AbstractLayered double hydroxides (LDHs) are regarded as potential adsorbents for water treatment from a wide range of pollutants. However, there are only a few studies concerning application of LDHs for elimination of microorganisms from aquatic systems. In this work, experiments were conducted to investigate the efficiency of commercial synthetic hydrotalcite Mg6Al2(CO3)(OH)16·4H2O as an adsorbent for water purification from fecal indicator bacteria Escherichia coli BIM B-378 and Enterococcus faecalis BIM B-1530. Our findings indicate that exposure for 4 h to hydrotalcite (5 g/L) in suspension resulted in the removal of about 40% of coliforms and 25% of enterococci from water, at a high bacterial load (2×1010 CFU/L), and the removal efficiency of E. coli and E. faecalis did not significantly change when both bacteria were present in water. In addition, the percentage of removed bacteria increased with increasing of hydrotalcite concentration in the suspension (0.5 to 10 g/L), contact time (1–7 h) and decrease of pH (5.5), and decreased at low incubation temperature (16°C). Finally, hydrotalcite did not exhibit bactericidal activity and retention of bacteria was found to be reversible. Therefore, our findings suggest that commercial synthetic hydrotalcite could be potentially used in technologies of water treatment from bacterial contamination.
Graphical Abstract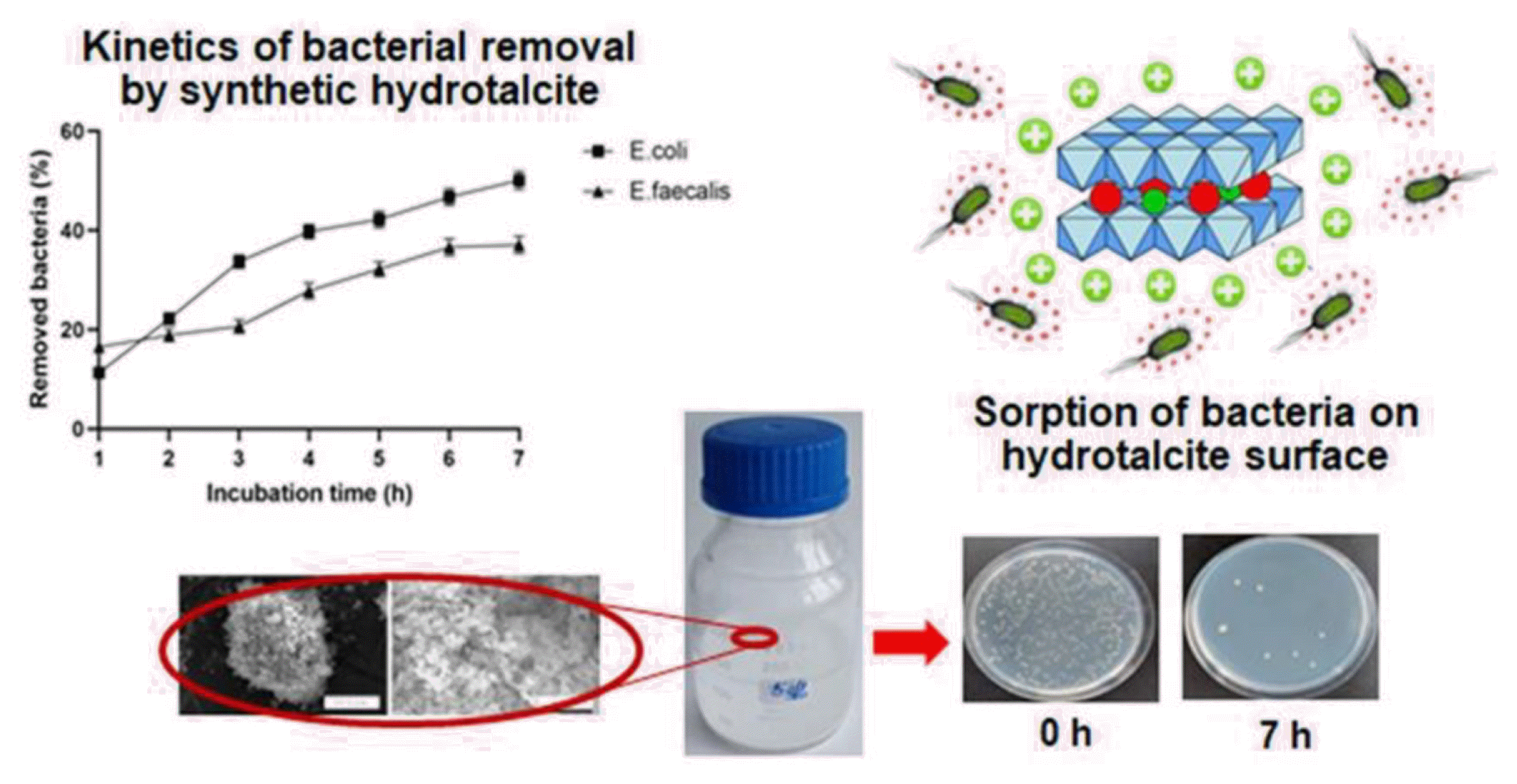
1. IntroductionContamination of drinking water with pathogenic bacteria is a serious threat to public health and safety in both developing and developed countries. Waterborne diseases (diarrhea, gastro-intestinal illness, etc.) affect about 38 million people and cause more than 3 million deaths over the world annually, mostly in Africa and South-East Asia. Children under 5 years are the main victims of waterborne infections, whose mortality rate reaches 4,000 cases daily [1–4]. As a result, the improvement of water quality, primarily its microbiological safety, can reduce the global disease burden by approximately 4% [3].
Different approaches have been used to purify drinking water from microbial contamination, varying from relatively simple and cheap procedures, such as boiling, to very complex and expensive ones (e.g., membrane filtration systems) [5]. In the last decades, there has been an increasing interest in the layered double hydroxides (LDHs) as promising nanomaterials for both bacteria and viruses removal from water [6–10].
LDHs belong to the family of anionic clays, which are composed of positively charged layers of mixed metal hydroxides, M2+ 1−xM3+ x(OH)2x+, and the interlayers intercalated with charge-compensating anions Ay− and water molecules [11]. Hereafter, a short form of the chemical formula, M2+ nM3+ −Ay−, where n=(1−x)/x is the atomic ratio of the cations in the layer, is used. LDHs with M2+ = Mg, Zn, Ca and M3+= Al, Fe are regarded as environmentally friendly, low cost and easy to synthesize nanomaterials [12,13]. These LDHs demonstrate a large surface area (20–120 m2/g), high anion-exchange capacity (200–500 cmol/kg), and selective affinity to negatively charged molecules [14].
Under physiological pH values, bacterial cells have a negative net electrostatic surface charge. The surface charge is determined by the composition of the cell envelopes, which are structurally and chemically different in gram-positive bacteria and gram-negative ones. The negative surface charge in gram-positive bacteria is due to virtue of phosphoryl groups in the substituent teichoic and teichuronic acid residues as well as carboxylate groups. In gram-negative bacteria, the negative charge of the cell surface is induced by the phosphoryl and 2-keto-3-deoxyoctonate carboxylate groups of lipopolysaccharide located in the outer membrane of the cell wall [15–17].
