Removal and fate of carbamazepine in the microbial fuel cell coupled constructed wetland system
Article information
Abstract
Carbamazepine (CBZ), which is difficult to remove in the wastewater treatment system and easily forms toxic transformation products during the treatment process, is one of the priority pollutants of pharmaceuticals and personal care products (PPCPs). Increasing attention has been paid to explore their treatment technology without side effects from the treatment products. This study aims to reveal the removal and transformation of CBZ in the microbial fuel cell coupled constructed wetland (CW-MFC) system. The CW-MFC system was operated continuously at room temperature for nearly 80 days. The results show that CW-MFC system can effectively remove CBZ with an average removal rate of 97%. Three transformation products were identified by liquid chromatography–high-resolution mass spectrometry: 2-(2-oxoquinazolin-1(2H)-yl) benzoic acid (TP267), methyl 2-(2-oxoquinazolin-1(2H)-yl) benzoate (TP281), 2-(2,4-dioxo-3,4-dihydroquinazolin-1(2H)-yl) benzoic acid (TP283). Except TP281 in the influent, the other transformation products were formed in the system, which indicated that TP267 and TP283 were the main transformation products of CBZ. The formation pathway of transformation products could be explained by reactions including oxidation, hydrolysis, bond rupture and intramolecular reaction. The results also indicate that the CW-MFC system might be a promising technology for PPCPs treatment.
1. Introduction
With the development of technologies related to industrial production, thousands of industrial chemicals are eventually discharged into the aquatic ecosystem. Their exposure may pose a major risk to aquatic ecosystem and human health due to their widespread presence in the environment [1–3]. Pharmaceuticals and personal care products (PPCPs) have received increasing attention in recent years because of their accumulation and persistence in aquatic environments [4, 5]. Carbamazepine (CBZ) is listed as an indicator of PPCPs pollution in the environment [6], and also one of the most concerned PPCPs with priority control [7]. The global annual consumption of CBZ is approximately 1,000 tons, which leads to the high accumulation of CBZ in the environment [8]. CBZ and its metabolites seriously threatens the ecological environment and human health by producing ecotoxicity and damage to the reproductive system of animals [9, 10], and inducing anticonvulsant hypersensitivity syndrome to human body [11].
Widely-used wastewater treatment processes in wastewater treatment plants (WWTPs), such as activated sludge (AS), anaerobic/anoxic/aerobic (A2/O), usually have little effect on the removal of CBZ, which leads to the concentration of CBZ in the effluent being equal to or even higher than that in the influent. Lee et al. [12] and Pivetta et al. [13] separately investigated the removal of CBZ in WWTP with the AS and A2/O treatment processes. Both the removal efficiencies of the two processes were less than 10%. Since WWTPs are generally designed to treat the overall organic matter in sewage, not specifically for PPCPs, the sludge retention time (SRT) and hydraulic retention time (HRT) of WWTP is not enough to form a higher microbial biomass concentration to reduce the ratio of food to microorganisms (F/M), which can increase microbial diversity and improve the removal rate of persistent pollutants, such as CBZ [14]. Therefore, further treatment is required to remove CBZ from the effluent of WWTPs.
The treatment techniques for CBZ are mainly divided into chemical and biological techniques. Chemical techniques, including ozone, photocatalysis, and electrochemical oxidation techniques, can efficiently degrade CBZ in wastewater with a removal rate higher than 90% [15–17]. However, these techniques are often costly, and toxic intermediate products (e.g. acridine) are easily produced during the process [18]. For biological techniques, membrane bioreactor (MBR) and constructed wetland (CW) are often used, except the above-mentioned conventional wastewater treatment processes (e.g. AS, A2O). Compared with MBR, CW has lower operating cost and no membrane pollution problem [19]. The related research of CW system on the treatment of sulfamethoxazole (SMZ), trimethoprim (TMP), tetracycline (TC) and other antibiotics has shown its application potential for advanced treatment of PPCPs [20]. However, CW has moderate performance in CBZ treatment with removal rate of 50%–70% [21, 22]. Microbial fuel cell coupled constructed wetland system (CW-MFC) is an alternative process technology in order to improve the treatment performance of CW for CBZ. MFC system is supposed to enhance the microbial activity in the system and thus the treatment performance [23–25]. MFC could be easily combined with CW because that they are both biological systems engaged in the degradation of organic matter, and the redox gradient required by the MFCs can be formed naturally in the CWs according to the flow direction and the wetland depth [26].
