The effect of ionic strength, pH and natural organic matter on heteroaggregation of CeO2 nanoparticles with montmorillonite clay minerals
Article information
Abstract
An accurate assessment of the fate and transport of engineered nanoparticles (ENPs) in aquatic environments hinges on developing an understanding of interaction between ENPs and natural colloids. In this study, we investigated the effects of pH and natural organic matter (NOM) on homoaggregation of CeO2 nanoparticles and their heteroaggregation with montmorillonite clay minerals. Time-resolved dynamic light scattering (TR-DLS) was employed to investigate the aggregation kinetics as function of electrolyte concentration in both homo- and heteroaggregation scenarios. The surface charge of CeO2 nanoparticles changed from positive to negative with pH increase while montmorillonite was negatively charged over the range of pH 4–11. At low pH, the critical coagulation concentration (CCC) of the montmorillonite-CeO2 mixtures shifted to a lower electrolyte concentration when compared to the homogenous system, indicating the negatively charged montmorillonite reduced the stability of positively charged CeO2 via heteroaggregation. In contrast, at high pH, the mixtures were stable because both montmorillonite and CeO2 nanoparticles were negatively charged. The presence of humic acid stabilized the systems against homo- and heteroaggregation. Our results suggest that the complex interactions between ENPs, natural colloids, and NOM should be considered to better understand the fate and transport of ENPs in realistic aquatic environments.
1. Introduction
The use of nanomaterials is significantly increasing as industries such as electronics, cosmetics, biotechnology and material science use these materials due to their high specific surface area and surface activity. During their use it is likely that products containing nanoparticles will release some of the particles into the environment [1, 2]. Once released, particles will interact with each other and with their surrounding environment. In aquatic systems aggregation strongly influences the environmental behavior of nanoparticles [3–6]. Particle aggregation, which depends upon the particles’ surface properties, will subsequently dictate particle settling and removal from the water column [7–11]. Therefore, a thorough understanding of how aggregation influences the fate and transport of nanoparticles is indispensable for predicting the potential risk of nanoparticles to human health and the environment.
Natural aquatic systems are heterogeneous and contain different mineral phases (e.g., clays, metal oxides and carbonates), cations (e.g., Ca2+, Mg2+, Na+, etc.) as well as natural organic matter (NOM), nevertheless most studies evaluating nanoparticle aggregation mainly focus on the influence of environmental parameters (i.e., ionic strength and pH) with a few including NOM [12–25]. A number of studies report that the type of cations (mono- vs. divalent) is a meaningful factor in determining nanoparticle aggregation [12, 13, 21, 22, 25]. In general, shielding of surface charge by multivalent cations is more effective than that by monovalent cations resulting in enhanced aggregation generally consistent with the Shulze-Hardy rule [22, 25, 26]. Several studies also addressed the behavior of nanoparticles as they interacted with NOM under different solution chemistries [7, 14, 15, 17, 27, 28]. The majority of these studies showed increased electrostatic and steric repulsion owing to the partial adsorption of NOM to the nanoparticle surface enhanced particle stability [27, 28]. Despite the abundance of clays in the environment, only a few studies have evaluated the aggregation of nanoparticles with clay in aqueous environments [20, 29–34]. These studies indicate the clays can destabilize positively and negatively charged nanoparticles across a range of pH and electrolyte conditions [20, 29, 31, 33, 34]. For example, Zhou et al. [29] studied on the interactions of two of nanoparticles, Ag and TiO2 and montmorillonite to understand how heteroaggregation can change the stability of nanoparticle/montmorillonite mixtures and Wang et al. [31] evaluated the effects of kaolin particles on the aggregation of TiO2 or AgNPs in aqueous environments. In addition, Li et al. [33] observed the heteroaggregation of CeO2 with natural colloids (kaolin and goethite). The abundance of natural clay particles and NOM nearly guarantees that their interactions will influence the fate of nanoparticles in the environment. As noted previously, however, there is insufficient information on how natural materials such as clay and NOM influence NP aggregation and fate.
In this study, the effect of pH and NOM on the homo- and hetero-aggregation of nanoparticles with clay was investigated. CeO2 NPs were selected as a model NP due to their commercial applications, being routinely used in fuel additives, catalytic converter, and other uses [35, 36]. Montmorillonite was chosen as a naturally occurring clay mineral, being the most often researched clay mineral [20, 27, 29, 32, 37]. Generally, montmorillonite has sheet-like structure and the faces of the lamellae have immutable negative charges owing to isomorphic substitutions, and pH-dependent charges develop on the surface hydroxyls at the edges [29, 32, 37]. The Suwannee River humic acid (SRHA) was chosen as a representative NOM. Time-resolved dynamic light scattering (TR-DLS) measurements were used to study the aggregation as a function of electrolyte concentration in homo- and hetero-aggregation scenarios. The results of this study suggest that heteroaggregation of nanoparticles with clay is influenced by aqueous environments such as ionic strength, pH and NOM.
