AbstractIn this study, we screened the performance of aqueous extracts of Talinum triangulare (AET) and soap solution prepared from Hura crepitans seed oil saponified by aqueous solution of ashed plantain peels (HCS) for removal of naphthalene and phenanthrene from soil. The distribution of trace metals in the soil before and after soil washing was also investigated. The results revealed maximum removal efficiencies after 25 min washing time of 96.25, 96.14, and 25.70% naphthalene, for Tween 80, AET and HCS, respectively. While for phenanthrene, the recorded maximum removal efficiencies after 25 min washing time were: 91.80, 26.00, and 94.0 for Tween 80, AET and HCS, respectively. Based on results from other experiments, AET generally showed slightly lower removal efficiencies compared to the commercial Tween 80 surfactant. Also, the results revealed that the three remediants affected the distribution of trace metals (Cd, Pb, and Zn) in the soil after surfactant washing. Based on the amount of trace metals extracted, the performance of the three remediants are as follows: AET > Tween 80 > HCS. The performance of AET has been attributed to the presence of multiple heteroatomic moieties for trace metals adsorption and moderation of the acidic conditions of the soil.
1. IntroductionGiven their persistence, mutagenic and carcinogenic potentials, polycyclic aromatic hydrocarbons (PAHs) contamination are regulated in many countries. Polluted soils such as oil spill sites are often required to be monitored for PAHs during clean-up and remediation exercises. Consequently, many attempts have been launched to remediate PAHs in crude oil contaminated soils. However, most of these attempts are largely impracticable, unsustainable or too expensive to be adopted in low economy countries [1, 2]. The new paradigm is towards the valorisation and utilisation of sustainable materials for cost-effective remediation [3].
Surfactant enhanced soil washing of PAH-contaminated soils have been widely investigated and many reviews published [4–11]. Due to environmental concerns about synthetic surfactants, researchers are now more focused on naturally derived biodegradable surfactants from plant sources [12–15]. Some reports of the application of plant-based surfactants include: solubilization and desorption of hexachlorobenzene (HCB) [16], and soil washing of naphthalene, phenanthrene as well as other PAHs [17–19]. In spite of the progress recorded, it is desirable to optimize conditions in order to achieve better efficiency. Some challenges in the selection of surfactants for soil washing protocol include coverage of multi-contaminants systems [19], overcoming negative interactions with soil washing agents (e.g., viscosity and clogging) and common ions effect from the environmental matrix [20–22]. Also, given the type of soil and target contaminants, a choice between non-ionic, cationic, anionic, gemini or cosolvent mixtures of surfactants may be required [11, 23–29]. In less developed countries, technical setbacks may not allow smooth applications of many of these methods. In the present study, our objective was to valorise wastes from common food and agricultural substrates (waterleaf and plantain peels) into surfactant products (aqueous extracts of waterleaf and saponified oil from Hura crepitans seeds using aqueous solutions of ashed plantain peels).
Plant-sourced precursors such as saponins and oleochemicals have been established to be useful as natural surfactants for soil remediation [18, 19, 30, 31]. Hura crepitans, otherwise known as the sandbox tree, is a tropical tree with many pharmaceutical and ethnobotanical applications [32–34]. The seeds of Hura crepitans have oil content within the range of 36–72% wt% [33]. However, the oil is non-edible [35]. As a consequence, the oils have been utilized for several other applications such as preparation of alkyd resins, methyl esters, biodiesel and surface-active materials such as soaps [33, 35, 36]. On the other hand, Talinum triangulare (common name: water leaf) is a tropical perennial herb with high surface-active saponin content [37, 38]. Aqueous extract of Talinum triangulare has been previously screened as candidate for soil washing of crude oil contaminated ultisol [39]. In the present study, we evaluated different substrate sources as plant-derived surfactants for soil washing of organic pollutants and examined the distribution of untargeted trace metals in such systems.
2. Materials and Methods2.1. Preparation of Bio-derived SurfactantsMethods for preparation of aqueous extracts of Talinum triangulare have been previously reported [39]. Matured dry fruits of Hura crepitans were harvested at Uyo Metropolis, Akwa Ibom State, Nigeria. The dry fruits were mechanically broken to remove the seeds. The seeds were air-dried and ground using a manual grinder. The Hura crepitans seed oil was extracted from the ground seeds through solvent extraction using petroleum ether as solvent at a temperature range of 60 to 80°C. Exactly, 500 g of the sample was soaked in petroleum ether, squashed in a borosilicate container, and filtered in order to separate the petroleum ether fraction. The filtrate was kept overnight in a fume cupboard, the settled debris at the bottom was removed by decantation. The filtrate was kept in an oven at the temperature of 70°C to get rid of petroleum ether leaving behind yellow-coloured oil with a pH of 5. Plantain peels were locally sourced and washed with running water to remove dirt, sun-dried for three (3) weeks and burnt to ash using a muffle furnace. The ash formed was cooled and stored in a sealed plastic container. About 500 g of the ash was added to a clean plastic container and 1,000 mL of deionised water added, stirred for 30 min and kept overnight. The resultant solution was filtered using Pall water filtration apparatus with 47 mm, 5-micron glass micro-filter membrane into a clean bottle using a vacuum pump. A pale brown filtrate with pH of 13 was collected and stored in a 250 mL volumetric flask. About 250 mL of the ash solution was added to a stainless-steel pan and boiled to 100°C and then 62 g of the Hura Crepitans seed oil was added and stirred thoroughly. The mixture foamed signifying saponification; the temperature was reduced to 60°C to control the foaming. Stirring continued until the foaming reduced leaving behind a grey soapy viscous liquid.