The first CW-MFC study was conducted by Yadav et al. [27], which showed its high efficiency in the treatment of refractory pollutants. CW-MFC was also used in the treatment of PPCPs in wastewater, including sulfadiazine, sulfamethoxazole, tetracycline, etc. [28–30]. It was found that the removal performance of CBZ in CW-MFC system was better than that of CW system [31]. However, most of studies focused on PPCPs treatment performance and its influencing factors, and relatively few studies were related to the transformation and fate of PPCPs, especially for CW-MFC. It is known that CBZ will form transformation products during the degradation process, some of which may be more reactive and toxic than the parent compound [18]. Therefore, the knowledge gap in identification and analysis of CBZ transformation products and degradation pathways in CW-MFC is critical to better assess the state-of-art and further application of CW-MFC [32–34].
In order to understand the transformation and fate of CBZ in CW-MFC, the CBZ removal performance of CW-MFC was investigated in a laboratory environment. While investigating the removal rate of CBZ, the transformation products of CBZ in the system were identified, and the degradation pathways were speculated according to the possible reactions.
2. Materials and Methods
2.1. Experimental Setting
As shown in Fig. 1, the reactor of CW-MFC is constructed using a cylindrical plastic container (20 cm in diameter and 40 cm in height). The inlet and outlet of wastewater are set at the bottom and top of the reactor, and the sampling points of water are set at three different depths viz. 10 cm (S1), 20 cm (S2) and 30 cm (S3) from the top of reactor. This set ensured that the water samples were taken from the areas between the inlet and the anode, around the anode, and between the anode and the cathode, respectively.
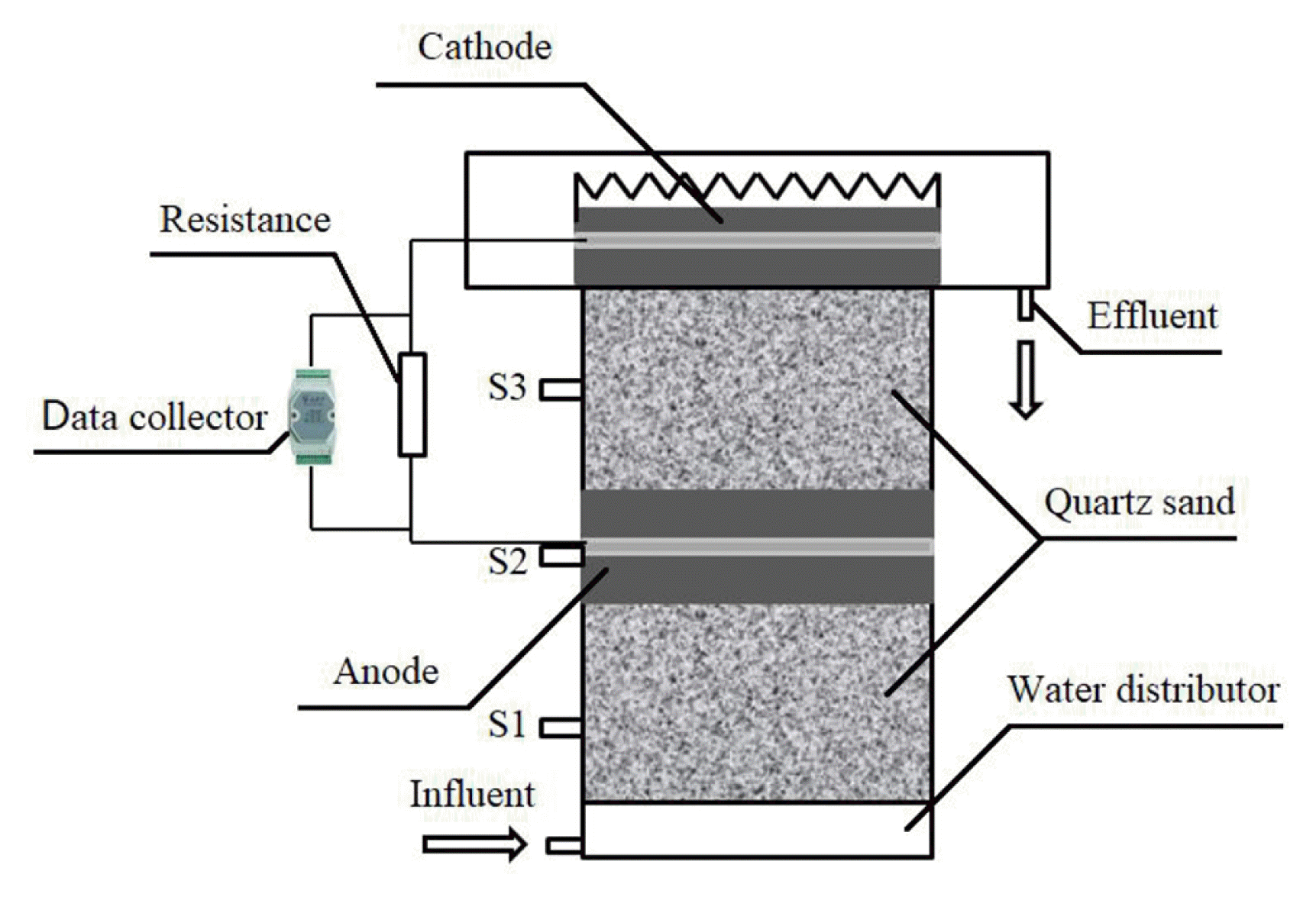
Schematic diagram of the CW-MFC system. S1–S3: Sampling points. Anode and cathode were composed of granular activated carbon and stainless-steel mesh.
