Synthesis of nanomaterial from industrial waste and its application in environmental pollutant remediation
Article information
Abstract
Increased productions of waste from industries have persuaded in sustainable and naturally stable methods to reuse the waste. Utilization of wastes for the synthesis of nanomaterial is of significant importance due to its extensive variety of uses in various industrial sectors. This review focuses on potential options available for nanomaterial synthesis from waste produced by industrial activities and manufacturing processes. Possible application of industrial waste derived nanomaterial for the expulsion of organic and inorganic contaminants is discussed. Furthermore, the future opportunities and challenges in this emerging research area of converting industrial waste to nanomaterials are addressed.
1. Introduction
Industrialization and urbanization has led to billion tons of toxic waste generation annually by creating significant burden on the environment [1]. Therefore effective treatment technology development and finite resources conservation is essential when the limiting resources of the earth, the limited land availability for waste disposal and its adverse impact on environment is taken into account [2]. In the waste generated from various industrial sector, there exist large quantities of secondary raw materials that could be used in wide range of applications [3]. In order to safeguard the environment, waste minimization, separation and recycling processes should be developed [4]. However industrial wastes released from different manufacturing processes exhibit hazardous characteristics and are mostly composed of heavy metals such as lead, cadmium, copper, arsenic, nickel, chromium, zinc and mercury creating significant burden on the environment. In particular, the main contributors for the release of toxic heavy metals in the waste streams include Mining, electroplating, metal processing, textile, battery manufacturing, tanneries, paint manufacturing, paper and pulp industry [5, 6]. The industrial wastes with heavy metals, toxic chemicals, and dyes and has the ability to accumulate in human bodies and cause serious diseases and physical disorders. Among which, heavy metals were considered as the most hazardous. For example, short term exposure to high dosage of chromium causes irritation at the site of contact, affects nasal mucosa and cause ulcers on the skin. Chromium utilizing industries such as paint pigment and paper industries, mining, electroplating, leather tanning, metal processing, textile, dyeing and steel fabrication are some of sources of chromium pollution. Lead pollution is caused from steel waste, battery waste and petrol where, lead gets accumulated in the kidney, brain, muscles, and bones causing hypertension, kidney and brain damage [7]. Nanomaterial synthesis from industrial waste is an effective way for treatment and recycle of waste which conforms to the concepts of minimum waste generation and wealth from waste [8]. Since, nanoparticles present unique properties like high specific surface area and reactivity, it helps in detection and removal of diverse targets, including chemicals, pollutant gases, organic pollutants, and biological substrates (e.g., bacteria, virus, antibiotics). Currently, industrial waste utilization as starting material for synthesizing nanomaterial has gained interest due to attractive economic returns and its path towards sustainable development [9]. Electroplating industrial waste is one of the highly used waste for the synthesis of waste derived nanomaterials due to high level enrichment with organic matter and metals (Fe, Cr, Cu, Ni, Pb, Mn, Sn, Ag etc.) in various forms such as mixed oxides, hydroxides, sulfates, silicates and phosphates, that are potential precursors to advanced catalysts [10]. At present, the conventional treatment methods for electroplating waste are landfill, brick making, stabilization with cement matrix etc. [11]. In like manner, fly ash produced during coal combustion in thermal power plants is one of the complex and abundant solid waste comprising major portion of Si and Al and small amounts of Fe, Ca, Na, P, K, Ti, and S. Fly ash are primarily in metal oxides forms which can easily agglomerate to form microspheres. Still another, Red mud produced during alumina generation from bauxite ores composed primarily of fine particles containing iron, aluminium, silicon, titanium oxides and hydroxides. Calcium is the main element of paper making industry with small number of other elements, including Si, Al, Mg, K, Na, Mn, and Cr [12].
Without proper treatment, the industrial waste can damage both environment and human. Conventional methods that are currently used for the treatment or disposal of industrial waste include landfill, incineration, curing and stabilization. However, all these technologies have certain disadvantages. For example, leakage of heavy metal and organics from the landfill potentially contaminates soil and groundwater; waste incineration reduces only the volume of waste polluting the air; high waste stabilization costs. According to the “3R” rules (Reduce, Reuse, and Recycle), it is urgently necessary to find a method for the utilization of industrial waste [13]. From a long term perspective, landfill is not environmentally benign method and may cause loss in huge amount of valuable resources and leaching of metals resulting serious secondary pollution. Thus the promising option for industrial waste treatment is to maximize the recovery and reusing of resource economically [14]. Many research works has been done to convert Industrial waste materials into valuable products such as ceramic [15], pigment [16–18], catalyst [14] etc. Recently it has gained interest in nanomaterial synthesis from waste and its application in pollutant removal due to its large surface area and properties. A survey of literature demonstrates that, this area is starting to attract more approach. The use of waste derived nanomaterials for the industrial application in pollution removal not only resolves the waste disposal problems but also helps in managing natural resource exploitation. Moreover, substituting the waste derived materials instead of virgin materials helps in improving the process economy, where the valuable materials are exploited in the production process. Therefore a great research interest is developing in usage of waste derived nanoparticle as an efficient and inexpensive material for the removal of pollutants from the environment. Previously, Yuan and Dai [19] has published a review paper which explains about the development of functional nanomaterials from sewage sludge and its applications. This review focuses on the nanomaterial synthesis from industrial waste streams highlighting the applicability of waste derived nanomaterial in pollution remediation.
