1. Introduction
Nitrogenous heterocyclic compounds (NHCs) releases into a water/wastewater stream due to its wide applicability in chemical industries such as coal tar, dye, fertilizer, insecticides, and pharmaceuticals [1]. Because of nitrogen-atom included in the ring system, these compounds have high water solubility. NHCs have been listed as hazardous and toxic chemical due to its adverse impact on the natural environment. These compounds are toxic, potentially carcinogenic and mutagenic properties even at low concentration of NHCs in the aqueous stream [2]. Among these compounds, Py and Qn selected as a model compounds under NHCs category. Furthermore, the Py has listed as a priority of organic pollutant by the United States Environmental Protection Agency (USEPA). It was reported that shale oil process water contains Py and Qn in the range of 20 ± 100 mg/L [3].
Numerous studies have been reported for the treatment of NHCs by biological processes such as aerobic and anaerobic process. Limited success was achieved by the biological process because it requires long residence time to degrade NHCs [4, 5]. Moreover, NHCs are hard to remove and are toxic to microbial communities. For medium strength wastewater, the thermal treatment process (incineration) is not a feasible option because of the high energy requirement [6].
Therefore, an advanced oxidation process is one of the alternative for the treatment of refractory organic compounds [7]. Among various advanced oxidation process, catalytic wet peroxidation (CWPO) received rapid attention because it may non-selectively degrade the non-biodegradable compounds into harmless or simple compounds in the presence of oxidant using homogeneous/heterogeneous catalyst under atmospheric pressure and moderate temperature (below 80°C) [8–10]. In recent years many researchers are focused on the development of a novel heterogeneous catalyst for CWPO process. Nanoscale iron particles (nFe0) stand for a new generation of environmental remediation technologies [11]. This nFe0 possess distinctive chemical, electronic, magnetic, mechanical, optical and catalytic properties have drawn immense attention on zero-valent iron [12]. And also, provide cost-effective solutions to most exigent environmental cleanup problems. Nowadays, most of the scientists are paying attention to dispersibility of nFe0 on various support materials, particularly on porous materials in order increase the available surface area for reaction, enhance the stability, and minimize the agglomeration and leaching [13, 14]. In the present study nanoscale zerovalent iron supported on granular activated carbon (nFe0/GAC or nZVI/GAC) was synthesized and subjected to CWPO of NHCs.
In recent years, researchers are focused on the statistical experimental approach. It is a very useful technique for the optimization of any process conditions using mathematical models and also to determine the significant parameters and their interaction with other parameters by conducting the minimum number of experiments. Traditional optimization methods are a time-consuming process; they can evaluate only one factor at a time. Further, it requires a large number of experimental runs to find out the optimum level [15]. Box-Behnken design (BBD) is one of the statistical optimization tools for analysis of multiple variables, with a limited number of experiments [16]. The merits of BBD over other design methods are it avoids the extreme conditions that can skew the outcome. Therefore, all factors are simultaneously tested at their lowest levels to the highest level.
The objective of this study is to investigate the significant operating parameters for the simultaneous degradation of NHCs (Py-Qn) by CWPO process using BBD. The design consists of five factors and three levels of each factor, which are used to optimize the TOC removal by nFe0/GAC.
2. Materials and Methods
2.1. Material
All chemicals used in these studies were of analytical reagent (AR) grade without any additional purification. Quinoline, pyridine, ferrous sulfate heptahydrate, ethanol, NaOH and H2SO4, were obtained from SD fine chemicals, India. Hydroxylamine hydrochloride, sodium borohydride, 1,10-phenanthroline were purchased from Sigma Aldrich. 30–33% hydrochloric acid, dichloromethane, acetic acid and 30% hydrogen peroxide were procured from Rankem, India. All analytical standards were prepared from Millipore water.
2.2. Synthesis of 20% nFe0/GAC
Sodium borohydride method was used for the synthesis of nanoscale zero-valent iron incorporated on granular activated carbon (20% nFe0/GAC) [14]. The commercial grade GAC was washed with distilled water to remove impurities and soaked in hot distilled water at 100°C to increase the activation of pores. Soaked activated carbon filtered and dried in an oven at 120°C for overnight. 9.956 g of FeSO4.7H2O was used as an iron precursor mixed with ethanol and water solution (80 mL ethanol + 20 mL water) with continuous stirring for 10 min. After complete dissolution of the iron precursor in the solution, 8 g of prepared GAC was added to iron precursor solution, in order to disperse the ion species uniformly over the GAC pores, the solution was kept for 20 min in an ultrasonic shaker. Gosu et al. [18] reported the iron reduction by borohydride is followed.
