Advancement in recycling waste tire activated carbon to potential adsorbents
Article information
Abstract
Waste tires have been identified as one of the contributors to environmental problems and the issue of inadequate landfill spaces. The lack of consistent and systematic approaches such as specific regulations/laws or mechanisms of waste management to waste tires, limited application of technology for recycling waste tires and lack of awareness on the impacts of waste tires problem, make waste tires a source of environmental pollution. Various researches have been conducted on recycling waste tires into polymer bends, and materials to harden concretes, fuels and adsorbent. Researchers suggested that pyrolysis is the current trend of recycling waste tire to harvest the saleable pyrolysis oil and the recycled carbon black. Therefore, this review attempts to compile relevant knowledge about the potential of adsorbent derived from waste tires to be applied in the removal of various types of pollutants like heavy metals, organic pollutants, dye and air/gaseous pollutant. Studies were carried out on revealing the properties and the characteristics of activated carbon derived from waste tire as effective adsorbent which influence the application performance at liquid or gas phase. In addition, the challenges in the production of activated carbon derived from waste tire were discussed.
1. Introduction
In the new global economy, the disposal of waste tires has become a crucial pressing issue as it contributes to the adverse environmental impacts. Recent researchers have pointed out that the passenger vehicles, trucks, motorcycles and bicycles are the main contributors to waste tire disposal [1]. The waste tire disposal, with current annual worldwide disposal of about one billion scrap tires, is expected to progressively increase as approximately 20 million waste tires are disposed annually [2]. Since waste tires cannot be degraded and contribute to the issue of insufficient landfill spaces, the conventional method of waste tire disposal could be a critical environmental concern [3]. Inappropriate treatment of the disposed waste rubber tire can cause many major environmental and health issues. Based to previous research, inappropriate disposal can cause water pipes clogging which can lead to flood risk. Besides that, it also brings the risk of fire, air pollution from the toxic fumes, which contain pollutants such as carbon and sulfur, and also water contamination from the release of oil [4]. According to the research, ~57,391 tonnes of waste tires are expected in the leading production countries like Malaysia annually [4] and the ultimate fate of these waste tires to be placed in the landfills. This could deplete the space in the landfill and causing the issue of inadequate landfill spaces for the domestic wastes from the residents [5]. Hence, the wasted tires shall be prohibited from the landfill to conserve the landfill spaces.
By referring the hierarchy in the waste management, recycling and recovery of these synthetic materials are seem to be the most favour route by most of the countries [6, 7]. Researchers have claim that the waste tires are the energy sources with 30–40 MJ/kg that can be used exclusively to support the combustion plant [1, 8]. Czajczyńska et al. [8] have discovered that the whole waste tires can be employed in the cement plant to overcome the combusting high temperature of the plant at 1,200°C. Most recent research shown that waste tires can be recycled by shredding or grinding the tires for producing rubber chips. Several researches have used rubber chips for concrete application, another common product produced from rubber chips such as noise barriers, floatation devices, mats, roofing materials, sports track, and so on [4].
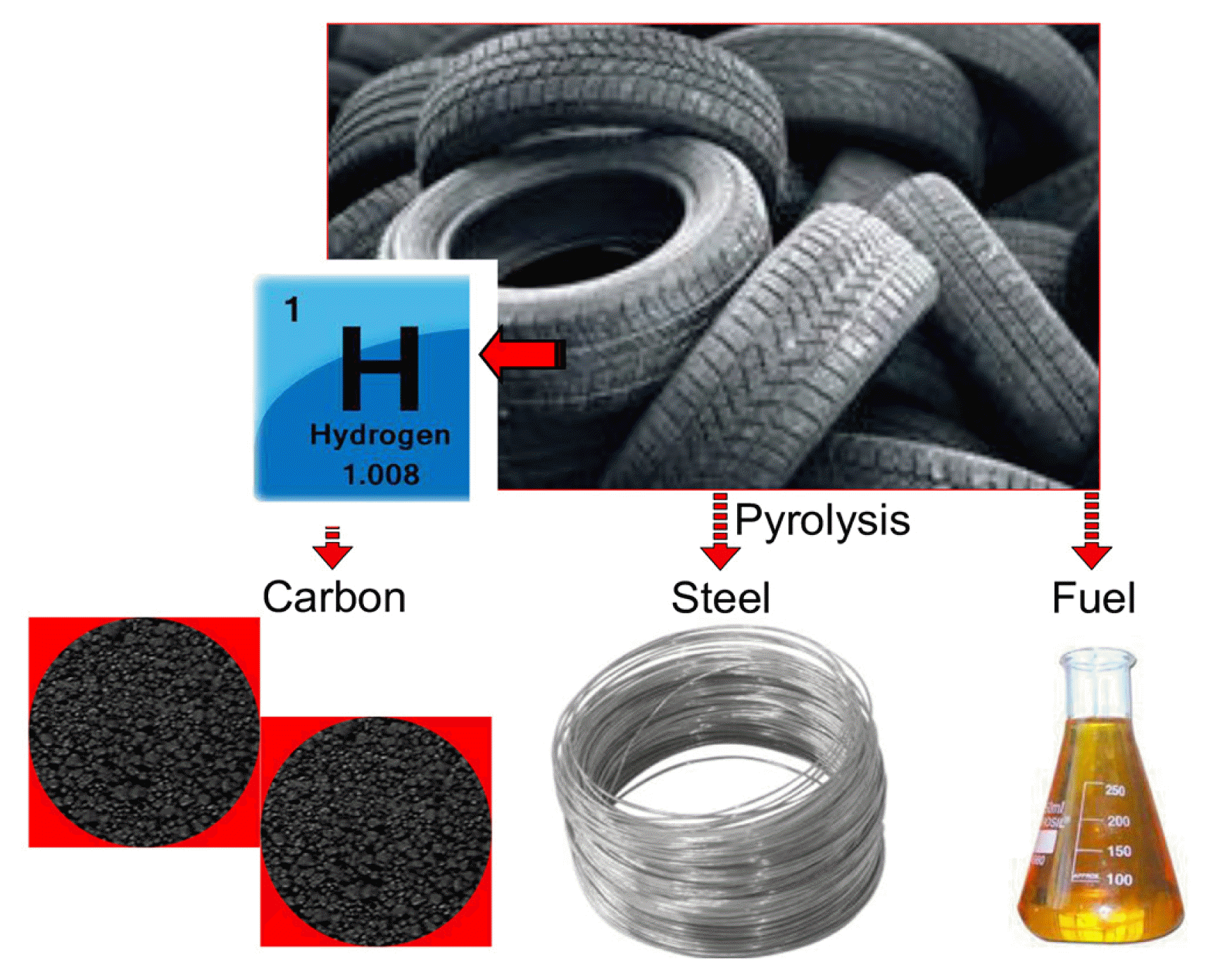
Recent literature offers the contradictory findings about the pyrolysis as the best technology in recovering the wasted tires into the valuable resources for the industrial activities [9] (Fig. 1). Pyrolysis of wasted tires could transform the used tires into gas, oil, steel and recycle carbon black waste (rCB) [10–12]. The common characteristic of the carbon black (CB) is the fluffy and fairly fine traits which provide a comparatively large total surface area [13]. However, CB from the pyrolytic process is not recommended to reuse as a pure CB for the downstream applications including the tire manufacturing industry, as the presence of the inorganic minerals in rCB would downgrade the products superiority, which originally made of pure CB [9].