2. Recovery/Synthesis of Nanomaterial from Industrial Waste
Conversion of regularly generated waste product into useful nanomaterial generally comes under the sustainable development [20]. A broad range of industrial waste has been thermo-chemically treated to recover valuable nanomaterials (Table 1). Different manufacturing techniques have been employed for the synthesis of nanomaterial with various morphology and size. Conventional technologies use a top-down method for construction of materials, where bulk materials are broken into smaller pieces using mechanical, chemical or other forms of energy. On the other hand, in bottom-up approach the ions are chemically combined together to form the particles [21, 22]. Industrial waste derived nanomaterials synthesis technique by bottom up approach includes co-precipitation [23–27], hydrothermal method [17, 28–33], Microwave irradiation [34] and by top down approach include ball milling technique [35–39]. The metal and their oxides recovered from industrial wastes include Cr, Cu, Fe, Ni, Ti, Al, Ni and the nanomaterials have been regenerated from electroplating waste, picking waste, tannery waste, fly ash, steel industry waste, paper mill sludge, phosphogypsum waste etc. A few examples of recovered nanomaterials are MFe2O4 (M: Mn, Cu, Zn, Fe, Ni, Ag), magnetic biochar, magnetic chitosan, TiO2 modified fly ash and zeolite [40, 41]
2.1. Pre-treatment of Industrial Waste
Industrial waste pre-treatment is a vital step for the production of nanomaterial from waste. Based on the composition of waste sample, preparation methods are categorized into physical treatment and chemical treatment methods. The chemical composition of the waste and the treatment method affect the physicochemical properties and surface functional group of synthesized nanomaterial. The process variables that has effect on synthesis include temperature, acid used for treatment and the chemicals used for activation [19, 42]. Initially industrial waste was directly used as catalyst without any pretreatment. The catalytic behaviour of Red mud and iron waste was studied for sulfadiazine degradation using H2O2 and persulfate with the generation of •OH and SO4 −. Then pretreatment of waste by physical and chemical activation was used to enhance the activity [12]. Ball milling of industrial waste is one of the physical treatment method which reduces the particle size to obtain homogenous mixture for further application [43, 44]. Lee et al. [45] studied the ball milling effect on particle size and its influence in calcium extraction from steelmaking slag. The finer the particle size, the reaction between the components becomes easier [46]. The efficiency of calcium extraction increased by ball milling effect (360 rpm for 72 h) [11]. Subsequently, the basic aim of chemical pre-treatment is to remove any contaminants present in the waste sample that can be made soluble by heating or treating with chemicals. The presence of soluble salts such as chlorides, sulfates, and carbonates in the waste can cause defects because of their dissociation thereby damaging the products during firing at high temperatures. Abreu et al. [47] reported that the particle size distribution of waste influenced the salt extraction due to the demand of great quantities of water and long exposure time. Strong acid hydrolysis is a kind of chemical pre-treatment where concentrated strong acids (e.g., HNO3, HCl, H2SO4) have been commonly applied for treating waste materials [48, 49]. For example, to prepare γ- and α-Al nanopowder from waste aluminum foils, waste materials were initially dissolved into aqua regia, after which an ammonia solution was added for maintaining the solution pH in the alkaline range [50].
2.2. Methods for Nanomaterial Synthesis
Several thermo chemical methods can be used to synthesize industrial waste derived nanoparticles such as co-precipitation, hydrothermal, carbothermal, solvothermal and microwave assisted methods [21]. The synthesis of waste derived nanoparticles is a complex process because of their characteristics. For pollutant removal applications, surface modification of nanoparticles is considered as a critical aspect regarding both selectivity and aqueous stability [51]. A brief description of the most widely used methods for preparing nanomaterials with advantages and disadvantages are discussed below.
2.2.1. Co-precipitation method
Co-precipitation method is one of the most common wet chemical methods for obtaining waste derived nanoparticle. This method is widely used among other methods due to its simple operation and high yield of nanomaterials [12]. The metal content in the industrial waste was leached with acids (HNO3, HCl, H2SO4). The acid extracted metal solutions are treated with alkali (NaOH, MgO,) to increase the pH ranging from 7 to 12 [52]. The size and shape of the nanoparticle depends on the type of extractant, molar ratio of the metal, reaction temperature, PH and other reaction parameters, e.g., stirring rate, dropping speed of basic solution [23, 26, 27]. Liang et al. [53] synthesised silica nano-particles by precipitating silica where, alkali extraction and acid treatment was done to bring up the pH to 7 and aging it for 12 h thereby dried at 100oC. The purity of the nanoparticles obtained by precipitating silica from biomass flyash ranged from 44.42 to 93.63%. The only disadvantage in precipitation method is, the products obtained seems to be less pure compared to the products obtained from other methods.