In the above equation, Eq. (1),
indicates iron species incorporated in the GAC framework and
indicates as nanoscale zero-valent iron species incorporated in the GAC framework.
The strong reducing agent NaBH4 was dissolved in 100 mL ultra-pure deionized water based on stoichiometric quantity and further added into the sonicated solution with continuous stirring. The black solid particles immediately appeared during the dropwise addition of the reducing agent, which indicates the formation of Fe0 on granular activated carbon. The complete solution filtered by using a vacuum filtration unit and the filtrate washed with ethanol for two to three times to remove water. At 50°C, the synthesized nFe0/GAC were dried and designated as 20% nFe0/GAC or nZVI/GAC.
2.3. Batch Experimental Program
The experiments were carried out in a three-necked round bottom glass reactor equipped with a reflux system to condense the vapor samples and to minimize the experimental error. The reactor was kept inside the oil bath to maintain the uniform desired temperature. The whole setup was mounted on a magnetic stirrer with a hot plate (2MLH, REMI). The temperature of the reaction mixture was increased using a proportional–integral–derivative controller. The reaction mixture was uniformly maintained with a magnetic stirrer at 300 rpm. For each experimental run, a 100 mL reaction mixture initial pH was adjusted (2–10 pH) by using 0.1 N HCl and 0.1 N NaOH solution and then charged into the reactor. After that, the reaction mixture heated to 60°C, with the help of oil bath, and required quantities of catalyst dose (nFe0/GAC) and oxidant (hydrogen peroxide) were added to the reaction mixture. During the reaction, pH was not controlled. After completion of reaction 5 h, the sample in the reactor was filtered using 0.45 μm syringe membrane filter (PTFE-2545, MOXCARE), and the filtrate samples were analyzed with a TOC analyzer.
2.4. Analytical Methods
Mineralization of pyridine and quinoline was quantified with the help of total organic carbon (TOC) conversion. TOC was quantified by catalytic oxidation of organic compounds into CO2, the formed CO2 quantified using non-dispersive infrared (NDIR) detector by TOC-VCPH-analyzer (Shimadzu 5500A, Germany). The instrument measure the total carbon by combustion of the sample at 700°C over a Pt catalyst bed and total inorganic carbon was measured by treating the sample with 25% phosphoric acid. TOC obtained by subtracting total inorganic carbon from total carbon. TOC values present the average of at least two measurements, in some cases samples were measured three times by injecting three times which is evaluated with the TOC apparatus. For the quantification of Fe in the aqueous solution, all the experimental samples were digested and further iron reduced to the ferrous state using hydroxylamine hydrochloride. The 1,10-phenanthroline was used as a ligand that reacts with metal (Fe) to form a strongly coloured complex. The coloured complex was measured at intensity 510 nm with the help of UV visible spectrophotometer (HACH, DR 5000, USA) [17]. Each sample was measured triplicate and results are presented with ± 5% deviation from the average value.
2.5. Catalyst Characterization
Micromeritics ASAP 2020 instrument was used to determine the surface area and pore volume with the help of N2-adsorption and desorption isotherm. Samples were pretreated by degassing the samples at 150°C under the vacuum of 10–3 torr for 6 h. Micropore volume was calculated using the t-plot and surface area was calculated using the Brunauer-Emmett-Teller (BET) equation by assuming that all pores in the sample are cylindrical and parallel.
Scanning electron microscopy (SEM-501 Phillips, Holland) employed to analyze the surface morphology of synthesized catalyst by operating at acceleration voltage 15–25 kV and magnification values up to 40000X. Before SEM analysis, samples were pretreated by gold coating with sputter coater thereby enhances the conductivity of the samples.
2.6. Box–Behnken Design
In this study, three-level and five-factorial BBD design were employed to optimize the process variables resulting in maximum TOC removal and minimum Iron leaching. BBD estimates the critical operating condition by second-order multivariate. BBD design consist of variable combinations at the center and at middle points of the edges by rotatable quadratic design [15].
