AbstractAnaerobic oxidation of methane (AOM) is capable of coupling the reduction of various substrates, which plays a crucial role in accelerating the abatement of pollution. In this study, we employed a sequencing batch reactor (SBR) to enrich a mixed consortium that included AOM microbes and examined the ensuing microbial reduction of tellurate. To obtain the mixed consortium, we enriched AOM microbes in anaerobic conditions utilizing methane as the only electron donor, with nitrate serving as the electron acceptor. We evaluated the abundance of typical methane-oxidizing microbes and associated genes in the reactor using quantitative PCR. Notably, the enriched microbes were able to achieve microbial tellurate reduction and produce elemental tellurium. An analysis of community structure further indicated the vital roles of methanotrophs, denitrifiers, and other heterotrophs in the reactor, although their molecular mechanisms require further investigation. These findings underscore the role of enriched microbial communities in tellurate reduction using a lab-scale SBR, laying the groundwork for future studies into the mechanism of this process, which utilizes methane as an electron donor.
Graphical Abstract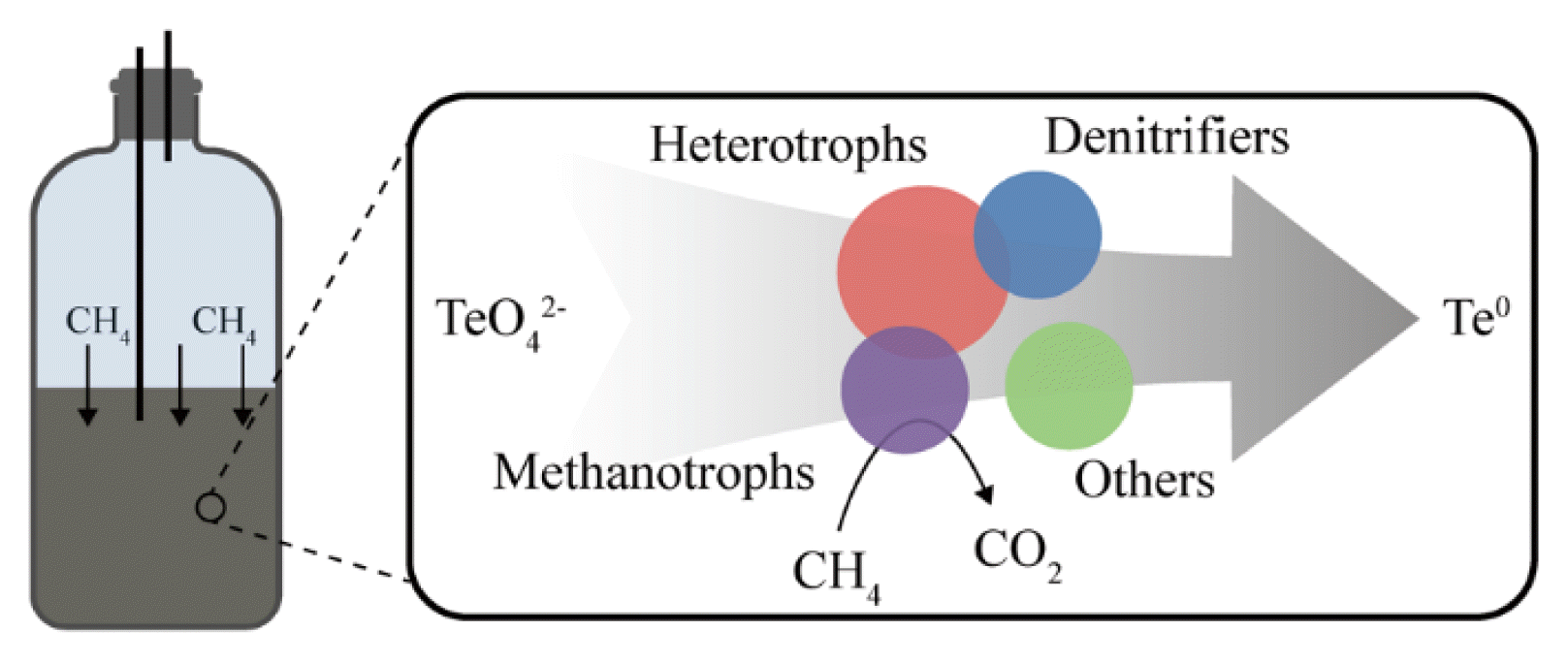
1. IntroductionTellurium (Te) is a scattered metal found in the chalcogenide group and typically forms compounds in association with other metals (e.g., AuTe2, AgAuTe4) [1]. In the natural environment, tellurium exists in various forms, including tellurate (TeO42−), tellurite (TeO32−), elemental tellurium (Te0), and telluride (Te2−) [2]; however, tellurium is most commonly found in the form of oxyanions in its toxic state. Tellurium and its compounds are commonly used in solar panels to improve thermal, optical, and electrical properties [3, 4], and as an additive, tellurium is also widely used in metallurgy [5], petrochemical [6], electronics and electrical [7], glass and ceramics [8], and medicine [9]. In addition, CdTe quantum dots are used as probes for biological detection systems [10]. Although approximately 90% of tellurium is currently recovered from copper anode slimes during the electrolytic refining of blister copper [5], the increasing demand for Te, mainly in renewable energy industries such as CdTe solar panels [11], could lead to significant limitations on production mode and contamination of industrial mining sites, particularly groundwater [12].
Tellurium distributes in the mineral deposits, solids, and sediments, for instance, 5 μg/kg in the Earth’s crust. However, because tellurium oxyanions tend to sorb onto mineral surfaces, tellurium is highly depleted in surface freshwater or seawater [13, 14]. The complicated interplay of various tellurium sinks causes tellurium to disperse in aerobic or anaerobic environments, including solids, sediments, and ocean floor [12, 15]. These environments provide a suitable opportunity to explore the interaction between microbes and tellurium oxyanions. The biogeochemical processes of tellurium can be divided into biooxidation, biosorption/bioaccumulation, and bioreduction. Bioreduction of tellurium oxyanions from oxidative states to inorganic or organic compounds is the more prevalent tellurium detoxification mechanism [12, 16]. Nonetheless, current research on tellurium biogeochemistry is still in its infancy. Microbes present in tellurium-enriched environments can transform various tellurium compounds, and their intracellular enzymes, such as nitrate reductase [17], dihydrolipoamide dehydrogenase [18], and catalase, can reduce tellurate (VI) and tellurite (IV) to elemental tellurium (0), which is an insoluble form in water with reduced toxicity. Several strains, for instance, Rhodobacter capsulatus [19], Methanogens [20], Shewanella [21], Pseudomonas [22], and Stenotrophomonas [23], can reduce tellurium oxyanion species to their elemental state, and thus, play significant role in the bioreduction and detoxification of the tellurium-contaminated environment. Recovery of insoluble elemental tellurium from wastewater is convenient; therefore, this microbial detoxification process offers a viable means to promote the recovery of tellurium from waste streams.