2. Materials and Methods
2.1. Materials
Montmorillonite (SWy-2) was purchased from the Clay Minerals Society (Wyoming, USA) and prepared by modifying Tombácz et al.’s method [20]. To begin, 10 g/L montmorillonite was dispersed in deionized water by sonicating for 15 min (60 W) with an ultrasonic probe (Ultrasonic Homogenizer, Cole-parmer Instruments, USA) to break agglomerates. The suspension was then mixed using magnetic stirrer for 4 h. The size fraction of < 2 μm was isolated by overnight gravity sedimentation. The supernatant was collected and the electrolyte concentration was adjusted to be 1 M NaCl to obtain the monocationic montmorillonite. After mixing this suspension for 2 h, the clay particles were separated through centrifugation at 2,000 g for 10 min (Sorvall Legend X1R, Thermo Fisher, Germany). The supernatant was discarded and replaced with fresh 0.01M NaCl. The suspension was vigorously shaken and subsequently centrifuged. This final washing procedure was repeated 5 times. The suspension was then transferred to dialysis tubing (10000 eMWCO, Spectrum Laboratories, USA) and dialyzed against 0.01M NaCl. The dialysate was changed daily until the conductivity of the clay suspension was the same as 0.01M NaCl. The concentration of montmorillonite stock suspension was determined by gravimetric analysis.
Bare CeO2 (99.9%, 10–30 nm) with spherical shape was obtained as powder from SkySpring nanomaterials (Houston, USA). CeO2 nanoparticle stock suspension (100 mg/L) was prepared by mixing with deionized water while sonicating for 30 min (56 W) [38]. The stock suspension was freshly prepared prior to experimental use.
Suwannee River humic acid II (SRHA) standard was purchased from the International Humic Substances Society (St. Paul, MN, USA). A SRHA stock solution was prepared by dissolving 300 mg/L of SRHA in deionized water and raising the solution pH to 10 with 0.1 N NaOH to increase the solubility of HA [39]. The SRHA stock was stirred overnight, and filtered through 0.45 um filter. The concentration of filtered SRHA was analyzed by a total organic carbon analyzer (TOC-VCPN, Shimadzu, Tokyo, Japan). The concentrated SRHA stock solution (121.1 mg/L TOC) was stored at 4°C in the dark.
2.2. Particle Characterization
The morphology and size of the montmorillonite and CeO2 particles were characterized using a transmission electron microscope (TEM, JEOL JEM-2010, Japan). About 10 μL of sample was dropped on the carbon coated copper TEM grid (CF200-Cu, Electron Microscopy Science, USA) and allowed to dry for the analysis. The electrophoretic mobility (EPMs) and hydrodynamic diameter of montmorillonite and CeO2 particles were measured using a ZetaSizer Nano ZS90 instrument (Malvern Instruments Inc, Worcestershire, UK) at 1 mM NaCl as a function of pH. The EPMs of the samples were measured in folded capillary cells (Malvern, Worcestershire, UK) and measurement were repeated 6 times. For the measurement of hydrodynamic diameter at each pH, montmorillonite or CeO2 suspensions were transferred to disposable cuvette (Kartell, Milan, Italy). Ten measurements were carried out for each sample.
2.3. Aggregation Kinetics
The effect of pH on the homoaggregation kinetics of montmorillonite and CeO2 was determined across a range of pH (4, 6, and 10). Samples were prepared by combining aliquots of the stock particle suspensions titrated to the appropriate pH with appropriate amounts of NaCl and deionized water. pH was controlling using 1 M HCl (pH 4) or 1 M NaOH (pH 10). In each case, the final concentrations of montmorillonite or CeO2 was 50 mg/L according to a previous study [29]. The environmental relevant concentrations of CeO2 was ug/l, but a high concentration of CeO2 NPs (50 mg/L) was used for accomplishing a proper measurement with DLS.
The aggregation of binary montmorillonite/CeO2 was determined on suspensions prepared in the same manner as those used in the single particle systems. When measuring heteroaggregation, the montmorillonite and CeO2 suspensions were added simultaneously. In addition, the effect of CeO2 concentration on the heteroaggregation was performed in systems with 50 mg/L montmorillonite and 5 to 50 mg/L CeO2. To investigate the impact of NOM on heteroaggregation, predetermined amounts of the SRHA stock solutions were added to the montmorillonite to get a final concentration of 5 mg/L TOC. The pH values of the suspensions were adjusted to the desired pH and allowed to equilibrate overnight. No aggregation of montmorillonite was observed during the equilibrium time.
