Recent advances in biological approaches towards anode biofilm engineering for improvement of extracellular electron transfer in microbial fuel cells
Article information
Abstract
Over the last two decades, scientific communities have been more interested in turning organic waste materials into bioenergy. Microbial fuel cells (MFC) can degrade organic wastewater and produce electrical power. Many constraints have limited the development of MFC. Among them, the anode biofilm development is one of the significant constraints that need to be improved. This review delineates the role of various biological components in the development of electroactive biofilm. The current article focuses on the numerous electron exchange methods for microbiome-induced electron transfer activity, the different proteins, and secretory chemicals involved in electron transfer. This study also focuses on several proteomics and genomics methodologies that have been adopted and developed to improve the extra electron transfer mechanism in electroactive bacteria. Recent advances and publications on synthetic biology and genetic engineering in investigating the direct and indirect electron transport phenomena have also been highlighted. This review helps the reader to understand the recent development in the genetic manipulations of the biofilm, electrode material modifications, EET mechanisms, and operational strategies for improving anode performance. This review also discusses the challenges in present technology and the future direction for improving biofilm production at the anode.
Abstract
Graphical Abstract
1. Introduction
Non-renewable energy sources are the most significant contributor to global energy production because they are potential carriers of energy that have been generated but cannot be replenished. Large-scale pollution and climate disruption are typically related to non-renewable energy utilization. Non-renewable resource depletion became an urgent issue in the 1970s when companies all over the world experienced abrupt shortages of vital raw materials. The need for energy around the world is rising fast due to factors such as population expansion and increased industrial production. Fossil fuels currently play a significant role in both human existence and industrial production. The rising global population necessitates more energy consumption, and the depletion of non-renewable energy supplies (coal, gas, and oil) poses a danger to the sustainability of the current way of life. One of the most significant obstacles to human survival and economic growth has been the insufficient availability of fossil fuels to meet the needs of the ever-increasing population and increased energy consumption. Problems such as global warming and pollution increase the need for renewable sources of energy that can be economical and sustainable. Urbanization, industrialization, depletion of non-renewable resources, and overpopulation have all emphasized the need for clean, sustainable, green, and renewable energy sources. The existence of wind, hydro, and solar power has satisfied this clause well enough. However, the equally promising idea of biological fuel cells as a renewable energy source is still under development and is least explored [1,2]. In a microbial fuel cell (MFC), the problem of treating wastewater can be solved by using certain electroactive microbes, which can perform wastewater treatment and simultaneous bioelectricity production [3–5]. MFC technology is a step ahead of other traditional bioenergy production technologies such as anaerobic digestion, gasification, and fermentation because it significantly reduces secondary pollutants’ levels and is comparatively more cost-effective [6].
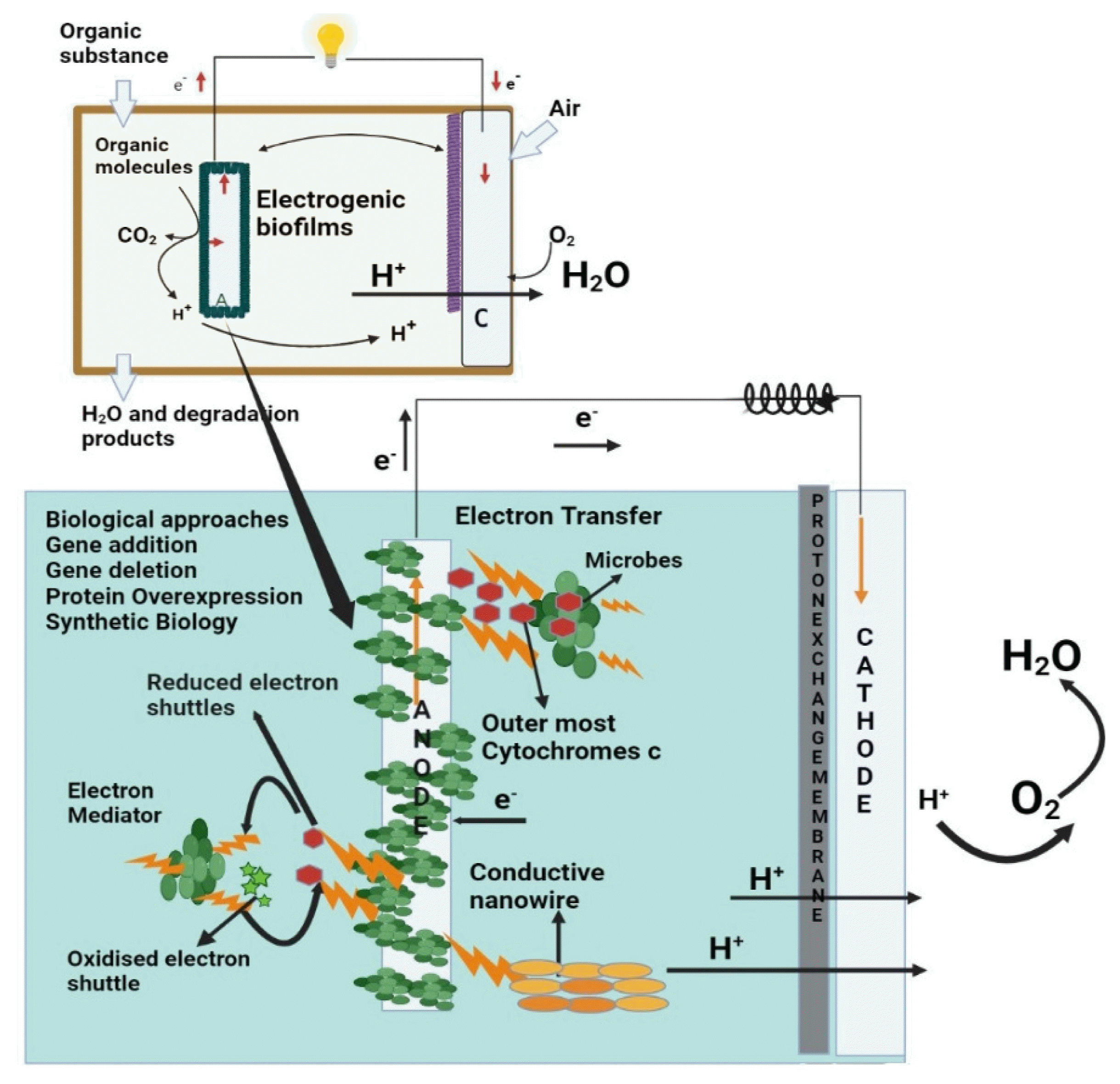
The MFCs comprises two electrodes—cathode and anode-often separated by a semi-permeable barrier or electrolyte. Electroactive microbes utilize the anode as a terminal electron acceptor [7]. The anode biofilm degrades organic substrates, produces electrons, and transports them to the anode surface (Fig. 1) [8]. The irreversible attachment of electroactive microorganisms to the electrode surface leads to the growth and development of electroactive biofilm (EAB). The microorganisms in the EAB use electrodes as electron acceptors for sustaining their metabolic or respiratory process. Microbial metabolism on the substrates provides energy for the anode biofilm and the MFC system. In single-chamber cubic MFCs, it takes 9 weeks to establish a stable cell performance, but it takes more than 17 weeks to get a mature anode biofilm [9]. These biofilms contain elements that conduct electrons like metal, and their conductivity is affected by various factors such as biofilm thickness, porosity, microbial composition, microbial viability, etc. Even in the identical system, the MFC power was significantly different up to 3.6 times depending on the anode biofilm properties.

Mechanisms of cyclic di-GMP signaling pathways (a) The relationship of C-di GMP signaling with DGC and QS; (b) Biochemical pathway for Geobacter sp. and Shewanella sp. carrying out Cyclic di-GMP signaling; (c) Regulation of P. aeruginosa biofilm growth by c-di-GMP signal transduction pathways via two network cascades. The upper section depicts PDE, DGC, and c-di-GMP receptors and the developmental period during which these are thought to function. The bottom section depicts two phase-specific different regulation mechanisms for biofilms. The range of yellow panels in the figure backdrop reflects growing internalized c-di-GMP concentrations (denoted by pink * with increasing amounts).
The electroactive bacteria transport electron to the electrode surface via extracellular electron transfer (EET). EET may be done through artificial mediators (e.g., Methylene blue), natural mediators (e.g., Pyocyanin), shuttles produced by the microbes (e.g., C-cytochromes), pili (also known as nanowires), etc [11]. The biofilm matrix is a heterogeneous medium made up of nucleic acids, polysaccharides, lipids, proteins, and so on and is collectively known as Exopolymeric substances (EPS). EPS provides a microenvironment for EAB for nutrient absorption and assimilation10. In EAB, EPS also provides an electronic medium for c-cytochromes and pili, which help discharge electrons and their transportation [9,11,12].
MFC’s mechanism deals with two types of microorganisms. One of them is electroactive microorganisms (EAMs), also known as exoelectrogens, electrogens, electricegens, exoelectrogenic bacteria, or anode-respiring bacteria, which oxidize organic material and release electrons onto the electrode [13]. The other type is non-EAMs, often referred to as endo-electrogens or non-exoelectrogens, because they either consume electrons or produce mediators that direct the electron route [14,15]. These non-EAMs can swim freely in the water as planktonic growth or stick together like a blanket in the EAB along with old cells [16]. When cells contact a solid and hard surface, they undergo a series of anatomical, biochemical, and physiological adaptations that mark the shift of planktonic growth into a sessile state. Non-EAMs are important in the MFC ecological system because they help produce dense biofilm and maintain an anaerobic condition by utilizing oxygen that would otherwise pollute the anode [13]. Compared to MFCs containing pure exoelectrogens, MFCs with mixed consortia exhibit increased power, durability, and the capacity to break down complex compounds and convert them into electricity [17].
