AbstractIn this paper, the chemical and physical effects generated during the dielectric barrier discharge plasma (DBDP) process, including O3, H2O2, light, e*, etc., were utilized to activate the peroxydisulfate (PDS) to form SO4−•. Then, the original reactive oxygen species (ROS), such as •OH, formed in the discharge system was combined to degrade the enrofloxacin (ENR) in water, and the corresponding influencing parameters and reactive substance in the DBDP/PDS system were analyzed. From the investigation, it was found that the neutral (pH =6.5) solution was more conducive to the ENR decomposition than the acidic and alkaline solution conditions. The presence of Fe2+ and Cu2+ in the reaction solution could hasten the ENR degradation, whereas the addition of Cl− and the HCO3− in the solution had a negative effect. Analysis of the reactive species and quenching tests were carried out to explore the generation of H2O2, O3, •OH, and SO4−• in the DBDP/PDS system and their effects on the ENR degradation. The UV-Vis and 3D fluorescence spectra analysis were applied to demonstrate the cooperative effects of the DBDP and the PDS. The TOC and COD removals of the ENR solutions in the DBDP and the DBDP/PDS systems were also compared. Based on the intermediates analysis of the ENR degradation, three possible pathways of ENR decomposition in the synergistic system have been inferred.
Graphical Abstract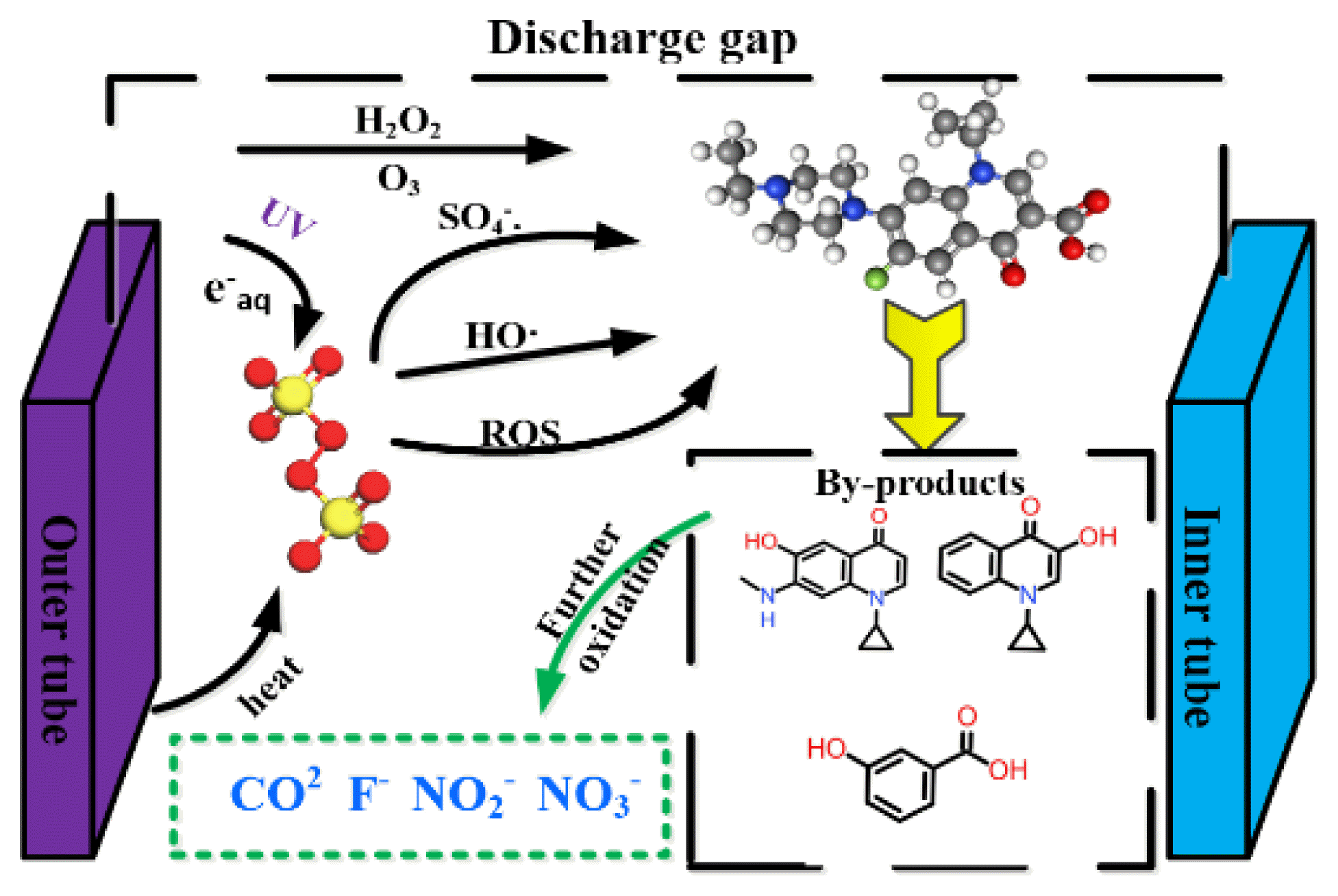
1. IntroductionAs a third-generation fluoroquinolone antibiotic, enrofloxacin (ENR) can persist in the form of an archetype or product of metabolism for a long time after entering the aquatic environment. Thus, ENR can often be detected in various surface waters. To resolve the probable environmental crisis induced by the ENR, developing promising technologies to remove the ENR from water has been necessary.
Advanced oxidation technologies (AOTs) have been demonstrated as efficient wastewater treatment technologies (including ozone oxidation [1], photocatalytic oxidation [2], electrochemical oxidation [3], Fenton oxidation [4], and non-thermal plasma, etc.) to oxidize the nondegradable pollutants. Among AOTs, non-thermal plasma technology has drawn significant attention because of its high efficiency and non-selectivity for recalcitrant degradation and organic wastewater treatment. During the discharge process, the physical effects of high-energy electrons and ultraviolet light could be formed, and the chemical effects of the reactive oxygen species (ROS) generation could also be obtained [5–7]. These physical and chemical phenomena induced by plasma could oxidize refractory organic pollutants directly or indirectly, potentially leading to mineralization. However, some physical (light) and chemical effects (O3 and H2O2) in the system have not been fully utilized, resulting in the relatively weak oxidation performance of pollutants in the discharge system, which limits the further development of the non-thermal plasma technology. In order to fully utilize the various physical and chemical effects and improve its treatment efficiency, establishing collaborative wastewater treatment systems combined with different technologies have become the good research direction.
