Different behaviours of biologically textile wastewater treatment using persulfate catalyzed electrochemical oxidation process on Ti/BDD and Ti/SnO2-Nb2O5 anodes
Article information
Abstract
Textile wastewater effluent includes high concentrations of pollutant such as dyes, detergents, and chemical auxiliaries that bring many negative impacts to humans and the environment. In this study, biologically textile wastewater effluent was first time treated using electrochemical oxidation catalyzed by persulfate on Ti/BDD and Ti/SnO2-Nb2O5 anodes. The results show that persulfate catalyst only significantly increases the electrochemical process efficiency at the Ti/BDD anode, but not at the Ti/SnO2-Nb2O5 anode. The optimized operation conditions at the Ti/BDD anode are: [NaCl] 3 g/L, [PS] 2 g/L, applied current density 4.16 mA/cm2, pH 7.5, stirring speed 200 rpm, electrolysis time 120 minutes that removed 94.78% color, 64.57% COD, and 41.57% TOC in textile wastewater. The optimized operation conditions at the Ti/SnO2-Nb2O5 anode are: [NaCl] 3 g/L, [PS] 1 g/L, applied current density 4.16 mA/cm2, pH 7.5, stirring speed 200 rpm, electrolysis time 150 minutes that removed 73.04% color, 41.32% COD, and 39.22% TOC. Detected oxidant radicals during the electrochemical oxidation processes could contribute to the oxidation of organic matters in biologically textile wastewater.
Abstract
Graphical Abstract

1. Introduction
The textile industry is essential for human life and has become a backbone for economic development, yet the amount of textile wastewater discharged to the environment is getting higher [1]. The dyes and chemicals in textile manufacturing induce a high coloration and suspension solids that are toxic to aquatic resources as they increase the osmotic pressure, reduce dissolved oxygen, inhibit the respiration of aquatic species, and affect cell metabolism. In addition, the contaminants in textile wastewater may accumulate in the organisms of the ecosystems, causing chronic diseases and cancer for humans [2]. There are many methods to treat textile wastewater, including physicochemical and biological methods [3]. Chemical methods and flocculation are often used to decolor textile wastewater, but these methods require large amounts of chemicals to treat pollutants and often cause secondary pollutants such as sewage sludge [4, 5]. Biological methods use the microorganisms present in wastewater to degrade organic compounds and nutrients. The products of the decomposition processes are CO2, H2O, N2, and sulfite ions [6]. However, these processes have some difficulty in degradation related to persistent organic matters in wastewater [3].
Among the strong oxidizing radicals, the free sulfate radical is a strong oxidizing agent that breaks down pollutants present in wastewater. It can be produced via activating persulfate anions via thermal [7], UV [8], alkalinity [9], and electron transfer activation of transition metal ions [10], as shown in Eq. (1–5):
The electrochemical oxidation method is based on the conversion principle of electrical energy decomposing water to produce activated hydroxyl radicals and other oxidizing agents to degrade organic compounds and nutrients in wastewater. The oxidant generation depends on the anode materials and electrolytes [11]. The electrochemical oxidation process comprises two mechanisms: direct oxidation and indirect oxidation. Direct oxidation processes decompose pollutants via an electrical energy current that produces charge transfer reactions. Indirect electrochemical oxidation occurs through strong oxidizing radicals formed in the solution [12]. Previous research on the textile wastewater treatment using the electrochemical method shows the optimal operating parameters to achieve a great COD removal efficiency at 96.2% and decolorization of 93% [13]. The oxidation of different active dyes by electrochemical active chlorine generation resulted in a COD decomposition 39.5%–82.8%, total organic carbon (TOC) decomposition 11.3%–44.7%, and complete color removal [14]. A study on active dye treatment in electrochemical textile wastewater showed COD and turbidity removal efficiencies were 32% and 82% for Levafix Blue CA active dye, 37% and 88% for Levafix Red CA active dye, 33%, and 94% for Levafix Yellow CA active dye, respectively [15]. Research on the toxicity depletion of Basic Red 29 (BR29) dye and textile wastewater effluent using the Ti/BDD anode showed that BR29 dye was eliminated almost completely up to 99%. Further, color and COD removal efficiencies were 97.2% and 91% in textile wastewater effluent at a current density of 1 mA/cm2 [16]. When an electrochemical is combined with persulfate (PS), TOC is degraded by over 60% [17]. The phenol decomposition in the solutions was high at 75.3% when using the persulfate/electrochemical/FeCl2 system (PS/Electro/Fe) at optimal conditions (1000 mg/L Na2S2O8 and 500 mg/L FeCl2 at 15 V) [18]. Optimization of atrazine depletion (> 99%) and TOC removal (22.1%) performance was achieved after 35 minutes of treatment with atrazine dose 3.0 g/L, PS concentration 4.0 mM at a current density 4 mA/cm2 and an initial pH of 6.3 [19].
To the best of our knowledge, this is the first study to fabricate Ti/SnO2-Nb2O5 anode for textile wastewater treatment and make the comparision with traditional superior Ti/BBD anode in the present of persulfate catalysts. In this study, the persulfate assisting electrochemical oxidation was used to treat biologically textile wastewater on Ti/BDD and Ti/SnO2-Nb2O5 anodes. Various factors affecting the electrochemical process, such as NaCl electrolyte concentration, persulfate catalyst concentration, current density, initial pH, stirring speed, oxidant generations, and energy consumption were studied on the pollutant removal efficiencies.
2. Materials and Methods
2.1. Textile Wastewater Effluent
The textile wastewater was taken directly after the biological tank (activated sludge) at the current wastewater treatment plant of ChyangSheng Textile company Co., Ltd, Thuan An, Binh Duong, Vietnam. The sampling method was followed to ISO 5667-1:2006 standard. Textile wastewater was then stored in a 30 L plastic in the refrigerator at a temperature 5°C. The main characteristics of textile wastewater are shown in Table S1 in Supporting information:
2.2. Research Models
The experimental model consists of a glass circular cylindrical reaction tank with a size of 8 cm × 5.5 cm (height × diameter) with a working volume 100 mL, as shown in Fig. 1. The capped reaction tank is designed to have two fixed slots for an electrode pair and an air outlet. A Ti/Pt commercial electrode (Permelec, Japan) with a size of 3 cm × 2 cm × 0.25 mm (length × width × thickness) was used as a cathode. Ti/BDD commercial electrode (Permelec, Japan) and fabricated Ti/SnO2-Nb2O5 electrode with dimensions of 3 cm × 2 cm × 0.25 mm (length × width × thickness) were used as anodes. The electrode pair was fixed to the reactor cover, and the distance between the two electrodes was kept at 2 cm. The reactor was then placed in a magnetic stirrer (Velp AREC.X, Italy) with a speed of 100 rpm to get a good diffusion and mass transfer. The Keithley's DC Power Supply 2260B-260-4 (DC, Taiwan) was used to control the current with a potential range of 0–150 V.