For the CW-MFC reactor, from bottom to top there are five layers: water distribution layer (4 cm in depth), bottom layer (11 cm in depth), anode (5 cm in depth), middle layer (11 cm in depth), and cathode (3 cm in depth). The bottom layer and the middle layer are filled with quartz sands of 2–3 mm in size. While the anode and cathode were made of granular activated carbon (GAC) (4 mm) with a stainless-steel mesh buried inside. Copper conductors are used to connect the electrodes at the stainless-steel mesh to an external resistance (1,000 Ω) and the electric circuit data collector (DAM-3032 and DAM-3057, Art Technology Co. Ltd., China).
2.2. Activated Sludge Inoculation
The functional microbial communities in CW-MFC system were brought by activated sludge inoculation. The activated sludge was collected from Tianjin Wastewater Treatment Plant. The concentration of the sludge was 6 g dry mass L−1. The sludge (0.3 L) was added to each reactor during the installation. For sludge acclimatization, synthetic wastewater was pumped into the two reactors with an up-flow mode by a peristaltic pump (BQ80S, Lead Fluid, China). The ingredients of the synthetic wastewater consisted of 0.2 g L−1 glucose, 0.135 g L−1 NH4Cl, 0.0277 g L−1 NaH2PO4·2H2O, 0.0138 g L−1 Na2HPO4·12H2O, 0.13 g L−1 KCl, 0.313 g L−1 NaHCO3, 0.025 g L−1 MgSO4, and 10 ml concentrated trace element solution (50 μg L−1 EDTA, 22 μg L−1 ZnSO4·7H2O, 8.2 μg L−1 CaCl2·2H2O, 5.1 μg L−1 MnCl2·4H2O, 5.0 μg L−1 FeSO4·7H2O, 1.1 μg L−1 (NH4)6Mo7O24·4H2O, 1.8 μg L−1 CuSO4·5H2O, 1.6 μg L−1 CoCl2·6H2O) for 1 L wastewater [35]. The flow rate of the peristaltic pump was set at 1 mL min−1 in order that the hydraulic retention time (HRT) keeps 2 d. During sludge acclimatization, the voltage of CW-MFC system was monitored by the electric circuit data collector. The sludge acclimatization lasted for 12 d at a room temperature (25°C) until the voltage of CW-MFC was stable.
2.3. Carbamazepine Treatment Experiment
After the sludge acclimatization, CBZ (over-the-counter) was added to the synthetic wastewater. In order to avoid the extremely high initial pollution load of CBZ for the microbes, the application rate of CBZ was slightly increased from 1.5 mg L−1 to 10 mg L−1 in the first 10 days. When the voltage of CW-MFC system for the CBZ concentration at 10 mg L−1 was stable, the microbial communities were considered to be adapted to the CBZ containing wastewater. Then, the CW-MFC system was operated continuously at room temperature (ca. 25°C) from June to September, 2019.
The water samples (50 mL) were collected every two days from the inlet (INF), three sampling points (S1, S2, and S3) and the outlet (EFF) to determine the CBZ removal in the system. CBZ content was measured by the method described in China Pharmacopeia using a spectrophotometer at 285 nm with a 1-cm cuvette [36]. As the CBZ used in the wastewater was the prescription drugs, the CBZ releasing characteristics in synthetic wastewater was analyzed through a supplementary experiment (Fig. S1). Then the CBZ concentration was corrected according to the increasing proportion of CBZ in the experiment.
2.4. Operational parameters
The voltage (V) data were recorded by a personal computer every 10 min to check the stability of the bioelectricity production. The current density (I) and power density (P) were calculated according to Eq. (1) and (2):
where U is the voltage, Rext is the external resistance, and A is the cross-sectional area of the anode.
The polarization curve and power density curve were established by varying the external resistance within a range of 5 Ω to 10000 Ω.
2.5. Identification of Transformation Products
After a stable operation for two months, 5 samples from influent, effluent, and three sampling points were collected, and then were stored in the −5°C refrigerator until solid phase extraction (within 24 h). The specific operation steps refer to the method described in Ashfaq et al. [37] with modifications. Oasis HLB cartridges (60 mg, 3 mL) from Waters Corporation (Milford, MA, USA) were conditioned with 5 mL of methanol followed by 5mL of deionized water (HPLC grade) at neutral pH, with a flow rate of l mL min−1. After the preparation, water samples were percolated through the cartridges with a flow rate of 10 mL min−1. After that, the cartridges were rinsed with 5 mL HPLC grade water, and then were dried in vacuum for 15–20 min to remove excess water. Elution was performed with 2–10 mL of methanol at 1 mL min−1 (CBZ concentration 10–100 ppb). Finally, the extraction solution was collected in a 2 mL amber glass bottle rinsed with ultrapure water for identification.