It is possible to classify the most common uses of MFCs that have emerged in the past few decades as follows:
Electricity generation: MFCs are designed to provide adequate power for low-powered electronic gadgets. Two days of operation of ten LED lights and one digital clock using a stackable MFC that Rahimnejad et al. depicted were successful [18].
Biohydrogen production: Simple modifications allow MFCs to collect biohydrogen instead of electricity generation. The hydrogen can be stored for future use [19]. MFCs provide a renewable hydrogen source in a hydrogen economy that can contribute to the total hydrogen demand [20,21].
Wastewater treatment: Wastewaters from a variety of industries, including sewage, the food processing industry, wastewater of swine, and the corn ethanol industry, all constitute biodegradable organic materials that can be converted into energy [19,22]. Long-detention-time anaerobic digestion processes are best suited for high-strength wastewater because they produce electricity and methane simultaneously from waste materials [23]. There have been reports of columbic efficiencies of up to 80% [24,25], allowing for the removal of up to 90% of the COD [26].
MFCs in biosensors: MFCs are convenient for fuelling electrochemical sensors and are compact telemetry systems for transmitting data to distant receivers due to the batteries’ limited lifetime and recharging requirements [27,28]. Using MFCs as a sensor for biological oxygen demand (BOD) has been shown to work [28]. This kind of sensor for BOD has excellent reproducibility and operational sustainability and can be kept running for 5 years [29].
Though the MFC technology has several advantages, its commercialization is yet to be seen. Therefore, it is critical to identify the flaws that impede MFC performance and its various applications. The role of biofilms in electricity production is necessary to understand the factors affecting biofilm production and the mechanism of EET [30]. While most studies focus on electron transfer techniques, the critical research questions for MFCs concern topics such as (1) redesigning exoelectrogens to make them more efficient and (2) developing inexpensive and highly efficient materials that can be used for power production and sewage treatment; 3) learning how to promote biofilm development in an MFC to enhance energy output; and 4) need to gain insight into a wide range of EAB phenomena, remained unanswered.
Biofilm-coated electrodes for bioelectricity production via MFCs have sparked much attention in the scientific community. Various experts recommend thick anode biofilm formation owes to the synergistic influence of microbial communities [17]. Even though MFC technology seems to be a partial solution to the current energy crisis, it is impossible not to view the MFCs as a sustainable energy source that can consistently power contemporary society while simultaneously treating wastewater [31]. Realizing the potential for extensive conversion of organic waste and biomass into bioenergy, this technology can be used to develop solutions for recharging biomedical devices, running home appliances, and other electrical devices [32]. However, only a handful of literature is presently discussing the challenges that affect the anode biofilm formation, which is one of the significant limitations of electricity production in MFCs. There is a need for a detailed discussion of the different strategies used to enhance biofilm formation and its future research direction. This review article aims to paint a detailed picture of the development of the EAB and the challenges involved, and the various strategies used to overcome the challenges. The present review focuses on the development of EAB and the microorganisms responsible. It will also focus on the various factors affecting biofilm formation, the hypothesized mechanisms by which the electrons are transported through the biofilm, the challenges that limit the electricity production by the biofilm, and the most recent advances in the strategies used to promote biofilm formation for enhanced electricity production. The present study will also discuss the future perspective of anode biofilm development for increased electricity production.
2. Microorganisms Generating Power in MFCs
The majority of MFCs generate electricity from sewage. Sediments occurring in open systems are prone to the occurrence of electrogenic community selection. Communities of electron-transferring microorganisms at the anode chamber’s electrode should perform actions similar to anaerobic digester methanogenic communities [33]. These microbiocenoses are known as anodophilic [34]. There are typically delta-Proteobacteria at the anode of sedimentary systems, ranging from 50% to 90%. Gamma-Proteobacteria (9%), Firmicutes (11.6%), and Cytophagales (33%) are also present, however, to a lesser extent [35]. Because of the unique abilities to generate EAB and highly efficient EPS, many photosynthetic and anaerobic biological microbes have been utilized in MFCs as donors and recipients of electrons, which include Saccharomyces cerevisiae [36], Rhodispirullum rubrum [37], Chlorella vulgaris [38], Thiobacillus ferrooxidance, Phormidium sp. [39], Leptothrix discophora [40], Desulfovibrio desulfuricans [41], Pseudomonas aeroginosa [42], Klebsiellapneumoniae [43], Geobacter metallireducens [14], Shewanella oneidensis along with a few other anaerobic microbes. The genetics of certain wild-type strains have been modified using recombinant DNA technology to improve current production and sustained biomass generation.
3. Types of EET
3.1. Direct Electron Transfer (DET)
Certain electroactive bacteria, especially Shewanella oneidensis, Rhodoferax ferrireducens and Geobacter sulfurreducens, use direct electron transfer (DET) for the transfer of electrons to the electrode surface via redox-active proteins or conductive pili (also known as nanowires). For DET, the microbes are closely associated with the electrode because only immobilized cells can effectively perform DET. This leads to a high rate of transfer of electrons from the microbes to the electrode, resulting in high power production. These bacteria used transmembrane proteins such as cytochrome c found on the cell wall to transfer electrons from their cell walls to the electrodes. The cell wall of the bacteria, or maybe the membranes of the organelle, must be directly connected to the electrodes of the MFC for DET to occur [44]. Exoelectrogens transport electrons to the anode via the help of suitable electron shuttles in two different methods: (i) the transfer of electrons across short distances by redox-active proteins on the cell wall of bacteria, such as cytochromes, and (ii) nanowires, or conducting pili, allow for electron transport across long distances [30]. Live cells are often assumed to remain electrically dormant owing to their non-conductive properties [45]. However, studies have shown that it’s possible to make good use of an organism inside an EAB since it has an electron transfer protein (ETP) bound by membranes [46]. Additionally, as shown by the literature, carrier proteins direct the transfer of electrons from the interior to the exterior of the cell. Redox proteins in the outer membrane, however, often carry external electrons to a solid final electron acceptor, such as an electrode [47]. Geobacter sp. [48], Rhodoferax sp. [49], and Shewanella sp. [50] have been reported to have multi-heme protein molecules along with c-type cytochromes and are also found with EAB [30]. In exoelectrogens, the flavins, as well as cytochromes, are found to be interconnected cyclic compounds with a strong redox capacity and are electron transporters. Shewanella oneidensis was shown to actively participate in electron transfer through electron shuttles and metallic reduction based on cyclic voltammetry (CV) study of the redox properties of flavins like FAD and vitamin B2 [51]. A hybrid strain MR-1 of Shewanella oneidensis, in which CymA is abundantly expressed, was also studied for its possible application in MFCs [52]. Recent research has also shown that Bacillus megaterium relies on flavins for electron transfer, joining the ranks of well-known flavin producers like Shewanella oneidensis [53]. Cytochromes are mainly proteins containing heme that shuttle electrons across the membrane and into the surrounding medium. Several studies have reported the presence of electron mediators in the EPS matrix of biofilms [54], the activity of outer membrane cytochromes in electrode and metal reduction, and the function of pili/nanowire in biofilm development [55].
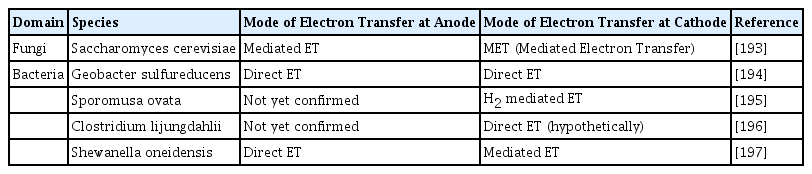
In addition to cytochromes, bacteria have sticky pili that are mostly made of sortase enzymes and proteins [56]. According to earlier research, several Geobacter and Shewanella strains develop electron-conducting pili. These pili permit such organisms to the long-range transfer of electrons to the electrode that is not in immediate contact with the microbes. These nanowires, or pili, attach to the cytochromes of cells and carry electrons from the outer membrane to the electrode [57]. These pili (nanowires) even promote the formation of thick EAB, further improving MFC performance [30]. Some of the microbes and their chosen methods of electron transfer are listed in Table 1.
3.2. Indirect Electron Transfer
Naturally produced redox mediators, known as secondary metabolites, are relevant in Indirect Electron Transfer (IDET) or mediated electron transfer (MET) [58]. In IDET, the synthesis of naturally produced mediators renders electron transfer without needing redox electron shuttles from the outside [59].
This can also be linked to soluble electron transporters released by microbes such as flavins and pyocyanin. The mediator accepts and donates electrons from the bacterial cell to a strong oxidant (anode) or into anaerobic biofilm sheets. They are reoxidized and made accessible for further redox activities. As a result, a molecule may support hundreds of redox cycles [60]. Thus, the microorganisms can dispose of electrons at increasing speed levels by making minute amounts of such compounds [44]. P. aeruginosa produces a pigment, Pyocyanin, that has previously been identified as required for conducting IDET in bacteria [61]. Shewanella oneidensis produced a mediator known as quinone. The quinone-treated version of MFC has shown double power output compared to the mediator-free version [61]. Pseudomonas alcaliphila can also produce redox mediators through bacterial synthesis. Thus, in addition to redox mediators, the oxidation of the metabolites also contributes to IDET. Bacterial fermentation causes the anode to produce hydrogen when oxidized [62]. Immediate bacterial interaction with the anode or soluble redox active mediators such as pigments, ubiquinone, metal complexes, and dyes, and readily soluble electron carriers are also viable options for transferring electrons from the electrode to the bacteria [44].