The design composed of 3 levels which were coded as −1 (low), 0 (central point or middle) and 1 (high). The process variables are initial pH (A), hydrogen peroxide dose (B), catalyst dose (C), Py concentration (CPy) (D) and Qn concentration (CQn) (E) shown in Table 1. The ranges of the process variables are selected based on the preliminary experiments. The statistical analysis was performed using Design-Expert™ software. The corresponding design matrix consists of 46 experiments with center point. The experimental design matrix by the BBD is tabulated in Table 2. To fit the mathematic model, analysis of variance (ANOVA) and multiple regression analysis were estimated. The Experimental data was analyzed with multiple regressions in order to fit the data in the second-order polynomial equation (Eq. (2)). It can be written as
Where Y is the response and A, B, C, D and E are process variables. A2, B2, C2, D2 and E2 are square of process variables, AB, AC, AD, AE, BC, BD, BE, CD and CE are interaction effect of process variables. β0 and ɛ are constants. β1, β2, β3, β4 and β5 are linear coefficients β12, β13, β14, β15, β23, β24, β25, β34 and β35 are interaction coefficients.
3. Result and Discussion
3.1. Catalyst Characterization
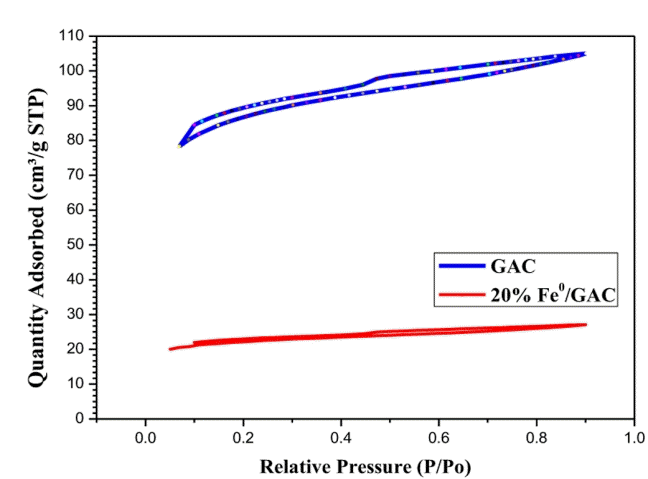
Surface area and textural properties of GAC and 20% nFe0/GAC was studied by N2 adsorption-desorption isotherms. Shape and behavior of the nitrogen adsorption-desorption isotherms of GAC and 20% nFe0/GAC are illustrated in Fig. 1. According to IUPAC classification, the GAC and 20% nFe0/GAC exhibits the type IV hysteresis based on nitrogen adsorption-desorption isotherms with the relative pressures (p/p0) in the range of 0.2 to 0.8 that may be attributed to the characteristic of mesoporous structure. In addition, the observations from the nitrogen adsorption-desorption loop that the adsorption branches of isotherm were horizontal and parallel to the desorption branches it indicates the possibility of the existence of slit pores in dominant form [19]. Passe-Coutrin et al. [20] reported that activated carbon exhibits type IV hysteresis due to the presence of ink-bottle type pore with slit type pore structure in the activated carbon framework. Table 3 depicted the textural properties such as surface area, pore volume and average pore diameter of the samples of bare GAC and nFe0/GAC. Moreover, nFe0 impregnated onto GAC decreased the corresponding BET surface area from 273 m2/g to 69 m2/g. In addition, the pore volume also decreased from 0.162 to 0.041 cm3/g. The above observation is due to the blocking of pores (growth of nFe0) of GAC by excess loading of nFe0 [18, 10].
Fig. 2 indicates the SEM image of GAC and 20% nFe0/GAC. The GAC images illustrate the porous nature and 20% nFe0/GAC shows the formation of the uniform film on the surface of GAC. At very high loading (above 10% weight) of active species (Iron) onto the porous support, the channels of porous material (GAC) get saturated with multilayer adsorption. Further, indicates the blocking of the channel which leads to the decrease in the surface area of the material. SEM image of 20% nFe0/GAC is good agreement with the results of the BET surface area. For bare GAC has a surface area of 273 m2/g and after loading of active metal the surface area was decreased drastically to 69 m2/g due to blocking of pores with an excess quantity of active metal. Subbaramaiah et al. [6] studied the different percentage (5%, 10% and 20%) of active metal (Cu) loading on SBA-15. At higher loading (20% Cu/SBA-15) of active metal the surface area was decreased drastically from 650 m2/g to 313 m2/g because of blocking of pores. The morphology of nFe0/GAC has been found as a uniform film, which is similar to trends reported in other studies [21].
