1. Introduction
Boezem is a temporary rainwater pool. Boezem of Kalidami is one of the boezem in Surabaya. The Surabaya Government does not yet have the domestic waste treatment so the majority of domestic waste is directly discharged into the water body and empty into the boezem. Domestic waste contains high nutrient and organic substances [1]. Increased of nutrients, especially Nitrogen (N) and Phosphor (P) in water, whether derived from waste or from the decomposition of organic substances can cause eutrophication [2–4]. Eutrophication causes an increase in algae and aquatic plants, ecological disturbances mainly related to food chain and adaptation of aquatic organisms [2].
The high rate algae pond (HRAP) is a waste treatment with shallow oxidation ponds and using a combination of algal bacterial [5, 6]. Photosynthesis of algae produces oxygen (O2) that will used by heterophilic bacteria to degrade organic substances. Nutrients and carbondioxide (CO2) from oxidation will be used by algae. HRAP waste treatment has some benefits that it is cost-effective and algae energy is converted to biofuel, biogas, and bioethane as biofuels [7], its reproduction is fast, non-pathogenic, the range of toxicity is extensive [8], useful for environmental sustainability [5], and environmentally friendly [9].
Several studies that have been conducted to examine the ability of microalgae in remediating polluted environments among others are HRAP in managing greywater on average can reduce biological oxygen demand (BOD5) to 69%, chemical oxygen demand (COD) to 62%, NO3 to 23%, NH4 to 52%, PO4 to 43% [7]. Bioremediation of Porphyra leucosticta red algae can reduce metals Cd(II) and Pb(I) with maximum capacities of 31.45 mg/g and 36.63 mg/g, respectively [10], Scenedesmus sp. can reduce Cr 98.63% and Cl− 54.18% [11]. Microalgae can reduce Cl− 66.98% [12]. Sago wastewater treatment with symbiosis of algal bacterial can decrease COD 90.29%, BOD 82.74%, TSS 84.52%, nitrate 82.85%, and phosphate 98.66%, increase in dissolved oxygen (DO) 73.82%, increase in pH 97.56% [13].
Algae growth affected by the availability of nutrients and the interaction of physical factors, such as pH, light intensity, aeration, and biotic factors [6, 14, 15]. The high concentration of ammonia (NH3-N) and organic substances in the body of water indicate the contamination of water bodies, one of them caused by domestic waste [16]. Organic compounds, such as COD or BOD5 and nutrients, such as N, K, P, carbon (C) are compounds needed for the growth of algae and bacteria [17]. Almost all types of algae have chlorophyll, so they can produce their own food through photosynthesis.
During the photosynthesis, algae needs CO2 and produce O2. Algae provide O2 for bacteria to carry out aerobic respiration so that it can degrade waste, then the bacteria produce CO2 which used for algae photosynthesis [13].
The study regarding the effect of adding K and C element to the reduction of NH3 and organic substances in the form of permanganate numbers (KMnO4) in boezem water treatment contaminated with domestic waste has never been done. Therefore, this study intended to (1) Determine the characteristics of Kalidami boezem water in Surabaya, Indonesia, (2) Examine the effect of adding K element form of potassium dihydrogen phosphate (KH2PO4) and C in the form of sucrose to the levels of NH3-N and organic substances in the form of permanganate numbers (KMnO4) of boezem water.
2. Materials and Methods
This research started with an analysis of the initial characteristics of Kalidami boezem water in Surabaya, including pH, DO, COD, BOD, NH3-N and number of KMnO4. Analysis of COD using closed reflux titrimetry, BOD and DO using winkler method, pH using pH meter, KMnO4 using titrimetry.
This research conducted in three stages. The first stage is algae culture, in order that the algae that used for bioremediation are truly ready. Algae culture carried out until reaching chlorophyll a concentration with amount of 3.5 mg/L [18]. Algae culture cultivation is done by adding the NPK fertilizers and aerated continuously. The analysis of chlorophyll a was conducted using spectrophotometric method [19, 20]. The algae that are grown derived from freshwater ponds in Wonorejo, Surabaya. Algae culture was carried out for 5 d and resulting in chlorophyll a with amount of 5.8 mg/L.
The second stage is range finding test (RFT). RFT aims to determine the comparison between the volume of boezem water and algae that can still be tolerated its existence by the algae. The volume comparison between boezem water and algae in RFT that is 25% boezem water: 75% algae, 50% boezem water: 50% algae, and 75% boezem water: 25% algae. The reactor for RFT is a glass reactor with a volume of 4 liters. RTF was done until obtained a concentration of chlorophyll a with a minimum of 3.5 mg/L. The best RTF is obtained in conditions with a comparison of boezem water: algae culture is 25%: 75%, for 7 (seven) d with concentration of chlorophyll a amounted to 3.8 mg/L.