4. Factors Affecting the Formation of Biofilm
Extracellular polymeric substances (EPS) and lipopolysaccharides (LPS) play a crucial role in biofilm development alongside exterior environmental circumstances and genomic pathways that ultimately result in biofilm formation in cells [63]. Signaling molecules of quorum sensing (QS) also control biofilm formation [64]. High temperatures, presence of heavy metals [65], salinity [66], pH [67], nutrient starvation [58], nutrient depletion, the flow rate of moving water bodies, growth substrate, and so on are some of the significant factors that affect the synthesis of EPS and signaling molecules, thus affecting the biofilm formation [68]. While genetically engineered molecules may have the upper hand over environmental factors, while forming biofilms, EPS and quorum-sensing molecules cannot be excluded from the limelight [30]. But further research into this domain is required for better understanding.
4.1. Role of Exopolysaccharides (EPS) and Lipopolysaccharides (LPS) in Electron Transfer
Changes in EPS contents seem to have significant ramifications in terms of surface energy and external adhesion, providing a foundation for preserving accessory peptides implicated in intercellular interaction, as seen in Shewanella sp [30]. In this regard, studies with Geobacter sulfurreducens have drawn attention, revealing the role of EPS as adhesive sites for exoelectrogens, allowing them to transport electrons. Results show that the formation of ion-conducting biofilms on the cathode and anode was absent in EPS-deficient mutants. Geobacter sulfurreducens, under standard conditions, produce peripheral anchoring polymers, which are required for electron transfer to the electrode and include c-type cytochrome sites [69].
EPS includes polysaccharides that improve biofilm formation, such as Alginate and Pel, which are responsible for generating high levels of c-di-GMP by diguanylate cyclases. Psl (a type of polysaccharide) is responsible for initiating and maintaining the biofilm structure [70]. At the same time, Pel, a matrix rich in glucose, helps biofilm formation associated with the solid surface for non-pileated organisms [71]. For example, in Pseudomonas aeruginosa, the algC gene produces an enzyme essential for the production of polysaccharides, namely, Psl, LPS, alginate, and Pel, which influence biofilm formation [72]. As mentioned earlier, most of the genes that make any of the polysaccharides are typically involved in producing biofilms [72].
A thorough examination of the microbial community in electrogenic multilayered populations provides information on the processes that convert complex compounds (organic) in industrial effluent to electric power in MFCs [73]. Direct interspecies electron transfer (DIET), occurring across bacteria or in combination with electromechanical particles, can be implemented in designed organizations to improve sewage treatment goals and for regenerative braking in bio-electrochemical advances [74]. As a result, EPS’s role in biofilm generated by microbes in MFCs has become a focus for scientists.
4.2. Cyclic di-GMP Signaling
C-di-GMP is a signaling component of a molecule that coordinates behavioral change from mobility to sessility and vice-versa (also called dispersion) [75]. Several bacteria, including Serovar typhimurium, Salmonella enterica, Pseudomonas aeruginosa, and Escherichia coli, have shown a connection between higher c-di-GMP concentrations in organism and biofilm generation or lower c-di-GMP concentrations and mobility [76]. The cyclic di-GMP controls biofilm production and dispersal via many genetic alterations [77]. Researchers report that MucR (membrane-associated regulator of alginate biosynthesis in biofilms, acts as Phosphodiesterase (PDE) and a positive controller of the dispersal of biofilms triggered by nitric oxide or glutamate [78].
Diguanylate cyclases (DGCs) and PDEs have been recognized as key players in the biofilm development of bacteria such as Pseudomonas aeruginosa. A thorough analysis of the characteristics of PDE and DGC mutants, together with epistasis research, revealed details regarding the participation of BifA (PDE) or RoeA (DGC) and SadC (DGC). As a result, there is a greater understanding of their perceived importance at various stages of the biofilm formation process [77]. In the given Fig. 1 (a) and (b), we demonstrate this notion developed by combining all the Pseudomonas aeruginosa’s PDEs and DGCs that have been found that take part in biofilm development. WspR, SadC, RoeA, SiaD, and YfiN/TpbB are the DGCs that are identified to be controlling the shift from planktonic growth to sessile cell growth of the bacteria, enhancing the biofilm formation [79]. The DGCs – NicD and GcbA, as well as the PDEs RbdA, NbdA, and DipA (Pch), are associated with the growth of the biofilm as represented diagrammatically in Fig. 1(c) [80].
4.3. Quorum Sensing
The microbial population interacts among itself via cell-to-cell association, coordinating their activities. Hence, this necessitates the generation of signaling molecules from the colonies, which results in the phenomena known as quorum sensing [30]. Quorum Sensing (QS) signaling molecules differ between Gram +ve and Gram −ve bacteria, as given in Table 2. The first question that arises when considering the relation between biofilm growth and QS is when the microbial density exceeds the critical level that permits QS signaling to engage in biofilm management. Because it restricts microbes that are moving freely in the medium, the preliminary adherence phase appears to be suboptimal for collecting QS. Later, whenever the attached microbes split and form microcolonies, the total population rises, leading to the QS reaching adequate levels to regulate the development and breakdown of the biofilm. Recent studies have shown that many bacterial species use QS to coordinate the dispersal of the biofilm. Dispersion of biofilms is required for bacteria to grow [81]. When nutrients and other elements become scarce and waste piles up, biofilm dispersal is critical for bacteria to break free and colonize habitats. Increasing the quantities of QS signals further may cause malnutrition and stress in the planktonic bacterium population, creating an unfavorable environment. Bacteria use biofilms to protect themselves from this stress. The environment on the biofilm’s surface is favorable for microbe growth [82].
In general, there are three kinds of QS systems: (1) AHL (Acylhomoserine lactones) are employed as signal molecules in gram-negative microorganisms, (2) oligopeptides are QS molecules generally found in gram-positive microorganisms, and (3) luxS-encoded autoinducers QS 2 (AI-2) are found in Gram −ve as well as Gram +ve microbes [83]. EPS production, chemotactic processes, and cell migration play an important role during the degradation of organic pollutants or purification. It is found that gene modification of QS may assist in creating designed biofilms with enhanced degradation kinetic models and increase the performance of MFCs [84].
Moreover, a rhamnolipid synthesis which QS controls contributes to biofilm design by maintaining open biofilm water channels during matrix growth [85]. Several studies indicated that rhlL a rhamnolipid synthesis gene, also regulates the production of secondary metabolites such as pyocyanin in P. aeruginosa [86]. It is still uncertain if population size affects the production of one of these polymers. As a result, the molecular pathways that explain the connection between QS and biofilm formation in Pseudomonas aeruginosa remain unclear. It is also found that Vibrio cholerae starts producing EPS even when in a low-density population, probably before even forming a heterogenous community. The QS compounds and the taxa that employ them are presented in Table 2.
Some anaerobic, facultative microorganisms, such as Pseudomonas aeruginosa along with Salmonella enteritidis, rely heavily on QS for their growth and biofilm formation. QS regulates the anaerobic growth of Pseudomonas aeruginosa PAO1, where the outer sheath proteins are Rhl and OprF. QS pathways have been identified to be essential for successful bacterial biofilm formation [87]. According to reports, bacterial growth is significantly reduced when OprF is not functional. However, a lacking rhlR or, sometimes, rhlurel leads microbes to undergo apoptosis owing to increased nitric oxide production. Moreover, the gene expression of Pseudomonas aeruginosa is usually regulated by AHL signals dependent on cell density, while QS in Salmonella enteritidis is mediated by signaling molecules AI-2, AI-1, and AI-3 [88].
4.4. Effect of Biosurfactants on Biofilm Formation
Biosurfactants are a type of surfactant that is produced by bacteria or fungi and have intense interfacial activity, emulsifying action, and foaming properties. Biosurfactants can change the ultrastructures of the cell membranes to form transmembrane channels, which are effective for enhancing cell permeability, reducing membrane resistance, and increasing the electron transport of the microbes in the EAB. It leads to an increase in substrate degradation, increasing the bioavailability of nutrients. According to recent studies, applying biosurfactants promotes microbial degradation of crude oil in MFCs, which may impact the taxonomic composition of EAB [89]. The researchers studied five detergents and discovered that the lecithosampholytic surfactants (primarily lecithin) exhibited high power output and high microbial degradation. The most selective alterations in bacterial populations were produced by Sodium dodecyl sulfate (SDS) and -cyclodextrin (biosurfactant). Another research found that the inclusion of Tween [80] improved polychlorinated biphenyl degradation in MFC by 43.5% [90]. The hydrophobic substrate’s bioavailability increases with the presence of biosurfactants [91]. Both biotic and abiotic surfaces can benefit from film conditioning when biosurfactants are released onto them [92]. The hydrophobicity of the cell surface increases with biosurfactants like rhamnolipids. Because of their ability to condition films and modify the hydrophobicity of bacterial surfaces, biosurfactants facilitate the adhesion of bacteria to surfaces, a necessary step in the development of biofilms [93]. Lipopeptide biosynthesis has been shown to reduce the hydrophobicity of cell surfaces and the Bacillus spp.’s adhesion to the stainless steel surface [94]. Biosurfactants were also shown to play a crucial role in the biofilm formation and adsorption of S. epidermidis, S. aureus, and P. aeruginosa at the -interface of ƞ-decane–water in another study [95]. Some of the surfactants and their impact on the EET of the microorganisms are given in Table 3.
