) landfills are more complex than those in wastewater treatment plants. In addition, the influence of the physical and chemical characteristics of waste and landfill operations on biological reactions may lead to unpredictable results. Therefore, it is necessary to understand the mechanisms of N2O emissions from traditional anaerobic and bioreactor landfills.
Several reviews have been published on the emission and dynamics of N2O in soils, municipal wastewater treatment plants, and groundwater [17–23]. However, these reviews do not comprehensively evaluate N2O dynamics in landfills or N2O emissions from such facilities.
In this study, an extensive keyword search was conducted across multiple databases (Web of Science, Scopus, Google scholar, etc.) and citation lists for the past 20 years (through 2022). Keywords used in the search were nitrogen, nitrification, denitrification, nitrous oxide/N2O emissions, landfill, leachate, landfill gas, bioreactor, and combinations thereof. The main objectives of this review were to:
2. N2O Production MechanismsThe nitrogen cycle is a critically important nutrient cycle in terrestrial ecosystems. It involves both nitrification and denitrification processes. Nitrification is a two-step process. In the first step, ammonia (NH3) is oxidized to nitrite (NO2−) by ammonia oxidizing bacteria (AOB) and ammonia oxidizing archaea (AOA). In the second step, NO2− is converted to nitrate (NO3−) by nitrite oxidizing bacteria (NOB). In addition, NH3 oxidized to NO3− by complete ammonia oxidation (comammox) bacteria has been observed [24, 25].
Facultative heterotrophic denitrifiers dominate N2O production under anoxic or anaerobic conditions. These bacteria sequentially reduce the enzymes of NO3− to NO2−, NO, N2O, and dinitrogen gas (N2) [26].
There are eight distinct processes for N2O production during nitrification and denitrification, as shown in Fig. 1. These processes are hydroxylamine (NH2OH) oxidation (a.k.a., nitrifier nitrification), heterotrophic nitrification, nitrifier denitrification by AOB, AOA, comammox, heterotrophic denitrification, simultaneous nitrification and denitrification (SND), and dissimilatory nitrate reduction to ammonia (DNRA).
2.1. N2O Production by Autotrophic AOBTwo pathways generate N2O by autotrophic AOB during nitrification, NH2OH oxidation, and AOB denitrification [27]. When AOB oxidizes NH3 to NO2−, NH2OH is produced as an intermediate product during nitrification. AOB utilizes the enzymes ammonia monooxygenase (AMO) and hydroxylamine oxidoreductase (HAO) to oxidize NH3 to NO2− by NH2OH. The autotrophic AOB produces N2O as a byproduct during the incomplete oxidation of NH2OH to NO2−. During the oxidation of NH2OH, both nitrosyl radical (NOH) and NO act as essential intermediates (Fig. 2), and N2O can be generated from both the decomposition of NOH and the reduction of NO [28]. Elevated O2 concentrations and increased NH3 loads significantly promote the oxidation pathway of NH2OH by AOB [22, 29].
As shown by Law et al. [30], the N2O production rate exponentially increases with the NH3 oxidation rate (AOR). The authors also found that N2O can be produced from the chemical decomposition of NOH (an intermediate product in the oxidation of NH2OH to NO2−) but not from the biological reduction of NO during the oxidation of NH2O to NO2− (Fig. 2). Also, during the transition from anoxic to aerobic conditions, a significant amount of N2O can be generated due to NH3 accumulation in the anaerobic condition [31].
It has also been suggested that NO is the end-product of NH2OH oxidation by AOB instead of NO2−, which results from the non-enzymatic reaction of NO with O2 [32]. N2O is usually produced by the reduction of NO (Fig. 2). Thus, N2O emissions at low O2 concentrations or during aerobic to anaerobic transitions can increase if the NO production rate exceeds NO oxidation rate to NO2−. Increased intracellular NO concentration leads to NO emissions or the conversion of NO to N2O via the respiratory NO reductase (Nor) for the AOB reaction. As shown by Law et al. [30], N2O can be generated through the oxidation pathway of NH2OH if the environmental conditions transition from aerobic to anaerobic.
Interestingly, N2O emitted under anaerobic conditions can be generated through NH2OH [33]. For example, Caranto et al. [34] found that the enzyme cytochrome (cyt) P460 from the AOB Nitrosomonas europaea can quantitatively convert NH2OH to N2O under anaerobic conditions based on Eq. (1).
The production of N2O can occur through the chemical decomposition of NH2OH (Eq. (2)) and a chemical reaction between NH2OH and NO2− (Eq. (3)) [35].
N2O can also be formed chemically through the abiotic reactions of NH2OH and NO2−, including the decomposition of NH2OH by oxidized iron (Fe) or manganese (Mn) or NO2− reaction with Fe2+ (Eq. (4)) [36, 37].
Heil et al. [38] found that the reaction of NH2OH with Mn was better than that with Fe. Thus, Mn has a stronger effect than Fe on NH2OH oxidation, despite its low abundance in soil. However, Su et al. [39] found that in the pH range of 6.5 to 8.0, abiotic N2O production contributes only slightly (<3%) to total N2O emissions. The abiotic contribution is considerably larger under acidic pH conditions (pH ≤ 5).
Another AOB pathway is nitrifier denitrification, in which N2O is generated as the final product via the sequential reduction of NO2− to NO [17]. Nitrifier denitrification differs from coupled nitrification–denitrification because the denitrifiers reduce the NO2− or NO3− produced by the nitrifiers [40]. Nitrifier denitrification is governed by the enzymes NO2− reductase (Nirk) and NO reductase for AOB (Nor).
Previous studies have proven that N2O production through nitrifier denitrification pathways increases as NO2− accumulates from decreasing O2 [29, 41, 42]. Other studies found that the production of N2O from NO2− reduction by AOB linearly increases with increasing NO2− concentration [35, 43]. The accumulation of NO2− can trigger AOB denitrification and N2O emissions due to low O2 concentrations [44, 45]. It has also been proposed that nitrifier denitrification may be inhibited at very high NO2− concentrations (>50 mg N L−1) [46]. Kampschreur et al. [19] have reported that NO2− is a terminal electron acceptor in nitrifier denitrification instead of O2 and that NH3 acts as an electron donor. However, Wunderlin et al. [35] suggested that NH3 was unlikely to act as an electron donor and that hydrogen, pyruvate, and other electron donors should be considered.
2.2. N2O Production through Heterotrophic NitrificationHeterotrophic nitrifiers are known for their ability to simultaneously nitrify and denitrify under aerobic conditions [47–50]. Alcaligenes faecalis is a common heterotrophic nitrifier. Otte et al. [51] reported that A. faecalis could generate N2O under any conditions ranging from fully anaerobic to fully aerobic. Nevertheless, it has been shown that A. faecalis is more sensitive to low O2 levels during N2O production than the autotrophic nitrifier N. europaea. Anderson et al. [47] found that A. faecalis produced 10 times more N2O per cell than N. europaea under aerobic conditions. However, N2O generation from heterotrophic nitrification is minimal compared to the total N2O production [52].
According to Schalk-Otte et al. [53], A. faecalis can activate Nirk and Nor to generate N2O via the nitrifier denitrification pathway. However, Otte et al. [51] reported very low NO2− reduction rates under aerobic conditions. This finding indicates that N2O may not be generated through the reduction of NO2− but is a byproduct of NH2OH oxidation.
2.3. N2O Production by AOA and the Comammox ProcessAOA produces less N2O through aerobic NH3 oxidation than AOB [26]. However, N2O production by AOA is not significantly affected by O2 concentration [26, 54]. AOA is believed to generate N2O from NO2−, but the mechanism of N2O production by AOA is unclear [54–56]. Two main theories can interpret the mechanism of N2O production by AOA. The first theory proposes that the AOA Nitrosocosmicus oleophilus produces N2O via the nitrifier denitrification pathway under aerobic conditions at low pH values [55, 56]. Jung et al. [56] found that N2O production by AOA and AOB increased with decreasing pH. This may have resulted from increased enzymes involved in N2O production or an optimally acidic pH condition for enzymatic function. In the second theory, Stieglmeier et al. [54] reported that AOA could not enzymatically reduce NO2− to N2O via the nitrifier denitrification pathway, while the AOB enzymatically produced N2O by nitrifier denitrification.
Therefore, the AOA Nitrososphaera viennensis fails to complete nitrifier denitrification and releases NO as an intermediate product during hypoxic conditions. Then, NO abiotically reacts with Cu2+ and Fe2+ in the medium to yield N2O [57]. Based on these findings, the mechanism of N2O production by AOA requires further investigation. The recent discovery of the comammox process, in which NH3 is completely oxidized to NO3− [58], has overturned the conventional concept of nitrification. Kits et al. [58] found that the comammox bacterium Nitrospira inopinata produces NO using NO2− as an electron donor rather than NH3 under aerobic conditions. However, Nitrospira inopinata cannot produce N2O through aerobic NH3 oxidation or during the transition from aerobic to anaerobic conditions. This is because Nitrospira inopinata lacks NO reductase for AOB (Nor), which is required for NO reduction to N2O in the nitrifier denitrification pathway. Therefore, comammox microbes may contribute to N2O production during nitrification.
2.4. N2O Production via Heterotrophic DenitrificationDenitrification is mediated by four key enzymes: NO3− reductase (Nar), NO2− reductase (Nir), NO reductase for denitrifiers (NOR), and N2O reductase (Nos). N2O is produced in the presence of NO2− reductase (Nir) and NOR, and consumed in the presence of N2O reductase (Nos) [59]. The majority of N2O emitted during heterotrophic denitrification is due to NO reduction catalyzed by NOR as in the nitrifier denitrification pathway [17]. Heterotrophic denitrification is the main pathway for N2O emission under anaerobic conditions [60]. Zhu et al. [42] found that in the absence of O2, N2O is produced only by heterotrophic denitrification. However, Hu et al. [61] observed that under optimal denitrification conditions, the N2O generated was immediately reduced to N2 by N2O reductase (Nos).
The optimal conditions for nitrification and denitrification are different; however, these processes can occur under the same environmental conditions due to different microhabitats [40]. Denitrification can also occur in the presence of O2, and many denitrifying organisms can produce N2O over a wide range of O2 concentrations [13, 41, 42]. During denitrification, low O2 concentrations can cause NO2− to accumulate, inhibiting the N2O reductase (Nos) and producing N2O [35, 62, 63].
The diffusion of O2 into the soil is restricted due to expanding the water-filled pore space (WFPS) and increasing the anaerobic fraction of the soil volume. Bateman and Baggs [52] reported that all the N2O emitted from the soil with 70% WFPS was generated by denitrification. Autotrophic nitrification is the predominant pathway for N2O emission from soils, with WFPSs ranging from 35% to 60%. Autotrophic nitrification accounts for 81% of the N2O emitted from soils with a WFPS of 60%, and O2 is limited for a short time. This study proposes an N2O emission model with contributions from both processes for soils with different WFPSs (Fig. 3). However, the WFPS does not provide information on the distribution of macropores and micropores associated with soil texture. Thus, O2 availability cannot be adequately estimated using WFPS alone [42].
Temperature can also affect N2O emissions through denitrification. For example, Benoit et al. [64] calculated an optimal denitrification temperature of 54°C for N2O production in soils. This optimal denitrification temperature was based on the results obtained at temperatures ranging from 5°C to 45°C [64]. However, others have found that N2O emissions from denitrification in wastewater and soil increase when the temperature drops from 20°C to 5°C [65, 66]. This increase in N2O emissions is due to the activity of all denitrification enzymes and a decrease in N2O reductase (Nos) with decreasing temperature.
Acidic pH also limits denitrification and promotes N2O production [67]. In addition, sulfide is a known heterotrophic denitrification inhibitor. Pan et al. [68] have found that hydrogen sulfide (H2S) strongly inhibits heterotrophic denitrification. They observed a 50% decrease in N2O reduction by methanol-utilizing denitrifiers exposed to 0.04 mg H2S L−1 before and 0.1 mg H2S L−1 after H2S adaptation.