The presence of a reliable carbon source as an electron donor will typically facilitate tellurium bioreduction [12]. Methane, a potent greenhouse gas with a global warming potential 28 times greater than that of carbon dioxide, can be used in the anaerobic oxidation of methane (AOM) to reduce its emission from anaerobic environments into the atmosphere. This process can consume up to 90% of the methane produced in deep ocean environments [24]. AOM was first reported in 1974, in which methane was oxidized by microorganisms as an electron donor, and sulfate was used as an electron acceptor [25]. The microorganisms responsible for this process are mainly thought to include a combination of anaerobic methanotrophic (ANME) archaea and sulfate-reducing bacteria (Deltaproteobacteria). ANME archaea are responsible for methane oxidation, often through the reverse-methanogenesis pathway, but can also independently couple methane oxidation with the reduction of sulfate to elemental sulfur (S0) [26, 27]. AOM has been observed in different geographical environments, such as marine sediment, freshwater environment silt, lake sediment, watersheds of forest valleys, and wetlands [27–31]. The enrichment of a mixed consortium of AOM from environmental inoculum is frequently conducted at the laboratory scale using anaerobic sequencing batch reactor (SBR) or membrane biofilm reactor (MBfR) [32–36]. For example, Candidatus Methanoperedens is an ANME archaea capable of reducing nitrate to nitrite [37], while Candidatus Methylomirabilis, affiliated with the NC10 Phylum, is responsible for converting nitrite to dinitrogen gas in SBR, jointly executing the microbial coupling of the AOM and nitrogen [36, 38]. Currently, great efforts have been devoted to investigating AOM showing that the coupling of AOM with various electron acceptors, such as nitrate, nitrite, iron/manganese, bromate, perchlorate, selenate, tellurite, etc [27, 35, 39–42]. Given that tellurium and methane co-occur in anaerobic environments, there will also be microbial tellurium oxyanions reduction based on AOM in such an environment.
In this study, we firstly enriched AOM microbes and determined the abundance of typical microbes and methane-oxidizing genes in an anaerobic SBR. Microbial tellurate reduction was demonstrated to be feasible by the enriched consortium. Then, the spectrophotometric method and X-ray diffraction (XRD) analysis were conducted to determine the transformation of tellurate. Finally, the community structure of this enrichment was investigated and the potential functional populations for tellurate reduction was also discussed.
2. Materials and Methods2.1. Reactor SetupThe enrichment of AOM microbes and further coupling with tellurate reduction was performed in an anaerobic SBR with a working volume of 500 mL and headspace of 600 mL. The schematic of the reactor configuration is illustrated in Fig. 1. The culture medium from the influence was replenished by a peristaltic pump, and the discharging liquid was controlled by a level pump. The SBR was connected to a gas cylinder (9×51-inch, 95% methane and 5% carbon dioxide), and methane pressure in the reactor headspace was maintained at 2 atm using a gas-pressure regulator. The channels were equipped with a luer-lock connector to ensure proper stages connection. A 100 mL sludge was collected from a lab-scale SBR, which had been inoculated using anaerobic composting beef manure and operated for more than 6 months using methane as carbon source. The initial mixed liquor volatile suspended solids (MLVSS) were diluted to 2 g/L. The solids in the SBR were completely mixed with a 150 rpm magnetic stirrer (220p, Fisher Scientific). To maintain anaerobic environment, the dissolved oxygen in SBR liquid was monitored by a dissolved oxygen meter (5000 series, YSI), and kept below 0.2 mg/L [43]. Methane concentration in the headspace was detected using GC (Micro GC 490, Agilent). The pH was measured using a pH meter (Orion 4 Star, Thermo Scientific) and maintained at 6.9~7.1 by adding 1 M HCl or 1 M NaOH stock solutions as necessary. The SBR was operated in a temperature-controlled lab at 23 ± 0.5°C.
2.2. Enrichment of AOM MicrobesThe anaerobic reactor was supplied with nitrate as the sole electron acceptor and methane as the only electron donor, to enrich AOM microbes in the sludge. The operation cycle of this reactor was 24-hour with a 40% volume exchange ratio. Within each cycle, the reactor ran continuously for 23 h, followed by 30 min of settling, 10 min of supernatant withdrawal, 10 min of fresh medium addition, and 10 min of methane purging. The medium consisted of the following components per liter: 0.5 g KHCO3, 0.05 g KH2PO4, 0.3 g CaCl2·2H2O, 0.2 g/L MgSO4·7H2O, 5 to 7 mg-N NaNO3, 0.5 mL acidic trace element solution, and 0.2 alkaline trace element solution as previously described [36]. The main ingredients of the medium were sterilized by autoclave, and the trace elements solution was treated with 0.2 μm filter. Before use, the medium was purged continuously with nitrogen to eliminate dissolved oxygen. Liquid samples were collected daily during the withdrawal step to monitor the concentrations of nitrate and nitrite. After the microbial activity reached steadily, solid samples were taken for DNA extraction and quantitative PCR analysis to assess the abundance of typical AOM microbes.
2.3. Microbial reduction of tellurateTo achieve the microbial reduction of tellurate in the enriched consortium, no replenishment was supplied to phase out the nitrogen in the SBR, and tellurate was used to replace nitrate as the sole electron acceptor. The concentration of tellurate in the influent was 2.5–5 mg-Te/L and the cycle time of this stage varied from 1 to 3 days depending on reactor performance. Liquid samples were collected daily during the withdrawal step to detect the concentrations of tellurate and tellurite. Solid samples from the reactor were collected for XRD analysis to confirm the generation of elemental tellurium. Furthermore, tests of methane deficiency and recovery were also conducted to confirm the role of methane oxidation in tellurate reduction. Methane was replaced with nitrogen and later replenished to examine its influence on tellurate reduction. The operation cycle of these processes was 72 h, with tellurate replenished at the beginning of each cycle. This test was performed with two independent replicates. An abiotic control that lacked microorganisms was also included. The abiotic control consisted of a 1-L serum glass bottle containing 500 mL of medium with 4 mg-Te/L tellurate. After purging the abiotic reactor with nitrogen gas, the bottle was filled with enough methane for 1 h. The operation conditions were pH 7.0, magnetic stirrer at 150 rpm, and a temperature of 23°C. Liquid samples were collected hourly to scrutinize variations in tellurate and tellurite. A solid sample at the final stage was taken to reveal the microbial community of the mixed consortium.
2.4. Analytical MethodsLiquid samples collected from the SBR were subjected to filtration using 0.2 μm membranes, prior to analysis for nitrate, nitrite, tellurate, and tellurite. Nitrate and nitrite were determined by an ion chromatograph (AS22, Dionex) employing 4.5 mM Na2CO3 and 1.4 mM NaHCO3 solutions as eluent, with a flow rate of 1.0 mL/min. To analyze tellurate and tellurite, the spectrophotometric method using diethyldithiocarbamate (DDTC) and KI was implemented [44, 45]. This approach entailed the reaction of tellurite with DDTC to form yellow colloidal at neutral pH, which were then via a spectrophotometer at 340 nm. This reaction is not interfered with by the presence of tellurate. In contrast, the reaction of tellurate with KI resulted in the reduction of tellurate to tellurite [45], with measurement of total tellurium (tellurite and tellurate) achieved through the same spectrophotometric procedure. The phase composition and mineral elements in the last period of reactor sludge were analyzed by X-ray diffraction (XRD, SmartLab, Rigaku).
2.5. qPCR and Metagenomic SequencingFor qPCR experiments, 5 mL solid samples were centrifuged at 5000 rpm for 10 min. After centrifugation, the supernatant was removed, and the pellet (approximately 0.3 g) was stored at −80°C until DNA extraction. DNA was then extracted from the collected pellets using the DNeasy PowerSoil Pro Kit (Qiagen, Germany) as per the manufacturer’s instructions. The extracted DNA samples were washed using a One Step PCR Inhibitor Removal Kit (Zymo, USA), and were quantified via Qubit (Invitrogen). The DNA samples were stored at −20°C until for use. qPCR experiments were performed to quantify the 16S rRNA gene of NC10 Phylum [36] and ANME-2d [46], as well as the methane-oxidizing genes pmoA [47] and mcrA [48]. The information on primers used in this study is provided in Supplementary Table S1. Six independent qPCR reactions of two DNA samples were performed for each time point. qPCR reaction was performed on an Eppendorf reaplex2 system. Each 20 μL reaction volume contained 10 μL SYBR Green qPCR Master Mix (Sigma-Aldrich), 1 μL of each forward and reverse primer (10 μM), and 1 μL DNA template. To establish standard curves, targeted DNA fragments for each primer pair were synthesized using G-blocks standard service (IDT, USA) and diluted in ddH2O from 101 to 108 copies/μL. Finally, the abundance of target genes was calculated based on the dry weight of the sample.
