Enhanced performance of constructed wetlands by incorporating magnetic Fe3O4 nanoparticles and the effects on the microbial community on the surface of the substrate
Article information
Abstract
Constructed wetlands (CWs) have been successfully employed to treat domestic sewage, while it is reported that inconsistent and low nutrient removal efficiency has been achieved. Due to the good performance in other treatment systems, magnetic Fe3O4 nanoparticles (Fe3O4 NPs) can provide a new pathway for the nutrient removal in CWs. In this study, the influences of Fe3O4 NPs on the nutrient removal and variation of microbial community were investigated. The CWs with Fe3O4 had better NH4+-N (ammonia nitrogen, 95.9%), NO3−-N (nitrate nitrogen, 83.8%), and TP (total phosphorus, 90.4%) removal percentages than that without Fe3O4. High-throughput sequencing analysis indicated that the microbial community structure could be changed by Fe3O4 NPs. For example, at class level, the major bacteria such as Betaproteobacteria, Gammaproteobacteria and Deltaproteobacteria were promoted by Fe3O4 NPs. On the other hand, at genus level, some abundant genera disappeared, while other abundant genera became dominant in CWs with Fe3O4 NPs, including Desulfuromonas (3.0%), Azospirillum (1.9%), Chryseolinea (1.5%), and Desulfomicrobium (1.8%). The latter genera essentially improved the microbial community involved in the nitrogen removal. Therefore, the CWs with Fe3O4 NPs showed higher NH4+-N and NO3−-N removal percentages than that without Fe3O4 NPs.
1. Introduction
Over the past few decades, excessive amounts of nitrogen and phosphorus from untreated wastewater caused serious ecological problems [1]. Especially, nitrogen compounds cause eutrophication in the water environment, resulting in bad water quality [2]. Therefore, effective wastewater management and treatment are critical for maintaining public health and environmental safety [3]. The scarcity of useable water necessitates sustainable wastewater treatment techniques, such as microbial fuel cell [4–6]. However, its upscaling is challenging primarily due to its capital expense and CWs are considered as an alternative to sewage treatment. [7–10]. It is reported that Constructed wetlands (CWs) have been successfully employed to treat domestic sewage for its easy operation and maintain [11, 12]. Consequently, the application of CWs for the purification of wastewater has been recently proposed as an alternative for the wastewater treatment and reuse [13–15].
The substrate plays a significant role in CWs, because can provide a suitable growth medium for plants and microorganism that play an important role in the removal of pollutants from wastewater. Some studies display that CWs with general media (e.g. sand and gravel) can meet the requirements for the reduction of organic matter and suspended solids [16]. However, it is also reported that inconsistent and low nutrient removal efficiency has been achieved [17]. Therefore, researchers have paid attention to other substrates that can be used to enhance the removal efficiency of nitrogen and organics [18]. Recently, magnetic Fe3O4 nanoparticles (Fe3O4 NPs) have been extensively applied to environmental remediation due to their small particle size, high surface area and good magnetism [19]. An appropriate amount of Fe3O4 NPs in CWs can improve biological activity. In addition, the magnetic property may provide some benefit for the microorganisms distribution and balancing the growth and death of microorganisms, which can lead to the improvement of pollutant treatment rate [20]. It has been reported that the TN removal efficiency (from 69 to 92%) can be significantly improved by the existed magnetic NPs [21–24]. Due to the good performance in other treatment systems, conventional substrates with Fe3O4 NPs may improve the performance of CWs, and the investigation can provide valuable information.
In CWs, microbial processes play a crucial role in the nutrient removal. However, most current studies on nutrient removal by CWs focused on the effects of different types of wetland configurations, plants and treatment strategies on the removal efficiency [25, 26]. Molecular biology techniques, including fluorescence in situ hybridization (FISH) and denaturing gradient gel electrophoresis (DGGE), have been used to monitor microbial community changes. However, these detection methods cannot obtain comprehensive information due to the insufficient sequences [27]. High-throughput 16S rRNA amplicon sequencing can help us better understand the microbial community structure and microbial diversity in CWs for the valuable information on bacterial community structures [28]. Therefore, Fe3O4 NPs can improve the performance of CWs by the mechanism, which can be effectively revealed.
In this study, the simulated domestic wastewater was treated in the CWs with and without Fe3O4 NPs. The removal efficiency of conventional wastewater quality and microbial parameters was investigated. The objectives of the present work were to (i) investigate the existence of Fe3O4 NPs on the performance of CWs, (ii) evaluate the influence of Fe3O4 NPs on microbial community structure and enzymatic activity. The investigations presented herein bring insights into the nutrient removal and transformation mechanisms from the aspects of microbes, and provide valuable information regarding the application of Fe3O4 NPs on the performance of CWs.