2.2. Laboratory Soil Washing ExperimentsThe soil washing experiments were done according to modified methods from the literature [4, 23, 40]. Soil samples were collected from an urban agricultural soil in Uyo, Nigeria. The air-dried homogenized soil samples (approx. 20 g) were added into 30-mL borosilicate round-bottom glass centrifuge tubes. Naphthalene and phenanthrene (AR, 99.9%) were purchased from British Drug House (now Merck, UK), while Tween 80 (extra pure grade) was acquired from Loba Chemie PVT. Ltd (Mumbai, India). Stock solutions of PAHs (naphthalene and phenanthrene) were prepared in volumetric flasks by dissolving appropriate amounts in methanol and stirred using a magnetic stirrer. A measured volume (15 mL) of the PAH stock solution was then added into the centrifuge tubes containing the thoroughly mixed soil samples such that the medium would be saturated. The spiking of the stock solution (concentrations: 0, 25, 50, 75, 100, and 125 mg/L) was done such that on evaporation, it would give a theoretical concentration in g/Kg of soil (0, 0.5, 1.0, 1.5, 2.0, 2.5 mg PAH/g soil). The mixture was then kept for 72 h to allow complete evaporation of methanol. Exactly, 20 mL of surfactant solutions prepared at appropriate concentrations were then added to the soil mixture. For aqueous extracts of waterleaf, a volume-by-volume aqueous concentrations were used (10, 20, 30, 40, and 50% vol/vol). Surfactant solution from saponified Hura Crepitans seed oil prepared using deionised water at different concentrations (5, 10, 15, 20, 30 mg/L). To compare the performance of bio-derived surfactants with a commercial surfactant, Tween 80 was used. Different concentrations (10, 20, 30, 40, 50 mg/L) of Tween 80 was prepared with deionised water from around its critical micellar concentration (CMC) which has been previously determined to be 13.10 mg/L according to Zhang and Zhu [41]. The amounts of soil and the surfactants were chosen so that the glass tubes would be filled up, and then sealed with Teflon-lined screw caps to avoid any loss. The mixtures were then agitated for required period in a thermostated shaker bath maintained at a speed of 120 rpm at room temperature (25°C). Thereafter, the samples were centrifuged at 12,000 g for 15 min. Aliquots (0.5 mL) of the supernatant were withdrawn using syringes, dissolved in 1.5 mL methanol, filtered through 0.22 μm PTFE membrane filters and placed in 1.5 mL vials for subsequent analysis.
2.3. Analysis of PAHsThe concentration of naphthalene and phenanthrene were determined using high performance liquid chromatograph (HPLC) (Shimadzu LC-20AT model) with an ultraviolet (UV) detector and a 5 μm 4.6 × 250 mm Agilent C18 reversed phase column. The following conditions were applied: flow rate of 1.0 mL/min, injection volume of 15 μL, under isocratic flow condition with acetonitrile and water (80:20) as mobile phase. The temperature was maintained at 30°C. The UV wavelength was fixed at 254 nm. The HPLC-UV method was a modification of a suitable method in the literature [42]. Blanks were prepared for all experiments. All aqueous solutions were prepared using deionised water. Experiments were carried out in triplicates.
2.4. Soil Washing Efficiency2.5. Characterization of Bio-derived SurfactantsThe prepared surfactant solutions without further purification except filtration, were characterised for functional group determination using Fourier-transform infrared (FTIR) spectroscopy analysis. The FTIR spectra were recorded using Thermo Scientific Nicolet iS5 spectrometer (Thermo Scientific, Waltham, USA).
2.6. Physicochemical and Trace Metals AnalysisPhysicochemical parameters of soils and trace metals analyses were carried out using standard analytical protocols according to the American Public Health Association [44]. Physicochemical parameters determined include pH, electrical conductivity, total organic carbon (TOC), cation exchange capacity (CEC), and particle size. Trace metals (Cd, Pb, Zn) were determined after nitric/hydrochloric acid digestion using an atomic absorption spectrometer (AA500 Atomic Absorption, PG Instruments Ltd, USA). The trace metals standards were purchased from Accustandard (New Haven, USA). The R2 for the standard calibration curves of the trace metals were greater than or equal to 0.995.
3. Results and Discussion3.1. Physicochemical Properties of SoilThe Physicochemical characteristics of the soil used for the present study are presented in Table 1. As expected, the soil is generally classed as ultisols which are characterised by low nutrient and common in tropical regions in both Nigeria and China [3, 45, 46]. The soil belongs to the sandy textural class.
3.2. Functional Group Characterization of Bio-derived SurfactantsFunctional group characterization of the prepared bio-derived surfactants is essential to provide insight into their possible chemical properties and behavior. The FTIR spectra of the aqueous extract of Talinum triangulare and saponified Hura crepitans seed oil are presented in Fig. 2. For the aqueous extract of Talinum triangulare (AET), the following key absorption peaks recorded were: 3362.1 cm−1 (O-H bond from alcohols), 1,621.4 cm−1 (C=C from alkenes), 1438.8 cm−1 (C=C from aromatic compounds), 1,304.6 cm−1 (O=C-O-C from aromatic esters), 1084.7 cm−1 (C-O from alcohols or esters), 1,036.2 cm−1 (=C-O-C from aromatic ethers), 898.3 cm−1 (C-H from geminal disubstituted alkenes), and 790.2 cm−1 (C-H from trisubstituted alkenes). On the other hand, the soap prepared from saponification of oil from seeds of Hura crepitans (HCS) recorded the following major absorption peaks: 3,473.9 cm−1 (N-H from amides), 3,011.7 cm−1 (C-H from aromatic compounds), 2,922.2 cm−1, 2,855.1 cm-1, 1,461.1 cm−1, 1,379.1 cm−1, 723.1 cm−1 (C-H from alkanes), 1,744.4 cm−1 (C=O from esters or ketones), 1,237.5 cm−1 (=C-O-C from aromatic ethers), 1,159.2 cm−1 (C-O-C from ethers), 1,095.8 cm−1 (C-O from ethers or alcohols) and 913.2 cm−1 (=C-H from alkenes). Generally, based on the FTIR spectra of the two surfactants, AET recorded more peaks assigned to heteroatomic functional groups than HCS. HCS contained more saturated functional moieties than AET. One major explanation for the differences is that AET is aqueous-based, while HCS is oil-based. Surfactants with multiple heteroatom functionalities usually show superior surface activity [47–50]. In addition, previous reports recorded similar absorption peaks for aqueous extracts of Talinum triangulare [14, 51].