The generation of rCB as solid waste has contributed to the escalating interest of researchers in the exploration of its sorption efficiency [14, 15] thermal and physiochemical properties [11, 16] as well as the beneficiation. Fazara et al. [11] has carried out a research and claimed that rCB has a higher specific surface area and oil adsorption number compared to the standard black. Besides that, the particle sizes of rCB is not distributed consistently (heterogeneous) and there is greater value of fines content. By raising the data from the previous researches, several studies have been done by applying the rCB as adsorbents with the modifications in the removal of organics [17–20] and inorganics from the aqueous solution [21–26]. This review focused on the production of the carbons from the waste tire and the potential applications of rCB in industrial sectors.
This review focused on the potential applications of carbon-based material derived from waste tire for the removal of pollutant in liquid and air. To the best of our knowledge there is a lack of research in the open literature focusing on the conversion of waste tires to carbon-based products for gas separation using the pyrolysis technique. Most of the studies so far emphasized on the potential of waste tires as adsorbent in the water and wastewater applications. This is a major research gap in this important and emerging technology, especially the application of pyrolysis technique in gas separation. The findings obtained can be related to recycling of waste into adsorbent and serve as a useful guideline to enhance the process so that it becomes more economically efficient for the removal of liquid and gas phase applications. This review article is relevant to the sustainable development goals on sustainable consumption and production/waste management (SDG 12). The review will have high impact on environment protection and waste management assessment. This review also intends to provide future researchers with the latest research findings, limitations and developments in the carbon-based material from waste tires. This article is structured as follow: After the Introduction in section 1, section 2 concentrates on the comparison of various pyrolysis techniques for carbon-based material production, followed by Section 3 describing the properties and characteristics of waste tires adsorbent. Section 4 presents the potential applications. Section 5 discuss on the challenges of activated carbon production from waste tires. Lastly, the conclusion and future perspectives are detailed out in section 6.
2. Uses of Waste Tire in Industry
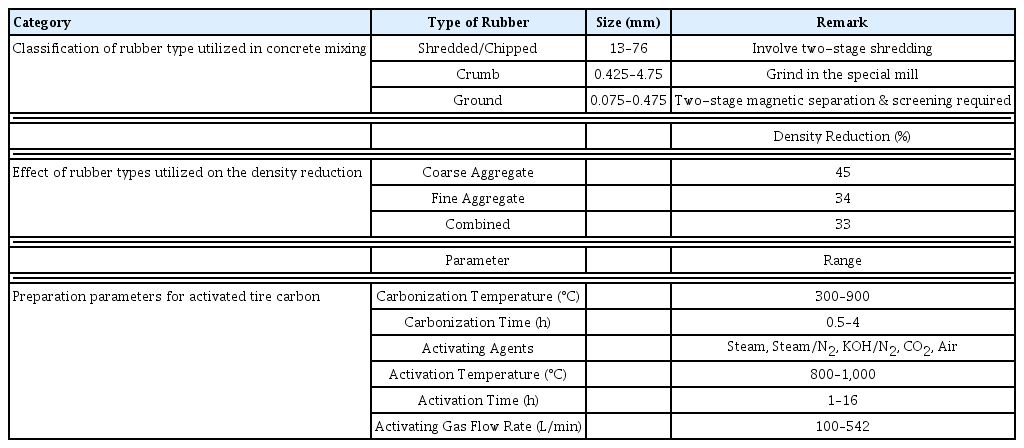
Recently, there have been increasing interests in recycling the waste tires in industry and waste tires had been employed in the polymer blend. According to Ramarad et al. [27], blending of waste tire and the polymer could help in reducing the price of polymer synthesis. Yet, the main issue faced in blending waste tires into the polymer blend is the waste tires would degrade the mechanical properties as the waste tires are not bond perfectly with the virgin matrix and modification technologies on the waste tires are needed. Apart from the polymer blend, researchers had also applied waste tire in the concrete. In 2016, Thomas & Gupta [28] discussed two types of cement which can be mixed with the waste tire, they are fresh cement and hardened concrete with the size-based classification (Table 1). In another study, Kashani et al. [29] reported type of rubber, size of particles and rubber contents significantly effects water absorption and permeability in concrete. The study also observed bigger rubber particles, small rubber particles fill the spaces among natural aggregates and thereby reduce permeability. Moreover, recycled rubber shreds/crumbs have potential to improve the mechanical properties such as tensile and compressive strength. The compressive strength decreased with increasing rubber crumb content [30].