2. Materials and Methods
2.1. Construction of the CWs
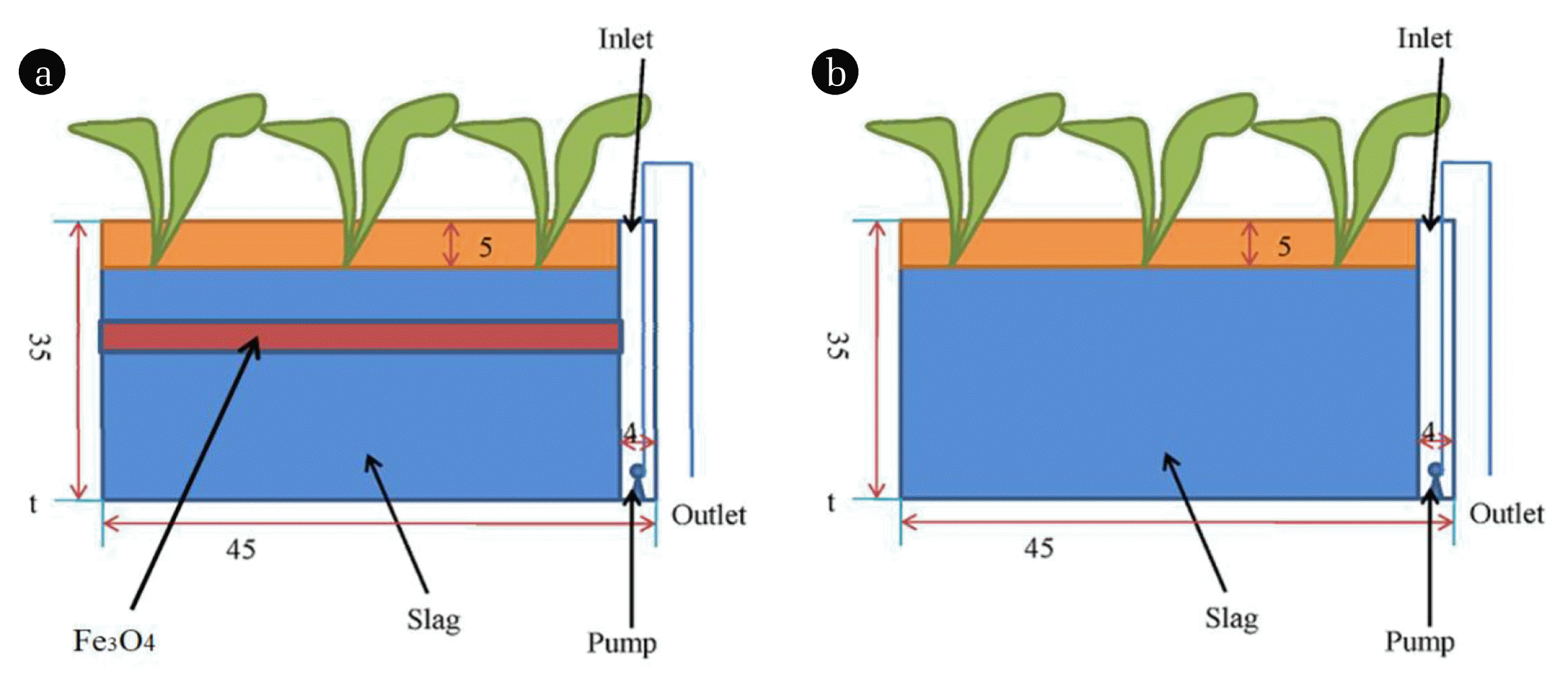
Parallel subsurface flow CWs were set up in the greenhouse at China Jiliang University, Hangzhou, China. The CWs system was constructed by polyvinyl butyral, and the length, width and height are 45, 30 and 35 cm, respectively, with a volume of 47 L. The slag (particle size: 10–15 mm, porosity 0.45 and thickness 30 cm) was chose as substrate (Fig. 1), and collected from a locally heated boiler and repeatedly cleaned with tap water. A 5-cm-deep layer of soil was covered on the top of the CWs and collected from the campus of China Jiliang University. In CWs with Fe3O4 NPs, 90 g Fe3O4 NPs (frequent strategy to lower the cost is the combination of a small number of Fe3O4 NPs and an excess of Fe3O4 NPs is a threat to microorganisms and plants in wetlands) was evenly distributed horizontally along the CW (at a distance of 20 cm from the bottom, because more plant roots and microbes concentrated at this distance) [22, 24]. One common type of plants Pontederia was planted in CWs (height 10 cm, depth 5 cm, and density 29.6 seedlings m−2), which could treat polluted urban river water with high removal efficiency of COD, TN and TP [29]. Tap water was used to enable the vegetation and microbial communities to adapt to their environment for the first two months. Three replicated mesocosms were established for each CW with and without Fe3O4 NPs.