N2O production significantly increases with increasing NO3− concentrations because NO3− inhibits N2O reduction [13, 69]. Limited organic carbon availability promotes N2O emission during denitrification. External organic carbon provides electrons for NO3− and NO2−. Consequently, internally stored compounds like polyhydroxyalkanoates (PHAs), glycogen, and poly-β-hydroxybutyrate (PHB) are oxidized when the external organic carbon is limited. These internally stored compounds also supply energy to sustain biomass, significantly reducing N2 production and increasing N2O generation [53, 70]. Thus, low O2 concentrations, limited available organic carbon, acidic pH, sulfide, high NO3− concentrations, and high or low temperatures suppress N2O reduction during denitrification, resulting in N2O emissions [13, 19].
2.5. N2O Production through Autotrophic DenitrificationAutotrophic denitrification does not require external organic carbon sources. Thus, it can be an alternative route for NO3− reduction. Autotrophic denitrification may occur in landfills (especially older landfills) possessing low C/N ratios [71, 72]. Chen et al. [73] reported that when the chemical oxygen demand (COD) in the effluent remained relatively constant during denitrification, a decrease in the NO3− concentration was coupled with an increase in the sulfate concentration. This indicates that NO3− consumption occurs primarily through autotrophic denitrification. Autotrophic denitrification is favored over heterotrophic denitrification if the C/N ratio is low. Autotrophic denitrification may account for 15 to 55% of total denitrification [74]. Thiobacillus and Ottowia are the most abundant autotrophic denitrifying bacteria [75]. In recent years, reduced inorganic sulfur compounds, such as sulfur (S0), sulfide (S2−), and thiosulfate (S2O32−), have been widely utilized as electron donors for autotrophic denitrification. In addition to N2O emission reduction, this method produces little excess sludge and does not require additional organic carbon sources [76].
Sulfide concentration is a limiting factor for sulfide-driven autotrophic denitrification. High initial sulfide-to-nitrate (S/N) ratios limit N2O emission, but operational costs are higher [76, 77]. Yang et al. [78] found that N2O emissions increased sharply when S/N ratios decreased from 2.1 to 1.4. Also, the percentage of nitrogen load that changed to N2O increased from 0.002% to 0.41%. This probably happens due to less competition between NO3− reductase (Nar), NO2− reductase (Nir), NOR, and N2O reductase (Nos). An excess supply of electrons limits N2O accumulation during NO2− reduction because N2O reductase (Nos) is the weakest competitor among the reductases [79].
2.6. N2O Production via SNDSeveral studies have found that nitrification and denitrification can simultaneously occur at low O2 concentrations [35, 80, 81]. Denitrifying bacteria can denitrify even in microaerobic environments. Thus, nitrification and denitrification can simultaneously occur under favorable conditions. According to previous studies, the presence of ammonium (NH4+) and NO2− in SND conditions increases N2O emissions [80, 81]. In addition, nitrification and heterotrophic denitrification pathways are mainly responsible for N2O emissions from the SND process. Nitrification and SND reactions are inversely related to the generation of N2O [82]. N2O production during the SND process is strongly affected by the accumulation of NO2−. Wunderlin et al. [35] found that N2O could be generated by heterotrophic NO2− reduction in the SND process because O2 inhibits N2O reductase (Nos). In the SND process, more N is converted to N2O than in the conventional nitrification and denitrification processes.
2.7. N2O Production via DNRADenitrification is favored over the DNRA process. However, under nitrate-limited and strongly reducing conditions, electron acceptor deficiency may limit microbial growth. When the C/NO3− ratio is high (>12), the electron acceptor NO3− is limited, allowing DNRA to gain an advantage over denitrification [83, 84]. In the DNRA process, NO3− is first reduced to NO2−. Then, NO2− is reduced to NH4+, and N2O is formed simultaneously [85]. However, when denitrification occurs simultaneously, the contribution of DNRA to total N2O production is negligible [83, 84].
2.8. AnammoxAnaerobic ammonium oxidation (anammox) is a unique type of denitrification that combines NO2− reduction and NH3 oxidation. The anammox process is not fully understood. However, anammox bacteria do not reduce NO3− by conventional denitrification with N2O. Consequently, the anammox bacteria likely do not generate N2O [19]. Li et al. [15] have proposed that denitrification is not the only pathway for N2O generation; however, N2O cannot be generated in the anammox process. The anammox bacterium Kuenenia stuttgartiensis consumes NO and convert it to N2, but not to N2O (Eq. (5)) [86, 87].
3. N2O Dynamics and Emissions from Anaerobic Landfills3.1. N2O Emissions from Anaerobic LandfillsN2O emissions from various anaerobic landfills are summarized in Table 1. N2O fluxes in these landfills vary significantly over space and time. The physical processes in the anaerobic landfills are governed by passive diffusion, gauge pressure, and atmospheric wind advection conditions [88, 89]. As a result, the production and emission of N2O are not equivalent in anaerobic landfills. Zhang et al. [90] found a strong negative correlation between atmospheric pressure and N2O fluxes. Field measurements have recorded negative N2O fluxes in several landfills where different mechanisms may have been responsible. Negative N2O fluxes indicate that the N2O reduction rate exceeds the N2O generation rate [82] or that N2O from the atmosphere has been absorbed by the cover soil [91]. The leachate head of a landfill can also affect the amount of N2O produced. In Japanese offshore landfills, waste is disposed of directly into the water until full. In these offshore landfills, high water contents exist in the waste layer, and the saturated conditions strongly promote N2O generation [5]. Hence, it is important to maintain a minimal leachate head on the liner at the base of a sanitary landfill to minimize N2O production and protect the landfill structure.
3.2. N2O Dynamics in Anaerobic LandfillsLandfill sites that receive fresh waste emit more N2O than older anaerobic landfills [6]. Wang et al. [82] found that closed landfills had lower N2O fluxes than operating landfills. After fresh waste is buried, anaerobic conditions develop rapidly within the waste. O2 in the void spaces of freshly deposited waste can be consumed in organic decomposition and nitrification processes during the initial aerobic phase. Wang et al. [82] reported that N2O emissions were usually high from the working faces of landfills. Previous studies have found that N2O emissions from landfills are highest during the initial phase of operation [94, 98]. Harborth et al. [94] confirmed this finding using measured NO2− and NO3− concentrations in the working face. They reported NO2− concentrations reaching 600 mg N kg−1 in dry matter (DM) and high NO3− concentrations in the working face of a mechanical-biological waste treatment (MBT) landfill. The maximum measured N2O flux in this MBT landfill was 428 mg N m−2 h−1. This study found strong negative correlations between the concentrations of N2O and CH4 at a depth of 50 cm from the landfill surface due to the nitrification process (initiated by O2 input during waste deposition).
The contribution of heterotrophic and autotrophic nitrification pathways (e.g., incomplete NH2OH oxidation) to N2O emissions increases with decreasing O2 concentration. When the O2 and NH3 concentrations are relatively high, incomplete NH2OH oxidation dominates nitrification [60]. As the O2 sources decrease, the environment promotes NO2− accumulation. Thus, the proportion of N2O produced by AOB denitrification increases. NO2− accumulation can result from the incomplete denitrification of NO3− in the waste, leading to N2O production [11]. O2 cannot be replenished once the waste is covered.
Consequently, the aerobic phase in a landfill lasts only for a few days. After O2 depletion, the pH decreases due to the production of organic acids during the initial stage of anaerobic organic decomposition. An increase in N2O emissions can be attributed to this phenomenon because the N2O reductase (Nos) is inhibited under these conditions [5, 67].
During the operational lifetime of a landfill, the contribution of nitrification to the total N2O emitted by the landfill is negligible. The intrinsic NO3− in fresh waste only undergoes denitrification and is responsible for most N2O emitted as waste stabilizes [82]. Positive correlations have been reported between N2O emission and CH4. This positive correlation indicates the generation of N2O under anaerobic conditions [5, 92].
3.3. Factors Affecting N2O EmissionsSeveral factors can affect N2O emissions from anaerobic landfills, including cover type, O2 levels, and climatic conditions [90]. For example, the O2 levels and climatic conditions strongly influence N2O production by microorganisms in uncovered landfills [5, 94]. Conversely, using high-density polyethylene (HDPE) cover in active landfill sites can prevent the emissions of N2O [10]. However, Bogner et al. [99] have found that daily cover can cause higher N2O fluxes than intermediate and final covers. This study reported a peak N2O flux of 7.5 mg N2O m−2 h−1 from fresh waste in a landfill. High N2O fluxes from daily cover can be attributed to the high moisture content in waste, readily available nitrogen, and limited aeration of the recently deposited waste.
Börjesson and Svensson [14] observed N2O concentrations close to atmospheric levels in the gas extraction system of a closed landfill in Sweden (Table 1). This study proposes that the gas extraction system may introduce O2 that oxidizes NH4+ to NO3−. Once NO3− reaches the anaerobic waste, it is denitrified and generates N2O. It has also been suggested that NO2− may act as an electron acceptor during AOB denitrification and promote N2O production under O2 deficient conditions [100].
High temperature conditions and precipitation may promote the conversion of nitrogen-bearing compounds to N2O [5, 96]. Benoit et al. [64] studied the effect of temperature on N2O production by nitrification and denitrification processes in luvisolic soil. This study suggests that the rate of N2O production by nitrification and denitrification continues to increase until the optimum temperature is reached. The optimum temperature for N2O production via nitrification is 42°C, and the optimum temperature for N2O production via denitrification is 54°C. Thus, a relative increase in N2O production under nitrifying and denitrifying conditions can be observed at temperatures above 20°C. Denitrification is the major N2O production pathway at temperatures below 20°C. However, based on data from monitored landfill sites, other studies found no correlation between N2O emission and environmental factors, including temperature and humidity [6, 14, 93]. These studies hypothesize that rather than environmental factors, the nitrogen content in waste, the age of the deposited waste, and O2 levels within landfills may affect N2O emissions [14, 93].
3.4. N2O Production Mechanisms in Cover SoilsThe physicochemical properties of soils are intrinsic factors that influence N2O emissions. Zhang et al. [90] proposed that most N2O emissions from landfills could be attributed to cover soils instead of the waste within the landfills. The authors indicate that when a landfill site is covered with soil containing lower carbon and nitrogen, the N2O flux decreases by one to two orders of magnitude relative to an uncovered area. The highest N2O fluxes reported by Börjesson and Svensson [14] were from a landfill covered with pure sewage, the substrate for N2O generation. As shown by Zhang et al. [92], clay soils emit higher N2O than sandy cover soils due to the lower pH of sandy cover soils (pH 4.89). The results of this study are attributed to the fact that AOA produced less N2O than AOB during NH3 oxidation and was superior to AOB under NH3 deficient and acidic conditions [26]. Zhang et al. [101] found strong positive correlations between NO3− concentrations and ammonia monooxygenase (AMO) abundance in AOA during active nitrification in acidic soils (pH < 4.50). However, this correlation was not observed in AOB. Long et al. [96] observed the same phenomenon in landfill cover soils. The results of this study indicate that selecting an appropriate cover soil can reduce N2O emissions [90].
Soil moisture has been identified as the most sensitive factor in N2O emissions because it directly regulates O2 availability in the soil pores. Thus, N2O emissions can be affected by WFPS in the cover soil. Large WFPSs in the cover soil can significantly increase N2O emissions after leachate irrigation or precipitation [90, 92, 102, 103]. For example, Gao et al. [102] reported that N2O emissions from aged refuse, clay soil cover, and sandy soil cover with 70% WFPS were 1.78–3.48 times higher than N2O emissions from soil cover with 46% WFPS. In addition, leachate irrigation can generate more N2O than distilled water due to the organic matter content of the leachate.
There are a few studies on the contribution of methane oxidizing bacteria (MOB) in cover soils to the production of N2O. N2O emissions are primarily attributed to nitrification and denitrification processes in the cover soil. In some cases, N2O emissions are directly related to methanotrophic activities [92]. MOBs can co-oxidize NH3 because of the similar size and structure of CH4 and NH3. This may be due to similarities between the physiology of methanotrophs and autotrophic nitrifiers and the enzymatic oxidation of these substrates. The simultaneous oxidation of NH3 and CH4 by methanotrophs is called methanotrophic nitrification. Zhang et al. [92] proposed that MOBs near the surface of landfills may generate N2O by using NH4+ as a surrogate substrate. Gao et al. [102] show that the co-oxidation of NH3 by methanotrophic bacteria accelerates N2O production by nitrifying NH4+ to NO3− in aged refuse.