Moreover, the workability of the fresh concrete decreases as the amount of crumb rubber grades and proportion added in the concrete increases. The poor inter-particle friction between rubber and other constituent is believed to the main factor contributing to the reduction of the workability of the fresh concrete. Previous researchers had explained that this phenomenon is caused by the high-water absorption of rubber particles within the concrete and further causing a reduction of slump of the rubberized concrete. The researchers also claimed that the rubberized concrete had a lighter density. They showed that the density of the concrete is reduced by 2–11% depends on the size of rubber used in the rubberized concrete compared to unrubberized concrete (Table 1)
For hardened concrete, Thomas & Gupta [28] had found that the compressive strength of the concrete decreased compared to the unrubberized concrete. This was further supported by Sofi [31] by pointed out that the compressive strength of the concrete decrease with an increasing percentage of crumb rubber mixed in the concrete. However, there were a few studies convinced that the compressive strength of the concrete was increased when tire rubber ash is utilized in the concrete mixing. Hence, Thomas & Gupta [28] concluded that the mixing of the crumb rubber shall not exceed 20% in order to preserve the compressive strength properties.
Waste tires had also been utilized as fuel in the cement industry [8], can be employed as energy source for the incinerator for the cement industry as it contains > 80 wt% of carbon contents, heating values of 30–40 MJ/kg as well as low moisture content compared to other solid waste. In addition, Hita et al. [1] had summarized the plants that were potential in utilizing waste tires as energy source.
The applications of waste tire rubber in the form of aggregates or binder in construction industry materials is advantageous includes lowering the CO2 emissions and increasing the greenness of the environment [32, 33]. He et al. [34] modified the surface properties of crumb waste tire rubber by impregnating the raw material with sodium hydroxide (NaOH) solution, potassium permanganate (KMnO4) solution and saturated sodium bisulfite solution (NaHSO3), respectively and observed that a large number of hydroxyl, carbonyl and sulfonate groups were introduced on the surface of the waste tire. Moreover, the cement containing the modified waste tires control the rate of compressive strength decrement, whereby the compressive strength decreased by 30%, contrast to the untreated waste tire which decreased to 52%. Fraile-Garcia et al. [35] investigated the thermal properties of light concrete construction elements produced using rubber particles from end-of-life tires. They reported that by adding scrap rubber into the composition of concrete will increase the energy and thermal strengths and recommended to be applied in making create material. In a similar study, Chaikaew et al. [36] prepared recycled crumb rubber particles to make concrete material. In order to enhance the mechanical properties of concrete pedestrian blocks, they mixed the waste crumb rubber with short steel fibres. They reported that by adding steel fibre into the rubber particle composition, the resistance to water absorption of blocks increase significantly.
Furthermore, several researchers have explored the influence of recycled waste tire in soil properties. For example, Bekhiti et al. [37] studied the beneficial use of waste tire rubber fiber mix with cement content to assess the strength of the stabilized soil. They discussed the effect of dry density, swelling behavior, unconfined compressive strength (UCS), and ductility behavior. The results showed that dry density decreases when waste tire rubber fiber ratio increases. Waste tire rubber fiber helps to reduce dry density and enhance its compressive strength and soil stability. They found that swell potentials and swelling pressure decrease slowly with increasing waste tire rubber fiber ratio. This parameter is important for the soil, where the focus of the study is to reduce the swell potential of the soil to remove damaging effects due to excessive heave and swell pressures, especially on geotechnical engineering applications. Therefore, waste tire rubber fibers have a beneficial effect when used as support materials to enhance the strength parameters, mitigating the swelling properties in soil. It was proven that waste tire rubber fiber can improve the UCS parameters. The obtained results also showed that ductility increases with increasing waste tire rubber fiber ratio. Similarly, Shukla et al. [38] conducted a study on the effect of waste tire fibres on swelling behavior and proved that waste rubber fibers successfully improved swelling behavior of the problematic soils.
Liu et al. [39] performed a review study on the application of recycled waste tire in soil and highlighted on compression behavior, shear properties, dynamic properties, thermal properties, hydraulic properties, and morphology characteristics. The use of different types of tire waste such as fibers, shreds, crumbles, particles, and chips can have an outstanding effect on the properties of soils. For example, waste tire shreds are suitable for use to enhance soil properties due to having low density, high shear strength, high hydraulic conductivity, and low thermal conductivity. Moreover, the effect of the particle size of tire waste influences the compression behavior. The compressibility of large size tire shreds was higher than that of the small size tire shreds [40, 41]. For shear properties, the shear strength significantly increases when the waste tire ratio in soil materials increases. There are very limited reports are available in literature on the effect of rubber mixture on shear strength parameters for enhancing soil strength and stability. For the dynamic properties, there is limited information reported in the literature on this parameter. Using rubber-soil mixture will help to increase the shear modulus damping ratio, thus improving dynamic strength [42]. There are few studies reported on the parameters of thermal properties. The combination of waste tire fibers or chips with soil can provide better thermal conductivity and hydraulic conductivity. The rubber-soil mixtures showed good thermal insulation, low thermal conductivity, and high hydraulic conductivity [39]. The surface morphology characteristics of rubber-soil mixtures also play an important role in stability performance. The surface morphology can be determined using Scanning Electron Microscopy (SEM) analysis.