Owing to their unique anion-exchange properties, LDHs are considered as very promising materials for removal of pollutants in forms of anions and anion complexes (both inorganic and organic) [18,19]. LDHs are capable of accommodating anionic species with the characteristic height up to few nanometers in the interlayer space. Bacteria display dimensions in the micrometer range; therefore, intercalation of LDHs with such large systems are not expected. Nevertheless, the large surface area, with uncompensated positive charge of the outer mixed metal hydroxide layers, makes LDHs suitable for removal of pathogenic bacteria from aquatic environment.
Nowadays, there are only a few studies concerning application of LDHs for water treatment from bacterial contamination. Jin et al. [6] reported a high efficiency of chloride-intercalated Mg2Al and Zn2Al LDHs for removal of Escherichia coli from synthetic ground water and heterotrophic bacteria from raw river water. Park et al. [9] investigated antimicrobial action of Mg2Al-NO3 LDHs entrapped into calcium-alginate beads using E. coli as test microorganism. The authors suggested that the observed low removal efficiency can be due to the small size of pores in the beads, limiting the diffusion of bacteria inside them.
It should be noted that all recent studies exploited LDHs synthesized in laboratory by co-precipitation method, which makes the scale up of water treatment technologies based on these nanomaterials difficult. In contrast, commercial synthetic hydrotalcite has some advantages as an adsorbent for water purification with respect to the laboratory-prepared ones. Its main benefits include stable morphological structure and chemical composition, large scale production and relatively low price.
Motivated by this issue, in the present study we examined the efficiency of commercial synthetic hydrotalcite, Mg3Al-CO3, as an adsorbent for removal of pathogenic bacteria from contaminated water in suspension experiments. We used E. coli and Enterococcus faecalis as model objects to compare removal efficiency of gram-negative and gram-positive bacteria. Furthermore, effect of contact time, solution pH and incubation temperature on retention of bacteria by hydrotalcite and potential bactericidal activity of this nanomaterial were evaluated.
2. Materials and Methods2.1. HydrotalciteSynthetic hydrotalcite with the nominal chemical composition of Mg6Al2(CO3)(OH)16·4H2O was purchased from Sigma-Aldrich (cat. no 652288). Phase content and crystal structure of the hydrotalcite powder were characterized using a PANalytical X'Pert Powder diffractometer (Ni-filtered CuKα radiation) at room temperature. Morphology of the powder was characterized using a Hitachi S-4100 scanning electron microscope (SEM) with an electron beam accelerating voltage of 20 kV. Surface area and pore size analysis were performed using the combined Brunauer-Emmet-Teller and Barrett-Joyner-Halenda (BET&BJH) experimental procedure. The adsorption/desorption isotherms were collected in a Gemini V2.0, model 2380 from Micromeritics Instrument Corp. at 77 K in nitrogen. The samples were degassed at 180°C before the measurements.
2.2. Bacterial Strains and Culture ConditionsBacteria E. coli and E. faecalis regarded as opportunistic pathogens and indicators of fecal pollution were used as model objects to evaluate the efficacy of commercial synthetic hydrotalcite for water treatment from bacterial contamination.
Bacterial strains E. coli BIM B-378 and E. faecalis BIM B-1530 were obtained from the Belarusian Collection of Non-Pathogenic Microorganisms (The Institute of Microbiology of the National Academy of Sciences of Belarus). Bacteria were stored at −80°C in Nutrient Broth N2 (NB) (Condalab) with 20% of glycerol. Frozen stock cultures were activated by streaking onto NB agar (NBA) and incubation at 37°C for 24 h. The same nutrient medium and growth conditions were used for cultivation of bacteria in subsequent experiments.
2.3. Suspension ExperimentsA set of experiments were conducted to investigate bacterial sorption by commercial synthetic hydrotalcite in suspension slurry and to determine the effect of selected external factors on this process.
Colonies of E. coli BIM B-378 or E. faecalis BIM B-1530 grown on NA for 24 h were suspended in 10 mL of artificial ground water (AGW: 0.075 mM CaCl2, 0.082 mM MgCl2, 0.051 mM KCl, 1.5 mM NaHCO3, pH 7.1) [6] to match the turbidity standard of 0.5 McFarland units (Pro-Lab Diagnostics, SD2350). Then 1 mL of bacterial suspension, containing ~2×107 colony-forming units (CFU)/mL, was diluted with 29 mL of AGW in 50-mL glass bottles and hydrotalcite was added in tested concentration (0.5, 1, 2, 3, 4, 5 and 10 g/L). AGW without hydrotalcite, contaminated with bacterial suspension, was used as a control sample. The bottles were shaken in horizontal position with an Multi–functional Orbital Shaker (BioSan) at 100 rpm and room temperature (20–22°C) for 1–7 h. Experiments targeting the effect of external factors on bacterial removal were carried out at pH 5.5, 7.1, 8.5 and temperatures 16, 22, 28 and 37°C.
2.4. Determination of Bacterial Cell CountConcentration of bacteria in water was quantified using plate count assay. After incubation with hydrotalcite, water was filtered through Whatman filter (Merck, WHA10300214), serially ten-fold diluted in physiological saline (PS) (0.9% NaCl, pH 7.2) followed by plating on NBA and incubation at 37°C for 48 h. Then the number of bacterial colonies was counted. The removal efficiency was calculated from the difference between the cell count of bacteria in suspension, before and after incubation with hydrotalcite, using the following equation:
where
2.5. Elution of Bacteria from Hydrotalcite SurfaceAfter separation from water, hydrotalcite was aseptically placed into tube with 10 mL of NB and incubated at room temperature with periodical shaking for 2 h. Then 0.1 mL of elution was plated on NBA and cultivated at 37°C for 48 h.
2.6. Evaluation of Bactericidal Action of HydrotalciteColonies of E. coli BIM B-378 or E. faecalis BIM B-1530 grown on NA for 24 h were suspended in 10 mL of PS to match the turbidity standard of 0.5 McFarland units. Flasks containing 100 mL of NB were inoculated with 0.1 mL of bacterial suspension and then different concentrations of LDH (0.5, 2 or 4 g/L) were aseptically added. NB without hydrotalcite, inoculated with bacterial suspension, was used as a control sample. After cultivation at 37°C and 180 rpm for 24 h bacterial cell count was enumerated using plate count assay.
3. Results3.1. Characterization of Synthetic HydrotalciteThe X-ray diffraction (XRD) pattern of synthetic hydrotalcite (Fig. 1) demonstrated that the only crystalline phase detected in this material is a layered double hydroxide. On the other hand, SEM studies revealed that the particles of synthetic hydrotalcite are quite homogeneous, with the average size around 200–300 nm (Fig. 2). It should be noticed here that the particles of synthetic hydrotalcite are rather round and not flake-like with the characteristic diameter-to-thickness aspect ratio of about 20:1 as those typical of the laboratory-prepared Mg-Al LDHs [20,21]. This morphological feature of the synthetic hydrotalcite particles is apparently related to specificities of production of this material.