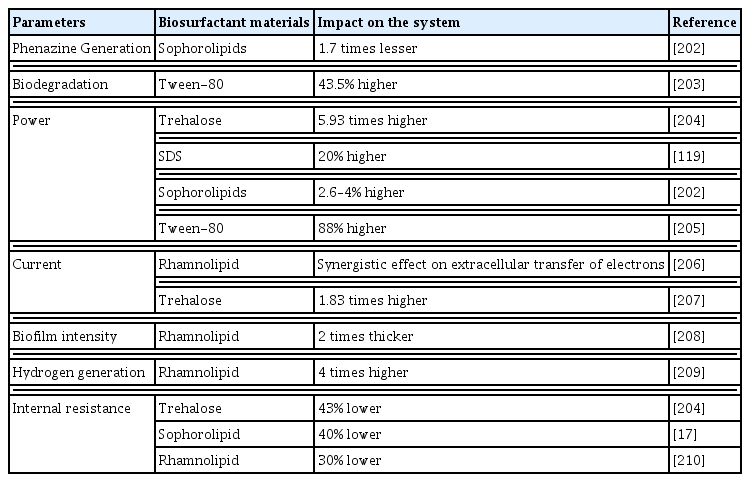
List of biosurfactant substances, the parameters they work under, and the impact they have on MFC and currents output
Only rhamnolipids and sophorolipids were discovered as biological detergents, even after multiple trials with various detergents. Reports state that biosurfactants may interact with electron transport channels such as phenazines, biofilm adherence structures, and survivability at the electrode. Adding biosurfactants and detergents in MFCs would’ve had the primary advantage of assuring an energy-neutral change, positively enhancing MFC’s performance in substrate utilization and energy output via multiple pathways.
4.5. External Resistance
External resistance (Rext) in an MFC regulates the anode availability to the exoelectrogens as an electron acceptor. It affects anode potential, EET rate, current production, and anodic microbial diversity. A lower Rext application increases electrode potential, EET rate, current production and microbial diversity in the anode [16]. It was reported that it’s not only electrochemistry hat gets affected by external resistance but the relative diversity of the microbial community also, as shown by analysis of ribosomal intergenic space [96]. Optimum external resistance, suggested by Jung’s group [97–99], is the external resistance at which an MFC produces its maximum power density according to the electric power relationship (P = IV). The maximum power point tracking method is also conducted by this algorithm that finds the external resistor that produces the maximum power.
When an anode biofilm adapts to its optimum external resistance in each stage, MFC power production gradually increases as the operation continues and reaches a stable performance [9]. Therefore, operating an MFC at its optimum external resistance is very important. Since optimal external resistance varies depending on cell conditions such as anode biofilm, MFC structure, medium properties, substrate concentration, etc., it is necessary to measure and apply optimal external resistance periodically. An optimum resistance value can be obtained by a polarization curve or an impedance test. The optimum external resistance is generally equal to the internal resistance of the system. When the external resistance is higher or lower than the optimal external resistance, the produced power will be lower than the maximum power.
For wastewater treatment, a lower external resistance can boost wastewater treatment. The low MFC power due to low external resistance application can be solved by utilizing a power-boosting electrical circuit.
4.6. Microbial Cooperation and Competition
Bacterial species have been found to have a wide variety of strategies for coexisting with or dominating other microbes competing over the same resources, as revealed by studies of interspecies competitive strategies [100]. Bacteria not only compete with one another within their species, but they can also participate in cooperative activities [101]. When present in mixed populations, these intraspecific processes can potentially affect the communication between species, such as by facilitating better strategies for competing [102]. In a growth environment with low nutrients level, the members of the mixed culture will compete for the same resource because of the depletion of resources due to a continuous increase in the population [103]. When there are several different ecological niches available, as in a biofilm or a static liquid culture, natural selection will favor the variants that are best able to colonize the various niches [104]. This antagonistic behavior in mixed culture can be tackled by selecting different organisms with different substrate requirements. This method of co-culturing has resulted in excellent outcomes in the degradation of waste materials and drug delivery [105].
5. Genetic Modifications
Genetically modifying the exoelectrogens can be done through several methods, such as metabolic engineering. Studies have, up to this point, mainly concentrated on the alteration of the cell membrane and the overexpression of redox mediators or electron-conducting proteins. Different genetic modification techniques to increase the performance of an MFC by improving EET can be seen in Table 4.

Various genetic modification techniques to enhance the functioning of MFC by improvising the mechanism of EET.
5.1. Genetic Modifications on Cytochrome to Improve Biofilm Production
In Shewanella oneidensis, EET transfer is done through metal reducing pathway consisting of cytochromes (Fcc, MtrB, OmcA, MtrF, MtrA, CymA, MtrC, etc.). Still, in Geobacter, the attached PCC (porin-cytochrome combination) in the outer surface helps in the EET [106]. Cytochromes are cell-bound multiheme-binding important for the DIET and oxidative phosphorylation. The heme group and the adaptable cytochrome-c [107] perform critical functions in the electron transport process. A CV study of c-type cytochrome electrochemical activity in Shewanella lochia PV-4 provided us with the redox maxima induced by the exchange of electrons between the electrodes and active redox complex on bacteria’s cellular membranes. Both cathodic and anodic fluxes in the multi-layered biofilm were more significant than those in the single-layer biofilm due to the enhanced transfer of electrons caused by more c-type cytochromes on the cell’s surface and matrix. Other studies show that a cytochrome in Shewanella oneidensis is transcribed by the OmcA gene. S. oneidensis, in a mutant devoid of the same OmcA, generated 40% less current [108]. In G. sulfurreducens, OmcZ encodes a cytochrome that helps in biofilm growth and is essential for electron transfer. The OmcZ gene deficiency reduced electrochemical activity compared to the wild type. OmcZ works as an electrochemical gateway, allowing electrons to travel from the biofilm to the electrode [109]. The most recent studies discovered that overexpression of CymA in S. oneidensis MR-1 had a greater power output and specific growth rate in an MFC over wild-type MR-1 [110].
5.2. Overexpression of Electron-Conducting Proteins
A study investigated the role of the PilA structural protein, linked to pilus formation, in altering the conductivity of G. sulfurreducens biofilms. This was done assuming that pili-based nanowires make up a significant component of the EET of G. sulfurreducens. The expression of PilA was shown to correlate with the filaments’ electron conductivity in the wild-type biofilm. PilA-deficient mutants exhibited decreased conductivity, confirming the role of the pilA gene in developing conducting biofilm networks and laying the groundwork for future genetic improvements [111]. Studies were carried out with mutants showing deletions in the regulatory pilZ gene to assess the favorable impact of PilA overexpression on the conductivity of G. sulfurreducens biofilms. This deletion produced noticeably more cohesive biofilms with higher conductivity in one of the mutants and enhanced PilA expression [112]. These findings show that a controlled increase in the number of nanowires within a biofilm that is electrochemically active can be a helpful technique for increasing the EET of EAB [113]. The question of whether c-type cytochromes, including OmcS filaments, or solely pili-based filaments, are in charge of long-distance electron transport is still up for debate. The overexpression of redox mediators like pyocyanin by P. aeruginosa can enhance the EET in addition to nanowires. P. aeruginosa’s biosynthesis pathway was genetically altered, leading to an increase in power density by four times and a 1.6-fold increase in pyocyanin concentration [114]. Additionally, overexpressing NAD synthase in P. aeruginosa increased the quantity of intracellular NAD+/NADH readily available, which boosted the production of pyocyanin by a factor of 1.5 and the power density by a factor of 3 [115].
5.3. Manipulation of the Cell Envelope
The impact of cell secretions and their overexpression of redox mediators on the EET pathways was studied. A study exposed P. aeruginosa to common membrane-disintegrating agents like EDTA (ethylenediamine tetra acetic acid) or surfactants and tested the membrane permeability of P. aeruginosa. It led to increased membrane permeability, leading to high power production in MFC. Similar experiments conducted by modifying sophorolipids (bio-surfactant) showed similar results in MFC. The rhlA gene, which is in charge of P. aeruginosa’s rhamnolipid synthesis, was overexpressed to avoid external surfactant addition, boosting the membrane permeability to pyocyanin and the power density and by 2.5-fold in contrast to the control. A transposon could be inserted into the S. oneidensis MR-1 polysaccharide biosynthesis gene cluster to affect the cell membrane. This method produced a power density that was 50% higher and a less noticeable polysaccharide capsule. A better cell adherence to the supplied graphite anode and a decrease in the development of non-conducting surface structures were found to be the causes of this variation [116].
6. Modification of Electrodes to Promote Anodic Biofilm Formation: A Strategy for Modifying Various Types of Electrodes
Electrocatalysts are often immobilized on electrodes used in MFCs; however, in other cases, the material of the electrodes acts as a catalyst. These electrodes should comprise a wide surface area, low resistance, high conductivity, along with low impedance. Furthermore, the electrode material should be biocompatible, allowing for the growth and adhesion of microorganisms on the surface of the electrode [117]. But somehow, some materials may fail to mediate electron transport and biofilm formation. These electrodes must be modified to promote EET and biofilm formation using various methods to enhance power production in MFC. The microenvironment produced by the coexistence of numerous bacterial species present in the biofilm drives its metabolism [97, 98]. Acidogenic and fermentative bacteria interact to break down complex organic molecules into molecular hydrogen. A perfect habitat for developing microbial symbiotic relationships is the micro-environmental niche found inside biofilms.