The third stage is the Kalidami Surabaya boezem water treatment. This study conducted with a batch system, in a laboratory scale using a glass reactor with a volume of 8 liters, with additional variations of K and C elements and without aeration. The source of K element used KH2PO4 salt with variations in concentrations of 0%, 1%, and 3%. The source of C element used sucrose, with variations of 0 mg/L and 29.4 mg/L. This study was done in two repetitions or duplo. This study also used a control reactor namely the reactor without the addition of algae, K and C elements. The reactor code is presented in Table 1. Bioremediation was done for 18 d, analysis of NH3-N and KMnO4 was carried out on day 0, 3, 6, 9, 11, 13, 16 and 18. The analysis of NH3-N with SNI method 06 6989.30–2005 [21] and analysis of organic substances in the form KMnO4 using SNI method 06 6989.22–2004 [22].
3. Results and Discussion
3.1. Initial Characteristics of Kalidami Boezem Water
The characteristics of Kalidami boezem water are presented in Table 2. The results of the initial analysis of Kalidami boezem water, pH ranging from 7.48 ± 0.00 shows that the pH of boezem water is at neutral pH so it allows microorganisms to grow normally. The concentrations of DO ranging from 0.00 ± 0.00 mg/L, the boezem water conditions was muddy, this show that boezem water contains a lot of suspended solids and microorganisms that can be used as seeding processes. From the results of the analysis of the initial characteristics of the Kalidami boezem water demonstrated that the activity of microorganisms in boezem water was quite high to decompose organic compounds and oxidate nutrients but the DO levels were low so that the life of micro-organisms was disturbed, therefore water treatment needed to increase the DO of boezem water.
The level of BOD5 52.70 ± 0.17 mg/L and COD 122.20 ± 0.20 mg/L. The ratio of BOD5/COD was 0.43, still in the range of 0.30–0.80, indicates that water is easy to decompose naturally [23]. This shows that 43% of Kalidami boezem water is biodegradable, a category of untreated water [24]. From the data of ratio number of BOD5/COD, it can be concluded that the Kalidami boezem water is suitable for biological treatment.
The level of NH3-N was 10.82 ± 0.70 mg/L. The NH3-N in boezem water shows that organic nitrogen is converted by bacteria into ammonia [16]. Nitrification bacteria decompose ammonia into nitrite and nitrate. Nitrate is used by algae and other plants to form proteins. The nitrite content in water shows the amount of oxidized nitrogen. Nitrite is one of the important elements for the synthesis of plant proteins. The high content of nitrite and phosphate in the water can stimulate unlimited algae growth, so DO of water will decrease.
The number of KMnO4 of Kalidami boezem water was 62.80 ± 0.09 mg/L, this condition shows that boezem water is contaminated by organic substances. Organic substances are food for microorganisms. The existence of organic substances caused the water to become muddy, have a color, taste and smells bad, and low of DO. The higher the content of organic substances shows that the water is increasingly polluted.