2.2. Experimental Operation and Sample Collection
The synthetic domestic sewage was prepared by composing NH4Cl (18 g/m3), KNO3 (35 g/m3), KH2PO4 (6.5 g/m3), CaCl2 (1.2 g/m3), CH3COONa (65 g/m3) and some trace element ingredients (MgSO4 0.2 mg/L and FeCl3 0.2 mg/L etc.). The influent concentrations of NH4+-N, NO3−-N, TN, TP and COD were 4.11, 4.53, 9.05, 1.21, and 50.54 mg L−1, respectively. The sequencing batch feeding influent mode (operation time 6 days) was adopted. The inflow rate of domestic sewage was 2.2 L for each CW. The treated water samples from outlet were collected every day and stored in brown reagent bottle in the refrigerator before detection. The amount of water extracted from each CW was 100 mL per day. The removal performance of CWs was evaluated in terms of NH4+-N, NO3−-N, TN, TP and COD in the influent and effluent concentration. The removal percentages were calculated by the influent and effluent concentration of NH4+-N, NO3−-N, TN, TP and COD. NH4+-N (HJ 536-2009), NO3−-N (HJ/T 346-2007), TN (HJ 636-2012), TP (HJ 671-2013) and COD (GB 11914-89) were determined as previous study [30, 31]. During the operation, soil samples were also collected every day to investigate the microbial activity that was consist of five soil samples from four corner directions and the center of each CW. The soil samples were immediately collected in separated plastic Ziploc bags and stored in a freezer at 4°C. Urease activity was measured by incubating 5 g dry soil with 10 mL 10% urea solution and 20 ml citrate buffer (pH 6.7) for 24 h at 37°C. In addition, sucrase activity was measured by incubating 5 g dry samples with 15 mL of 8% sucrose solution, 5 mL phosphate buffer (pH 5.5) and 5 drops of toluene for 24 h at 37°C. Then, 1 mL of the supernatant was treated with 3 mL of 3,5-dinitrosalicylic acid for 5 min at 100°C. The urease activity and sucrase activity were spectrophotometrically determined at 578 nm and 508 nm, respectively. At the end of the experiment, substrate samples were also collected. A total of 5g substrate samples were captured from each CW and collected from four corner directions and the center of each CW. The substrate samples from CWs with and without Fe3O4 NPs were respectively combined into one sample for the analysis of microbial structure and sent to Lianchuan Biology Technology Co. Ltd., China.
2.3. High-throughput Sequencing Analysis
In this study, the high-throughput sequencing was used to analyze the microbial structure of CWs. The extraction steps for DNA were based on the methods described in previous studies [26, 32]. According to the manufacturer’s instructions, DNA was extracted from substrate with PowerSoil® DNA Isolation kit (MoBio Laboratories, Catalog No. 12888–50, USA). During the PCR amplification process, the V3 and V4 hypervariable regions of bacterial 16S rRNA were amplified. The first amplification PCR conditions were 95°C for 5 min and 27 cycles of three steps, 95°C for 30 s, 55°C for 30 s, and 72°C for 30 s, followed by a final step at 72°C for 10 min. The universal bacterial primers were 338F (5′-ACTCCTACGGGAGGCAGCAG-3′) and 806R (5′-GGACTACHV GGGTWTCTAAT-3′). The amplification process and the analysis of the pyrosequencing data were similar to previous studies [33–35]. The extraction and amplification of DNA samples were performed with an Illumina MiSeq PE300 platform (Hangzhou Lianchuan Biology Technology Co. Ltd., China). A similarity of 97% was used to illustrate the same operational taxonomic units (OTUs). The biodiversity of microbial communities in CWs was analyzed by OTUs, Shannon and Simpson estimators [36].
2.4. Statistical Analysis
According to the influent and effluent concentration of NH4+-N, NO3−-N, TN and COD, the removal efficiency of pollutants was calculated. The statistical difference of the mean removal efficiency and enzymatic activity values were investigated (The urease and sucrase activity were expressed as NH4+-N mg g−1 soil and mg glucose g−1 soil, respectively.) in CWs with and without Fe3O4 NPs. Meanwhile, the significance difference was calculated using ANOVA. Statistical analyses were performed using Origin 8.0 for Windows.