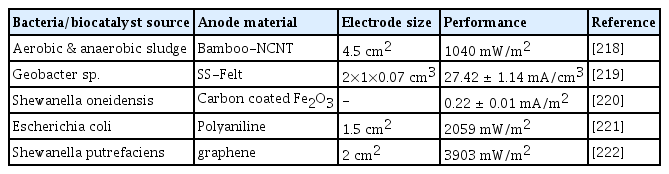
Moreover, interactions between populations of metabolically cooperative microbes support the growth of colonies inside the biofilm. One or more microbial species may build colonization depending on the food and metabolite exchanges. Several anode electrode modifications were studied in MFC. Carbon nanotubes (CNT) have been used as a promising electrode material. The CNT is a carbon electrode material with a vast surface area that is formed like a cylinder. It also has excellent electrical conductivity, is physically stable, and is chemically inert. Researchers created a modified anode composed of Carbon cloth (CC) that was embellished with nitrogen-doped CNT (NCNT) that resembles bamboo to increase the biocompatibility of the CNT to encourage microbial attachment111. Compared to unmodified ones, the bamboo-like NCNT considerably improved MFC performance and decreased the internal resistance of the anode. It is evident that modifying the anode electrode with bamboo-like NCNT considerably increased the anodic potential and current density. This was attributed to the increased number of active sites brought on by various joint structures as well as the nitrogen dopant’s improved biocompatibility. Ohmic and non-ohmic losses have been linked to high internal resistance. By simply covering the electrode with a biofilm, it is possible to reduce the distance between the bacterial cells and the electrode and therefore reduce the charge transfer loss.
To lower the MFC’s operational costs, metal was used as the electrode material. They are reported to be corrosion-resistant and reasonably affordable. Metals like stainless steel fiber felt (SSFF), and stainless-steel mesh (SSM) have been used to operate as the MFC’s anodic current collector and as a 3D support, respectively [118]. However, the metal-based fiber has muscular strength, conductivity, and corrosion resistance; its low biocompatibility and significant over-potential limit its applicability in more MFC applications. Therefore, using binder-assisted pasting, the performance of SSFF was enhanced through modification with either graphene, CNT, or activated carbon. When MFC is used with SSM as the anode, Song et al. found that the start-up time and activation resistance are reduced. Screen printing sodium dodecyl sulfate (SDS) composited with exfoliated graphene-multiwalled CNT onto the SSM was used to modify the electrode. Their research revealed that the redesigned anode had better hydrophilicity and biocompatibility [119].
Due to a reduction in ohmic loss resulting from improved bacterial cell adherence, electrode modification with nanocomposite metal or its oxides considerably improved MFC performance. Along with improving long-range EET in MFC, metal oxides like goethite and rutile also promote the growth of chemotrophic and heterotrophic bacteria by harnessing solar energy. The carbonaceous material has been combined with the oxides of titanium (Ti), tin (Sn), manganese (Mn), iron (Fe), and other metals to create nanocomposite, which has been used as an anode electrode modifier.
Conductive polymers have gained popularity as anode doping materials in MFC because of their conductivity. They are usually used to modify anode electrodes to improve the biocompatibility of the anodes. When the polymer is doped with a nanomaterial in the form of composites, enhanced anodic performance is reported. To increase bacterial cell adhesion and promote EET of EAB, certain functional groups are introduced onto the anode surfaces via electrochemical oxidation, a quick and inexpensive way of anode material modification. The technique calls for a low treatment time and ambient temperature. For electrolysis-based surface modification of the carbon cloth anode, three alternative electrolytes have been investigated. The adjustment resulted in a quicker start-up time while increasing the anode electrode’s wettability parameter and electrochemically accessible surface area. However, this benefit gradually disappears with time, most likely due to the declining metabolic activity of the biomass adhering to the electrode surface [120]. It has been demonstrated that amide groups added to the surface of activated carbon felt (ACF) modified with ethylenediamine promote EET and increase microbial cell adhesion more than unmodified ACF changed with nitric acid. Although the start-up time of MFC was sped up by both techniques, compared to unmodified ACF, the amide group was more biocompatible in modified ACF with ethylenediamine. Compared to pristine carbon paper (CP), the MFC operated with CP/PANI electrode modified with HNO3, ethylenediamine, and diethanolamine used less substrate to reach greater power densities. Due to adequate microbial cell adhesion on the electrode, their experimental findings demonstrated that the MFC operated with CP/PANI modified with ethylenediamine outperformed the others [121]. Some of the electrode modifications are tabulated in Table 5.
Together, the inoculum source, reactor configuration, electrode materials, and operational circumstances all impact on MFC’s performance and output. Due to the broad and abundant microbial populations and their interactions in the EAB, mixed consortia have become widespread. However, unexplained and frequent changes in microbial populations throughout an experiment and adjustments to the media’s nutritional balance affect the systems’ ability to work consistently. Numerous electroactive microbial communities used for bioelectricity production and other potential uses in BESs have been isolated over time from enriched MFCs. Some of these electrode materials which could be used and their pros and cons are discussed below.
6.1. Electrodes Composed of Various Metals
According to various literature on electrode materials, copper, gold, and silver had the best performance among some metals tested with variable current densities. Biofilms with polarization potentials of 0.2 V were developed on silver, gold, and carbon anode materials. On the other hand, copper, steel, nickel, and titanium anode materials may be developed with a −0.2 V potential. Gold, silver, copper electrodes, nickel, stainless steel, Titanium, and cobalt, have all been extensively researched to construct MFCs [122]. Because of compatibility, silver, copper, and gold provide better current intensities than other metals. Numerous metallic electrodes have a current transmission magnitude nearly twice that of conventional graphite electrodes. If economic feasibility is considered, stainless steel is an enticing resource for MFC as an electrode source due to its reasonable price and outstanding conductivity. However, due to the poor biocompatibility of metal electrodes to microorganisms, the use of metal electrodes in MFCs is limited; therefore, thermal oxygenation was used to improve their functional qualities.
6.2. Electrodes Composed of Carbon or Graphite
Over the years, various carbon materials have been utilized in MFCs, including carbon felt, graphite, carbon cloth, charcoal foam, graphene, carbon paper, carbon brush, and graphene felt [123]. These materials allow microbes to mediate high-speed electron transfer, making them an intriguing category of electrode materials for MFC. In this regard, biofilm and interfacial colonization significantly increase when such carbon compounds are utilized, resulting in enhanced power output [124]. Electrochemical pre-processing with graphene sheets to improve biofilm development has been mentioned earlier in the literature. Modification of graphite surface yields carboxyl-containing side chains, which boosts microbial adherence solely at the active electrode material junction and improve electrochemical characteristics of the MFC. The graphene oxide layer typically contains atmospheric O2, which forms various reactive compounds on the graphene sheets layer, including phenolic, quinoline, carboxylic acids, and acetic acids, which promote charge transfer across the biofilm [125].
6.3. Electrodes Composed of Porous Ceramic
When graphite fiber electrodes were utilized, no biofilms were generated in some microorganisms, such as Chlorella vulgaris. But using porous ceramic electrode materials in Chlorella vulgaris, such as tin oxide with fluorine-doped coating on nanoporous TiO2 ceramic materials, can be employed for MFC biofilm formation. The fluorine doping TiO2-coated ceramic electrode had a fibrous exterior network, allowing Chlorella vulgaris to cling to it [126]. As a result, the EPS became less fibrous. In addition, studies regarding biofilms produced on carbon felt wires, exposed disoriented cells, and a lacking EPS are required for biofilms’ formation and their growth. Inside the carbon felt, a dense aggregate of cells of Chlorella vulgaris was present, but they did not adhere properly to the electrode [44].
6.4. Electrodes Composed of Titanium
According to the latest studies, the performance of the titanium (Ti) anode based on the types of materials can be classified as Ti without any coating > Ti with Pt-coating > or flat graphite [127]. Even though Ti has great resistance against corrosion, the poor conductivity of Ti, its scalability, and its less biocompatibility make it inappropriate for application as electrode material in MFC. Because of its broad bandgap, TiO2 in nanotubes is widely used on the positive electrode surfaces of MFC to enhance biofilm formation. For example, carbon cloth, paint, and graphite felt are not feasible for photosynthetic biofilm formation. At the same time, this has been successfully proven by utilizing carbonic barriers and carbonic sheets [128].
One of the drawbacks of MFCs is that power generation does not exponentially rise with adequate contact coverage of the electrodes to accommodate for increased EET. Approaches including lowering oxidation and reducing overpotentials, enhancing fluid conductance, decreasing mass transfer resistances, shortening the distance between electrodes, employing novel gas electrode materials, and layering have all been used to help scale up the MFCs from the laboratory level to the industrial level [129]. While improvements in the scaling up of MFCs and their architecture have resulted in increased power output of the MFC, they have not addressed the current state of electrode development used in the fundamental scaling of such devices. The EET techniques employed by EABs are linked closely to the scaling up of anodes in MFCs and other bioelectrochemical systems [130]. Considering each electrode’s contribution to the overall energy transmission is crucial.
7. Processes Concerning Biofilm Attachment and Detachment: Challenges
Advective flow and three forms of non-covalent interactions influence the initial adhesion of microorganisms to a solid surface: (1) Lewis’s acid-base (L-AB), (2) Lifshitz version of Vander Waals (LVW) (also called electrodynamic connections), (3) double electrical layer (EL). Such relationships are thought to be in the extended DLVO theory (EDLVO, where DLVO stands for Derjaguin-Landau-Verwey-Overbeek) [131]. All liberated energies for each type of relationship as a distance variable can be calculated and kept separate before combining all three (expressed in the same energy forms) to get the ultimate XDLVO graph [132]. Metal ions with valency two (Mg2+ and Ca2+) can create ionic bridges between negative molecules or substances, which could also affect initial attachment. Cell mobility by flagella might propel cells in routes towards potential gradient (chemotactic way), electrostatic interactions (electrogalvanotaxis), electromagnetic field (magneto-taxis), and illumination (photo-taxis), which also acts as additional forces affecting initial adhesion [133], when a pioneer species of microbes connects to a substrate surface, the number of active sites for microbial adhesion of other species increases.