Ti/SnO2-Nb2O5 electrode was fabricated using the sol-gel method based on the hydrolysis and condensation reactions of the precursors. 1 mL of mixing SnCl4 0.1 M and NbCl5 0.1 M solution was prepared to get a molar ratio between SnO2:Nb2O5 was 4:6, then mixed for 120 min at 60°C. Finally, the precusor solution was coated on the Titanium substrates and calcinated at a temperature of 500°C in 1 hour. The process repeated 10 times until the desired thickness of SnO2-Nb2O5 was obtained.
2.3. Analytical Methods
Chemical Oxygen Deman (COD), Total Organic Carbon (TOC) and color were analyzed based on the Standard Method for the Examination of Water and Wastewater [20]. The true color of water was determined using HI 727, Hanna colorimeter (Romania). COD concentration was determined using reflux heating of K2Cr2O7 with Ag2SO4 in H2SO4 concentrated acid using RD - 125 heater, Lovibond (Germany). Absorbance was measured at wavelength 604 nm using UV–1800 spectrophotometer Shimadzu (Japan). TOC was determined using a Shimadzu TOC-L (Japan). In situ Cl2 free chlorine present in the effluent water after electrolysis was determined using DPD method using a DR900 multi-index portable colorimeter (USA) as suggested by Dr. Joonseon Jeong and Prof. Yoon Jeyong’s group at the Seoul National University, South Korea [21]. HO• and SO4•−radicals produced at the anode surfaces during electrochemical processes were consumed in the presence of tert-Butyl alcohol (TBA) and ethanol [22]. The nPS/nEtOH ratio was 1/250 and the nPS/nTBA ratio was 1/250. H2O2 concentration was determined using DMP reagent and Cu(II) sulfate 0.1M solution. Ozone concentration was determined using the Indigo Colorimetric Method [21]. The data were then statistically analyzed using an ANOVA two-factor replication, with a significance level of 0.05 (p = 0.05). The hypothesis H0 is insignificant different and H1 is significant different; if Ffisher > Fcrit then accept H0, in contrast, if Ffisher < Fcrit then reject H0.
3. Results and Discussion
3.1. Effects of NaCl Electrolyte Concentration
Fig. 2 shows the effects of NaCl electrolyte concentration on the pollutant decomposition performance in textile wastewater treatment using electrochemical oxidation without the presence of the persulfate catalyst at both the Ti/BDD anode (A–C) and Ti/SnO2-Nb2O5 anode (D–F). As can be seen in Fig. 2, color, COD, and TOC removal performances in both anodes significantly increase with the increase of NaCl concentration as the supporting electrolyte. Since the textile wastewater was collected after the activated