3. Pyrolysis
The most recommended method for recycling the waste tires by several researchers was pyrolysis and would produce gas, liquid and solid fractions (Fig. 2). The products produced from pyrolysis were utilized fully in recent industries. The pyrolytic oil contains high calorific values can be recovered and used as alternative fuel for the transportation industry. The presence of the valuable chemicals such as d-limonene within the pyrolytic oil had also draw the attention of the researchers in investigating the values of the pyrolytic oil. However, the high sulphur content, heavy molecules and high concentration of aromatics compounds were the major drawbacks of the pyrolytic oil as these compounds were unacceptable in the environmental perspective [36]. The general pyrolysis process can be divided into three main types (such as flash, fast, and slow pyrolysis) and each type have different reaction duration, heating rate, and main product yield. For flash and fast pyrolysis processes, there are operated at high heating rate, at a medium temperature range, and shorter reaction time. This method produces a minor yield of solid char materials and high yield of fuel oil. Moreover, for slow pyrolysis, this method performed at low heating rate, at a low temperature range, and longer reaction time. This method produces a high yield of solid char materials [43]
3.1. Solid Pyrolysis
The solid waste (char) produced from the waste tire pyrolysis has no further application in current industry. Hence, researchers were in the midst of exploring the potential of the char as useful adsorbents in the wastewater industry. The adsorbents were mainly produced from the char and the original waste tires. There were several ways in formulating the waste tires into the carbon as well as the activation methods are discussed. The previous methods used for preparing the waste tires into adsorbent are summarized in Fig. 2(c).
Mui et al. [44] summarizes that the physical activation of waste tires commonly including utilization of CO2 or steam. Generally, the preparation of the adsorbent from waste tires can be performed in two steps as follows (Thermal pyrolysis at low temperature (400–700°C) and activation at 800–1,000°C). The thermal pyrolysis is performed under the helium or nitrogen atmosphere attempting to break down the cross-linkage within the waste tire sample [44, 45]. There is a prominent preparation method is highlighted by Mui et al. [44] as this method demonstrates an adsorbent with SBET of 1,260 m2/g. The pyrolysis and activation of the waste tire is carried out in helium environment with steam at 900°C for 1 h. However, the carbon yield for this method is only 9 % from the initial mass.
Demineralization of the waste tire sample can also help in developing the micropores and mesopores. Acid treatment using HCl solution is essential to remove the inert mineral matter, blocking the pore structure of pyrolyzed waste tire sample. A study has shown that acid treatment of the sample by using 1 M HCl solution for 24 h at room temperature before activation under steam/nitrogen (77: 23, v/v) atmosphere contribute to a specific area of 1,119 m2 and mesopore volume of 1.62 cm3/g.
3.2. Gas Pyrolysis
To produce char or activated carbon from waste tires by pyrolysis, there are two stages that need to be carried out. The first step is heating the waste tires in an inert atmosphere (pyrolysis), which produce a yield of char, oil and gas depends on the pyrolysis temperature, heating rate and duration of the pyrolysis process. The second stage is to activate or modify char material with physical or chemical activation to enhance surface properties of activated carbon material. The physical activation normally uses CO2 and/or steam. For chemical activation, acid (such as sulfuric acid, nitric acid) and/or alkali (such as potassium hydroxide and sodium hydroxide) are usually used as an activating agent [46]. This section will only discuss gas pyrolysis or physical activation. Activation of carbon-based material with CO2 will help in increasing the surface area of activated carbon. Mui et al. [44] found that steam is a potential activating agent for preparing activated tire carbon. This study has revealed that tire chars present higher capacity with steam than with CO2. It was proved that steam activation can cover a surface area up to 1,000 m2/g. On the other hand, Betancur et al. [46] performed a study to produce activated carbon from waste tire using CO2 activation. The benefit of physical activation is that it is a simple and low-cost process and can produce a high surface area. To obtain activated carbons with high surface area and porosity, they investigated the effect of CO2 flow rate, heating temperature, and reaction time. They found that heating temperature and the reaction time showed a significant effect in porosity development. The highest surface area of 414 m2/g was obtained at 900°C of temperature, 150 mL/min of CO2 flow rate and 180 min of treatment time. Similarly, Choi et al. [47] studied the potential of converting waste tire rubber into activated carbon through pyrolysis with CO2 activation. Pyrolysis was performed at a temperature of 950 °C using CO2 activation and at 3 h. The highest surface area was 437 m2/g. Activated chars formed using CO2 activation had high surface areas and porosity. To produce a large surface area of char using steam or CO2 activation, the char has to be heated at a high temperature (> 800°C) to give a successful performance. Steam or CO2 activation can enhance porosity development of the produced char and can produce a high surface area of up to 1,000 m2/g [48]. In their latest study, González-González et al. [49] used a combination of chemical activation and physical activation to produce high char yield using pyrolysis. They produced 52% of char yield from waste tire. The highest surface area was 339 m2/g and with an average pore size of 3.6 nm.
3.3. Carbon Production from Waste Tire
Carbons can be effectively developed through both chemical and physical activations on the waste tire crumbs or char produced from the waste tire pyrolysis. Only with the physical activation manipulated by researchers was somewhat longer than the methods which utilized both chemical and physical activation processes. Several researches have implemented this technique [44, 45] and overall have concluded that physical activation take longer time than chemical and physical activation process where the SBET of the activated carbons mostly found in the range of 200 to 814 m2/g.
3.4. Pyrolysis by Microwave Activation
In previous years, the microwave activation had emerged and became one of the most operative methods in high total surface area AC production. Gupta et al. [19] have recently developed a study for the microwave activation on the pyrolyzed char. The pyrolyzed char was primarily impregnated with KOH, at the KOH to char ratio of 1.5. The resulting carbon was the microwaved at 600 W for 10 min. The SBET of the WT-AC produced by using this method is comparatively high at 1,802 m2/g which is the highest value among the previous studies reviewed.