3.2. Fitting Model and Analysis of Variance (ANOVA)
The optimum process parameters such as initial pH, catalyst dose, oxidant dose, the concentration of Py and Qn determined by BBD analysis. TOC removal and Fe leaching were used as response value to perform the analysis using RSM. The BBD design matrix for real values together with experimental and predicted values in terms of percentage removal of TOC and total Fe leaching are tabulated in Table 2. The results of BBD were used to perform the analysis of variance (ANOVA) using Expert Design software (trial version). A polynomial regression model was found to be the best suitable fit between the response variables and input process variables. The calculated regression model was a function of initial pH (A), hydrogen peroxide dose (B), catalyst dose (C), Py conc. (D), and Qn conc. (E). The best fit of the responses with coded factors are given below (Eq. (3) and (4)).
For each response, the adequacy of the model was justified with the analysis of variance (ANOVA) and the results are shown in Table S1(a) and (b), that the liability of the model was extremely significant. In the obtained regression models (Eq. (3) and (4)), the positive sign indicates the positive effect on the removal of TOC and total iron leaching, respectively. Similarly, negative coefficients signify the negative effect on the removal of TOC, and total iron leaching, respectively. The “Prob > F” is the probability that all the variation in the results are due to random error [22]. When “Prob > F” is less than 0.05, terms in the model have a significant effect on response listed in the Table S1(a) and (b). The significant model terms for TOC removal are A, B, C, E, AB, AC, AD, AE, BC, BD, BE, CD, DE, A2, B2, C2, D2 and E2. While, for total Fe leaching A, B, C, E, AB, AC, AD, AE, BE, CD, CE, DE, A2, B2, C2, D2 and E2 are significant model terms. In addition, values of “Prob > F” greater than 0.1 specifies the insignificant model terms. The F-values for TOC and total Fe leaching are 1594.82 and 75.89, which suggested that the terms in the model have a significant effect on response. The F-values could be larger due to noise. And the desirable adequate precision is always greater than four, and for TOC and total iron leaching it was found to be 198.018 and 38.75, respectively. Further affirmed the convenience of the model to navigate the design space. The P value represents the lack of fit which corresponds to 1.00 entail the insignificant as compared to the net error. Further regression model provides better knowledge in connection with five factors and their response [23].
3.3. Adequacy of the Test Model
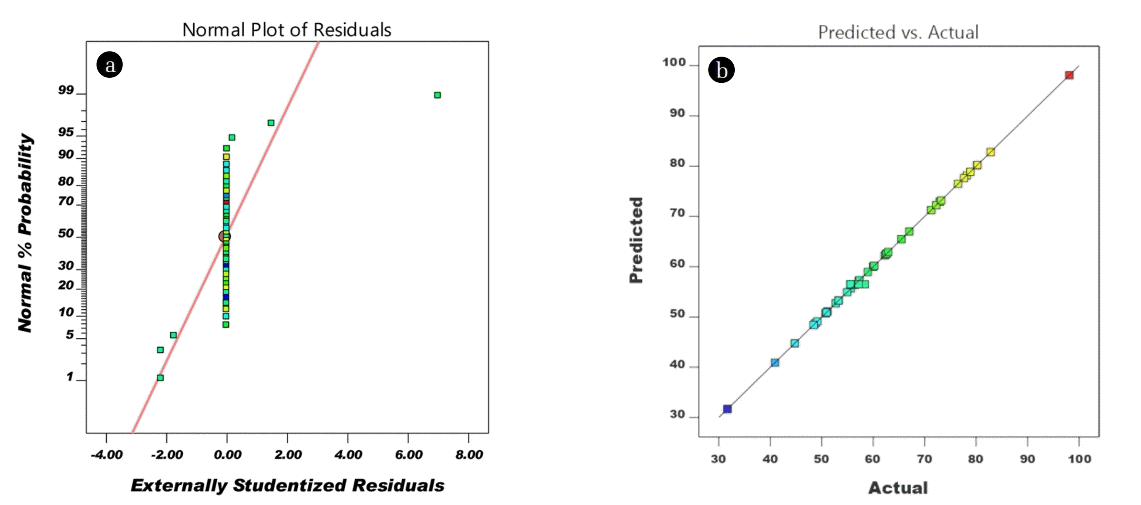
The diagnostic plot of actual versus predicted and normal probability plots are acquired from the adequacy of the model. These plots provide information between actual values and predicted values and also assist to evaluate the model fitness. From Fig. 3(b), the data points between the actual value and predicted values, located closed to a diametrical line attributed to good concurrence with the fitted model [24]. In addition, the prediction of the adequacy of the model can be determined by residual normality, Fig. 3(a) depicted the studentized residuals of a normal probability plot. No deviation was observed between observed residual and expected values [23, 25]. Two models namely i) the sequential model sum of squares, and ii) sequential model summary statistics were assessed to evaluate the adequacy of the test models (Table S2). Based on Table S2, the quadratic model is the best fit among other models. Furthermore, the R2 and adjusted R2 are closely related to each other which indicate a good correlation between the experimental and predicted values.