3.3. Surfactant Soil WashingThe effect of washing time was determined and the results are presented in Fig. 3. Generally, removal efficiencies tend to increase with increase in washing time. HCS recorded very low removal efficiencies (< 30%), while AET and Tween 80 recorded approximately 70–96% (Fig. 3). For HCS, as it can be observed in Fig. 3, there was no clear difference between the removal efficiencies of naphthalene and phenanthrene. However, AET tends to remove phenanthrene faster, although naphthalene recorded higher removal efficiency. Given the plateau nature of the graph for Tween 80 data, it seems that equilibrium was achieved earlier due to the fact that naturally derived surfactants possess lower CMCs compared to synthetic surfactants [52]. On the other hand, little changes are observed for the removal efficiencies of HCS over time. This implies that the performance of HCS is not influenced by time. As observed in a previous study, the efficiency of removing contaminants by soil washing increases with increase in washing time, but is later stabilized [7]. According to the study, the time-dependent relationship is caused by rate-limited dissolution and desorption of contaminants.
In an actual contaminated site, it may be necessary to determine the amount of remediant materials required for a certain level of contamination. We measured the removal efficiencies of different concentrations of naphthalene and phenanthrene in soils and the results are presented in Fig. 4. Generally, more contaminants led to higher removal efficiencies. Although beyond 100 mg/Kg the graphs flattened, the result is unusual since the same concentration was used. As explained in a previous study, certain amounts of contaminants could partition into the soil matrix, such that the amount removed depends on the partitioning of the overall concentration of the contaminant at any given time [22]. More so, the present soil used in this study recorded TOC > 0.30 (Table 1). Usually, for soils with organic content > 0.1, PAHs get partitioned into the soil organic matrix [53]. This observation was pronounced in AET and Tween 80 which showed high reactivity during the study. Therefore, to ensure complete removal, it is recommended to carry out soil washing more than once [18]. Mostly, naphthalene, which is of a lower molecular weight, was removed with higher removal efficiencies compared to phenanthrene. This may be due to easier diffusion. The adsorption data was fitted with first and second order pseudo-kinetic models and the results are presented in Fig. S2. From the results, we observed that the pseudo second order show better fitting with R2 > 0.98 for both naphthalene and phenanthrene in all applied surface-active materials. This implies that PAHs-soil-surfactant interactions were beyond physisorption and could be better classified as chemisorption process.
Different surfactant concentrations were used to test the removal efficiencies after 15 min washing time. We observed that the removal efficiencies increased with surfactant concentrations (Fig. 5). In this case, phenanthrene recorded higher removal efficiencies compared to naphthalene. One explanation to this observation could be that phenanthrene with higher octanol-water partition coefficient (log Kow = 4.46) than that of naphthalene (log Kow 3.36) will likely dissolve more in organic phase of the surfactant solutions and gets easily dissolved while some part of naphthalene remains in the aqueous phase [54]. Another explanation is that appreciable amounts of naphthalene could have been lost by evaporation since it is lighter than phenanthrene such that the amounts available for removal was less [55]. Again, higher removal efficiencies were recorded by Tween 80, followed by AET, and lastly HCS. The results demonstrate the superior performance of AET which has been characterized to contain saponins. This implies that saponin-based surfactants may be more suitable for removal of PAHs from contaminated soils compared to oil-based soaps. In a previous study, saponin-based surfactant has been shown to compete favorably with Tween 80 in soil washing of phenanthrene [18]. Other researchers have reported the use of oil alone from plant substrates to achieve removal efficiencies above 80% [53, 56]. When soybean oil was used for removal of anthracene from contaminated soil, it was reported that removal efficiencies decreased with increase in initial anthracene concentration [57]. It seems therefore, that preparation of the soap further introduces additional materials that may lead to high viscosity of the materials and consequent clogging. Furthermore, previous studies have established that light permeability, hydrogen donor capability of soil washing agents significantly influenced their performance for removal of organic pollutants from soils [58]. In this study, AET is more transparent than HCS, which may permit photo-induced attenuation of the contaminants.
3.4. Trace Metals DistributionThe trace metals characteristics of the soil before and after surfactant washing are presented in Table 2. The choice of the three trace metals was based on their usual occurrence in urban agricultural soils. With the exception of zinc, it can be observed from the results that cadmium and lead exceeded the World Health Organization (WHO) allowable levels in agricultural soils (Table 2). Since it is an urban area, the source of contamination may be attributed to anthropogenic activities such as transport-related emissions and atmospheric deposition from industrial processes [59–61]. The soil washing experiments significantly affected the distribution of trace metals levels. A cursory visualization of the data reveals that after soil washing, the cadmium levels significantly reduced in all treatments. In the case of lead (Pb) and zinc levels, only treatments with AET and Tween 80 recorded significantly lower values. Generally, the levels of trace metals were lowest in soils treated with AET, followed by Tween 80 and HCS. Tween 80 is a hydrophilic nonionic surfactant that has been used mostly for remediation of organic pollutants [62]. The nonionic nature of Tween 80 may have limited its reactivity with the negatively charged soil matrix [63]. As such, the acidic nature of the soil (Table 1) may not have changed significantly which allowed a significant amount of the trace metals to remain mobile in the soil. On the other hand, AET with a lot of heteroatomic moieties as established from the FTIR spectra (Fig. 2) provides a lot of adsorption sites for metal adsorption and subsequent extraction [64]. Also, it has been established from previous research that AET demonstrates acidic soil pH moderation which may result in immobilization of the trace metals [39]. For HCS, clogging of the soil matrix and weak reactivity may have accounted for the low metal extraction.
4. ConclusionsThe removal of trace metals and PAHs from soil using aqueous extracts of Talinum triangulare (AET) and soap solution prepared from Hura crepitans seed oil saponified by aqueous solution of ashed plantain peels (HCS) was investigated. Based on the results obtained, AET recorded removal efficiencies for naphthalene and phenanthrene slightly comparable with commercial Tween 80. Clogging of soil matrix and limited heteroatomic moieties in HCS may have resulted in poor removal efficiencies for naphthalene and phenanthrene. In most cases, the lower molecular weight naphthalene tends to record more removal efficiencies compared to phenanthrene. Similarly, AET recorded better removal of trace metals due to its soil pH moderation ability and availability of more adsorption sites.