Bogner et al. [104] show that N2O concentrations in the final cover soil peak at a depth of about 100 cm, indicating that denitrification restricts aeration and increases N2O production. This study found that the minimum O2 content in the soil air was about 11% (v/v) at depths of 50 to 100 cm. Furthermore, a linear relationship was observed between N2O fluxes and N2O concentrations at a depth of 100 cm. However, the optimum depth for N2O production varies due to seasonal changes in soil moisture and aeration.
3.5. N2O Emissions through Landfill Leachate TreatmentN2O emissions from landfills are assumed to be negligible and are not considered in the 2006 IPCC Guidelines for National Greenhouse Gas Inventories [105]. The contribution of N2O from landfills to the greenhouse effect is only about 3% [6]. However, nitrification and denitrification reactions within landfills are minimal. Nitrogen is primarily emitted from landfills through leachate in the form of ammonia (NH3–N) or ammonium (NH4+–N), implying that N2O is mainly generated from leachate treatment.
N2O emissions from various leachate treatment facilities are listed in Table 2. Lin et al. [106] estimated annual N2O emissions from the five landfills to be between 0.002 to 0.479 Mg N2O. The percentages of nitrogen converted to N2O in the leachate from Datianshan, Likeng, Shuikukeng, Guoqiaowo, and Xiaping landfills were 0.0002%, 0.0054%, 0.0252%, 0.0160%, and 0.0531%, respectively. Wang et al. [10] estimated that the average N2O emission factors (emission per unit of nitrogen) removed from the leachate of Dongbu, Dongfu, and Nanjing landfills were 8.9%, 11.9%, and 10.2%, respectively. Law et al. [107] reported that N2O emission factors from wastewater treatment plants (WWTPs) varied widely and ranged from 0% to 25%. This variability was attributed to the different WWTP configurations and operating conditions [21]. Sun et al. [108] found that the influent N2O emission factors for the anoxic-oxic process, sequencing batch reactor process, and oxidation ditch process were 1.37%, 2.69%, and 0.25%, respectively.
The annual estimated N2O emissions from these sites range from 0.15 to 34.50 Mg N2O. The leachate treatment facility at the Nanjing landfill (0.15 Mg N2O) released much lower N2O than the Dongbu (34.50 Mg N2O) and Dongfu (14.94 Mg N2O) landfills. This study concluded that the low nitrogen removal rate at the Nanjing landfill (14.5%) was due to inadequate oxidation ditch management [82]. Regarding CO2 equivalent, N2O emissions from the leachate treatment systems at the Dongbu and Dongfu landfills account for 76.2% and 44.4%, respectively, of the total N2O emissions from the landfill reservoirs and leachate treatment systems. Therefore, the annual total amount of N2O emitted from leachate treatment facilities in the Dongbu and Dongfu landfills is estimated to be 8.55 g N2O–N per capita. This exceeds the IPCC recommended emission factor of 3.2 g N2O–N per capita per year [105]. This recommended emission factor corresponds to the emission of about 0.035% of the nitrogen load from a WWTP as N2O [82].
The emission of N2O primarily occurs due to aerobic processes in aeration tanks. The contribution from anoxic zones (e.g., denitrifying tanks) is negligible because the gas transfer rate in these zones is very low [109], as shown in Fig. 4. However, due to intensive stripping in the WWTPs, aeration leads to N2O emissions [108, 110]. The solubility of N2O in water is relatively high; thus, stripping is not a rapid process. Consequently, dissolved N2O in effluents can be emitted from the receiving rivers [111].
AOBs are the main aerobic N2O producers, generating N2O over a wide range of DO and NO2− concentrations and low COD loads. Biological N2O production via NH2OH oxidation is favorable at high NH3 and DO, and low NO2− concentrations when combined with high nitrogen oxidation rates [35, 112]. It has been suggested that highly saline landfill leachate inhibits biological nitrogen removal [113]. Studies have shown that high salt concentrations can promote N2O production, with NO2− and NO3− serving as terminal electron acceptors [70, 114]. Liu et al. [113] found that the relative proportion of N. europaea in the microbial population increased with increasing salinity (from 10 g L−1 to 30 g L−1). This may be an important factor in promoting N2O production by salinity.
4. N2O Dynamics and Emissions from Bioreactor Landfills4.1. Bioreactor LandfillsBioreactor landfill operations use technologies (such as water or air injection, leachate recirculation, and various combinations of in-situ and ex-situ treatments) to facilitate nitrification and control redox conditions for accelerating waste stabilization [115, 116]. Bioreactor landfills are commonly classified based on their respective metabolic pathways. These bioreactors include anaerobic, aerobic, and hybrid bioreactors [115]. Leachates can be recirculated through solid waste in anaerobic bioreactor landfills. However, leachate recirculation increases in the NH3–N concentrations due to the lack of a pathway for NH3–N removal under anaerobic conditions [117].
Air is injected into landfills for several reasons. These include accelerating waste stabilization, reducing leachate contamination, removing NH3–N through nitrification, extending the operational lifespan of the landfill, and mitigating CH4 emission [118–120]. However, aerobic bioreactor landfills have high operating and capital costs due to energy consumption and the need for aeration systems [121]. An alternative to this is semi-aerobic landfills that circumvent the economic disadvantages of aerobic bioreactor landfills by eliminating aeration system [122]. However, as demonstrated by Matsuto et al. [123], high ambient temperatures cause a slight difference between the temperature of the landfilled waste and ambient temperatures, which may reduce the buoyancy force. They also suggest that connecting the gas vents to the leachate collection pipe is unnecessary. Connecting the vents only increases the airflow through the pipe and has little impact on aeration.
Hybrid bioreactor landfills have been developed to circumvent NH3–N accumulation and reduce energy consumption. Hybrid bioreactor landfills are operated under various aerobic and anaerobic conditions to achieve nitrification and NH3 denitrification. There are two types of hybrid bioreactor landfills. One is the in-situ nitrification–denitrification bioreactor landfill, in which nitrification and denitrification are promoted by intermittent aeration in the landfill [116, 121]. The other is the ex-situ nitrification and in-situ sequential denitrification bioreactor landfill method, in which leachate is nitrified and then introduced back into the landfill for denitrification [116, 124, 125]. Benson et al. [117] virtually found no NO3− in leachate collected from a bioreactor landfill, resulting from nitrified leachate recirculation. This is because NO3− is highly favored as an electron acceptor in the absence of O2, and the anaerobic landfill functioned as a denitrification bioreactor [13]. However, Zhong et al. [126] reported that CH4 production decreased with increasing NO3− loading during nitrogen removal by ex-situ nitrification and in-situ denitrification in a bioreactor landfill. This occurred because the processes in which NO3− acted as a terminal electron acceptor were more energetically favorable than the acetogenic, sulfate reducing, and methanogenic processes [71]. Thus, methanogenesis was inhibited by both NO and N2O produced during denitrification [73]. Sun et al. [127] proposed using a hybrid bioreactor landfill, in which ex-situ SND and in-situ denitrification overcome the methanogenic inhibition and accelerate CH4 production.
4.2. Features of N2O Emissions from Bioreactor LandfillsRecently, N2O emissions from bioreactor landfills have attracted strong interest because they can remove nitrogen and promote N2O emissions [72, 100]. The reported N2O emission factors of each type of landfill are summarized in Table 3, though the available information is limited. Laboratory studies of N2O emissions from each category of bioreactor landfill estimated N2O emission factors from negligible to a maximum of 0.670 g N2O kg DM−1. The aeration rate (low or high) and the mode of aeration (continuous or intermittent) have significant impacts on N2O emission levels [11, 83]. Elevated moisture content due to leachate recirculation can result in denitrification, leading to high N2O emissions. O2 diffusion is controlled by moisture, substrate availability, and microbial activity [42]. Aerobic-anaerobic-aerobic transitions emit more N2O than aerobic conditions [11]. It has been proposed that NO2− may accumulate due to incomplete nitrification or denitrification during aerobic-anaerobic transitions, thus promoting N2O production.
The CDM Method AM0083, “Avoidance of landfill gas emissions by in-situ aeration of landfill”, elaborates on N2O emission factors based on waste composting. These N2O emission factors range from 0.2 to 1.6 g N2O kg DM−1 [128]. However, landfill conditions (such as waste properties, density, and aeration rate) can differ significantly from compost. Thus, it is important to track the N2O production pathways in bioreactor landfills and determine the sources of N2O generated through various microbial processes. In addition, proper control of operating parameters minimizes N2O production and emissions, and facilitates assessing N2O emission factors [100, 129].
4.3. Anaerobic Bioreactor Landfills (Leachate Recirculation)N2O is produced by heterotrophic and/or autotrophic denitrification pathways in anaerobic bioreactor landfills. The pathways depend on the age of the waste and the C/N ratio. The COD/NH4+–N ratio in recirculated leachate is the primary factor controlling N2O emissions during denitrification [131]. Li et al. [60] have found that low COD/NH4+–N ratios in recirculated leachate are more likely to promote N2O emissions by denitrification. Less N2O is emitted when more biodegradable organic compounds contribute to COD at the same COD/NH4+–N ratio. This is particularly true when the COD/NH4+–N ratio is low. Thus, it can be concluded that sufficient amounts of carbon in the leachate (indicated by high C/N ratios) may be beneficial for complete denitrification and result in reduced N2O emissions [130].
Leachate irrigation and flowing leachate combined with vertical injection wells have been employed to recirculate leachate in landfills. However, these leachate recirculation methods can influence N2O emissions. In situations where O2 from the ambient air is readily available, leachate irrigation and flowing leachate with high NH3 concentrations were found to promote significant levels of N2O production in landfill topsoil [7, 103]. Therefore, these studies propose installing underground pipes or vertical wells to distribute the leachate evenly and prevent excessive N2O production. These studies also recommended using an impermeable top liner to control gas emissions.
4.4. Aerobic Bioreactor Landfills4.4.1. N2O dynamicsIn batch experiments, significantly more N2O is produced under anaerobic conditions than aerobic conditions [42, 82]. This result may be due to a rapid transition to anoxic/anaerobic conditions, which promotes N2O production via denitrification [82]. However, aerated landfills emit more N2O than anaerobic landfills [4, 11]. In aerated landfills, the introduction of air increases the overall volume of gas, but the N2O concentration remains unchanged [8]. More N2O is produced in highly aerated landfills than in non-aerated landfills. According to Kampschreur et al. [111], high aeration flow dramatically increases the emissions of both NO and N2O. Without adding NO3−, N2O emissions from denitrification decrease as the intrinsic NO3− is depleted in the anaerobic landfill waste. When air is introduced into these landfills, different N2O production pathways often simultaneously occur due to spatial heterogeneity in O2 concentrations [11]. These are the reasons that aerobic bioreactor landfills emit significantly more N2O than anaerobic landfills.
The production of N2O due to aeration varies significantly with waste stabilization. Nag et al. [11] found that in-situ aeration with or without leachate recirculation considerably promotes N2O production in new waste landfills compared to old landfills. Aeration of a landfill can facilitate waste biodegradation, promoting the dissolution of NH3–N in the leachate. This process is called ammonification [132]. According to Li et al. [16], O2 increases the concentration of NH4+–N in the leachate from fresh waste during the initial stages of in-situ aeration. The concentration of NH4+–N may increase due to insufficient O2 in aerobic bioreactor landfills to convert NH4+–N by nitrification. It may also be due to the presence of readily available COD substrates that inhibit autotrophic nitrification [71]. At this stage, N2O is not generated via nitrification. This is presumably because nitrification is suppressed, and denitrification is promoted at high NH4+ concentrations and low DO levels [11].