3.4. Effect of Parameters on Response
Simultaneous mineralization of Py and Qn by catalytic wet peroxidation process was carried out in a batch study in the presence of the heterogeneous catalyst (nFe0/GAC) using hydrogen peroxide as oxidant. In this process nanoscale zero-valent iron utilizes the available dissolved oxygen converted into hydrogen peroxide and Fe2+. The formed hydrogen peroxide, Fe2+, and externally added hydrogen peroxide lead to generation of hydroxyl radical and Fe3+, the generated hydroxyl radical has high oxidation potential and is responsible for the oxidation/mineralization of organic compound (NHCs) (R) present in the reaction mixture. The generated hydroxyl radicals are non-selectively degrade the organic compound (NHCs) (R) into intermediate compounds (R*) and further oxidation of these intermediate compounds (R*) into simple harmless compounds which are illustrated in below reaction scheme (Eqs. (5), (6) and (7)) [9, 18].
Where: R: Organic compound (NHCs); R*: Intermediate compounds
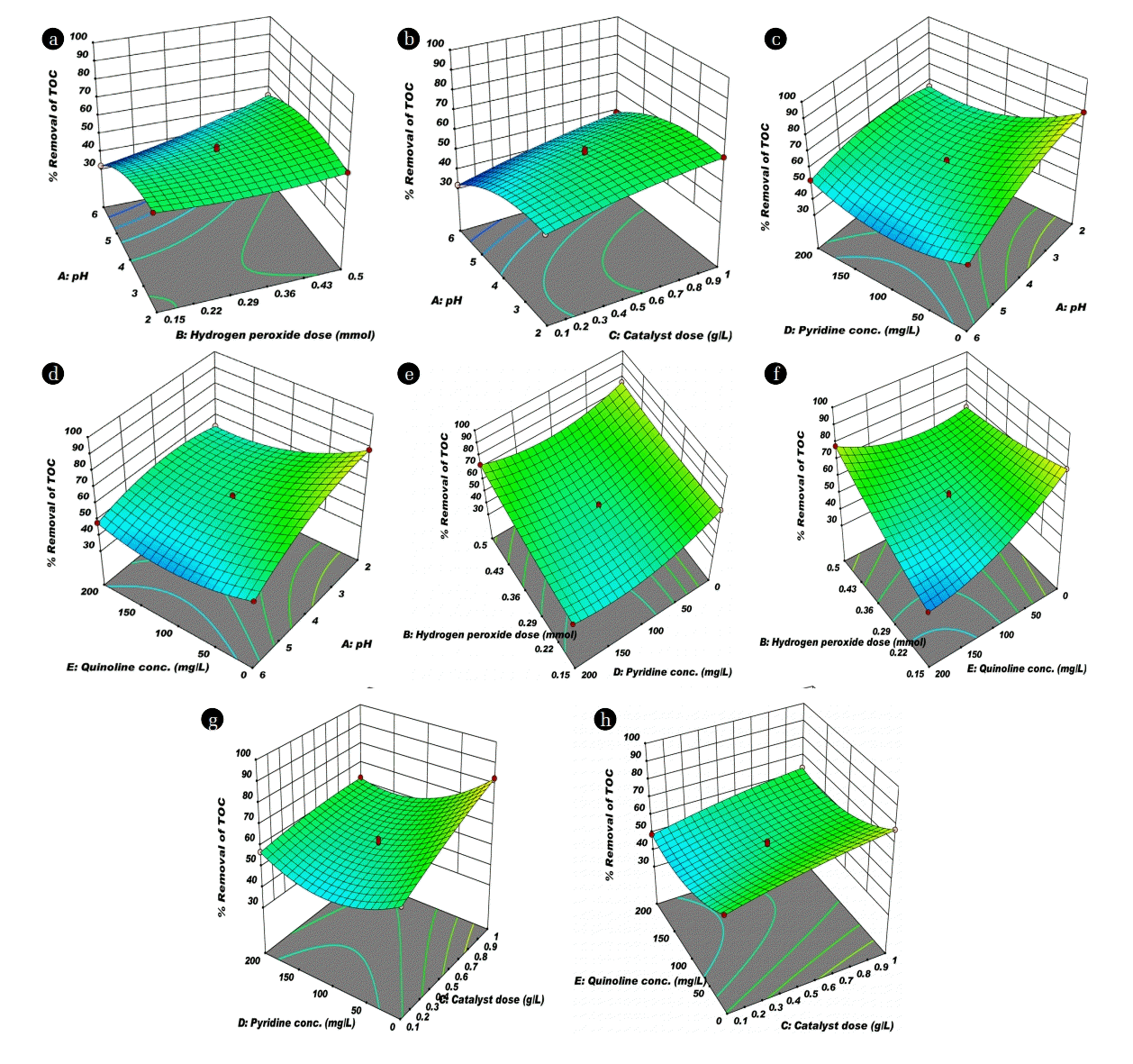
In order to gain the better understanding of the effects of independent variables and their interaction on TOC removal and total Fe leaching, 3D surface response plots and 2D contour plots were constructed based on the quadratic model. Moreover, 2D contour plots provide a straightforward assessment of the effects of experimental factors on the responses. In addition, an elliptical or saddle nature of the contour plots indicates the Interaction between the corresponding variables are significant [26]. Furthermore, each contour line designates the infinite number of arrangement of the selected process variables. The displayed plots reasonably interact with the process variables. All plots are nonlinear with selected process variables. Detailed effects of the process variable are discussed in the following section.