sludge process of the current wastewater treatment plant of the factory, it was still lacking in conductivity for electrochemical reactions. In overview, high color, COD, and TOC removal efficiencies at 3 g/L and 5 g/L NaCl were achieved with both anodes. However, the Ti/BDD electrode at NaCl concentration 3 g/L produced an equivalent removal yield at NaCl 5 g/L. The removal yields on the Ti/BDD anode increased from 39.69 ± 2.16% to 100% for color, 29.78 ± 1.57% to 54.66 ± 1.44% for COD, and 11.95 ± 1.31% to 27.77 ± 1.07% (Ffisher < Fcrit) for TOC when measured at 30 minutes and 180 minutes of electrolysis at 3 g/L NaCl. When using the Ti/SnO2-Nb2O5 anode, the removal efficiencies increased from 33.91 ± 1.27% to 80.43 ± 2.15% for color, 19.83 ± 1.14% to 49.82 ± 0.85% for COD, and 3.43 ± 1.99% to 36.63 ± 1.23% (Ffisher < Fcrit) for TOC at NaCl concentration of 3 g/L after 30 minutes to 180 minutes of electrolysis. The color, COD, and TOC removal efficiencies when using the Ti/BDD anode were higher than when using the Ti/SnO2-Nb2O5 anode. At the anode surface, chlorine is made up of chloride ions, which forms hypochlorous acid and is further dissociated into hypochlorite ions, as shown in Eq. (6–8) [23]:
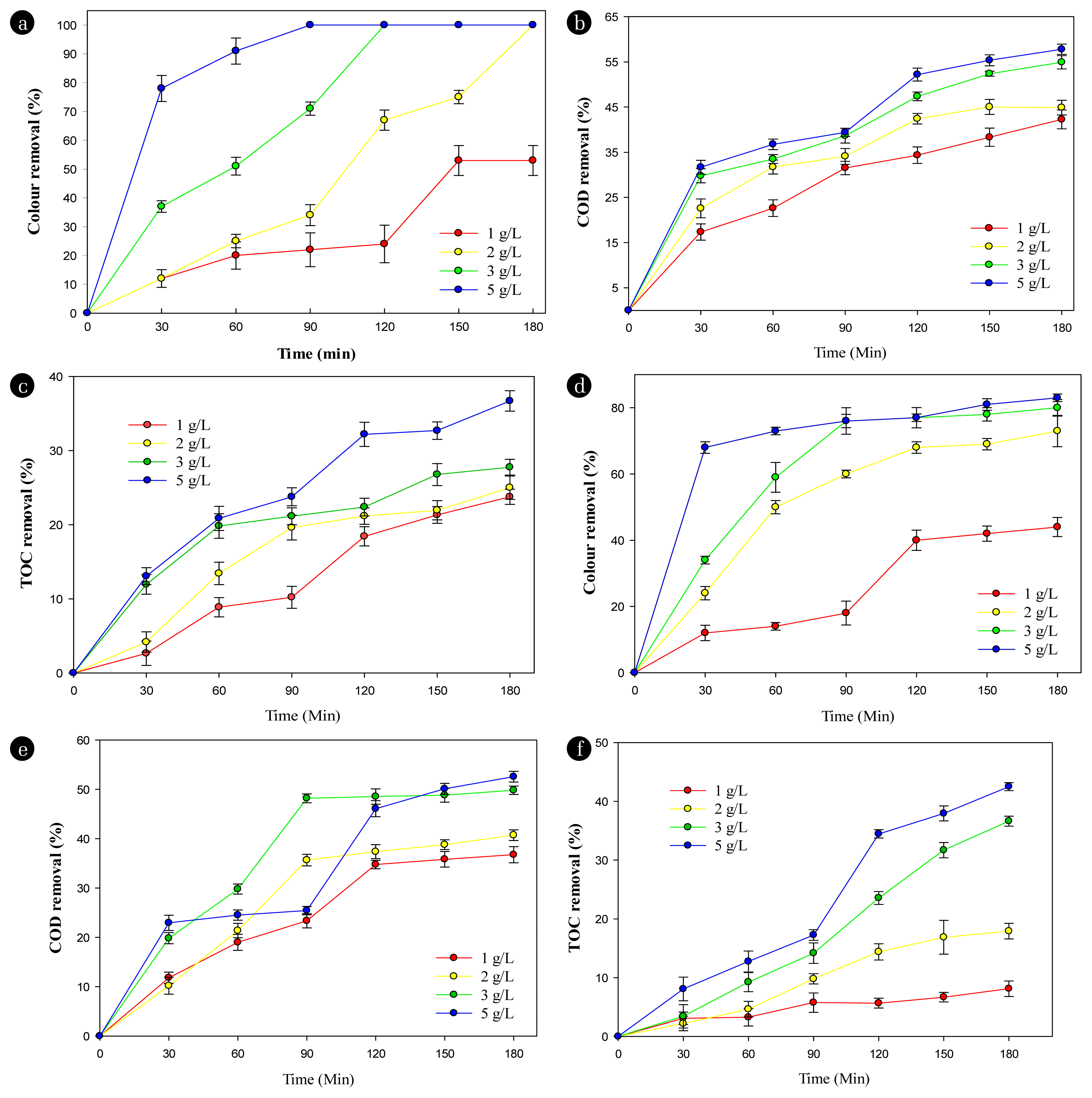
Effects of NaCl concentration on the color, COD, and TOC removal efficiencies in textile wastewater at Ti/BDD anode (A–C) and Ti/SnO2-Nb2O5 anode (D–F). Experimental conditions: current density 6.67 mA/cm2, PS = 0 mg/L, pH 7.5, stirring speed 100 rpm.
The hypochlorous acid and hypochlorite ions then react with the dye and degrade to CO2 and H2O which causes a decrease in color, COD, and TOC values after the electrochemical process, as shown in Eq. 9–10:
These results are comparative with previous reports when increasing the NaCl to 2 g/L reached 95% elimination of RY3 dye color [2]. Yong et al. (2011) [24] reported the effect of NaCl on the COD decomposition efficiency of methyl orange (MO) dye solution after 20 minutes of electrolysis for 15%, 23%, 37%, 45%, 58%, 57.5% and 58% NaCl concentrations of 0.02, 0.04, 0.06, 0.08, 0.1 and 0.12 mol/L, respectively.
3.2. Effects of Persulfate Concentration
Fig. 3 shows the influence of sodium persulfate (PS) concentration on the pollutant decomposition performance in electrochemical textile wastewater treatment at the Ti/BDD anode (A–C) and Ti/SnO2-Nb2O5 anode (D–F). Fig. 3A shows that the decolorization efficiency of Ti/BDD anodes was very high, almost reached 100%. When increasing PS concentration, the decolorization rate occurs quickly. At a [PS] 2 g/L after 