Precursors of the AC have been identified as a major contributing factor for the quality of AC yielding [50]. The precursor used in the past studies can be mainly categorized into two: tire crumbs and pyrolyzed char. By comparing the activation results from previous studies, the tire crumbs were slightly inferior to the pyrolyzed char as precursor for the AC production. Dimpe et al. [51], used tire crumbs as precursor for production of the WT-AC. The carbon was produced by chemical impregnation of the crumbs in 10 wt% HNO3 followed by thermal activation at 900°C for 2 h. The resulting WT-AC had demonstrated SBET of 397 m2/g, which is lower than the carbon produced by Saleh et al. [52], they employed char as a precursor. The SBET of the WT-AC produced was 493 m2/g which was chemically impregnated by 4 M HNO3 followed by thermal activation at 900°C for 5 h. By comparing both works, the pyrolyzed char can be said to be a better precursor that can be employed for the AC yielding process. However, both studies had kind of different process conditions. Hence, a thorough investigation of the consequence of precursor used on the capability of spawning high-quality WT-AC is required.
By the reason of the poor performance of WT-AC produced in the past research, the WT-AC produced can be blended with the other materials to boost the performance of the carbon. This method is believed to yield a comparable characteristic of AC to the commercial AC in the recent market. A work has been conducted by Acevedo et al. [53] on the production of high-performance AC. In their works, the blend of waste tire and coal are activated and the total surface area of the resulting product is found out to be increase to about 840–1,125 m2/g which is proportionate with the commercial AC. The production of the activated carbons derived from the waste tires in the previous studies are summarized in Table 2.
4. Properties and Characteristics
4.1. Surface Area
Researchers identified that the shape of the adsorption isotherm can provide qualitative information on the adsorption process and the extent of the surface area available to the adsorbate [63]. Fig. 3(a) shows the nitrogen adsorption isotherm of activated carbon that was prepared from waste tire. According to the results in figure, the isotherm exhibits a type IV indicating the presence of mesopores and H4 hysteresis loop, characteristic of a porous carbon containing slit-shaped pores.

Characteristics of waste tire activated carbon. (a) Adsorption/desorption isotherms of nitrogen at 77 K of activated carbon (Adapted from: Acevedo et al. [53]); (b) FTIR spectrum of activated carbon prepared from used tire (Adapt from: Gupta et al. [49]). (c) SEM micrographs of activated carbon (a) x400 (b) x20,000 (Adapted from: Belgacem et al. [56])
Acevedo et al. [64] reported that carbon-based material from waste tires modified with acid activation, showed insignificant effect on the textural characteristics of the produced adsorbent. Only slight increase in surface area were detected, which may be due to the fact that the pore entrances were blocked by ash. As reported by Saleh et al. [65] a fast adsorption rate of methyl orange obtained within 30 min due to the large surface area of adsorbent (465 m2/g) resulted in the increase of the number of active sites on the surface of adsorbent which increases the removal efficiency. Similarly, Alexandre-Franco et al. [66] stated that chemical treatment and heat-chemical treatments of used tire rubber do not significantly affect their porosity or decrease it. However, significant porosity developments are achieved only when the used tire rubber is heat-treated using steam activation.
Siddiqui et al. [67] investigated the potential of waste rubber tire as effective adsorbent for nickel ion removal from aqueous solution. The activated carbon was produced by physical and chemical activation method. The surface area of the activated carbon was measured to be 465 m2/g. This large surface area of the activated carbon could play a vital role in enhancing the removal of Ni(II). Mashile et al. [68] prepared a magnetic waste tire activated carbon-chitosan (MWTACC) composite as adsorbent for the removal of organic pollutants. The BET surface area of MWTAC was 1387 m2/g. The results showed that the MWTAC surface area increased with the addition of magnetic nanoparticles. However, after the combination of chitosan and MWTAC, few pores were found clogged. Thus, the BET surface area and pore volume decreased.
4.2. Surface Functional Group
Fourier-transform infrared (FTIR) spectroscopy is employed as a powerful tool and applied for qualitative and quantitative identification of chemical species or functional groups located on the activated carbon surface with the assistance of infrared spectra shone on the sample. Gupta et al. [59] analyze the FTIR spectrum of activated carbon prepared from used tire as shown in Fig. 3(b). A large absorption band around 3,400 was presents which corresponded to the stretching vibration of OH groups that can come from phenol groups that present on the surface or some adsorbed water. While a peak at 2,900 shows −CH stretching vibration in methyl group. In accordance to the results which is the present of weak peak at 2,845 can be analyses as -O-stretching vibration in aldehyde group. A peak at 1,620 can represents C–O stretching of carboxyl or carbonyl groups.
Saleh et al. [65] studied the FTIR spectrum of the tire-derived activated carbon using KBr pellets over the range 4,000–400 cm−1. A strong band around 1,180 cm−1 which corresponded to the carbonyl band to an ester is confirmed present on the surface. The peak at 1,580 cm−1 is attributed to C=C double bonds. The peak at 1,710 cm−1 and 1,650 cm−1 are presents the vibrations of carboxyl and carbonyl in acidic oxygen surface groups. The peak at 3,400 cm−1 is ascribed to stretching (O-H) vibration in hydroxyl groups. The peak at 2,350 cm−1 is ascribed as the alkyne group. Babiker et al. [69] used modified nano-waste tire rubber for investigating the removal of boron by adsorption method. The FTIR peaks of the waste tire rubber samples at 3,037 cm−1 were associated to the polyisoprene vibrations. The presence of CH symmetrical stretching was observed at 2,917 cm−1. A large absorption band was detected at peak 779 cm−1 which attributed to the functional group. They reported that the two peaks between 1,380 cm−1 and 160 cm−1 are confirmed formation of new peaks due to acid activation treatment. Thus, enhancing the surface properties of the carbon-based material will increase adsorption capacity.