Due to the stronger oxidation ability (E0=2.5~3.1 V) and longer half-life of the sulfate radical (SO4−•) [8], the AOT based on the SO4−• is becoming increasingly popular in the field of refractory organic wastewater treatment [8]. Multiple methods could activate peroxydisulfate (PDS) to produce SO4−•, including heat [9], light [10], ozone (O3) [11], hydrogen peroxide (H2O2) [12], electrochemistry [13], transition metals [14], and alkali [15], etc. As the physical effects (heat and light) and chemical effects (O3 and H2O2) could be formed simultaneously in the non-thermal plasma system, research on the PDS activation by non-thermal plasma has been attracted attention recently. Furthermore, the generation of •OH in the system could also be promoted by PDS activation [16]. The generation of the new kind of active substance (SO4−•) as well as the enhancement of the critical reactive species (•OH) could lead to the efficient decomposition and mineralization of the organic pollutants. Though researchers have combined the dielectric barrier discharge plasma (DBDP) and PDS to apply in the different organic compounds’ degradation, the detailed analysis is still insufficient. To figure out the influence of more parameters on the oxidation in the collaborative process of the DBDP/PDS, there is still much work to be done.
In this study, the DBDP was chosen to produce the non-thermal plasma and the DBDP/PDS cooperative system was established to treat the ENR wastewater. Different influencing factors, including the initial pH value and the different inorganic ions’ (Fe2+, Cu2+, Cl− and HCO3−) addition on the ENR degradation in the DBDP/PDS system were studied. The cooperative mechanism was also studied by analyzing the critical reactive substances (O3, H2O2, •OH, and SO4−•) through concentration detection or electron paramagnetic resonance (ESR) analysis. The degradation of the ENR solution was characterized by UV-Vis spectroscopy and 3D fluorescence spectrum analysis, and the removals of COD and TOC of the ENR solutions were studied to illustrate the mineralization of the ENR. The intermediates of ENR degradation were analyzed by HPLC-MS, and then possible pathways of the ENR degradation in the DBDP/PDS system were speculated.
2. Experimental2.1. MaterialThe acetonitrile (≥99.9%, Macklin Biochemical Technology Co., Ltd. Shanghai) used in the research was HPLC-pure. All other reagents were analytical pure. ENR (≥98%), DMPO (≥97%), ferrous sulfate (≥98%), copper sulfate (≥99%) were purchased from Aladdin Biochemical Technology Co., Ltd. Peroxydisulfate (≥98%), sodium chloride (≥99.5%), ethanol (≥99.8%), tert-butyl alcohol (≥99%), and sodium hydroxide (≥96%) were purchased from the China National Pharmaceutical Group Corporation. All solutions were prepared with deionized water.
2.2. DBDP Reaction System
Fig. S1 shows the experimental system of the study. A cyclic water-falling film DBDP reactor was used as the source for the non-thermal plasma generation (purchased from Nanjing Suman Plasma Technology Co., Ltd., PG-3000). In the DBDP reactor, the stainless-steel mesh covered on the surface of the outer quartz tube served as the high-voltage electrode and the solution flowing between the outer and inner quartz tubes served as the grounding electrode. The detailed parameters of the DBDP reactor were given in our former research [17, 18]. The target solution entered the reactor from the bottom of the inner quartz tube and then flew out through the uniform flow port above it. Finally, the solution flew along the outer wall of the inner quartz tube in the form of a water film. The higher gas-liquid ratio between the discharge electrode and the ground electrode was favorable for the O3 formation of the water-falling film DBDP reactor. The voltage and current waveforms were recorded by an oscilloscope (Tektronix DPO 2014b) and probes (Tektronix, P6015A, and TPP0200). During the experiments, the injection power was 0.8 kW and was kept as a constant parameter for each experiment.
2.3. Experimental MethodThe whole reaction solution was circulated with a peristaltic pump (Baoding Lead Fluid, BT600/YZ15, China) and the ENR solution (3 L, 20 mg L−1) was pumped into the reactor at the 1.5 L min−1 flow rate was. 3 mM PDS was added to the ENR solution. A magnetic stirrer (China, Xinrui, DJ-1) was used to mix the solution evenly. For the reactive substances analysis, deionized water was applied as the reaction solution. In all experiments, the total reaction time was controlled at 25 min and the samples were taken at 5 min intervals. The reaction temperature was controlled below 35°C throughout the experiments.
2.4. Analytical MethodThe HPLC (Lunan Ruihong, P1010, China) was used for analyzing the ENR concentration. 0.1 M NaOH and 0.1 M HCl solutions were used to adjust the pH value of the solution. A pH meter (INESA, PHS-3E, Shanghai) and a conductivity analyzer (INESA, DDS-307A, Shanghai) were applied to measure the pH and conductivity of the solutions. An UV-Vis full band scanner (Shimadzu, UV-2600, Japan) was used for the full-wave band scanning of the solutions during the treatment. The TOC and COD values of the ENR solution were measured using TOC (German element, Vario TOC select, German) and COD (Lianhua, 5B-3A, China) instruments, respectively. A 3D fluorescence spectrophotometer (Hitachi, FL4500) was used to measure the molecular fluorescence properties of the different samples. Analysis of the sulfate and the hydroxyl radicals was performed by an ESR (Bruker, Micro ESR, Germany). The O3 concentration of the solution was detected using an ozone analyzer (Shanghai Haiheng, CY-1A, China). The TiOSO4 method was used to identify H2O2 concentrations. A LC-MS (America, Thermo LXQ) was used to detect the byproducts formed during the ENR degradation. The degradation efficiency (removal percentage) of the ENR was calculated by comparing the change of the concentration during the treatment. The corresponding kinetics analysis was fit based on the pseudo first-order equation. The energy efficiency (G50, mg (k Wh)−1) was also investigated. The corresponding calculation methods are given in Table S1. The experimental results are presented by the average value of the three parallel experiments with an error bar.
3. Results and Discussion3.1. Influence of the Initial pH ValueFirstly, the influence of the solution initial pH (3, 5, 6.5, 9 and 11) on the ENR degradation in the DBDP/PDS system was studied. The degradation efficiency, k value and G50 were calculated, and the results are shown in Fig. 1. The figure showed that the degradation efficiency, k value, and G50 were the highest at the initial pH of 6.5. The ENR degradation under the acidic and alkaline solution conditions was relatively lower. For the reason of the results, it can be ascribed as follows: the ENR is an amphoteric compound, it has a positively charged piperazine moiety and a negatively charged carboxyl group. The two pKa values of the ENR are pKa1=6.1 and pKa2=7.7 [19]. Therefore, when the ENR solution was at its initial pH value of 6.5 (the value was between pKa1 and pKa2), the smaller charge on the ENR molecules would be favorable for the mass transfer of the oxidation reaction, which was conducive to the ENR degradation. Furthermore, •OH could be captured by the CO32− (formed by the cleavage of ENR molecules) in the alkaline solution [20], which would weaken the effectiveness of the formed •OH for the ENR degradation.
3.2. Influence of the Inorganic Ion AdditionAs multiple ions often coexist in the practical wastewater, the influence of different ions’ addition on the ENR degradation in the DBDP/PDS was investigated. The four chemicals, FeSO4, CuSO4, NaCl and NaHCO3 were dissolved in the solution to simulate the effect of the Fe2+, Cu2+, Cl− and HCO3− existence in the practical water environment, respectively. The ENR degradation under different conditions of inorganic ion addition is displayed in Fig. 2.
