Iron Mixed Ceramic Pellet for Arsenic Removal from Groundwater
Article information
Abstract
In this study, an innovative media, iron mixed ceramic pellet (IMCP) has been developed for arsenic (As) removal from groundwater. A porous, solid-phase IMCP (2–3 mm) was manufactured by combining clay soil, rice bran, and Fe(0) powder at 600°C. Both the As(III) and As(V) adsorption characteristics of IMCP were studied in several batch experiments. Structural analysis of the IMCP was conducted using X-ray absorption fine structure (XAFS) analysis to understand the mechanism of As removal. The adsorption of As was found to be dependent on pH, and exhibited strong adsorption of both As(III) and As(V) at pH 5–7. The adsorption process was described to follow a pseudo-second-order reaction, and the adsorption rate of As(V) was greater than that of As(III). The adsorption data were fit well with both Freundlich and Langmuir isotherm models. The maximum adsorption capacities of As(III) and As(V) from the Langmuir isotherm were found to be 4.0 and 4.5 mg/g, respectively. Phosphorus in the water had an adverse effect on both As(III) and As(V) adsorption. Scanning electron microscopy results revealed that iron(III) oxides/hydroxides are aggregated on the surface of IMCP. XAFS analysis showed a partial oxidation of As(III) and adsorption of As(V) onto the iron oxide in the IMCP.
1. Introduction
Arsenic (As) contamination in groundwater has caused a devastating health crisis all over the world, especially in developing countries [1]. Today, in Bangladesh and India, over 100 million people are consuming drinking water with As concentrations exceeding the guideline values of the World Health Organization and Environmental Protection Agency (10 μg/L) and of the Bangladesh standard (50 μg/L) [2]. This crisis is expanding to Southeast Asia, affecting several countries including China, Cambodia, and Vietnam [3]. Drinking As-contaminated water can result in various adverse health effects, including cancers of the skin, bladder, kidneys, and lungs [4].
Any system for removing arsenic from drinking groundwater in developing countries should meet several critical requirements: 1) easy manufacturing of the process; 2) efficient removal of highly contaminated groundwater; 3) efficient removal of both arsenite (As(III)) and arsenate (As(V)); 4) low-cost and easy maintenance; 5) high adsorptive capability; 6) sufficient mechanical strength and large surface area; 7) long life expectancy; 8) limited toxic sludge production; 9) safe disposal of sludge; and 10) limited secondary problems such as changes in pH or microbiology that affect the water quality [5]. Different techniques for the removal of As from groundwater have been developed and tested. These include oxidation and sedimentation, coagulation and co-precipitation (e.g., with iron or aluminium salts), ion exchange, adsorption by activated carbon, and membrane processes [5]. In most treatment methods, the oxidation of As(III) into As(V) is usually needed for the effective removal of arsenic from groundwater. Precipitation, co-precipitation, and adsorption are involved in the removal of As from groundwater in the process of coagulation. In the adsorption process, dissolved As is removed by attaching onto the supportive media at the molecular level. Commonly used supportive media include modified activated carbon, iron-coated sand, and iron-coated pottery granules [5–7]. Analysis of these techniques shows that none of these can presently satisfy all of the aforementioned criteria [8]. For instance, Dong et al. [7] developed an iron-coated pottery granule and investigated the efficiency of As removal from relatively low-contaminated water (150 μg/L of As). However, any developed adsorption media would have the capability of removing As from highly contaminated groundwater. In some of the As-contaminated regions of Bangladesh, groundwater contains high levels of As (>400 μg/L) [9].
It is necessary to develop a simple, inexpensive, and effective As adsorption media that would be affordable for the removal of arsenic from highly contaminated groundwater in developing countries. In this study, an innovative medium in the form of an iron mixed ceramic pellet (IMCP) has been developed for As removal from groundwater. We successfully developed the IMCP by combining clay soil, rice bran, and Fe(0) powder, and investigated both its As(III) and As(V) adsorption capability. Accordingly, batch study was conducted using the IMCP in various experimental conditions, including pH batch experiments, batch kinetics experiments, batch isotherm experiments, and experiments to examine the effect of a co-existing anion. Moreover, structural analysis of the IMCP was conducted by X-ray absorption fine structure (XAFS) analysis to understand the mechanism of As removal by the IMCP.