3. Results and Discussion
3.1. The Removal Performance of CWs
Fig. 2 shows the removal efficiency of nutrients and COD in CWs with and without Fe3O4 NPs. Overall, the nutrient and COD removal efficiency gradually increased with the operation time. CWs with Fe3O4 NPs achieved higher removal efficiency of NH4+-N, NO3−-N, TN, TP and COD. When the operation time was at 6th day, the removal efficiency of NO3−-N, TN and COD in CWs with Fe3O4 were 83.8, 91.2 and 90.1%, respectively, that were 14.6, 15.4 and 8.1% higher than those of CWs without Fe3O4 NPs. Previous study also proved that the presence of magnetic NPs increased TN removal efficiency (94.4%) compared with the control (80.3%) [21]. Fe3O4, known as a magnetic material, can produce magnetic field and be ionized into Fe (II) and Fe (III) and a certain concentration of Fe3+ can promote the growth of anammox bacteria [37–40]. In this study, Fe-anammox bacteria (such as, Desulfosporosinus) was increased by the existence of Fe3O4 NPs, which could explain why higher TN removal efficiency was achieved. In the reduction of TP, mesocosms with Fe3O4 NPs also exhibited significantly higher (p < 0.05) TP (90.4%) removal percentages than mesocosms without Fe3O4 NPs. Ma et al. [41] also proposed that Fe3O4 NPs could promote the phosphorus removal due to the dissolution of Fe ion from Fe3O4, which could be effective precipitants for phosphorus removal. Overall, the results demonstrated that Fe3O4 NPs could significantly improve the removal efficiency of NH4+-N and TP. The microbial growth and activity were accelerated, which could affect the release and uptake rate of PO43− in CWs.
3.2. Enzymatic Activity in CWs
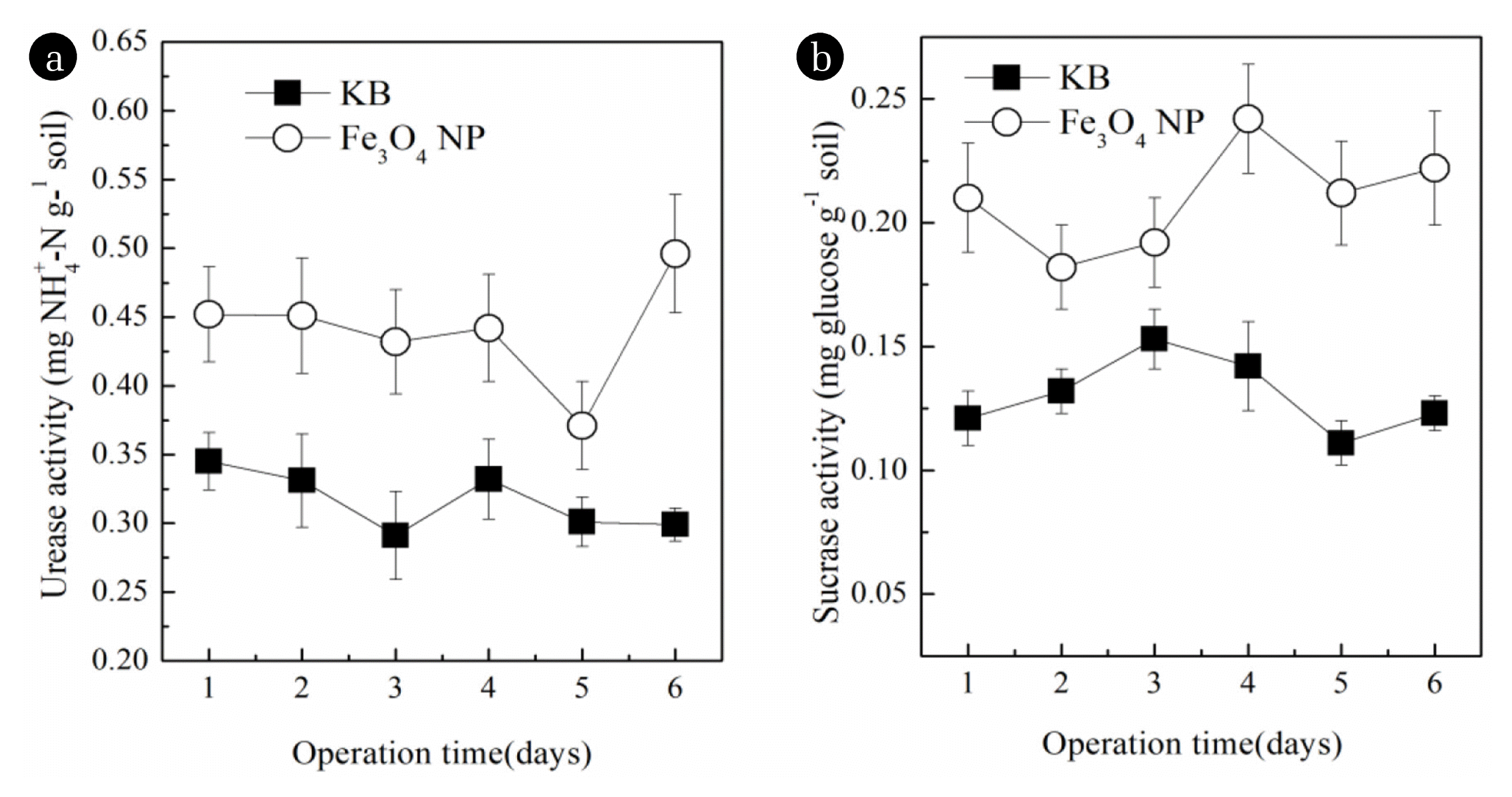
In this study, the enzymatic activity was quantified to compare the effects of Fe3O4 NPs on microbial activity in CWs (Fig. 3). The urease activities in CWs with Fe3O4 NPs were higher than those of CWs without Fe3O4, ranging from 0.37 to 0.50 and 0.29 to 0.35 mg NH+4-N g−1 soil, respectively. This observation might illuminate why CWs with Fe3O4 NPs had higher removal percentage of NH4+-N. He et