Fig. 2a shows that the addition of Fe2+ was favorable for enhancing the ENR degradation, and the effect of the 2 mM Fe2+ addition was more evident than the 1 mM and 3 mM Fe2+ additions. The positive effect of the Fe2+ addition can be explained for the following two reasons: one was the promotion of the Fe2+ for the SO4−• production, and the other was the production of •OH stimulated by the Fenton reaction between Fe2+ and H2O2 (Eqs. (1) and (2)) [21]. However, Fe2+ could also consume the SO4−• and the •OH (Eqs. (3) and (4)) [22], and the processes might result in a gradual change of the ENR degradation with the extension of the reaction. Fig. 2b shows that the ENR removal was enhanced by increasing the Cu2+ concentration in the reaction solution. When 2 mM Cu2+ was added, the degradation efficiency of ENR could reach 100%. The results were owing to the formation of oxidative free radicals by reactions of Cu2+/Cu+ with the PDS and the H2O2 (Eqs. (2), (5)) [23]. Fig. 2c shows that the addition of Cl− could inhibit the ENR degradation because the Cl− could react with the SO4−• or the •OH to produce Cl•, which had less oxidizing capacity (Eqs. (6)) [24]. The results shown in Fig. 2d revealed that there was an increase of the ENR degradation in the first 10 min when there was the HCO3− in the reaction system, while the degradation rates decreased in the following reaction period. The reductions were 10.79% (1 mM) and 40.73% (2 mM) at the 25 min. The initial increase might be due to the extra production of •O2− and •CO3− (Eq. (7)) [25], which have certain oxidation capacities. Furthermore, the following formation of the 1O2 and the •OH by the •O2− could also oxidize the ENR (Eq. (8)). However, the inhibition reactions of HCO3− with SO4−• and •OH would result in a decrease of the ENR removal in the following reaction stages (Eqs. (9) and (10)) [26].
3.3. Reactive Species Analysis3.3.1. O3 and H2O2The cooperative effect of the DBDP and the PDS could be indicated by comparing the changes of O3 and H2O2 concentrations in demonized water in the DBDP system with and without the PDS addition. Fig. 3a shows that the O3 concentration in the two reaction systems increased first and then decreased during the whole reaction time. The reason for the increase might be attributed to the increased solubility of ozone at lower pH values, which was induced by the formation of organic acid byproducts [27]. The following decrease was because of the increase in solution temperature during the reaction, which led to the decomposition of O3. The changes of H2O2 concentration are shown in Fig. 3b. As shown in the figure, the H2O2 concentration decreased when the PDS was present in the reaction system. The results could be explained as the PDS promoted the decomposition of H2O2 into HO2• (Eqs. (11)–(13)) [28, 29], which in turn induced the generation of SO4−•. Furthermore, H2O2 could be generated by the reaction of HO2• and O2−•, which could also react with SO4−• when PDS was added (Eqs. (14)–(15)) [30]. All the above reactions led to the reduction of the formed H2O2.
3.3.2.•OH and SO4−•The ESR analyzer could identify the active substances using the different characteristic signals exhibited by the compounds formed by the reaction of DMPO with •OH and SO4−• [31, 32]. The analysis results are displayed in Fig. 4. In the two reaction systems, the linear relationship between the characteristic peak intensities of DMPO-•OH was shown as 1:2:2:1. Meanwhile, the linear relationship of DMPO-SO4−• peak intensity would also exhibit an approximate relationship of 1:1:1:1:1:1. From the characteristic peaks shown in Fig. 4a, it can be observed that the •OH signal was able to be detected in the DBDP-alone system, but the intensity was relatively low. Fig. 4b shows that both SO4−• and •OH could be formed in the system of DBDP/PDS, and the intensity of •OH was higher in the cooperation system. The results indicate that the activation of the PDS in the DBDP system and the chain reactions could induce the formation of SO4−•. It was found that a stronger DMPO-•OH peak was generated in the DBDP/PDS system compared to the DBDP-alone system. The results verified the cooperative effect of the DBDP and the PDS on the reactive species and accordingly the ENR degradation.
3.3.3. Quenching testThe quenching test was carried out in the research to determine the contribution of different active substances to the ENR degradation in the DBDP/PDS system. In this part, TBA was used as a scavenger for •OH, and EtOH was applied to quench SO4−• [33]. The results are displayed in Fig. 5. As displayed in Fig. 5a, the addition of TBA and EtOH reduced the degradation rates of ENR by 39.33% and 18.15%, respectively. The corresponding k values also performed the same trend of change (Fig. 5b). The different oxidation results induced by Et-OH and TBA in the DBDP/PDS system also indicated that the •OH and SO4−• played an important role in the ENR degradation.
3.4. ENR Degradation3.4.1. UV-Vis analysisThe UV-Vis scanning of ENR solutions in both DBDP-alone and DBDP/PDS systems at different reaction periods is shown in Fig. 6. It can be observed that the characteristic peak of ENR at 276 nm was progressively decreasing, and the reduction was more pronounced in the DBDP/PDS system than that in the DBDP-alone system. Moreover, the characteristic peaks at 185–250 nm were increasing due to the accumulation of many byproducts in this region as the ENR degradation [34]. The results also confirmed the cooperative effect of DBDP and PDS on the ENR degradation.
3.4.2. 3D fluorescence spectra analysisThe fluorescence intensity of organic compounds detected by 3D fluorescence spectroscopy was used to characterize the change of the ENR solution during the degradation process. The weaker the fluorescence intensity presents the lower the concentration of organic substances [35, 36]. The analysis methods were also used to analyze the composition and concentration changes of the ENR solution during the degradation in the DBDP/PDS system, and the results are displayed in Fig. 7. It can be observed from Fig. 7 that the fluorescence intensity at Ex/Em = (400–558 nm)/(281–392 nm) was gradually disappearing when the ENR solution was treated in the DBDP/PDS cooperative process, which demonstrated that the chemical bond of ENR was decomposed and the functional group was replaced. The target chemicals decomposed continuously into the small molecular compounds by the oxidation of •OH, SO4−•, O3, and H2O2 generated in the discharge process. It was noteworthy that after 25 min, all fluorescence belonging to the ENR region disappeared, indicating that ENR was effectively degraded in the DBDP/PDS system.