To investigate the microbial community of the mixed consortium, a solid sample was taken by the end of the tellurate reduction. The sample was centrifuged, pelleted, and stored for DNA extraction. The DNA of the sample was isolated and purified, as described above. DNA libraries were prepared using Nextera XT kits and analyzed using the Illumina sequencing platform (San Diego, CA) for high throughput metagenomic sequencing at the University of Nebraska Medical Center Genomics Center, and the details as previously described [49]. The sequencing data has been deposited at the NCBI Sequence Read Archive with accession numbers PRJNA970988.
3. Results and Discussion3.1. Enrichment of AOM Microorganisms in Anaerobic SBRAOM microbes were initially determined to couple with nitrate or nitrite reduction [36–38], and the enrichment of associated consortium was regularly with the supplement of nitrate as the electron-electron acceptor [41, 43]. In this study, a mixed consortium of AOM microorganisms was enriched in an anaerobic SBR using nitrate as the only electron acceptor, and methane as the sole electron donor in the sludge. The SBR was initially established with 20 mg-N/L nitrite and 20 mg-N/L nitrate without replacing the solution for 7 days during the startup; however, no significant nitrate or nitrite reduction was observed in the reactor after seeding, likely due to inhibition from high nitrogen concentrations [50]. Hence, to activate the microbes, both nitrate and nitrite in the reactor were reduced to 10 mg-N/L on Day 8. Microbial activities resumed, and denitrification began to appear after 4 days of operation. Thereafter, the culture medium was periodically replaced from 5 mg-N/L to 7 mg-N/L nitrate/nitrite with a cycle of 24 h. However, the concentration of nitrite increased with the excess addition of nitrite, which indicated that microorganisms could not consume the amount of either external addition or internal nitrate conversion. Therefore, the influent concentration of nitrate was adjusted to 7 mg-N/L nitrate. The anaerobic SBR operated for 46 d until achieving steady microbial activity (Fig. 2a). The removal efficiency of nitrate elevated continuously from 0.1 mg-N/L/d to 1.6 mg-N/L/d (Fig. 2b). The SBR effectively consumed the external addition of nitrate, and the stability and denitrification activity of the SBR operation continuously improved. After replenishing the fresh medium on Day 44 for the next step of tellurate reduction, no replenishment was conducted for four days to phase out the nitrate and nitrite in the SBR, resulting in a slight decrease in the removal efficiency of nitrate (Fig. 2b).
Based on steadily increasing the microbial activity of AOM, quantitative PCR tests were employed to quantify the 16S rRNA gene of NC10 Phylum [36] and ANME-2d [46], and also the functional genes comprising particulate methane monooxygenase pmoA [47] and methyl-coenzyme M reductase mcrA [48]. Candidatus Methylomirabilis oxyfera (M. oxyfera), a gram-negative bacterium that belongs to the NC10 phylum [38]. It contains particulate methane monooxygenase (pmoA gene) and can oxidize methane and reduce nitrite to nitrogen gas by intracellular aerobic denitrification pathway. Candidatus Methanoperedens nitroreducens (M. nitroreducens) is a typical methane oxidation archaea affiliated to ANME-2d cluster, which oxidizes methane using the reverse methanogenic pathway and reduces nitrate to nitrite using the nitrate reductase gene narGH [51]. The characteristic enzyme of methanotrophic and methanogenic pathways is methyl-coenzyme M reductase (MCR). The primers, McrA159F and McrA345R, are specific for detecting mcrA from reactor sludge or environmental sample [48]. The abundance of the functional genes and the 16S rRNA genes increased in the SBR when nitrate was used as the electron acceptor (Fig. 3). The abundance of mcrA and pmoA increased by 9.5 and 1.25 folds, respectively, during the SBR operation, indicating an increased abundance of AOM microbes, particularly ANME-2d [37]. Taking together the above results, the increasing abundance of methane oxidation genes and related microorganisms indicate the increasing abundance of AOM microbes.
3.2. Achieving Microbial Tellurate Reduction in Established SBRTo achieve reduction of tellurate in the SBR using the enriched microbial consortium, the sole electron acceptor was switched from nitrate to tellurate (Fig. 4a). The influent concentration ranged from 2.5 to 5 mg-Te/L, and the cycle time was set between 1 to 3 d to match the microbial activity. Tellurate reduction in the SBR began immediately even without prior adaptation, and the reduction rate of tellurate was 0.25 mg-Te/L/d (Fig. 4b). The reduction rate reached 0.55 mg-Te/L/d on Day 5 and the highest reduction rate of tellurate, 0.65 mg-Te/L/d, was achieved on Day 22 (Fig. 4b). Tellurite was nearly undetected throughout the operation with only two occasions at 0.11 mg-Te/L and 0.15 mg-Te/L. These results indicated that the AOM-enriched reactor was effective in reducing tellurate to tellurite, and the latter could be further transformed into elemental tellurium. Thus, the XRD analysis of the biomass composition during confirmed the presence of elemental tellurium in the system (Fig. 5). The intensity peaks of tellurium were weak, due to the low abundance of tellurium. Further, the tellurate reduction could not be performed in an abiotic experiment (data not shown), thereby demonstrating that microbial nature of tellurate reduction. The detection of elemental tellurium in the SBR confirmed the integrated microbial reduction of tellurate to elemental tellurium by the mixed consortium.
In this study, we established an anaerobic SBR for the enrichment of methanotrophic microbes using methane as the sole carbon source and nitrate as the electron acceptors. As a result, denitrifying capacity was enhanced with sustained operation of the constructed reactor, owing to the efficient consumption of provided nitrate. Further, the removal rate of tellurate reached its peak at 0.65 mg-Te/L/d when nitrate was purged from the reactor and the electron acceptor was systematically switched to tellurate, with virtually no tellurite detected throughout the operation. This finding supports the complete transformation of tellurate, directly converting it to elemental tellurium within the reactor. Nitrate is a common contaminant responsible for eutrophication soil or water environment [52], yet some microorganisms, including the denitrifying microbes, can tolerate and thrive in such conditions. Moreover, microorganisms used in this study came from nitrate-cultivated sludge, and these kinds of denitrifying microbes would preferentially utilize nitrate as the electron acceptor. Nevertheless, tellurate and tellurite are toxic compounds that occur in many environments and adversely affect microbial metabolic activity [53]. The doses of tellurate used in this study were higher than previously reported [53], thereby slowing down the reduction rate, or even leading to the reduction being incomplete.