2.2.2. Hydrothermal method
Hydrothermal processing can convert waste to value added products by heating at high temperature (120–550°C) and pressure (20–150 bar) with oxygen or an inert gas such as nitrogen. [54]. Solvothermal method is similar to hydrothermal method, but the only difference is in this method different solvents are used instead of water [55]. The materials prepared by this method contains many oxygen containing functional groups like C=O, C–O and O–H groups. Herein, C=O could generate oxygen (1O2) species which degrades the organic pollutant by solving the environmental problem of carbon rich waste [56]. Ferrite catalyst from electroplating sludge [14], Mganetic biochar from ferric sludge and biological sludge [33], sludge carbon/ TiO2 nanocomposite from anaerobic sludge [32], TiO2–FA nano-composites [30] and Tungsten oxide-FA nanocomposite [29] was prepared by hydrothermal method. It is considered to be an efficient method in treating wet solid waste rich in carbon, because it has more advantages than high temperature pyrolysis. The energy consumptions are low and the reaction conditions are mild and it has no requirement on the moisture content of the raw materials. So there is no need to dry the waste before hydrothermal treatment [56]. Importantly, the advantage of hydrothermal method is, it can be used to control material size, particle morphology, crystalline phase, and surface chemistry by controlling the reaction temperature, pressure and solvent properties and the main disadvantage is its high reaction temperature and pressure [55].
2.2.3. Microwave assisted synthesis
Microwave assisted synthesis method have been used in the synthesis of diverse inorganic materials such as metallic nanoparticles, amorphous and nanoporous materials, core-shell particles, semiconductors, bioceramics, pure and mixed metal oxides [57]. During synthesis process microwave energy strikes the material through molecular and ionic interaction of the precursor solvents and reducing agents [58]. Microwave radiation has also been used to detoxify sediment sludge and immobilization of metal ion within the sediment [59]. This method has many advantages over other techniques which includes, rapid heating, fast reaction, high yield and excellent thermal stability thus being one of the most effective method for nanoparticle synthesis [34]. Duan et al. [60] synthesized magnetic biochar from iron sludge and cotton stalk for the removal of Cr(VI) using microwave chemical reactor. Zhang et al. [34] successfully synthesized hydroxyapatite nanoparticles from phosphogypsum waste produced from phosphorus fertilizer factory and checked its application for fluoride removal from aqueous solution. This method is upright compared to other synthesis methods due to their low temperature operation and its quick method of producing crystalline nanomaterials [55].
2.2.4. Pyrolysis
Pyrolysis is one of the most effective methods which helps to produce materials with satisfactory surface area, stable structure, great ion exchange capacity and value added surface functional groups like −OH, −COOH, C-O/C=O [61]. Pyrolysis temperature and reaction time plays a major role in altering the surface chemistry of the material. It affects the surface affinity to adsorb water, which proves to be an important factor in adsorption reaction. In a study conducted by Bandosz [62], temperature increase from 650 to 950oC resulted in increase of surface pH. Thus, the surface properties, such as porosity, selectivity, or catalytic activity can be modified by changing the pyrolysis conditions. The catalytic activity of the product is directly related to the new surface chemistry formed by solid-state reactions during pyrolysis. The disadvantages of this method are release of toxic gas release during material synthesis process and high energy consumption [12].
2.2.5. Sol-Gel method
Sol-gel method is in existence since 1800s and is applied in the development of materials for catalysis, chemical sensors, membranes, fibres and used in scientific and engineering fields. In this technique, a colloidal suspension is formed from the precursors by hydrolysis, polycondensation, aging, drying and thermal decomposition. The materials synthesized by Sol-gel method were highly crystalline with smaller crystallite size when compared to materials prepared by hydrothermal method under similar reaction conditions [63, 64]. Inorganic metal salts or metal organic compounds are the mostly used precursors in this method. Concerning the gel formation, the factors that impact the properties include solvent type, water content, acid or base content, precursor concentration and temperature [55]. The most important advantage of this method is that, the nanomaterial with high surface area and stable surfaces are formed at the end of the process [65]. As in, single phase chromium doped α-alumina nanoparticles was synthesized using sol-gel method by recycling chromium containing tannery waste using an initial solution pH of 4.0 and calcined at 1,100°C [66]. Similarly, coral like hierarchical magnesium oxide incorporated fly ash composite is formed by agglomeration by sol-gel method and is used as an adsorbant for Reactive Black 5 azo dye from aqueous solution.
2.2.6. Calcination
Calcination is done for the structural transformation of waste, which focuses on final product of oxide and mixed oxide. When the calcination temperature is below 400°C, the simple metal oxide was formed. However, at calcination temperature higher than 600°C spinel oxide was formed. By controlling the calcination temperature interesting functional materials were potentially obtained. When the precursor is calcined at lower temperature it gets transformed into layered double hydroxide. And, when this product was immersed in water or placed in moist gas, it reconstructed and the process is called “memory effect.” Conversely, if the calcination temperature was too high, the spinel will become unrecoverable [13].