3.4.3. Change of solution pH and conductivityIn the DBDP/PDS system, a series of chain reactions could lead to the formation of different intermediates and then the change of solution properties. To illustrate the change, the pH and conductivity values of the ENR solution in the DBDP and DBDP/PDS reaction systems at different reaction stages were measured. The changing rules are displayed in Fig. 8. The figure shows that the pH value of the ENR solution in the two systems decreased, while the conductivity value showed the opposite trend. In addition, when PDS was introduced into the DBDP system, the changing trend of pH and conductivity values was more obvious. As ENR molecules could dissociate to form a series of intermediates, including organic acids and CO32−, HCO32− during the reaction process [37, 38], it would affect the pH and conductivity of the solution. The addition of PDS could further accelerate the generation of these substances and ultimately affect the solution's properties. Besides that, ionization of air induced by the plasma could also generate HNO3 and HNO2 and lead to the change of solution properties [39].
3.4.4. TOC and CODThe removal of solution COD and TOC of the ENR solutions after treating in the DBDP-alone and DBDP/PDS systems are shown in Fig. S2. It can be seen from the figure that the removal rates of TOC and COD in the DBDP/PDS system were 14.34% and 43.53% after 25 min of treatment, which were 7.66% and 19.58% higher than the DBDP-alone system. The results evidenced that the addition of PDS could reduce improve the mineralization of the ENR solution, which also further proved the cooperative effect of the DBDP and the PDS.
3.5. Degradation Pathway AnalysisThe byproducts formed in the DBDP/PDS system were analyzed and identified through LC-MS analysis. Based on the 14 identified products and related references [40–42], three degradation pathways were speculated, as shown in Fig. S3.
Pathway I: Once the F atom of the ENR molecule was substituted by •OH, the piperazine ring was oxidized and opened to form the E1 and the following E2, which were further transformed to the E3 by losing the piperazine ring. Finally, E4 was formed through the decarboxylation reaction.
Pathway II: The E5 was first formed through the decarboxylation and the •OH substitution reactions that took place on the quinolone ring. Then the E6 was produced through the oxidation and ring-opening of the piperazine ring. Under the continuous attack of reactive oxygen species, the piperazine ring disappeared and the E6 transformed into the E7, then the F and NH2 groups were dropped to form the E8.
Pathway III: Firstly, the E9 was formed after the F atoms were substituted by the •OH. Then, the quinolone ring cleavage reaction and decarboxylation occurred, and the E10 and the E11 were generated. In addition, the N atom between the carbon group and the piperazine ring and the Quinolone ring were attacked, and the E12 was formed by losing the aldehyde groups and the piperazine ring branches. Finally, the E13 and the E14 were generated through the cleavage of the quinolone rings and the loss of N atoms.
When there are sufficient reactive oxygen species in the DBD/PDS system, the intermediate products will further oxidize to CO2, H2O, and inorganic ions after sustained attack, ultimately achieving complete removal of ENR.
4. ConclusionsIn the study, a DBDP/PDS water treatment system was constructed and applied to degrade ENR. In the cooperation system, neutral (pH=6.5) solution conditions were more advantageous for the decomposition of ENR compared to the acidic and alkaline solutions; Fe2+ and Cu2+ ions’ addition could promote the formation of active substance in the system and then accelerate the ENR degradation, while Cl− and HCO3− would hinder the active substances formation. The analysis and quenching experiments of active substances also confirmed that the addition of PDS was conducive to the generation of active substances. The results of the UV-vis analysis verified that there was a better degradation of the ENR in the DBDP/PDS system. 3D fluorescence spectroscopy analysis indicated that the ENR was effectively degraded in the DBDP/PDS system. Three pathways of ENR degradation in the DBDP/PDS cooperative system were finally speculated. In summary, the addition of PDS could improve the removal rate and mineralization degree of ENR in the DBDP system. The results achieved from the study will provide some references for the possible application of the DBDP/PDS cooperative process in practical wastewater treatment in the future.
AcknowledgementsThis work was supported by the National Natural Science Foundation of China (No. 21876070) and Jiangsu Collaborative Innovation Center of Technology and Material of Water Treatment.
NotesAuthor Contributions S. S. (Master student) made the experiment plan, data analysis and Writing-Original Draft the experiment in the whole process. H. W. (Professor) conducted all the experiments and wrote the manuscript. Y. H. (Master student) was responsible for water quality analysis. Y. M. (Associate Professor) wrote and revised the manuscript. References1. Guo H, Li D, Li Z, et al. Promoted elimination of antibiotic sulfamethoxazole in water using sodium percarbonate activated by ozone: Mechanism, degradation pathway and toxicity assessment. Sep. Purif. Technol. 2021;266:118543.
https://doi.org/10.1016/j.seppur.2021.118543
2. Shurbaji S, Huong P, Altahtamouni T. Review on the visible light photocatalysis for the decomposition of ciprofloxacin, norfloxacin, tetracyclines, and sulfonamides antibiotics in wastewater. Catalysts. 2021;11:437.
https://doi.org/10.3390/catal11040437
3. Zeng W, Liang H, Zhang H, Luo X, Lin D, Li G. Efficient electrochemical oxidation of sulfamethoxazole by a novel reduced TiO2 nanotube arrays-based flow-through electrocatalytic membrane. Sep. Purif. Technol. 2022;289:120720.
https://doi.org/10.1016/j.seppur.2022.120720
4. Wu Q, Siddique M, Guo Y, Wu M, Yang Y, Yang H. Low-crystalline bimetallic metal-organic frameworks as an excellent platform for photo-Fenton degradation of organic contaminants: Intensified synergism between hetero-metal nodes. Appl. Catal. B-Environ. 2021;286:119950.
https://doi.org/10.1016/j.apcatb.2021.119950
5. Wang H, Shen Z, Yan X, Guo H, Mao D, Yi C. Dielectric barrier discharge plasma coupled with WO3 for bisphenol A degradation. Chemosphere. 2021;274:129722.
https://doi.org/10.1016/j.chemosphere.2021.129722
6. Hu K, Xie Q, Wang H, et al. Synergistic catalysis of Cu-CeO2@CA composite film in a circulating DBD plasma system and its effect on ciprofloxacin degradation. Chem. Eng. J. 2023;455:140895.
https://doi.org/10.1016/j.cej.2022.140895
7. Mao D, Yan X, Wang H, Shen Z, Yi C. Catalysis of rGO-WO3 nanocomposite for aqueous bisphenol A degradation in dielectric barrier discharge plasma oxidation process. Chemosphere. 2021;262:128073.
https://doi.org/10.1016/j.chemosphere.2020.128073
8. Guo H, Pan S, Hu Z, et al. Persulfate activated by non-thermal plasma for organic pollutants degradation: A review. Chem. Eng. J. 2023;470:144094.
https://doi.org/10.1016/j.cej.2023.144094
9. Dominguez C, Fernandez A, Romero A, Santos A. Degradation of HCHs by thermally activated persulfate in soil system: Effect of temperature and oxidant concentration. J. Environ. Chem. Eng. 2021;9:105668.
https://doi.org/10.1016/j.jece.2021.105668
10. Cao Z, Li J, Zhao Y, Mei Q, Wang Q, Cheng H. Metakaolinite/LaFeCoO3 microsphere catalyst for photocatalytic persulfate activation: Enhanced removal of tetracycline hydrochloride. Chem. Eng. J. 2023;466:143076.