A Linear Ion Trap-Orbitrap Mass Spectrometer (LTQ OrbitrapVelos Thermo Scientific, Bremen, Germany) in positive ESI mode was utilized and the MS source parameters for the analysis were set as follows: ion voltage: 3,500V; ion transfer tube temperature: 300°C; vaporizer temperature: 350°C; default charge state: 1; sheath gas flow rate 50 arbitrary units (arb. unit); aux gas flow rate 10 arb. unit; sweep gas flow rate 1 arb. unit; RF lens: 60%, orbitrap resolution: 120K; scan range: 50–350 m/z; AGC target: 400000; method duration: 8 min. Chromatographic separation was performed using an Acquity UPLC BEH C18, 1.7 μm, analytical column, 2.1 × 50 mm (Waters). The mobile phase was purified water (A) and methanol (B), both with 0.01% formic acid. The percentage of B was changed as follows: 0 min, 5%; 4 min, 90%; 5 min, 90%; 5.1 min, 10%; and 8 min, 5%. The flow rate was 0.4 mL/min, and the run time was 8 min. The column temperature was set to 30°C. The transformation products MS2 spectra were conducted at which the collision energy was set to 30 eV (CID, collision induced dissociation).
The final identification of the transformation products was obtained by the following analysis. Firstly, the retention time and accurate mass of the transformation products are obtained by chromatogram and mass spectrometry (Fig. S2 and S3), compared with the database of CBZ transformation products created by collecting literature (Table S1). The structure of the transformation products was further determined by the fragmentation pattern obtained by MS2 spectra (Fig. S4). The mass error (ME) is calculated by [38]:
where M1 is the measured mass, and M0 is the theoretical mass.
Quality assurance and quality control (QA/QC) was conducted to ensure the accuracy of CBZ identification and quantitative results. The limit of detection (LOD) was calculated at S/N ratio of 3:1 and the quality control (QC) was based on the standard recovery rate of the effluent and influent samples with spiked concentration of 1 mg L−1 and 10 mg L−1, respectively. LOD and standard recovery rate have been presented in Table S2. In addition, procedural blank was applied for each batch.
2.6. Bacterial Community Analysis
Microbial DNA was extracted from electrode substrate (cathode and anode) samples using the E.Z.N.A.® soil DNA Kit (Omega Bio-tek, Norcross, GA, U.S.) for high-throughput sequencing analysis. Bacterial 16 S rRNA V4–V5 regions were amplified using primers 515F (5′-GTGCCAGCMGCCGCGG-3′) and 907R (5′-CCGTCAATT CMTTTRAGTTT-3′) [39]. For the 16S rRNA polymerase chain reaction (PCR) reactions were performed at 95°C for 3 min followed by 27 cycles of 30 s at 95 °C, 30 s for annealing at 55°C, and 45s for elongation at 72°C, and a final extension at 72°C for 10 min. The PCR products were extracted from a 2% agarose gel and further purified using the AxyPrep DNA Gel Extraction Kit (Axygen Biosciences, Union City, CA, USA). High-throughput sequencing was performed by the Illumina Miseq platform at Majorbio Bio-Pharm Technology Co. Ltd. (Shanghai, China). Raw fastq files were quality-filtered by Trimmomatic and merged by FLASH. At the 97% similarity level, Operational taxonomic units (OTUs) were clustered with a novel ‘greedy’ algorithm that performs chimera filtering. The sequence of merge partition was used for analysis of the status identification classification, and a bar graph of phylum class level was created to reveal the bacterial composition of the samples.
2.7. Statistical Analysis
ANOVA was conducted using the MIXED procedure in SAS 9.4 (SAS Institute Inc., USA) to evaluate data of CBZ of the water samples. Treatment effects were deemed to be significant when p<0.05. Differences among the treatments were identified by multiple comparisons with t-test using the LSMEANS statement in SAS 9.4. The differences between the measured variables of different treatments were significant when p < 0.05.