Several factors influence cell adhesion and biofilm production, such as roughness, hardness, wettability, cell surface features, and types of substratum [134]. Flagella, fimbriae, pili, slime layer, capsule production (EPS), as well as the physiochemical parameters such as nutrients, pH, temperature, O2, etc., consist of cell surface features. Some of the distinct mechanisms in a biofilm detachment process are - a) Erosion and shearing, where tiny blotchy sections of such biofilm are continually eliminated; b) sloughing (rapid and comprehensive clearance); c) abrasion (detachment owing to the impact of mass fluid molecules on the biofilm interface); d) natural shedding. These dispersal models may have an impact on the species’ phenotypic traits. Eroded or sloughed biofilm aggregates are particularly prevalent in denser biofilms that form in nutritionally rich environments [135]. Several biofilm features, such as EPS and antibacterial resistance, are likely to be retained in the biofilm, while colonies shedding as a result of growth may swiftly convert to planktonic morphology. As expected, the biofilm detachment rate increases with the depth of the biofilm cover, associated with severe fluid shearing at the microbial biofilm liquid interface. Sloughing in this state is much less predictable than degradation and is thought to be triggered by a lack of nourishment or oxygen inside the biofilm matrix. At high hydrodynamic shear stress, harsh granules of minerals in the intake medium may damage biofilms by abrasion [136].
8. Insertion of Synthetic Amino Acids (AAs) to Improve the Performance
Within the Schultz laboratory [137], tools for integrating artificial amino acids (AAA) onto proteins of bacteria in vivo have been developed. By employing tRNA-NH2-acyl tRNA synthetase pairings, this approach allows for the preferential integration of unusual amino acids (AAs) into polypeptides in yeast [138], mammalian cells [139], and E. coli [140]. These pairings do not bridge with intrinsic parts of translating mechanism but rather identify the target AAA and integrate this into proteins with reaction to TAG, a nonsense codon in yeast and E. coli, or TGA, another opal codon in animal cells. To improve system selectivity, tRNA synthetases are now being created to integrate only AAAs into proteins while ignoring native AAs. Interaction between redox enzymes and the electrodes can be increased by adding different AAAs upon that site of the enzyme, allowing electrons to transit between the enzyme’s active site and its surface and then to the electrodes. Redox enzymes would gain stabilization and stay functioning for prolonged periods if they are expressed at the surface, which offers a stabilizing microenvironment for the microbes. 3-amino tyrosine has been recently integrated further into E. coli RR (ribonucleotide reductase)’ s A2 subunit, and it has been conclusively proved that 3-AT (aminotyrosine) continues to serve as a radical pit instead of a single tyrosine radical, showing the presumed electron transfer route in this enzyme involved [141]. This study has also provided evidence for immediate electron transport communication between the redox AAA and the sulfur-iron group in this enzyme, which motivates us to use AAA as electron transport.
9. Bacteria Coated with Gold and Silver Nanoparticles
Studies have utilized nanoparticles (NPs) as electrical bridgers between the electrodes and enzymes’ active sites [142]. The Willner group achieved an eight-fold increase in electron turnover rate from the original enzyme’s cofactor to its natural receiver. FAD-reformed GOx was employed, where the FAD got altered with gold nanocrystals and subsequently connected to electrodes. Its alignment concerning the electrode aided in achieving the highest transfer of electrons possible. Over the years, various approaches have been used to proficiently wire oxidation/reduction enzymes, such as encapsulation in conjugated polymers [143], trying to combine nanotubes with redox enzymes [144,145] or even site-targeted mutagenesis to ensure alignment of proteins with diodes146. Several attempts to cover microorganisms with metallic NPs have been attempted. One example is the manufacture of gold nanoparticles (GNPs) with the help of E. coli, in which the use of microbiological NP hybrids was proven in the direct electrochemical processes of hemoglobin [147]. There is a distinction between the manufacture of GNPs by microbes and the adhesion of GNPs generated in an inorganic setting and afterward attached to the bacteria. Tufted organisms with two lengths of fibrils, such as Streptococcus sanguis, contain a charge and thus are hydrophobic on their short and long filament fibrils [148]. So, because the bald region of the surface of the microbes is hydrophilic, colloid gold, which is hydrophobic and is attracted to the negative, did not cling to it throughout a pH of 3.7 to 9.0. The attachment of colloidal gold to lengthy fibrils happened regardless of pH. When the pH is 3.7, colloidal gold adhered to short fibrils. The electrostatic interaction between the negative charges short fibrils and GNPs grew at higher pH levels, preventing colloidal gold from attaching to the short fibers [149]. In the mid-1980s, it was discovered that bacteria collect metals like gold, and silver, which are recoverable from various bacterial species by methods that are yet unknown [150]. Many research groups have recently proven the production of GNPs by various bacterial species [151–153]. The Raman spectra of silver metal produced by bacteria were reported by Zeiri et al. The bacteria were exposed to sodium borohydride, which acted as a nucleating substrate for silver ion reduction, and a rough silver coating developed all around microorganisms [154]. Several microorganisms have been employed to generate defined as follows nanostructures composed of metallic nanoparticles [149,155,156]. R. F. Saraf’s team applied extremely negative charges of the teichoic acid brush to the surfaces of the Gram −ve bacterium Bacillus cereus.
Furthermore, nanorod-coated bacteria have shown greater conductivity than nanospheres at just approximately 10% coverage, far below the threshold of almost 45%. The high electrical conductivity makes it possible to build electronic circuitry on top of the bacterium without smothering it [156]. These findings imply that site-specific adhesion of nanorods to microorganisms may result in more efficient electron transport between bacteria and electrodes than spherical attachment would.
10. Advances in Biofilm Engineering to Enhance the MFC Performance
Given the importance of EPS in microbial EET and biofilm development on the electrode, controlling the biofilm formation techniques for enhanced adherence and EET is indeed the MFC’s future goal. Several MR-1 excision mutants of Shewanella oneidensis were generated and evaluated for electricity production and oxide reduction [157]. Shewanella oneidensis MR-1’s EET process includes MtrC, OmcA, and decaheme ccyts in transferring electrons to heavy steel oxides and MFC electrode materials; however, Shewanella loihica PV-4 exhibited a distinct method for current production [158]. In another study using the Shewanella oneidensis MR-1 strain, it was found that the conducting EPS found in the biofilm affects the cell attachment to the graphite anode material and current production in MFCs. At the same time, the electromagnetically non-conductive polysaccharides can start interfering with the electron transfer when in contact with cytochromes. Cell surface treatment has therefore been offered as a potential technique for boosting power in an MFC system [159]. In a model organism Shewanella oneidensis MR-1, genetic engineering approaches were used for the biosynthesis of flavin metabolizing enzymes gene cluster ribCribD -ribE-ribA and a metal-reducing channel biosynthetic gene group mtrA-mtrC-mtrB, resulting in an increased EET rate in MFC with approximately 110% increase in the current production [160].
Similarly, the use of partial degradation of graphite composite substrate by Ultraviolet/Ozone exposure resulted in changes in the surface properties of the electrode, allowing for enhanced EAB growth on the electron, which increased EET. This Lead to high power production in MFC. Shewanella oneidensis MR-1 microbial community establishment was advanced on UV/O3-medicated graphite felt anode and cathode at 0.3V versus Ag/AgCl, with the graphite terminals of electrodes treated to 45 minutes of ultraviolet and ozone intervention which improved the microbial adhesion on the electrode surface [161]. Furthermore, the influence of specific working conditions on biofilm development and nitrogen fixation in three moving-bed biofilm reactors (MBBRs) was also investigated. Scientists used genetics and gene suppression techniques to study the relevance of such attachments, revealing a comprehensive depiction of the EET pathway from bacteria to the positive electrode. Yet, minimal reports of phytoplankton that were genetically modified or biochemical processes of phytoplankton, along with their voltage productivity in energy generation, were reported.
However, enhancing EPS synthesis using modification in the substrate strategies has boosted power output in phytoplankton [30]. Aside from its role in the complex creation of amylopectin and the development of starch synthase, new capsules, a GT5 enzyme, have been studied because of their contribution to carbohydrate synthesis. There is currently no indication that these polymer-producing enzymes are being upregulated in algae at the moment. Transcription factors responsible for EPS production in microalgae would thus require extensive investigation before becoming a feasible strategy for enhanced power production in photosynthesis processing algae MFC (PAMFCs), which are more lasting than MFC.
The science of bio-electrochemistry has faced a significant issue for many years: improving the current generation of MFCs. There have been numerous papers and patents as a result of this endeavor. Electrode materials, electrode spatial layout, electrolytes, current collectors, reactor architecture, separation membranes, and microbial catalysts are just a few of the MFC components that have been optimized. The three methods, screening, adaptation, and synthetic biology, are frequently utilized to create effective microbial catalysts for MFCs. Four categories can be made from the synthetic biology techniques used to increase the EET rates between microorganisms and anodes:
optimization of substrate transport and cytoplasmic metabolism of electrogenic microbes,
reengineering of DET mechanisms,
reengineering of IDET mechanisms,
optimization of cell adhesion and biofilm formation
To increase the availability of electrons in the form of NADH for subsequent EET to electrodes, the first technique manipulates the electrogenic microorganisms’ core metabolism. DET and IDET in the second and third techniques can be strengthened and optimized to increase the flux of electrons from the cytoplasmic membrane to the electrode. Finally, strong micrometer-thick biofilms can be formed by electrogenic bacteria such as Geobacter spp. [162]. EET proteins are used by cells from the biofilm’s edge to the electrode surface to generate current. Therefore, microorganisms that have been altered to be better at attaching to cells and forming biofilms ought to be better at transferring electrons to the anode.