AcknowledgmentsThe first author (N.O.O.) acknowledges the Petroleum Technology Development Fund (PTDF) of Nigeria for foreign scholarship award (Award Ref: PTDF/ED/PHD/NPO/5/18).
This project was financially supported by the National Key R&D Program of China (No. 2020YFC1808202) and National Natural Science Foundation of China (Grant No. 42077167).
NotesAuthor Contributions N.O.O. (Ph.D) visualised and carried out the experiments, literature search, data curation, analysis, interpretation, and wrote the original draft. G.J.U. (Ph.D) carried out some analysis, carried out data interpretation, and contributed to the final manuscript. E.J.I. (Associate Professor) provided resources for the experiments and contributed to the final manuscript. A.N.E. (Ph.D) provided resources for the experiments and contributed to the final manuscript. J.J.A. (Ph.D) provided resources for the experiments and contributed to the final manuscript. E.J.U. (Ph.D) provided resources for the experiments and contributed to the final manuscript. J.D. (Professor) conceptualised and supervised the project, acquired funding, provided resources for the experiments and contributed to the final manuscript. All authors read and approved the final version. References1. Offiong NAO, Inam EJ, Etuk HS, et al. Current status and challenges of remediating petroleum-derived PAHs in soils: Nigeria as a case study for developing countries. Remediation. 2019;30:65–75.
https://doi.org/10.1002/rem.21630
2. Prendergast DP, Gschwend PM. Assessing the performance and cost of oil spill remediation technologies. J Clean Prod. 2014;78:233–242.
https://doi.org/10.1016/j.jclepro.2014.04.054
3. Offiong N-AO, Inam EJ, Etuk HS, et al. Biochar and humus sediment mixture attenuates crude oil-derived PAHs in a simulated tropical ultisol. SN Appl Sci. 2020;2:
https://doi.org/10.1007/s42452-020-03744-5
4. Liu Z, Laha S, Luthy RG. Surfactant solubilization of polycyclic aromatic hydrocarbon compounds in soil-water suspensions. Water Sci Technol. 1991;23:475–485.
https://doi.org/10.2166/wst.1991.0447
5. Sahle-Demessie E, Grosse DW, Bates ER. Solvent extraction and soil washing treatment of contaminated soils from wood preserving sites: Bench-scale studies. Remediation. 2000;10:85–109.
https://doi.org/10.1002/rem.3440100308
6. Mulligan CN, Yong RN, Gibbs BF. Surfactant-enhanced remediation of contaminated soil: A review. Eng Geol. 2001;60:371–380.
https://doi.org/10.1016/S0013-7952(00)00117-4
7. Peng S, Wu W, Chen J. Removal of PAHs with surfactant-enhanced soil washing: Influencing factors and removal effectiveness. Chemosphere. 2011;82:1173–1177.
https://doi.org/10.1016/j.chemosphere.2010.11.076
8. Cheng M, Zeng G, Huang D, et al. Advantages and challenges of Tween 80 surfactant-enhanced technologies for the remediation of soils contaminated with hydrophobic organic compounds. Chem Eng J. 2017;314:98–113.
https://doi.org/10.1016/j.cej.2016.12.135
9. Li Y, Liao X, Huling SG, et al. The combined effects of surfactant solubilization and chemical oxidation on the removal of polycyclic aromatic hydrocarbon from soil. Sci Total Environ. 2019;647:1106–1112.
https://doi.org/10.1016/j.scitotenv.2018.07.420
10. Karthick A, Roy B, Chattopadhyay P. A review on the application of chemical surfactant and surfactant foam for remediation of petroleum oil contaminated soil. J Environ Manage. 2019;243:187–205.
https://doi.org/10.1016/j.jenvman.2019.04.092
11. Ali N, Bilal M, Khan A, et al. Effective exploitation of anionic, nonionic, and nanoparticle-stabilized surfactant foams for petroleum hydrocarbon contaminated soil remediation. Sci Total Environ. 2020;704:135391.
https://doi.org/10.1016/j.scitotenv.2019.135391
12. Tmáková L, Sekretár S, Schmidt Š. Plant-derived surfactants as an alternative to synthetic surfactants: Surface and antioxidant activities. Chem Pap. 2015;70:188–196.
https://doi.org/10.1515/chempap-2015-0200
13. Wisetkomolmat J, Suppakittpaisarn P, Sommano SR. Detergent plants of Northern Thailand: Potential sources of natural saponins. Resources. 2019;8:1–14.
https://doi.org/10.3390/resources8010010
14. Wisetkomolmat J, Suksathan R, Puangpradab R, et al. Natural surfactant saponin from tissue of litsea glutinosa and its alternative sustainable production. Plants. 2020;9:1–15.
https://doi.org/10.1080/10408398.2022.2025574
15. Pradhan A, Bhattacharyya A. Quest for an eco-friendly alternative surfactant: Surface and foam characteristics of natural surfactants. J Clean Prod. 2017;150:127–134.
https://doi.org/10.1016/j.jclepro.2017.03.013
16. Kommalapati RR, Valsaraj KT, Constant WD, et al. Aqueous solubility enhancement and desorption of hexachlorobenzene from soil using a plant-based surfactant. Water Res. 1997;31:2161–2170.
https://doi.org/10.1016/S0043-1354(97)00052-3
17. Roy D, Kommalapati RR, Mandava SS, et al. Soil washing potential of a natural surfactant. Environ Sci Technol. 1997;31:670–675.
https://doi.org/10.1021/es960181y
18. Zhou W, Wang X, Chen C, et al. Enhanced soil washing of phenanthrene by a plant-derived natural biosurfactant, Sapindus saponin. Colloid Surfac A Physicochem Eng Asp. 2013;425:122–128.