3. Results
3.1. Electricity Generation of CW-MFC
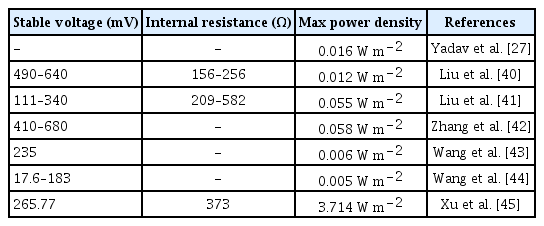
The CW-MFC system in this study maintained a stable voltage of 315 mV after two weeks (Fig. 2(a)). The polarization curve and power density curve of the CW-MFC system showed that the internal resistance and the maximum power density of the system were 257 Ω and 0.330 W m−2 (the corresponding current density was 2.293 A m−2) (Fig. 2(b)). As shown in Table 1, the electricity generation data of the CW-MFC system are within the range reported in the relevant literatures [27, 39–44].

Electrical performance of CW-MFC. (a) Bioelectricity generation of CW-MFC, (b) Polarization curves and power density curve of CW-MFC.
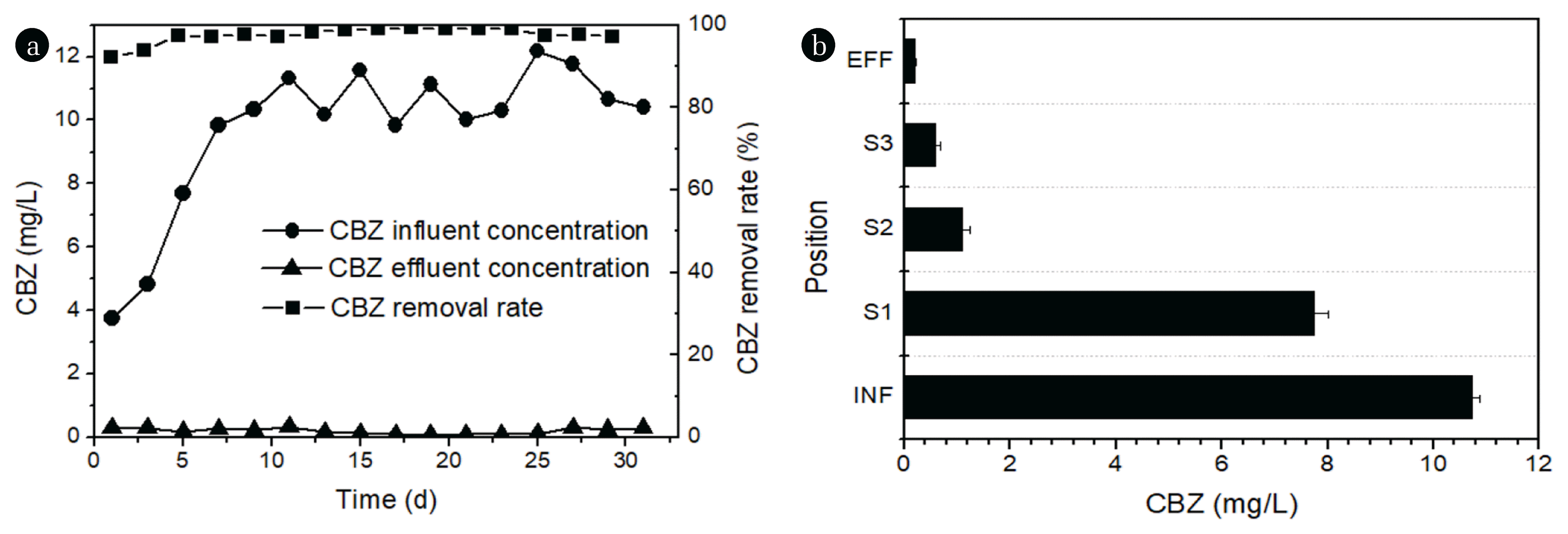
3.2. Evolution of Carbamazepine
The average removal rate of CBZ in the CW-MFC system was 97% (Fig. 3(a)). In the CW-MFC system, the CBZ concentrations decreased along with the upward water flow direction, and this tendency remained from the start to the end of the experiment, especially the CBZ concentrations of the water in S2, S3 and EFF decreased significantly (Fig. 3(b)).