4.3. Morphology
Scanning electron microscopy (SEM) was used to examine surface morphology of activated carbon. Belgacem et al. [56] reported that the surface of WT-AC (Fig. 3(c), (d)) shows a heterogeneous distribution of grain and a well-developed porous structure with a small size pore. It also showed presence of cavities and rough texture which can be attributed to the evaporation of volatile compound due to the KOH reaction at activation temperature under nitrogen atmosphere. Babiker et al. [69] characterized the surface morphology of waste tire rubber sample using scanning electron microscopy (SEM). They showed that the untreated adsorbent presented morphology image with more homogeneity and less rough on the surface than modified adsorbents. While, the morphology image of the modified samples (activation with HNO3 and H2SO4) had a coarser surface and more pores on the surface compared to untreated sample. They concluded that the acid activation would help in enhancing the surface area of the produced samples. Similarly, Mashile et al. [68] observed the surface morphology of adsorbent and showed that a rough surface with more porous structure was detected on the surface of magnetic waste tire activated carbon (MWTAC). As reported by Karmacharya et al. [23] the morphology image of adsorbent showed pore become larger and massive after adsorption of Cr(VI). Furthermore, the surface texture of the adsorbent is quite agglomerated after adsorption of Mn(II).
4.4. X-ray Diffraction (XRD)
X-ray diffraction (XRD) analysis is a unique method to determine the crystallinity structure of a compound [70]. Mashile et al. [68] analyzed the XRD patterns of waste tire activated carbon, chitosan and magnetic waste tire activated carbon-chitosan (MWTACC). The results showed that the presence of Fe3O4 at peaks 2θ with the values of 30.1°, 35.4°, 43.1°, 53.4°, 56.9°, 62.5°. The XRD patterns observed at peaks 2θ=10° for pure chitosan and 2θ=20° due to crystalline structure. Karmacharya et al. [23] used waste tire rubber for the removal of Cr(VI) and Mn(II) ions from aqueous solution. They analyzed the XRD patterns of the produced sample and found broad and intense peaks at 2q=24°–25° and 42°–43° matching to amorphous structure. A similar observation was reported by Jusoh et al. [71] The intense peaks observed at 2θ=25.0° and 42.5° for waste tire adsorbent were associated to amorphous carbon and graphite structure. This confirmed that carbon present in waste tire plays an important role for the adsorption of pollutant. Islam et al. [72] synthesized waste tire rubber by impregnating the raw sample with acid solution and namely as modified waste tire rubber adsorbent (WTR-SO3H). The results showed that, two peaks at ~24.78° and 42.17° corresponding to (002) and (101) reflections of amorphous carbon.
5. Potential Applications
An effort taken by the researchers to ensure sustainable management of waste tires through several utilization methods such as reuse, recycling, recovery, and pyrolysis. Waste tires can be recycled and utilized in various applications such as [73–76]:
Use in civil application (as filler in road construction, as modifier for asphalt, as a filler in concrete)
Use as adsorbent in water treatment applications (for organic and inorganic removal)
Flue gas cleaning
Air pollution control applications
Recovery of oil spills
Energy storage (as fuel source to produce electricity for cement kilns, steam production, electrical energy
In this review, the waste tire-derived activated carbon has been focused on the applications of liquid and gas separation using the pyrolysis technique.
5.1. Liquid Phase
5.1.1 Organic pollutants adsorbent
WT-AC had been employed by previous researchers in the removal of the organic pollutants that can be found in the recent industry. The performance of the WT-AC in the past studies was persuading comparable removal efficiency as the commercial AC (Fig. 4).
Makrigianni et al. [18] utilized WT-AC prepared by impregnating the pyrolyzed char in 1-butanol: distilled water (1:5) solution and calcinated at 500°C for 1 h. The resulting carbon had demonstrated an increasing phenol adsorption of 6 mg/g (pyrolyzed char) to 51.92 mg/g. The author stated that it was considerably efficient adsorbent for phenol as the carbon showed the highest adsorption of phenol by comparing the results to the earlier researches performed. The adsorptions of the phenolic compounds on the WT-AC were also performed by Gupta et al. [21] The phenolic compounds that tested in their work were phenol and p-cresol. The carbon that utilized in their work was activated by consuming microwave energy at 600 W for 10 min. According to Gupta et al. [21] the adsorption of p-cresol (250 mg/g) on the microwaved WT-AC is higher than the phenol with only 100 mg/g. The author explained that the low adsorption of phenol was due to phenol’s high solubility and kinetic rate as its molecular structure is smaller compared to p-cresol. Apart from that, the author also declared that the microwaved WT-AC removal ability on both compounds is superior compared to the other material-derived AC. The other organic compounds such as aniline groups were studied by Gupta et al. [60]. It was found out that the overall performance of the carbons on the removal of the pollutants is as follow: RTACOX > RTAC > Commercial AC. The performance of the RTAC and commercial AC demonstrated the similar removal ability on the pollutants in the aqueous solution as follow: aniline > p-toulidine > p-anisdine > p-Cl-aniline. The removal percentage of the aniline by the commercial AC reported was 40.53% compared to the removal percentage of RTAC produced (76.14%). This exposed that the RTAC produced by only employing physical activation was superior enough in the adsorption of aniline.
5.1.2. Dye adsorbent
The performance of the AC is mostly determined by adsorption of the dyes by most of the researchers. The dyes that commonly used for the WT-AC adsorption are methylene blue [18] and methylene blue [20]. The adsorption of the methylene blue studied by Makrigianni et al. [18] shown a maximum MB adsorption of 65.8 mg/g for WT-AC. This carbon was chemically activated by 2 M HNO3 followed by the calcination process at 500°C. The author claimed that WT-AC produced had demonstrated a better performance on the MB adsorption compared to un-activated pyrolyzed char, which is originally 53.4 mg/g [62]. However, the performance of the WT-AC produced is incomparable with the commercial AC. Hence, the WT-AC shall be activated in another method to boost the MB adsorption.