The surface area of the synthetic hydrotalcite was estimated from the BET&BJH experiments to be in the range of 6–12 m2/g, depending on the experimental method and the model used (Table 1). The pore volume and the pore size values found from the same experiments were ~0.02 cm3/g and ~7 nm, respectively. We would like to point out again that the commercial synthetic hydrotalcite is morphologically different from LDHs of the same chemical composition prepared the conventional laboratory routes. Aramendia et al. [22] have shown that the Mg3Al-CO3 LDH synthesized using a co-precipitation method has the surface area of about 50 m2/g and the pore volume of about 0.23 cm3/g. The latter is about one order of magnitude larger than that measured in the synthetic hydrotalcite under study in this work.
3.2. Removal of Fecal Indicator Bacteria from Water Using Synthetic HydrotalciteThe ability of commercial synthetic hydrotalcite to sorb gram-negative and gram-positive fecal indicator bacteria, E. coli BIM B-378 and E. faecalis BIM B-1530, respectively, from contaminated water with a high bacterial load (~2×1010 CFU/L) was tested under different concentrations of LDH and contact time.
The suspension experiments demonstrated that the removal efficiency of the model bacteria from water after 4-h exposure depends on concentration of the hydrotalcite in the suspension. An increase in the hydrotalcite concentration from 0.5 to 10 g/L resulted in a rise of the percentage of removed bacteria from 20.5±1.6 to 58.4±1.8% and from 8.9±0.3 to 26.0±1.1% for E. coli BIM B-378 and E. faecalis BIM B-1530, respectively (Fig. 3). The percentage of removed enterococci under all studied concentrations of hydrotalcite was significantly lower as compared to coliforms, and the effect of hydrotalcite concentration for E. faecalis BIM B-1530 was less pronounced. Experiments were not done to assess the maximum sorption capacity of hydrotalcite. However, the observed sorption capacity of this LDH after 4-h incubation was about 1.1×108 CFU/kg for E. coli BIM B-378 and 1.2×107 CFU/kg for E. faecalis BIM B-1530. Therefore, the sorption capacity of hydrotalcite was significantly higher for coliforms than for enterococci.
The results of removal kinetics of E. coli BIM B-378 and E. faecalis BIM B-1530 from water by synthetic hydrotalcite are presented in Fig. 4. High removal rate of coliforms was observed during the first 3 h of incubation, when around 33.7±1.2% of bacteria were removed from the model aquatic system. Further incubation with hydrotalcite resulted in a gradual decrease in concentration of E. coli BIM B-378 in water up to 50.1±1.8% after 7 h. Maximal removal of E. faecalis BIM B-1530 occurred during the first 1 h of incubation, when 16.5±1.8% of bacteria were eliminated from water. The percentage of removed enterococci gradually increased during the incubation period, reaching 27.8±1.6% and 37.1±1.7% after 4 h and 7 h, respectively.
3.3. Effect of pH and Temperature on Bacterial Removal by Synthetic HydrotalciteThe effect of abiotic factors, such as pH and temperature, on bacterial elimination from water using commercial synthetic hydrotalcite was also evaluated. The removal efficiency of E. coli BIM B-378 and E. faecalis BIM B-1530 increased as pH decreased (Fig. 5). After 4-h incubation, the removal percentage of coliforms was 44.0±1.9% at pH 5.5, while at pH 7.1 and 8.5 the removal percentage of coliforms corresponded to 39.2±1.9% and 34.9±1.9%, respectively. The percentage of removed enterococci was 31.8±1.9%, 27.4±1.8% and 24.8±1.9% at pH 5.5, 7.1 and 8.5, respectively.
Incubation temperature in the range of 22–37°C did not affect the removal efficiency of E. faecalis BIM B-1530 and E. coli BIM B-378 by synthetic hydrotalcite, while the percentage of removed cells of both strains significantly decreased when incubated at 16°C (Fig. 6).
3.4. Competitive Removal of Coliforms and Enterococci by Synthetic HydrotalciteBoth E. coli BIM B-378 and E. faecalis BIM B-1530 were added to the model aquatic system to observe if any competitive sorption occurred. A competitive removal experiment demonstrated that about 37.4±2.3% of coliforms and 22.1±2.1% of enterococci were removed from 5 g/L hydrotalcite suspension, containing both bacteria, after 4-h incubation. These results were close to the removal values measured in the suspension experiments with individual strains, indicating that no competitive sorption of coliforms and enterococci occurs on the surface of synthetic hydrotalcite.
3.5. Evaluation of Bactericidal Activity of Synthetic HydrotalciteTo investigate if retention of E. coli BIM B-378 and E. faecalis BIM B-1530 by synthetic hydrotalcite could be due to their inactivation (which is preferred), bacteria were eluted from hydrotalcite surface by NB for 2 h and plated on NBA. Bacterial lawn was formed after 24 h of incubation for both coliforms and enterococci, suggesting that their attachment to hydrotalcite particles was reversible, bacterial cells were still viable after attachment-detachment process, and this nanomaterial itself possesses no bactericidal activity.
Evaluation of bactericidal action of synthetic hydrotalcite by broth method confirmed that it is inactive against E. coli BIM B-378 and E. faecalis BIM B-1530. No inhibition of bacterial growth was detected, as well as there were no significant differences in viable cell count of coliforms and enterococci after 24 h of incubation in NB with 0.5, 2 and 4 g/L of hydrotalcite (Table 2).
4. DiscussionInfectious diseases caused by pathogenic bacteria and viruses are the most widespread and serious health risk associated with drinking water. Therefore, many attempts have been made to develop effective treatment technologies to reduce the concentration of microbial contaminants in aquatic systems. The unique structure and physicochemical properties of LDHs make these nanomaterials promising candidates for application in water purification processes.
