Anaerobic membrane bioreactors for wastewater treatment: mechanisms, fouling control, novel configurations, and future perspectives
Article information
Abstract
As the world focuses on reducing greenhouse gas emissions and recovering energy from sewage, technologies that can recover energy and reuse wastewater are becoming increasingly important. Anaerobic membrane bioreactor (AnMBR) technology has recently attracted attention, as it can achieve energy recovery and yields high-quality effluent. However, the large-scale commercial application of AnMBRs requires that certain barriers be overcome, including membrane pollution and dissolved methane recovery. In this review article, we summarize the basic principles of anaerobic wastewater digestion and discuss the excellent performance of AnMBRs for wastewater treatment. Various factors, membrane types, and pollution are discussed, and control strategies for dealing with membrane pollution are put forward. In addition, a variety of new AnMBRs are described that may better control membrane pollution. Finally, future research directions for AnMBRs are presented to promote the large-scale integrated application of AnMBRs for industrial wastewater treatment.
1. Introduction
Wastewater treatment has attracted attention in recent years due to a growing lack of water resources and concurrent increase in environmental pollution caused by wastewater discharge [1]. Wastewater contains an abundance of materials, including carbohydrates, proteins, lipids, and other reducing organic substances [2], all of which are an untapped resource once the wastewater has been treated. Wastewater utilization mainly depends on efficient, economical, and environmentally friendly treatment technologies [3]. Conventional anaerobic digestion technologies cannot overcome long sludge retention times (SRT) and hydraulic retention times (HRT), and have unstable digestion processes, resulting in poor results. Anaerobic membrane bioreactor (AnMBR) technology, which is a promising wastewater treatment and resource recovery technology, can capture almost all microorganisms and solids by introducing membrane systems [4]. Thus, AnMBRs can overcome the limitations of traditional anaerobic digestion methods. They can effectively separate SRT and HRT, significantly reduce HRT, and increase the organic loading rate [5–7], while also improving treatment efficiency and energy recovery using wastewater. This is due to the efficient retention of the membrane modules, which makes the AnMBR a relatively closed system for microorganisms. The microorganisms in the reactor can be completely retained, achieving a complete separation of HRT and SRT, thus enabling the bioreactor to maintain a high sludge concentration, a short HRT and a long SRT, improving shock resistance and pollutant removal efficiency [8]. Moreover, AnMBRs improve the competitiveness and applicability of anaerobic systems for mainstream wastewater treatment processes [9–11].
As of 2020, AnMBRs have achieved remarkable success in treating wastewater. Compared to conventional aerobic technologies, AnMBRs can reduce energy consumption and sludge production. However, AnMBRs have many disadvantages, including susceptibility to severe membrane contamination, difficulty in recovering dissolved methane, insufficient alkalinity, and low COD/SO42− recovery [12, 13].
The aim of this paper is to provide a comprehensive review of the membrane contamination mechanism of AnMBR and membrane contamination control strategies by reviewing the AnMBR literature published in recent years, in order to provide a reference for AnMBR in future large-scale applications. The objectives of this manuscript are : (1) to provide an overview of AnMBR technology and mechanism; (2) to summarise the current performance of AnMBR, such as the removal of COD and methane yield; (3) to review and discuss the membrane contamination mechanism, membrane contamination influencing factors, membrane contamination control strategies and novel process configurations; (4) to highlight the need to address in order to facilitate large-scale commercial operation of AnMBR key issues to be addressed in order to promote large-scale commercial operation of AnMBR.
2. AnMBR Overview
2.1. Technology Overview
By definition, an AnMBR is a device that performs biological treatment in an anaerobic environment, utilizing various types of membranes for solid–liquid separation, including membrane separation and anaerobic digestion systems. The characteristics of conventional aerobic and conventional anaerobic treatments, as well as aerobic and anaerobic membrane bioreactors are summarized in Table S1. As shown in Table S1, AnMBRs have certain advantages in terms of anaerobic biological treatment technology and membrane separation, and they also are superior in terms of their high effluent quality, low energy consumption, and energy recovery. The anaerobic membrane bioreactor has its own shortcomings: (i) it has limited ability to remove inorganic nutrients such as nitrogen and phosphorus from the wastewater, and when the discharged water is sensitive to nitrogen and phosphorus, it cannot be discharged directly and needs to be treated in depth before it can meet the discharge standards. (ii)The start-up time of the reactor is longer than that of the aerobic reactor, and it usually takes at least 2 to 3 months before it can be started up properly. (iii) The treatment of SO42− containing wastewater produces hydrogen sulphide gas with an odour.
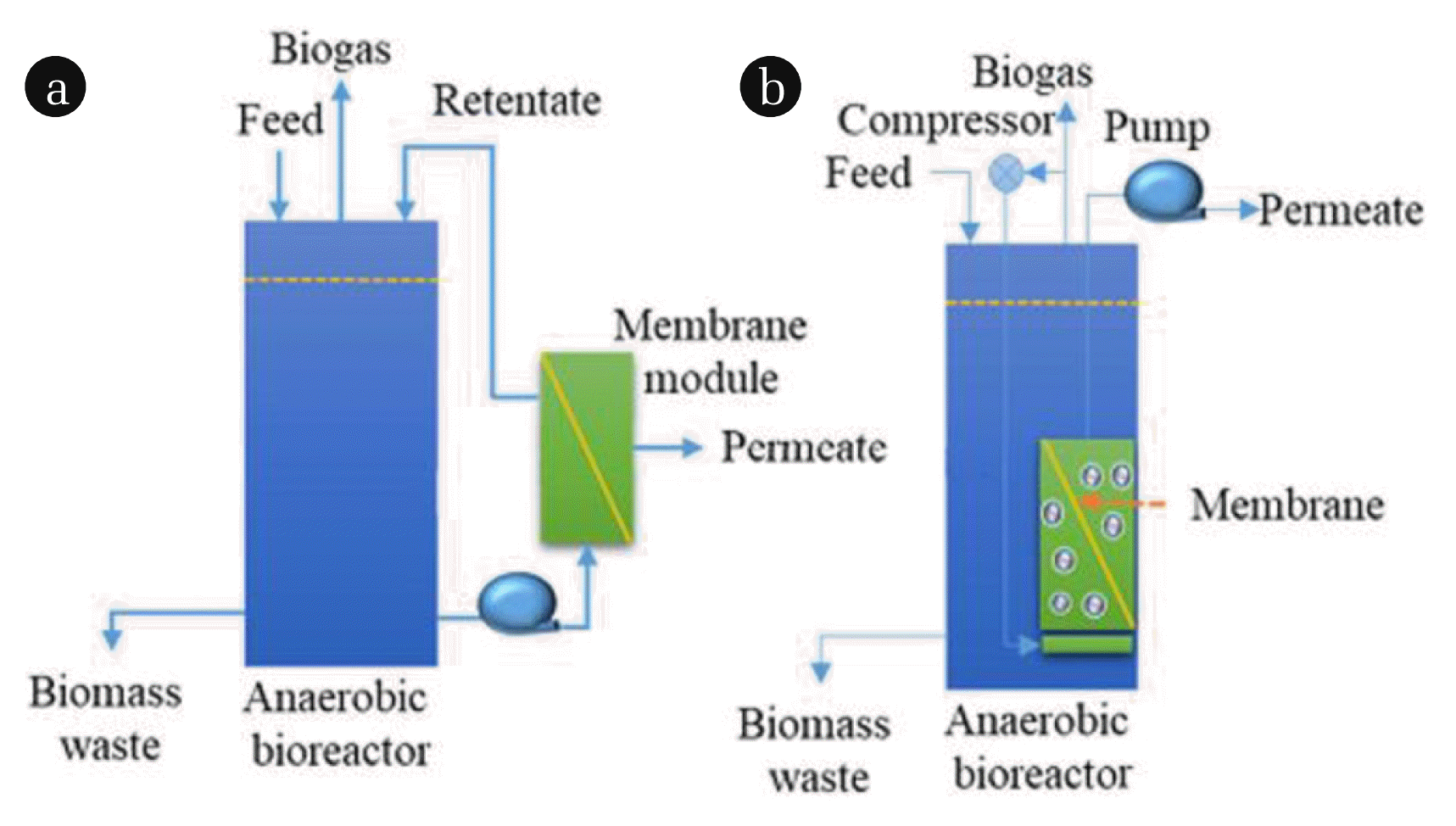
Depending on the relative position of the membrane modules and bioreactors, AnMBRs can be divided into two categories (Fig. 1): sidestream-AnMBRs and submerged-AnMBRs [13–20]. The membrane module in sidestream-AnMBRs is located outside the anaerobic reactor, which facilitates membrane cleaning and replacement [21]. However, the microbial flow-measured activity of side-stream-AnMBRs is low due to the shearing effect of the hydraulic cycle, resulting in broken sludge flocs [16]. Furthermore, the membrane module in submerged-AnMBRs is located inside the anaerobic reactor. Submerged-AnMBRs do, however, have various advantages, including their compact size, small footprint, and low energy consumption. However, membrane contamination is more problematic in submerged-AnMBRs than in sidestream-AnMBRs. This is because in sidestream-AnMBRs the membrane module is located outside the bioreactor and the sludge mixture in the reactor is transferred to the external membrane module by circulation. The circulation of the mix creates strong staggered shear, which can mitigate membrane contamination of the membrane surface to some extent. In contrast, the submerged AnMBR membrane module is in direct contact with the sludge mixture and the membrane module filters the water directly from the mixture, so membrane contamination in submerged-AnMBRs is a more serious problem than in sidestream-AnMBRs.