3. Application for Pollutant Remediation
Different types of nanocatalysts such as semiconductors and metal oxides are used for wastewater treatment herein the photocatalysts, electrocatalysts and Fenton-based catalysts are used to improve chemical oxidation of organic pollutants and antimicrobial actions [127]. Recently various research was carried out for the removal of organic and inorganic contaminants by the nanomaterials obtained through processing of industrial waste. In this part the application of industrial waste derived nanomaterial for pollutant remediation is discussed. Table 2 summarizes the method of removal of pollutant and their removal capacities using industrial waste based nanomaterial.
3.1. Heterogeneous Phototcatalytic Degradation
Heterogeneous photocatalysis is recognized as a very potential technology for the removal of organic contaminants from water [128]. Heterogeneous photocatalysis takes place on the surface of catalyst and the pre-adsorption of pollutants are essential for their degradation. This is attained by using large surface area supports for adsorption and high adsorption capacity for target material. Adsorption strength of the substrate should be fair enough for its diffusion into the loaded TiO2. Many support materials such as activated carbon, stainless steel, silica, zeolites or clay materials were used to prepare hybrid photocatalysts [129]. Recently, utilization of waste material as surface support of TiO2 based photocatalyst has paid attention.
3.1.1. Photocatalytic activity of Fly ash/TiO2 nanocomposites
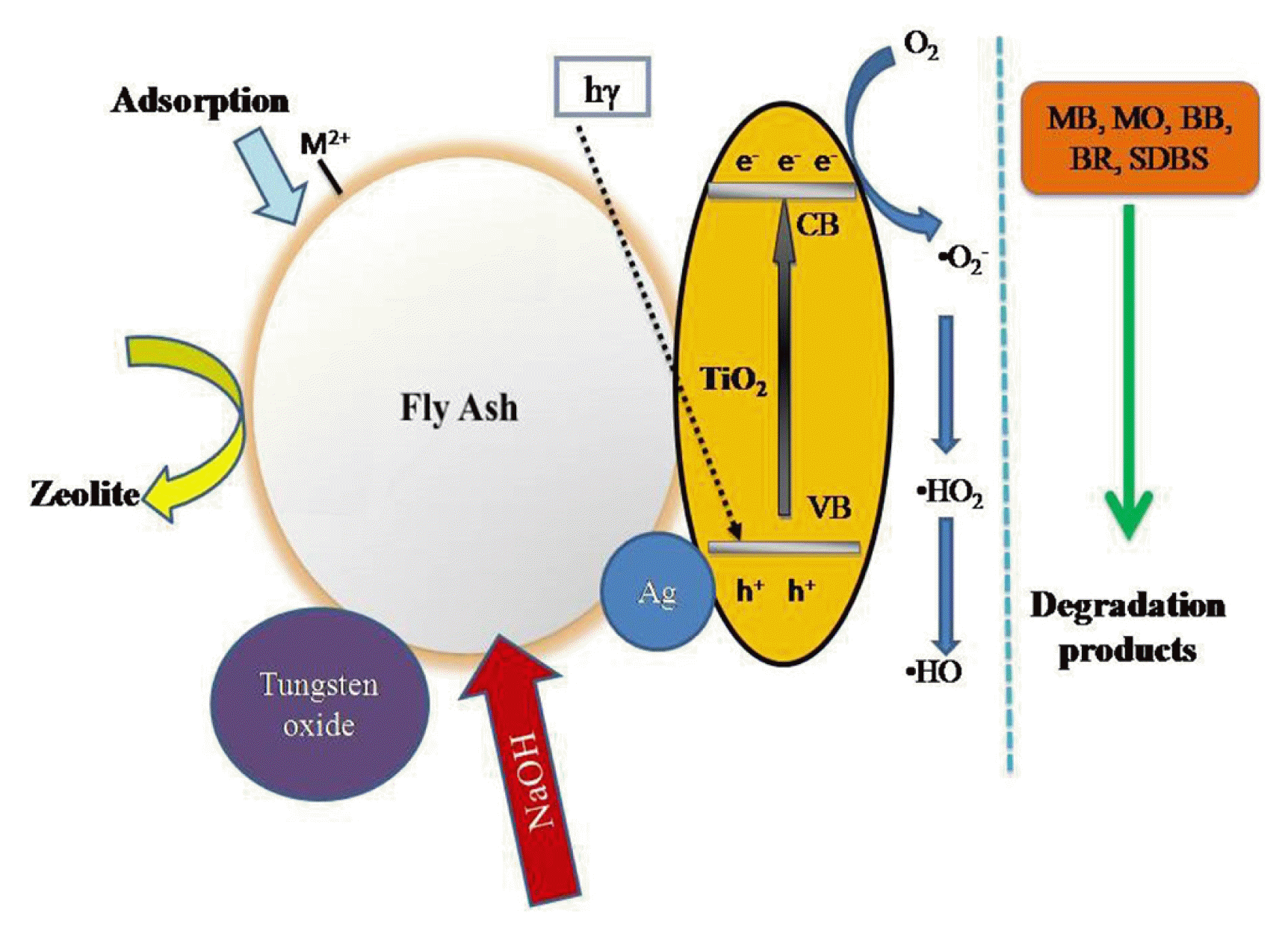
Fly ash serves as adsorption substrate along with TiO2 photocatalyst which represents an efficient and environmental friendly solution. The nanocomposites simultaneously removes two pollutants with different chemical structure and is accounted for the treatment of wastewaters loaded with heavy metals, dyes and surfactants [28, 127]. Fly ash is a mixture of unburned carbon and metal oxides in which Fe2O3 and MnO can act as in situ Fenton-systems and TiO2 being likely active in photocatalysis. Waste fly ash are promising support materials because of their surface composition, charge and morphology [80]. The photo-degradation and adsorption of Methylene blue, Bemacid Blau and Bemacid Rot, surfactant SDBS, Cd and Cu by Fly ash/TiO2 nanocomposites was extensively studied by Visa et al. [30, 79, 80, 82] The alkali activation can develop new active site (≡≡SiO−) and (≡≡AlO−) on FA surface which allows metals to be complexes at the surface. The change in chemical and structural property induce surface modification resulting in difference in surface affinity for Cd2+ and Cu2+ before and after treating with 2N NaOH [81]. Yang et al. [84] investigated the degradation effeciency of Rhodamine B by Zeolites fly ash bead (ZFAB)/TiO2 composite and benzene by Ag@TiO2/ZFAB. The composite was prepared by alkali activation by NaOH and mixing with TiO2 nanomaterial. The degradation efficiency of RhB dye reaches 83.8% after five cycles. This indicates that the prepared material has a good recyclability for RhB degradation. The removal rate of benzene gas reached 96.3% after three cycles, indicating the Ag@TiO2/ZFAB modified photocatalytic cementitious material exhibits constancy.