One of the main requirements to sorbents used in drinking water treatment facilities is high removal efficiency of biological infectious agents. It was reported that Mg-Al and Zn-Al LDHs can adsorb more than 99% of E. coli and E. coli phage MS2 from a synthetic ground water [6,7]. In the present study, we found that only about 40% of E. coli BIM B-378 and 25% of E. faecalis BIM B-1530 are removed from model aquatic system containing synthetic hydrotalcite (Mg3Al-CO3) in the conditions similar to those used in the aforementioned references. Apparently, the difference in the sorption efficiency of synthetic hydrotalcite and that of the laboratory-prepared Mg-Al LDHs reported in Refs [6] and [7] should be analysed in relation to differences in the LDH materials involved, namely to their chemical compositions and morphological features. Commercial synthetic hydrotalcite, which is chemically a carbonate-intercalated Mg-Al LDH with the Mg/Al atomic ratio of 3:1, is prepared using aluminium hydroxycarbonate and magnesium hydroxide. A mixture of the precursors is subjected to hydrothermal treatment at elevated pressure [23]. The LDHs applied in the previously reported water treatment experiments were prepared using the most conventional laboratory method, a co-precipitation from the solutions containing magnesium and aluminium in forms of either nitrates [6] or chlorides [7] in the ratio of 2:1. Thus, we compare the bacterial removal efficiency of the following absorbents: Mg2Al-NO3 [6], Mg2Al-Cl [7] and Mg3Al-CO3 (this study). After an LDH is immersed in the model aquatic system, a substitution of the intercalated anion by other anionic species available (e.g., OH−) occurs. Kinetics of this anion exchange depends on many external factors (temperature, pH, concentration of the replacing anionic species). Besides, and considering all the external conditions being equal, the maximum anion release will happen in nitrate-intercalated LDHs, while in LDHs intercalated with carbonate the effect will be negligible [24]. Although no particular study of the possible effect of the environment-neutral intercalated anions (NO3−, OH−, Cl−, CO32−) has been undertaken, such an effect (if any) is unlikely to be considerable. Since sorption is the main process in the antimicrobial effect of LDH, let us consider the distinctive features relevant to this process. In synthetic hydrotalcite considered in this work, the Mg/Al ratio is 3:1, while in the LDHs prepared via co-precipitation [6,7], the ratio is 2:1. According to the general formula of a mixed metal hydroxide layer indicated in the Introduction section, the layer charge (per formula unit), x+, is 0.25|e| and 0.33|e| for Mg3Al and Mg2Al compositions, respectively. Therefore, the difference in the layer charge and thereby in the specific surface charge between synthetic hydrotalcite and the laboratory-prepared Mg2Al LDHs reported in Refs [6] and [7] is about 30%.
The larger size of bacterial cells relative to the interlayer spacing of the LDHs make it impossible the intercalation of bacteria into LDHs structure. Consequently, sorption of bacteria could only occur on the external surface of the hydrotalcite and the surface area significantly influenced the sorption capacity of this nanomaterial. According to our data, commercial synthetic hydrotalcite has a considerably smaller surface area than that of the LDH of the same compositions prepared via co-precipitation (see the Results section), which correlates well with its relatively small sorption capacity (108 CFU/kg compared to 1013 CFU/kg reported in Ref [6]).
A percentage of removed E. coli BIM B-378 was significantly higher than that of E. faecalis BIM B-1530, which is apparently due to the different cell surface charge of these bacteria, associated with cell wall structure and chemical composition, and their cell size. E. coli BIM B-378 cells (straight rods 1.0–1.5 μm in length and 0.5 μm in diameter) have smaller size compared to E. faecalis BIM B-1530 (round-shaped cells with diameter 1.5–2.0 μm). The shape of bacterial cells may also influence their removal from water by porous materials [26, 27], and preferential retention of long rod-shaped cells (like E. coli) has been demonstrated. These facts may also explain the less pronounced effect of hydrotalcite concentration and contact time on the removal efficiency of E. faecalis BIM B-1530 than E. coli BIM B-378. Thus, increase in hydrotalcite concentration in water suspension from 1 to 10 g/L led to the increase of removed coliforms and enterococci by a factor of 2.5 and 1.7, respectively. The percentage of removed E. coli BIM B-378 and E. faecalis BIM B-1530 has risen by a factor of 2.2 and 4.4, respectively, when compared 1 h and 7 h of incubation. Taking into account that contaminated water contains many bacterial species (e.g. E. coli, Salmonella sp., Shigella sp., Vibrio cholerae, Aeromonas hydrophila, Clostridium perfringens) that can be hazardous to human health [25], careful studies on selective sorption efficiency of LDHs are required to develop applicable sorbents for water treatment.
As more than one type of bacteria is usually present in natural aquatic environment, they could compete for adsorption on the LDH surface, resulting in decrease or even inhibition of adsorption of particular microbes. Thus, a minor decrease in the retention of bacteriophage MS by Mg2Al-Cl LDH was detected in presence of additional bacteriophage ɸX174 in water suspension [7]. In our study, the percentage of removed E. coli BIM B-378 and E. faecalis BIM B-1530 did not significantly change when both bacteria were present in aquatic system. This finding indicates the absence of competitive sorption of coliforms and enterococci on the synthetic hydrotalcite surface, which may be an advantage of this nanomaterial as an adsorbent for water treatment from microbiological contamination.
Due to the nature of sorption process, especially when electrostatic interactions are involved, a wide range of environmental factors influence sorption capacity of LDHs [19]. Many studies report that the removal efficiency of dyes, nitrates and phosphates is higher at acidic pH (4.0–5.0) decreasing as the pH becomes alkaline (10.0–11.0) [28–30]. The impact of pH is due to the change of the surface charge of LDHs and functional groups of pollutants. The extent of bacterial adsorption onto solid surfaces also depends on the pH of liquid phase. pH and ion concentration in solution affect cell surface charge of bacteria because of the dissociation of carboxylic and amino groups located on the cell wall [26]. In a previous work, it was found that the retention of bacteria on cation- and anion-exchange beads and mineral surfaces was not affected by changes in pH in the range 4.0–9.0 [31]. On the contrary, the decrease of the suspension pH from 7.5 to 5.0 resulted in the increase in the retention of a ground-water bacterium on quartz chips [32]. Herein, we revealed only a slight increase in the removal efficiency of E. coli BIM B-378 and E. faecalis BIM B-1530 at pH 5.5, which may be related to the increase in electrostatic interactions between synthetic hydrotalcite and bacterial cells.
Temperature may also impact sorption of chemical pollutants on LDH surface, mainly due to the influence on their mobility and solubility. Previously, the removal efficiency of chemical dyes Congo Red and Methyl Orange onto Mg-Fe LDHs has been found to increase by 3% and 5%, respectively, over a temperature increase of 30°C. Contrastingly, the sorption of Methyl Orange onto Co-Cu LDH surface decreased by 171 mg/g with an increase in temperature from 25°C to 40°C [19]. Concerning bacteria, their adsorption is believed to be substantially greater at high temperatures. The decrease in bacterial sorption at low temperatures may be caused by the reduced chemisorption and certain types of physical adsorption, and changes in the physiology of microorganisms [26]. In this study, we found a significant decrease in percentage of removed E. coli BIM B-378 and E. faecalis BIM B-1530 at 16°C, whereas other tested temperatures (22, 28 and 37°C), regarded as physiological for coliforms and enterococci, did not affect the removal efficiency.
The bactericidal activity of LDHs, attributed to metallic ions or hydroxides released from the layered structure to the medium, has been demonstrated in several studies and has garnered much attention [10,33,34]. Disinfection action of LDHs is generally associated with incorporation of copper, zinc and some other metallic ions, which can be easily released, promoting a rapid and efficient attack to the microorganisms [35,36]. In particular, copper-containing hydrotalcite proved to possess strong antimicrobial action against E. coli and E. coli phage Qβ and was regarded as promising for use as an alternative disinfectant to chlorine in a water purification system [10]. Bactericidal activity of hydroxide ions, which are strong oxidant species, is due to their ability to cause protein denaturation, damages in the cytoplasmic membrane and DNA molecules [34,37]. In the present study, synthetic hydrotalcite itself possessed no bactericidal action against E. coli BIM B-378 and E. faecalis BIM B-1530. This finding is related to the absence in its structure of so-called antibacterial metals and poor release of hydroxides into the model aquatic system (the pH of hydrotalcite suspension did not significantly change during the time of the experiment).