2.2. Methane Production from Anaerobic Digestion
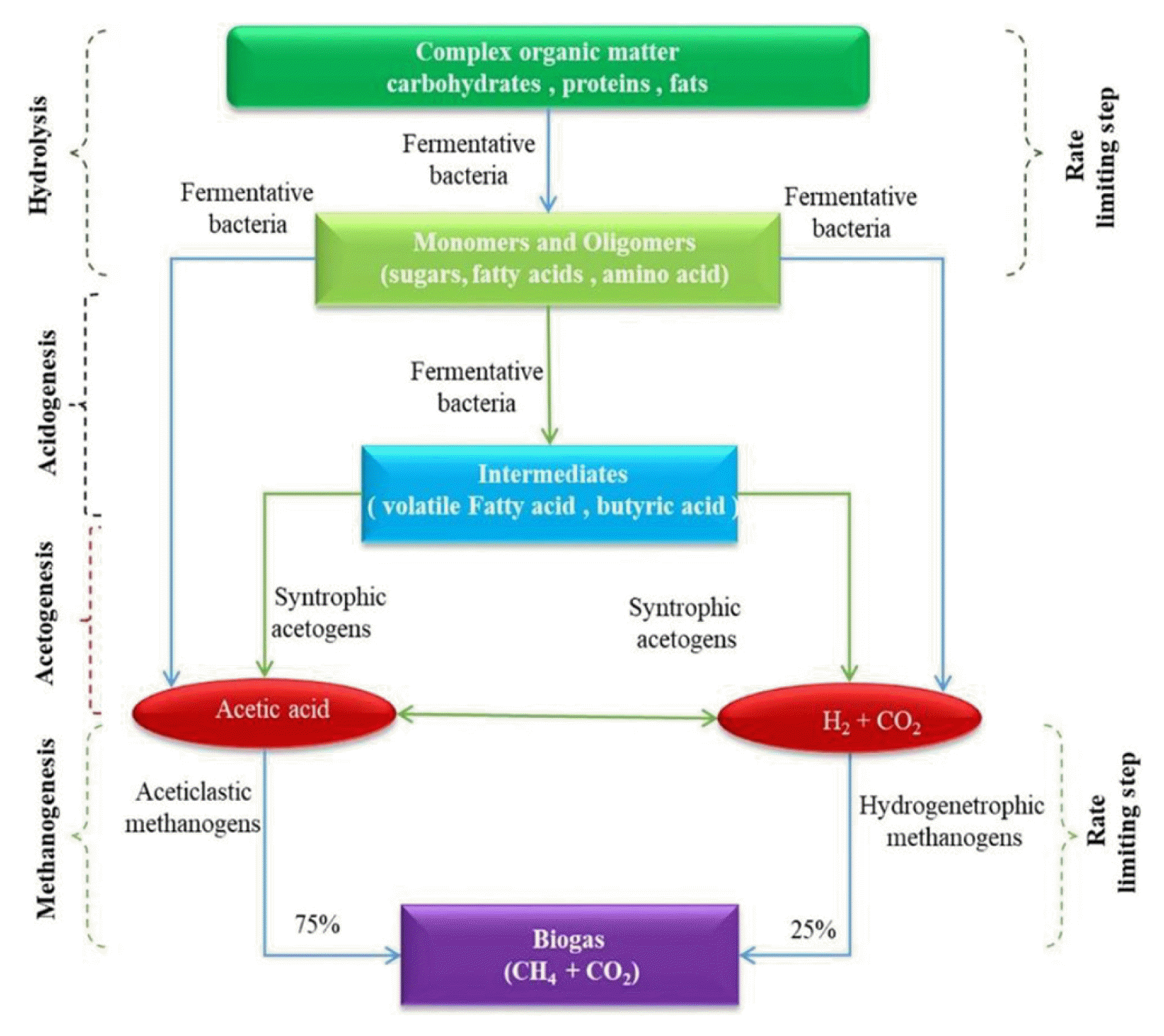
In anaerobic digestion, organic matter is broken down into H2O and CO2 through the combined action of multiple (anaerobic or parthenogenic) microorganisms in anaerobic conditions. Compared to aerobic treatments, the hydrogen receptor used in anaerobic conditions consists of chemosynthetic oxygen, carbon, sulfur, or hydrogen. Many scholars believe that methane production from anaerobic digestion can be divided into three stages: the hydrolytic fermentation stage, the hydrogen and acetic acid production stage, and the methane production stage [22].
The anaerobic digestion process for wastewater is shown in Fig. 2. During the hydrolytic fermentation stage, under the action of hydrolytic acidifying bacteria, complex organic matter is decomposed into soluble organic matter and then converted into fatty acids (propionic, butyric, and lactic acids), as well as alcohols (ethanol and other small molecules) [23]. The hydrolysis rate and the degree of hydrolysis are determined by the hydrolysis temperature, pH, organic matter composition (such as lignin, or the mass fraction of protein, fat, and carbohydrates), residence time, and the concentration of hydrolysis products. During the hydrogen and acetic acid production stage, the oxidative decomposition of organic acids and alcohols occurs, specifically using molecules with three or more carbon atoms, which are produced from the hydrolytic fermentation stage, and are then converted into acetic acid, H2, and CO2. In addition, the H2 and CO2 are converted into acetic acid through the metabolism of homotypic acetic acid-producing bacteria. During the methane production phase, methane can be produced in two ways. In the first pathway, acetic acid trophic methanogenic bacteria produce methane using acetic acid as a substrate. In anaerobic reactors, about 70% of the methane is produced from the oxidative decomposition of acetic acid [24, 25]. In the second pathway, hydrogenotrophic methanogenic bacteria convert CO2 and H2 into methane, producing approximately 30% methane [26].
2.3. Membrane Materials and Modules
To mitigate membrane contamination and extend membrane life, a variety of membrane materials have been developed for AnMBRs. Membrane materials can be divided into two main categories: organic polymers and inorganic membranes. Table 1 summarizes the membrane types, pore sizes, and other properties commonly found in AnMBRs. Organic polymer membranes are produced from readily available materials, and mainly include polyethersulfone (PES), polytetrafluoroethylene (PTFE), and polyvinylidene fluoride (PVDF). PVDF and PES have made up 75% of the film market over the past few decades due to their excellent thermal stability, resistance to acid and alkali corrosion, and significant mechanical properties [2, 27]. Recently, PTFE materials with higher membrane flux and better fouling resistance have been increasingly used as membrane materials, as membranes with thinner fibrous mesh pores have stronger adsorption properties and are less affected by irreversible contamination over the long term [28, 29]. In addition, polymeric materials such as polyethylene (PE) [30], polypropylene (PP) [31, 32], and polysulfone (PSF) [33, 34] have been used as anaerobic bioreactor membranes. Inorganic membranes can be divided into metal, alloy, ceramic, and glass membranes, among which ceramic and metal membranes are the main types of inorganic membranes. Compared to organic polymer membranes, metal membranes have good plasticity, toughness, and strength, and are well-suited to harsh environments [35, 36]. Ceramic membranes, however, have advantages such as high mechanical strength, corrosion resistance, and high membrane flux. Furthermore, the super-hydrophilic nature of ceramic membranes can effectively mitigate membrane contamination by hydrophilic-hydrophobic repulsion, as determined based on a comparative analysis of filtration and treatment performance of polyvinylidene fluoride with ceramic membranes [37]. However, most currently commercialized ceramic membranes use expensive materials such as alumina, titanium dioxide, and silicon carbide, which limits their large-scale application.