al. [42]. also proposed that urease activities in Fe3O4 NPs treatments were significantly higher than those without Fe3O4 NPs. The sucrase activities in CWs with and without Fe3O4 NPs were ranged from 0.18 to 0.11 and 0.291 to 0.15 mg glucose g−1 soil, respectively. The results suggested that the Fe3O4 NPs also had effects on sucrase activities in CWs. Li et al. [43] also held the view that sucrase activity was positively correlated with the rate of C mineralization and accelerate COD removal, which might imply that the removal of organic matter was positively consistent with sucrase activity. Therefore, the results confirmed that Fe3O4 NPs played a certain role in NH4+-N and COD removal efficiency due to the changes in enzymatic activity in CWs.
3.3. Biodiversity and Distribution of Microorganisms in CWs
Table 1 presents the richness and evenness of microorganisms in the CWs. The total number of usable sequences for all the samples was 112310, and the number of operational taxonomic units (OTUs) was 8741 with a similarity level of 97%. Compared with CWs with Fe3O4 NPs, the samples collected from CWs without Fe3O4NPs yielded a higher OTU number. The richness of the microbial community was evaluated by Ebrahimi et al. [44]. In this study, Chao estimator indicated that the richness of microorganisms in CWs without Fe3O4 NPs was higher than that in CWs with Fe3O4 NPs, which was similar to OTUs. The Shannon indexes contain information about the diversity of the microbial structure [45]. The Shannon value of CWs without Fe3O4 NPs (10.13) was also higher than that of CWs with Fe3O4 NPs (9.97), which indicated that Fe3O4 NPs could reduce the bacterial diversity. A previous study also indicated that short-term exposure to magnetic NPs could result in acute toxicity to microorganisms [46]. Therefore, the richness of bacteria in the CWs with Fe3O4 NPs was much lower. On the contrary, some bacteria, such as denitrification, nitrification, or COD-reducing microorganisms, could thrive after long exposure to magnetic NPs [47]. Hence, the CWs with Fe3O4 NPs could still show better performance.