60 minutes of electrolysis, the color decreased from 230 Pt-Co to 0 with a removal yield of 100%. The COD removal efficiency when using PS 3 g/L after 30 minutes to 180 minutes of electrolysis increased from 64.47 ± 1.29% to 69.26 ± 1.09% (Ffisher < Fcrit), and in the absence of PS, changed to 54.66 ± 1.44% (Ffisher < Fcrit). TOC decomposition efficiency was highest at 2 g/L PS, and the removal yield increased from 22.66 ± 1.88% to 49.48 ± 2.42% (Ffisher < Fcrit) after 30 minutes to 180 minutes of electrolysis. The oxidants generated in the electrochemical processes as sulfate radicals and hydroxyl radicals could oxidize organic compounds in wastewater, as shown in Eq. 11–13 [25]:
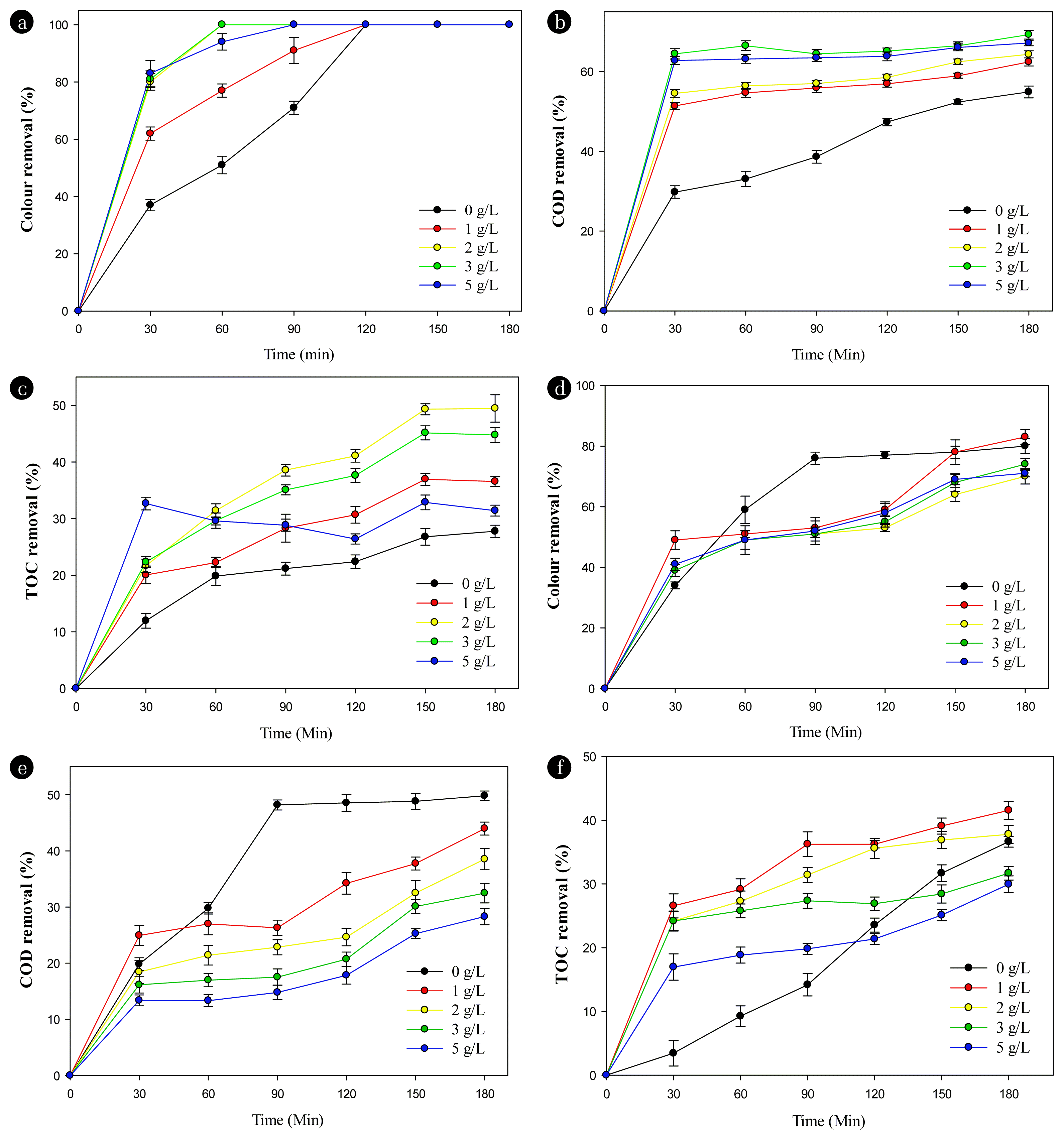
Effects of sodium persulfate concentration on the performance of textile wastewater treatment at Ti/BDD (A–C) and Ti/SnO2-Nb2O5 anodes (D–F). Experimental conditions: current density 6.67 mA/cm2, [NaCl] = 3 g/L, pH 7.5, stirring speed 100 rpm.
PS anions are precursors for the SO4•− radical which is a strong oxidizing agent capable of oxidizing refractory pollutants in wastewater. However, increasing the PS concentration beyond the optimal concentration will cause competition between strong oxidizing radicals SO4•−, HO• and the mutual self-reduction of SO4•− radicals, as shown in Eq. 14 [26]. In addition, a significant excess of the S2O82− anion will consume the HO• generated at the anode, as shown in Eq. 15, which reduces the COD removal efficiency in the electrochemical process [27]:
Contrary to the Ti/BDD anode, the efficiency of the electrochemical process gradually decreases when increasing the PS concentration at the Ti/SnO2-Nb2O5 anode. Fig. 3D shows that after 180 minutes of electrolysis, the decolorization yields at Ti/SnO2-Nb2O5 anode were 80.43 ± 2.15%, 82.6 ± 2.2%, 69.57 2.15%, 73.9 ± 2.2%, and 70.87 ± 1.21% (Ffisher < Fcrit), corresponding to PS concentrations 0, 1, 2, 3, and 5 g/L. The decline in COD removal efficiency with increasing PS concentration is evident in Fig. 3E, when the COD decomposition efficiency decreased from 49.82 0.85% at PS 0 g /L to 43.97 ± 1.15% (Ffisher < Fcrit) while adding PS 1 g/L, and further decreased to 28.31 ± 1.45% (Ffisher < Fcrit) when PS concentration increased to 5 g/L. The same trend was reported by Silveira et al. (2017) [17] who studied the decolorization of disperse Blue azo dye 3 (DB3 - C17H16N2O3) and reached 96% removal efficiency after 60 minutes of electrolysis when using DSA electrode at a current density of 40 mA/cm2 at 50°C, [DB3] = [PS] of 80 mg, [Na2SO4] = 0.05 M.