Li et al. [16] demonstrate that the peak of NH4+–N arrival is delayed by a decrease in COD due to the slow growth rate of autotrophic nitrifiers and potential competition with heterotrophs. Once the peak levels of NH4+–N are reached, it decreases rapidly due to the volatilization and nitrification of NH3. Tong et al. [12] have also reported that NH3 volatilization drastically reduces NH4+–N concentrations after in-situ aeration. Under aerobic conditions, nitrification occurs after NH3 volatilization [4, 130]. NH3 volatilization is affected by air stripping, pH, and temperature [71]. NH3 volatilization can be further promoted at higher temperatures and pH [12, 71].
At the beginning of the nitrification process, the N2O emission is controlled by the accumulation of NO2− and NO3− [11]. However, the accumulation of NO2− and NO3− lags behind the decrease in NH4+–N concentrations [16]. This is due to the depletion of organic substrates for denitrification in aerobic bioreactor landfills [12, 16]. Therefore, nitrification may occur as the liquid passes through the aerated waste mass [133].
It is challenging to ensure completely aerobic conditions throughout a landfill. Li et al. [16] measured NOx−–N concentrations and found that the concentrations were consistently lower than expected based on stoichiometric NH4+–N nitrification calculations. The low measured NOx−–N concentrations are probably due to the SND process in an aerated system where aerated and anoxic regions exist simultaneously. The simultaneous presence of aerated and anoxic regions in aerobic bioreactor landfills is attributed to the transportation of air through preferential flow paths, which seems reasonable given the pronounced heterogeneity of the waste. According to van Turnhout et al. [134], O2 is readily consumed near the preferred channels when the waste contains sufficient biodegradable carbon. This generates an anaerobic zone within the landfill. NO3− is produced by nitrification in the aerobic zone, diffuses into the anaerobic zone, and is used for denitrification. However, when the amount of biodegradable carbon falls below a certain threshold, O2 penetrates deeper into the landfill, denitrification ceases, and NO3− concentration increases. This finding corresponds to the results reported by Berge et al. [135] and Raga and Cossu [118]. Nag et al. [11] showed that the N2O production rate peaks as temperature and DO increase because SND can occur on the microscale. This phenomenon is critical for reaching high N2O concentrations. O2 is partially distributed in the waste layer in passively aerated landfills, such as semi-aerobic landfills. The partial distribution of O2 in the waste layer accelerates waste stabilization and promotes SND, which can reduce the nitrogen content [9]. Therefore, under aerobic conditions, N2O emissions probably occur through all possible pathways such as NH2OH oxidation, nitrifier denitrification, and heterotrophic denitrification.
4.4.2. Factors affecting N2O productionVariations in O2 concentration and temperature significantly affect nitrification and denitrification in aerobic bioreactor landfills [100]. Due to the inherent heterogeneity of the landfills, variations in O2 concentration and temperature occur simultaneously [136]. The high N2O production at O2 concentrations above 15% (v/v) may be due to the relatively high NH3 oxidation rate (AOR), which stimulates the formation of N2O from the oxidation of NH2OH [30, 100]. O2 concentrations above 15% (v/v) favor nitrification [11]. These findings are consistent with the results reported by Law et al. [30]. The results of several studies indicate that N2O production from NH2OH oxidation is significantly promoted by increased NH3 loadings and elevated O2 concentrations [22, 29–31, 60, 129]. NH3 accumulates in anaerobic landfills because there is no pathway for NH3 oxidation under anaerobic conditions. Thus, the amount of N2O produced by NH2OH oxidation can be increased by injecting sufficient O2 for the nitrification of accumulated NH3. NH2OH oxidation is always dominant in nitrification compared to heterotrophic nitrification [129]. However, it must be noted that N2O is not only generated by nitrification during aeration. It can also be produced by denitrification [4]. Hwang and Hanaki [137] found that nitrification and denitrification simultaneously occur at O2 levels of 10% to 15%, though nitrification predominates at higher O2 concentrations. Zhu et al. [42] studied the influence of O2 availability on N2O production in agricultural soils. This study showed that as the O2 concentration decreased from 21 to 3%, the amount of N2O produced increased 19-fold. Three to six times more N2O was produced at O2 levels between 0.0% and 0.5% than N2O produced at 3% O2. At low O2 concentrations of 0.5% and 3%, N2O was primarily produced from nitrifier denitrification and heterotrophic denitrification. While the lack of O2 may inhibit nitrification, nitrifier denitrification and heterotrophic denitrification may lead to the generation of N2O [42, 130].
The temperature of the waste in the landfill is another factor controlling the dynamics of N2O. When the temperature in the waste layer is too low during aeration, the aeration rate will be too low for the decomposition of organic carbon. This may happen because O2 in the landfill is solely consumed by CH4 oxidation [138]. High temperatures can stimulate the enzymatic activity of nitrifiers and denitrifiers and enhance the microbial production of N2O [139]. NO2− is generated as an intermediate product in both nitrification and denitrification. NO2− rarely accumulates in the environment, but its concentration can increase at high temperatures. Increased NO2− concentrations at high temperatures may be due to reduced NOB activity, leading to increased emission of N2O [64].
In-situ aeration of landfills can accelerate the decomposition of organic matter [100]. This energy released can increase the temperature and promote N2O production. The optimal temperatures range for nitrification (30°C–40°C) induces the formation of N2O at high O2 concentrations as a byproduct of nitrogen turnover. However, in the thermophilic range of 50°C–60°C, nitrification may be inhibited, possibly due to thermally-induced death of Nitrosomonas cultures [71].
The oxidation of organic materials is an exothermic process. Thus, fire is a potential disadvantage in aerobic bioreactor landfills. The temperature in aerobic landfills can reach 80°C [138]. The temperature should be controlled by injecting water or leachate and cessation of aeration, thus minimizing the risk of combustion in a landfill. However, N2O production may be accelerated by the decrease in O2 concentration in a landfill after aeration is stopped. N2O emissions due to nitrifier denitrification can be noticeable if nitrification occurs at low O2 concentrations [41, 42]. Moreover, during denitrification, the rate of N2O production is accelerated at higher temperatures (i.e., 50°C) compared to the rate at lower temperatures (i.e., 20°C) with low O2 concentrations (<5%) [13]. Thus, intermittent aeration may not be a suitable countermeasure against rising temperatures in landfills to lower N2O emissions [13].
4.5. Hybrid Bioreactor Landfills4.5.1. Intermittently aerated bioreactors with leachate recirculation4.5.1.1. N2O dynamicsAn intermittently aerated bioreactor creates a hybrid oxidation-reduction environment in a landfill. This alternative aerobic-anoxic-anaerobic environment may attenuate nitrogen pollutants from the SND process [119, 120]. However, frequently switching between aerobic and anoxic conditions can significantly produce N2O by AOB. NOB activity is inhibited when the O2 concentration is too low, leading to NO2− accumulation [62, 63]. NO2− may also accumulate due to the incomplete denitrification of NO3− in the waste [11]. Tran et al. [131] found that NO2− concentrations were higher than NO3− concentrations in a hybrid bioreactor. Thus, a high NO2− level is an essential factor for N2O production by AOB under limited aerobic conditions. Aeration interval also affects N2O emissions. He et al. [130] determined a positive correlation between N2O production and prolonged aeration.
Conversely, frequent switching between anaerobic and aerobic conditions suppresses the activity of heterotrophic denitrifying bacteria and inhibits N2O production via heterotrophic denitrification [140]. Since N2O formation occurs primarily during aerated periods and increases during denitrification, sufficient time is needed to remove nitrogen-bearing pollutants. For example, Yang et al. [140] showed that in a full-scale, one-step intermittent aeration process, more than 90% of N2O emissions could occur during the aeration period. However, less than 8% of emissions occurred during the non-aeration period. It should be noted that the switching interval between oxidizing and reducing conditions in landfills needs to be further studied to reduce N2O formation and enhance the efficiency of nitrogen removal.
4.5.1.2. Factors affecting N2O productionLow organic carbon and C/N ratios during denitrification suppress N2O reduction, leading to N2O emissions [13, 72]. Li et al. [60] demonstrated that the COD/NH4+–N ratio in recirculated leachate is important for N2O emissions. Nitrous oxide reductase (Nos) competes less for carbon sources than nitrate reductase (Nar) or nitrite reductase (Nir) at low COD/NH4+–N ratios, inhibiting N2O reduction. Moreover, the availability of biodegradable carbon substrates at the same COD/NH4+–N ratio during denitrification directly affects N2O emissions. These results are consistent with He et al. [130] and Li et al. [16], who found a negative correlation between N2O production and bioavailable C/N ratio under limited aerobic degradation conditions in recycled leachate.
Li et al. [16] showed limited emissions of N2O during the initial stages of waste stabilization in intermittent aeration and continuous micro-aeration bioreactors. However, N2O emissions increased by one to three orders of magnitude in the later stages due to a lack of carbon sources in the recycled leachate. Therefore, during the operation of a bioreactor with intermittent aeration and leachate recirculation, fresh waste replenishment provides carbon for denitrification and reduces N2O production. Suppose the bioreactor system is used in a closed landfill site. In that case, the recirculation volume and the number of cycles should be reduced during the later stages to impede NH3 accumulation, eventually decreasing N2O production [11].
4.5.2. Combined ex-situ nitrification and in-situ denitrification bioreactor landfills4.5.2.1. N2O dynamicsWu et al. [13] found that using NO3−-type leachate in an anaerobic landfill resulted in significantly less N2O emissions than using NO2−-type leachate. The maximum specific N2O emissions were below 4 mg nitrogen per kilogram of waste when NO3− leachate was used for five days, corresponding to about 1.5% nitrogen removal. However, the total N2O emissions were equivalent to about 50 mg nitrogen per kg of waste containing NO2− leachate. This corresponds to approximately 40% nitrogen removal. Wang et al. [116] propose a bioreactor landfill that combines ex-situ nitrification and in-situ denitrification as an improved alternative to intermittent aeration to control N2O emissions.
N2O emissions are related to the denitrification capacity of the waste. Chen et al. [73] reported that partially degraded one-year-old waste is suitable as a denitrification medium. Due to the relatively low denitrification capacity of the aged refuse, the aged waste produces more N2O than one-year-old waste when NO3− is introduced to the waste mass. Wang et al. [116] proposed using a combined bioreactor landfill consisting of an anaerobic reactor loaded with fresh waste for denitrification and an aerobic reactor loaded with aged waste for nitrification. However, He et al. [130] found no positive correlation between N2O emissions and nitrification–denitrification capacity and concluded that N2O production is influenced by other environmental factors.
4.5.2.2. Factors affecting N2O productionCombined ex-situ nitrification/in-situ denitrification bioreactor landfills are problematic in terms of N2O emissions. Variations in N2O emissions in combined bioreactor landfills are closely related to changes in leachate quality. It is recognized that N2O emissions from denitrification are affected by the C/N ratio and NO3− concentration [13, 19]. Wang et al. [116] found that as the COD/TN ratio decreases during waste degradation, N2O emissions from denitrification increase due to the inhibition of the denitrification process. During the initial stage of leachate recirculation, when the leachate is enriched in organic matter, a complete and intense denitrification reaction occurs, and N2O is completely reduced to N2 [116].
On the other hand, if the COD/TN ratio of the leachate decreases during the later stages of waste stabilization, the bioreactor landfill may enter a microaerobic or anoxic state immediately after recirculating the nitrified leachate, followed by a transition to an anaerobic state [116]. This phenomenon is attributed to the inhibition of DO and oxidation-reduction potential (ORP) in the nitrified leachate during denitrification, resulting in N2O emissions. Nitrous oxide reductase (Nos) activity is inhibited by the addition of molecular oxygen and induction of NO3− adaptation [132]. At this point, a large amount of N2O is produced as the COD/TN ratio decreases [116, 130].