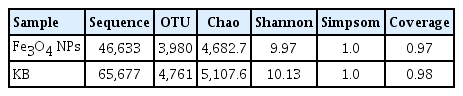
The Richness and Evenness of Bacterial Samples Collected from the CWs Incorporated with and without Fe3O4 NPs
The relative abundance of bacteria at the phylum level is summarized in Fig. 4 and Fig. S1. The most abundant groups (relative abundance of > 5%) in CWs with Fe3O4 NPs were Proteobacteria (55.4%), Acidobacteria (8.9%), Cyanobacteria (5.9%) and Bacteroidetes (7.9%), accounting for 78.1% of the total effective reads. For CWs without Fe3O4 NPs, the major dominant group (relative abundance of > 5%) was different and Actinobacteria became a dominant bacterium. The results suggested that the relative abundance of bacteria was changed in CWs for the existence of Fe3O4 NPs. Notably, the content of Proteobacteria and Bacteroidetes in the CWs with Fe3O4 NPs increased compared with that of CWs with Fe3O4 NPs, and the content of Acidobacteria and Cyanobacteria decreased. Li et al. [46] also proposed that the abundance of Proteobacteria and Bacteroidetes can be significantly increased from 37.15% (control, R1) to 60.72% (1 mg/L Fe3O4 NPs, R2), which suggested that some bacteria (called Fe-anammox attach to Proteobacteria and Bacteroidetes) that oxidized NH4+-N coupled to the reduction of ferric iron (Fe3+) could be thrived by the presence of low dose Fe3O4 NPs [38, 46]. In a previous study, it was also reported that Proteobacteria and Bacteroidetes were denitrobacteria and ubiquitous organic nutrient bacteria, respectively, which explains why CWs with Fe3O4 NPs displayed higher removal percentages of NO3−-N and COD [48].
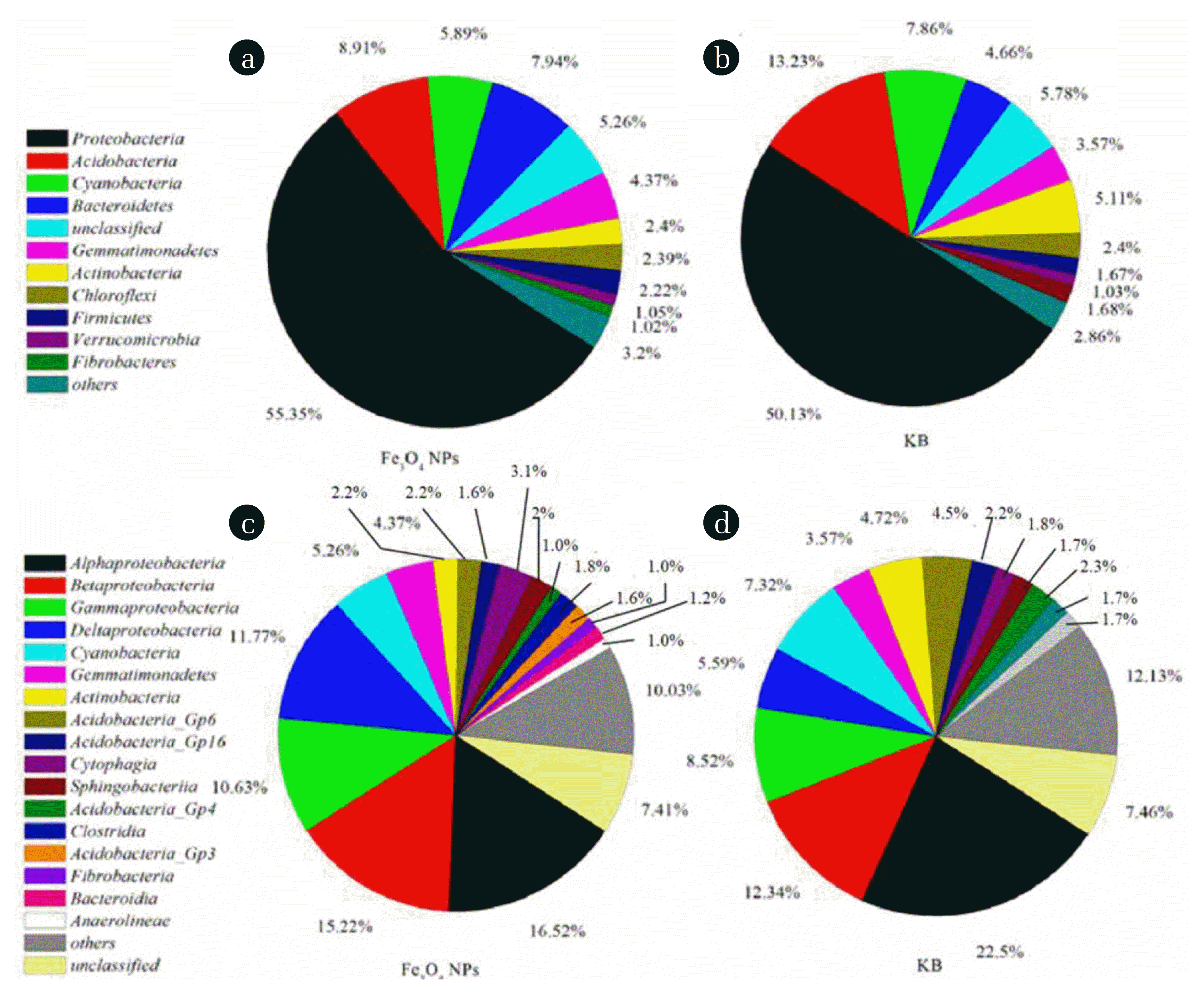
Bacterial communities at the phylum (a) and (b) and class level (c) and (d) from the microbial DNA sequences in the CWs. (Fe3O4 NPs represents CW with Fe3O4 NPs; KB represents CW without Fe3O4 NPs)
The relative abundance of bacteria at the class level is shown in Fig. 4 and Fig. S2. The results indicated that the presence of Fe3O4 NPs could change the relative abundance of bacteria at the class level. For CWs with and without Fe3O4 NPs, five major dominant bacteria, namely Alphaproteobacteria, Betaproteobacteria, Gammaproteobacteria, Deltaproteobacteria and Cyanobacteria, were found. Overall, Betaproteobacteria (15.3%), Gammaproteobacteria (10.5%) and Deltaproteobacteria (11.8%) in CWs with Fe3O4 NPs were higher than that of CWs without Fe3O4 NPs. In contrast, the relative abundance of Alphaproteobacteria (16.5%) and Cyanobacteria (5.3%) in CWs with Fe3O4 NPs