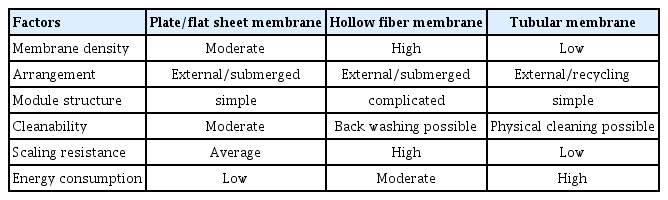
AnMBR effluents can be obtained by microfiltration (MF) or ultrafiltration (UF), and there are three general membrane module installation types: plate-and-frame/plate (FS), hollow fiber (HF), and multi-tube (MT) [38, 39], where the membrane module installation is selected based on the influent flow rate and anaerobic sludge particle size [40]. A comparison of flat, hollow fiber, and tubular membranes is shown in Table 2 [41].
Flat membranes, which have high stability, and are easy to clean and replace, have recently attracted attention from many groups [38, 42–46]. Tubular membranes are composed of multiple tubular membranes, which are arranged in a tubular pattern, and have certain advantages over other membrane types, such as their low component pressure loss, high filtration efficiency, high fouling resistance, and they are easy to clean. However, the high operation and maintenance costs of flat and tubular membranes limits their large-scale commercial application [47, 48]. Furthermore, compared to flat and tubular membranes, hollow fiber membranes are most widely used in AnMBRs due to their high loading densities and high output efficiencies [38, 49].
3. Reactor Performance
3.1. COD Removal Effect
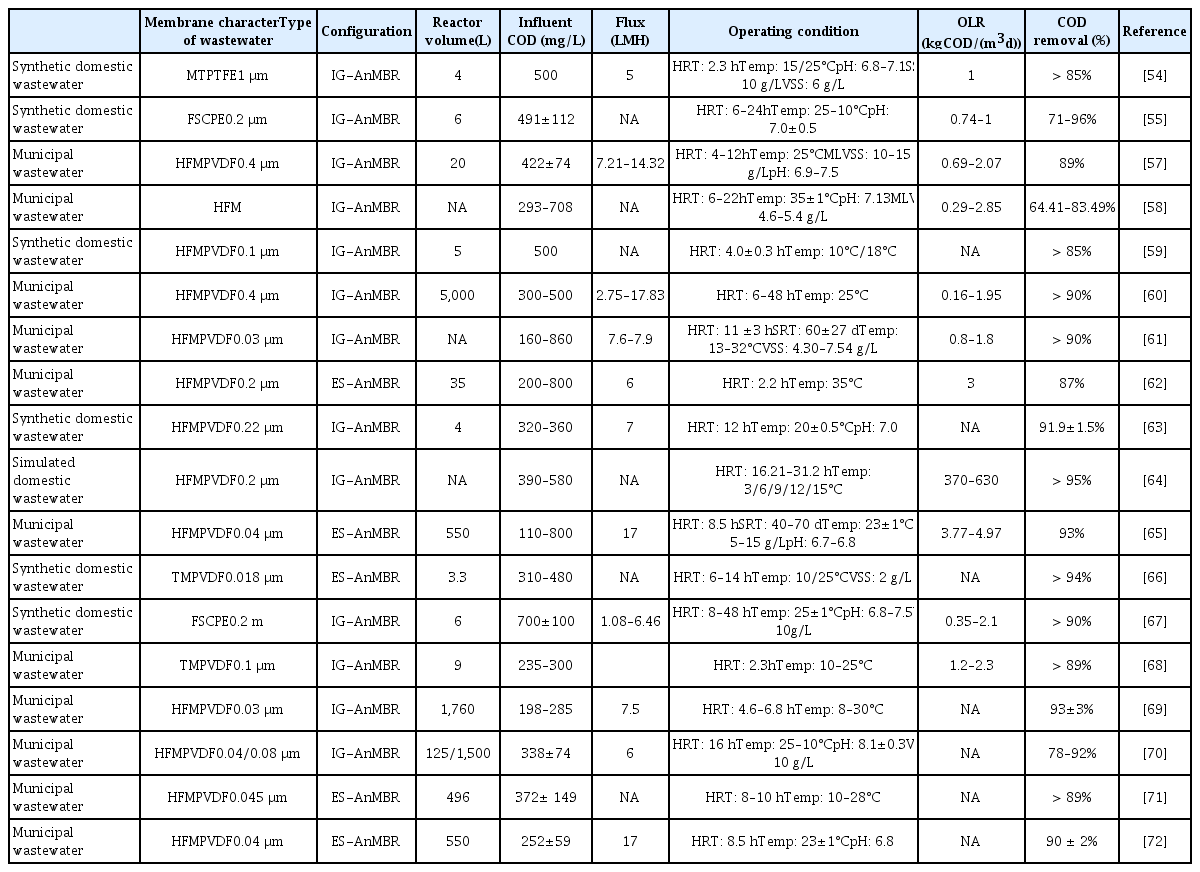
Early anaerobic processes were mainly used to treat industrial wastewater or highly-concentrated organic wastewater, and have been rarely used to treat municipal wastewater [50], for several reasons. For example, controlling the hydraulic retention time reduces the process volume due to the high volume of domestic wastewater However, shorter hydraulic retention times can lead to a loss of anaerobic microorganisms, and the effluent water quality means that it cannot be reused for human consumption [38, 51]. Recently, AnMBRs have become increasingly popular for municipal wastewater treatment, as they can solve these problems. Table 3 shows the operating conditions and treatment performance of an AnMBR for municipal wastewater treatment, where the COD influent concentration was in the range of 110–860 mg/L, and the hydraulic retention time was approximately 2.2–48 h. When the influent COD concentration of municipal wastewater is high (> 500 mg/L), the hydraulic retention time is generally greater than 10 h [12, 52]. The longest hydraulic retention time for this configuration is 48 h, while the shortest hydraulic retention time is 2.2 h. In addition, the maximum organic loading rate (OLR) for this configuration ranged from 3.77–4.97 kg COD/(m3/d), while the minimum OLR for this configuration ranged from 0.16–1.95 kg COD/(m3/d).
Municipal domestic wastewater temperature varies with climate, and the water temperature can drop to as little as 5–10°C during winter [53]. Temperature is an important factor that affects anaerobic microorganism activity, which in turn affects the removal rate of organic matter during anaerobic digestion [23, 54, 55], sludge production, and membrane contamination [56]. Microorganisms respond to a decrease in temperature by exhibiting self-protective behavior, including the release of soluble microbial products and extracellular polymers, and as a result, lower temperatures can lead to higher membrane contamination [55]. As we can see from Table 3, numerous scholars have conducted studies on anaerobic membrane organisms for domestic wastewater treatment at different temperatures [54, 55, 57–72]. Ho and Sung [54] compared the operational performance of two AnMBRs with synthetic municipal wastewater as feedstock at operating conditions of 15°C and 25°C, respectively. The COD removal rate was greater than 95% at 25°C, while the COD removal rate was only 85% at 15°C, indicating the same trend for specific methanogenic activity. Watanabe et al. [55] increased pore plugging from 17% to 45% and transmembrane pressure (TMP) increased from 0.1701 to 0.7231 kPa/d when the operating temperature decreased from 25°C to 15°C. Shin et al. [69] studied a pilot-stage anaerobic fluidized membrane bioreactor (SAF-AnMBR) for more than six months, and found that membrane contamination was more severe in winter under ambient temperature (8–30°C) using TMP as an indicator. Activated carbon (GAC) and ferric chloride (FeCl3) can also be added to resolve some of the issues related to the poor performance of AnMBRs at low temperatures, as well as serious membrane pollution. Yoo et al. [68] used GAC to dose a graded anaerobic fluidized membrane bioreactor (SAF-MBR), and treated municipal domestic wastewater at 10–25°C, achieving more than 89% COD removal with a hydraulic retention time of 2.3 h. The researchers speculated that the high COD removal rate at low temperatures was related to the formation of a biofilm on the surface of the activated carbon and membranes [12]. Dong et al. [72] increased the COD removal rate from 79.9% to 93.7% with the addition of FeCl3 as a coagulant. However, the addition of FeCl3 also caused an increase in sludge concentration and a decrease in methane production. Seib et al. (2016a) [66] ran four different AnMBR configurations at 10°C and 25°C, in which all of the reactors were treated with synthetic wastewater first for the first 320 days. After this priming period, the reactors began treating municipal wastewater, and all four AnMBRs achieved more than 94% COD removal for both synthetic and actual wastewater. Chen et al. (2017a) [63] compared the performance of a side-flow granular AnMBR with a submerged granular AnMBR in a laboratory setting at 25°C. Both systems exhibited similar COD removal efficiencies (> 91%), although the submerged granular AnMBR accumulated volatile fatty acids.