High NO3− concentrations also increase N2O production during denitrification because NO3− inhibits N2O reduction [13, 69]. According to Tallec et al. [125], enhanced NO3− leachate recirculation induces a 100-fold increase in N2O concentrations in the gas collection pipe. In this study, the N2O concentration reaches 23 ppm in the collection pipe. He et al. [130] injected leachate containing KNO3 into a lab-scale reactor to simulate the leachate after nitrification. The results confirmed that 0.011% of the externally added nitrogen was converted to N2O. Results from a lab-scale reactor simulating a traditional landfill estimated that 0.00003% of the initial nitrogen in the waste was converted to N2O. Thus, high concentrations of NO3−–N resulting from ex-situ nitrification can stimulate N2O production and inhibit CH4 production during in-situ denitrification in bioreactor landfills. Sun et al. [127] proposed combining ex-situ SND in an aged refuse bioreactor with in-situ denitrification in a fresh refuse bioreactor to reduce the amount of NO3−–N in the recirculated leachate.
Finally, leachate for ex-situ nitrification should be treated in a nitrification system (e.g., aged refuse bioreactor [116], aerobic biofilter, sequential batch reactor [124], or continuous stirred tank reactor [126]). However, significant N2O can be emitted from aerobic processes in ex-situ nitrification systems, as shown in Fig. 4. Therefore, combined bioreactors may not be suitable for controlling N2O emissions.
5. Summary of Major Pathways and Factors Affecting N2O ProductionThe main pathways and factors affecting N2O production in each type of landfill are summarized in Table 4. Bioreactor landfills emit larger amounts of N2O than anaerobic landfills. N2O production in bioreactor landfills typically occurs via different pathways, including NH2OH oxidation, nitrifier denitrification, and heterotrophic denitrification. The dynamics of N2O in bioreactor landfills are controlled by several factors, including aeration mode and rate, C/N ratio, AOR, redox conditions, and temperature. At high aeration rates, larger amounts of N2O may be produced than at low aeration rates. When air is introduced into a landfill, different N2O production pathways often occur simultaneously due to spatial heterogeneity in the O2 concentrations. The transition from aerobic to anaerobic conditions may result in the accumulation of NO2− due to incomplete nitrification or denitrification, leading to increased N2O production. Under limited aerobic conditions, high NO2− concentrations are the main factor controlling N2O production by AOB. Lack of available organic carbon and low C/N ratios suppress N2O reduction during denitrification, resulting in increased N2O emissions.
6. Conclusions and Future PerspectivesThe decomposition of waste in landfills takes place in several phases. The formation of N2O is influenced by biogeochemical parameters that affect nitrification and denitrification processes during decomposition. As a result, it is challenging to control N2O emissions. Landfills can release large amounts of N2O into the atmosphere. N2O emissions from landfills are typically characterized by significant spatial and temporal variations in N2O fluxes. N2O emissions from anaerobic landfills are related to the nitrogen content, waste age, and O2 content in landfilled waste. Denitrification is the main pathway for N2O production in anaerobic landfills because the O2 is not replenished after the waste is covered. Thus, intrinsic NO3− in the waste is utilized for denitrification and may be responsible for most of the N2O emitted during the stabilization of the buried waste. Nitrification occurs only in the oxygenated cover soil layer and at the working face, and may contribute to N2O emissions from landfills. Aerobic decomposition may occur in areas exposed to environmental conditions, increasing the generation of N2O. Thus, leachate irrigation or flowing leachate may generate substantial N2O through nitrification.
The data indicate that significant amounts of N2O are emitted from aerobic processes during leachate treatment. Thus, it is essential for national GHG inventory reporting to estimating the GHGs (i.e., N2O and CH4) contribution from leachate storage and treatment.
The urgency to address N2O emissions has become apparent for integrated nitrogen management in landfills and obtaining minimal N2O emissions. Knowledge of the mechanisms involved in N2O production is rapidly expanding. However, further investigation is needed to quantify the contribution of participating microbial communities. For instance, AOAs may be present in significant numbers under certain conditions, but their contribution to N2O emissions from landfills has not been investigated. Moreover, no relevant information on N2O emissions during autotrophic denitrification in landfills has been reported.
Quantifying N2O fluxes involves a high level of uncertainty due to the spatial and temporal variability of landfills. The high variability of N2O emissions from different landfill types underscores the need for on-site measurements. Therefore, full-scale studies of landfills are needed to assess N2O production in bioreactor landfills and identify mechanisms of N2O production under specific conditions. Such studies are required to improve operating conditions to mitigate N2O emissions from bioreactor landfills. For example, anammox bacteria may consume NO and convert it to N2 instead of N2O. Sun et al. [141] proposed that ex-situ leachate nitrification based on partial nitritation and the anammox process is an attractive alternative for combined bioreactor landfills.
AcknowledgmentsThis work is supported by the “R&D Center for Reduction of Non-CO2 Greenhouse gases” (2017002410008), funded by the Korea Ministry of Environment (MOE) as the “Global Top Environment R&D Program.”
NomenclatureAMO ammonia monooxygenase Anammox anaerobic ammonium oxidation AOA ammonia oxidizing archaea AOB ammonia oxidizing bacteria AOR ammonia oxidation rate C&D construction and demolition waste COD chemical oxygen demand Comammox complete ammonia oxidation DNRA dissimilatory nitrate reduction to ammonia DM dry matter DO dissolved oxygen GHG greenhouse gas HAO hydroxylamine oxidoreductase HDPE high density polyethylene MBT mechanical-biological waste treatment MOB methane oxidizing bacteria MSW municipal solid waste N nitrogen Nar nitrate reductase (for denitrifiers) Nir nitrite reductase (for denitrifiers) NirK nitrite reductase (for AOB) NOB nitrite oxidizing bacteria NOH nitrosyl radical Nor nitric oxide reductase (for AOB) NOR nitric oxide reductase (for denitrifiers) Nos nitrous oxide reductase (for denitrifiers) ORP oxidation-reduction potential PHAs polyhydroxyalkanoates PHB poly-β-hydroxybutyrate SND simultaneous nitrification and denitrification WFPS water-filled pore space WWTPs wastewater treatment plants NotesAuthor Contributions N.-H.L. (Professor) conceptualized and wrote the original manuscript. S.-H.S. (Ph.D. student) conducted data interpretations, visualized, and wrote the original manuscript. M.-J.J. (Professor) reviewed and edited the manuscript. R.-H.K. (Ph.D. student) reviewed and edited the manuscript. J.-K.P. (Ph.D.) conceptualized, reviewed, and edited the manuscript. References1. IPCC. Climate Change 2021: The Physical Science Basis, Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge University Press; 2021. p. 1017–1018.
2. Strokal M, Kroeze C. Nitrous oxide (N2O) emissions from human waste in 1970–2050. Curr. Opin. Environ. Sustain. 2014;9–10:108–121.
https://doi.org/10.1016/j.cosust.2014.09.008
3. Syakila A, Kroeze C. The global nitrous oxide budget revisited. Greenh. Gas Meas. Manag. 2011;1:17–26.
https://doi.org/10.3763/ghgmm.2010.0007
4. Nag M, Shimaoka T, Komiya T. Nitrous oxide production during nitrification from organic solid waste under temperature and oxygen conditions. Environ. Technol. 2016;37:2890–2897.
https://doi.org/10.1080/09593330.2016.1168485
5. Ishigaki T, Nakagawa M, Nagamori M, Yamada M. Anaerobic generation and emission of nitrous oxide in waste landfills. Environ. Earth Sci. 2016;75:750.
https://doi.org/10.1007/s12665-016-5543-3
6. Rinne J, Pihlatie M, Lohila A, et al. Nitrous oxide emissions from a municipal landfill. Environ. Sci. Technol. 2005;39:7790–7793.
https://doi.org/10.1021/es048416q
7. Lee CM, Lin XR, Lan CY, Lo SCL, Chan GYS. Evaluation of leachate recirculation on nitrous oxide production in the Likang landfill. China. J. Environ. Qual. 2002;31:1502–1508.
https://10.2134/jeq2002.1502
8. Powell J, Jain P, Kim H, Townsend T, Reinhart D. Changes in landfill gas quality as a result of controlled air injection. Environ. Sci. Technol. 2006;40:1029–1034.
https://doi.org/10.1021/es051114j
9. Sun Y, Wang Y-N, Sun X, Wu H, Zhang H. Production characteristics of N2O during stabilization of municipal solid waste in an intermittent aerated semi-aerobic bioreactor landfill. Waste Manage. 2013;33:2729–2736.
https://doi.org/10.1016/j.wasman.2013.08.013
10. Wang X, Jia M, Zhang C, Chen S, Cai Z. Leachate treatment in landfills is a significant N2O source. Sci. Total Environ. 2017;596–597:18–25.
https://doi.org/10.1016/j.scitotenv.2017.04.029
11. Nag M, Shimaoka T, Komiya T. Optimization of operations for nitrous oxide production and mitigation in aerobic and aerobic-anaerobic landfill method on MSW degradation. Sci. Total Environ. 2019;696:134045.
https://doi.org/10.1016/j.scitotenv.2019.134045
12. Tong H, Yin K, Giannis A, Ge L, Wang J-Y. Influence of temperature on carbon and nitrogen dynamics during in situ aeration of aged waste in simulated landfill bioreactors. Bioresour. Technol. 2015;192:149–156.
https://doi.org/10.1016/j.biortech.2015.05.049
13. Wu C, Shimaoka T, Nakayama H, Komiya T. Kinetics of nitrous oxide production by denitrification in municipal solid waste. Chemosphere. 2015;125:64–69.
https://doi.org/10.1016/j.chemosphere.2015.01.025
14. Börjesson G, Svensson BH. Nitrous oxide emissions from landfill cover soils in Sweden. Tellus B: Chem. Phys. Meteorol. 1997;49B:357–363.
https://doi.org/10.3402/tellusb.v49i4.15974
15. Li K, Fang F, Wang H, et al. Pathways of N removal and N2O emission from a one-stage autotrophic N removal process under anaerobic conditions. Sci. Rep. 2017;7:42072.
https://doi.org/10.1038/srep42072
16. Li W, Sun Y, Wang H, Wang Y-N. Improving leachate quality and optimizing CH4 and N2O emissions from a pre-aerated semi-aerobic bioreactor landfill using different pre-aeration strategies. Chemosphere. 2018;209:839–847.
https://doi.org/10.1016/j.chemosphere.2018.06.148
17. Duan H, Ye L, Erler D, Ni B-J, Yuan Z. Quantifying nitrous oxide production pathways in wastewater treatment systems using isotope technology – A critical review. Water Res. 2017;122:96–113.
https://doi.org/10.1016/j.watres.2017.05.054
18. Jurado A, Borges AV, Brouyère S. Dynamics and emissions of N2O in groundwater: A review. Sci. Total Environ. 2017;584–585:207–218.
https://doi.org/10.1016/j.scitotenv.2017.01.127
19. Kampschreur MJ, Temmink H, Kleerebezem R, Jetten MSM, van Loosdrecht MCM. Nitrous oxide emission during wastewater treatment. Water Res. 2009;43:4093–4103.
https://doi.org/10.1016/j.watres.2009.03.001
20. Li L, Ling Y, Wang H, et al. N2O emission in partial nitritation-anammox process. Chin. Chem. Lett. 2020;31:28–38.
https://doi.org/10.1016/j.cclet.2019.06.035
21. Massara TM, Malamis S, Guisasola A, Baeza JA, Noutsopoulos C, Katsou E. A review on nitrous oxide (N2O) emissions during biological nutrient removal from municipal wastewater and sludge reject water. Sci. Total Environ. 2017;596–597:106–123.
https://doi.org/10.1016/j.scitotenv.2017.03.191
22. Ren Y, Ngo HH, Guo W, Ni B-J, Liu Y. Linking the nitrous oxide production and mitigation with the microbial community in wastewater treatment: A review. Bioresou. Technol. Rep. 2019;7:100191.
https://doi.org/10.1016/j.biteb.2019.100191
23. Signor D, Cerri CEP. Nitrous oxide emissions in agricultural soils: a review. Pesqui. Agropecu. Trop. 2013;43:322–338.
https://doi.org/10.1590/S1983-40632013000300014
24. Hayatsu M, Tago K, Saito M. Various players in the nitrogen cycle: Diversity and functions of the microorganisms involved in nitrification and denitrification. Soil Sci. Plant Nutr. 2008;54:33–45.