3.2. Changes in NH3-N Levels
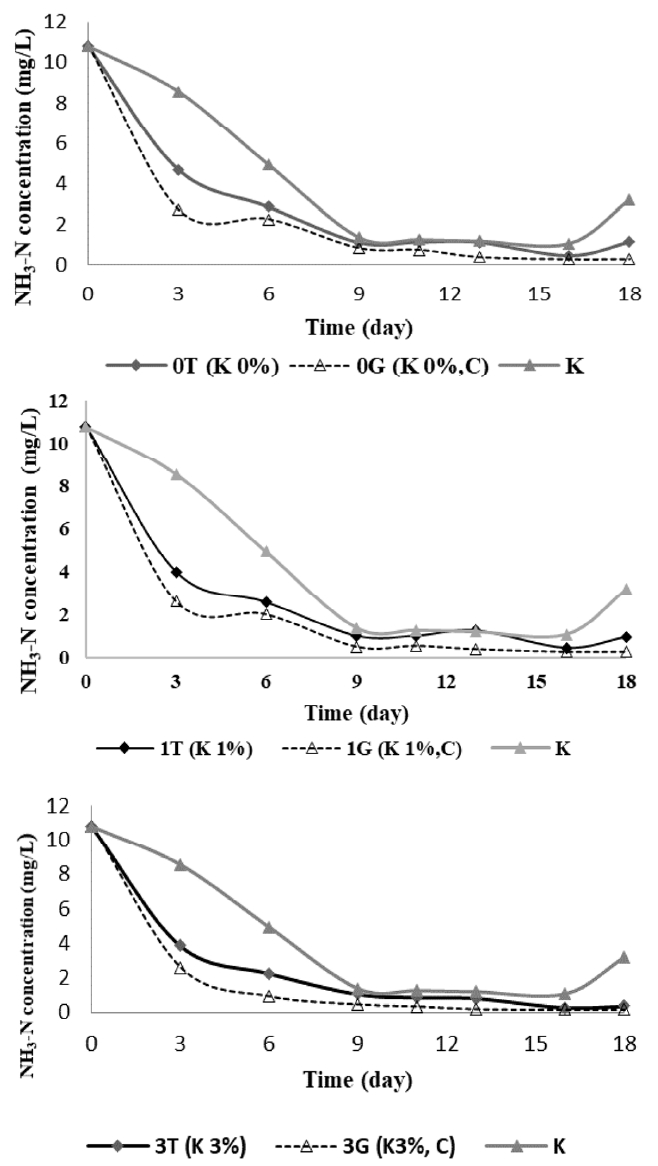
The effect of adding K and C elements to NH3-N levels is presented in Fig. 1. This showed that the addition of K (in the form of KH2PO4) and C (in the form of sucrose) elements affect the levels of NH3-N. At the beginning of the study, the NH3-N levels were 10.82 ± 0.70 mg/L, then it decreased fluctuatively. On day 3, the NH3-N levels experienced reduction drastically, especially in reactor with the addition of K elements without the addition of C element (0T, 1T, 3T) the average decline was 61% and the reactor with the addition of K and C (0G, 1G, 3G) elements was 76%. In the control of reactor (K) the reduction of NH3-N on day 3 was only 21%.
The highest control reactor and treatment reactor reduction of NH3-N were achieved on day 16. The highest control reactor reduction of NH3-N was 90%, the reactor with the addition of K element (0T, 1T, 3T) was the highest decrease on an average by 96% and the reactor with the addition of K and C elements (0G, 1G, 3G), the highest reduction of NH3-N on average by 98%. On the 18th day, the control reactor and the treatment reactor experienced an increase in the levels of HN3-N.
The reduction of NH3-N happened because of the nitrification process, nitrite bacteria (nitrosomonas) oxidate ammonia into nitrite [5, 16]. Nitrosomonnas sp. functions as ammonia converters to nitrite. Nitrobacter sp. functions as a converter of nitrite to nitrate. The high nitrate content will be used by Chlorella sp. as nutrient [13]. The reduction of NH3-N in the control reactor is caused by the process of decomposition and absorption of organic materials by bacteria. The reduction of NH3-N levels in the treatment reactor because of the symbiotic process of algal bacterial in biodegradation of organic substance of boezem water. In the metabolic process, heterotrophic bacteria degrade organic substances into inorganic substances which absorbed by algae during photosynthesis. The results of photosynthesis in the form of water (H2O), O2 and energy. O2 is used by bacteria to decompose organic substances in boezem water [13]. The more the algae that grow, then the more the inorganic compounds are used as algae nutrients so that NH3-N is decreasing.
Fluctuations of the reduction of NH3-N levels happen because of the availability of nutrients in boezem water. At the beginning of the study is adaptation phase (lag), bacteria acclimatize to pH, temperature and nutrient, not much use of nutrients for its growth, bacteria metabolize but the fission has not been significant so that the increased number of bacteria is slow [14, 25].
On the 3rd to 9th day, the reduction of NH3-N occur drastically, this phase is referred to as the exponential phase. The exponential phase of bacterial growth is very fast, because nutrients are still high so microorganisms are used for maximum growth [14, 25]. The exponential phase is stopped due to the decreasing nutrient so that the population of bacteria and algae also decreases.
On the 9th day until the 16th day was the stationary phase, the concentrations of NH3-N tended to be permanent, though the reduction was not too large. This phase demonstrated that bacteria do not experience exponential growth and bacterial growth is balanced by cell death, due to the reduction of nutrients and energy reserves in the media [14, 25].
On the 18th day is the phase of death, marked by the rate of death which is faster than the rate of bacterial growth [14]. The increase of NH3-N levels happen because of ammonification by bacteria that converts N-organic to ammonium and decomposition of living things that has been dead [26].