https://doi.org/10.1016/j.colsurfa.2013.02.055
19. YE M, SUN M, XIE S, et al. Feasibility of Tea Saponin-Enhanced Soil Washing in a Soybean Oil-Water Solvent System to Extract PAHs/Cd/Ni Efficiently from a Coking Plant Site. Pedosphere. 2017;27:452–464.
https://doi.org/10.1016/S1002-0160(17)60341-2
20. Greish S, Rinnan Å, Marcussen H, et al. Interaction mechanisms between polycyclic aromatic hydrocarbons (PAHs) and organic soil washing agents. Environ Sci Pollut Res. 2018;25:299–311.
https://doi.org/10.1007/s11356-017-0374-7
21. Liang X, Dong J, Wei G, et al. Colloidal biliquid aphron demulsification using polyaluminum chloride and density modification of DNAPLs: Optimal conditions and common ion effect. Environ Sci Process Impacts. 2020;22:1908–1915.
https://doi.org/10.1039/D0EM00248H
22. Chi FH, Leu MH, Tsao CW, et al. Removal of anthracene contaminated soil using microemulsified solvent and mixed surfactant. Sustain Environ Res. 2011;21:181–186.
23. Dar AA, Rather GM, Das AR. Mixed Micelle Formation and Solubilization Behavior toward Polycyclic Aromatic Hydrocarbons of Binary and Ternary Cationic–Nonionic Surfactant Mixtures. J Phys Chem B. 2007;111:3122–3132.
https://doi.org/10.1021/jp066926w
24. Ashraf U, Lone MS, Masrat R, et al. Co-solubilization of polycyclic aromatic hydrocarbon mixtures in aqueous micellar systems and its correlation with FRET for enhanced remediation processes. Chemosphere. 2020;242:125160.
https://doi.org/10.1016/j.chemosphere.2019.125160
25. Sales PS, Fernández MA. Synergism in the desorption of polycyclic aromatic hydrocarbons from soil models by mixed surfactant solutions. Environ Sci Pollut Res. 2016;23:10158–10164.
https://doi.org/10.1007/s11356-016-6242-z
26. Liang X, Zhang M, Guo C, et al. Competitive solubilization of low-molecular-weight polycyclic aromatic hydrocarbons mixtures in single and binary surfactant micelles. Chem Eng J. 2014;244:522–530.
https://doi.org/10.1016/j.cej.2014.01.097
27. Liang X, Guo C, Liao C, et al. Drivers and applications of integrated clean-up technologies for surfactant-enhanced remediation of environments contaminated with polycyclic aromatic hydrocarbons (PAHs). Environ Pollut. 2017;225:129–140.
https://doi.org/10.1016/j.envpol.2017.03.045
28. Wei J, Huang G, Zhu L, et al. Enhanced aqueous solubility of naphthalene and pyrene by binary and ternary Gemini cationic and conventional nonionic surfactants. Chemosphere. 2012;89:1347–1353.
https://doi.org/10.1016/j.chemosphere.2012.05.091
29. Yadav T, Tikariha D, Lakra J, et al. Solubilization of polycyclic aromatic hydrocarbons in structurally different gemini and monomeric surfactants: A comparative study. J Mol Liq. 2015;204:216–221.
https://doi.org/10.1016/j.molliq.2015.01.015
30. Böttcher S, Drusch S. Saponins — Self-assembly and behavior at aqueous interfaces. Adv Colloid Interface Sci. 2017;243:105–113.
https://doi.org/10.1016/j.cis.2017.02.008
31. Davin M, Starren A, Deleu M, et al. Could saponins be used to enhance bioremediation of polycyclic aromatic hydrocarbons in aged-contaminated soils? Chemosphere. 2018;194:414–421.
https://doi.org/10.1016/j.chemosphere.2017.11.174
32. Vassallo A, Armentano MF, Miglionico R, et al. Hura crepitans L. Extract: Phytochemical Characterization, Antioxidant Activity, and Nanoformulation. Pharmaceutics. 2020;12:553.
https://doi.org/10.3390/pharmaceutics12060553
33. Oraegbunam JC, Oladipo B, Falowo OA, et al. Clean sandbox (Hura crepitans) oil methyl esters synthesis: A kinetic and thermodynamic study through pH monitoring approach. Renew Energy. 2020;160:526–537.
https://doi.org/10.1016/j.renene.2020.06.124
34. Oniya OO, Oyelade JO, Ogunkunle O, et al. Optimization of Solvent Extraction of Oil from Sandbox Kernels ( Hura Crepitans L.) by a Response Surface Method. Energy Policy Res. 2017;4:36–43.
https://doi.org/10.1080/23815639.2017.1324332
35. Ibrahim AP, Omilakin RO, Betiku E. Optimization of microwave-assisted solvent extraction of non-edible sandbox (Hura crepitans) seed oil: A potential biodiesel feedstock. Renew Energy. 2019;141:349–358.
https://doi.org/10.1016/j.renene.2019.04.010
36. Ezeh IE, Umoren SA, Essien EE, et al. Studies on the utilization of Hura crepitans L. seed oil in the preparation of alkyd resins. Ind Crops Prod. 2012;36:94–99.
https://doi.org/10.1016/j.indcrop.2011.08.013
37. Swarna J, Lokeswari TS, Smita M, et al. Characterisation and determination of in vitro antioxidant potential of betalains from Talinum triangulare (Jacq.) Willd. Food Chem. 2013;141:4382–4390.
https://doi.org/10.1016/j.foodchem.2013.06.108
38. Ikewuchi CC, Ikewuchi JC, Ifeanacho MO. Bioactive phytochemicals in an aqueous extract of the leaves of Talinum triangulare. Food Sci Nutr. 2017;5:696–701.
https://doi.org/10.1002/fsn3.449
39. Offiong N-AO, Fatunla OK, Essien JP, et al. Soil washing of total petroleum and polycyclic aromatic hydrocarbons from crude oil contaminated ultisol using aqueous extracts of waterleaf. Environ Technol. 2021;1–24.
https://doi.org/10.1080/09593330.2021.1961875
40. López-Vizcaíno R, Sáez C, Cañizares P, et al. The use of a combined process of surfactant-aided soil washing and coagulation for PAH-contaminated soils treatment. Sep Purif Technol. 2012;88:46–51.