3.3. Effects of Current Density
Fig. 4 shows the effects of current density on the pollutant decomposition performance in textile wastewater using the electro- persulfate process at the Ti/BDD anode (A–C) and Ti/SnO2-Nb2O5 anode (D–F). Clearly seen in Fig. 4A and Fig. 4D, the higher the current density, the greater the decolorization efficiency. Decolorization performance after 90 minutes of electrolysis at current densities 2.50, 4.16, 5.83, and 7.50 mA/cm2 were 63.91 ± 1.26%, 84.78 ± 2.18%, 100%, and 100% (Ffisher < Fcrit) with the Ti/BDD anode, and 29.57 ± 1.21%, 45.65 ± 2.18%, 58.70 ± 2.2%, and 63.04 ± 3.75% (Ffisher < Fcrit) with Ti/SnO2-Nb2O5 anode, respectively. The decolorization efficiency when using the Ti/BDD anode is higher than that of the Ti/SnO2-Nb2O5 anode. Fig. 4B and Fig. 4E show clearly that at the Ti/BDD anode, the COD removal efficiency at current densities 4.16, 5.83 and 7.5 mA/cm2 were the same. The COD removal efficiency from the first 30 minutes to 120 minutes of electrolysis significantly decreased, but after 120 minutes of electrolysis, the COD removal efficiencies at these three current densities appeared stable. The highest COD removal efficiency at the current densities 4.16, 5.83 and 7.5 mA/cm2 were 66.91 0.93%, 69.66 ± 0.55%, and 69.54 ± 0.96% (Ffisher < Fcrit), respectively. This result showed that when the critical current density is reached at the Ti/BDD anode, the pollutant removal efficiency of the electrochemical process does not increase further. In contrast to the Ti/BDD anode, the COD removal efficiency on the Ti/SnO2-Nb2O5 anode increased sharply after 120 minutes of electrolysis. The peaks of the COD removal efficiencies were 36.03 ± 1.19%, 43.850.83%, 46.211.04% and 47.29 0.60% (Ffisher < Fcrit) at current densities of 2.50, 4.16, 5.83 and 7.50 mA/cm2. The COD removal efficiency has an insignificant difference at current densities 4.16, 5.83 and 7.50 mA/cm2. In another aspect, at a current density of 4.16 mA/cm2, TOC removal efficiencies between 30 minutes to 180 minutes increased from 36.88 ± 1.14% to 43.78 ± 1.09% (Ffisher < Fcrit) at the Ti/BDD anode and 15.11 ± 1.58% to 34.64 ± 0.73% (Ffisher < Fcrit) on the Ti/SnO2-Nb2O5 anode. Free HO• and persulfate radicals were generated more and more at the anode surface as the current density gradually increased, becoming involved in the oxidation of pollutants, as shown in Eq. 16–17 [28]:
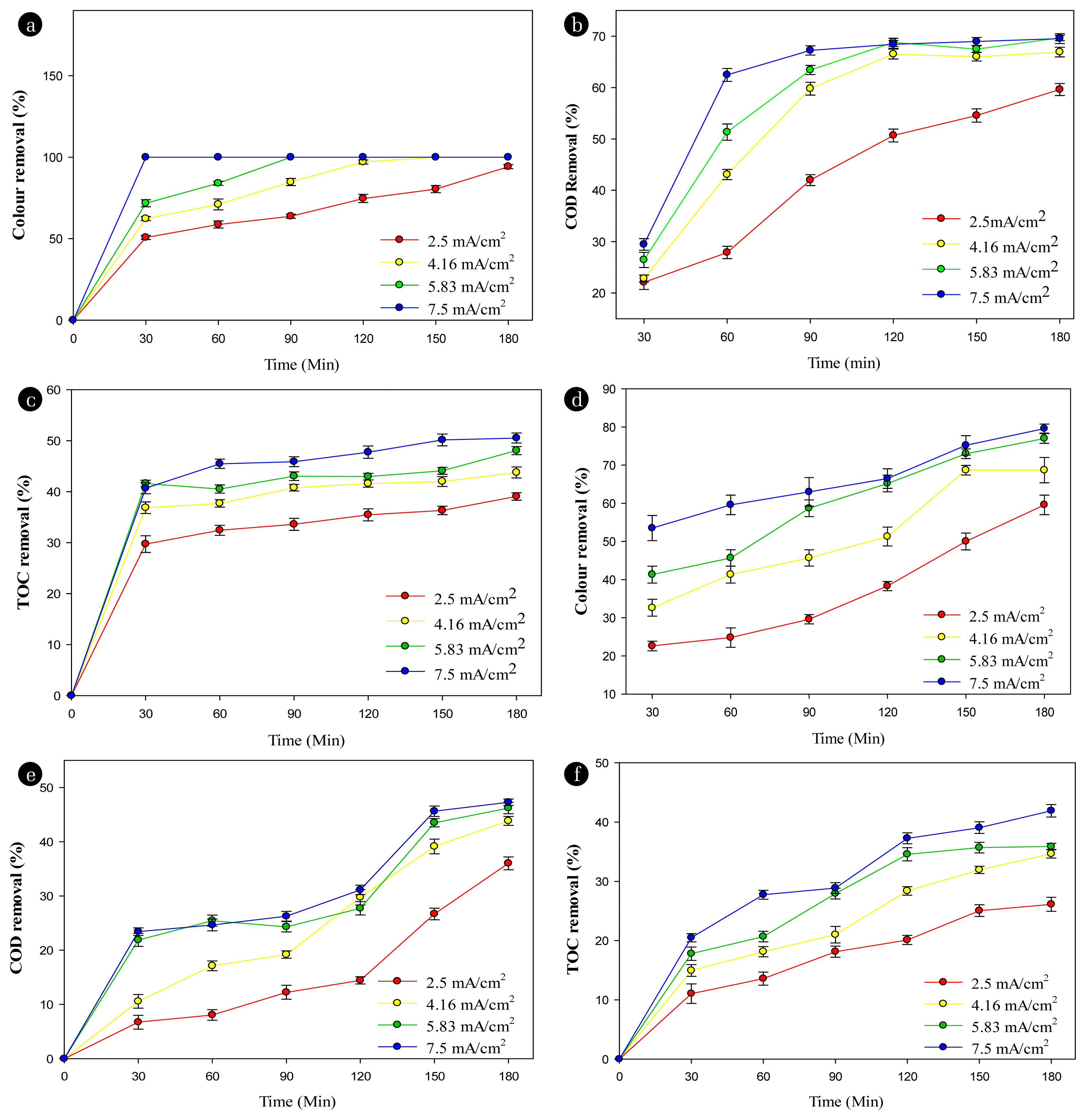
Effects of current density on the performance of the electrochemical treatment of textile wastewater with the Ti/BDD anode (A–C) at [NaCl] = 3 g/L, [PS] = 2 g/L, pH 7.5, stirring speed of 100 rpm; and Ti/SnO2-Nb2O5 anode (C–F) at [NaCl] = 3 g/L, [PS] = 1 g/L, pH 7.5, stirring speed of 100 rpm.