3.3. Transformation Products of Carbamazepine
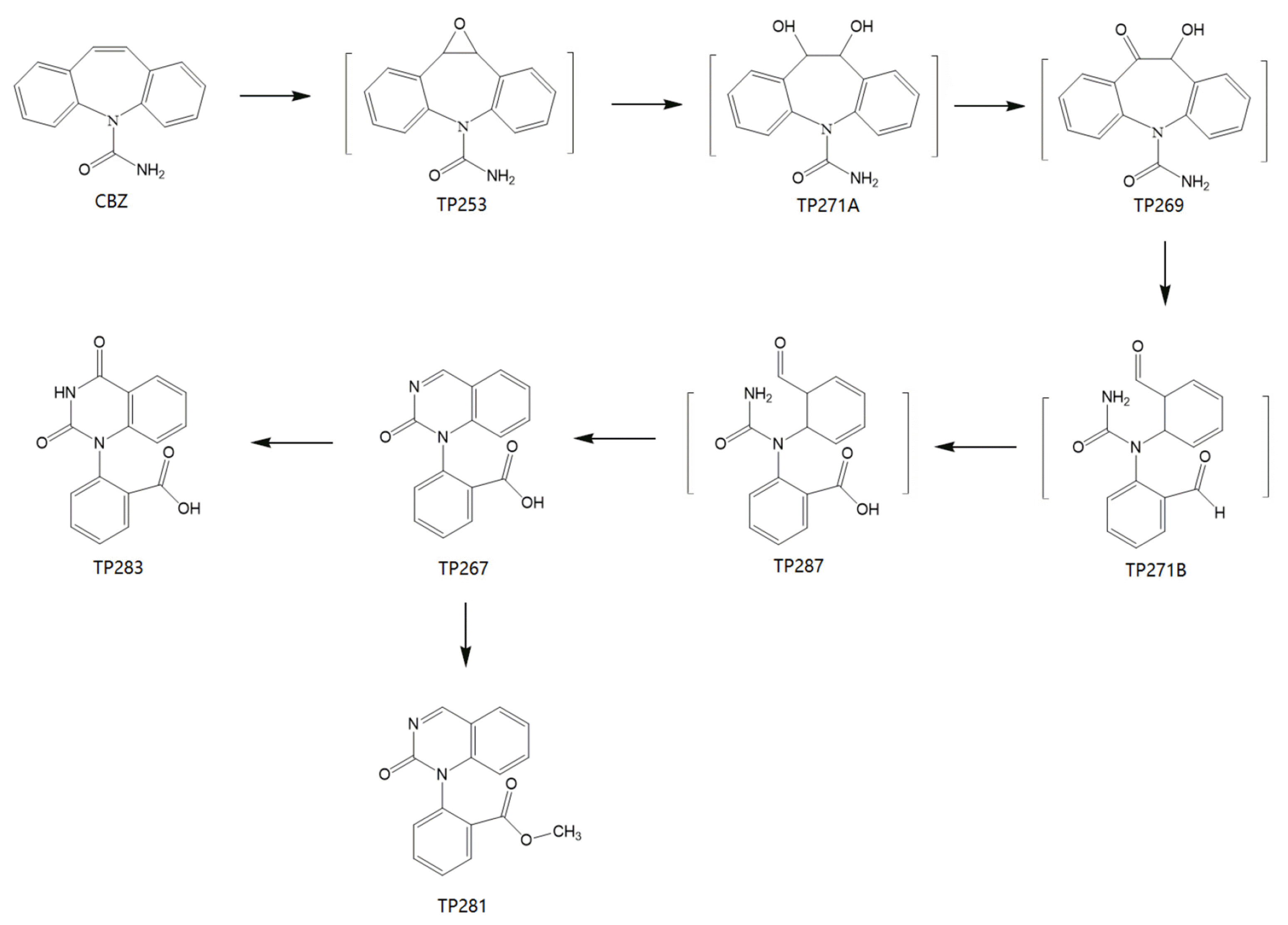
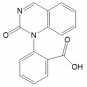
According to the results of LC-MS of samples at different locations of the CW-MFC system and influent, three major transformation products from CBZ were identified: 2-(2-oxoquinazolin-1(2H)-yl) benzoic acid (TP267), methyl 2-(2-oxoquinazolin-1(2H)-yl) ben zoate (TP281), 2-(2,4-dioxo-3,4-dihydroquinazolin-1(2H)-yl) benzoic acid (TP283) (Table 2, Fig. S2, S3 and S4). Table 1 shows the identification information of these transformation products and the parent compound CBZ. The CBZ and its transformation products were found in the water samples at the positions of S1, S2, S3, and EFF, and the characteristic peaks of CBZ and TP281 were also observed in the LC-MS results of the influent samples (INF) (Fig. 4).
3.4. Microbial Community Composition
The microbial community composition of the CW-MFC system is shown in Fig. 5. At the phylum level, the microorganisms on the electrodes of the CW-MFC system mainly include Proteobacteria, Bacteroidota, Firmicutes, and Chloroflexi. For the cathode, the predominant phylum of the microbe was Proteobacteria with a relative abundance of 63%, followed by Firmicutes with a relative abundance of 20%. For the anode, the predominant phyla of the microbes were Bacteroidota and Proteobacteria with relative abundance of 45% and 22%, respectively.
4. Discussion
4.1. Electricity Generation of CW-MFC
The power generation performance of CW-MFC system in this study was at a median level compared with other related studies, but it was relatively excellent in related studies under similar configurations and operating conditions. The highest power density of our CW-MFC system (0.330 W m−2) is higher than other reported CW-MFC systems (0.005–0.055 W m−2) with the configuration and operating conditions similar to ours [40, 41], likely because of the lower electrode spacing (11 cm) promotes the flow of electrons in our system compared to other systems (20–25 cm) [40, 41]. Xu et al. [45] found the highest power density decreased from around 0.050 W m−2 to 0.037 W m−2 when the electrode spacing increased from 2 cm to 5 cm.