https://doi.org/10.1111/j.1747-0765.2007.00195.x
25. Lancaster KM, Caranto JD, Majer SH, Smith MA. Alternative Bioenergy: Updates to and challenges in nitrification metalloenzymology. Joule. 2018;2:421–441.
https://doi.org/10.1016/j.joule.2018.01.018
26. Hink L, Nicol GW, Prosser JI. Archaea produce lower yields of N2O than bacteria during aerobic ammonia oxidation in soil. Environ. Microbiol. 2017;19:4829–4837.
https://doi.org/10.1111/1462-2920.13282
27. Ni B-J, Peng L, Law Y, Guo J, Yuan Z. Modeling of nitrous oxide production by autotrophic ammonia-oxidizing bacteria with multiple production pathways. Environ. Sci. Technol. 2014;48:3916–3924.
https://doi.org/10.1021/es405592h
28. Chandran K, Stein LY, Klotz MG, van Loosdrecht MCM. Nitrous oxide production by lithotrophic ammonia-oxidizing bacteria and implications for engineered nitrogen-removal systems. Biochem. Soc. Trans. 2011;39:1832–1837.
https://doi.org/10.1042/BST20110717
29. Peng L, Ni B-J, Erler D, Ye L, Yuan Z. The effect of dissolved oxygen on N2O production by ammonia-oxidizing bacteria in an enriched nitrifying sludge. Water Res. 2014;66:12–21.
https://doi.org/10.1016/j.watres.2014.08.009
30. Law Y, Ni B-J, Lant P, Yuan Z. N2O production rate of an enriched ammonia-oxidising bacteria culture exponentially correlates to its ammonia oxidation rate. Water Res. 2012;46:3410–3419.
https://doi.org/10.1016/j.watres.2012.03.043
31. Yu R, Kampschreur MJ, van Loosdrecht MCM, Chandran K. Mechanisms and specific directionality of autotrophic nitrous oxide and nitric oxide generation during transient anoxia. Environ. Sci. Technol. 2010;44:1313–1319.
https://doi.org/10.1021/es902794a
32. Caranto JD, Lancaster KM. Nitric oxide is an obligate bacterial nitrification intermediate produced by hydroxylamine oxidoreductase. Proc. Natl. Acad. Sci. U. S. A. 2017;114:8217–8222.
https://doi.org/10.1073/pnas.1704504114
33. Ribera-Guardia A, Pijuan M. Distinctive NO and N2O emission patterns in ammonia oxidizing bacteria: Effect of ammonia oxidation rate, DO and pH. Chem. Eng. J. 2017;321:358–365.
https://doi.org/10.1016/j.cej.2017.03.122
34. Caranto JD, Vilbert AC, Lancaster KM.
Nitrosomonas europaea cytochrome P460 is a direct link between nitrification and nitrous oxide emission. Proc. Natl. Acad. Sci. U. S. A. 2016;113:14704–14709.
https://doi.org/10.1073/pnas.1611051113
35. Wunderlin P, Mohn J, Joss A, Emmenegger L, Siegrist H. Mechanisms of N2O production in biological wastewater treatment under nitrifying and denitrifying conditions. Water Res. 2012;46:1027–1037.
https://doi.org/10.1016/j.watres.2011.11.080
36. Zhu-Barker X, Cavazos AR, Ostrom NE, Horwath WR, Glass JB. The importance of abiotic reactions for nitrous oxide production. Biogeochemistry. 2015;126:251–267.
https://doi.org/10.1007/s10533-015-0166-54
37. Taghizadeh-Toosi A, Elsgaard L, Clough TJ, Labouriau R, Ernstsen V, Petersen SO. Regulation of N2O emissions from acid organic soil drained for agriculture. Biogeosciences. 2019;16:4555–4575.
https://doi.org/10.5194/bg-16-4555-2019
38. Heil J, Liu S, Vereecken H, Brüggemann N. Abiotic nitrous oxide production from hydroxylamine in soils and their dependence on soil properties. Soil Biol. Biochem. 2015;84:107–115.
https://doi.org/10.1016/j.soilbio.2015.02.022
39. Su Q, Domingo-Félez C, Jensen MM, Smets BF. Abiotic nitrous oxide (N2O) production is strongly pH dependent, but contributes little to overall N2O emissions in biological nitrogen removal systems. Environ. Sci. Technol. 2019;53:3508–3516.
https://doi.org/10.1021/acs.est.8b063193
40. Wrage N, Velthof GL, van Beusichem ML, Oenema O. Role of nitrifier denitrification in the production of nitrous oxide. Soil Biol. Biochem. 2001;33:1723–1732.
https://doi.org/10.1016/S0038-0717(01)00096-7
41. Khalil K, Mary B, Renault P. Nitrous oxide production by nitrification and denitrification in soil aggregates as affected by O2 concentration. Soil Biol. Biochem. 2004;36:687–699.
https://doi.org/10.1016/j.soilbio.2004.01.004
42. Zhu X, Burger M, Doane TA, Horwath WR. Ammonia oxidation pathways and nitrifier denitrification are significant sources of N2O and NO under low oxygen availability. Proc. Natl. Acad. Sci. U. S. A. 2013;110:6328–6333.
https://doi.org/10.1073/pnas.1219993110
43. Sutka RL, Ostrom NE, Ostrom PH, et al. Distinguishing nitrous oxide production from nitrification and denitrification on the basis of isotopomer abundances. Appl. Environ. Microbiol. 2006;72:638–644.
https://doi.org/10.1128/AEM.72.1.638-644.2006
44. Gao J, Wang R, Li Y, et al. Effect of aeration modes on nitrogen removal and N2O emission in the partial nitrification and denitrification process for landfill leachate treatment. Sci. Total Environ. 2022;853:158424.
https://doi.org/10.1016/j.scitotenv.2022.158424
45. Wrage-Mönnig N, Horn MA, Well R, Müller C, Velthof G, Oenema O. The role of nitrifier denitrification in the production of nitrous oxide revisited. Soil Biol. Biochem. 2018;123:A3–A16.
https://doi.org/10.1016/j.soilbio.2018.03.020
46. Law Y, Lant P, Yuan Z. The confounding effect of nitrite on N2O production by an enriched ammonia-oxidizing culture. Environ. Sci. Technol. 2013;47:7186–7194.
https://doi.org/10.1021/es4009689
47. Anderson IC, Poth M, Homstead J, Burdige D. A comparison of NO and N2O production by the autotrophic nitrifier Nitrosomonas europaea and the heterotrophic nitrifier Alcaligenes faecalis
. Appl. Environ. Microbiol. 1993;59:3525–3533.
https://doi.org/10.1128/aem.59.11.3525-3533.1993
48. Fu Y, Yue Q, Luo S, Tian X, Zheng J, Yang Y. Two kinds of behavior of fruit peel coagulant in treating low carbon source wastewaters. Environ. Eng. Res. 2023;28:220223.
https://doi.org/10.4491/eer.2022.223
49. Choi D, To TP, Yun W, Ju D, Kim K, Jung J. Effect of nitrogen loading rate and alkalinity on partial nitritation in a continuous stirred tank reactor. Environ. Eng. Res. 2022;27:200573.
https://doi.org/10.4491/eer.2020.753
50. Khanichaidecha W, Nakaruk A, Ratananikom K, Eamrat R, Kazama F. Heterotrophic nitrification and aerobic denitrification using pure-culture bacteria for wastewater treatment. J. Water Reuse Desalin. 2019;9:10–17.
https://doi.org/10.2166/wrd.2018.064
51. Otte S, Schalk J, Kuenen JG, Jetten MS. Hydroxylamine oxidation and subsequent nitrous oxide production by the heterotrophic ammonia oxidizer Alcaligenes faecalis
. Appl. Microbiol. Biotechnol. 1999;51:255–261.
https://doi.org/10.1007/s002530051390
52. Bateman EJ, Baggs EM. Contributions of nitrification and denitrification to N2O emissions from soils at different water-filled pore space. Biol. Fertil. Soils. 2005;41:379–388.
https://doi.org/10.1007/s00374-005-0858-3
53. Schalk-Otte S, Seviour RJ, Kuenen JG, Jetten MSM. Nitrous oxide (N2O) production by Alcaligenes faecalis during feast and famine regimes. Water Res. 2000;34:2080–2088.
https://doi.org/10.1016/S0043-1354(99)00374-7
54. Stieglmeier M, Mooshammer M, Kitzler B, et al. Aerobic nitrous oxide production through N-nitrosating hybrid formation in ammonia-oxidizing archaea. ISME J. 2014;8:1135–1146.
https://doi.org/10.1038/ismej.2013.220
55. Jung M-Y, Well R, Min D, et al. Isotopic signatures of N2O produced by ammonia-oxidizing archaea from soil. ISME J. 2014;8:1115–1125.
https://doi.org/10.1038/ismej.2013.025
56. Jung M-Y, Gwak J-H, Rohe L, et al. Indication for enzymatic denitrification to N2O at low pH in an ammonia-oxidizing archaeon. ISME J. 2019;13:2633–2638.
https://doi.org/10.1038/s41396-019-0460-6
57. Kozlowski JA, Stieglmeier M, Schleper C, Klotz MG, Stein LY. Pathways and key intermediates required for obligate aerobic ammonia-dependent chemolithotrophy in bacteria and Thaumarchaeota. ISME J. 2016;10:1836–1845.
https://doi.org/10.1038/ismej.2016.2
58. Kits KD, Jung M-Y, Vierheilig J, et al. Low yield and abiotic origin of N2O formed by the complete nitrifier Nitrospira inopinata
. Nat. Commun. 2019;10:1836.
https://doi.org/10.1038/s41467-019-09790-x
59. Bian R, Sun Y, Li W, Ma Q, Chai X. Co-composting of municipal solid waste mixed with matured sewage sludge: The relationship between N2O emissions and denitrifying gene abundance. Chemosphere. 2017;189:581–589.
https://doi.org/10.1016/j.chemosphere.2017.09.070
60. Li W, Sun Y, Bian R, Wang H, Zhang D. N2O emissions from an intermittently aerated semi-aerobic aged refuse bioreactor: Combined effect of COD and NH4
+–N in influent leachate. Waste Manage. 2017;69:242–249.
https://doi.org/10.1016/j.wasman.2017.08.022
61. Hu Z, Zhang J, Xie H, Li S, Wang J, Zhang T. Effect of anoxic/aerobic phase fraction on N2O emission in a sequencing batch reactor under low temperature. Bioresour. Technol. 2011;102:5486–5491.
https://doi.org/10.1016/j.biortech.2010.10.037
62. Alinsafi A, Adouani N, Béline F, Lendormi T, Limousy L, Sire O. Nitrite effect on nitrous oxide emission from denitrifying activated sludge. Process Biochem. 2008;43:683–689.
https://doi.org/10.1016/j.procbio.2008.02.008
63. Gong Y-K, Peng Y-Z, Yang Q, Wu W-M, Wang S-Y. Formation of nitrous oxide in a gradient of oxygenation and nitrogen loading rate during denitrification of nitrite and nitrate. J. Hazard. Mater. 2012;227–228:453–460.
https://doi.org/10.1016/j.jhazmat.2012.05.002
64. Benoit M, Garnier J, Billen G. Temperature dependence of nitrous oxide production of a luvisolic soil in batch experiments. Process Biochem. 2015;50:79–85.
https://doi.org/10.1016/j.procbio.2014.10.013
65. Holtan-Hartwig L, Dörsch P, Bakken LR. Low temperature control of soil denitrifying communities: Kinetics of N2O production and reduction. Soil Biol. Biochem. 2002;34:1797–1806.
https://doi.org/10.1016/S0038-0717(02)00169-4
66. Maag M, Vinther FP. Nitrous oxide emission by nitrification and denitrification in different soil types and at different soil moisture contents and temperatures. Appl. Soil Ecol. 1996;4:5–14.
https://doi.org/10.1016/0929-1393(96)00106-0
67. Okabe S, Oshiki M, Takahashi Y, Satoh H. N2O emissions from a partial nitrification-anammox process, and identification of a key biological process of N2O emission from anammox granules. Water Res. 2011;45:6461–6470.