where S is the anode surface, R is a pollutant compound, and RO is an oxidized pollutant compound. Large volumes of generated HO• radicals may combine to form hydrogen peroxide, as seen in Eq. 18:
Water decomposes at the anode to produce molecular oxygen according to Eq. 19, which itself is reduced at the cathode to produce hydrogen peroxide (H2O2) according to Eq. 20 [29]:
The water oxidation at the anode surface can also produce ozone, and the concentration of the produced ozone depends more or less on the material of the anode, as shown in Eq. 21 [30]
The electrochemical process produces ozone faster at a higher applied current intensity, because, under such conditions, the electrode potential achieved higher. This promotes the production of ozone that participates in the oxidation of pollutant compounds according to Eq. 22 [31]:
This hydrogen peroxide will be strongly involved in the oxidation of pollutants, especially organic pollutants. However, when the current density increases beyond the optimal level, the high current density will decompose H2O2, according to Eq. 23 [32]:
Pillai and Gupta (2017) [33] investigated the influence of current densities at 0.38, 0.77, 1.15, and 1.54 mA/cm2 to achieve COD removal efficiency of 62%, 70%, 72% and 68% (Ffisher < Fcrit) in textile effluent electrolysis at 28°C, pH 9.5 with a support electrolyte NaCl concentration 1 g/L. It is also comparative to the treatment of coking wastewater using electrochemical oxidation. Here, COD removal efficiencies were 66.4%, 78.7%, 82.3% and 84.8% (Ffisher < Fcrit) after 60 minutes of electrolysis at current densities of 6, 8, 10 and 12 mA/cm2 [34]. TOC decomposition efficiency upon electrochemical oxidation of methylene blue dye solution in 20 minutes were 40%, 56%, 73%, 78%, 82% and 83% (Ffisher < Fcrit) at current densities 2, 4, 6, 8, 10 and 12 mA/cm2, respectively [35].
3.4. Effects of Initial pH
Fig. 5 shows the effect of the initial pH on the pollutant decomposition performance in electrochemical textile wastewater treatment at Ti/BDD and Ti/SnO2-Nb2O5 anodes. In general, the electrochemical pollutant removal efficiencies in a weak base environment better than in the acid environment. Fig. 5 (A) and Fig. 5 (D) show that the decolorization of textile effluent on the Ti/BDD anode occurs very quickly with 100%, 94.35 ± 1.27%, 81.74 ± 1.27%, 79.57 ± 1.21%, and 63.91 ± 1.27% (Ffisher < Fcrit) after 30 minutes of electrolysis at pH 2–10. The decolorization yields at Ti/SnO2 -Nb2O5 anode were 81.71 ± 1.27%, 77.39 ± 1.25%, 72.61 ± 1.25%, 66.09 ± 125%, and 63.04 ± 2.15% (Ffisher < Fcrit) after 180 minutes of electrolysis at pH 2–10. The highest COD and TOC decomposition yields at pH 2 were 56.51 ± 1.1% and 57.12 ± 0.82% (Ffisher < Fcrit) at the Ti/BDD anode and 51.18 ± 1.18% and 51.76 ± 1.5% (Ffisher < Fcrit) at the Ti/SnO2-Nb2O5 anode after 180 minutes of electrolysis. At a higher pH (6–10), COD, and TOC removal efficiencies decreased to 43.36 ± 1.18% and 50.08 0.57% at the Ti/BDD anode and 37.26 ± 0.8% and 31.06 ± 0.81% (Ffisher < Fcrit) at the Ti/SnO2-Nb2O5 anode after 180 minutes of electrolysis. This is because OCl− radicals and HClO acid exist and function best in a low pH environment [2]. This hypochlorous acid is a strong oxidizing agent as a major agent in the oxidation of dyes leading to the high decolorization of textile effluents, as shown in Eq. 24 [36].

Effects of initial pH on the performance of the electrochemical textile wastewater at the Ti/BDD anode (A–C) at [NaCl] = 3 g/L, [PS] = 2 g/L, current density 4.16 mA/cm2, stirring speed 100 rpm; and at the Ti/SnO2-Nb2O5 anode (D–F) at [NaCl] = 3 g/L, [PS] = 1 g/L, current density 4.16 mA/cm2, stirring speed 100 rpm.
Further, the level of H2O2 generated during the electrochemical process decreases as the pH of the solution increases, according to Eq. 25 [36]:
Rosales et al. (2012) [37] studied the Azure B dye decolorization and showed that after 15 minutes of electrolysis at pH 2, the dye was degraded to 85%, at pH 4 to 70%, and pH 8 to 55%. Zhou et al. (2012) [38] obtained the highest COD decomposition efficiency after 60 minutes of electrolysis at pH 3 with 42%, decreasing to pH 5 with 36% and further decreasing to pH 7 with 28%. At a low pH, the pollutants are quickly degraded by active chlorine. In particular, chlorine will prevail at a pH close to 3, HClO acid will predominantly exist at pH 3–8, and the ClO− ion dominates at pH over 8 [39].
3.5. Effects of Stirring Speed
Fig. 6 shows the effects of stirring speed on the pollutant decomposition performance in textile wastewater effluent. When using the Ti/BDD anode, the color removal efficiencies were 100%, 100%, and 96.96 ± 1.26%; COD removal efficiencies were 67.36 ± 1.31%, 68.36 ± 0.53%, and 66.10 ± 0.98%; and TOC removal efficiencies were 46.97 ± 0.72%, 47.84 ± 0.65%, and 45.35 ± 0.51% (Ffisher < Fcrit) at the stirring speeds 200, 400, 600 rpm after 180 minutes of electrolysis. The results show that the pollutant removal efficiencies at the Ti/BDD anode had an insignificant difference depending on the stirring speed, meaning that the mass transfer at the electrode surface is high. When using the Ti/SnO2-Nb2O5 anode, the decolorization yields were 74.78 ± 1.26%, 68.70 ± 1.26%, and 64.35 ± 1.26% (Ffisher < Fcrit); COD removal yields were 44.22 ± 0.61%, 40.94 ± 0.4%, and 37.95 ± 1.17%; and TOC removal yields were 39.05 0.68%, 38.07 0.92%, and 35.56 0.74% (Ffisher < Fcrit) at stirring rates 200, 400, 600 rpm after 180 minutes of electrolysis. The results show that the pollutant removal efficiencies decrease as the stirring speed at the Ti/SnO2-Nb2O5 anode increases too much. The influence of the stirring speed on the methylene blue (MB) dye decolorization was also reported through the color removal increases alongside the increased the stirring speed [40]. After 240 minutes of electrolysis, the color removals reached 91.84%, 99.35%, and 99.49% at stirring speeds 200, 400, and 700 rpm, respectively. However, the color removal efficiency between the stirring speed 400 rpm and 700 rpm showed an insignificant improvement.