3.4.1. Effect of pH
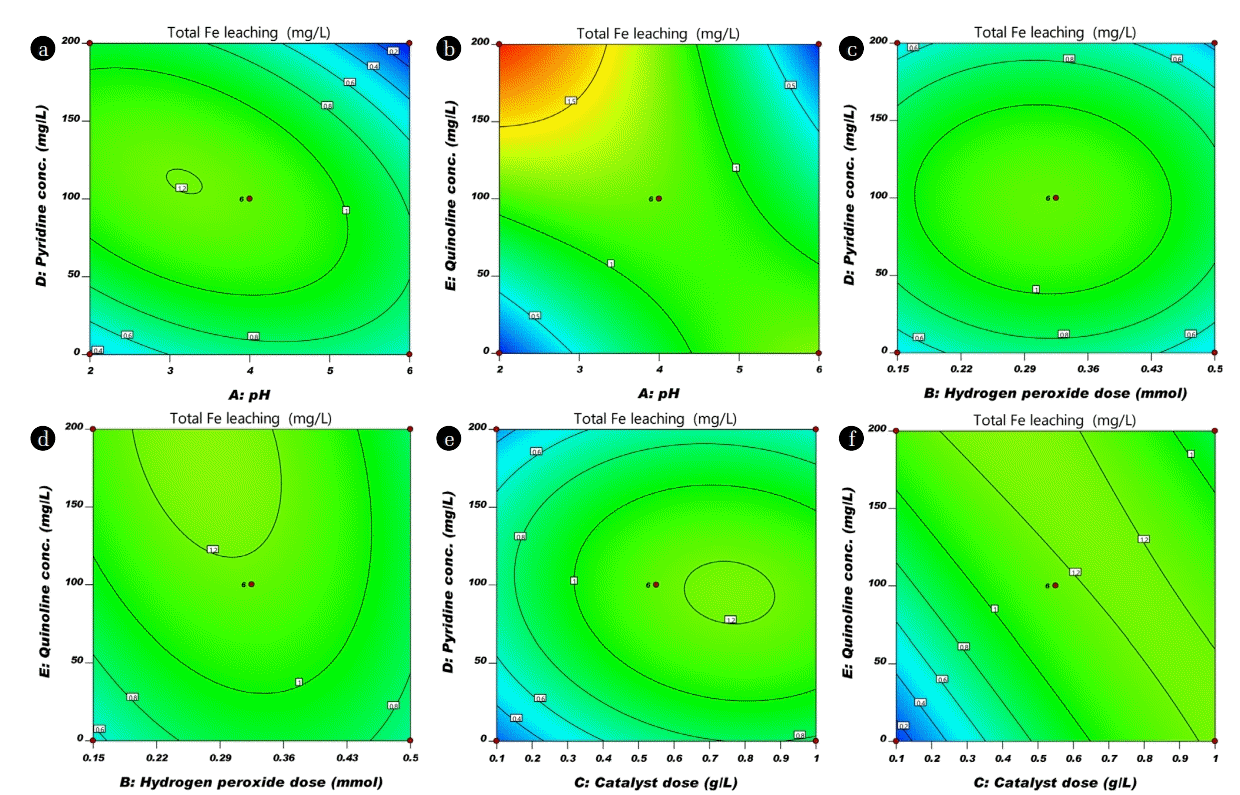
pH is one of the significant factor in CWPO process, which influences the performance of the oxidation process in the degradation of organic compounds. It plays an important role in controlling catalytic activity, stability of hydrogen peroxide and activity of iron species [27]. It can be seen in Fig. 4(a), (b), (c) and (d), that removal of TOC increased when initial pH value increased from 2–4. The low removal of TOC below pH 3 might be attributed to scavenging of hydroxyl radical with H+ ions ( ) [9]. Maximum removal was observed around 4 initial pH. While the initial pH increased from 4 to 6 the TOC removal rate was declined, which limits the production of hydroxyl radical on the surface of nFe0. Thus, lead to slow mineralization of organic compound (low TOC removal) [28]. The total Fe leaching of NHCs are depicted in Fig. 5. Fig. 5(a) of pH vs Py concentration contour plots on total Fe leaching shows an elliptical nature attributed to the significant interaction between the pH and Py concentration [26]. The total Fe leaching was within the range of permissible limit (3 mg/L) of wastewater discharge in India (CPCB 2001). Therefore, it is concluded that from the above study that the initial pH value greater than 4, more hydrogen peroxide was decomposed without appreciable improvement of CWPO.
3.4.2. Effect of catalyst dose