https://doi.org/10.1016/j.cej.2023.143076
11. Amr S, Aziz H, Adlan M, Bashir M. Pretreatment of stabilized leachate using ozone/persulfate oxidation process. Chem. Eng. J. 2013;221:492–499.
https://doi.org/10.1016/j.cej.2013.02.038
12. Adil S, Maryam B, Kim E, Dulova N. Individual and simultaneous degradation of sulfamethoxazole and trimethoprim by ozone, ozone/hydrogen peroxide and ozone/persulfate processes: A comparative study. Environ. Res. 2020;189:109889.
https://doi.org/10.1016/j.envres.2020.109889
13. Li J, Liang Y, Jin P, et al. Heterogeneous Metal-Activated Persulfate and Electrochemically Activated Persulfate: A Review. Catalysts. 2022;12:1024.
https://doi.org/10.3390/catal12091024
14. Luo J, Yi Y, Fang Z. Effect of Mn-based magnetic biochar/PS reaction system on oxidation of metronidazole. Chemosphere. 2023;332:138747.
https://doi.org/10.1016/j.chemosphere.2023.138747
15. Santos A, Fernandez J, Rodriguez S, et al. Abatement of chlorinated compounds in groundwater contaminated by HCH wastes using ISCO with alkali activated persulfate. Sci. Total. Environ. 2018;615:1070–1077.
https://doi.org/10.1016/j.scitotenv.2017.09.224
16. Shang K, Wang X, Li J, et al. Synergetic degradation of Acid Orange 7 (AO7) dye by DBD plasma and persulfate. Chem. Eng. J. 2017;311:378–384.
https://doi.org/10.1016/j.cej.2016.11.103
17. Song S, Zhang H, Han S, et al. Activation of persulfate by a water falling film DBD process for the enhancement of enrofloxacin degradation. Chemosphere. 2022;301:134667.
https://doi.org/10.1016/j.chemosphere.2022.134667
18. Song S, Huang Y, Du Y, et al. Oxidation of ciprofloxacin by the synergistic effect of DBD plasma and persulfate: reactive species and influencing factors analysis. Plasma Sci. Technol. 2023;25:025505.
https://doi.org/10.1088/2058-6272/ac8dd4
19. Jiang C, Ji Y, Shi Y, Chen J, Cai T. Sulfate radical-based oxidation of fluoroquinolone antibiotics: Kinetics, mechanisms and effects of natural water matrices. Water. Res. 2016;106:507–517.
https://doi.org/10.1016/j.watres.2016.10.025
20. Han S, Mao D, Wang H, Guo H. An insightful analysis of dimethyl phthalate degradation by the collaborative process of DBD plasma and Graphene-WO3 nanocomposites. Chemosphere. 2022;291:132774.
https://doi.org/10.1016/j.chemosphere.2021.132774
21. Shang K, Li W, Wang X, et al. Degradation of p-nitrophenol by DBD plasma/Fe2+/persulfate oxidation process. Sep. Purif. Technol. 2019;218:106–112.
https://doi.org/10.1016/j.seppur.019.02.046
22. Monteagudo J, Durán A, González R, Expósito A. In situ chemical oxidation of carbamazepine solutions using persulfate simultaneously activated by heat energy, UV light, Fe2+ ions, and H2O2
. Appl. Catal. B-Environ. 2015;176–177:120–129.
https://doi.org/10.1016/j.apcatb.2015.03.055
23. Xu Z, Shan C, Xie B, Liu Y, Pan B. Decomplexation of Cu(II)-EDTA by UV/persulfate and UV/H2O2:Efficiency and mechanism. Appl. Catal. B-Environ. 2017;200:439–447.
https://doi.org/10.1016/j.apcatb.2016.07.023
24. Kaur B, Kuntus L, Tikker P, Kattel E, Trapido M, Dulova N. Photo-induced oxidation of ceftriaxone by persulfate in the presence of iron oxides. Sci. Total. Environ. 2019;676:165–175.
https://doi.org/10.1016/j.scitotenv.2019.04.277
25. Chen S, Hou Y, Rong Y, et al. Hydroxyl radical and carbonate radical facilitate chlortetracycline degradation in the bio-photo-electrochemical system with a bioanode and a Bi2O3/CuO photo-cathode using bicarbonate buffer. Chemosphere. 2022;296:134040.
https://doi.org/10.1016/j.chemosphere.2022.134040
26. Zhang T, Yang Y, Gao J, et al. Synergistic degradation of chloramphenicol by ultrasound-enhanced nanoscale zero-valent iron/persulfate treatment. Sep. Purif. Technol. 2020;240:116575.
https://doi.org/10.1016/j.seppur.2020.116575
27. Shang K, Morent R, Wang N, et al. Degradation of sulfamethoxazole (SMX) by water falling film DBD Plasma/Persulfate: Reactive species identification and their role in SMX degradation. Chem. Eng. J. 2022;431:133916.
https://doi.org/10.1016/j.cej.2021.133916
28. Boukari S, Pellizzari F, Leitner N. Influence of persulfate ions on the removal of phenol in aqueous solution using electron beam irradiation. J. Hazard. Mater. 2011;185:844–851.
https://doi.org/10.1016/j.jhazmat.2010.09.097
29. Ren J, Zhen Y, Wang J, Li J. Catalytic degradation of caffeic acid by DBD plasma and Mn doped cobalt oxyhydroxide catalyst. Chemosphere. 2021;275:130101.
https://doi.org/10.1016/j.chemosphere.2021.130101
30. Herrmann H, Ervens B, Jacobi H, Wolke R, Nowacki P, Zellner R. CAPRAM 2.3: A Chemical Aqueous Phase Radical Mechanism for Tropospheric Chemistry. J. Atmos. Chem. 2000;36:231–284.
https://doi.org/10.1023/A:1006318622743
31. Xu Y, Tang X, Xiao Y, et al. Persulfate promoted visible photocatalytic elimination of bisphenol A by g-C3N4-CeO2 S-scheme heterojunction: The dominant role of photo-induced holes. Chemosphere. 2023;331:138765.
https://doi.org/10.1016/j.chemosphere.2023.138765
32. Zhang H, Xiao S, Du Y, et al. Catalysis of MnO2-cellulose acetate composite films in DBD plasma system and sulfamethoxazole degradation by the synergistic effect. Sep. Purif. Technol. 2022;298:121608.
https://doi.org/10.1016/j.seppur.2022.121608
33. Matta R, Tlili S, Chiron S, Barbati S. Removal of carbamazepine from urban wastewater by sulfate radical oxidation. Environ. Chem. Lett. 2011;9:347–353.
https://doi.org/10.1007/s10311-010-0285-z
34. Hu K, Song S, Zhang H, et al. Degradation of sulfadiazine in a cyclic V-SDBD plasma system: Parameters analysis and degradation pathway. J. Environ. Chem. Eng. 2022;10:107415.