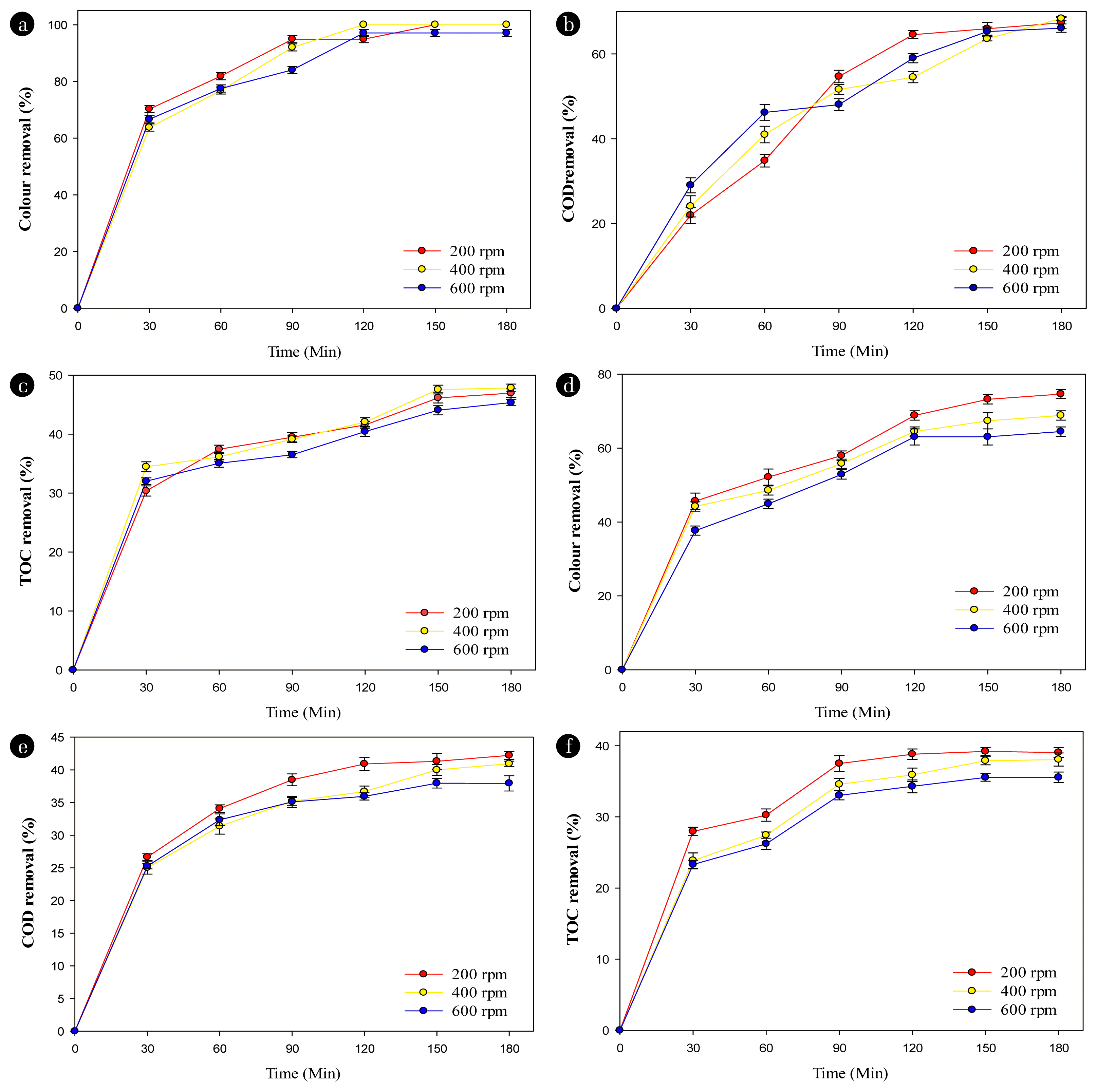
Effects of stirring speed on the performance of the electrochemical textile wastewater treatment at the Ti/BDD anode: (A–C) at [NaCl] = 3 g/L, [PS] = 2 g/L, current density = 4.16 mA/cm2, pH = 7.5; and at the Ti/SnO2-Nb2O5 anode (D–F) at [NaCl] = 3 g/L, [PS] = 1 g/L, current density 4.16 mA/cm2, pH = 7.5.
3.6. Oxidants Radical Generation
Fig. 7 shows the generation of oxidants during the electrochemical process at Ti/BDD and Ti/SnO2-Nb2O5 anodes. The results of Fig. 7 (A) show that when only NaCl 1g/L is present in the electrolyte, the Cl2 concentration produced at the Ti/BDD positive electrode is higher than at the Ti/SnO2-Nb2O5 anode. Generated Cl2 concentration was 0.62 ± 0.025 mg/L and 0.36 0.042 mg/L at the Ti/BDD and Ti/SnO2-Nb2O5 anodes. In the presence of PS concentration 2 g/L, the capability of both electrodes to produce Cl2 is roughly the same. When the PS in the textile wastewater effluent was added, Cl2 concentrations at Ti/BDD and Ti/SnO2-Nb2O5 anodes were 0.43 ± 0.02 mg/L and 0.46 ± 0.035 mg/L. In this case, PS could be a scavenger to consume Cl2. The free Cl2 produced during the electrochemical process is an important agent for producing hypochlorous acid and hypochlorite ions that are involved in the oxidation of organic compounds in textile wastewater effluents.

Oxidants generation during the electrochemical process at Ti/BDD and Ti/SnO2-Nb2O5 anodes (A) Cl2; (B) O3; (C) Color number removal (CN); (D) H2O2
Fig. 7 (B) shows that the produced ozone concentrations were 0.12 ± 0.01 mg /L and 0.09 0.01 mg/L at the Ti/BDD and Ti/SnO2-Nb2O5 anodes after 20 minutes of electrolysis. This demonstrates that in the electrochemical process, ozone is produced and will also participate in the oxidation of pollutants. The concentration of ozone produced at the Ti/BDD anode was higher than that at the Ti/SnO2-Nb2O5 anode under the same electrochemical condition. This could contribute to the higher pollutant decomposition at the Ti/BDD anode than seen at the Ti/SnO2-Nb2O5 anode.