Therefore, regardless of the AnMBR type, the configuration, or the membrane characteristics, AnMBRs have good COD removal rates using municipal biological wastewater in laboratory or pilot settings. However, most AnMBR systems operate at 25°C (17–35°C) or room temperature, and in many temperate or cold regions, the temperature fluctuates significantly. In the face of the increasing energy crisis, the study of AnMBR for domestic wastewater treatment at low temperatures is of particular importance, especially for temperate and cold regions. Smith [64] showed that the COD removal rate of an anaerobic membrane bioreactor could reach more than 95% at 6°C, but decreased to 86% at 3°C. The decrease in dissolved COD content in the sludge mixture indicated a decrease in microbial activity. The high COD removal rate at low temperatures in the reactor was attributed to the biofilm effect on the surface of the membrane module. Although the start-up period of the anaerobic membrane bioreactor at low temperatures is longer and the treatment effect and gas production is lower compared to medium temperature conditions (33–35°C), the electrical energy consumption required to operate the anaerobic membrane bioreactor is also relatively lower.
Therefore, additional research is needed to address temperature and energy consumption issues, to promote the widespread applications of AnMBRs. The adverse effects of low temperatures on the AnMBR can be compensated for by extending the hydraulic residence time, but also by increasing the operating costs if the residence time is too long. To solve these problems, the shortcomings of low temperature operation can be compensated for by developing new membrane modules, improving start-up operation at low temperatures and using the biogas produced for reactor warming.
3.2. Methane Production
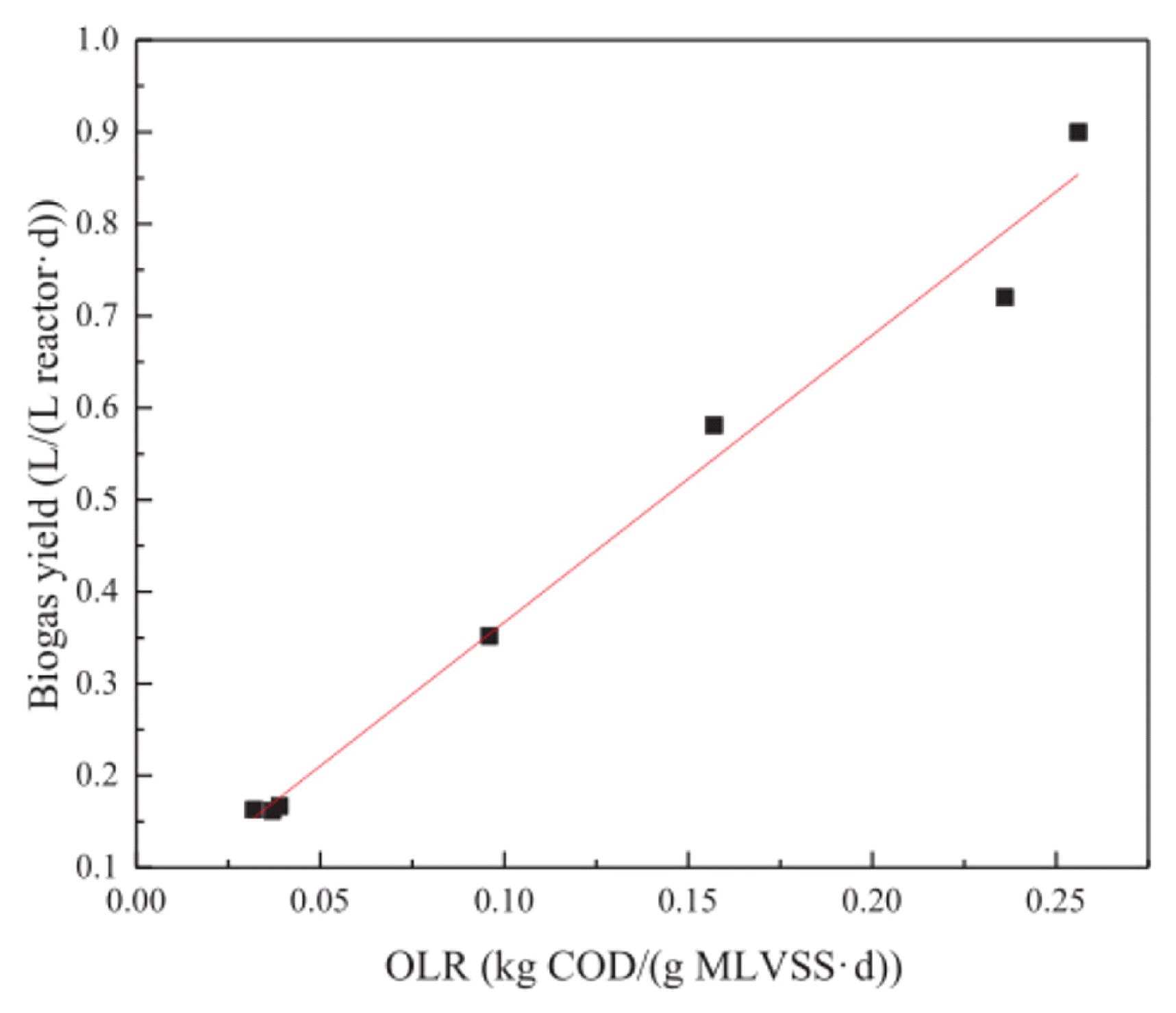
Biogas is a mixture of gases that are produced by the biological decomposition of organic matter under anaerobic conditions, and typically consists of methane (CH4), hydrogen (H2), carbon dioxide (CO2), and nitrogen (N2). Generally, methane accounts for 40–90% of biogas content, and under optimal conditions, energy output/input can reach 28.8 MJ/mol, which would efficiently utilize this biomass energy and help to alleviate the global energy crisis [23, 38, 73–79]. As shown in Table 4, biogas and methane production varies greatly depending on the reactor type. Methane yield is typically in the range of 0.08–0.338 L CH4/g COD. However, the theoretical value of methane yield is 0.382 L CH4/g COD (25°C) [38]. In practice, methane is partially dissolved in water [80]; therefore, the presence of inhibitors may inhibit anaerobic reactions [81], leading to actual biogas yields that are much lower than theoretical values. Inhibitors are substances that inhibit the metabolic activity of microorganisms in the anaerobic digestion process. Wastewater often contains toxic inhibitors and a wide variety of them, including ammonia, sulphides, heavy metals, salts, etc. They mainly reduce or even inhibit the metabolic action of methanogenic bacteria by affecting the activity of their enzymes, and the inhibition is usually manifested by a decrease in methane production rate and the accumulation of organic acids [81]. During AnMBR treatment, the sustainable OLR for reactors is subjected to the activity of bulk sludge, the activity of bulk sludge also acts on OLR in turn, CH4 yield will increase linearly with organic loading rate (OLR) [82], so a proper OLR is of great significance for an efficient methane conversion rate [23]. As shown in Fig. 3, there is a good linear relationship between OLR and methane production regardless of reactor structure, influent COD concentration, ambient temperature, and sludge concentration, which indicates that OLR is the main limiting parameter for methane production [23].
Dissolved methane in effluent can cause two problems. First, it reduces the methane yield of an AnMBR and affects the energy efficiency of the reactor, and second, methane is a greenhouse gas, and contributes significantly to global warming, especially as the greenhouse impact of CH4 is 25 times greater than the impact of CO2 [83]. Therefore, minimizing and recovering dissolved methane in wastewater is a critical requirement for the large-scale application of AnMBRs in municipal wastewater treatment.