The homo- and hetero-aggregation kinetics were determined by monitoring the hydrodynamic diameter as a function of time through time-resolved DLS measurement (ZetaSizer Nano ZS90, Malvern Instruments). The instrument was equipped with a 633 nm laser with a detection angle of 90 degrees and the experiments were conducted at a fluid viscosity of 0.8872 cP and a temperature of 25 ± 0.5°C. The total volume of the prepared suspensions for these measurements remained fixed at 1 mL. Immediately upon sample preparation, the samples were rapidly mixed by hand and placed in the sample holder. The measurements of hydrodynamic diameter (Dh) were started immediately after sample insertion and continued for 25 min at intervals of 15 s. The initial aggregation rate (k) of suspensions can be determined by fitting the initial linear increase [40] using the following equation:
where N0 is the initial particle concentration. In this study all samples have the same particle concentration (i.e., 50 mg/L) and thus the attachment efficiency (α) was calculated by normalizing the initial rate of increase in Dh(t) in a given electrolyte concentration by the initial rate of increase in Dh(t) under favorable (fast) aggregation conditions (i.e., diffusion-limited conditions), where the rate is independent of electrolyte concentration, as follows [40] :
3. Results and Discussion
3.1. Material Characterization
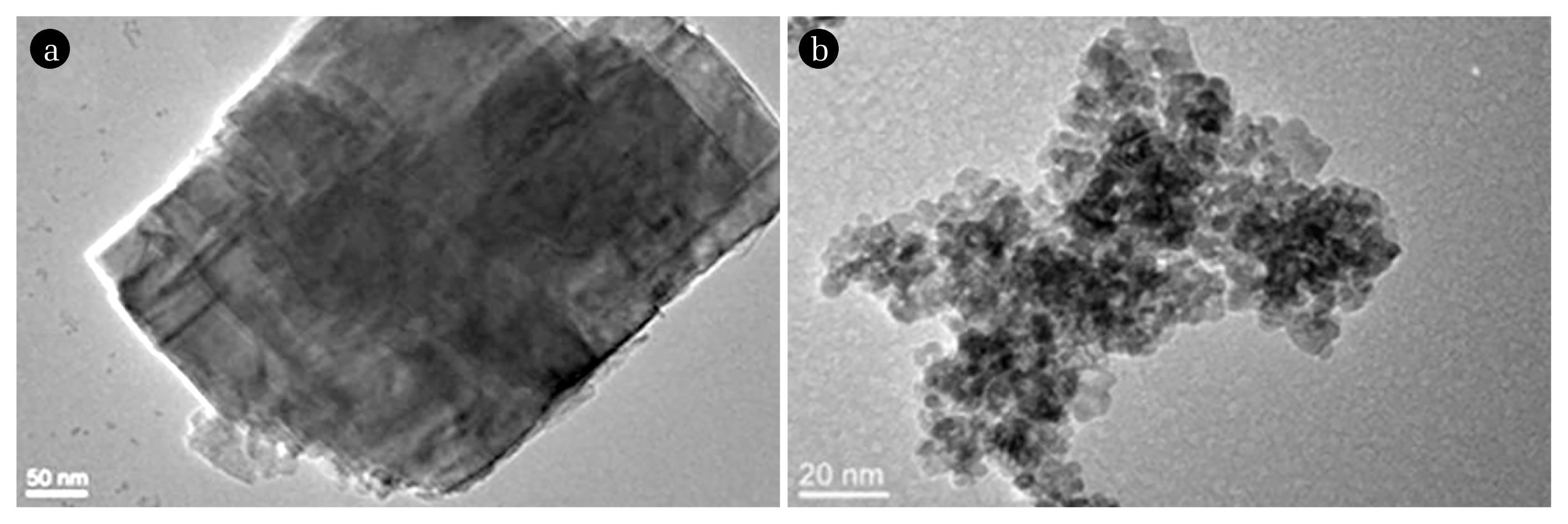
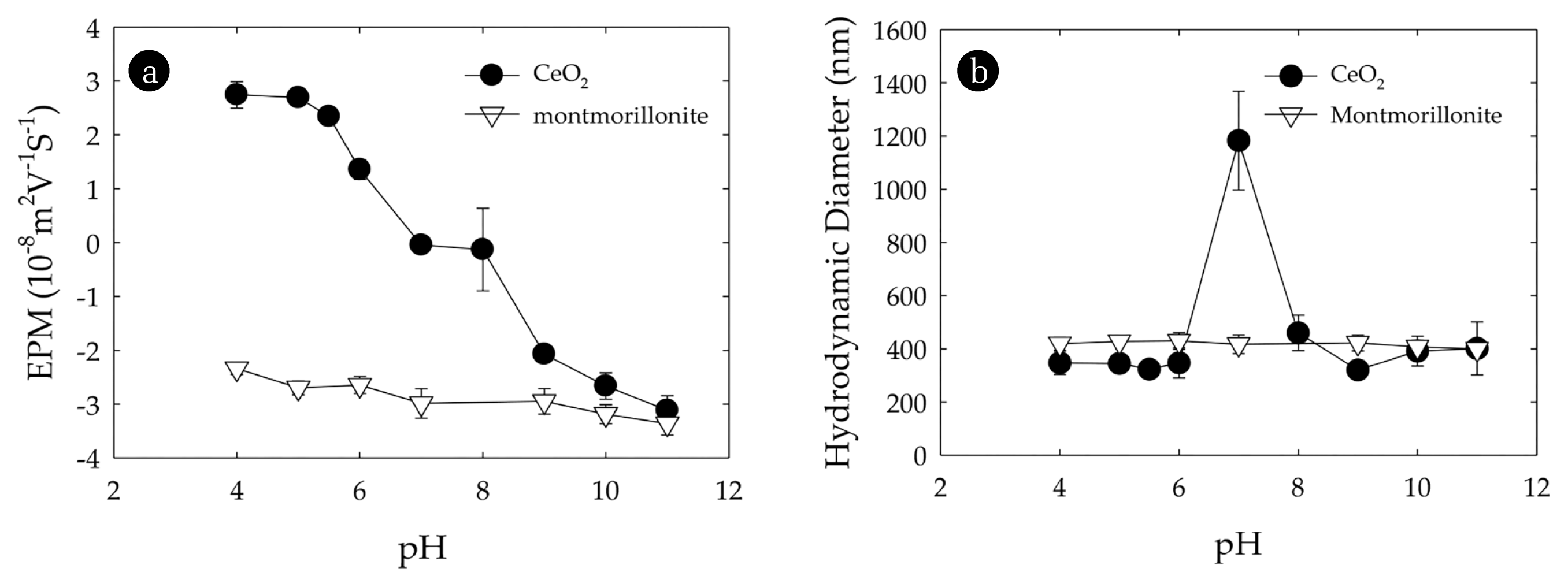
Transmission electron microscopy (TEM) was used to characterize the morphology of the montmorillonite and CeO2 particles (Fig. 1). Montmorillonite particles were present as lamellae structure with roughly 300 nm to 500 nm in size (Fig. 1(a)). This is consistent with the method of preparation and reported particle morphology [20]. Montmorillonite was negatively charged over the range of pH studied and exhibited a slight decrease from −2.3 × 10−8 m2/Vs to −3.4 × 10−8 m2/Vs as the pH increased from 4 to 11 (Fig. 2(a)). This was consistent with reported trends and reflects changes dissociation of the silica and alumina sites at the edges of the clay particles with increasing