4.2. Removal of Carbamazepine
The CBZ removal effect of the CW-MFC system in this study is comparable to the results of other CW-MFC systems, and is higher than the results of the CW system. The CBZ removal rates of the CW-MFC system in related studies are 99% [31], while the CW system is 50–70% [21, 22]. In order to better compare the removal effect, we also constructed a pure CW system without adding electrodes to evaluate its CBZ removal rate, and the result showed that its average CBZ removal rate of 32% (not shown as results) was significantly lower than that of the CW-MFC system (Fig. S5(a)). The results indicate that the CW-MFC system constructed in this study has the potential to remove CBZ in wastewater, and some characteristics of the CW-MFC system different from the CW system may be the reason for its better CBZ treatment effect. The first reason is the electrode material. By investigating the removal of CBZ in different positions of the system and comparing with the results in the CW system (Fig. S5(b)), it can be observed that the electrode (S2) was the main area for CBZ removal, which was mainly related to the application of GAC. GAC is considered to be an ideal CW-MFC electrode material, its high specific surface area and porosity can provide a good environment for the attachment and growth of microbial communities [46]. The anode with a larger area than the cathode is more suitable for the growth of biofilm. It was observed that the average bacterial cell density of the anode (5.13 × 107 cells g−1) was higher than that of the cathode (3.85 × 107 cells g−1) [40]. In addition to the electrode material, the connection of the circuit can promote the growth of the biofilm on the electrode, and affect the structure of the microbial community and increase the enrichment of the functional microbial community. It was observed that the diversity and abundance of microorganisms in the closed-circuit CW-MFC system were higher than those in the open-circuit CW-MFC system and the CW system [25, 39, 47]. The functional microorganisms enriched by CW-MFC electrode mainly include Proteobacteria, Firmicutes, Pseudomonas, Rhodocyclaceae, Nitrospira, etc. [25,39]. Among them, Proteobacteria and Firmicutes at the phylum level and Pseudomonas at the genus level were related to the biodegradation of CBZ [48–50]. Proteobacteria and Firmicutes not only play an important role in the degradation of organic pollutants, but are also important electrochemically active bacteria, which are the main enriched microorganisms in the CW-MFC system [39]. In this study, the microbial composition shows that Proteobacteria and Firmicutes are the main microorganisms at the phylum level (Fig. 5). The enrichment of functional microorganisms helps the effective removal of CBZ. In addition, the electric field in CW-MFC can also promote the interface exchange process between pollutants and microorganisms, which will strengthen the diffusion and mass transfer of organic matter in the biomass, and promote the entry of organic matter into the bottom layer of the biofilm without destroying the microorganisms in the membrane, thereby improving the removal effect of CBZ [51]. The structure and circuit operating environment of CW-MFC can increase the biomass of microorganisms and enrich functional microorganisms. This also explains why the CBZ removal is significantly different between the CW-MFC system and the normal WWTP system.
It should be noted that although CBZ has a low octanol-water partition coefficient (logKow = 2.7), the application of GAC may cause significant differences in the removal effect of CBZ in different systems due to adsorption in the short term [52]. For this reason, the study tested the adsorption performance of GAC on CBZ and the results showed that the equilibrium absorption capacity of CBZ on the unit mass GAC was 0.111 mg g−1. According to this result and the GAC mass in the CW-MFC (1,194 g) and the CBZ mass load in the influent (0.019 g d−1), the adsorption can affect the removal of CBZ within a week, and our system maintains a long-term stable high CBZ removal rate. Adsorption onto the substrates was limited compared to the total mass removal during operation. In addition, previous studies have demonstrated that CBZ is relatively stable in the environment, and its half-life in wetland sediments is 165–264 d [53]. Therefore, the long-term stable high CBZ removal rate of the CW-MFC system indicates that the biodegradation of the abundant functional microorganisms on the electrode is the main reason for the effective removal of CBZ other than the adsorption by GAC.
4.3. The Degradation Pathways of Carbamazepine
Previous studies have reported two main degradation pathways of CBZ. The first degradation pathway is as briefly described below. As the high frontier electron density of the double bond in the nitrogen-containing heterocycle results in high reactivity [54], it is easily oxidatively cleaved, and then the intramolecular rearrangement reaction causes ring contraction of CBZ and loss of carbamoyl groups. The nitrogen-containing heterocyclic ring shrinks from a seven-membered ring to a six-membered ring, and forms an aldehyde group at the rearranged position. The aldehyde group is further oxidized to carboxyl, and then decarboxylated to form ketone. This degradation pathway mostly occurs in the photodegradation, electrochemical oxidation and active chlorine oxidation processes of CBZ [55–57]. This pathway is known to form acridine that is more toxic than the parent compound [18, 58]. The second degradation pathway is different from the above pathway. The intramolecular cyclization reaction occurs after the nitrogen-containing heterocyclic ring of CBZ is opened. The carbamoyl group bonds with the aldehyde group to form a new substance, and then further oxidation reaction occurs on the newly formed nitrogen heterocyclic ring and the aldehyde group. This pathway was found upon ozonation of CBZ, ferrate oxidation and biodegradation of activated sludge and sand filters [54, 59, 60].