https://doi.org/10.1016/j.jece.2022.107415
35. Guo H, Jian N, Wang H, et al. Enhanced catalytic performance of graphene-TiO2 nanocomposites for synergetic degradation of fluoroquinolone antibiotic in pulsed discharge plasma system. Appl. Catal. B-Environ. 2019;248:552–566.
https://doi.org/10.1016/j.apcatb.2019.01.052
36. Xiao S, Shen Z, Song S, Han S, Du Y, Wang H. Enhanced sulfadiazine degradation in a multi-electrode paralleling DBD plasma system coupled with ZnO/cellulose acetate films. J. Environ. Chem. Eng. 2023;11(1)109063.
https://doi.org/10.1016/j.jece.2022.109063
37. Li X, Xiao C, Ruan X, et al. Enrofloxacin degradation in a heterogeneous electro-Fenton system using a tri-metal-carbon nanofibers composite cathode. Chem. Eng. J. 2022;427:130927.
https://doi.org/10.1016/j.cej.2021.130927
38. Zhang H, Song S, Xie Q, et al. DBD plasma coupling MnO2-Fe3O4-cellulose acetate films for sulfamethoxazole degradation: Insight for catalytic ozonation and Fenton effect. J. Water Process Eng. 2023;53:103819.
https://doi.org/10.1016/j.seppur.2022.121608
39. Wang L, Jiang X, Liu Y. Degradation of bisphenol A and formation of hydrogen peroxide induced by glow discharge plasma in aqueous solutions. J. Hazard. Mater. 2008;154:1106–1114.
https://doi.org/10.1016/j.jhazmat.2007.11.016
40. Chen T, Zhang Q, Xie Z, et al. Carbon nitride modified hexagonal boron nitride interface as highly efficient blue LED light-driven photocatalyst. Appl. Catal. B-Environ. 2018;238:410–421.
https://doi.org/10.1016/j.apcatb.2018.07.053
41. Lukes P, Dolezalova E, Sisrova I, Clupek M. Aqueous-phase chemistry and bactericidal effects from an air discharge plasma in contact with water: evidence for the formation of peroxynitrite through a pseudo-second-order post-discharge reaction of H2O2 and HNO2
. Plasma Sources Sci. T. 2014;23:015019.
https://doi.org/10.1088/0963-0252/23/1/015019
42. Hai H, Xing X, Li S, Xia S, Xia J. Electrochemical oxidation of sulfamethoxazole in BDD anode system: Degradation kinetics, mechanisms and toxicity evaluation. Sci. Total. Environ. 2020;738:139909.
https://doi.org/10.1016/j.scitotenv.2020.139909
Fig. 1Impact of the initial pH (a) oxidation rate, (b) k value, (c) G50. Experimental conditions: P= 0.8 kW, PDS=3 mM, C0=20 mg L−1. 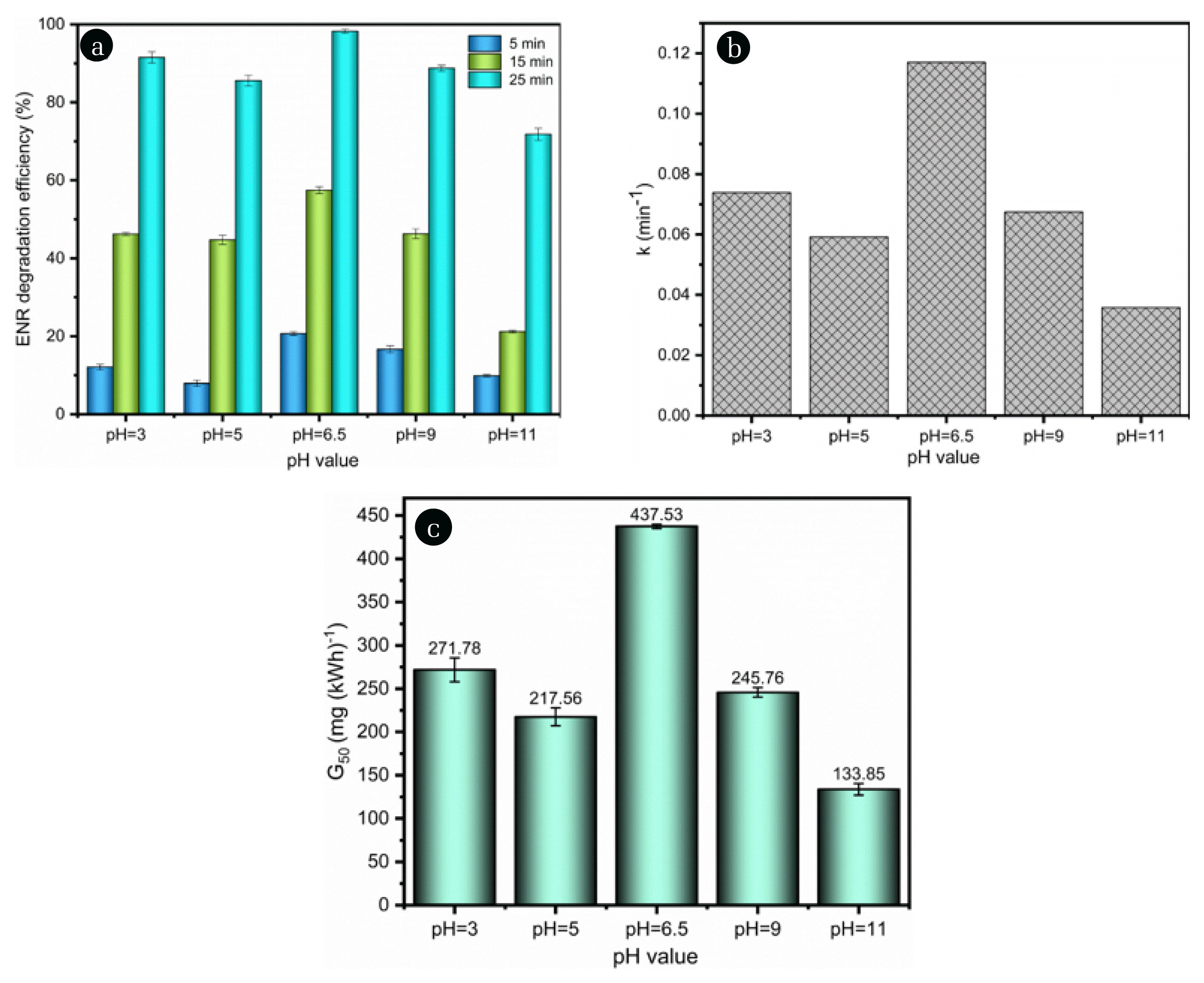
Fig. 2Impact of inorganic ions (a) Fe2+, (b) Cu2+, (c) Cl−, (d) HCO3−. Experimental conditions: P= 0.8 kW, PDS=3 mM, C0=20 mg L−1. 