Furthermore, the relationship between the microbial reduction of tellurate and methane oxidation was not confirmed. Therefore, tests of methane deficiency and recovery were conducted. During this experiment, methane served as the sole electron donor and was replenished at a pressure of 2 atm immediately following the replenishment of fresh medium, ensuring that there was excess methane in both the sludge and headspace. When methane was sufficiently supplied, tellurate was constantly reduced, with an average reduction rate of 0.017 mg-Te/L/h within 70 h (Fig. 6a). To confirm that methane in the bioreactor played a role in tellurate reduction, methane was replaced by pure nitrogen gas in the replenishing step (Fig. 6b). After purging methane from the reactor completely with nitrogen gas for 1 h, only 0.01% (v/v) methane remained in the headspace. Despite this, tellurate unexpectedly decreased from 1.83 mg-Te/L to 1.62 mg-Te/L over the next 14 h. High methane pressure in the headspace of SBR could not effectively increase the mass transfer of methane between the microbial consortium, and therefore could not significantly increase the activity of microorganisms to oxidize methane [54]. However, the microbial reduction of tellurate was subsequently abolished due to the shortage of methane (Fig. 6b). Upon reestablishing the methane supplement, the reduction of tellurate resumed with a reduction rate of 0.025 mg-Te/L/h. Overall, these results suggest that the enriched consortium relied on methane as the carbon source for the microbial reduction of tellurate to elemental tellurium.
3.3. Microbial Community of the Tellurate Reducing ConsortiumIn the final stage of SBR, a solid sample was collected for high-throughput sequencing to reveal the microbial community. The structure of this microbial community is illustrated in Fig. 7 at the family and genus levels. Comamonadaceae was found to be the most abundant family in the reactor, and it includes several denitrifying species typically found in activated sludge [55]. Other identified families, such as Rhodobacteraceae, Rhodocyclaceae, and Pseudomonadaceae, also contained nitrate-reducing bacteria commonly present in wastewater or activated sludge [35]. Although detected in small quantities (Fig. 7a), known methanotrophic bacteria, such as Methylobacteriaceae, Methylococcaceae, Methylocystaceae, and Methylophilaceae, were identified, with a combined abundance of 0.90%. These microbes can couple methane oxidation with the reduction of oxyanions like nitrate, selenate, or perchlorate [35, 43, 56]. The presence of methane-oxidizing microbes in the SBR highlights their role in the nitrate and tellurate reduction processes (Fig. 7b). The dominant genus Methylobacterium, which belongs to Methylobacteriaceae, can transform perchlorate and chlorite to chloride using the electron from methane oxidation [35]. The enriched methanotrophs of the genera Pseudoxanthomonas and Candidatus Methylomirabilis were also identified. Specifically, Pseudoxanthomonas sp. Q3, one species of Pseudoxanthomonas, can grow with methane as the sole carbon source [57]. Although Candidatus Methylomirabilis can conduct anaerobic methane oxidation coupled to nitrite [36], there are currently no reports of it being able to utilize electron acceptors other than nitrite [41]. Despite being detected by qPCR, Candidatus Methanoperedens was not observed through high-throughput sequencing, which implies that the sensitivities of the two methods may differ.
The functional, dominant microbes in this study are denitrifiers (Comamonadaceae, Rhodobacteraceae, Rhodocyclaceae and Pseudomonadaceae), methanotrophs (Methylobacteriaceae, Methylococcaceae, Methylocystaceae and Methylophilaceae), as well as heterotrophs. The presence of Candidatus Methanoperedens and Candidatus Methylomirabilis was detected using qPCR analysis based on the abundance of correlative genes. Given the coexistence of methanotrophs, denitrifiers, and other heterotrophs, we propose that the bioreduction of tellurate is a complex process, possibly driven by the diverse microbes in the reactor (Fig. 3 and Fig. 8). Archaea of methanotrophs independently oxidize methane at anaerobic conditions via a reverse methanogenesis pathway and may drive tellurate reduction [37]. Oxygenic methanotrophs might utilize extracellular/intracellular oxygen released from oxygenic bacteria for intra-aerobic pathways and oxidize methane via initial monooxygenation reaction and may produce organic intermediates, which serve as the carbon sources for denitrifiers [35]. In the future, additional studies are needed to investigate the reduction mechanism of tellurates by the enriched consortium in the reactor.
AOM archaea possess versatile abilities to use a range of electron receptors, including promoting the reduction of nitrate, iron/manganese, and selenate [27, 37, 41]. Given some of the similar physicochemical properties of selenium and tellurium, these archaea may play an important role in the microbial reduction of tellurate. Tellurium has multiple forms, such as tellurate (VI), tellurite (IV), elemental tellurium (0), and telluride (−II) in the natural environment [2]. Oxygenated tellurate and tellurite are noxious to archaea and bacteria, with tellurite exhibits harmfulness at a concentration of 1 μg/mL [58]. Since a bulky amount of tellurium compound was introduced into the reactor, it had an inevitable negative effect on the relevant consortium of AOM (Fig. 3). Luo et al. [41] investigated the microbial reduction of selenate in MBfR and they found that the persistent attachment of elemental selenium precipitate, produced by reduction, could cause adverse effects on microorganisms. This may be due to elemental selenium hindering the inter-microbial transfer of selenate or binding with extracellular polymers, resulting in decreased activity. Therefore, the resulting elemental tellurium in the reactor might also affect microbial activity for continued attachment to the cell surface [42]. However, due to its weak adhesion, elemental tellurium can be continuously removed by drainage in a continuous flow reactor, causing only a feeble effect on microbial activity [20]. In the current study, the generated elemental tellurium was dispersed in the sludge for discontinuous running. Thus, the toxicity of elemental tellurium and its compounds should be the main factor affecting the activity of AOM.
Many microorganisms, such as bacteria, fungi, and archaea, have demonstrated their ability to convert toxic tellurium oxyanions into low-toxicity elemental tellurium or nanoparticles [4, 12, 59]. For example, Rhodobacter capsulatus [19], Methanogens [20], Shewanella [21], Pseudomonas [22], and Stenotrophomonas [23] can reduce tellurium oxyanion species to their elemental state. In our earlier work, we isolated a Rhodopseudomonas palustris strain from wastewater that exhibited tellurite reduction ability [60]. In a CH4-based MBfR, Shi and colleagues demonstrated the feasibility of nanoscale Te0 formation by tellurite. The researchers found that Thermomonas and Hyphomicrobium in the mixed culture might conduct the tellurite reduction [42]. However, only a few strains are known to produce nanoparticles from tellurate, potentially because tellurite reduction to elemental tellurium is more prevalent than tellurate reduction in microorganisms [61]. In this study, we first prove the microbial tellurate reduction in an enriched consortium using methane as the carbon source. Further studies are needed to investigate the mechanism of tellurate reduction by the enriched consortium using methane as the carbon source and the formation of elemental tellurium.
4. ConclusionsA mixed consortium of AOM microbes was enriched to conduct the reduction of tellurate. Microbial tellurate reduction was achieved in the enriched consortium using methane as the carbon source. The analysis of community structure further indicated the coexistence of methanotrophs, denitrifiers, and other heterotrophs in the reactor. However, further investigation is required to determine the molecular mechanism of the enriched consortium driving the microbial reduction of tellurate into elemental tellurium. These findings confirm the role of enriched microbial communities using a lab-scale SBR in tellurate reduction and lay the foundation for future studies on the mechanism of tellurate reduction using methane as an electron donor. This study advances our understanding of tellurium cycles and may facilitate the future study of the biogeochemistry cycle of tellurium.
AcknowledgmentsThis work was supported by the Double First-Class Environmental Science and Ecology of Chengdu University of Technology (No. 10800-18Z0101) and the Open Project of Key Laboratory of Development and Application of Rural Renewable Energy, Ministry of Agriculture, China (No. 2017011).