Lin et al. [20] had conducted a study on ethylene blue (EB) adsorption on the WT-AC and the outcomes of the study were delighting. WT-AC resulted from the thermal activation at 900 °C for 4 hours with CO2 flow demonstrate SBET of 1048 m2/g. The optimum EB adsorption of the carbon was 323 mg/g which is higher than the other AC. WT-AC also performed the removal of the dyes in aqueous solution by adsorption in contrasting behavior. Acevedo et al. [64] had conducted a research by utilizing activated carbons prepared from wasted tires reinforcing fiber. In their research, they found out that the carbons demonstrated the eminent performance on the removal of Basic Astrazon Yellow 7GLL (AY) in higher pH while Reactive Rifafix Red 3BN (RR) in a lower pH. The main reason due to the distinctive behavior of the adsorption is the ionization of the compound.
Nogueira et al. [77] prepared effective adsorbents from waste tire rubber for remazol yellow dye removal from aqueous solution. The produced sample was characterized by relatively high ash content (12.9% wt), high fixed-carbon content (69.7% wt), a surface area of 69 m2/g, and total pore volume of 0.14 cm3/g. The results showed that adsorption isotherm was better fit for the Langmuir model and the maximum adsorption capacity was 11.9 mg/g. Mashile et al. [68] showed a relatively high removal efficiency up to 100% removal of parabens and high adsorption capacity of parabens as 85.9 mg/g and 90.0 mg/g for methylparaben and propylparaben, respectively using waste tire rubber as adsorbent. The produced sample in this study was claimed to follow pseudo-second-kinetic model. Zhang et al. [78] prepared porous carbons from heavy residue of tire pyrolysis oil for methylene blue removal. The produced porous carbon showed high adsorption capacity of 843.5 mg/g at 25°C and the adsorption equilibrium data fitted the Freundlich isotherm model well.
5.1.3. Heavy metals adsorbent
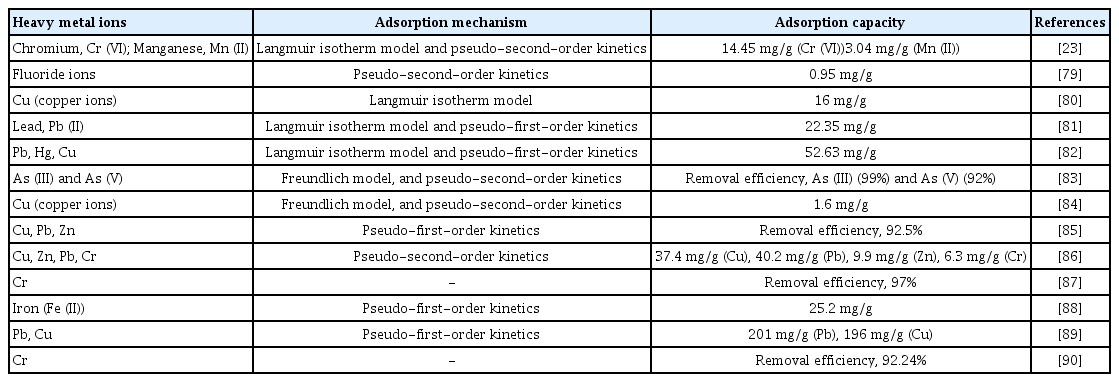
The inorganic pollutants were also employed by most researchers in the determination of the removal capability of WT-AC produced. The inorganic pollutants that were utilized in previous studies were common heavy metal ions (such as Cu2+, Zn2+, Pb2+, Cr3+, Mn2+, Hg+) that are present in recent wastewater industry. Among the heavy metals, the chromium ions and lead ions were the most-picked ions employed in previous studies due to their toxicity and diverse oxidation number. WT-AC will help in increasing surface area and porosity, thus increasing the adsorption capacity. In addition, important parameters such as pH, mass of adsorbent, contact time, initial concentration of metal ions and activating agents also influenced the removal efficiency and adsorption capacity. The applications of waste tire-based activated carbon to eliminate heavy metal ion from synthetic and wastewater treatment are summarized in Table 3.
5.2. Gas Phase
Waste tire derived carbon is potential in the removal of gas phase pollutants such as sulfur dioxide (SO2) and nitrogen oxides (NOx). According Nieto-Márquez et al. [91] the waste tire activated with KOH solution can be utilised in the removal of SO2 in the air. The waste tire activated with KOH at the mass ratio of 4:1 (KOH: tire weight) demonstrated the highest SO2 uptake with approximately 42.5 mg SO2/g carbon at 45°C. An identical research had also carried out by Brady et al. [92], regarding the adsorption kinetics on the SO2 removal. In their research, they found out that the steam-activated sample with total surface area of 1,000 m2/g is the most efficient WT-AC in SO2 removal. However, the rapid removal reaction had causing excessive heat generation throughout the SO2 removal process. Another research was also carried out by Al-Rahbi et al. [54] on the NOx uptake by utilizing activated waste tire carbon. The most eminent carbon in their work was the KOH activated waste tire at a mass ratio of 3:1 (KOH: char) and pyrolyzed under N2 atmosphere at 900°C yielded SBET of 621 m2/g. This KOH activated carbon demonstrates NO removal up to ~75% at 25°C. Tang et al. [93] prepared different types of municipal solid waste-based carbons for CO2 capture. The optimum conditions were at 25°C adsorption temperature, CO2 concentration of 14% CO2 and flow rate of 100 mL/min. They reported that the triple components of municipal solid waste-based carbons (pinewood, acrylic textile, and tire) showed the superior adsorption performance, which is 1.522 mmol/g. In a similar study, Krishnaiah et al. [94] conducted a study to modify waste tire derived activated carbon KOH to enhance CO2 adsorption capacity. They reported that CO2 adsorption was enhanced up to 238.7 mg/g at 30°C using impregnation ratio of 1.5 molar of KOH solution.