Methods used to treat dissolved methane include membrane separation, aeration, and blowdown [84–87], among which membrane separation is the most effective method for recovering dissolved methane [85, 88]. Cookney et al. [89] found that more than 99% of dissolved methane could be recovered from anaerobic effluent using a hollow fiber membrane contactor system. However, the recovery of dissolved methane from anaerobic wastewater by membrane separation is still in the proof of concept stage, and its economic feasibility and process safety have not been fully evaluated. Therefore, in-depth studies on the membrane separation of dissolved methane for methane recovery have received significant attention from researchers as of late.
4. Membrane Fouling Issues
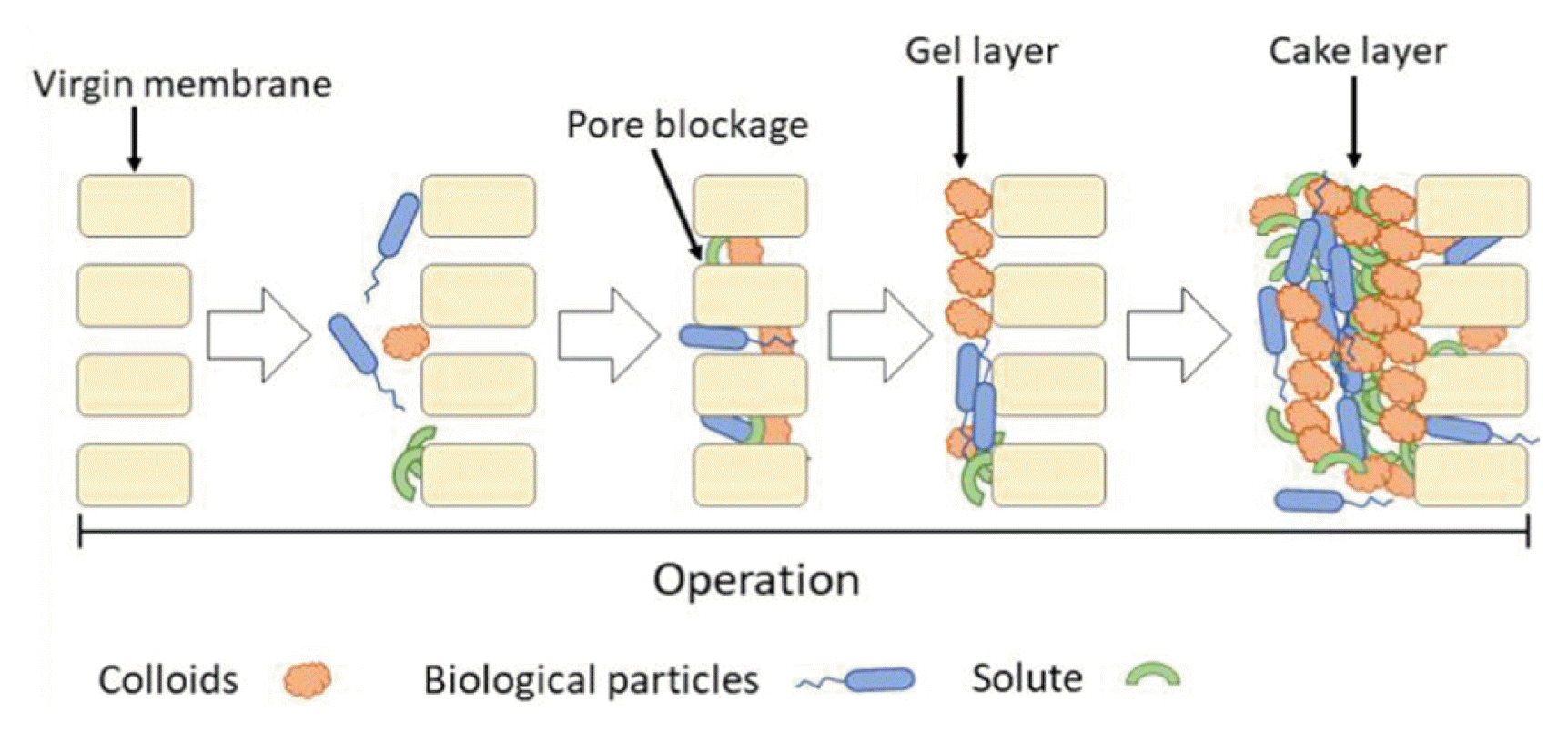
Membrane fouling is a key challenge that limits the applications of AnMBRs in municipal wastewater treatment, and one of the main reasons why membrane bioreactors cannot be widely used on an industrial scale. Membrane fouling refers to the phenomenon where colloids, suspended solids, sludge flocs, dissolved organic matter [90, 91], and other particles in wastewater are adsorbed and deposited on a membrane surface and block membrane pores, eventually causing decreased membrane flux [2, 92]. Considering the various pollutants that contribute to membrane fouling, membrane fouling mechanisms include membrane pore clogging, gel layer clogging, and cake clogging [48]. The common membrane fouling mechanisms affecting AnMBRs are shown in Fig. 4.
Membrane pore clogging occurs via membrane pore blockages, which mainly occurs during the initial stage of filtration [93, 94]. In membrane pore clogging, small particle sizes (smaller than the membrane pore size) will deposit in the membrane pores during filtration, eventually clogging the membrane pore. The main contaminants that cause membrane pore blockage include macromolecular organics and colloids, and these pollutants cannot be completely removed by physical cleaning, thus causing irreversible pollution [2, 95].
In gel layer blockage, a dense colloidal substance forms on the surface of the fouling layer close to the membrane, which is called the gel layer. This process forms via complexation reactions involving dissolved organic matter and metal ions during the filtration process [96].
In cake clogging, solid substances with larger particle sizes (larger than the membrane pore size) accumulate on the membrane surface during filtration, forming a cake layer after enrichment and concentration [2]. In AnMBRs, the large particles that form a cake layer mainly consist of sludge flocs [97], and sludge properties (including electrification, hydrophobicity, and EPS concentration) directly affect sludge aggregation in the mixture, as well as the formation of the cake layer [98]. The mud cake layer can be easily removed by backwashing and aeration, which causes shearing forces due to the large particle size, porosity, and the low viscosity of the filter cake layer. Therefore, filter cake clogging is considered a type of reversible pollution.
Membrane contamination can reduce membrane flux when an AnMBR is operated at a constant pressure. However, membrane contamination can cause an increase in differential pressure across the membrane, especially when an AnMBR is operated at a constant flow. A three-stage trans-membrane pressure (TMP) curve can signify constant-flow operating conditions [48, 99, 100]. In stage one, a rapid rise in TMP is typically observed at the beginning of a run (pore plugging). In stage two, a slow increase occurs in TMP (gel layer plugging), and stage three is known as the late TMP surge (cake plugging) (Fig. 4). Various hypotheses exist regarding the formation of stage three, all of which are consistent with the self-acceleration of membrane contamination at a constant flow rate, while some suggest that the stage three TMP jump is related to membrane surface contamination or an uneven distribution in membrane pore blockage. Furthermore, a larger drop in membrane flux may signify a more heavily contaminated local area. Therefore, to maintain a constant flow state, the flux in some areas of the membrane surface will operate at higher than critical flux, resulting in accelerated deposition of sludge floc particles [38, 48, 101–103]. Other researchers have suggested that the TMP jump is related to microorganism activity at the bottom of the membrane surface, and bacterial death may release a large amount of EPS and SMP [87], causing the TMP jump [104]. As membrane fouling is a very complicated process, a combination of these explanations may provide more accurate assessments what is occurring during fouling [38].
4.1. Membrane Fouling Classification
Membrane fouling can be classified according to contaminant composition as biological, organic, or inorganic [90, 93].
4.1.1. Biological fouling
Biological fouling, also known as biofilm fouling, occurs when bacteria or bacterial colloids produce a biofilm that is adsorbed on a membrane surface through proliferation and metabolism, and this is the main cause of membrane filtration performance degradation [90]. Large-sized sludge flocs are intercepted by the membrane and adsorbed on the surface, resulting in serious membrane fouling, which affects normal AnMBR operations [105]. Biological fouling is a two-step process, starting with initial bacterial attachment and followed by bacterial proliferation and metabolism on a membrane surface [106].