Fig. 3Formation of O3 and H2O2 (a) O3, (b) H2O2. Experimental conditions: P= 0.8 kW, PDS=3 mM, deionized water. 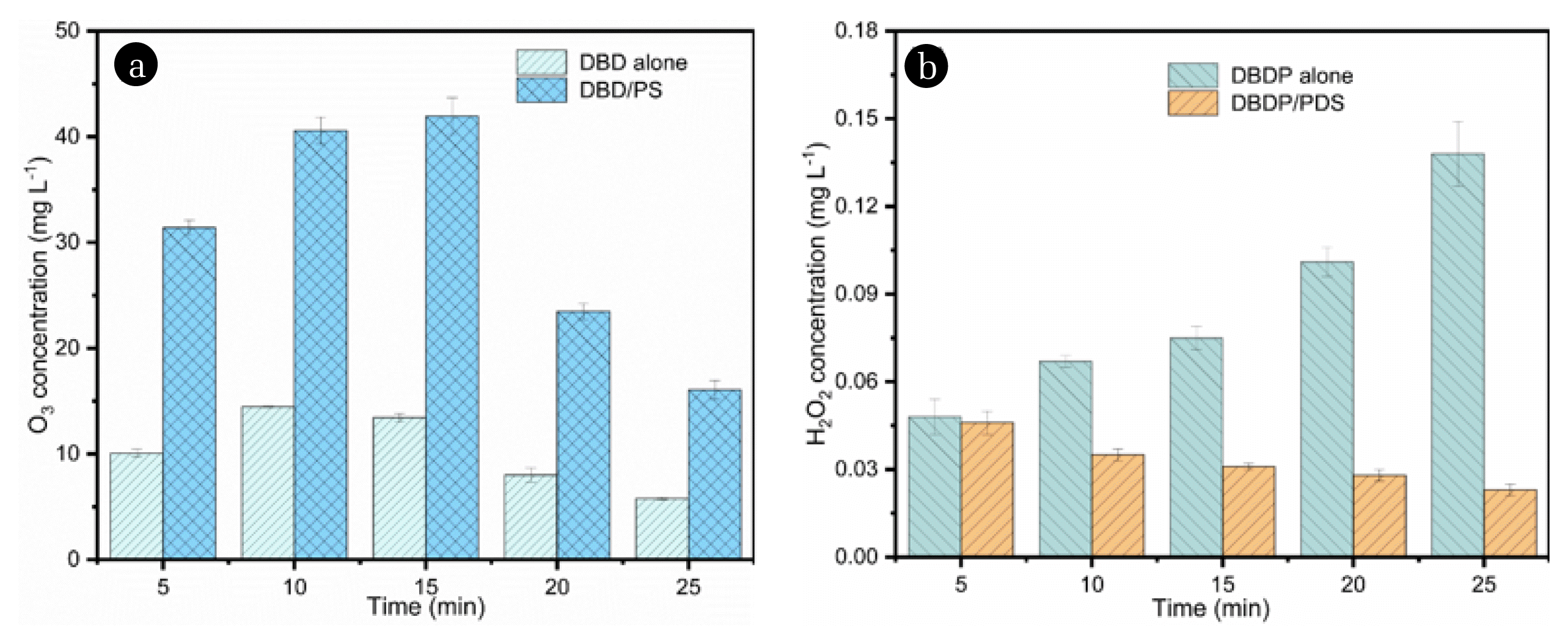
Fig. 4ESR analysis of DMPO/·OH and SO4−• (a) DBDP alone, (b) DBDP/PDS. Experimental conditions: P=0.8 kW, PDS=3 mM, deionized water. 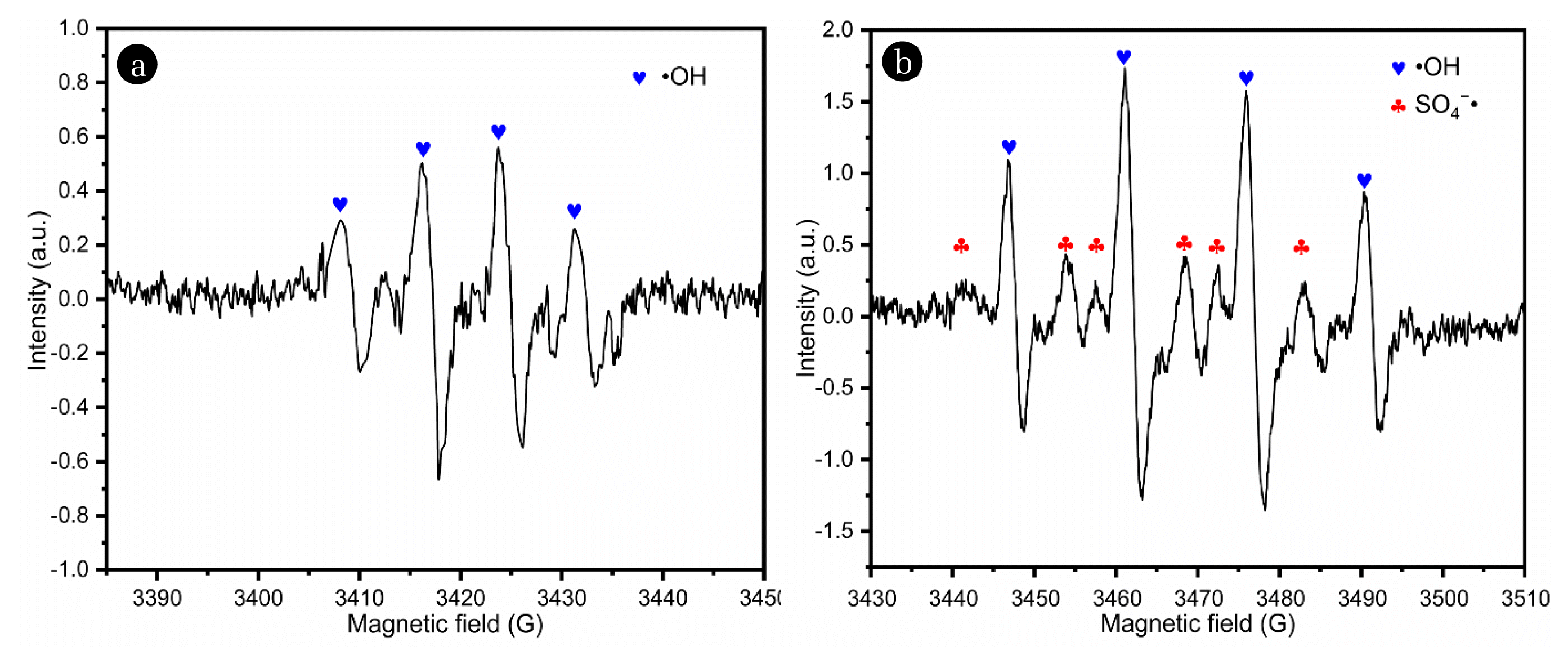