2. Materials and Methods
2.1. Synthesis of IMCP
IMCPs were manufactured by mixing clay soil, rice bran, and Fe(0) powder. This is the first novel method of manufacturing IMCP materials. The soil sample and the rice bran were collected from a local brick field and rice mill, respectively, in Khulna, which is in the south-western region of Bangladesh. The soil sample was dried, ground with a hammer, and passed through a 0.5-mm sieve. The rice bran was also dried and passed through a 1-mm sieve. The particle size distribution of the collected soil was measured by the laser diffraction method (SALD-3000; Shimadzu Corp., Kyoto, Japan). The results showed that the soil sample contained approximately 40% sand, 52% silt, and 8% clay. The Fe(0) powder (mesh size 200) was supplied by Wako Pure Chemical Industries, Ltd. (Osaka, Japan). To manufacture the IMCP, dry clay soil, rice bran, and Fe(0) powder were mixed homogeneously at a ratio of 80:20:40, respectively, on a weight basis. This dry mixture was then combined with de-ionized water to make a paste. The IMCPs were made manually from the paste with 2- to 3-mm diameter. The obtained IMCPs were then dried at 105°C for 24 hr and fired at 600ºC for 1.5 hr.
2.2. Arsenic Adsorption Experiments
The chemicals were all reagent-grade and obtained from Wako or Nacalai Tesque Inc., Japan. As(III) and As(V) stock solutions (100 mg/L) were prepared by dissolving arsenic oxide (As2O3) in de-ionized water with 5 mL/L of HCl and sodium arsenate (Na2HAsO4·7H2O) in de-ionized water, respectively. Phosphorus phosphate (PO4-P) stock solution (1,000 mg/L) was prepared by mixing reagent-grade K2HPO4 with de-ionized water. All the solutions for arsenic removal experiments and analysis were prepared by an appropriate dilution of stock solutions. Synthetic groundwater representing the geochemistry of Bangladesh groundwater [9] was prepared in the laboratory by dissolving the appropriate chemicals. Each litre of synthetic groundwater consisted of 10 mg of NH4C1, 500 mg of MgSO4·7H2O, 1.2 mg of NaCl, 400 mg of CaCl2·2H2O, 2 mg of MnSO4·5H2O, and 500 mg of NaHCO3. Synthetic groundwater with a desired concentration of As(III), As(V), and P was prepared by an appropriate dilution of stock solutions. Synthetic groundwater was prepared just before starting the experiments to avoid any pre-oxidation of the As(III) into As(V).
Both the As(III) and As(V) adsorption characteristics of the IMCP were studied in several batch systems, including pH experiments, kinetics experiments, isotherm experiments, and an experiment showing the effect of a co-existing anion (PO4-P) with the synthetic groundwater. The effect of pH on the As adsorption by IMCP was also carried out in a separate batch experiment by adjusting the pH from 3 to 11 using HCl or NaOH solutions. The adsorption kinetics were carried out at pH 7.0–7.5 at different time intervals with an initial As concentration of 750 μg/L and IMCP concentration of 2.5 mg/L. An isotherm study at pH 7.0–7.5 was carried out by varying the IMCP amount from 2.5 to 20 mg/L with a fixed amount of As (4 mg/L). Finally, the effects of P on As adsorption by IMCP were observed by varying the P concentration of 1 to 10 mg/L in synthetic groundwater containing 2 mg/L of As and 2.5 mg/L of IMCPs. All batch experiments were performed in a 100-mL Teflon bottle and shaking at 80 rpm at room temperature for a desired duration. All experiments were carried out in triplicate to ensure the reproducibility.