3.1.2. Photocatalytic activity of other nanocomposites
Photocatalytic mechanism involves the generation of electron - hole pairs upon irradiation of light which results in the formation of oxidizing and reducing agents on the surface of semiconductors. These oxidizing and reducing agents favour the degradation of organic pollutants in the presence of photons. Several semiconductor metal oxide photocatalysts including CdO-SnO2, ZnO-Cu2O, Cu2O/ TiO2, SnO2-TiO2, SnO2-Graphene, SnS2-SnO2, TiO2/CuS, NaNbO3/ CdS etc., have been developed, which can extend the absorption range to the visible region thereby suppressing the recombination of electron-hole pairs to improve the photocatalytic efficiency of the photocatalyst [132]. The zeta potential value of Magnetic iron oxide nanomaterial derived from industrial waste was positively charged at pH 2 and 3 and negatively charged from pH 5 to 10. The catalyst surface adsorbed H+ ions in the acidic medium and the basic medium due to the presence of OH− ion thus two anionic dye (Methylene blue and Congo Red) and cationic dye (Rhodamine B) were degraded. The photocatalytic degradation of magnetic iron oxide nanopowder showed a better recyclable capacity of 78% upto 10 cycles [67].
Tungsten oxide (WO3), a semiconductor photocatalyst with 2.8 eV band gap, is activated by a portion of the visible light irradiation (λ < 442 nm). It has drawn interest as an alternative for TiO2. The tandem’s concept could be extended by mixing WO3 with other semiconductors, with suitably aligned band gap. As fly ash (FA) contains traces of a TiO2, it is used as photocatayst along with Fenton precursors (Fe2O3, MnO) which can participate in the hydroxyl generation process. Visa et.al., explored novel composite based on tungsten oxide and fly ash by hydrothermal synthesis and used as a substrate to treat Bemacid Blau - BB and Bemacid Rot – BR dye and Cu2+ heavy metal ion [29]. Zeolites fly ash bead/TiO2 composite was prepared by Alkali activation and mixing with TiO2 and is used for Rhodamine B dye degradation [84]. Additionally, Carbon based nanomaterial prepared from anaerobic sludge impregnated with TiO2 serves as a catalyst to degrade Bisphenol A. TiO2 addition on the surface of sludge carbon increase the mobility of charges, electro negativity and electro affinities, which in turn enhances photocatalytic activity [32].
3.2. Heterogeneous Fenton and Photo-Fenton Degradation
Fenton’s reagent is a mixture of ferrous ions and hydrogen peroxide (H2O2), which forms active oxygen species capable of oxidizing organic contaminants. Photo-Fenton process combines the effect of the Fenton’s reaction and UV-Vis to produce hydroxyl radicals along with the photo-reduction of the ferric ions to form ferrous ions, which in turn contribute to the Fenton’s reaction. This creates more hydroxyl radicals compared to the classical Fenton and hence accelerates the oxidation process, which is considered as an advantage of this process [133].