pH [29, 31]. The hydrodynamic diameter of the clay particles determined by DLS was approximately 410 nm (Fig. 2(b)) and this size did not vary across the range of pH values as the significant EPMs of these particles providing electrostatic stability. The individual CeO2 particles were spherical and about 5–10 nm (Fig. 1(b)). The EPMs of CeO2 was positive at low pH and with increasing pH it gradually decreased becoming negative at pH greater than about 7 (Fig. 2(a)). This pH corresponded with reported values of the pHPZC of CeO2 [17]. At this pH, the absence of electrostatic interactions results in an unstable suspension and the formation of aggregates with hydrodynamic diameter greater than 1,000 nm (Fig. 2(b)).
3.2. Homoaggregation of Montmorillonite and CeO2
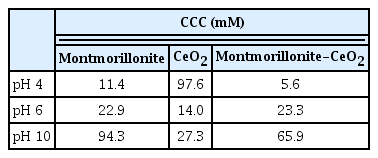
The attachment efficiency of montmorillonite and CeO2 at pH 4, 6 and 10 are shown in Fig. 3. The stability of montmorillonite increased with increasing pH (Fig. 3(a)). When the pH increased from 4 to 10, the CCC of montmorillonite increased from 11.4 mM NaCl to 94.3 mM NaCl (Table 1). The observed trend in particle stability was counter to the measured EPMs, which of montmorillonite was more negative at pH 10 than it was at pH 4. As is well known, montmorillonite has a structure with permanent negative charges on basal plane (face) and pH-dependent charges on edge [20, 29, 37]. At low pH the basal plane of montmorillonite carries a negative charge while the edge carries a positive charge (pHPZC, edge~6.5) [29, 37]. However, the positive electric double layer (EDL) near the edge is hidden at low ionic strength due to the spillover of the dominant basal plane (face) EDL. The positive edge EDL can emerge at high ionic strength, where the EDL is sufficiently compressed [29, 37]. This charge heterogeneity can induce edge-to-face particle interactions which enhance aggregation and likely produced the relatively low CCC at pH 4 [29, 37]. At high pH values, the basal plane and edges both carry a negative charge and thus in the face of enhanced electrostatic repulsion the clay suspension was more stable at pH 10 than it was at pH 4 or 6.