https://doi.org/10.1016/j.seppur.2011.11.038
41. Zhang D, Zhu L. Effects of Tween 80 on the removal, sorption and biodegradation of pyrene by Klebsiella oxytoca PYR-1. Environ Pollut. 2012;164:169–174.
https://doi.org/10.1016/j.envpol.2012.01.036
42. Lan Chun C, Lee JJ, Park JW. Solubilization of PAH mixtures by three different anionic surfactants. Environ Pollut. 2002;118:307–313.
https://doi.org/10.1016/S0269-7491(01)00304-9
43. Peng S, Wu W, Chen J. Removal of PAHs with surfactant-enhanced soil washing: Influencing factors and removal effectiveness. Chemosphere. 2011;82:1173–1177.
https://doi.org/10.1016/j.chemosphere.2010.11.076
44. APHA. Standard Methods for the Examination of Water and Wastewater. 23rd EditionWashingtin DC: American Public Health Association (APHA); 2017.
45. Peng X, Ye LL, Wang CH, et al. Temperature-and duration-dependent rice straw-derived biochar: Characteristics and its effects on soil properties of an Ultisol in southern China. Soil Tillage Res. 2011;112:159–166.
https://doi.org/10.1016/j.still.2011.01.002
46. Offiong N-AO, Inam EJ, Etuk HS, et al. Trace Metal Levels and Nutrient Characteristics of Crude Oil-Contaminated Soil Amended with Biochar-Humus Sediment Slurry. Pollutants. 2021;1:119–126.
https://doi.org/10.3390/pollutants1030010
47. Alami EO, Holmberg K. Heterogemini surfactants. Adv Colloid Interface Sci. 2003;100–102:13–46.
https://doi.org/10.1016/S0001-8686(02)00072-6
48. Liu S, Sang R, Hong S, et al. A novel type of highly effective nonionic gemini Alkyl O-glucoside surfactants: A versatile strategy of design. Langmuir. 2013;29:8511–8516.
https://doi.org/10.1021/la401569n
49. Ao M, Huang P, Xu G, et al. Aggregation and thermodynamic properties of ionic liquid-type gemini imidazolium surfactants with different spacer length. Colloid Polym Sci. 2009;287:395–402.
https://doi.org/10.1007/s00396-008-1976-x
50. Karaborni S, Esselink K, Hilbers PAJ, et al. Simulating the Self-Assembly of Gemini (Dimeric) Surfactants. Science. 1994;266:254–256.
https://doi.org/10.1126/science.266.5183.254
51. Almutairi MS, Ali M. Direct detection of saponins in crude extracts of soapnuts by FTIR. Nat Prod Res. 2015;29:1271–1275.
https://doi.org/10.1080/14786419.2014.992345
52. Pradhan A, Bhattacharyya A. Quest for an eco-friendly alternative surfactant: Surface and foam characteristics of natural surfactants. J Clean Prod. 2017;150:127–134.
https://doi.org/10.1016/j.jclepro.2017.03.013
53. Gong Z, Alef K, Wilke B-M, et al. Dissolution and removal of PAHs from a contaminated soil using sunflower oil. Chemosphere. 2005;58:291–298.
https://doi.org/10.1016/j.chemosphere.2004.07.035
54. Inam E, Etuk I, Offiong N-A, et al. Distribution and ecological risks of polycyclic aromatic hydrocarbons (PAHs) in sediments of different tropical water ecosystems in Niger Delta, Nigeria. Environ Earth Sci. 2018;77:
https://doi.org/10.1007/s12665-018-7396-4
55. Karaca G, Cindoruk SS, Tasdemir Y. Migration of polycyclic aromatic hydrocarbons (PAHs) in urban treatment sludge to the air during PAH removal applications. J Air Waste Manage Assoc. 2014;64:568–577.
https://doi.org/10.1080/10962247.2013.874380
56. Pannu JK, Singh A, Ward OP. Vegetable oil as a contaminated soil remediation amendment: application of peanut oil for extraction of polycyclic aromatic hydrocarbons from soil. Process Biochem. 2004;39:1211–1216.
https://doi.org/10.1016/S0032-9592(03)00254-1
57. Chi F-H, Leu M-H, Lee R-C. Removal of anthracene contaminated soil using soybean oil. Sustain Environ Res. 2010;20:275–280.
58. ISOSAARI P, LAINE O, TUHKANEN T, et al. Photolysis of polychlorinated dibenzo-p-dioxins and dibenzofurans dissolved in vegetable oils: influence of oil quality. Sci Total Environ. 2005;340:1–11.
https://doi.org/10.1016/j.scitotenv.2004.08.007
59. Davydova S. Heavy metals as toxicants in big cities. Microchem J. 2005;79:133–136.
https://doi.org/10.1016/j.microc.2004.06.010
60. Ercilla-Montserrat M, Muñoz P, Montero JI, et al. A study on air quality and heavy metals content of urban food produced in a Mediterranean city (Barcelona). J Clean Prod. 2018;195:385–395.
https://doi.org/10.1016/j.jclepro.2018.05.183
61. Luo X, Yu S, Zhu Y, et al. Trace metal contamination in urban soils of China. Sci Total Environ. 2012;421–422:17–30.
https://doi.org/10.1016/j.scitotenv.2011.04.020
62. Udo GJ, Offiong N-AO, Nwadinigwe A, et al. Efficiency and Kinetics of Total Petroleum Hydrocarbons (TPHs) Removal from Crude Oil Polluted Arable Soil using Palm Bunch Ash and Tween 80. Chem Africa. 2021;4:333–337.
https://doi.org/10.1007/s42250-020-00219-3
63. LEE J, HSU M, CHAO H, et al. The effect of surfactants on the distribution of organic compounds in the soil solid/water system. J Hazard Mater. 2004;114:123–130.
https://doi.org/10.1016/j.jhazmat.2004.07.016
64. Feng C, Chen Y, Zhang S, et al. Removal of lead, zinc and cadmium from contaminated soils with two plant extracts: Mechanism and potential risks. Ecotoxicol Environ Saf. 2020;187:109829.