4.1.2. Organic fouling
Organic fouling is mainly caused by the metabolic products of microorganisms, which can adsorb on a membrane surface, forming a gel layer or deposit in membrane pores; thereby causing blockages or membrane fouling. Extracellular polymers (EPS) and soluble microbial products (SMP) are the main components that lead to organic fouling [107–112]. Due to their small size, these organic products can easily deposit on membrane surfaces and within membrane pores, resulting in organic fouling that is more difficult to remove than biological fouling [113].
4.1.3. Inorganic fouling
Inorganic fouling is the deposition of inorganic substances on a membrane surface or along the inner wall of membrane pores, through chemical precipitation or bioprecipitation. This process results in membrane pore occlusion or pore blockages, and causes a decrease in membrane flux [2, 48]. Chemical precipitation mainly occurs due to concentrated polarization, and bioprecipitation is mainly caused by metallic ions, which act as bridges between the deposited microorganisms and organic polymers, forming a dense filter cake layer [114]. Ions related to inorganic fouling include Ca2+, Mg2+, Fe3+, Al3+, SO42−, PO43−, CO32−, and OH− plasma [48, 115, 116]. Furthermore, at appropriate concentrations, metallic ions, such as Ca2+, will combine and bridge with extracellular polymers and soluble microbial products, increasing the floc size. This can effectively reduce the deposition of organic fouling on membrane surfaces, but excessive metal ion concentrations can also aggravate inorganic fouling [117]. Hence, to remove inorganic contamination deposits on membrane surfaces, the surfaces must be chemically cleaned.
4.2. Factors that Affect Membrane Fouling in AnMBRs
Many factors can influence membrane fouling, and these can be broadly classified according to membrane characteristics, operating conditions, and sludge properties, as shown in Fig. S1.
4.2.1. Sludge properties
Mixed liquid suspended solids (MLSS) significantly affect membrane flux, as the higher the MLSS concentration, the faster the membrane fouling rate [118]. A higher MLSS concentration results in increased deposition of colloids, sludge flocs, macromolecules, and microbial products on membrane surfaces [1].
Wu and Huang [119] reported that AnMBRs operating at MLSS concentrations above 10,000 mg/L deal with increased sludge viscosity, and membrane permeability decreases with increasing sludge viscosity. Therefore, EPS and SMP are the most significant factors that contribute to membrane fouling compared to other sludge characteristics (such as MLSS, sludge particle size, and sludge viscosity) [107–109]. Due to the deposition and adsorption of EPS and SMP on membrane surfaces or pores, faster Brownian diffusion of liquid will occur [1, 107, 120]. This also causes the formation of a gel contamination layer, which can significantly increase membrane filtration resistance.
4.2.2. Operation conditions
The mode of operation is closely related to the membrane fouling rate, and in AnMBRs, there are two modes of operation: constant pressure and constant flux (constant flow) operations [48]. AnMBRs are commonly operated in constant flux mode, as they better handle fluctuations in influent hydraulic load [121]. In addition, the flux is critical to the long-term stability of the membrane, and operating at a higher flux will accelerate the formation of a filter cake layer. However, low temperatures and an increase in organic loading can promote the release of EPS, resulting in faster membrane contamination rates [122, 123]. Hydraulic retention time (HRT) and sludge retention time (SRT) can also influence membrane contamination by changing the nature of the sludge mix in a reactor [48]. Furthermore, longer SRTs can cause an increase in sludge concentration and the release large amounts of EPS due to the decomposition of dead cells, resulting in faster membrane fouling rates [120]. Although a shorter HRT can increase the sludge growth rate and effluent treatment efficiency, it can also cause an increase in sludge concentration and increased membrane fouling [124].
4.2.3. Membrane properties
Membrane properties such as membrane surface morphology (roughness), hydrophilicity/hydrophobicity, surface charges, and membrane pore size, can have varying degrees of influence on membrane fouling [1, 125]. Membrane fouling can be more severe with hydrophobic membranes than for hydrophilic membranes, due to hydrophobic interactions between membrane materials, microorganisms, and solutes [97–99, 107]. Membrane surface roughness and surface charges will have a greater impact on membrane fouling, as opposed to hydrophilicity/hydrophobicity. Thus, the greater the membrane surface roughness, the greater the specific surface area of a corresponding membrane, although more contaminants will be adsorbed on the membrane surface. However, the increased roughness will also increase the degree of hydrodynamic disturbance on a membrane surface, which will hinder the adsorption and deposition of contaminants on a membrane surface and thus delay membrane fouling [126]. In addition, colloidal particles in aqueous solutions are generally negatively charged, and using membrane materials with the same charges as the solute in a mixture can improve and alleviate membrane fouling via electrostatic repulsions, thus improving membrane flux. As a result, choosing membrane materials with a certain degree of roughness may result in negative potential membrane fouling that will be relatively minimal [125]. The pore size of a membrane can also significantly affect membrane fouling, with larger pore sizes having higher fouling rates compared to smaller pore sizes. In general, AnMBRs use membranes with pore sizes of 0.02–0.5 μm [127].
4.3. Controlling Membrane Fouling
Membrane fouling is inevitable in practical applications, and therefore, strategies to control or mitigate membrane fouling are important to the operation of a reactor and to address the causes and mechanisms of membrane fouling. Based on the factors that affect membrane fouling, control strategies can be divided into the following categories.
4.3.1. Membrane cleaning
Depending on the reversibility of membrane fouling, or the degree to which membrane fouling can be removed, membrane module cleaning can be divided into either physical or chemical cleaning. Physical cleaning methods mainly focus on removing reversible contamination, and the main physical cleaning methods include isobaric rinsing, backwashing, gas-liquid mixture shock cleaning, negative pressure cleaning, mechanical scraping, and electric cleaning [38, 128]. Recently, controlling membrane fouling by applying shear forces via biogas injection has been widely studied and applied, especially for particle injection and rotating membranes [1, 12, 52]. Evans et al. [61] tested the performance of biogas injection for controlling membrane fouling in AnMBRs and found a decrease in membrane surface fouling and an increase in membrane permeability at higher injection rates. Particle jet membrane bioreactors, also known as anaerobic fluidized bed membrane bioreactors, consist of fluidized granular activated carbon (GAC). These solutions not only offer a scouring effect on the membrane surface in the form of a scouring agent, but also provide a location for microbial growth [129–131]. Compared to biogas jet reactors, particle jet reactors have relatively low energy consumption and can control membrane fouling well [131–134]. The rotating membrane creates turbulent flow on the membrane surface through the rotation of the membrane module itself, which slows down the formation of the pollution layer on the membrane surface. Additionally, rotating the membrane module can agitate the sludge mixture. Although a rotating membrane module was designed by Belford et al. [135] 28 years ago, this method has attracted extensive attention from researchers in recent years due to its low energy consumption [136]. One advantage of physical cleaning is that cleaning is simple, no new pollutants are introduced, and the likelihood of chemical damage to the membrane is low. However, the physical cleaning method is only effective for the membrane modules during the initial pollution stage, and the cleaning effect does not last long. Chemical cleaning is required for irreversible pollution.
Chemical cleaning agents include acids (citric acid, oxalic acid, and hydrochloric acid), alkali products (sodium hydroxide), oxidants (sodium hypochlorite, and hydrogen peroxide), and chelating agents (EDTA) [83]. Acid washing mainly removes chemical precipitation between biopolymers and salts, and inorganic contamination caused by bio-induced mineralization [115, 137, 138], while alkaline washing mainly removes organic contaminants deposited on the membrane surface [139]. In addition, organic matter such as proteins and carbohydrates will hydrolyze into small molecules under alkaline conditions [140]. Oxidants primarily remove biological and organic contamination, leading to interactions between contaminants and membrane components being weakened and contaminants becoming more easily dislodged from the membrane surface [107]. Chelating agents such as EDTA have strong binding abilities for metal cations and can remove organic pollutants formed by metal cations through bridging [38]. However, frequent exposure to chemical reagents will compromise membrane integrity and alter membrane surface properties [141], which will affect normal membrane filtration behavior. Therefore, a combination of physical and chemical cleaning is an effective strategy for mitigating membrane fouling.