NotesAuthors Contributions H.G.X. (Ph.D.), M.C. (Ph.D.), and X.L. (Professor) contributed to the study conception. M.C. (Ph.D.) performed all the experiments and wrote the first draft of the manuscript. H.G.X. (Ph.D.), M.C. (Ph.D.) and X.F.L. (M.S. student) interpreted the data and edited the entire draft manuscript. G.Q.H. (Professor) and M.X.H. (Professor) reviewed the manuscript and participated in helpful the discussion section. X.L. (Professor) provided a critical review and substantially revised the manuscript. All authors read and approved the final manuscript. References1. Ba L, Doring M, Jamier V, Jacob C. Tellurium: an element with great biological potency and potential. Org. Biomol. Chem. 2010;8:4203–4216.
https://doi.org/10.1039/c0Ob00086h
2. Zannoni D, Borsetti F, Harrison JJ, Turner RJ. The bacterial response to the chalcogen metalloids Se and Te. Adv. Microb. Physiol. 2008;53:1–72.
https://doi.org/10.1016/S0065-2911(07)53001-8
3. Bureau B, Boussard-Pledel C, Lucas P, Zhang X, Lucas J. Forming glasses from Se and Te. Molecules. 2009;14:4337–4350.
https://doi.org/10.3390/molecules14114337
4. Zare B, Faramarzi MA, Sepehrizadeh Z, Shakibaie M, Rezaie S, Shahverdi AR. Biosynthesis and recovery of rod-shaped tellurium nanoparticles and their bactericidal activities. Mater. Res. Bull. 2012;47:3719–3725.
https://doi.org/10.1016/j.materresbull.2012.06.034
5. Makuei FM, Senanayake G. Extraction of tellurium from lead and copper bearing feed materials and interim metallurgical products - a short review. Miner. Eng. 2018;115:79–87.
https://doi.org/10.1016/j.mineng.2017.10.013
6. Fan Y, Yang Y, Xiao Y, Zhao Z, Lei Y. Recovery of tellurium from high tellurium-bearing materials by alkaline pressure leaching process: thermodynamic evaluation and experimental study. Hydrometallurgy. 2013;139:95–99.
https://doi.org/10.1016/j.hydromet.2013.07.005
7. Zhang T, Kong L, Dai Y, et al. Enhanced oils and organic solvents absorption by polyurethane foams composites modified with MnO2 nanowires. Chem. Eng. J. 2017;309:7–14.
https://doi.org/10.1016/j.cej.2016.08.085
8. Binnemans K, Jones PT, Blanpain B, Van Gerven T, Pontikes Y. Towards zero-waste valorisation of rare-earth-containing industrial process residues: a critical review. J. Clean. Prod. 2015;99:17–38.
https://doi.org/10.1016/j.jclepro.2015.02.089
9. Gupta PK, Sharma PP, Sharma A, Khan ZH, Solanki PR. Electrochemical and antimicrobial activity of tellurium oxide nanoparticles. Mater. Sci. Eng. B. 2016;211:166–172.
https://doi.org/10.1016/j.hydromet.2013.07.005
10. Deng Z, Zhang Y, Yue J, Tang F, Wei Q. Green and orange CdTe quantum dots as effective pH-sensitive fluorescent probes for dual simultaneous and independent detection of viruses. J. Phys. Chem. B. 2007;111:12024–12031.
https://doi.org/10.1021/jp074609z
11. Philip N. Losses and environmental aspects of a byproduct metal: tellurium. Environ. Chem. 2019;16:243–250.
https://doi.org/10.1071/EN18282
12. Missen OP, Ram R, Mills SJ, et al. Love is in the Earth: A review of tellurium (bio)geochemistry in surface environments. Earth-Science Rev. 2020;204:103150.
https://doi.org/10.1016/j.earscirev.2020.103150
13. Belzile N, Chen YW. Tellurium in the environment: A critical review focused on natural waters, soils, sediments and airborne particles. Appl. Geochemistry. 2015;63:83–92.
https://doi.org/10.1016/j.apgeochem.2015.07.002
14. Filella M, Reimann C, Biver M, Rodushkin I, Rodushkina K. Tellurium in the environment: current knowledge and identification of gaps. Environ. Chem. 2019;16:215–228.
https://doi.org/10.1071/EN18229
15. Gil-Díaz T, Schäfer J, Dutruch L, et al. Tellurium behaviour in a major European fluvial-estuarine system (Gironde, France): fluxes, solid/liquid partitioning and bioaccumulation in wild oysters. Environ. Chem. 2019;16:229–242.
https://doi.org/10.1071/EN18226
16. Presentato A, Turner RJ, Vásquez CC, Yurkov V, Zannoni D. Tellurite-dependent blackening of bacteria emerges from the dark ages. Environ. Chem. 2019;16:266–288.
https://doi.org/10.1071/EN18238
17. Avazeri C, Turner RJ, Pommier J, Weiner JH, Giordano G, Vermeglio A. Tellurite reductase activity of nitrate reductase is responsible for the basal resistance of Escherichia coli to tellurite. Microbiology. 1997;143:1181–1189.
https://doi.org/10.1099/00221287-143-4-1181
18. Castro ME, Molina R, Díaz W, Pichuantes SE, Vásquez CC. The dihydrolipoamide dehydrogenase of Aeromonas caviae ST exhibits NADH-dependent tellurite reductase activity. Biochem. Biophys. Res. Commun. 2015;375:91–94.
https://doi.org/10.1016/j.bbrc.2008.07.119
19. Borghese R, Baccolini C, Francia F, Sabatino P, Turner RJ, Zannoni D. Reduction of chalcogen oxyanions and generation of nanoprecipitates by the photosynthetic bacterium Rhodobacter capsulatus
. J. Hazard. Mater. 2014;269:24–30.
https://doi.org/10.1016/j.jhazmat.2013.12.028
20. Ramos-Ruiz A, Sesma-Martin J, Sierra-Alvarez R, Field JA. Continuous reduction of tellurite to recoverable tellurium nanoparticles using an upflow anaerobic sludge bed (UASB) reactor. Water Res. 2017;108:189–196.
https://doi.org/10.1016/j.watres.2016.10.074
21. Kim DH, Kim MG, Jiang S, Lee JH, Hur HG. Promoted reduction of tellurite and formation of extracellular tellurium nanorods by concerted reaction between iron and Shewanella oneidensis MR-1. Environ. Sci. Technol. 2013;47:8709–8715.
https://doi.org/10.1021/es401302w
22. Chien CC, Jiang MH, Tsai MR, Chien CC. Isolation and characterization of an environmental cadmium-and tellurite-resistant Pseudomonas strain. Environ. Toxicol. Chem. 2011;30:2202–2207.
https://doi.org/10.1002/etc.620
23. Kagami T, Fudemoto A, Fujimoto N, et al. Isolation and characterization of bacteria capable of reducing tellurium oxyanions to insoluble elemental tellurium for tellurium recovery from wastewater. Waste Biomass Valor. 2012;3:409–418.
https://doi.org/10.1007/s12649-012-9145-3
24. Knittel K, Boetius A. Anaerobic oxidation of methane: progress with an unknown process. Annu. Rev. Microbiol. 2009;63:311–334.
https://doi.org/10.1146/annurev.micro.61.080706.093130
25. Martens CS, Berner RA. Methane production in the interstitial waters of sulfate-depleted marine sediments. Science. 1974;185:1167–1169.
https://doi.org/10.1126/science.185.4157.1167
26. Milucka J, Ferdelman TG, Polerecky L, et al. Zero-valent sulphur is a key intermediate in marine methane oxidation. Nature. 2012;491:541–546.