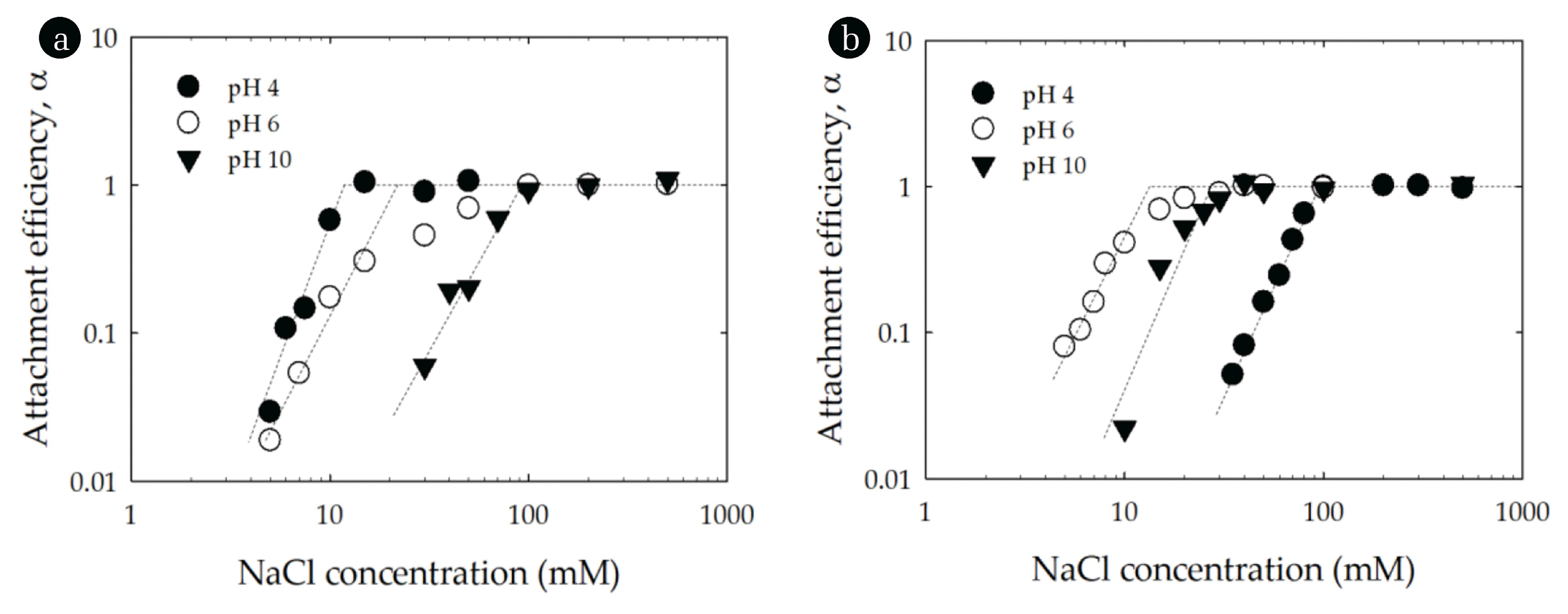
Attachment efficiencies of (a) montmorillonite and (b) CeO2 as functions of NaCl concentration and pH. Lines are provided to guide the eye.
The attachment efficiency of CeO2 as function of NaCl concentration exhibited different trends with pH than did montmorillonite (Fig. 3(b)). At pH 6, the CCC of CeO2 was lower than at pH 4 or 10 (Table 1). This reflects that CeO2 at low and high pH was more highly charged than at pH 6 (see Fig. 2(a)). The more extensive charge prevented particle aggregation due to the higher repulsive interactions. Thus, the more the pH of the solution departs from the particle’s PZC the higher the surface charge and the interparticle electrostatic repulsion. The reaction-limited and diffusion-limited regimes can be observed from the inverse stability profiles for both montmorillonite and CeO2 (Fig. 3) indicating that their aggregation behavior was in qualitative agreement with DLVO theory.