4.3.2. Membrane material selection and membrane modification
Hydrophilic modification of the membrane surface is a common strategy for controlling membrane fouling in AnMBRs [142, 143]. Currently, polar organic functional groups on membrane surfaces can be modified by plasma treatment, surface grafting, surface coating, or surface blending. Plasma treatment forms hydrophilic functional groups on a membrane surface [144]. As an example, Yu et al. [145] used plasma technology to modify the surfaces of polypropylene membranes, and found that NH3 and CO2 plasma-treated membranes exhibited a significant increase in hydrophilicity. Furthermore, the newly modified membranes had better filtration and flux recovery performance than unmodified membranes [145, 146]. Surface polymer grafting is also an effective method to improve membrane hydrophilicity. By grafting and polymerizing methoxypolyethylene glycol on the surface of polypropylene membranes, Wang et al. observed that with increased grafting degree, the flux of the modified membrane was twice that of an unmodified membrane [147]. Surface coatings are also a surface modification method, and Pi et al. demonstrated that TiO2 nanoparticles can improve the surface wettability and permeability properties of hydrophobic membranes, specifically when TiO2 was loaded onto the polypropylene microfiltration membrane surfaces using the sol-gel method [148].
The use of engineered nanomaterials (ENMs) as membrane materials has become an area of recent intense research. Engineered nanomaterials, including metal oxide-based nanomaterials, carbon nanomaterials, and silver nanoparticles, which have good antibacterial and hydrophilic properties, may offer great benefits in alleviating membrane fouling, especially biological fouling [149].
4.3.3. Optimization of operating parameters
Optimizing AnMBR operating parameters is essential for controlling membrane fouling, with key operating parameters including hydrodynamic conditions, membrane flux, HRT, SRT, MLSS, pH, and temperature [2, 38]. Increasing the intensity and duration of gas flushing in submerged AnMBRs, as well as the flow rate of mixed liquids in sidestream-AnMBRs, can provide the optimal hydrodynamic conditions to mitigate membrane fouling. An important strategy for mitigating membrane fouling involves membrane filtration at a filtration flux below the critical flux. Thus, when the filtration flux is below the critical flux, membrane fouling is less severe. However, when the filtration membrane flux is above the critical flux, more severe membrane fouling can be observed [150]. In addition, recent results indicate that the operating parameters greatly affect the metabolism of microorganisms, especially the release of SMP. Longer SRTs can promote the complete degradation of solid matter, but will reduce floc size, and increase the sludge and SMP concentration, which can cause increased membrane fouling [128]. Shorter HRTs can reduce the reactor volume, but will result in insufficient metabolism of organic matter, and higher organic matter concentrations also aggravate membrane fouling [151, 152].
4.3.4. Regulation of sludge mixture characteristics
Currently, the most direct and effective way to improve sludge mixture characteristics involves the use of additives, such as adsorbents and flocculants [38]. In AnMBRs, adsorbents will adsorb colloids and dissolve organic compounds in the sludge mixture, which reduces membrane fouling. Powdered activated carbon (PAC) is a widely used adsorbent in AnMBRs, and PAC addition can control scaling, including dirt adsorption and its subsequent biodegradation, as well as increase the critical flux, improve the strength of microbial flocs, and enhance the scouring effect of particles on membrane surfaces. In 1999, it was reported for the first time that PAC can reduce fouling in membrane bioreactors, and the results showed that as the PAC dosage increased to 5 g/L, the fouling resistance and filter cake resistance continued to decrease. In addition, PAC provides a carrier for the growth of microorganisms, avoiding the rupture of sludge flocs under the action of hydraulic shear. However, the adsorption capacity of activated carbon is limited, and therefore, the saturated portion needs to be discharged during operation and newly activated carbon must be simultaneously added [153]. Unfortunately, excessive PAC can also increase membrane fouling, as excessive PAC can act as a pollutant [154, 155]. Other adsorbents such as zeolite, bentonite, and vermiculite can also be used to reduce membrane fouling [156]. Coagulant addition is also an effective strategy for controlling membrane fouling. Commonly used flocculants include iron salts (FeSO4 and FeCl3), aluminum salts (AlCl3), inorganic polymers (polyferric sulfate and polyaluminum chloride), and organic polymers (such as starch and chitosan). Flocculant addition can also increase the particle size of sludge and reduce the concentration of organic matter in the supernatant, thus reducing membrane fouling [157]. Zhang et al. [133] observed that the addition of ferric chloride increased floc particle size and reduced the dissolved organic matter in the supernatant, which alleviated membrane fouling. However, flocculant addition reduces the pH of the mixture, and a decrease in pH affects microorganism activity. In addition, excessive flocculants can deposit on the membrane surface, which can aggravate membrane fouling [48].
5. Novel AnMBRs for Fouling Mitigation
5.1. Anaerobic Electrochemical Membrane Bioreactors (AnEMBRs)
Recently, to solve the problems of membrane fouling in AnMBRs, many scholars have sought to combine membrane separation technology, anaerobic microbiology, and microbial electrolysis cell technology to construct a new type of anaerobic electrochemical membrane bioreactor (AnEMBR) in an attempt to control membrane fouling and promote methane production [52]. As shown in Fig. S2(a), sludge loss can be reduced by the interception of the membrane. However, an electrical bias needs to be applied to the membrane surface to generate electrochemical auxiliary conditions. Membrane fouling can be alleviated by the electrostatic forces between pollutants and the membrane surface, as well as the electro-flocculation between pollutants, the electrochemical reactions on the membrane surfaces, and the scouring effect of the gas produced by the electrode [158]. The introduction of an electric field stimulated the growth and metabolism of electroactive microorganisms in the reaction zone and electrode surface, which increased the population of hydrolytic acidifying and methanogenic bacteria [159]. In addition, this also promoted the extracellular electron transfer of anaerobic microorganisms between the electrodes. This enhanced the transformation efficiency of the organic matter, intermediates, and cellular metabolites in anaerobic reactions, improving treatment efficiency and biogas yield. As a result, this method could recover energy from sewage via biogas production, while also reducing energy consumption.
Yang et al. [160] designed a new type of conductive carbon nanotube hollow fiber membrane (CNT-HFMs) as an AnMBR, in which the CNT-HFMs had dual functions as cathodes and membrane filters. Compared to conventional AnMBRs, and CNT-HFM AnMBRs without voltage, the transmembrane pressure differential rate of this new type of anaerobic electrochemical membrane bioreactor increased slowly throughout the nearly 100 day operation period, and the COD removal rate in the effluent reached more than 95%, due to the actions of the electrical field. In addition, negatively charged membrane fouling substances (including microorganisms and their secretions) moved away from the membrane surface. This reduced membrane pore blockage in the anaerobic electrochemical membrane bioreactor, and the application of the electric field effectively improved microorganism activity in the mixture and improved COD removal efficiency. Katuri et al. [161] also used atomic layer deposition (ALD) to deposit platinum on a metal catalyst. On the porous skeleton membrane surface, the researchers used a biological cathode and a carbon fiber brush as an anode, to study the performance of this new anaerobic electrochemical membrane bioreactor. The experimental results showed that the COD removal rate of simulated domestic sewage was more than 95% under a voltage of 1.0 V, and the COD removal rate of actual wastewater reached 83%. In addition, membrane fouling was well alleviated, and TMP after long term operation was only 1/3 of the control group. However, the magnitude of the applied voltage had to be reasonably regulated according to the experimental conditions, purpose, and raw water quality, to achieve better sewage treatment effect and minimize membrane fouling.