According to our data, the main limitations of the application of commercial synthetic hydrotalcite as an adsorbent in water purification technologies include rather high concentration of this nanomaterial (5 g/L or more) and long contact time (~4 h) needed for the effective removal of pathogenic bacteria in suspension slurry. The retention of bacteria on the hydrotalcite surface was reversible and special measures should be developed for inactivation (disinfection) of attached microbes. Besides pH and temperature, the effect of anions present in water and their consequent competitive sorption, may significantly decrease the overall sorption capacity of bacteria on LDH, and needs to be examined. Considering that suspension slurries are not feasible for large treatment volumes, additional studies should be carried out to evaluate the feasibility of using hydrotalcite as an adsorbent for filtration systems. Moreover, one crucial point requiring a special attention for development of hydrotalcite-based technologies of water treatment is the reusability, regeneration or utilization of spent hydrotalcite.
5. ConclusionsTo the best of our knowledge, the current study represents the first attempt to evaluate commercial synthetic hydrotalcite as an adsorbent for removal of bacteria from contaminated water. Our findings indicate that exposure to an aqueous suspension of hydrotalcite (5 g/L) for 4 h resulted in the removal of about 40% of E. coli BIM B-378 and 25% of E. faecalis BIM B-1530 from water, at a high bacterial load (2×1010 CFU/L). In addition, the sorption efficiency of coliforms and enterococci was not affected by the presence of both bacteria in contaminated water. The absence of competitive sorption may be an advantage of hydrotalcite as an adsorbent for water purification, as more than one type of bacteria is usually present in natural aquatic environment. Furthermore, hydrotalcite itself possesses no bactericidal activity and the retention of bacteria was due to reversible sorption than inactivation, which would be a preferable process. The percentage of removed bacteria increased with increasing of hydrotalcite concentration in the suspension (0.5 to 10 g/L), contact time (1–7 h) and under acidic conditions (5.5), although decreasing at low incubation temperatures (16°C).
The findings presented in this study along with more practical questions such as reusability, regeneration or utilization of spent hydrotalcite, its feasibility as an adsorbent for filter systems, the effect of abiotic and biotic factors on the efficiency of bacterial removal, should be addressed for developing of profitable hydrotalcite-based technology of water treatment.
AcknowledgmentsD.E.L.V and J.P.V.C acknowledge the financial support of FCT – the Portuguese Foundation for Science and Technology through the individual PhD grants PD/BD/143033/2018 and SFRH/BD/ 145281/2019, respectively. A.N.S. acknowledges the financial support of national funds (OE) through FCT - Portugal in the scope of the framework contract foreseen in the numbers 4, 5, and 6 of the article 23, of the Decree-Law 57/2016, of August 29, changed by Law 57/2017, of July 19. The research done in University of Aveiro was supported by project COAT4LIFE. This project has received funding from the European Union’s Horizon 2020 research and innovation programme under the Marie Skłodowska-Curie grant agreement No 101007430. The authors from University of Aveiro acknowledge the support from the project CICECO - Aveiro Institute of Materials, UIDB/50011/2020, UIDP/50011/2020 & LA/P/0006/2020, financed by national funds through the FCT/MEC (PIDDAC).
NotesAuthor Contributions A.V.S. (Assistant Professor) conceptualization, microbiological investigations, data curation, writing original draft, review and editing the manuscript; A.N.S. (Assistant Professor) conceptualization, formal analysis, methodology, review and editing the manuscript; S.I.L. (M.Sc.) microbiological investigations, data analysis, visualization; D.E.L.V. (Ph.D. Student) data curation, BET&BJH and SEM analysis, visualization; J.P.V.C. (Ph.D. Student) data curation, BET&BJH and SEM analysis, visualization; J.T. (Assistant Professor) project administration, formal analysis, validation, review and editing the manuscript; E.I.K. (Professor, Academician) resources, supervision, validation. References1. Pal M, Ayele Y, Hadush M, Panigrahi S, Jadhav VJ. Public health hazards due to unsafe drinking water. Air Water Borne Dis. 2018;7:1000138. doi:10.4172/2167-7719.1000138
2. Ouf SA, Yehia RS, Ouf AS, Abdul-Rahim RF. Bacterial contamination and health risks of drinking water from the municipal non-government managed water treatment plants. Environ. Monit. Assess. 2018;190:685. doi:10.1007/s10661-018-7054-z
3. Pandey PK, Kass PH, Soupir ML, Biswas S, Singh VP. Contamination of water resources by pathogenic bacteria. AMB Express. 2014;4:51. doi:10.1186/s13568-014-0051-x
4. Prüss-Ustün A, Bartram J, Clasen T, et al. Burden of disease from inadequate water, sanitation and hygiene in low- and middle-income settings: a retrospective analysis of data from 145 countries. Trop. Med. Int. Health. 2014;19(8)894–905. doi:10.1111/tmi.12329
5. Lawrence B. Water Purification: Treatment of microbial contamination. Satinder A, editorAdvances in water purification techniques. Elsevier; 2019. p. 385–395.
https://doi.org/10.1016/B978-0-12-814790-0.00015-6
6. Jin S, Fallgren PH, Morris JM, Chen Q. Removal of bacteria and viruses from waters using layered double hydroxide nanocomposites. Sci. Technol. Advanced Mater. 2007;8(1–2)67–70.
https://doi.org/10.1016/j.stam.2006.09.003
7. You Y, Vance GF, Sparks DL, Zhuang J, Jin Y. Sorption of MS2 bacteriophage to layered double hydroxides: effects of reaction time, pH, and competing anions. J. Environ. Qual. 2003;32(6)2046–2053. doi:10.2134/jeq2003.2046
8. Kim JH, Park JA, Kim SB. Mg/Al layered double hydroxide for bacteriophage removal in aqueous solution. Water Sci. Technol. 2012;66(4)761–767. doi:10.2166/wst.2012.239
9. Park JA, Lee CG, Park SJ, Kim JH, Kim SB. Microbial removal using layered double hydroxides and iron (hydr)oxides immobilized on granular media. Environ. Engineer. Res. 2010;15(3)149–156. doi: https://doi.org/10.4491/eer.2010.15.3.149
10. Sunayama S, Sato T, Kawamoto A, Ohkubo A, Suzuki T. Disinfection effect of hydrotalcite compounds containing antimicrobial metals against microorganisms in water. Biocontrol Sci. 2002;7(2)75–81.
https://doi.org/10.4265/bio.7.75
11. Goh KH, Lim TT, Dong Z. Application of layered double hydroxides for removal of oxyanions: a review. Water Res. 2008;42:1343–1368.