2.3. Solid Phase Characterization of IMCP
Morphological analysis of the samples was performed by emission scanning electron microscopy (SEM; JSM-6330F; JEOL Ltd., Tokyo, Japan). To understand the mechanism of As removal by the IMCP, iron and arsenic speciations of IMCP media were examined by Fe K-edge and As L3-edge spectra of XAFS. Samples were prepared by adding 5 g of IMCP to 1 L of synthetic groundwater containing 50 mg/L of As(III) and As(V) and shaking at 80 rpm. After shaking for 120 hr, IMCP media were taken out, air-dried, and ground to 0.5 mm. The XAFS analysis was undertaken on BL-3 for Fe and BL-10 for As at the Synchrotron Radiation Centre of Ritsumeikan University. The measurement was done using BERYL(10-10) in a high vacuum. Na2HAsO4 ·7H2O and KH2AsO4 were respectively used as a standard specimen of As(V) and As2O3, and NaAsO2 was used as a standard specimen of As(III). Fe(0), Fe2O3, and FeSO4·7H2O were used as standard specimens of Fe. The specific surface area (SBET) of the IMCP was measured by Brunauer-Emmett-Teller (BET) N2 method. The apparent porosity of the filter was tested according to the method presented by Yang et al. [10].
2.4. Analytical Methods
Arsenic was analyzed using a polarized Zeeman atomic absorption spectrophotometer (Z-2700; Hitachi, Tokyo, Japan) or an inductively coupled plasma-mass spectrometry (HP4500; Yokogawa, Tokyo, Japan). P was measured with the molybdenum blue colorimetric method (JIS K 0102: Japanese Industrial Standards). The pH was measured with a pH meter (D-54S; Horiba, Kyoto, Japan).
3. Results and Discussion
3.1. Solid-phase Characterization of IMCP before and after Adsorption
The SEM images of unused IMCPs and after 120 hr of adsorption are shown in Fig. 1(a) and (b), respectively. Particle aggregation of iron(III) oxide/hydroxide was observed after 120 hr of adsorption of As in Fig. 1(b). SEM pictures also clearly show the growth of a concentrated crystallite, which transform to an apparent amorphous phase in Fig. 1(a) and (b). Unused IMCP had a specific surface area of 112 m2/g. The porosity and pore size of the IMCP were 60±1% and 1–5 μm, respectively.

Scanning electron microscopy image of iron mixed ceramic pellet (IMCP): (a) unused IMCP and (b) after 120 hr of adsorption of arsenic by IMCP.
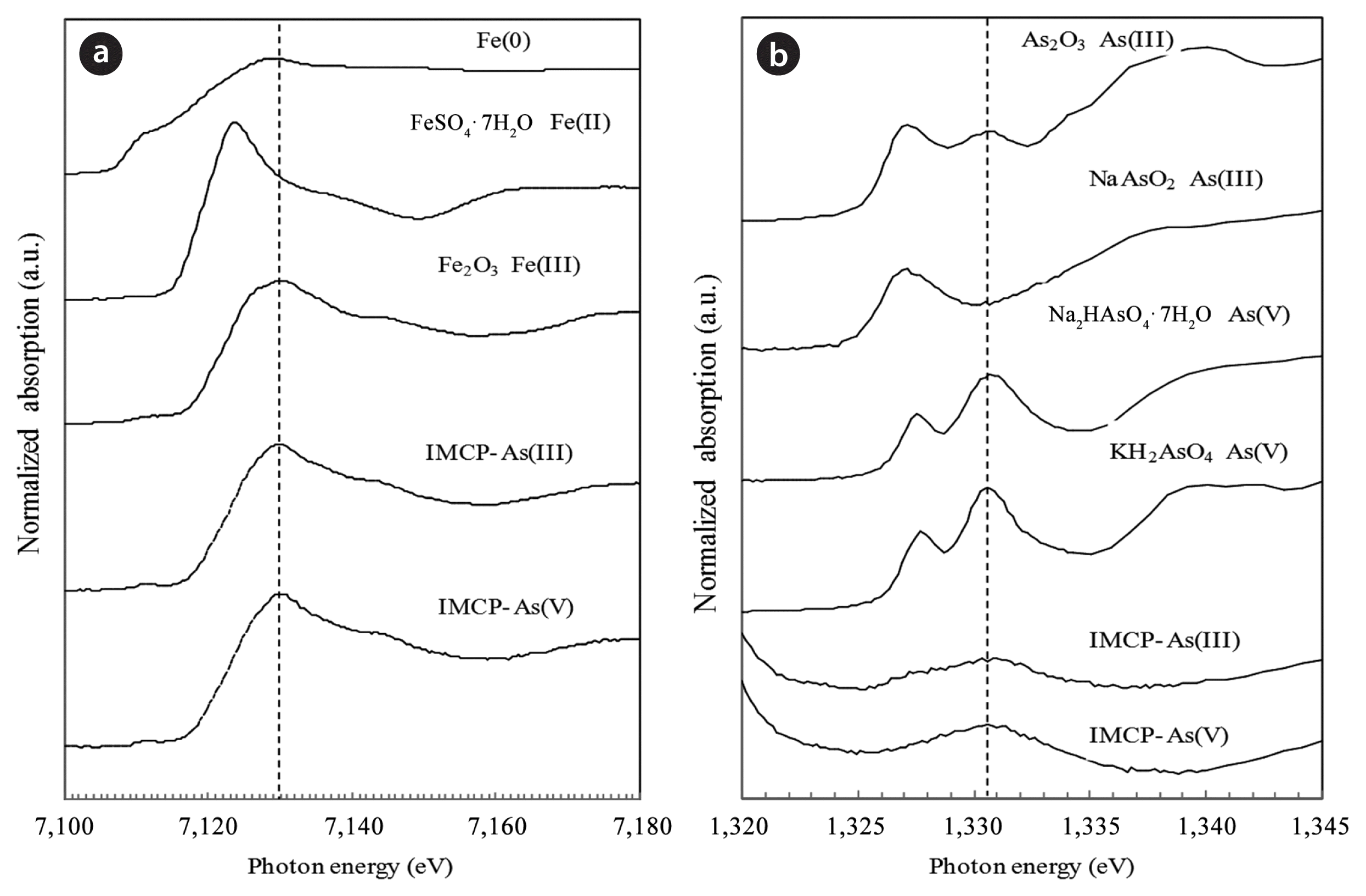