3.3. Heteroaggregation of CeO2 with Montmorillonite
Experiments evaluating the heteroaggregation of mixed CeO2-montmorillonite particle systems were conducted across the same range of NaCl concentrations and pH values as those evaluated for the single-particle systems (Fig. 4). The CCC values of the montmorillonite-CeO2 mixtures at pH 4, 6 and 10 were about 5.6, 23.3 and 65.9 mM NaCl, respectively, which signifies that the pH has an influence on the CCC (Table 1). At pH 4, the CCC of the montmorillonite-CeO2 shifted to a lower electrolyte concentration when compared to the homogenous montmorillonite and CeO2 suspensions (Fig. 4(a)). The EPMs of CeO2 particles at pH 4 was 2.7 × 10−8 m2/Vs while that for montmorillonite was −2.3 × 10−8 m2/Vs (Fig. 2) and as a result the positively charged CeO2 was able to interact with the negatively charged montmorillonite and thus enhanced aggregation. In contrast, at pH 10, the CCC of the two-particle system was between that for the montmorillonite and CeO2 systems (Fig. 4(c)). Likely this reflects that at pH 10 both montmorillonite and CeO2 are negatively charged and mixing the two does not significantly enhance aggregation. At pH 6, the montmorillonite-CeO2 mixture stability was nearly identical to that for the single-particle systems (Fig. 4(b)). Since this pH was near the pHPZC of CeO2, the CeO2 particles were unstable and as mentioned in homoaggregation, the aggregation of montmorillonite was favorable due to edge-to-face particle interactions at this pH. Therefore the CCC of the mixture was similar to that of the single-particle systems of montmorillonite and CeO2.
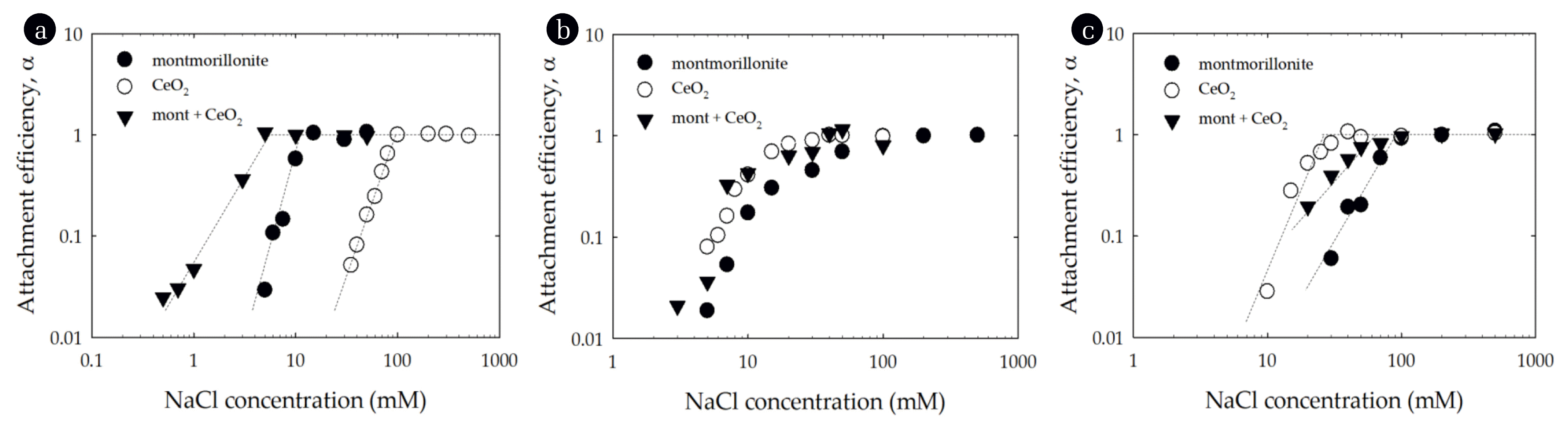
Attachment efficiency of montmorillonite, CeO2 and a mixture, as a function of NaCl concentration at (a) pH 4, (b) pH 6, and (c) pH 10. Lines are provided to guide the eye.