https://doi.org/10.1016/j.watres.2007.10.043
12. Mohapi M, Sefadi JS, Mochane MJ, Magagula SI, Lebelo K. Effect of LDHs and other clays on polymer composite in adsorptive removal of contaminants: a review. Crystals. 2020;10:957.
https://doi.org/10.3390/cryst10110957
13. Gu P, Zhang S, Li X, et al. Recent advances in layered double hydroxide-based nanomaterials for the removal of radionuclides from aqueous solution. Environ. Pollut. 2018;240:493–505. doi:10.1016/j.envpol.2018.04.136
14. Jin S, Brown TH, You YW, Vance GF. Charge-based water filtration systems. Canadian Patent. 2005. WO2005/012194 A1
15. Wilson W, Wade MM, Holman SC, Champlin FR. Status of methods for assessing bacterial cell surface charge properties based on zeta potential measurements. J. Microbiol. Methods. 2001;43(3)153–164.
https://doi.org/10.1016/S0167-7012(00)00224-4
16. Ayala-Torres C, Hernandez N, Galeano A, Novoa-Aponte L, Sot CY. Zeta potential as a measure of the surface charge of mycobacterial cells. Ann. Microbiol. 2014;64:1189–1195.
https://doi.org/10.1007/s13213-013-0758-y
17. Gottenbos B, Grijpma DW, van der Mei HC, Feijen J, Busscher HJ. Antimicrobial effects of positively charged surfaces on adhering Gram-positive and Gram-negative bacteria. J. Antimicrob. Chemother. 2001;48(1)7–13. doi:10.1093/jac/48.1.7. PMID:11418507
18. Nichitus S, Calin G, Burlui A, Stadoleanu C, Burlui V. Layered double hydroxides (LDHs) type materials used in water treatment. Key Eng. Mater. 2015;660:273–278. doi:10.4028/www.scientific.net/KEM.660.273
19. Johnston AL, Lester E, Williams O, Gomes RL. Understanding layered double hydroxide properties as sorbent materials for removing organic pollutants from environmental waters. J. Environ. Chem. Eng. 2021;9:105197. doi:10.1016/j.jece.2021.105197
20. Serdechnova M, Salak AN, Barbosa FS, et al. Interlayer intercalation and arrangement of 2-mercaptobenzothiazolate and 1,2,3-benzotriazolate anions in layered double hydroxides: in situ x-ray diffraction study. J Solid State Chem. 2016;233:158–165. doi:10.1016/j.jssc.2015.10.023
21. Sokol D, Vieira DEL, Zarkov A, et al. Sonication accelerated formation of Mg-Al-phosphate layered double hydroxide via sol-gel prepared mixed metal oxides. Sci. Rep. 2019;9:10419. doi:10.1038/s41598-019-46910-5
22. Aramendia MA, Borau V, Jimenez C, Marinas JM, Ruiz JR, Urbano FJ. Comparative study of Mg/M(III) (M=Al, Ga, In) layered double hydroxides obtained by coprecipitation and the sol-gel method. J. Solid State Chem. 2002;168:156–161. doi:10.1006/jssc.2002.9655
23. He J, Wei M, Li B, Kang Y, Evans DG, Duan X. Preparation of layered double hydroxides. Struct. Bond. 2006;119:89–119. doi:10.1007/430_006
24. Miyata S. Anion-exchange properties of hydrotalcite-like compounds. Clays Clay Miner. 1983;31:305–311. doi:10.1346/CCMN.1983.0310409
25. Aghalari Z, Dahms HU, Sillanpää M, Sosa-Hernandez JE, Parra-Saldivar R. Effectiveness of wastewater treatment systems in removing microbial agents: a systematic review. Global. Health. 2020;16:13.
https://doi.org/10.1186/s12992-020-0546-y
26. Stevik TK, Aa K, Ausland G, Hanssen JF. Retention and removal of pathogenic bacteria in wastewater percolating through porous media: a review. Water Res. 2004;38(6)1355–1367. doi:10.1016/j.watres.2003.12.024
27. Weiss TH, Mills AL, Hornberger GM, Herman JS. Effect of bacterial cell shape on transport of bacteria in porous media. Environ. Sci. Technol. 1995;29(7)1737–1740. doi:10.1021/es00007a007
28. Hassani KE, Beakou BH, Kalnina D, Oukani E, Anouar A. Effect of morphological properties of layered double hydroxides on adsorption of azo dye Methyl Orange: a comparative study. Appl. Clay Sci. 2017;140:124–131.
https://doi.org/10.1016/j.clay.2017.02.010
29. de Sa FP, Cunha BN, Nunes LM. Effect of pH on the adsorption of sunset yellow FCF food dye into a layered double hydroxide (CaAl-LDH-NO3). Chem Eng J. 2013;215–216:122–127.
https://doi.org/10.1016/j.cej.2012.11.024
30. Alagha O, Manzar MS, Zubair M, Anil I, Mu’azu DN, Qureshi A. Magnetic Mg-Fe/LDH intercalated activated carbon composites for nitrate and phosphate removal from wastewater: insight into behavior and mechanisms. Nanomaterials. 2020;10:1361.
www.mdpi.com/journal/nanomaterials
31. Stenstrom TA. Bacterial hydrophobicity, an overall parameter for the measurement of adhesion potential to soil particles. Appl. Environ. Microbiol. 1989;55:142–147. doi:10.1128/aem.55.1.142-147.1989
32. Jewett DG, Hilbert TA, Logan BE, Arnold RG, Bales RC. Bacterial transport in laboratory columns and filters: influence of ionic strength and pH on collision efficiency. Water Res. 1995;29:1673–1680.
doi.org/10.1016/0043-1354(94)00338-8
33. Segura-Perez V, Lobo-Sanchez M, Velazquez-Herrera FD, Frias-Vazquez DA, Reyes-Cervantes E, Fetter G. Hydrotalcite/hydroxyapatite composites with high bacterial activity against clinical bacteria. A new alternative to prevent osteomyelitis diseases. Micropor. Mesopor. Mater. 2020;298:110069.
https://doi.org/10.1016/j.micromeso.2020.110069
34. Lobo-Sánchez M, Najera-Melendez G, Luna G, et al. ZnAl layered double hydroxides impregnated with eucalyptus oil as efficient hybrid materials against multi-resistant bacteria. Appl. Clay Sci. 2018;153:61–69. doi:10.1016/j.clay.2017.11.017
35. Velazquez-Herrera FD, Fetter G, Rosato V, Pereyra AM, Basaldella EI. Effect of structure, morphology and chemical composition of Zn-Al, Mg/Zn-Al and Cu/Zn-Al hydrotalcites on their antifungal activity against A. niger
. J. Environ. Chem. Eng. 2018;6:3376–3383.
https://doi.org/10.1016/j.jece.2018.04.069
36. Parello ML, Rojas R, Giacomelli CE. Dissolution kinetics and mechanism of Mg-Al layered double hydroxides: a simple approach to describe drug release in acid media. J. Colloid Interface Sci. 2010;351:134–139.
https://doi.org/10.1016/j.jcis.2010.07.053
Fig. 1XRD pattern of the synthetic hydrotalcite with the most intense reflections of layered double hydroxide structure indicated. 