Fe K-edge XAFS spectroscopy was used to determine the oxidation state of iron after the adsorption of As by the IMCP. Fig. 2(a) shows the XAFS spectra for Fe. The peak energy values for both the As(III)-treated IMCP (IMCP-As(III)) and the As(V)-treated IMCP (IMCP-As(V)) adsorption experiments (7,130.4 eV) were the same as the peak of Fe2O3 at 7,130.45 eV, indicating that the Fe(0) added to the IMCP had been oxidized into iron oxide during the firing period. The As L3-edge XAFS spectra exhibit a well-resolved edge structure with an adsorption maximum at 1,327.3 eV and 1,330.8 eV, corresponding to As(III) and As(V), respectively (Fig. 2(b)). The energy of the first derivative peaks for both IMCP-As(III)- and IMCP-As(V)-treated media corresponded closely to the energy values for the As(V) standard. This indicated that As was presented only as As(V) in the IMCP in the batches with a contact time of 120 hr at an initial solution pH of 7.0–7.5. Therefore, XAFS analysis of As(III)-treated IMCP media gave clear evidence of the partial oxidation of As(III) to form As(V) during the oxidation of Fe(0) in IMCP media. The intermediate reactive oxidant (•O2−, H2O2, and •OH or Fe(IV)) can be produced during the physicochemical oxidation of Fe(0). Some of the reactive oxidants are able to oxidize As(III) into As(V) [11, 12]. Therefore, the mechanism of As removal involved the oxidation of As(III) into As(V) and the adsorption of As(V) onto the iron oxides in our study.
3.2. Effect of Solution pH
The effect of pH on As removal from synthetic groundwater by the IMCP was examined at various pH values (ranging from 3 to 11). Fig. 3 shows the effect of pH on both As(III) and As(V) removal. The IMCPs exhibited strong adsorption of both As(III) and As(V) when the pH was between 5 and 7. As the added Fe(0) was oxidized into iron oxide through the firing of IMCPs in our study—which will be discussed later—the higher adsorption in this pH range may be due to the electrostatic attraction between the positively charged iron oxide and the As anions [13]. On the other hand, the adsorption of As(V) was higher than that of As(III). The difference in the removal of As(III) and As(V) can be explained by the respective speciation differences. Under these pH conditions, the predominant As(V) species exist as negatively charged H2AsO4− and HAsO42−, whereas the predominant As(III) species exist as H2AsO3, which has no charge [13]. Therefore, the adsorption of As(V) by Fe(III), which has a positive charge, was higher than that of the As(III).