https://doi.org/10.1016/j.ecoenv.2019.109829
65. Kinuthia GK, Ngure V, Beti D, et al. Levels of heavy metals in wastewater and soil samples from open drainage channels in Nairobi, Kenya: community health implication. Sci Rep. 2020;10:8434.
https://doi.org/10.1038/s41598-020-65359-5
Fig. 2FTIR spectra of (a) aqueous extract of Talinum triangulare and (b) saponified Hura crepitans seed oil. 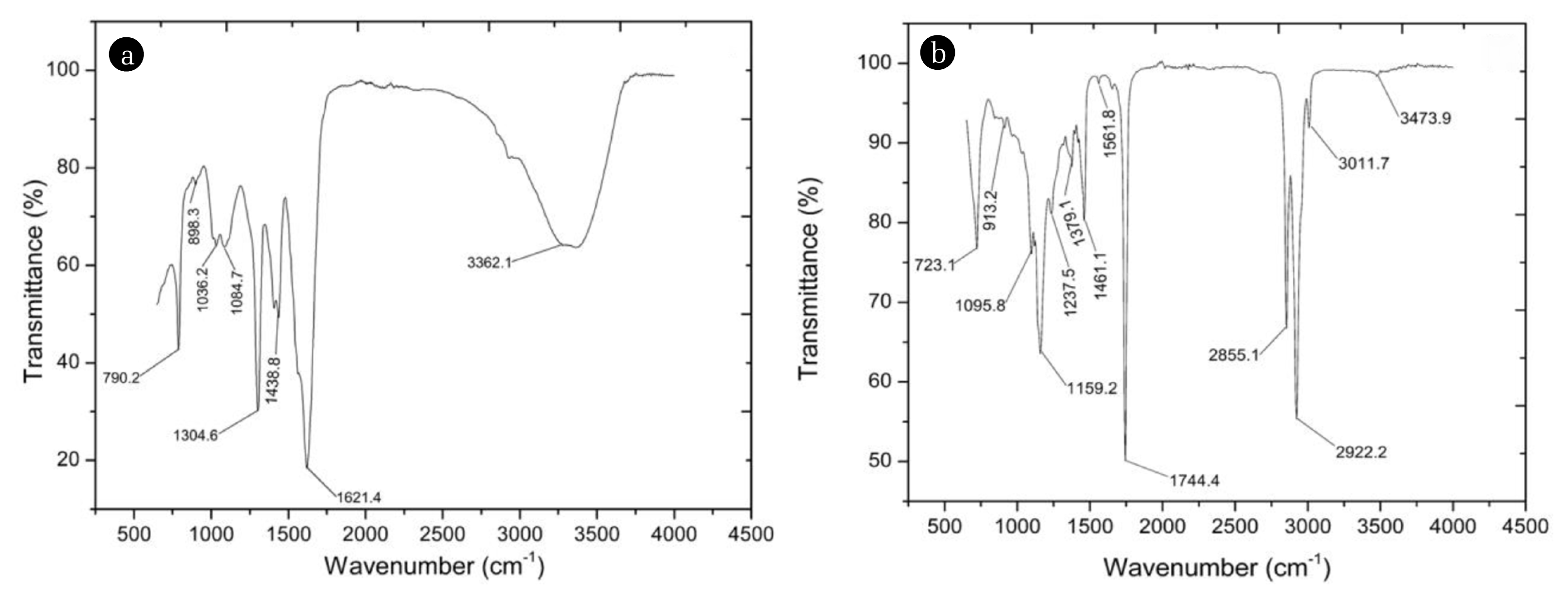
Fig. 3Effect of washing time on removal efficiencies of naphthalene (Naph) and phenanthrene (Phen) using different surface-active agents. Experimental conditions: 100 mg/Kg of contaminants, 30 mg/L of both HCS and Tween 80 and 50% (vol/vol) of AET. 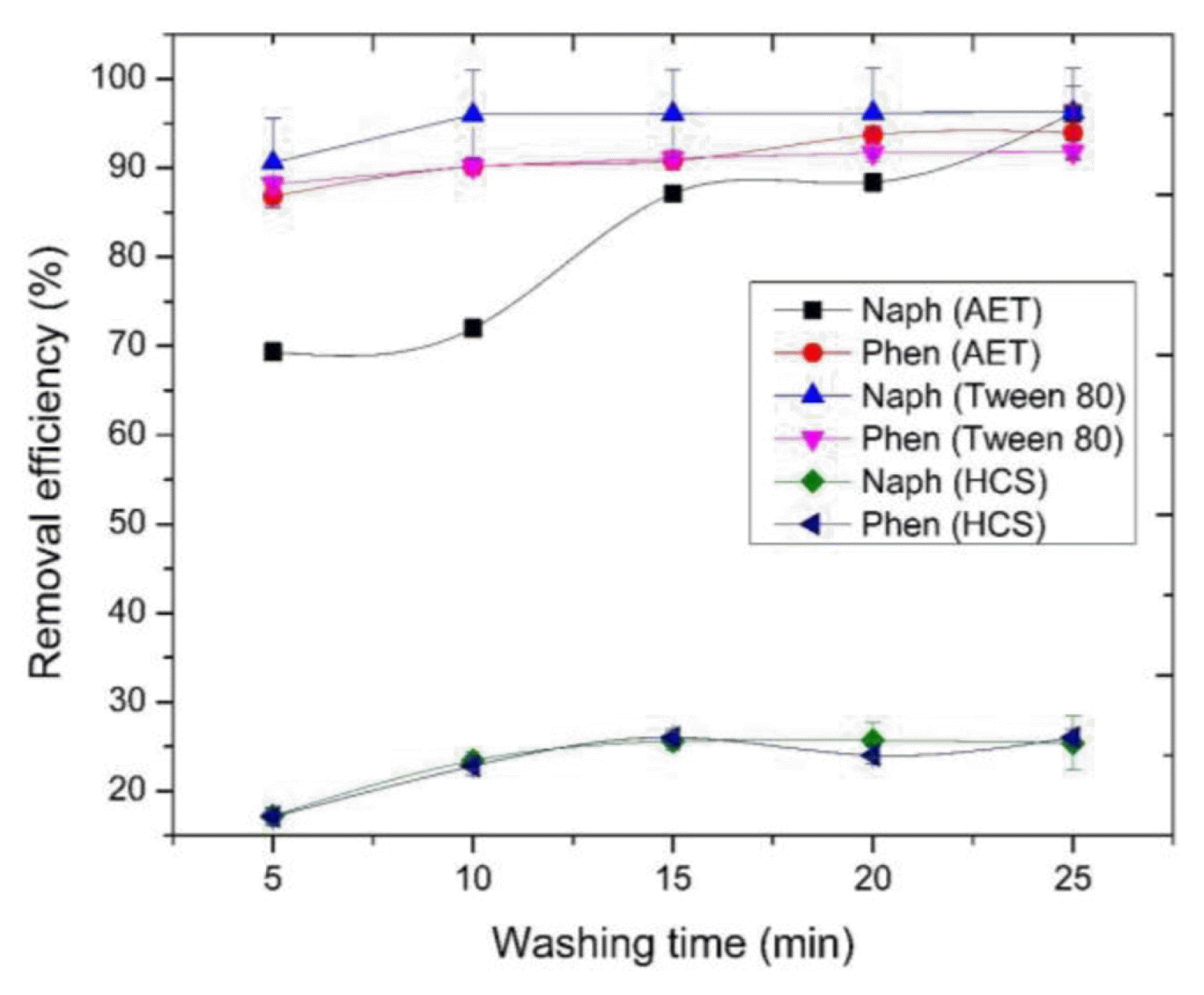