Fig. 3Percentage of removed E. coli BIM B-378 and E. faecalis BIM B-1530 after exposure to hydrotalcite (0.5–10 g/L) in suspension slurry for 4 h. Incubation was performed at room temperature (~20–22°C) and pH 7.1, initial concentration of bacteria was ~2×1010 CFU/L. Each point is an average of three independent experiments, error bars (too small to see on some bars) are the standard deviation of the mean. 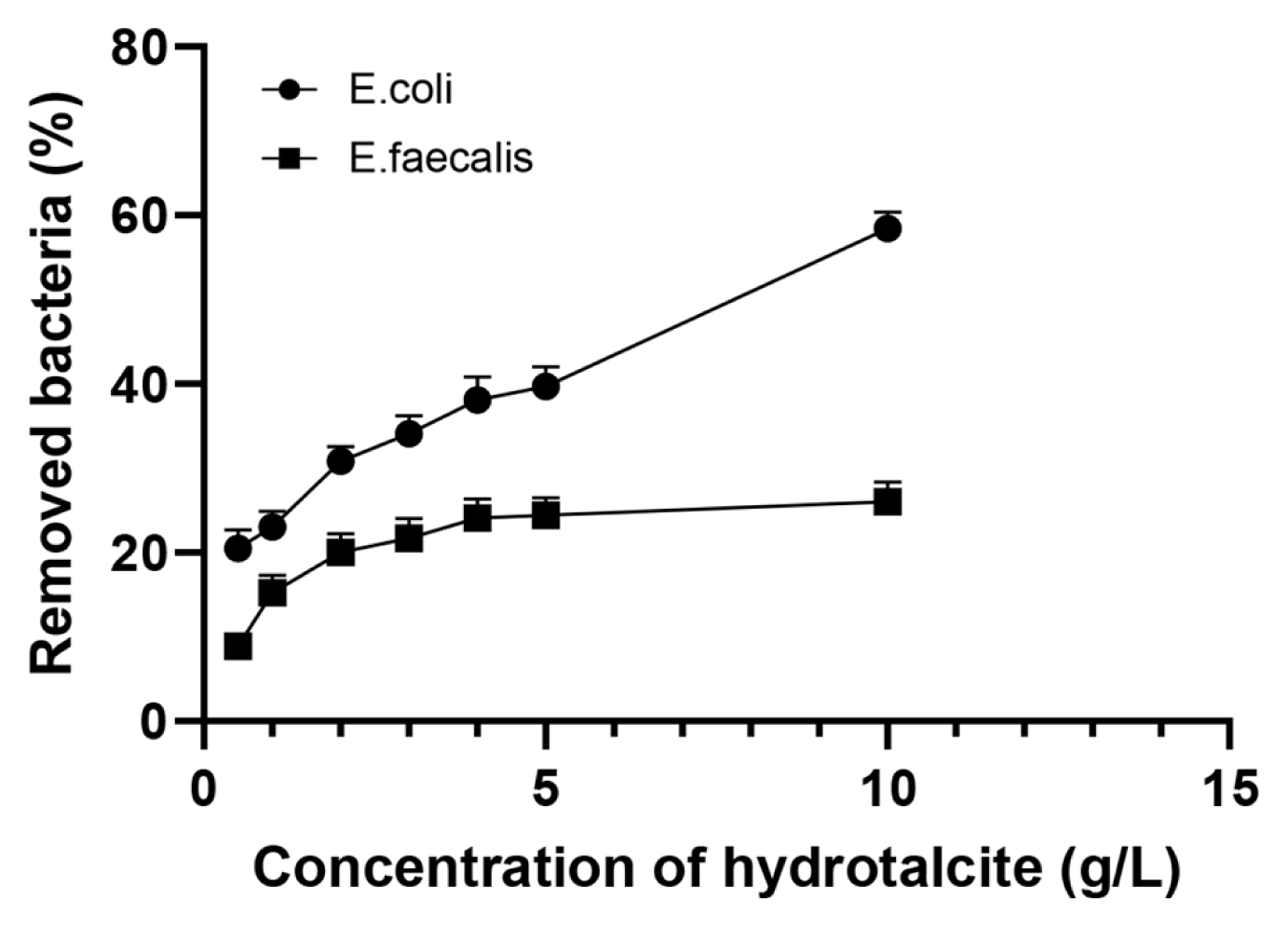
Fig. 4Removal kinetics of E. coli BIM B-378 and E. faecalis BIM B-1530 during exposure to hydrotalcite (5 g/L) in suspension slurry for 7 h. Incubation was performed at room temperature (~20–22°C) and pH 7.1, initial concentration of bacteria was ~2×1010 CFU/L. Each point is an average of three independent experiments, error bars (too small to see on some bars) are the standard deviation of the mean. 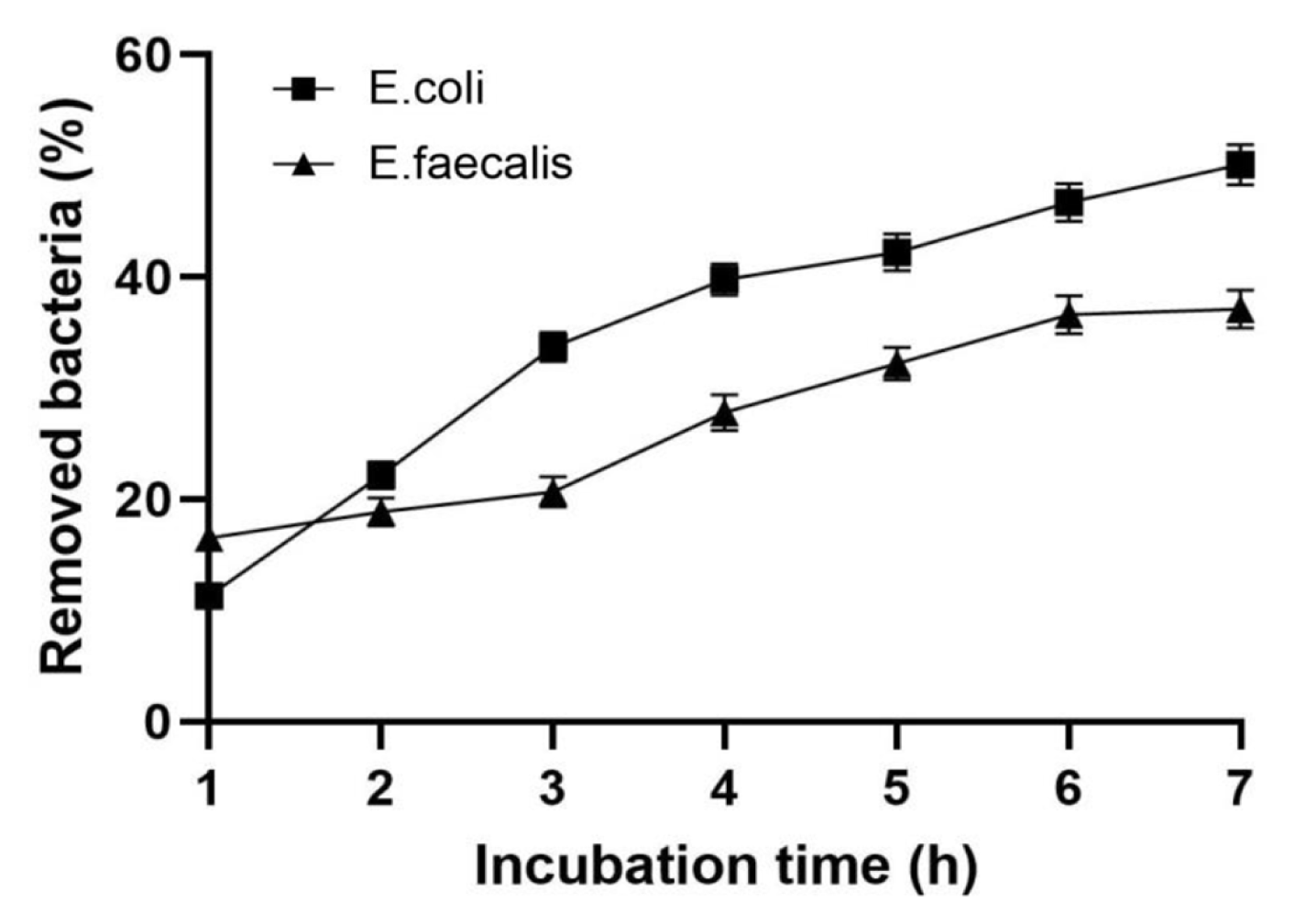