Effect of solution pH on Arsenic (As) adsorption by iron mixed ceramic pellet (IMCP). Conditions: As, 2 mg/L; IMCP, 2.5 mg/ L; pH, 3–11. The error bar shows the standard deviation of triplicate tests.
The removal efficiency of both the As species fell sharply as the pH decreased below 5 or increased above 8. The sharp decrease in the amount of As adsorbed under the alkaline pH conditions was due to the competition of adsorption sites between the As species and hydroxyl ions [14]. The equilibrium pH after 120 hr of shaking increased at the initial pH range of 3–7, while it was kept constant at pH 8 and decreased at pH 9. However, it was observed that IMCPs had a significant capacity to buffer highly acidic and alkaline solutions. No Fe(II) or Fe(III) was released into the final solutions when the pH was in the range of 5 to 9, indicating that no harmful metal ions were released into the treated water in these ranges of pH.
3.3. Adsorption Kinetic Studies
Pseudo-first-order equations are widely used to investigate adsorption kinetics, while pseudo-second-order equations allow for the evaluation of effective adsorption capacity. The adsorption capacity and the constants of adsorption are determined using the pseudo-first-order equation and the pseudo-second-order kinetics equation, which are shown in Eqs. (1) and (2), respectively [15]:
where qt (mg/g) is the amount of As adsorbed at contact time t; qe (mg/g) is the amount of As adsorbed at equilibrium time; k1 (1/hr) is the rate constant of the pseudo-first-order model; k2 (g/mg·hr) is the rate constant of the pseudo-second-order model.
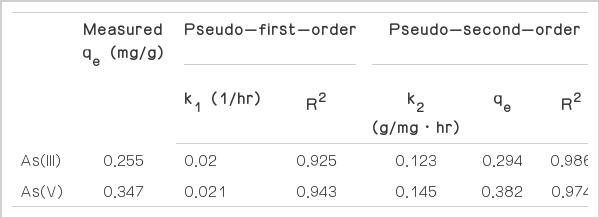
The kinetic models adequately fit the data obtained in the batch experiments. The model parameters, including kinetic constants, equilibrium adsorption capacities, and correlation coefficients, are presented in Table 1. The correlation coefficient values for As(III) and As(V) obtained for the pseudo-second-order adsorption model (0.986 and 0.974, respectively) are higher than those obtained from the first-order kinetics (0.925 and 0.943, respectively). Therefore, it is concluded that the adsorption kinetics can be better described in terms of the pseudo-second-order rate model than the first-order rate model. The calculated equilibrium adsorption capacity (qe) values of 0.294 and 0.384 mg/g for As(II) and As(V), respectively, are consistent with the experimentally measured values of 0.255 and 0.347 mg/g, respectively. Furthermore, a comparison of the k2 values indicates that the adsorption rate of As(V) is greater than that of As(III). The adsorption of monodentate non-ionized As(III) happens only through a Lewis acid-base (ligand-exchange) reaction, and is favorably adsorbed onto the non-ionized surface functional group at neutral pH. In contrast, the adsorption of As(V) forms, such as H2AsO4− or HAsO42−, is known to take place via Coulombic as well as Lewis acid-base interactions, and to form monodentate and bidentate inner sphere complexes [16]. This is a possible reason for the higher adsorption rate of As(V) on the IMCP.
3.4. Adsorption Isotherm Studies
Table 2 shows the results of equilibrium experiments. To evaluate the As adsorption capacity of IMCP media, a Freundlich isotherm and Langmuir isotherm model were used to describe the arsenic adsorption on solids in Eqs. (3) and (4), respectively:
Parameters of Langmuir and Freundlich isotherms for the adsorption of As(III) and As(V) by iron mixed ceramic pellet
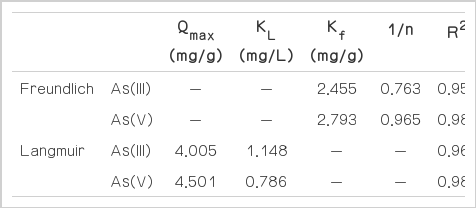
where qe is the amount of solute adsorbed per unit dry weight of IMCP (mg/g); Ce is the equilibrium concentration of As (mg/L); qm is the maximum adsorption capacity (mg/g); kf is the Freundlich sorption coefficient (mg/g); 1/n is the adsorption intensity of the Freundlich isotherm; and KL is the Langmuir constant (L/mg). The values of the Freundlich parameters Kf and 1/n were evaluated from the linear plot of logqe vs. logCe, while the Langmuir constants KL and qm were evaluated from the linear plot of Ce/qe vs. Ce. These parameters are presented in Table 2. The results show that correlation coefficients (R2) for all cases are more than 0.950, representing an excellent fit of observed data using both isotherm models. It could therefore be speculated from our data that both monolayer and multilayer adsorption would be involved in the both As(III) and As(V) adsorption on the IMCP.