Hydrothermal synthesis of Ferric sludge and biological sludge derived from the dyeing wastewater treatment process yielded magnetic biochar and degradation efficiency Methylene blue was catalyzed by Fenton process (Fig. 2). Dyeing wastewater was treated with Magnetic biochar, removal efficiency of Methylene blue was 98% and the catalytic efficiency was stable upto 4th cycle. The COD removal rate was 47 ± 3.3% and TOC removal was observed to be 49 ± 2.7% [33]. Previously Zhou at al. [76] synthesized magnetic biochar, a heterogeneous catalyst derived from paper mill sludge for the Fenton-like degradation of methylene blue with 93.3% removal efficiency. For the degradation of Bisphenol A, ferric sludge was used as catalyst source along with calcium peroxide and the ability of chelating agents, such as citric acid, oxalic acid, ethylene diamine tetra acetic acid and tartaric acid to hasten the BPA degradation and to reduce the Fe3+ dosage was assessed [88]. Meanwhile, Kong et al. [68] reported the conversion of iron rich sludge into Fe-SC hybrid Fenton-like catalyst by carbothermal process for the degradation of AOII. The efficiency AOII degradation achieved within 20 min at 30oC temperature and pH 7. Magnetic separable MnFe2O4 nanoparticle and CuFe2O4 was obtained from synthesised by co precipitation of heavy metal containing pickling waste liquor (PWL) and electroplating wastewater (EPW). The fenton like degradation of Rhodamine B removed up to 99% of dye and remained unchanged upto 8 cycles [23]. Similarly, Huang et al. [26] reported removal fenton like degradation of Bisphenol A, close to 100% and TOC 45%, by Fe3O4 nanoparticles from steel pickling waste liquor.
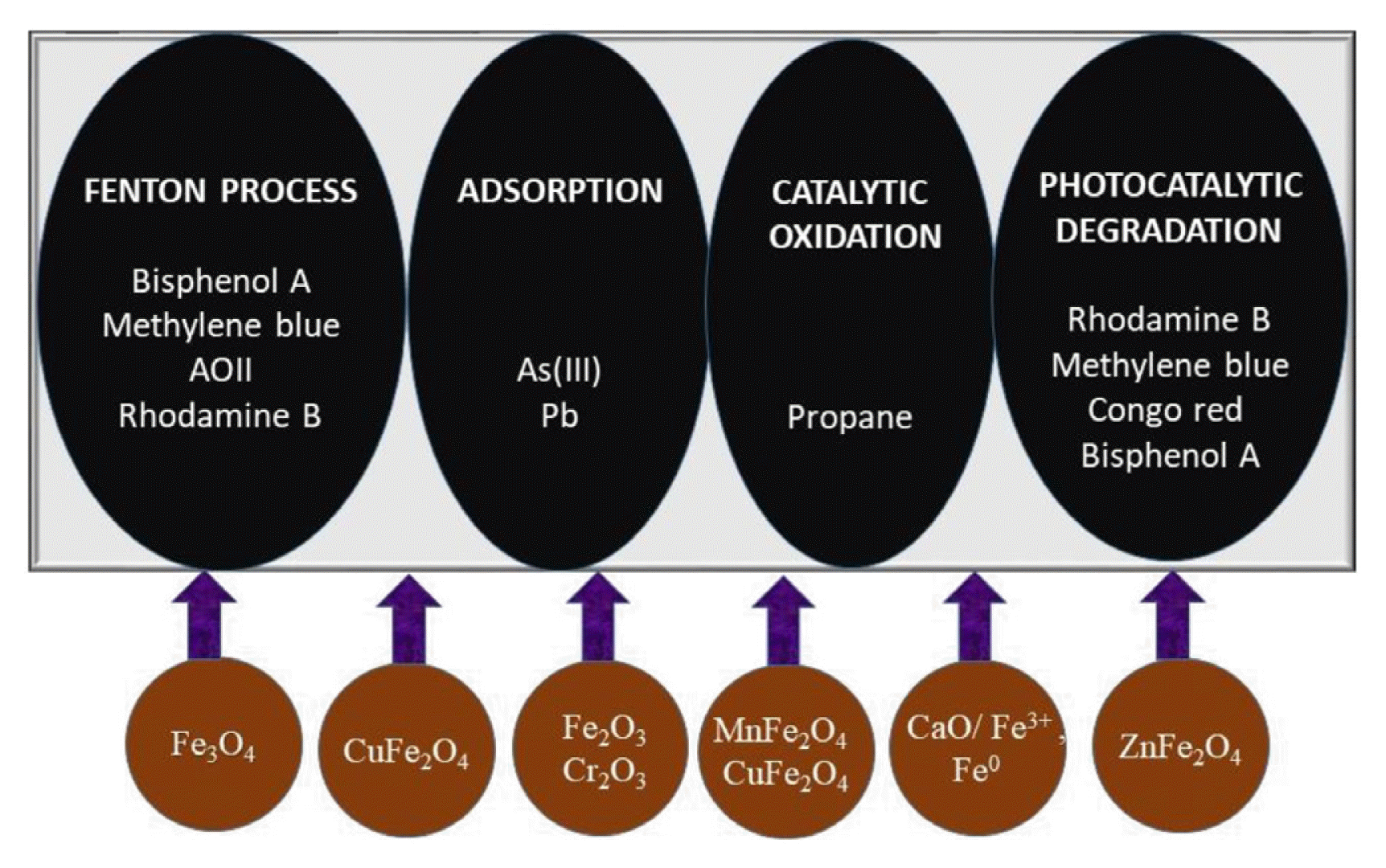
Schematic representation of iron waste derived catalyst and its application in pollutant remediation.