Fig. 7 (C) shows that in the absence of an inhibitor, HO• and SO4•− radicals were generated in the oxidation of organic pollutants resulting in a high CN yield: 68.54 0.61% and 65.76 ± 1.58% at the Ti/BDD and Ti/SnO2-Nb2O5 anodes, respectively. With the addition of tert-Butyl alcohol (TBA) inhibitor, CN removal on both anodes were markedly reduced, indicating that the produced HO• radical in both anodes were consumed in the oxidation of pollutants. The CN removal efficiency at Ti/BDD and Ti/SnO2-Nb2O5 anodes decreased to 31.73 ± 0.39% and 23.22 ± 2.81% (Ffisher < Fcrit), respectively. Furthermore, when the inhibitor of both HO• and SO4•− radicals is ethanol (EtOH), the CN removal efficiencies were 29.74 ± 0.2% and 19.56 2.59% (Ffisher < Fcrit) at the Ti/BDD and Ti/SnO2-Nb2O5 anodes. This demonstrates the presence of the SO4•− radical at both anodes. Since EtOH further inhibits the SO4•− radical, the CN removal efficiency is lower than when inhibited with TBA. Upon continuing to add both inhibitors (TBA and EtOH) in the textile wastewater effluent, the CN removal at both anodes continues to decrease. Specifically, CN removal efficiency decreased to 22.420.53% and 16.27 ± 2.6% (Ffisher < Fcrit) at the Ti/BDD and Ti/SnO2-Nb2O5 anodes, which demonstrated the HO• residue was produced more than the SO4•− residue in both anodes and that persistent HO• radicals were partly consumed by the inhibitors still involved in the oxidation of the pollutant. When using both TBA inhibitor and EtOH, the SO4•− radical inhibitor does not change, the HO• inhibitor increases, and the CN decreases, meaning that the HO• radical is still inhibited by TBA but not totally, so that upon addition of EtOH, the HO• radical continues to be inhibited. Can-Güven et al. (2021) [41] reported the changes in CN removal when TBA and EtOH were added under optimal operating conditions. When TBA was added at a ratio of 1/250 to total oxidizer, CN efficiency decreased from 92.1% to 56.8% after 120 minutes of electrolysis. When EtOH was added at a ratio of 1/250 to total oxidizer, the CN removals reduced to 73.8%.
Fig. 7 (D) shows that at the Ti/BDD anode, in the presence of persulfate, the amount of H2O2 produced was 17.5 ± 0.5 mL, more than twice as in the presence of NaCl 1g/L, which was 7.5 ± 0.5 mL. At the Ti/SnO2-Nb2O5 anode, when there was no persulfate in the electrochemical process, the volume of H2O2 was generated at 7.5 ± 1 mL. However, similar to the Ti/BDD anode, when PS was added to the electrochemical process, the volume of produced H2O2 increased very little, from 7.5 ± 1 mL to 8.5 ± 1 mL. In that case, PS could be a scavenger to consume ozone in the degradation process.
3.7. Energy Consumptions
Fig. 8 shows the energy consumption at the Ti/BDD (A) and Ti/SnO2-Nb2O5 (B) anodes at different current densities. The energy consumption increases as the current density increases in both anodes. When using the Ti/BDD anode at a current density of 2.5 mA/cm2, energy consumption increased from 4.8–10.9 kWh/kgCOD with a COD removal efficiency of 22.1% to 59.6%. Energy consumption at the current density of 7.5 mA/cm2 increased from 13.8–36.4 kWh/kgCOD with a COD decomposition efficiency of 29.46% to 69.54% (Ffisher < Fcrit). At the Ti/SnO2-Nb2O5 anode, the highest energy consumption was achieved when a current density of 7.5 mA/cm2 was applied, at which the power consumption increased from 27.5 kWh/kgCO to 128.9 kWh/kgCOD with a COD decomposition efficiency of 23.42% to 47.29% (Ffisher < Fcrit). The applied current density of 2.5 mA/cm2 resulted in the lowest energy consumption. Here, power consumption increased from 28.4 to 43.3 kWh/kgCOD with a COD decomposition efficiency of 6.73% to 36.03% (Ffisher < Fcrit). The results reveal that the energy consumption at the Ti/SnO2-Nb2O5 anode is approximately four times higher than at the Ti/BDD anode. The energy consumption appears to be different when various experimental conditions are applied. The energy consumption was found to be 11.9 kWh/kg COD for the textile effluent upon removal of 52.86% COD under a circulating reactor with a recirculation flow rate of 100 L/h, a discharge flow rate of 3 L/h, an input COD concentration of 560 mg/L, and an applied current density of 5 A/cm2 [42]. COD removal efficiency was achieved at 90% for synthetic dyed textile wastewater (initial COD 780 mg/L) at a flow rate of 500 mL/h (retention time 6 h) and a flow density of 1.15 mA/cm2 to get an energy consumption of 9.2 kWh/kg COD [33].
4. Conclusions
The study evaluated the pollutant removal efficiency of biologically textile wastewater using electro-persulfate oxidation at the Ti/BDD and Ti/SnO2-Nb2O5 anodes. The results show that the Ti/BDD anode exhibits a higher pollutant removal efficiency and has less energy consumption than the Ti/SnO2-Nb2O5 anode at optimal operating conditions. The Ti/BDD anode achieved the best color, COD, and TOC removal efficiencies at 94,781.25%, 64,570.95%, and 41,570.56% (Ffisher < Fcrit), respectively, and energy consumption at 11.1 kWh/kgCOD. At the Ti/SnO2-Nb2O5 anode, the best color, COD, and TOC removal efficiencies were 72,041.25%, 41,321.21%, and 38,220.55% (Ffisher < Fcrit), respectively, and the best energy consumption was 51.3 kWh/kgCOD,. Persulfate presents the different trends in the electro-oxidation behavior of Ti/BDD and Ti/SnO2-Nb2O5 anodes in biologically textile wastewater degradation.
Supplementary Information
Acknowledgement
This research is funded by Vietnam Ministry of Education and Training (MOET) under grant number B2021-VGU-07.
Notes
Conflict-of-Interest Statement
None.
Author Contribution
N.C.T. (Bachelor student): Collecting data, doing experiments, preparing for the manuscript draft; T. L. L. (Associate Professor): Sketch the idea, conceptualization, methodology, investigation, revise the manuscript, final checking.