In our study, the As(III) and As(V) maximum adsorption capacities (qm) of the IMCP media estimated from the Langmuir isotherm are 4.005 and 4.501 mg/g, respectively. It has been reported that the values of qm, the Langmuir constant related to the saturated monolayer adsorption capacity for iron-coated pottery granules [7], nanoscale Fe(0) [17], and iron-oxide-coated sand [6] are 1.74, 1.8, and 0.026 mg/g, respectively. The IMCP has a 2–3 times higher adsorption capacity for As than these adsorption materials. The 1/n value of the Freundlich isotherm is related to the strength of adsorption. The calculated 1/n for values for both the As are less than <1.0, denoting favorable adsorption of the As onto the IMCP. However, the As removal involved the oxidation of As(III) followed by subsequent adsorption of As(V). Therefore, the Langmuir-Hinshelwood isotherm model would be used to describe the As adsorption on IMCPs.
3.5. Effect of a Co-existing Anion (P) on As Adsorption
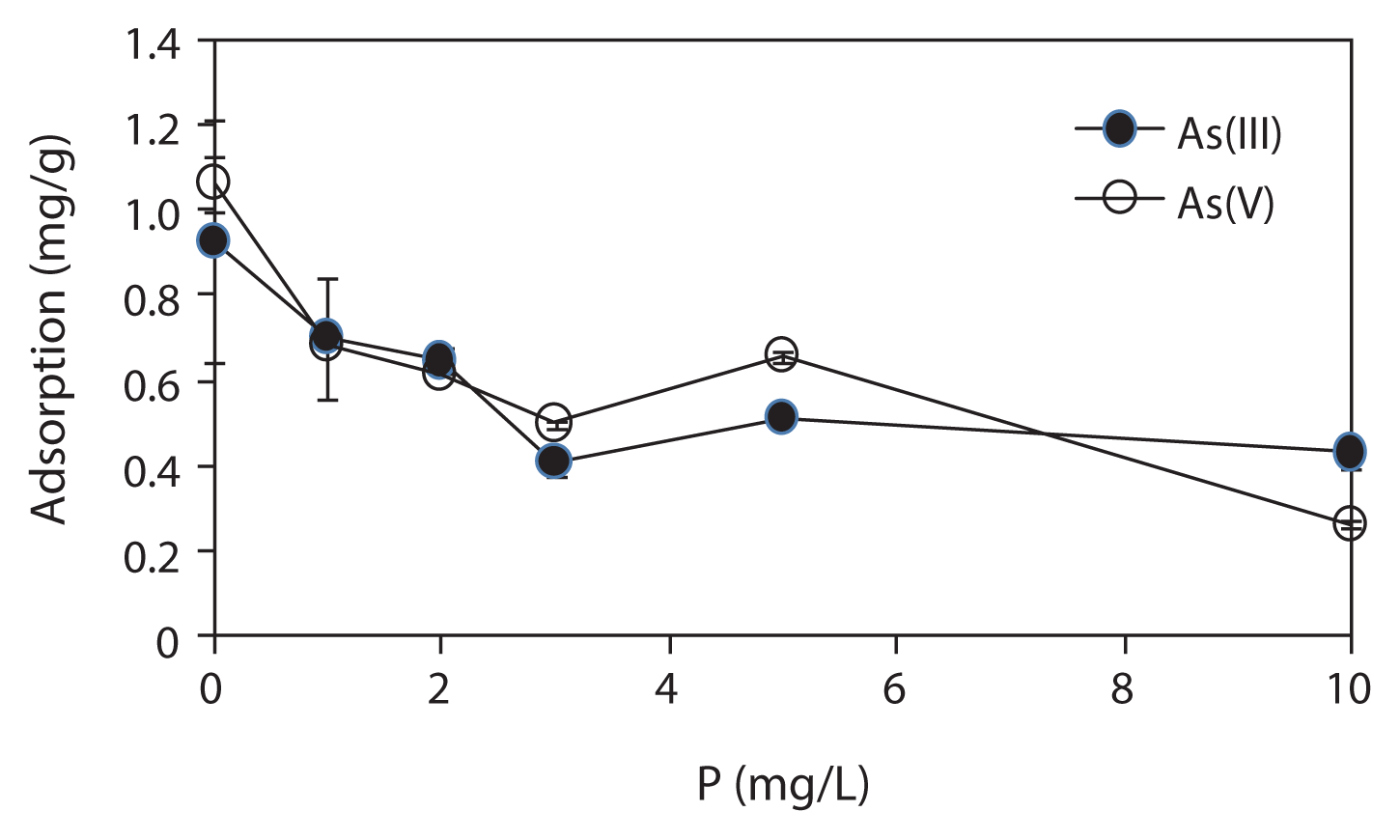
Fig. 4 shows the effect of P on As adsorption by the IMCP. As shown in Fig. 4, both the As(III) and As(V) adsorption capacities of IMCP were significantly reduced when the P concentration in the solution was increased from 1 to 10 mg/L. The results indicate that P in groundwater would cause a significant decrease in the As removal efficiency by the IMCP. P was thought to strongly influence and reduce the effectiveness of iron for As removal. As and P have similar chemical properties, and are known as an inner-sphere complex forming anions that are strongly sorbed onto the surfaces of minerals such as Fe oxides [18]. Therefore, the decrease in As adsorption in the presence of P was due to the fact that they compete for each other for sorption sites on the precipitated iron hydroxide surfaces.
3.6. Key Characteristics of IMCP