6. Challenges in Waste Tire Derived Activated Carbon Production
The major challenge of adsorbent production of waste tire is to increase surface area of activated carbon during activation process. Many studies of derivation of new activated carbon have been published with low surface area, which results in truncated adsorption capacity. In activation phase, the waste tire contacted with an activation gas such as steam, CO2, N2 and air at slower rate of heating with isothermal holding. During heating process, reaction between waste tire and activation gas can cause pore opened and enlarge existing pores. The disadvantage of the conventional slow rate heating is greater in energy consumption and increases duration of heating process. In addition, slow rate heating leads to low total pore volume and surface area and non-homogenous microstructure of waste tire activated carbon. The temperature used for heating process range of 500–975°C and majority of studies yield surface area of WT-AC less than 600 m2/g. One of the alternative techniques adapted for conventional heating process is microwave heating, but no study that used microwave technique in activation of waste tire has been published. It is recognized that using microwave heating to provide new challenges to develop more surface area of WT-AC, which results in good adsorption capacity.
The modification of WT-AC was carried out by the formation of different types of surface groups using various methods. The main purpose of surface modification is to increase adsorption capacities which yield in long lifespan of activated carbon. Future studies of surface modification can be applied sulfurization and impregnation methods to form new functional groups that favour pollutant’s uptake and enhance lifespan of activated carbon. The management of exhausted adsorbent is one of challenge in activated carbon treatment to ensure their sustainable application. Thus, the preferred method for regeneration of WT-AC should be considered for future research.
6.1. Environmental Impact of Waste Tire Derived Activated Carbon
The large amount of solid waste generated from tires is becoming a major environmental issue. This is due to improper waste management treatment. Waste tires have high contents of combustible composition and potential for valuable materials and energy resources [95]. Waste tires have a severe negative impact on the environment in terms of air, water, and soil pollution [96]. There are several issues should be addressed concerning waste tires including open dumping (requires large space in landfills), open burning (release harmful chemicals into the air), presence of disease vectors, such as insects, rodents or mosquitoes, leaching (soil contamination). The high composition of harmful chemicals (such as cyanhydric acid, hydrochloric acid, toluene, polycyclic aromatic hydrocarbons, polychlorinated biphenyls) and gaseous (such as carbon dioxide, carbon monoxide, nitrous oxide, nitric oxide, sulfur oxide) released through the combustion of tyres is a big concern [97].
As mentioned earlier, waste tire-derived activated carbon has produced high quality activated carbons and achieves high removal efficiency. The waste tire-derived activated carbons contained high concentrations of sulphur and zinc, as well as traces of other metals such as lead, cadmium, chromium and molybdenum. Thus, the leaching of some of these species may limit their use in various applications specifically in drinking or water treatment. However, this problem can be controlled by carried out leaching test to remove the inorganic species. For example, San Miguel et al. [98] performed a leaching test of inorganic species for waste tire-derived activated carbons at various pH values. The authors confirmed that the values of concentration levels of all species were below limit values of WHO drinking waters standard. In another study, Selbes et al. [99] investigated the leaching of selected inorganic and organics constituents from waste tire. They performed a systematic study of leaching test and suggested the best alternative solutions for managing of waste tires.
7. Conclusion and Future Perspectives
The conversion of waste material to valuable products could reduce the environmental pollution and disposal problem. The application of modified activated carbon from waste tire rubber has been widely studied at the laboratory scale for the removal of dye and heavy metals through the adsorption method. However, there is still diminutive information on a full study pertaining to the waste tire as adsorbent in the gas separation application to remove air pollutants. This would be useful in the development of a detailed techno-economic analysis of waste tire. In this review, a substantial number of relevant published articles of waste tired activated carbon for the removal of pollutant in liquid and air have been reviewed. Waste tire activated carbon has been found to be efficient in removing different type of organic, dyes and heavy metals from wastewater. Furthermore, waste tire activated carbon has been verified as good adsorbent to remove air pollutants such as CO2, SO2 and NOx. The chemical modification of surface waste tire activated carbon can increase the surface are, thereby, increasing adsorption capacity. In future research, the studies should be scaled-up to a large-scale using waste tire activated carbon to evaluate their performance in removing pollutants from wastewater or air. Pilot scale studies can also be used to complete the life cycle assessment of modified waste tire adsorbent to be compared with commercial sorbents including adsorbent stability and regeneration/reuse cycles. In addition, the cost of the precursor, final product of waste tire activated carbon and regeneration process is important to evaluate. If the waste tire activated carbon is not regenerated, the exhausted adsorbent is required handled as hazardous waste. However, cost comparison between the commercial carbon and porous-carbon adsorbents produced from waste rubber tires is crucial in order to evaluate their utility in adsorption process from the economic point of view. Still, more research efforts have to be made to significantly improve the properties and performance of waste rubber tire. Overall, waste tire activated carbon has significant advantages as alternative activated carbon for liquid and air pollution control and can minimize the waste volume produced from the disposed tires.
Acknowledgment
The authors acknowledged the financial support from the CRG-UTP-UniMAP grant (9023-00023) and Loreal-UNESCO FWIS 2020 grant (9008-00018).
Notes
Author Contributions
U.F.M.A (Associate Professor) visualized, wrote, reviewed and edited the original draft. F.H. (Ph.D) wrote and edited the manuscript. S.C.B.G (Associate Professor) wrote and edited the manuscript. M.K.A (Professor) reviewed and edited the manuscript. M.H.K (Ph.D) wrote and revised the manuscript. N.J. (Ph.D) reviewed and proofread the manuscript. N.I. (Associate Professor) reviewed and edited the manuscript. S.F.K.A (MSc) wrote and edited the manuscript.