Fig. 5Analysis of quenching experiments (a) TBA (200 mM) and EtOH (200 mM), (b) k value, Experimental conditions: P= 0.8 kW, PDS=3 mM, C0=20 mg L−1. 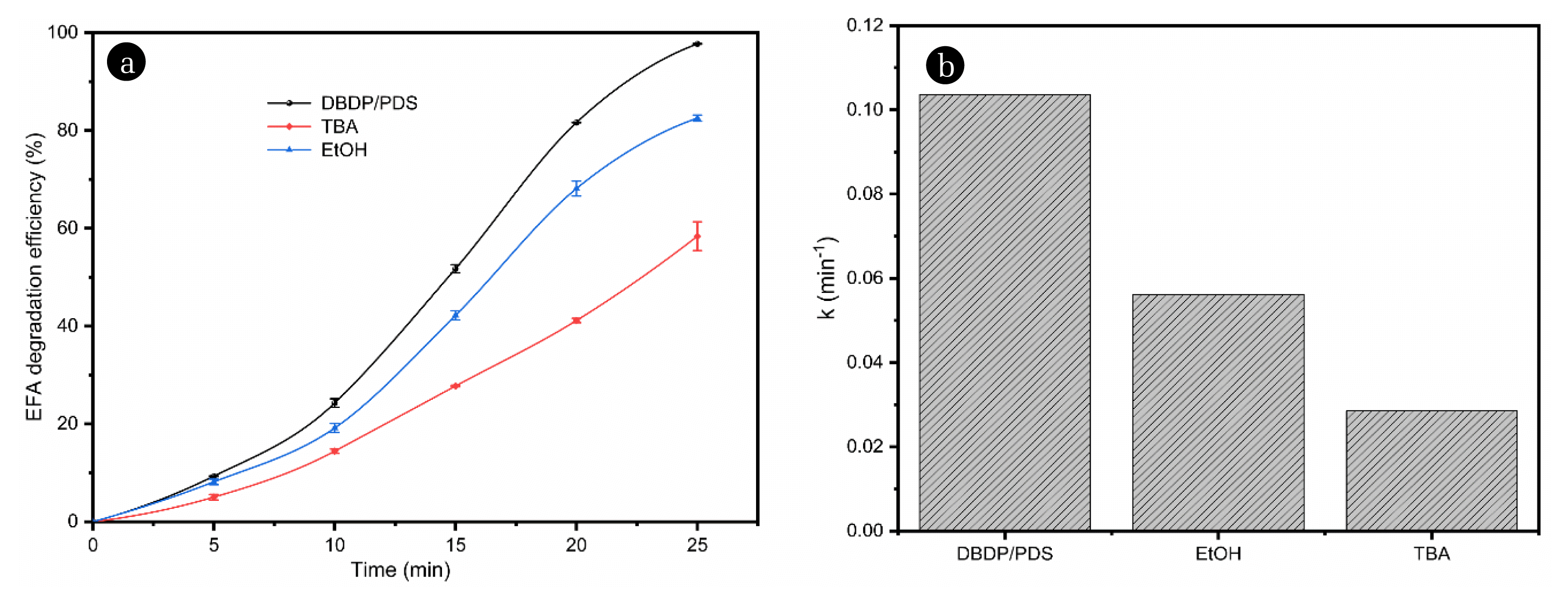
Fig. 6UV-Vis analysis (a) DBDP alone, DBDP/PDS. Experimental conditions: P= 0.8 kW, PDS=3 mM, C0=20 mg L−1. 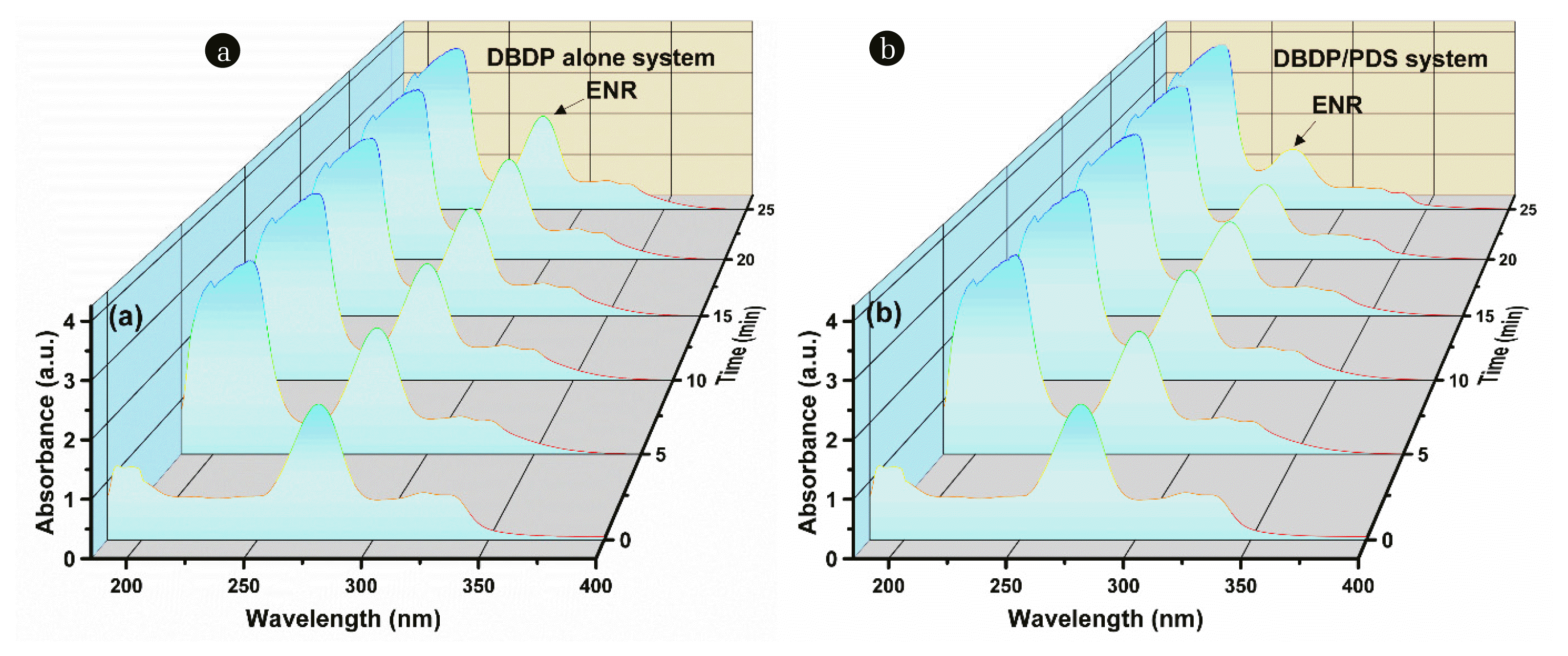
|
|
||||||||||||||||||||||||||||||||||||