were 6.0% and 2.0% lower, respectively, than that of CWs without Fe3O4 NPs. Additionally, it was also found that the number of Proteobacteria was increased the from 50.1% to 55.3%. Microorganisms from Proteobacteria might be involved in the biodegradation or biotransformation of numerous organic compounds or nitrate in natural or manmade ecosystems [49]. For example, Gammaproteobacteria was recognized as a major colony responsible for nitrate removal and Betaproteobacteria played an essential ecological function in reducing phosphorous [33, 50]. Our results illustrated that the major bacteria (e.g. Betaproteobacteria, Gammaproteobacteria, and Deltaproteobacteria) in CWs could be promoted by Fe3O4 NPs, thus improving the removal efficiency of nitrate and phosphorous.
The distribution of bacteria at the genus level is presented in Figs. 5 and 6. Overall, more abundant genera were achieved in the CWs with Fe3O4 NPs. The genera (relativqkre abundance of > 1%) in CWs without Fe3O4 NPs included Gemmatimonas (3.6%), Gp6 (4.5%), Gp16 (2.2%), Hydrogenophaga (3.0%), Sphingomonas (1.0%), Rhodobacter (2.2%), Pseudomonas (1.1%), Aridibacter(1.7%), Derxia (1.9%), Ilumatobacter (1.7%) and Desulfomicrobium (1.0%). However, the relative abundance of Gemmatimonas, Gp6, Gp16, Hydrogenophaga, Sphingomonas, Rhodobacter, Pseudomonas, Aridibacter, Derxia, Ilumatobacter and Desulfomicrobium in CWs incorporated with Fe3O4 NPs was changed, with the values of 4.4, 2.2, 1.6, 2.3, 0, 1.1, 2.0, 0, 1.6, 0 and 1.8%, respectively. Some abundant genera disappeared, and only Gemmatimonas and Desulfomicrobium increased. Gemmatimonas had been identified in enhanced biological phosphorus removal, and Desulfomicrobium was a kind of microorganism related to the process of nitrification and denitrification [26, 45]. The nitrification, denitrification and phosphorus-accumulating bacteria thrived, which was closely correlated with the phylum-level results. Most importantly, some new genera became the most abundant genera, including Desulfuromonas (3.0%), Azospirillum (1.9%), Chryseolinea (1.5%), Geobacter (1.5%), GpXIII (1.9%), Desulfomicrobium (1.8%), Schlegelella (1.0%) and Fibrobacter (1.0%). Some studies had revealed that Desulfuromonas, Azospirillum and Desulfomicrobium were involved in nitrogen removal and Chryseolinea was involved in the process of reducing COD [33, 51]. Therefore, our results proved that the bacteria at the genus level were also changed by the existence of Fe3O4 NPs.