The influence of the catalyst dose in the range of 0.1–1 g/L was studied with respect to the removal of TOC. Fig. 4(b), (g), and (h) demonstrate the removal of TOC with catalyst dose. Mineralization increased with increment of catalyst dose. At higher catalyst dose, the greater number of active sites are available which are responsible to generate the more hydrogen peroxide by oxidation of Fe0 with available dissolved oxygen into Fe2+ (Eq. (5)). The formed oxidized species (Fe2+) again utilizes the available hydrogen peroxide (generated and external added hydrogen peroxide) convert into Fe3+ and hydroxyl radical (Eq. (6)). Further, presence of more number of active species (Fe0 or Fe2+) leads to the generation of more quantity of hydroxyl radical. This hydroxyl radical oxidized the organic molecules into various organic acids and further these organic acids utilize the hydroxyl radical to convert into harmless compounds. Gosu et al. [18] proposed the degradation mechanism of Qn with hydroxyl radical using TOC removal. Fig. 5(f) shows the contour plot of catalyst dose vs Qn on iron leaching; it was observed that almost parallel line in 2D contour plot which represents the non interactive influence of these two parameters on iron leaching. Similarly catalyst dose vs Py on iron leaching in contour plots shows the elliptical nature due to the significant interaction between the catalyst dose and Py concentration on Fe leaching (Fig. 5(e)). Even though, the catalyst dose of nFe0/GAC is increased in the range of 0.1–1 g/L, the total Fe leaching is within the range of permissible limit of wastewater discharge in India shown in Fig. 5 [29].