Microbial adherence to the anode surface is critical in boosting MFC’s productivity and higher energy production [163]. Pseudomonas aeruginosa’s biofilm formation and cell attachment ability improved when genetic manipulation was done. PilT mutants in P. aeruginosa lack twitching motility because their pili are permanently stretched [164]. PilT is an ATPase required for the depolymerization of pilin subunits in P. aeruginosa type IV pili, which are non-conductive [165]. Type IV pili concentrate on the surface of less mobile P. aeruginosa cells in the absence of PilT, resulting in increased biofilm development, a cell with cell adherence, and cell adhesion to electrodes [164].
To date, no reports of phytoplankton that have been genetically altered or that have biochemical pathways, as well as their potential productivity in energy cultivation, have been reported. However, improving EPS synthesis utilizing substrate modification approaches has been demonstrated to enhance power production in phytoplankton. Starch synthase, a glycosyltransferase 5 (GT5) enzyme, has been looked at for its potential role in increasing carbohydrate production and its involvement in the complex formation of amylopectin and the creation of new capsules. There is yet no proof that these polysaccharide-producing enzymes in algae are being up-regulated. To date, no reports of phytoplankton that have been genetically altered or that have biochemical pathways, as well as their potential productivity in energy cultivation, have been reported. However, improving EPS synthesis in an individual utilizing substrate modification approaches has been demonstrated to enhance power production in phytoplankton. Starch synthase, a glycosyltransferase 5 (GT5) enzyme, has been looked at for its potential role in increasing carbohydrate production and its involvement in the complex formation of amylopectin and the creation of new capsules. There is yet no proof that these polysaccharide-producing enzymes in algae are being up-regulated.
The attempts to improve the performance of electroactive bacteria have focused on altering the genetics of the EET phenomenon due to developments in genetic engineering and synthetic biology. The rate of electron transfer and the efficiency of electroactive bacteria is generally low, except for Shewanella and Geobacter. Due to the rapid development of analytical tools and advancements in biotechnology, researchers have started changing strains to create modified electroactive bacteria to improve electron transport. Exoelectrogens that have been developed have also been found to improve target product performance and achieve various bioprocess goals in microbial system-based processes. The power density is doubled when glycerol dehydrogenase (GldhA) is overexpressed in genetically altered Escherichia I compared to wild-type ones. This was due to the production of redox mediators from the GldhA-catalyzed metabolism of aminopropanol, threonine, L-1,2-propanediol, and glycerol [54]. In similar research, heterogeneous overexpression of cyclic-di-GMP in Shewanella sp. dramatically increased biofilm formation, resulting in almost threefold greater power density output 166. A non-metal-reducing E. coli strain created by adding MtrCAB genes from Shewanella oneidensis can reduce metallic ions eight times greater than the original strain conduit [167]. The expression of key enzymes for glucose and glycerol fermentation and metabolite synthesis is influenced by electrode-based electron transfer in MFCs [168]. In producing effective electroactive bacteria, a recent study of engineered electrogens emphasized the techniques, genetic modification of electrochemical genes, and a synthetic biology approach [169].
11. Strategies of Synthetic Biology
Microbial catalysts, separation membranes, reactor configuration, current collectors, electrolytes, electrode spatial design, and electrode materials have all been optimized in MFCs to enhance their performance. Four categories can be established for the synthetic biology approaches used to increase ET rates between the anode and the microbes [170]:
Electrogenic bacteria’ cytoplasmic metabolism and substrate transport are optimized.
Improvements to the Direct EET Mechanism.
Improvements to the indirect EET Mechanism (Fig. 3).
Development of biofilm formation and cell adhesion.
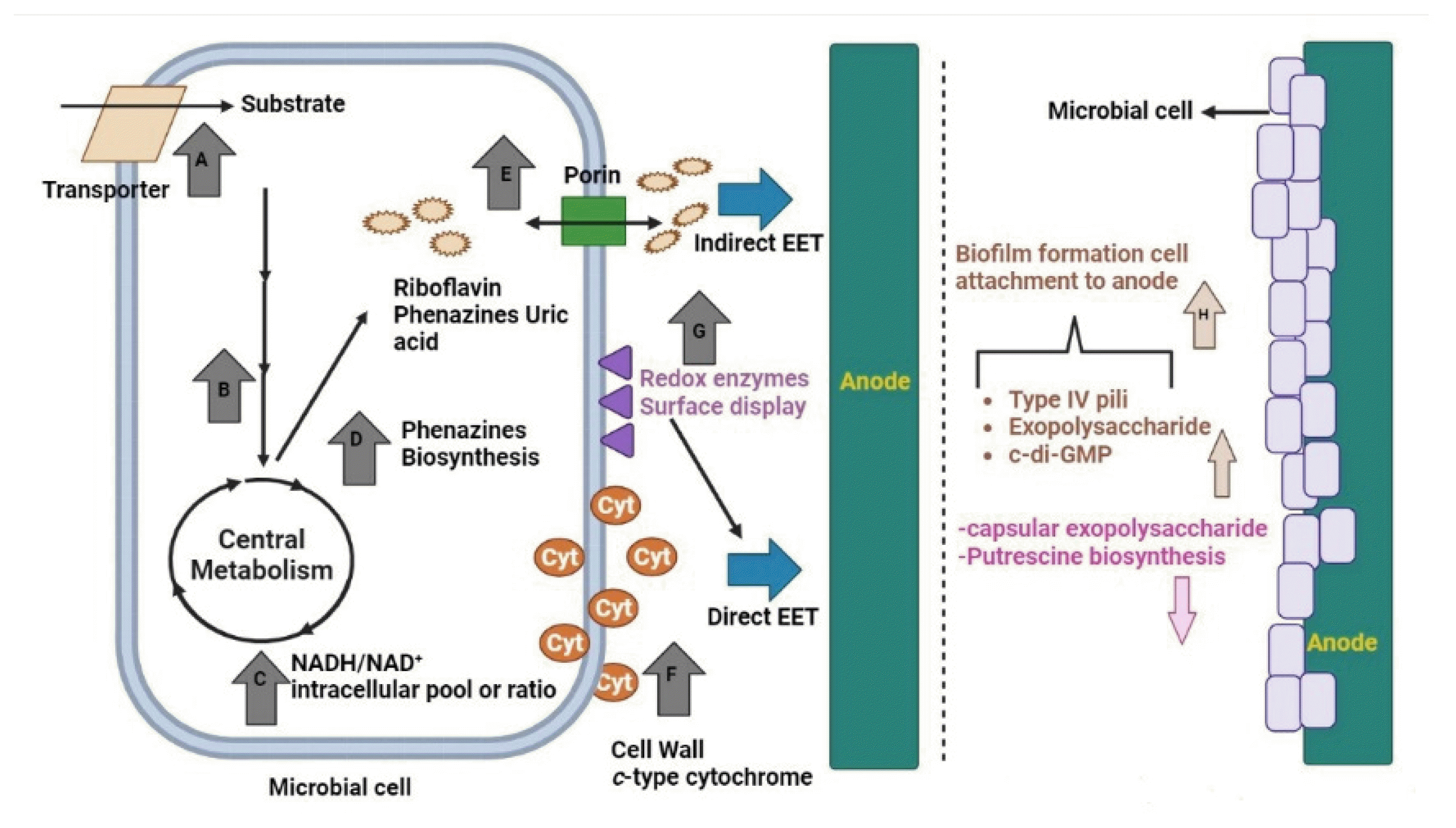
The efficiency of microbial fuel cells (MFCs) can be enhanced by using synthetic biology to target specific cellular processes of microorganisms. (a) Increased substrate uptake is achieved by overexpressing the transporter protein. (b) Substrate oxidation speedup by genetic engineering. (c) Genetic modification of central metabolism to boost NADH/NAD+ ratio or pool. (d) Regulatory network’s genetic modification or phenazines’ biosynthesis-related gene overexpression to boost the production of phenazines. (e) Soluble electron shuttle (ES) transport via the cell wall can be improved by overexpressing porin. (f) Improving direct extracellular electron transport by c-type cytochrome’s overexpression. (g) Redox enzyme’s surface display. (h) The synthetic biology method improves biofilm growth and cell adhesion to an electrode surface.
The first case involves modifying the core metabolism of electrogenic bacteria to increase the amount of NADH available for EET to the electrodes. The EET mechanisms can be optimized in the second and third approaches to increase the flow of electrons to the electrode from the cytoplasmic membrane [171,172]. Last but not least, exoelectrogens like Geobacter spp. can produce biofilm of several micrometers in thickness. Cells from the biofilm’s periphery to the electrode surface can produce current using a system of extracellular ET proteins. Thus, microorganisms engineered to bind to cells and produce biofilms more effectively should be better at electron transfer to the anode. Antibiotic selection markers, plasmid shuttle machinery, Promoters, and replication origins have been recently identified in G. sulfurreducens and S. oneidensis and can be employed in either the orthogonal or native host to regulate EET pathways [173]. The severe anaerobic cultivation requirements of G. sulfurreducens have hampered the speed of bioengineering, despite the availability of a molecular toolbox for G. sulfurreducens. To modify the genome of S. oneidensis with great precision and specificity, using ssDNA oligos was necessary, and creating a highly efficient electrotransformation process made this possible. Corts et al., who attempted and optimized recombineering technology in S. oneidensis, found that ssDNA oligos can recombine site-specifically in the chromosome of S. oneidensis in the control reactions in the absence of recombinase but at a somewhat lower frequency than W3Beta-mediated recombination [173]. CRISPR/Cas9 is another step forward in genome engineering for S. oneidensis. In order to get rid of the unedited cells once recombineering was produced, Corts et al. employed CRISPR/Cas9 [174]. By targeting a specific wild-type (unedited) gene in the chromosome with a single guide RNA, Cas9 may create a double-stranded break that is lethal for many bacteria, making it a powerful tool for use in counter-selection [175].