From this study it can be seen that the addition of K and C elements has an effect to the levels of NH3-N though there are little differences. The reactor with the addition of 3% K and C elements 29.4 mg/L (3G reactor) had the largest reduction value of NH3-N by 98% on the 16th day with the NH3-N levels was 0.16 ± 0.01 mg/L. This happen because in the reactor the nutrients for the growth of symbiotic algae-bacterial are sufficient so the process of decomposing organic substances in the boezem water is optimal. The addition of KH2PO4 and K2HPO4 functions as a buffer, namely the pH controller affects bacterial cell density. The higher the cell density, then the more acid is released into the media, with buffer then the pH can be maintained [17]. Sucrose as a food source and bacterial energy [25]. The addition of N, C and P sources will increase cell growth and density [17].
3.3. Changes in KMnO4 Levels
The effect of adding K and C elements to the number of KMnO4 is presented in Fig. 2. The initial number of KMnO4 for boezem water was 62.80 ± 0.09. All treatment reactors experienced reduction of KMnO4 numbers drastically on day 3. The reactor with the addition of K and C (0G, 1G, 3G) on the 3rd day experienced the highest average reduction of KMnO4 number by 45% and the reactor with the addition of K without the addition of C (0T, 1T, 3T) has the highest reduction on average by 38%. Whereas the control reactor reduced by only 21%. It started on the 6th day, the number of KMnO4 in all reactors are fluctuated but still experienced reduction until the 13th day. All reactors on day 13 experienced an increase in the number of KMnO4.
The reduction of the number of KMnO4 is drastically happen on the 3rd day was due to the availability of organic substances in boezem water so the microorganisms grow rapidly, so that the organic substances will also degraded quickly. The exponential phase occurs at the beginning of the study because of the abundance of nutrients [14]. The reduction in organic substances in the reactor treatment happens because of the symbiosis of algae with bacteria. The photosynthesis of microalgae will produce O2, which will be used by bacteria for their life and degrade organic substances into CO2, then CO2 is used by microalgae for photosynthesis [15]. Heterotrophic bacteria convert elements in boezem water into organic substances, which will degraded by algae in the process of photosynthesis into H2O, O2 and energy [13]. Organic substances in boezem water are also used as a nutrient source for bacterial growth [15], so there is a reduction in organic substances in the form of KMnO4 numbers.
The highest reduction in the number of KMnO4 was occur in the reactor with the addition of 3% K element and the addition of C element 29.40 mg/L (3G reactor) on day 11, with an efficiency reduction of KMnO4 numbers by 52% and the final KMnO4 number was 29.50 ± 0.70 mg/L. This happens because the addition of K element 3% and C element 29.40 mg/L causes adequate nutrient requirements for the growth of algae-bacterial symbiosis. The nutrients needed by myroorganisms for growth are C, N, S, P, Ca, Zn, Na, K, Cu, Mn, Mg, vitamins, water and energy [27]. The addition of C element (sucrose) functions as a source of energy [17], and co-substrate that can increase bacterial growth so that it can reduce the number of KMnO4. Potassium is a macro nutrient that functions to change the physical form of enzyme molecules, exposing active chemical sites which is suitable for reaction. The potassium also neutralizes various organic anions and other compounds in plants, which help stabilize the pH between 7 and 8, which is optimal for most of enzyme reactions. The potassium also plays a major role in the transportation of water and nutrients throughout the plants in xylem [28].
The increased of organic substances in boezem water is caused by the death of microorganisms that are no longer able to absorb nutrients in boezem water. The dead microorganisms will be released into boezem water so that the concentration of organic substances increases. This effect is referred to as depuration, that is the return of pollutants to the environment.
4. Conclusions
This research concludes that the boezem water of Kalidami Surabaya has pH value of 7.48 ± 0.00, DO of 0.00 ± 0.00 mg/L, NH3-N of 10.82 ± 0.70 mg/L, COD of 122.20 ± 0.20 mg/L, BOD5 of 52.70 ± 0.17 mg/L, and KMnO4 number of 62.80 ± 0.09 mg/L. These characteristics show that boezem water is possible to conduct a bioremediation of bacterial algae. The highest NH3-N reduction efficiency occurs in the reactor with the addition of K 3% and C 29.40 mg/L on day 16 with the decreasing efficiency of 98% and the final NH3-N level was 0.164 ± 0.01 mg/L. the highest decreasing efficiency of KMnO4 number occurs in the reactor with the addition of K 3% and C 29.40 mg/L on day 11 with the decreasing efficiency of 52% and the final KMnO4 number was 29.50 ± 0.70 mg/L.