The homo- and hetero-aggregation rates of montmorillonite, CeO2 and the clay-NP mixtures are presented in Fig. S1. At pH 4, the homoaggregation rates of montmorillonite increased from 0.13 to 4.77 nm/s, as the NaCl increased from 5 to 50 mM, and that of CeO2 increased from 0.03 to 0.50 nm/s, as the NaCl increased from 35 to 500 mM. Thus, under diffusion-limited conditions the aggregation rate of CeO2 was lower than that of montmorillonite. On the other hand, the heteroaggregation rates of montmorillonite-CeO2 mixtures increased from 0.04 to 2.83 nm/s, as the NaCl increased from 0.3 to 50 mM. As previously mentioned, due to the interaction between the positively charged CeO2 and the negatively charged clay, the aggregation rate of montmorillonite-CeO2 mixtures was higher than that of CeO2 or montmorillonite under reaction-limited regime. The aggregation rate of the mixtures under the diffusion-limited regime approximated to that of pure montmorillonite. Similar trends in the aggregation rates were observed at pH 6 and pH 10. The Hamaker constant of CeO2 (5.57 × 10−20 J) in water is lower than that of montmorillonite (2.54 × 10−15 J) [4, 22]. Therefore, consistent with its smaller Hamaker constant, the aggregation rate of CeO2 was less than montmorilonite.
A similar phenomenon was reported for an oppositely charged nanoparticles and clay mixture, where pH is a key factor to determine the heteroaggregation. According to Zhou et al. [29], at pH 4, the CCC (10 mM) of the montmorillonite-TiO2 shifted to a lower electrolyte concentration when compared to the homogenous montmorillonite (35 mM) and TiO2 (25 mM) suspensions. In another study, kaolin clay particles reduced the energy barrier and increased the aggregation rate of TiO2 nanoparticles, particularly at pH 4 [31].
As described in Section 2.3, the effect of nanoparticle concentration on heteroaggregation was also carried out at three different CeO2 concentrations (i.e., 5, 25, and 50 mg/L). The NaCl concentrations were selected to correspond to conditions that provided similar attachment efficiencies (0.3~0.4) at pH 4, 6 and 10 in the mixed systems with equal particle concentrations. They were 3, 7, 20 mM NaCl, respectively for systems at pH 4, 6 and 10 (Fig. S2). At pH 4, the heteroaggregation of CeO2 was not influenced by CeO2 concentration (Fig. S2(a)), because the attractive electrostatic interactions due to the oppositely charged montmorillonite and CeO2 dominates. At pH 6, the concentration of CeO2 has little influence on the rate of particle aggregation. Likely, this reflects the instability of the negligibly charged CeO2 particles at pH 6 was controlling. Only at pH 10 was any difference observed. At this pH, decreasing CeO2 concentration decreased aggregation rate. This was consistent with the impact particle concentration has on the probability of particle collisions [41].
3.4. Role of HA
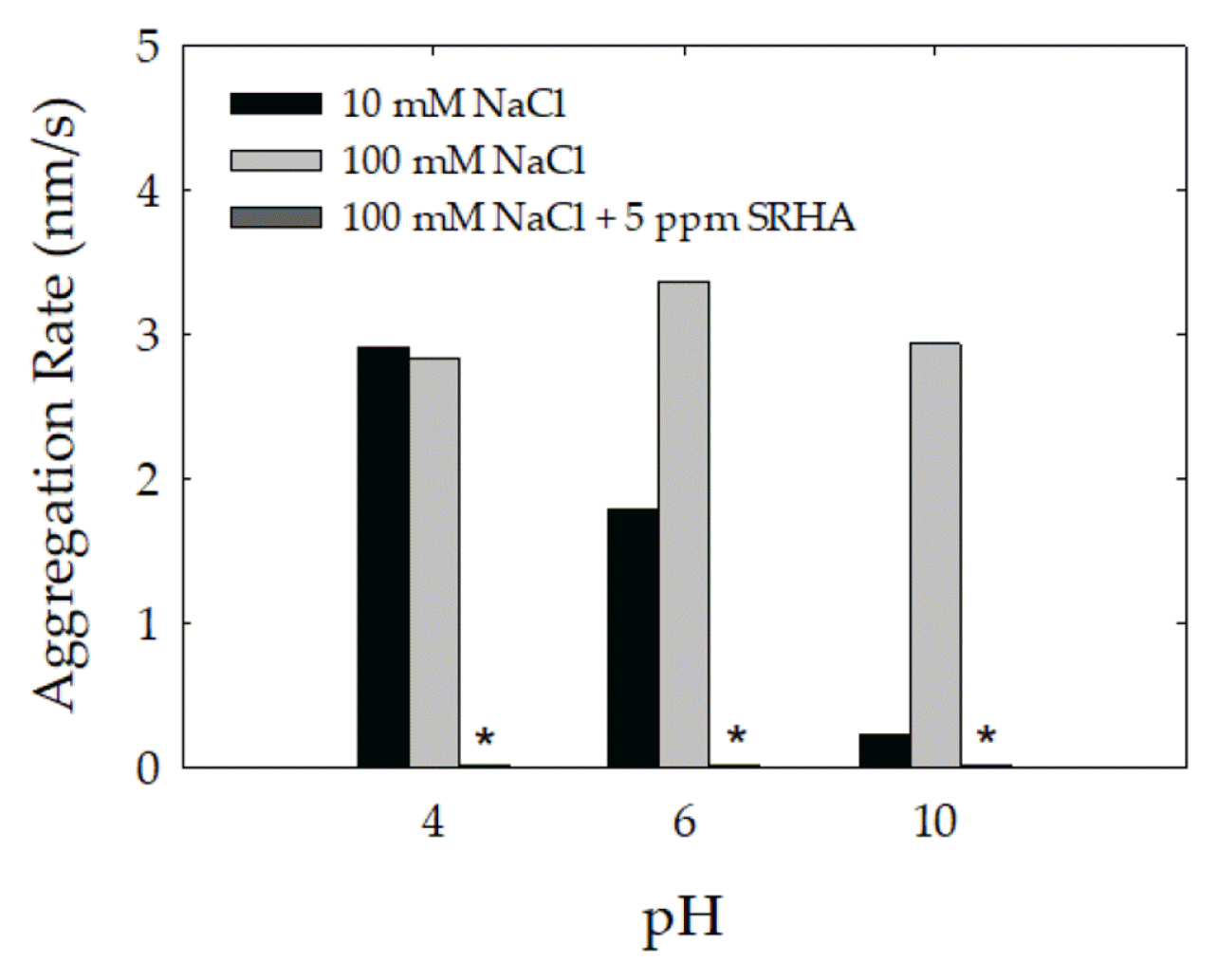