The expression of numerous multiheme cytochromes c was a significant technological barrier in developing EET-capable E. coli strains (Fig. 2). Kinetic equilibrium between maturation, secretion, translation, and transcription is necessary for multiheme c cytochromes expression [176]. CcmH recognizes and binds the apo cytochrome c protein, which then undergoes a thioether bond formation with heme b to create the holoprotein [177], which is the end product of the cytochrome c maturation (ccm) system.
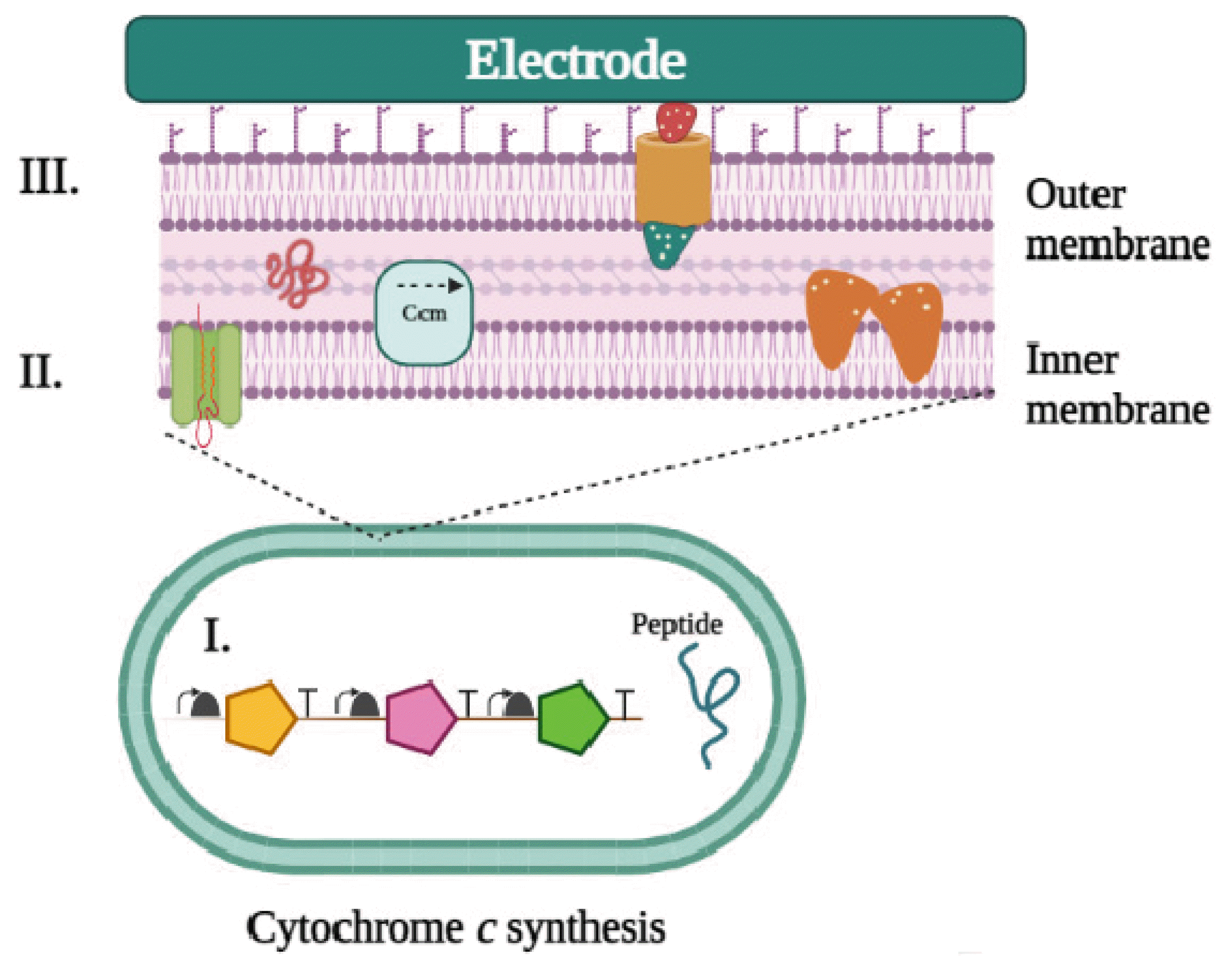
Electron transfer (EET) and electronic regulation in non-native hosts engineered via genetic engineering. For an orthogonal expression to occur in a non-native host, specific conditions must be met using engineered expression vector transfer (EET) pathways: i) Maintaining correct cytochrome expression levels. ii) The correct cytochrome c maturation process allows optimal protein expression and localization without causing unnecessary toxicity. iii) Proper folding of the proteins that were expressed into their complexes.
12. Research Directions for Electroactive Biofilms in the Future
A first step could be to look into different measuring methods for quantifying electron fluxes and archiving in EAB. Electrochemical tests should preferably be carried out in combination with visual observations obtained in the field to measure the surface area and biofilms’ densities as a variable dependent on time [178]. This would enable the study of bioactive compounds per unit of organic matter under various situations. CLSM (confocal laser scanning microscopy) [179] and Raman spectroscopy [180] are two techniques that could be used to determine which parts of the cell are essential for electron storage and transit.
Across these lines, UV-Vis light spectroscopy [181] and fluorescence spectroscopy could be used to evaluate the cytochrome heme concentration and the degree of cytochrome pool declines. Hence, the redox state of cytochromes can be used for predicting energy output [182]. Likewise, cyclic voltammetry may calculate the mediatory voltage of operational charge carrier cell components [183]. Furthermore, combining such methodologies allows the properties of various components of MFCs to be studied in greater depth [184].
Accessibility of the electrolytes and electrogenic bacteria in MFCs is a fresh field of investigation with many possibilities [185]. In this aspect, oxygen has the potential to break down complex organic material into power-generating compounds as by-products. It is also critical to assess the feasibility of mutualistic relationships between stringent anaerobic organisms and aerotolerant anaerobic microorganisms and the biofilm role in safeguarding electrodes against contamination [116]. One example can be researching the EET processes in somewhat under aerobic conditions. These themes should be the focus of exciting research in the future. Another set of concerns emerges from using MFC technology to generate power and by-products by treating wastewater as a commodity while also seeking to collect and treat wastewater [186–190].
EABs’ ability to oxidize a wide range of biomolecules and the transport of electrons are some critical aspects. Furthermore, converting specific and complex natural substances are significant issues that may demand pretreatment to be used as an anolyte in MFC to produce power [124]. Research in this field will increase as systems achieve commercially relevant performance. Although much is known about the methods allowing EABs to transfer electrons toward the positive electrode, there are numerous uncertainties. The significance of biofilm structure and its components, including EPS and nanotubes, in regulating electron conductivity in EAB is unknown; nonetheless, knowing how they operate and how they could be improved is crucial. The utilization of fluid chambers based on microplates and miniature MFCs, in combination with current- and voltage-sensitive pigments and complex bio-molecules, appears to possess the capacity to simplify research, offering a better knowledge of EABs’ role in MFC. Research regarding biocathodes, whether the microorganisms at the cathode are electro-chemolithotrophs or mixotrophs, is underway [191].
13. Conclusion
This paper describes various ways to improve biofilm formation to increase the effectiveness of MFCs and generate high current output. Under this situation, biofilm engineering may enhance electron transport in electrode-electrolyte conjunctions. Improved microbial activity and electrical conductivity can lower charge transmission resistance at the anode. Furthermore, new materials for electrolyte and electrode functionalization technologies could significantly help in customizing biofilms and increasing rates of bio-electrocatalysis. Exogenous supplementation of biological mediators that boost the signaling cascades necessary for biofilm establishment can improve biofilm development. The final strategy, which involves manipulating genes encoding biofilm-related proteins, necessitates expertise and elegance. An intelligent blend of the physicochemical, biological, and computational approaches discussed in this review will need to be applied to improve biofilm growth and activity for specific applications. However, significant obstacles remain until electrogenic microorganisms for MFCs may be significantly genetically engineered in a custom-tailored way. However, our understanding of the EET mechanism of microorganisms to anodes remains inadequate, despite tremendous advances in this area in recent years. It was also discovered that MFCs might be used to reliably power low-power medical equipment implanted in humans over the long term. These miniaturized MFCs, bio-catalyzed by Saccharomyces cerevisiae, can convert the glucose from the blood into electricity. It was discovered that the current generation rises in synchrony with the level of pollutants.
Consequently, this method can help detect and count microbes in food [192]. It is necessary to thoroughly understand the metabolic activity and anatomy of electrogenic microorganisms to develop effective synthetic biology techniques. Nonetheless, the work done so far is impressive, and it appears that using synthetic biology methods gives us a chance to enhance the performance of the MFC as well as genuinely comprehend it.
Acknowledgements
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korean government (MSIT) (NRF-2021R1A2C1013989).
Notes
Conflicts of Interest
The authors declare no conflict of interest.
Author Contributions
Conceptualization. S.P.J, and S.P.; writing—original draft preparation, C.P, A.K, T.N, and S.P.J; writing—review and editing, N.S, K.P and S.P.; supervision, S.P, K.P and S.P.J; funding acquisition, S.P.J. All authors have read and agreed to the published version of the manuscript.