https://doi.org/10.1038/nature11656
27. Beal EJ, House CH, Orphan VJ. Manganese- and iron-dependent marine methane oxidation. Science. 2009;325:184–187.
https://doi.org/10.1126/science.1169984
28. Segarra KEA, Comerford C, Slaughter J, Joye SB. Impact of electron acceptor availability on the anaerobic oxidation of methane in coastal freshwater and brackish wetland sediments. Geochim. Cosmochim. Acta. 2013;115:15–30.
https://doi.org/10.1016/j.gca.2013.03.029
29. Sivan O, Antler G, Turchyn AV, Marlow JJ, Orphan VJ. Iron oxides stimulate sulfate-driven anaerobic methane oxidation in seeps. Proc. Natl. Acad. Sci. U. S. A. 2014;111:E4139–E4147.
https://doi.org/10.1073/pnas.1412269111
30. Wankel SD, Adams MM, Johnston DT, Hansel CM, Joye SB, Girguis PR. Anaerobic methane oxidation in metalliferous hydrothermal sediments: influence on carbon flux and decoupling from sulfate reduction. Environ. Microbiol. 2012;14:2726–2740.
https://doi.org/10.1111/j.1462-2920.2012.02825.x
31. Xie F, Ma A, Zhou H, et al. Niche differentiation of denitrifying anaerobic methane oxidizing bacteria and archaea leads to effective methane filtration in a Tibetan alpine wetland. Environ. Int. 2020;140:105764.
https://doi.org/10.1016/j.envint.2020.105764
32. Yan H, Li J, Meng J, Wang X, Tang L, Jha AK. A mixed culture performing nitrite-dependent anaerobic methane oxidation and the nitrite removal mechanism revealed by high-throughput sequencing. Environ. Eng. Res. 2022;27:230135.
https://doi.org/10.4491/eer.2021.135
33. Shi LD, Lv PL, Wang M, Lai CY, Zhao HP. A mixed consortium of methanotrophic archaea and bacteria boosts methane-dependent selenate reduction. Sci. Total Environ. 2020;732:139310.
https://doi.org/10.1016/j.scitotenv.2020.139310
34. Mal J, Nancharaiah Y, van Hullebusch ED, Lens PNL. Biological removal of selenate and ammonium by activated sludge in a sequencing batch reactor. Bioresour. Technol. 2017;229:11–19.
https://doi.org/10.1016/j.biortech.2016.12.112
35. Xie T, Yang Q, Winkler MKH, et al. Perchlorate bioreduction linked to methane oxidation in a membrane biofilm reactor: Performance and microbial community structure. J. Hazard. Mater. 2018;357:244–252.
https://doi.org/10.1016/j.jhazmat.2018.06.011
36. Ettwig KF, Van Alen T, Van De Pas-Schoonen KT, Jetten MSM, Strous M. Enrichment and molecular detection of denitrifying methanotrophic bacteria of the NC10 phylum. Appl. Environ. Microbiol. 2009;75:3656–3662.
https://doi.org/10.1128/AEM.00067-09
37. Haroon MF, Hu S, Shi Y, et al. Anaerobic oxidation of methane coupled to nitrate reduction in a novel archaeal lineage. Nature. 2013;599:567–570.
https://doi.org/10.1038/nature12375
38. Ettwig KF, Butler MK, Le Paslier D, et al. Nitrite-driven anaerobic methane oxidation by oxygenic bacteria. Nature. 2010;464:543–548.
https://doi.org/10.1038/nature08883
39. Shi LD, Lv PL, McIlroy SJ, et al. Methane-dependent selenate reduction by a bacterial consortium. ISME J. 2021;15:3683–3692.
https://doi.org/10.1038/s41396-021-01044-3
40. Lai CY, Lv PL, Dong QY, Yeo SL, Rittmann BE, Zhao HP. Bromate and nitrate bioreduction coupled with poly-β-hydroxybutyrate production in a methane-based membrane biofilm reactor. Environ. Sci. Technol. 2018;52:7024–7031.
https://doi.org/10.1021/acs.est.8b00152
41. Luo JH, Chen H, Hu S, Cai C, Yuan Z, Guo J. Microbial selenate reduction driven by a denitrifying anaerobic methane oxidation biofilm. Environ. Sci. Technol. 2018;52:4006–4012.
https://doi.org/10.1021/acs.est.7b05046
42. Shi LD, Du JJ, Wang LB, et al. Formation of nanoscale Te0 and its effect on TeO32− reduction in CH4-based membrane biofilm reactor. Sci. Total Environ. 2018;655:1232–1239.
https://doi.org/10.1016/j.scitotenv.2018.11.337
43. Lai CY, Wen LL, Shi LD, et al. Selenate and nitrate bioreductions using methane as the electron donor in a membrane biofilm reactor. Environ. Sci. Technol. 2016;50:10179–10186.
https://doi.org/10.1021/acs.est.6b02807
44. Turner RJ, Weiner JH, Taylor E. Use of diethyldithiocarbamate for quantitative determination of tellurite uptake by bacteria. Anal. Biochem. 1992;204:292–295.
https://doi.org/10.1016/0003-2697(92)90240-8
45. Chen M, Wu L, Yi X, Yang K, Xie H. Tellurium speciation in a bioleaching solution by hydride generation atomic fluorescence spectrometry. Anal. Methods. 2017;9:3061–3066.
https://doi.org/10.1039/C7AY00866J
46. Ding J, Ding ZW, Fu L, Lu YZ, Cheng SH, Zeng RJ. New primers for detecting and quantifying denitrifying anaerobic methane oxidation archaea in different ecological niches. Appl. Microbiol. Biotechnol. 2015;99:9805–9812.
https://doi.org/10.1007/s00253-015-6893-6
47. Han P, Gu JD. A newly designed degenerate PCR primer based on pmoA gene for detection of nitrite-dependent anaerobic methane-oxidizing bacteria from different ecological niches. Appl. Microbiol. Biotechnol. 2013;97:10155–10162.
https://doi.org/10.1007/s00253-013-5260-8
48. Vaksmaa A, Jetten MSM, Ettwig KF, Lüke C. McrA primers for the detection and quantification of the anaerobic archaeal methanotroph ‘Candidatus Methanoperedens nitroreducens’. Appl. Microbiol. Biotechnol. 2017;101:1631–1641.
https://doi.org/10.1007/s00253-016-8065-8
49. Baral D, Dvorak BI, Admiraal D, Jia S, Zhang C, Li X. Tracking the sources of antibiotic resistance genes in an urban stream during wet weather using shotgun metagenomic analyses. Environ. Sci. Technol. 2018;52:9033–9044.
https://doi.org/10.1021/acs.est.8b01219
50. Ding J, Fu L, Ding ZW, Lu YZ, Cheng SH, Zeng RJ. Environmental evaluation of coexistence of denitrifying anaerobic methane-oxidizing archaea and bacteria in a paddy field. Appl. Microbiol. Biotechnol. 2016;100:439–446.
https://doi.org/10.1007/s00253-015-6986-2
51. Hatamoto M, Kimura M, Sato T, et al. Enrichment of denitrifying methane-oxidizing microorganisms using up-flow continuous reactors and batch cultures. PLoS One. 2014;9:e115823.
https://doi.org/10.1371/journal.pone.0115823
52. Deng F, Hou L, Min L, et al. Dissimilatory nitrate reduction processes and associated contribution to nitrogen removal in sediments of the Yangtze Estuary. J. Geophys. Res. Biogeosciences. 2015;120:1521–1531.