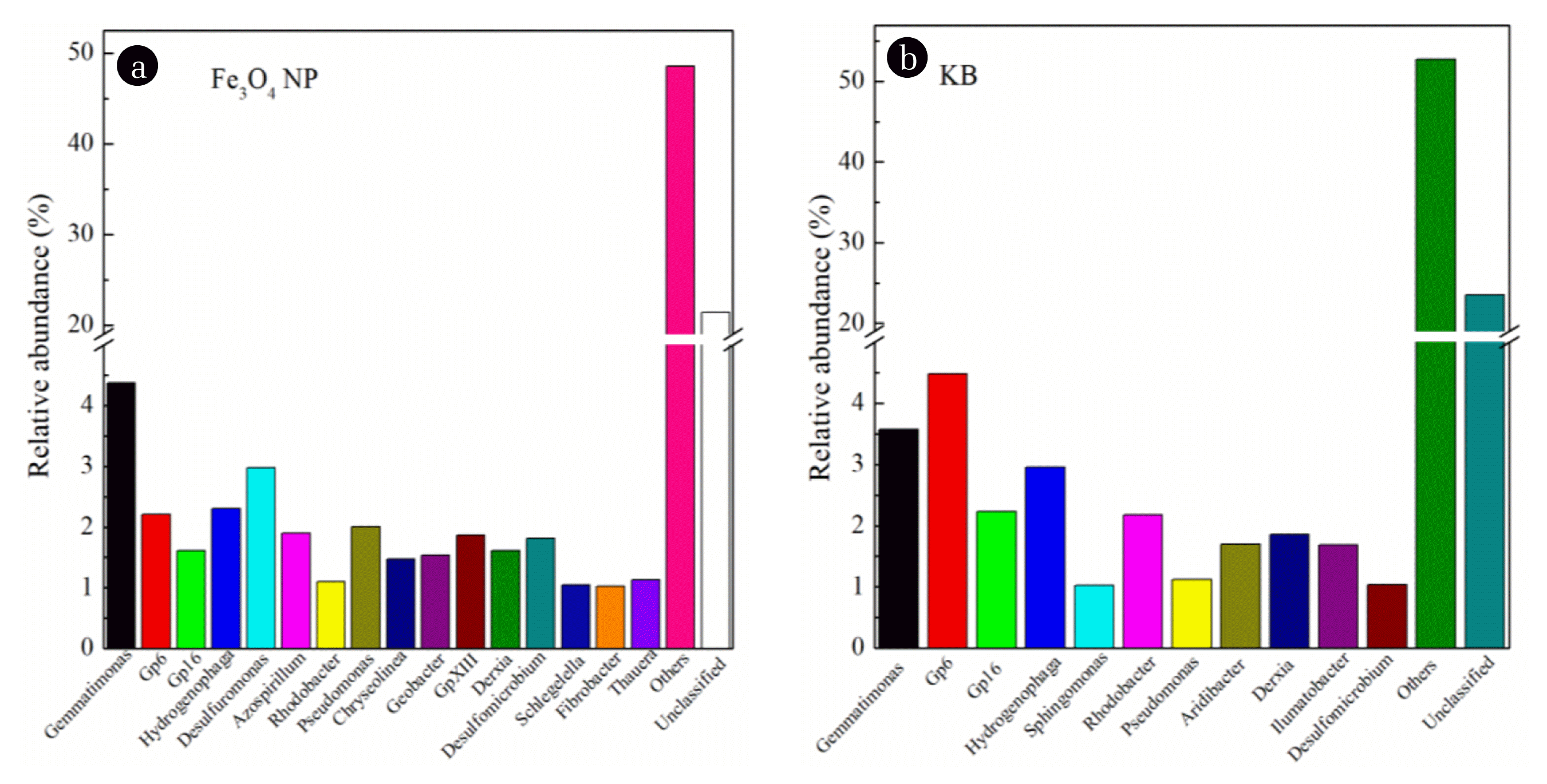
Bacterial communities at the genus level from the microbial DNA sequences in the CWs. (Fe3O4 NPs represents CW with Fe3O4 NPs; KB represents CW without Fe3O4 NPs)
4. Conclusions
In this study, Fe3O4 NPs were employed to treat the simulated wastewater in CWs. The results indicated that the simulated wastewater can be better treated by the CWs with Fe3O4 NPs. At the same time, the results also showed that the magnetic Fe3O4 NPs could improve the enzymatic activity and stimulate the development and growth of nitrification, denitrification and polyphosphate-accumulating microorganisms, thereby improving the performance of CWs, which could contribute to the large-scale application of CWs in rural China.
Supplementary Information
Acknowledgment
The authors would like to acknowledge the financial support from the National Nature Science Foundation of China (NO.42173073) and Zhejiang Huachuan Industrial Group Co. LTD.
Notes
Author Contributions
Z.Q. (Ph.D. lecturer) conducted all the experiments and wrote the manuscript. W.W. (Ph.D. Professor) revised the manuscript.