A zinc ferrite catalyst was prepared by the calcination of electroplating sludge (ES) for efficient degradation of methylene blue. The catalyst displayed 88.3% decolourization efficiency of methylene blue in the presence of H2O2 combined with UV irradiation. The small amount Al and Mg present in the sludge might also contribute to the catalysis[11]. The ferrite based fenton catalyst synthesized from industrial waste proves to be efficient in removing dye from wastewater and stable after recycling.
3.3. Catalytic Degradation
Waste as raw material for the synthesis of catalyst has gained interest due to the demand of catalyst in various industrial sectors. Catalytic reduction of 4-nitrophenol to aminophenols is investigated in the study conducted by Cunha et al. [66]. The chromium waste from tannery industry is doped with Alumina to synthesize catalyst to reduce 4-nitrophenol to aminophenols, which has wide application in the synthesis of industrial dyes, pharmaceuticals, and other biologically active compounds. The kinetics of the catalytic reduction of 4-nitrophenol was very fast, varying from 45 s to 40 min that are strongly influenced by the concentration of chromium ions in the α-alumina matrix. The efficiency of the recycled wastes in reducing 4-nitrophenol was dependent on their purity, with the conversion rates for the wastes varying from 55% to 98%. The catalytic capacity of waste derived catalyst is similar to those reported for high-purity catalyst [66]. Recently Sulfate radicals and hydroxyl radicals are commonly used as oxidizing agents for degradation of organic compounds. Usually, SO4 − are produced by the activation of peroxymonosulfate (PMS). Zhang et al. [56] fabricated sludge-based biochar material doped with Fe3O4 by hydrothermal carbonization and activated by peroxymonosulfate for degradation of Rhodamine B of 50 ppm in 10 min. Catalytic oxidation of Volatile organic compound employing propane as a model gas was explored by Sushil et al. [6]. Wastes from the aluminium industry, the tannery industry and the electroplating industry were tested as precursors for catalysts. The quantity of ferrite in red mud and chromite in tannery shavings governs in determining the catalytic reduction of propane. By adding chromium containing waste to red mud, the catalytic activity increased. A 50% conversion of propane was achieved in the range 320–380oC. Much less studies were reported that deals with the catalytic conversion by pollutants by utilizing waste derived materials as catalyst.
3.4. Electrocatalysis
Electrocatalysis is accompanied by charge transfer processes that play important role in sustainable development and technological advancement. The major electrochemical processes include, electrochemical reduction of CO2 into synthetic liquid fuels, water splitting to generate molecular hydrogen from water, energy recovery in fuel cells and solar cells. The development of nanomaterials for electrocatalysis is a vibrant research field [134]. Waste containing high organic content as well as low content of metals is generally used to synthesize electrocatalyst, since metal dopant played an important role in enhancing electrocatalytic activity. The sludge with complex organics are possibly used as a low cost precursor to synthesize electrocatalysts for H2O2 generation. Using high organic content porous carbon electrode was formed. The components present in waste such as N, S and P are transformed to heteroatoms on the electrode surface [13]. The performance of electrocatalysts and its efficiency depend upon its structure, components and surface characteristics, which depend on the method of synthesis. Catalyst derived from thermally treated electroplating sludge is used in the electrochemical reduction of CO2 with a peak potential of −0.3 V, as measured using a linear sweep voltammogram proves to pocess good electrocatalytic activity. Additionally, it is used in a microbial electrolysis cell in converting CO2 to methane, ethylene, carbon monoxide and acetate with faraday efficiencies of 37.3%, 25.9%, 7.8% and 6.8% at an external potential of 0.6 V, respectively [10].