https://doi.org/10.1002/2015JG003007
53. Taylor A. Biochemistry of tellurium. Biol. Trace Elem. Res. 1996;55:231–239.
https://doi.org/10.1007/BF02785282
54. Hu B, He Z, Geng S, et al. Cultivation of nitrite-dependent anaerobic methane-oxidizing bacteria: impact of reactor configuration. Appl. Microbiol. Biotechnol. 2014;98:7983–7991.
https://doi.org/10.1007/s00253-014-5835-z
55. Osaka T, Yoshie S, Tsuneda S, Hirata A, Iwami N, Inamori Y. Identification of acetate-or methanol-assimilating bacteria under nitrate-reducing conditions by stable-isotope probing. Microb. Ecol. 2006;52:253–266.
https://doi.org/10.1007/s00248-006-9071-7
56. Chen R, Luo YH, Chen JX, et al. Evolution of the microbial community of the biofilm in a methane-based membrane biofilm reactor reducing multiple electron acceptors. Environ. Sci. Pollut. Res. 2016;23:9540–9548.
https://doi.org/10.1007/s11356-016-6146-y
57. Kong SQ, She YH, Jia CZ, Wang C, Vauesa CCR. Physiological characteristics and primary identification of a methane-fed bacterium from a natural gasfield in China. Int. Biodeterior. Biodegrad. 2013;76:67–70.
https://doi.org/10.1016/j.ibiod.2012.06.006
58. Presentato A, Piacenza E, Anikovskiy M, Cappelletti M, Zannoni D, Turner RJ.
Rhodococcus aetherivorans BCP1 as cell factory for the production of intracellular tellurium nanorods under aerobic conditions. Microb. Cell Fact. 2016;15:204.
https://doi.org/10.1186/s12934-016-0602-8
59. Ao B, He F, Lv J, et al. Green synthesis of biogenetic Te(0) nanoparticles by high tellurite tolerance fungus Mortierella sp. AB1 with antibacterial activity. Front. Microbiol. 2022;13:1020179.