https://doi.org/10.1016/j.watres.2011.09.040
68. Pan Y, Ye L, Yuan Z. Effect of H2S on N2O reduction and accumulation during denitrification by methanol utilizing denitrifiers. Environ. Sci. Technol. 2013;47:8408–8415.
https://doi.org/10.1021/es401632r
69. Senbayram M, Chen R, Budai A, Bakken L, Dittert K. N2O emission and the N2O/(N2O+N2) product ratio of denitrification as controlled by available carbon substrates and nitrate concentrations. Agric. Ecosyst. Environ. 2012;147:4–12.
https://doi.org/10.1016/j.agee.2011.06.022
70. Zhao W, Wang Y, Liu S, Pan M, Yang J, Chen S. Denitrification activities and N2O production under salt stress with varying COD/N ratios and terminal electron acceptors. Chem. Eng. J. 2013;215–216:252–260.
https://doi.org/10.1016/j.cej.2012.10.084
71. Berge ND, Reinhart DR, Townsend TG. The fate of nitrogen in bioreactor landfills. Crit. Rev. Environ. Sci. Technol. 2005;35:365–399.
https://doi.org/10.1080/10643380590945003
72. Wu D, Wang C, Dolfing J, Xie B. Short tests to couple N2O emission mitigation and nitrogen removal strategies for landfill leachate recirculation. Sci. Total Environ. 2015;512–513:19–25.
https://doi.org/10.1016/j.scitotenv.2015.01.021
73. Chen YX, Wu SW, Wu WX, Sun H, Ding Y. Denitrification capacity of bioreactors filled with refuse at different landfill gas. J. Hazard. Mater. 2009;172:159–165.
https://doi.org/10.1016/j.jhazmat.2009.06.150
74. Onay TT, Pohland FG. Nitrogen and sulfate attenuation in simulated landfill bioreactors. Water Sci. Technol. 2001;44:367–372.
https://doi.org/10.2166/wst.2001.0791
75. Lan L, Zhao J, Wang S, et al. NO and N2O accumulation during nitrite-based sulfide-oxidizing autotrophic denitrification. Bioresour. Technol. Rep. 2019;7:100190.
https://doi.org/10.1016/j.biteb.2019.100190
76. Liu Y, Peng L, Ngo HH, et al. Evaluation of nitrous oxide emission from sulfide- and sulfur-based autotrophic denitrification processes. Environ. Sci. Technol. 2016;50:9407–9415.
https://doi.org/10.1021/acs.est.6b02202
77. Yang W, Lu H, Khanal SK, Zhao Q, Meng L, Chen G-H. Granulation of sulfur-oxidizing bacteria for autotrophic denitrification. Water Res. 2016;104:507–519.
https://doi.org/10.1016/j.watres.2016.08.049
78. Yang W, Zhao Q, Lu H, Ding Z, Meng L, Chen G-H. Sulfide-driven autotrophic denitrification significantly reduces N2O emissions. Water Res. 2016;90:176–184.
https://doi.org/10.1016/j.watres.2015.12.032
79. Shao M-F, Zhang T, Fang HHP. Sulfur-driven autotrophic denitrification: Diversity, biochemistry, and engineering applications. Appl. Microbiol. Biotechnol. 2010;88:1027–1042.
https://doi.org/10.1007/s00253-010-2847-1
80. Zhang F, Li P, Chen M, et al. Effect of operational modes on nitrogen removal and nitrous oxide emission in the process of simultaneous nitrification and denitrification. Chem. Eng. J. 2015;280:549–557.
https://doi.org/10.1016/j.cej.2015.06.016
81. Meyer RL, Zeng RJ, Giugliano V, Blackall LL. Challenges for simultaneous nitrification, denitrification, and phosphorus removal in microbial aggregates: Mass transfer limitation and nitrous oxide production. FEMS Microbiol. Ecol. 2005;52:329–338.
https://doi.org/10.1016/j.femsec.2004.11.011
82. Wang X, Jia M, Zhang H, Pan S, Kao CM, Chen S. Quantifying N2O emissions and production pathways from fresh waste during the initial stage of disposal to a landfill. Waste Manage. 2017;63:3–10.
https://doi.org/10.1016/j.wasman.2016.08.007
83. Li Q, Wang F, Yu Q, Yan W, Li X, Lv S. Dominance of nitrous oxide production by nitrification and denitrification in the shallow Chaohu Lake, Eastern China: Insight from isotopic characteristics of dissolved nitrous oxide. Environ. Pollut. 2019;255:113212.
https://doi.org/10.1016/j.envplo.2019.113212
84. Sun Y, De Vos P, Willems A. Influence of nitrate and nitrite concentration on N2O production via dissimilatory nitrate/nitrite reduction to ammonium in Bacillus paralicheniformis LMG 6934. MicrobiologyOpen. 2018;7:e00592.
https://doi.org/10.1002/mbo3.592
85. Denk TRA, Mohn J, Decock C, et al. The nitrogen cycle: A review of isotope effects and isotope modeling approaches. Soil Biol. Biochem. 2017;105:121–137.
https://doi.org/10.1016/j.soilbio.2016.11.015
86. Hu Z, Wessels HJCT, van Alen T, Jetten MSM, Kartal B. Nitric oxide-dependent anaerobic ammonium oxidation. Nat. Commun. 2019;10:1244.
https://doi.org/10.1038/s41467-019-09268-w
87. Kartal B, Tan NCG, Van de Biezen E, Kampschreur MJ, Van Loosdrecht MCM. Effect of nitric oxide on anammox bacteria. Appl. Environ. Microbiol. 2010;76:6304–6306.
https://doi.org/10.1128/AEM.00991-10
88. Park J-K, Chong Y-G, Tameda K, Lee N-H. Applying methane and carbon flow balances for determination of first-order landfill gas model parameters. Environ. Eng. Res. 2020;25:374–383.
https://doi.org/10.4491/eer.2019.074
89. Park J-K, Kang J-Y, Lee N-H. Estimation of methane emission flux at landfill surface using laser methane detector: Influence of gauge pressure. Waste Manag. Res. 2016;34:784–792.
https://doi.org/10.1177/0734242X16654976
90. Zhang H-H, He P-J, Shao L-M. N2O emissions from municipal solid waste landfills with selected infertile cover soils and leachate subsurface irrigation. Environ. Pollut. 2008;156:959–965.
https://doi.org/10.1016/j.envpol.2008.05.008
91. Wang YS, Xue M, Zheng X, Ji B, Du R, Wang Y. Effects of environmental factors on N2O emission from and CH4 uptake by the typical grasslands in the Inner Mongolia. Chemosphere. 2005;58:205–215.
https://doi.org/10.1016/j.chemosphere.2004.04.043
92. Zhang H, He P, Shao L. N2O emissions at municipal solid waste landfill sites: Effects of CH4 emissions and cover soil. Atmos. Environ. 2009;43:2623–2631.
https://doi.org/10.1016/j.atmosenv.2009.02.011
93. Zhang C, Guo Y, Wang X, Chen S. Temporal and spatial variation of greenhouse gas emissions from a limited-controlled landfill site. Environ. Int. 2019;127:387–394.
https://doi.org/10.1016/j.envint.2019.03.052
94. Harborth P, Fuss R, Münnich K, Flessa H, Fricke K. Spatial variability of nitrous oxide and methane emissions from an MBT landfill in operation: Strong N2O hotspots at the working face. Waste Manage. 2013;33:2099–2107.
https://doi.org/10.1016/j.wasman.2013.01.028
95. Zhang H, He P, Shao L, Qu X, Lee D. Minimizing N2O fluxes from full-scale municipal solid waste landfill with properly selected cover soil. J. Environ. Sci. 2008;20:189–194.
https://doi.org/10.1016/S1001-0742(08)60030-3
96. Long X-E, Huang Y, Chi H, Li Y, Ahmad N, Yao H. Nitrous oxide flux, ammonia oxidizer and denitrifier abundance and activity across three different landfill cover soils in Ningbo, China. J. Clean. Prod. 2018;170:288–297.
https://doi.org/10.1016/j.jclepro.2017.09.173
97. McBain MC, Warland JS, McBride RA, Wagner-Riddle C. Micrometeorological measurements of N2O and CH4 emissions from a municipal solid waste landfill. Waste Manag. Res. 2005;23:409–419.
https://doi.org/10.1177/0734242X05057253
98. Sormunen K, Einola J, Ettala M, Rintala J. Leachate and gaseous emissions from initial phases of landfilling mechanically and mechanically-biologically treated municipal solid waste residuals. Bioresour. Technol. 2008;99:2399–2409.
https://doi.org/10.1016/j.biortech.2007.05.009
99. Bogner JE, Spokas KA, Chanton JP. Seasonal greenhouse gas emissions (methane, carbon dioxide, nitrous oxide) from engineered landfills: Daily, intermediate, and final California cover soils. J. Environ. Qual. 2011;40:1010–1020.
https://doi.org/10.2134/jeq2010.0407
100. Nag M, Shimaoka T, Nakayama H, Komiya T, Xiaoli C. Field study of nitrous oxide production with in situ aeration in a closed landfill site. J. Air Waste Manag. Assoc. 2016;66:280–287.
https://doi.org/10.1080/10962247.2015.1130664
101. Zhang L-M, Hu H-W, Shen J-P, He J-Z. Ammonia-oxidizing archaea have more important role than ammonia-oxidizing bacteria in ammonia oxidation of strongly acidic soils. ISME J. 2012;6:1032–1045.
https://doi.org/10.1038/ismej.2011.168
102. Gao X, Tenuta M, Nelson A, et al. Effect of nitrogen fertilizer rate on nitrous oxide emission from irrigated potato on clay loam soil in Manitiba, Canada. Can. J. Soil Sci. 2013;93:1–11.
http://doi.org/10.4141/cjss2012-057
103. Zhang H-H, He P-J, Shao L-M, Yuan L. Minimisation of N2O emissions from a plant-soil system under landfill leachate irrigation. Waste Manage. 2009;29:1012–1017.
https://doi.org/10.1016/j.wasman.2008.08.005
104. Bogner JE, Spokas KA, Burton EA. Temporal variations in greenhouse gas emissions at a midlatitude landfill. J. Environ. Qual. 1999;28:278–288.
https://doi.org/10.2134/jeq1999.00472425002800010034x
105. IPCC. 2006. IPCC Guidelines for National Greenhouse Gas Inventories. 5:Waste, IGES; Japan: 2006. p. 6.25–6.26.
106. Lin L, Lan CY, Huang LN, Chan GYS. Anthropogenic N2O production from landfill leachate treatment. J. Environ. Manage. 2008;87:341–349.
https://doi.org/10.1016/j.jenvman.2007.01.014
107. Law Y, Ye L, Pan Y, Yuan Z. Nitrous oxide emissions from wastewater treatment processes. Philos. Trans. R. Soc. B Biol. Sci. 2012;367:1265–1277.
https://doi.org/10.1098/rstb.2011.0317
108. Sun S, Bao Z, Sun D. Study on emission characteristics and reduction strategy of nitrous oxide during wastewater treatment by different processes. Environ. Sci. Pollut. Res. Int. 2015;22:4222–4229.
https://doi.org/10.1007/s11356-014-3654-5
109. Liu M, Yang Q, Peng Y, Liu T, Xiao H, Wang S. Treatment performance and N2O emission in the UASB-A/O shortcut biological nitrogen removal system for landfill leachate at different salinity. J. Ind. Eng. Chem. 2015;32:63–71.
https://doi.org/10.1016/j.jiec.2015.07.017
110. Wang X, Jia M, Chen X, et al. Greenhouse gas emissions from landfill leachate treatment plants: A comparison of young and aged landfill. Waste Manage. 2014;34:1156–1164.
https://doi.org/10.1016/j.wasman.2014.02.004
111. Kampschreur MJ, Poldermans R, Kleerebezem R, et al. Emission of nitrous oxide and nitric oxide from a full-scale single-stage nitritation-anammox reactor. Water Sci. Technol. 2009;60:3211–3217.