Fig. 5Percentage of removed E. coli BIM B-378 and E. faecalis BIM B-1530 after exposure to hydrotalcite (5 g/L) in suspension slurry at different pH values (5.5–8.5). Incubation was performed at room temperature (~20–22°C) for 4 h, initial concentration of bacteria was ~2×1010 CFU/L. Each column is an average of three independent experiments, error bars are the standard deviation of the mean. 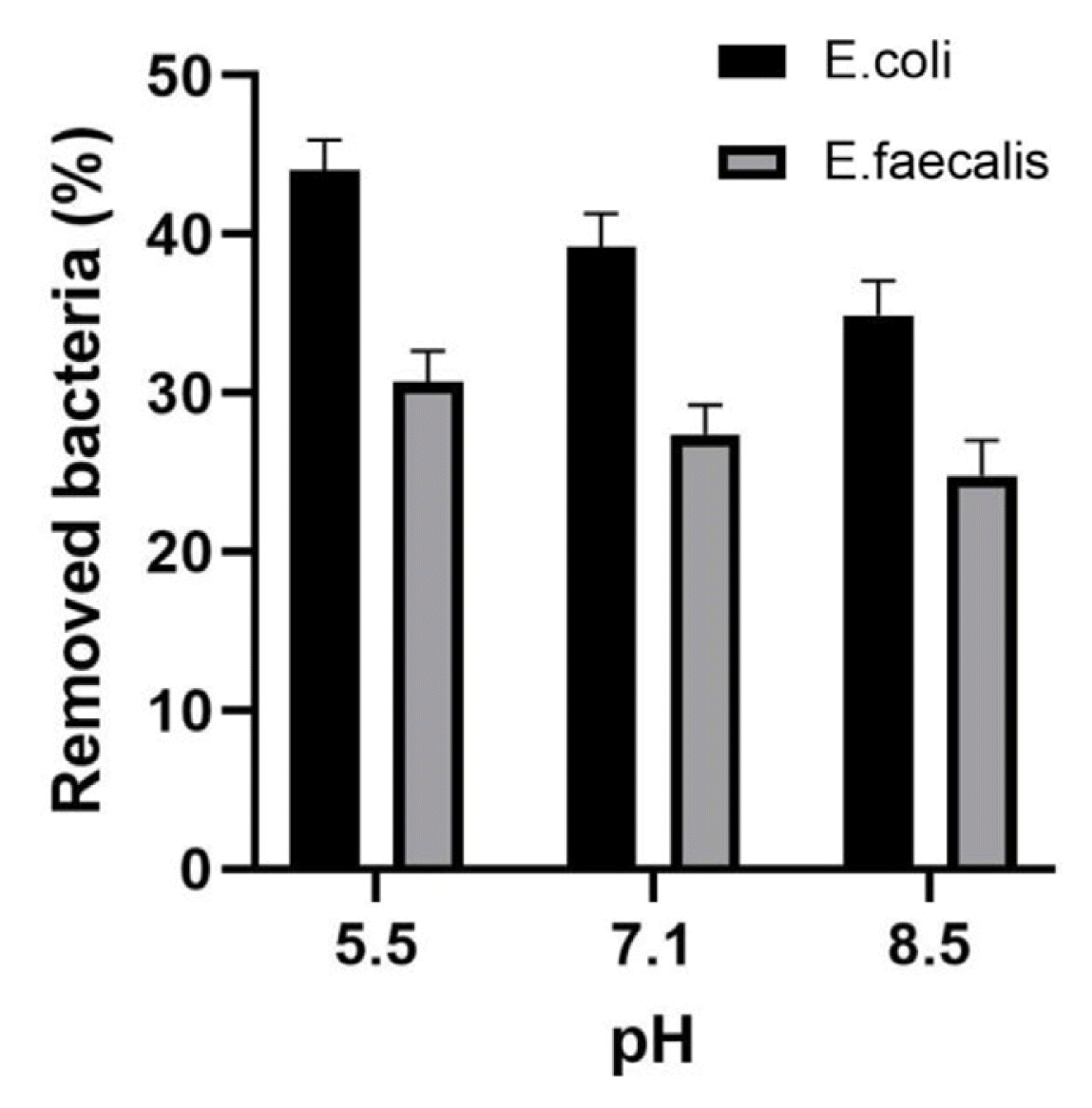
Fig. 6Influence of temperature on the percentage of removed E. coli BIM B-378 and E. faecalis BIM B-1530 after exposure to hydrotalcite (5 g/L) in suspension slurry for 4 h. Incubation was performed at pH 7.1, initial concentration of bacteria was ~2×1010 CFU/L. Each column is an average of three independent experiments, error bars are the standard deviation of the mean. 
Table 1Summary of the Surface Area and the Pore Size Measurements of Commercial Synthetic Hydrotalcite Using the Combined BET&BJH Experimental Procedure Table 2Viable Cell Count of E. coli BIM B-378 and E. faecalis BIM B-1530 after 24-h Cultivation in NB with Different Concentrations of Commercial Synthetic Hydrotalcite |
|
||||||||||||||||||||||||||||||||||