https://doi.org/10.3389/fmicb.2022.1020179
60. Xie HG, Xia W, Chen M, Wu LC, Tong J. Isolation and characterization of the tellurite-reducing photosynthetic bacterium, Rhodopseudomonas palustris strain TX618. Water. Air. Soil Pollut. 2018;229:158.
https://doi.org/10.1007/s11270-018-3817-y
61. Maltman C, Donald LJ, Yurkov V. Tellurite and tellurate reduction by the aerobic anoxygenic phototroph Erythromonas ursincola, strain KR99 is carried out by a novel membrane associated enzyme. Microorganisms. 2017;5:20.
https://doi.org/10.3390/microorganisms5020020
Fig. 2The concentration of nitrogen (a) and nitrate removal rate (b) during the enrichment process of AOM microbes. 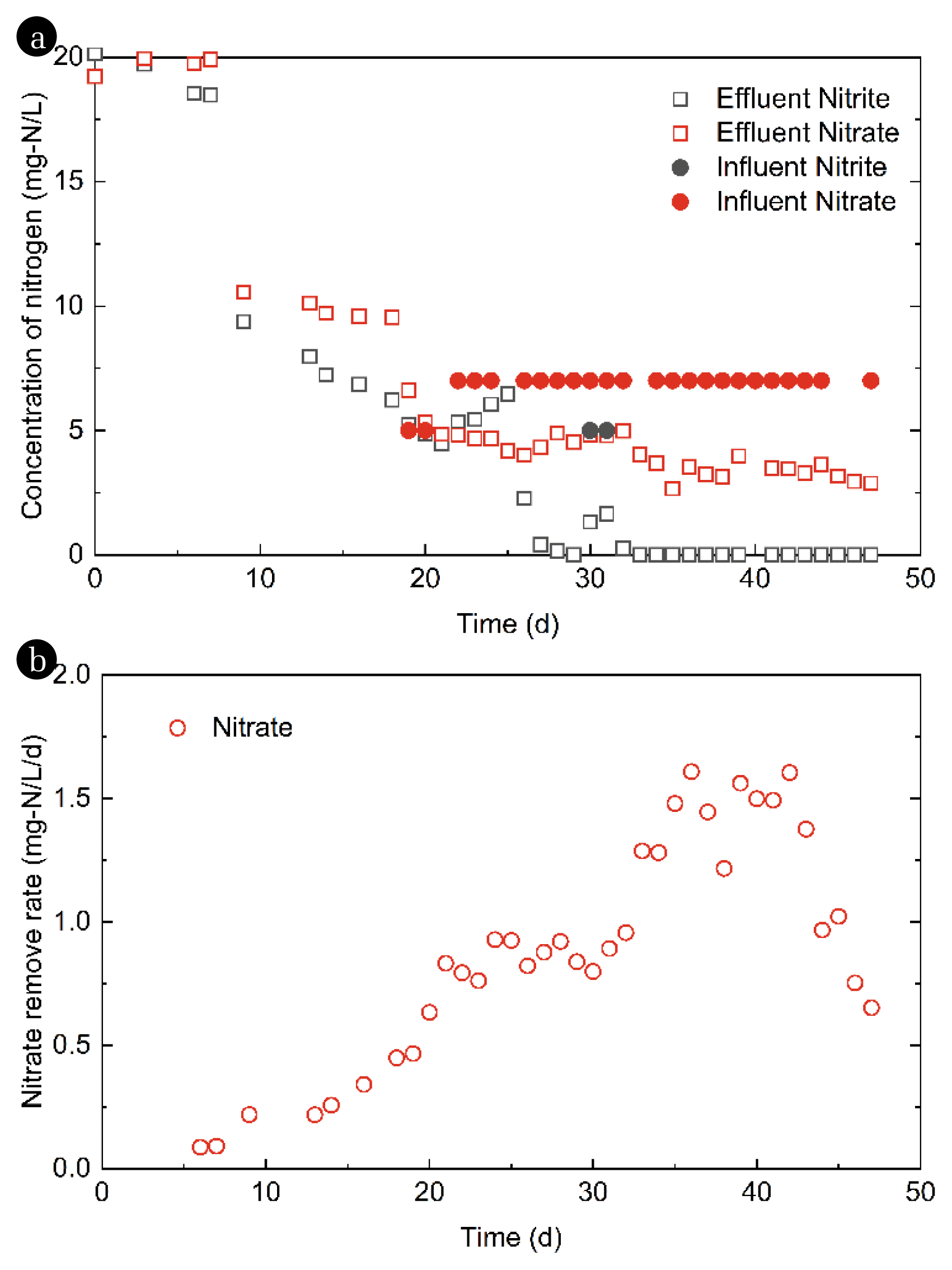
Fig. 3The abundance of the methane-oxidizing genes, and the archaeal and bacterial 16S rRNA gene for methane oxidation during the enrichment of AOM microbes and after tellurate reduction. The abundance of genes was calculated based on the volume of the sample. ** means p<0.01. 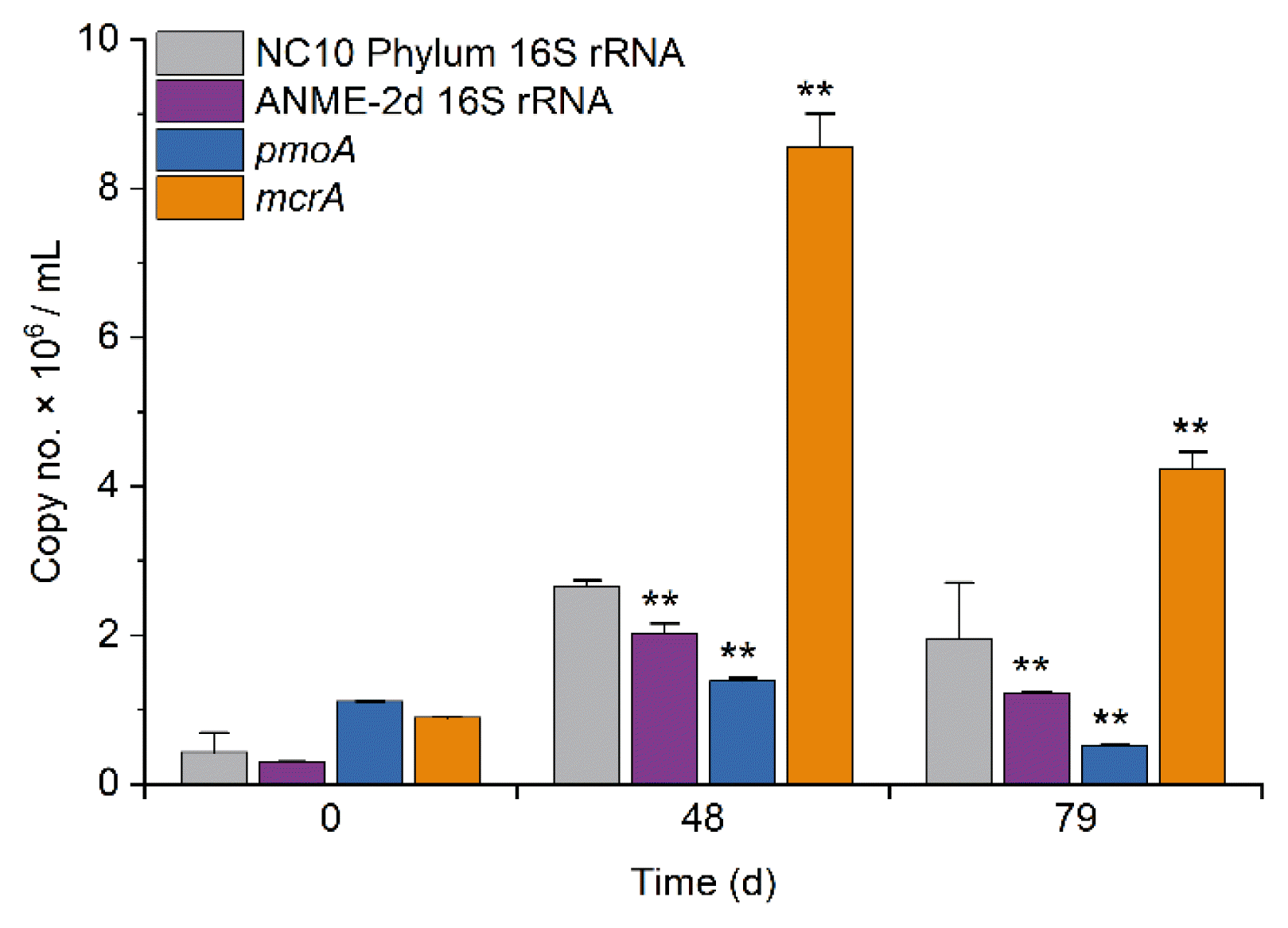
Fig. 4The concentration of tellurium (a) and tellurium removal rate (b) in the AOM microbes enriched SBR. 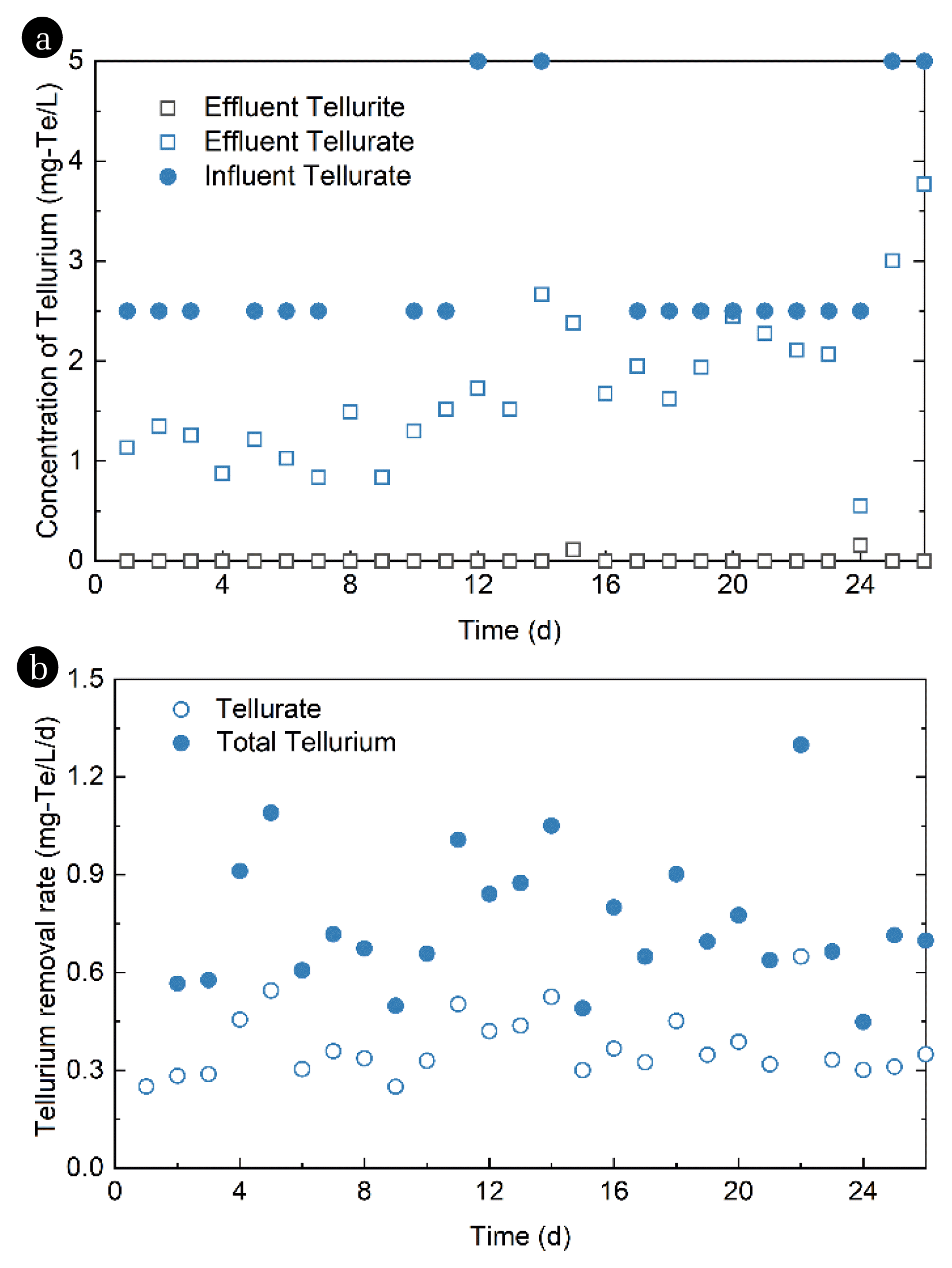
Fig. 7Relative abundance of dominating populations in the SBR on Day 79 at the family level (a). Others indicated the sum of the genus whose relative abundance was below 1%. Genera that are the methanotrophs and methylobacterium detected in the metagenomic library, which were speculated for tellurate reduction in SBR (b). 
|
|
||||||||||||||||||||||||||||||||||||