According to the CBZ transformation products obtained after identification, the degradation pathway of CBZ in our study may be similar to the second degradation pathway, indicating that the CW-MFC system contains bacteria that can effectively degrade CBZ. The presumed degradation pathway is that CBZ was transformed by biological oxidation into reactive and pharmacologically active 1-(2-(3-(o-tolyl) oxiran-2-yl) phenyl) urea (TP253). Then, TP253 was hydrolyzed into 1-(2-(1,2-dihydroxy-2-(o-tolyl) ethyl) phenyl) urea (TP271A). intermediate product 1-(2-(1-hydroxy-2-oxo-2-(o-tolyl) ethyl) phenyl) urea (TP269) was formed by dehydrogenation of TP271A. TP269 may be transformed to 1-(6-formylcyclohexa-2,4-dien-1-yl)-1-(2-formylphenyl) urea (TP271B) through the cleavage of the nitrogen-containing heterocycle. TP271B can be further oxidized to 2-(1-(6-formylcyclohexa-2,4-dien-1-yl) ureido) benzoic acid (TP287). Then, TP267 was formed by the intramolecular reaction of the carbamoyl with the aldehyde group. TP267 can be further oxidized to TP283 or methylated to TP281 (Fig. 6). In addition, the characteristic peak of TP281 observed in the influent indicates that it was not formed by CBZ degradation in the system, but may be transformed from TP267 during the extraction procedure with methanolation [18]. The transformation products formed during the degradation of CBZ have relatively low toxicity and reactivity. The HPTLC bioassay with V. fischeri indicated the microbial toxicity of TP267 and TP283 was not higher than that of the parent compound, and the inhibitory effect of TP283 is less than that of CBZ [59].
Based on the degradation pathways reported in previous studies, the formation process of the transformation products in this study and the possible intermediate products in the process were speculated. No difference was found in the transformation products observed between different positions of the CW-MFC system. Since the relatively aerobic environment in the upper layer of the system may contribute to the degradation of CBZ, the proposed degradation path is most likely to occur in this area. It has been observed in related biological treatment systems that aerobic conditions are more suitable for CBZ degradation than anaerobic conditions [53, 61]. The reason why the same transformation products are observed at different positions may be attributed to the transformation products produced by CBZ degradation that ionize in the solution, and then the ionized ions migrate to the vicinity of the anode area due to electrostatic adsorption, causing them to diffuse in different positions [62].
5. Conclusions
This study demonstrates that the CW-MFC system has good performance in CBZ treatment while generating electric energy. The higher removal rate of CBZ in CW-MFC was mainly reflected in the significant decrease of CBZ concentration at the electrode, which indicates that the electrode promotes the transformation and removal of CBZ. Three transformation products were identified by LC-MS in the CW-MFC system, which were 2-(2-oxoquinazolin-1 (2H)-yl) benzoic acid (TP267), methyl 2-(2-oxoquinazolin-1(2H)-yl) benzoate (TP281), 2-(2,4-dioxo-3,4-dihydroquinazolin-1(2H)-yl) benzoic acid (TP283). TP267 and TP283 were the main transformation products. The formation of transformation products could be explained by proposed degradation pathways via intramolecular reactions after cleavage of the double bond in the nitrogen-containing heterocycle of CBZ. Furthermore, the high removal rate of CBZ and low ecological risk of its transformation products for the CW-MFC system found in this study indicate that the CW-MFC system could be a promising technology to remove PPCPs in sewage, although further study is needed to test its performance for co-existence of various PPCPs.
Supplementary Information
Acknowledgments
This work was supported by Major Science and Technology Program for Water Pollution Control and Treatment of China (2018ZX07110-007) and the Tianjin Science and Technology Program (18PTZWHZ00110, 18ZXSZSF00250 and 19YFZCSF 00840).
Notes
Author Contributions
J.X. (Ph.D. student) conducted all the experiments and wrote the manuscript. H.L. (Post-Doc.) assisted the data analysis and manuscript writing. S.W. (M.Sc. student), H.C. (Ph.D.), W.J. (Engineer), L.Z. (Engineer), L.W. (Engineer), Y.W. (Engineer), and L.L. (Engineer) supported the experiments. X.L. (Professor) initiated and supervised the work.