5.2. Anaerobic dynamic membrane bioreactors (AnDMBRs)
Dynamic membranes were first reported by Oak Ridge National Laboratory in the United States in the early 1960s. When conducting seawater desalination experiment, the researchers found that a physical dynamic zirconia membrane with reverse osmosis would form when the porous material was exposed to a specific solution [162, 163]. The dynamic membrane bioreactor was intercepted by cheap coarse porous filter substrates, such as non-woven cloth, and solid–liquid separation performance was similar to microfiltration and ultrafiltration membranes, when a sludge layer (dynamic membrane) was attached to the filter membrane module [164]. The application of a dynamic membrane to an aerobic membrane bioreactor was also assessed in the early 2000s. Currently, AeDMBRs have developed into a relatively mature technology. However, the application of dynamic membranes for AnMBRs is still developing, and was first proposed by David Jeison et al. [165] in 2008, which confirmed the feasibility of dynamic membranes for anaerobic bioreactors. Compared to AnMBRs, the coarse porous filter material in AnDMBRs replaces the traditional microfiltration/ultrafiltration membrane, greatly reducing the initial cost (Fig. S2(b)). Secondly, the dynamic membrane that forms on the coarse porous filter material can be regenerated in situ and recycled, without requiring frequent cleaning or replacement of expensive membrane components, giving it better removal performance for pollutants in water (60–90% COD removal rate, 90–100% turbidity, and suspended solids removal rate). Li et al. [166] used a stainless-steel mesh with a pore diameter of 40 μm as a filter material to conduct a study on the treatment of low concentration domestic sewage using an AnDMBR. The results showed that the removal efficiency of COD was more than 90%, and the methane yield was 0.24 L CH4/g CODremoved. In addition, when a carbon fiber–coupled AnDMBR was used to treat the wastewater with different organic loads (0.46–17 kg COD/ (m3d)), satisfactory and stable process performance was achieved. The COD removal efficiency was 95% and the methane yield was 0.29 L CH4/g CODremoved. Alibardi et al. [167] used an external AnDMBR with a 200 μm aperture grid to treat low concentration wastewater, and the average total COD and soluble COD removal efficiencies were higher than 80% and 90%, respectively. Ersahin et al. [168] studied the effect of AnDMBR configuration (immersion type and external type) on the treatment and filtration performance, and found that both devices achieved high COD removal rates of more than 90%. Further studies showed that submerged AnDMBR had a faster start-up time, better effluent quality, and higher biogas production than other types of AnMBRs.
5.3. Anaerobic Osmotic Membrane Bioreactors (AnOMBRs)
Forward osmosis (FO) refers to the transport of water from areas of higher hydration potential to areas with lower hydro-chemical potential, using selective osmosis membranes. This process is driven by the solute concentration differences on both sides of the membrane, and selective permeation membrane allows for the water molecules to pass through, while repelling most solute molecules or ions [169]. AnOMBR introduces forward osmosis into an AnMBR. Under the action of osmotic pressure differences between the mixture and suction, most of the water molecules in sewage will spontaneously enter the absorption liquid through an FO membrane, and pollutants will be intercepted by the FO membrane in the reactor, thus concentrating the wastewater [170]. In contrast to a conventional AnMBR, the AnOMBR membrane filtration process does not require any energy input (Fig. S2(c)). Chen et al. [171] treated domestic sewage with an AnOMBR at 25°C and found that the COD, ammonia nitrogen, and total phosphorus removal rates in the reactor were 96, 62, and 100%, respectively. In addition, the treatment effectiveness was much higher than for conventional AnMBRs, and the methane yield of the reactor was approximately 0.21 L CH4/g CODremoved. Although a portion of the methane was dissolved in the mixture and entered the absorption solution, resulting in a methane yield of only 58–76% of the maximum theoretical value, the methane production rate was still much higher than conventional AnMBRs. Gao et al. [172] studied the treatment effect of an AnOMBR on urban domestic sewage using different concentrations of extracted solutions (NaCl solution). The results showed that this type of reactor was effective in treating municipal domestic sewage under different working conditions, and the removal rates of COD, NH4+-N, TN, and TP were 96, 88, 89, and nearly 100%, respectively. Although AnOMBRs have shown excellent performance in treating domestic wastewater, enriched solutes in domestic sewage and salt solute back mixing of an FO membrane can cause salt accumulation in this type of reactor, which adversely affects the membrane flux and microbial activity. Therefore, reducing salt accumulation in an AnOMBR reactor should be studied in the future.
5.4. Anaerobic Membrane Distillation Bioreactors (AnMDBRs)
Membrane distillation is a membrane process where the vapor pressure difference produced by the temperature gradient on both sides of the hydrophobic microporous membrane is used to power a separation effect [173, 174]. Anaerobic membrane distillation bioreactors (AnMDBRs) are a new type of AnMBR that specifically integrates membrane distillation into the AnMBR for wastewater treatment and reuse (Fig. S2(d)). According to the principles of membrane distillation, hydrophobic microporous membranes are used for mud–water separation, and mass transfer is the driving force in such an anaerobic membrane distillation bioreactor, in turn driven by the temperature differences between the two sides of the membrane. Through this temperature difference, water vapor evaporates through the membrane and the organic matter is intercepted to optimize effluent quality. Song et al. [175] used an anaerobic membrane distillation bioreactor to treat simulated highly concentrated domestic sewage. The removal rates of TOC and COD in this reactor were more than 98%, the biogas yield was 0.3–0.5 L/g CODadded, and the methane content was stable at about 65%. Furthermore, Kim et al. [176] coupled an anaerobic moving bed membrane bioreactor and membrane distillation to treat domestic sewage, and the COD and TP removal rates reached 92% and 100%, and methane production accounted for approximately 58–72% of total biogas production using this bioreactor.
6. Future Perspectives
Over the past few decades, many scholars have investigated new techniques to mitigate membrane fouling and improve the performance of AnMBRs. Compared to conventional AnMBRs, coupled processes such as AnEMBRs, AnDMBRs, AnOMBRs, and AnMDBRs have higher pollutant removal rates, methane yields, and lower membrane fouling rates. However, these processes are still limited to the laboratory, and the membrane fouling control mechanisms and energy balance for treatment processing need to be studied further. In addition, the results of laboratory-scale studies should be validated on a large scale, and long-term studies need to be conducted for practical applications.
Another aspect that needs to be studied is the recovery of dissolved methane using AnMBRs with high efficiency and at a low cost. This process will require maximum production and minimum dissolution of methane at low temperatures (10–30°C), the development of methane recovery technology, the design of methane recovery equipment, improved cost control, and more efficient energy consumption during the recovery process.
In addition, to further solve reliability and large-scale commercial application issues for AnMBRs, breakthroughs need to be made in the following areas.
-
Improvements in the performance of membrane materials and modules
Develop and research new separation membranes with superior performance that are not only pollution resistant, long-lasting and mechanically strong, but also chemically stable and inexpensive.
-
Development of environmentally friendly membrane cleaning strategies
Development of green cleaning agents to minimise the impact of chemical cleaning on the membrane and microbial activity; research into new membrane cleaning strategies such as biological cleaning, ultrasonic cleaning, etc.
-
Membrane contamination mechanism and prevention technology
Further in-depth research into the effects and mechanisms of membrane filtration and membrane contamination processes; develop more effective, easy-to-use methods to control and mitigate membrane contamination; establish effective contamination models for domestic wastewater to avoid long and costly tests.
-
Optimisation of process flow and operating conditions of anaerobic membrane bioreactors
Optimisation is carried out in terms of both pollutant treatment effects and membrane pollution control to ensure both stable treatment effects and long operation of the AnMBR process. Specific studies include strengthening denitrification to improve nitrogen removal; reducing energy consumption during operation; studies on sludge retention control measures and time; and optimisation studies on the combination of membrane modules and new wastewater treatment technologies.
7. Conclusions
This review article summarized the recent progress in AnMBR technology for wastewater treatment, offering a promising alternative to traditional wastewater treatment technology with various application prospects. However, there are still many challenges that limit the large-scale commercial applicability of AnMBRs. Membrane fouling is the main obstacle for large-scale commercial AnMBR applications, and membrane materials, membrane modules, sludge properties, and operating conditions are the main factors that affect membrane fouling. In recent years, many new types of AnMBRs have been developed to control membrane fouling and have achieved satisfactory results in the lab. Therefore, future research should focus on the following areas: First, these treatments need to be scaled to reach a level that is suitable for practical applications. In addition, simpler and more effective strategies to control membrane fouling control need to be developed. Finally, the recovery of dissolved methane in water must be maximized and the energy consumption during reactor operation must be reduced.
Supplementary Information
Acknowledgments
We thank the staff of the School of Energy and Environment, the Shenyang University of Aeronautics and Astronautics and the Environmental Pollution Control Research Center, and the Chinese Academy of Environmental Sciences for their assistance. We acknowledge financial support from the National Key Research and Development Program of China (No. 2018YFD1100505).
Notes
Conflict-of-Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Author Contributions
L.W. (Professor) supervised the students, read, wrote and edited the manuscript. Jinyuan Jiang (Professor) provided funding, wrote and edited the manuscript. J.L. (M.E. student) conducted the investigation process and wrote the original draft. J.c.L. (M.E. student) read, wrote, reviewed and edited the manuscript. C.H. (M.E. student) and Y.L. (M.E. student) curated the data.