Fig. 4Effect of contaminant dose on removal efficiencies of naphthalene (Naph) and phenanthrene (Phen) using different surface-active agents. Experimental conditions: washing time of 25 min, 30 mg/L of both HCS and Tween 80 and 50 % (vol/vol) of AET. 
Fig. 5Effect of surfactant concentration on removal efficiencies of naphthalene (Naph) and phenanthrene (Phen) using different surface-active agents. Experimental conditions: washing time of 15 min, 30 mg/L of both HCS and Tween 80 and 50 % (vol/vol) of AET. 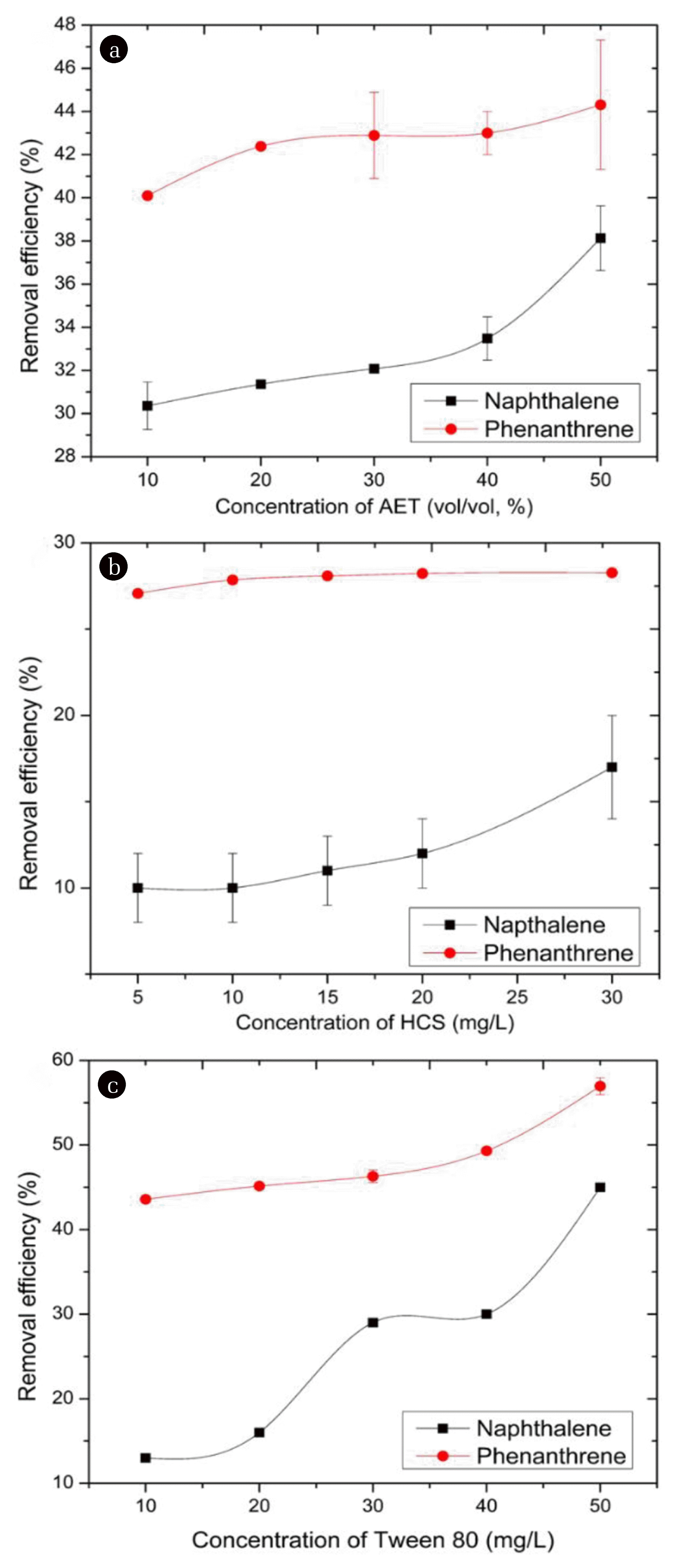
Table 1Physicochemical Properties of Soil
Table 2Trace Metal Levels in Soil before and after Washing Treatments
Source: Kinuthia et al. [65]; values with the same letter are not significantly different (p > 0.05); values with different letters are significantly different (p < 0.05) |
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||