3.4.3. Effect of hydrogen peroxide
CWPO effectiveness mainly depends on the generation efficiency of oxidizing species such as hydroxyl radicals. Moreover, powerful radicals are produced by the decomposition of hydrogen peroxide in the presence of nFe0 catalyst. Fig. 4(a), (b), (e), and (f) depicted the impact of hydrogen peroxide concentration on TOC removal. Furthermore, the TOC removal was improved as increase in the dose of hydrogen peroxide significantly because increase the number of hydroxyl radical generation for the mineralization of organic compound which leads the high TOC removal (Eq. (7)). Fig. 5(c) depicted the circular pattern of the lines in the contour plot suggested for the weak interactive influence of hydrogen peroxide vs. Py on total iron leaching. Further, indicate that Py mineralization path generates less number of acid intermediates that lead to low interaction of iron leaching into the aqueous solution. The elliptical view of lines in 2D contour plot (Fig. 5(d)) suggested for the significant interactive influence of hydrogen peroxide dose vs Qn concentration on total Fe leaching. This may depend on degradation path and type of intermediate compounds formation, if more number of intermediate acids are formed that leads to more leaching of iron into solution.
3.4.4. Effect of initial concentration
Organic compound concentration is an important operational factor as it influences the removal of TOC in the CWPO process. Fig. 4(c), (d), (e), (f), (g) and (h) describes the declining trend of TOC with an increase in the initial concentration of NHCs (Py and Qn concentration). As the more NHCs molecules may be adsorbed on active site of nFe0/GAC, which become unavailable for hydrogen peroxide molecules to reach the active site for the generation of hydroxyl radical, and thus leads to lower degradation. The same observation was observed in interaction plot of Fig. 4(e), and (f), as TOC removal rate increased with an increase in the initial concentration of NHCs along with increase in hydrogen peroxide dose with their respective experimental ranges. Among these, Quinoline degradation shown the better efficiency when compare with pyridine. Higher rate of quinoline degradation can be attributed due to more electron density of nitrogen in quinoline molecule with respect to pyridine molecule. Longuet-Higgins and Coulson [30] calculated the Π-electron density of pyridine and quinoline, it was found that quinoline have higher Π-electron density which leads the greater the ease and rate of cationoid substitution at that position.
Effect of Initial concentration of organic molecules on total Fe leaching was observed in 2D contour plot, Fig. 5(a), (c), (d), and (e) shows catalyst dose and hydrogen peroxide concentration interaction with pyridine concentration depicted circular pattern of the lines, which attributed to the weak interaction of two variables on total Fe leaching [26]. Furthermore, for Qn concentration with initial pH and catalyst dose shows an almost parallel line in 2D contour plot (Fig. 5(b) and (c)), it indicates that almost no interactive influence of these two variables on the total Fe leaching. It was found that, all treated sample Fe leaching was below 2 mg/L. The permissible limit of Fe in wastewater discharge is 3 mg/L (CPCB, Environment (protection) rules 1986). According to the present study, further Fe treatment is not necessary. In case if it is exceeded any other process by active metal leaching beyond permissible limit one can recommend simple adsorption process as simple post-treatment process.
3.5. Optimization Using Desirability Function
In the present study, multi-response optimization by desirability function approach was used for the optimization of the process variables by Derringer’s desirability function. As shown in Table S3, the desired goal was selected for each process variable and response. The each goal was changed with respect to our desired goals. Further, 10 optimum points are generated through numerical simulation. Among those, the best optimum conditions are initial solution pH of 3.5, hydrogen peroxide dose of 0.34 mmol, catalyst dose of 0.55 g/L, Py concentration of 200 mg/L and Qn concentration of 200 mg/L, at which TOC removal of 84% has suggested with the desirability of 0.7. The experiments are conducted in duplicate to validate the model (Table S4). The predicted values nearly close to experimental values obtained from optimization analysis (Table S4).
4. Conclusions
Catalytic wet peroxidation process was used for the simultaneous oxidation of Py and Qn containing wastewater in the presence of nanoscale zero-valent iron with the help of Box-Behnken design. The five variables including initial pH, oxidant dose, catalyst dose and concentration of Py and Qn were evaluated based on TOC removal. From BBD, concluded that all five parameters are effective parameters on TOC removal. The F-values of TOC and total iron leaching are 1,594.82 and 75.89, which suggested that the terms in the model have a significant effect on response. ~84% TOC removal was observed in binary compounds oxidation at initial solution pH of 3.5, catalyst dose of 0.55 (g/L), hydrogen peroxide dose of 0.34 mmol, Py concentration of 200 mg/L and Qn concentration of 200 mg/L.