The IMCP developed in this study could serve as a simple and low-cost technology. All materials are locally available at little to no cost, and the manufacturing procedure is simple and easy. Moreover, it is easy to identify the advantages of IMCP media over other similar major technologies, such as iron-coated pottery granules, iron coagulation, iron-coated sand, and lime softening. For instance, the manufacturing process of iron-coated pottery granules is quiet complicated compared to that of IMCPs, and involves the addition of zerovalent iron in the final stage by a continuous agitation process that needs skill and extra care [7]. In contrast, the addition of Fe(0) powder is involved in the first stage of IMCP manufacturing, and does not require any skill or extra care. For the iron coagulation method, pre-oxidation may be required if arsenic is present as As(III). Moreover, experiments with IMCP showed that neither Fe(II) nor Fe(III) were released into the effluent solutions when the pH ranged from 5 to 9. Therefore, neither sedimentation nor filtration were required to separate Fe ions from the effluent water. Iron-coated sand is an effective and low-cost method, and removes both As(III) and As(V), but requires filtration to remove extra iron ions in the effluent [5]. In the lime-softening method, pH adjustment is required [5], whereas the IMCP method can be operated under natural pH and without changing the pH of water.
4. Conclusions
IMCP, a new adsorption media, has been successfully developed for arsenic removal from groundwater. The IMCP media showed several advantages, such as 1) an inexpensive and simple manufacturing process using locally available materials, and 2) an ability to absorb both As(III) and As(V) effectively in neutral pH. The As adsorption was found to be pH-dependent, and exhibited strong adsorption of both As(III) and As(V) at pH 5–7. The adsorption process was observed to follow a pseudo-second-order reaction, and the adsorption rate of As(V) was greater than that of As(III). The adsorption process followed both Freundlich and Langmuir isotherm models. The presence of P in the water decreased the adsorption efficiency of both As(III) and As(V). XAFS analysis showed a partial oxidation of As(III) and the adsorption of As(V) onto the iron oxide in the IMCP. It was shown that neither ferric nor ferrous ions were leached out from the IMCP. Therefore, the new IMCP would be a promising material for As removal from groundwater.