To investigate the NOM on the heteroaggregation behavior of CeO2, the aggregation of CeO2 in systems with montmorillonite precoated with SRHA was examined. When the effect of SRHA concentration on aggregation of montmorillonite was tested up to 10 ppm, the aggregation did not occur above 5 ppm (data not shown). The EPM values of montmorillonite precoated with SRHA exhibited a decrease of about 1 × 10−8 m2/Vs (Fig. S3(a)). This suggests the negative charge of the adsorbed SRHA was sufficient in magnitude to shift the EPMs to lower values. This was consistent with observations by Tombácz [42]. The hydrodynamic sizes of montmorillonite precoated with SRHA was remained stable over the range of pH (Fig. S3(b)). In the absence of SRHA at 10 mM NaCl, the heteroaggregation rate decreased with increasing pH reflecting the CCC values of 5.6, 23.3 and 65.9 mM NaCl at pH 4, 6 and 10, respectively (Fig. 5). At a higher NaCl concentration of 100 mM NaCl, the heteroaggregation rate was consistently high, reflecting that this concentration exceeds the CCC value of the mixture. In the presence of SRHA, however, the heteroaggregation rate sharply decreased at 100 mM NaCl to less than 0.1 nm/s (Fig. 5). Since extremely low aggregation kinetics was observed even at high NaCl concentrations (over 0.1 M NaCl), the attachment efficiency profiles were not produced with NaCl in the presence of SRHA. Both particles in the presence of SRHA are highly negatively charged, preventing their homo- and hetero-aggregation due to the formation of electrostatic and steric repulsion. The stabilizing effect of NOM has also been reported in other studies [14, 17, 27–29, 31, 33, 34]. For example, according to Wang et al. [34], the CCC of the TiO2-montmorillonite in the presence of NOM increased to 312 mM NaCl compared to that (40 mM NaCl) of the mixture in the absence of NOM. For this reason, the effect of natural clay colloids on stability of nanoparticles in aquatic environment depends considerably on the absence/presence of NOM.
4. Conclusions
The heteroaggregation between CeO2 nanoparticles and montmorillonite clay minerals were significantly affected by pH and NOM. In particular, at low pH, the negatively charged montmorillonite significantly reduced the stability of positively charged CeO2 nanoparticle via heteroaggregation behavior of oppositely charged particles. On the other hand, the presence of NOM greatly enhanced the stability of the montmorillonite-CeO2 mixture due to increased electrostatic repulsion and steric hindrance. These results provide a basis for predicting the environmental fate and transport of nanoparticles in aquatic environments. However, further investigations are needed to fully elucidate the combined effects that multiple environmental factors may have on the fate and behavior of nanoparticles.
Supplementary Information
Acknowledgments
This research was supported by a fund (No. 2019R1A2C1089726) by the National Research Foundation of Korea (NRF) and by a fund (No. RE202101055) by the Korea Environment Industry & Technology Institute (KEITI).
Notes
Author Contributions
S.B. (Researcher) conducted the experiments, performed the analyses, and wrote the manuscript. J.J.L. (Professor) reviewed and edited the manuscript. Y.H. (Principal Researcher) interpreted the data and edited the manuscript. All authors have read and agreed to the published version of the manuscript.