https://doi.org/10.2166/wst.2009.608
112. Peng L, Ni B-J, Ye L, Yuan Z. The combined effect of dissolved oxygen and nitrite on N2O production by ammonia oxidizing bacteria in an enriched nitrifying sludge. Water Res. 2015;73:29–36.
https://doi.org/10.1016/j.watres.2015.01.021
113. Liu M, Liu T, Peng Y, Wang S, Xiao H. Effect of salinity on N2O production during shortcut biological nitrogen removal from landfill leachate. J. Biosci. Bioeng. 2014;117:582–590.
https://doi.org/10.1016/j.jbiosc.2013.10.011
114. Tsuneda S, Mikami M, Kimochi Y, Hirata A. Effect of salinity on nitrous oxide emission in the biological nitrogen removal process for industrial wastewater. J. Hazard. Mater. 2005;B119:93–98.
https://doi.org/10/1016/j.jhazmat.2004.10.025
115. Morello L, Raga R, Lavagnolo MC, et al. The S.An. A.® concept: Semi-aerobic, Anaerobic, Aerated bioreactor landfill. Waste Manage. 2017;67:193–202.
https://doi.org/10.1016/j.wasman.2017.05.006
116. Wang YN, Sun YJ, Wang L, et al. N2O emission from a combined ex-situ nitrification and in-situ denitrification bioreactor landfill. Waste Manage. 2014;34:2209–2217.
https://doi.org/10.1016/j.wasman.2014.06.023
117. Benson CH, Barlaz MA, Lane DT, Rawe JM. Practice review of five bioreactor/recirculation landfills. Waste Manage. 2007;27:13–29.
https://doi.org/10.1016/j.wasman.2006.04.005
118. Raga R, Cossu R. Bioreactor tests preliminary to landfill in situ aeration: A case study. Waste Manage. 2013;33:871–880.
https://doi.org/10.1016/j.wasman.2012.11.014
119. Shao L-M, He P-J, Li G-J.
In situ nitrogen removal from leachate by bioreactor landfill with limited aeration. Waste Manage. 2008;28:1000–1007.
https://doi.org/10.1016/j.wasman.2007.02.028
120. Sun X, Zhang H, Cheng Z, Wang S. Effect of low aeration rate on simultaneous nitrification and denitrification in an intermittent aeration aged refuse bioreactor treating leachate. Waste Manage. 2017;63:410–416.
https://doi.org/10.1016/j.wasman.2016.12.042
121. Nag M, Shimaoka T, Komiya T. Impact of intermittent aerations on leachate quality and greenhouse reduction in the aerobic-anaerobic landfill method. Waste Manage. 2016;55:71–82.
https://doi.org/10.1016/j.wasman.2015.10.018
122. Grossule V, Morello L, Cossu R, Lavagnolo MC. Bioreactor landfills: Comparison and kinetics of the different systems. Detritus. 2018;3:100–113.
https://doi.org/10.31025/2611-4135/2018.13703
123. Matsuto T, Zhang X, Matsuo T, Yamada S. Onsite survey on the mechanism of passive aeration and air flow path in a semi-aerobic landfill. Waste Manage. 2015;36:204–212.
https://doi.org/10.1016/j.wasman.2014.11.007
124. Chung J, Kim S, Baek S, et al. Acceleration of aged-landfill stabilization by combining partial nitrification and leachate recirculation: A field-scale study. J. Hazard. Mater. 2015;285:436–444.
https://doi.org/10.1016/j.jhazmat.2014.12.013
125. Tallec G, Bureau C, Peu P, et al. Impact of nitrate-enhanced leachate recirculation on gaseous releases from a landfill bioreactor cell. Waste Manage. 2009;29:2078–2084.
https://doi.org/10.1016/j.wasman.2009.01.006
126. Zhong Q, Li D, Tao Y, et al. Nitrogen removal from landfill leachate via ex situ nitrification and sequential in situ denitrification. Waste Manage. 2009;29:1347–1353.
https://doi.org/10.1016/j.wasman.2008.10.014
127. Sun X, Zhang H, Cheng Z. Use of bioreactor landfill for nitrogen removal to enhance methane production through ex situ simultaneous nitrification-denitrification and in situ denitrification. Waste Manage. 2017;66:97–102.
https://doi.org/10.1016/j.wasman.2017.04.020
128. UNFCCC. AM0083: Avoidance of landfill gas emissions by in-situ aeration of landfill. [cited 29 September 2022]. Available from: https://cdm.unfccc.int/methodologies/DB/R8O6P4ANGE24L9067H08TYVPOM5Q7P
129. Li W, Sun Y, Li G, Liu Z, Wang H, Zhang D. Contributions of nitrification and denitrification to N2O emissions from aged refuse bioreactor at different feeding loads of ammonia substrates. Waste Manage. 2017;68:319–328.
https://doi.org/10.1016/j.wasman.2017.06.037
130. He P, Yang N, Gu H, Zhang H, Shao L. N2O and NH3 emissions from a bioreactor landfill operated under limited aerobic degradation conditions. J. Environ. Sci. 2011;23:1011–1019.
https://doi.org/10.1016/S1001-0742(10)60574-8
131. Tran HN, Münnich K, Fricke K, Harborth P. Removal of nitrogen from MBT residues by leachate recirculation in combination with intermittent aeration. Waste Manag. Res. 2014;32:56–63.
https://doi.org/10.1177/0734242X13512892
132. Xu Q, Jin X, Ma Z, Tao H, Ko JH. Methane production in simulated hybrid bioreactor landfill. Bioresour. Technol. 2014;168:92–96.
https://doi.org/10.1016/j.biortech.2014.03.036
133. Ritzkowski M, Heyer K-U, Stegmann R. Fundamental processes and implications during in situ aeration of old landfills. Waste Manage. 2006;26:356–372.
https://doi.org/10.1016/j.wasman.2005.11.009
134. van Turnhout AG, Brandstätter C, Kleerebezem R, Fellner J, Heimovaara TJ. Theoretical analysis of municipal solid waste treatment by leachate recirculation under anaerobic and aerobic conditions. Waste Manage. 2018;71:246–254.
https://doi.org/10.1016/j.wasman.2017.09.034
135. Berge ND, Reinhart DR, Dietz J, Townsend T. In situ ammonia removal in bioreactor landfill leachate. Waste Manage. 2006;26:334–343.
https://doi.org/10.1016/j.wasman.2005.11.003
136. Berge ND, Reinhart DR, Dietz JD, Townsend T. The impact of temperature and gas-phase oxygen on kinetics of in situ ammonia removal in bioreactor landfill leachate. Water Res. 2007;41:1907–1914.
https://doi.org/10.1016/j.watres.2007.01.049
137. Hwang S, Hanaki K. Effects of oxygen concentration and moisture content of refuse on nitrification, denitrification and nitrous oxide production. Bioresour. Technol. 2000;71:159–165.
https://doi.org/10.1016/S0960-8524(99)90068-8
138. Ban J-K, Park J-K, Ahn Y-M, Yoon S-P, Lee N-H. Assessment of temperature variations in an aerated landfill. J. Korea Soc. Waste Manag. 2015;32:335–341.
https://doi.org/10.9786/kswm.2015.32.4.335
139. Reino C, van Loosdrecht MCM, Carrera J, Pérez J. Effect of temperature on N2O emissions from a highly enriched nitrifying granular sludge performing partial nitritation of a low-strength wastewater. Chemosphere. 2017;185:336–343.
https://doi.org/10.1016/j.chemosphere.2017.07.017
140. Yang J, Trela J, Plaza E. Nitrous oxide emissions from one-step partial nitritation/anammox processes. Water Sci. Technol. 2016;74:2870–2878.
https://doi.org/10.2166/wst.2016.454
141. Sun F, Su X, Kang T, et al. Integrating landfill bioreactors, partial nitritation and anammox process for methane recovery and nitrogen removal from leachate. Sci. Rep. 2016;6:27744.
https://doi.org/10.1038/srep27744
Fig. 3A model summarizing the contribution of autotrophic nitrification, heterotrophic nitrification, and denitrification to N2O emissions with variations in soil WFPS [52]. 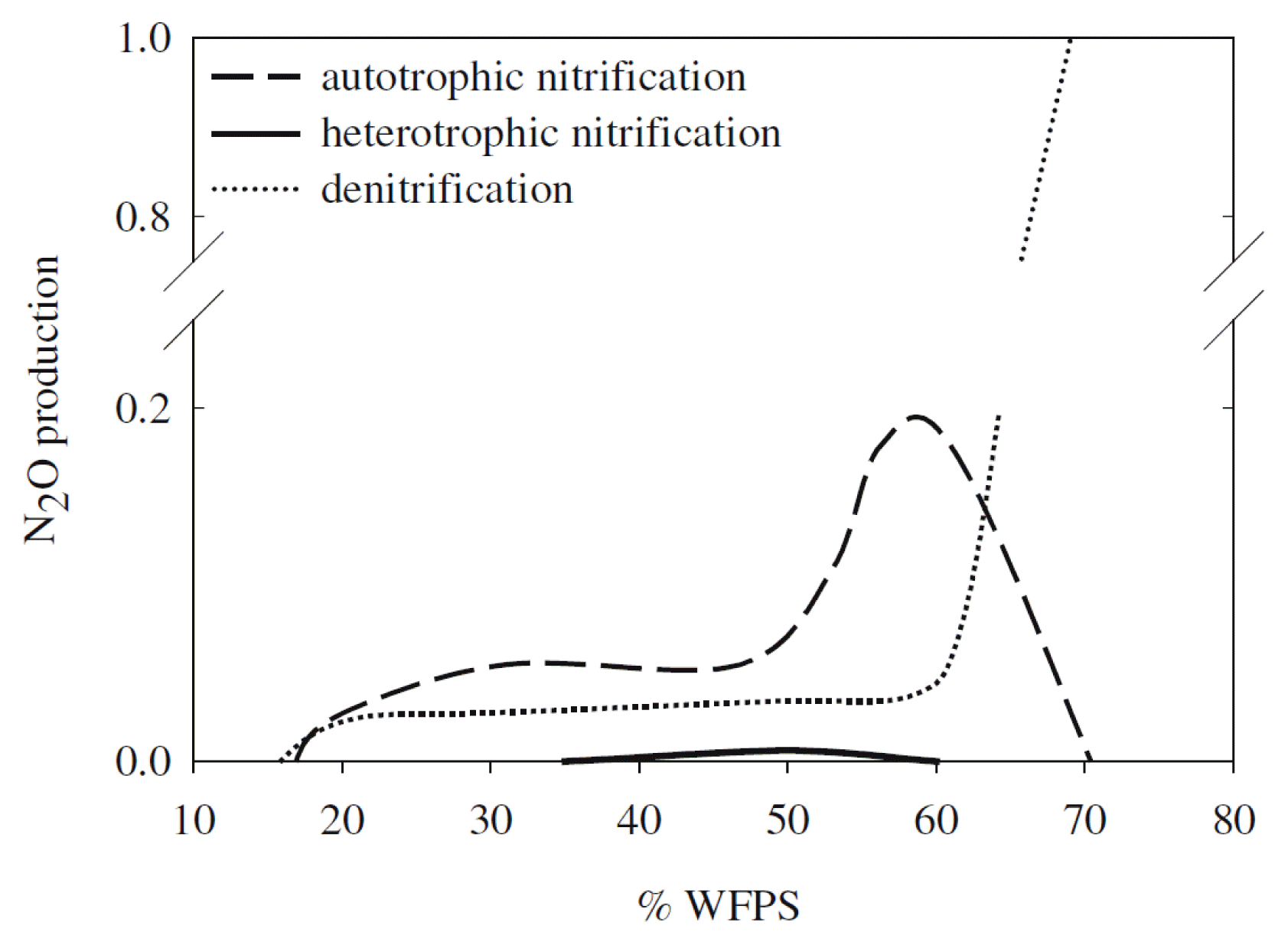
Table 1N2O fluxes from anaerobic landfills reported in the literature
Table 2N2O emissions from leachate treatment facilities reported in the literature
Table 3N2O emission factors for each landfill category reported in the literature
Table 4Summary of major pathways and factors affecting N2O production in each landfill type |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||