3.5. Adsorption of Pollutant
Pollution was recognized as one of the significant threat to human-kind; in this manner an ever increasing number of examinations are committed to executing the idea of expelling waste by utilizing squanders [130]. To avoid the environmental pollution many methods were developed for removing contaminants. Adsorption is widely considered to be an important physicochemical process and its practical application relies upon recovering and reusing the spent adsorbents [135]. Non-conventional adsorbents usage is getting importance and several low cost materials including wood dust, neem bark, rice bran, leaf dust, agricultural wastes, biological wastes, clarified sludge, flyash, kaolinite clay and zeolite are used [78]. Utilization of waste as an adsorbent to remove the pollutants from the environment has gained interest in recent years. It has gained much attention because it simultaneously solves two problems: pollutant removal and application of waste as adsorbent [79]. Nano-sized particles are attracting considerable interest as adsorbent due to their high surface area and thus enhancing the adsorption of pollutant on their surface [136]. Many researches have been carried out to remove heavy metal and dyes using industrial waste derived nanomaterials as an adsorbent. Removal of Cr(VI) by tea waste/ Fe3O4 nanocomposite [24] and Fe loaded magnetic biochar [60], As(V) removal by alum based adsorbent [73], Pb(II) removal by Calcium hydroxyapatite nanomaterial [75] and Fe2O3 in red mud and Cr2O3 in tannery shavings [61], Pb(II) and Cu(II) removal by Magnetic chitosan sludge composite [41] Methylene blue and Congo red removal by Fe3O4 [70], F−removal by Hydroxyapatite Nanoparticles (nHAp) [34] and H2S removal by sludge based nanocomposite [62,86]. Steel slag has potential to adsorb Ni(II) from aqueous solutions due to its high concentration of magnetite and its adsorption rate increased with initial concentration but decreased with increase in temperature, due to competition by other ions dissolved from steel slag [127].
The adsorption mechanism of pollutants on the nanocomposite denotes to physical adsorption, electrostatic interaction, reduction, ion exchange, surface complexation, etc. Tu et al. [48] studied the adsorption behaviour of As(III) and Pb on to copper ferrite nanomaterial synthesized from the sludge of printed circuit board (PCB) industry. He found that higher the pH value decreased the adsorption capacity of copper ferrite due to enhanced repulsion between As(III) and the adsorbent surface [48, 69]. Manna et al. [98] synthesized graphene oxide coated over pre-treated carbonaceous industrial for the separation of phenol which sowed better separation performance than that of the graphene oxide. Similarly, meso-macropore adsorbents were prepared from hybrid of biological sludge and chemical sludge that showed higher adsorption than that of the commercial activated carbon due to the well-developed mesopore and macropore structure, as well as abundant acidic surface functional groups [87]. While, Cation adsorption involves mainly electrostatic forces therefore the surface energy of the substrate strongly influences the adsorption process [131]. The pH is one of important parameter which significantly affect the adsorption capacity by influencing (1) the surface charge of the adsorbent, (2) the degree of ionization of the adsorbate and (3) extent of dissociation of functional groups [70]. The maximum adsorption capacity of As(III) was found to be 41.2 mg/g at pH 4.2, much higher than most of the adsorbents. Subsequently, over 90% of lead was removed at pH 4.5 using copper ferrite nanoparticle [48, 69]. Visa, 2016 investigated zeolite as adsorbents for the simultaneous removal of heavy metals (Cd2+, Cu2+, Ni2+, Zn2+ and Pb2+). The increase in the substrate amount creates more active centres favourable for adsorption of heavy metals. New active centres (≡ SiO−) and (≡AlO−) has developed on zeolite surface forming complex structures with metal cations thereby replacing the exchangeable Na+, K+ cations [31]. Increased ion exchange capacity was observed by S.Fan et al. [24] when the Cr(VI) was removed by the Tea Waste/Fe3O4 nanocomposite.
3.6. Disinfection Using Industrial Waste Derived Nanomaterial
Antibacterial agents are compounds that kill bacteria or slow down their growth and have been used in many fields, such as, textile industry, water disinfection, food packaging, and medicine [137]. Antibacterial activity of Ag-Fe3O4 nanoparticle synthesized from iron ore tailing was carried out by using spot inoculation method using Escherichia coli, a gram-negative bacterium cultured in agar media and compared with Fe3O4, hydrophobic and hydrophilic Ag-Fe3O4 nanomaterials. The result revels that Fe3O4 does not show any zone of inhibition area whereas hydrophobic and hydrophilic Ag-Fe3O4 exhibit significant zone of inhibition area from 60–120 minutes confirming the role of Ag. Silver and its compounds are shown to be effective against both aerobic and anaerobic bacteria by precipitating bacterial cellular proteins and by blocking the microbial respiratory chain system. Hence, magnetite nanoparticles derived from iron ore tailings can act as a good support for silver nanoparticles to use it effectively in the water disinfection [35]. Similarly, Boruah et al. [67] studied about the inactivation of biological contaminants by photocatalytic method under irradiation of natural sunlight in an aqueous medium. Magnetic mixed metal oxide nanomaterial synthesized from industrial waste showed 96% inactivation of E.coli bacteria within 120 min at pH 5 with the catalytic loading rate of 1 g/L and E.coli concentration 200 mg/L.
The above equation explains the inactivation mechanism, where, •OH formed during the reaction acts as an oxidizing agent. Hydroxyl radical is responsible for the oxidation of polyunsaturated phospholipids as well as destruction of bacterial cell membrane resulting cell death [67].
4. Conclusions and Recommendations
The increasing generation of industrial waste has forced the researchers to work on effective utilization of waste to achieve the concept of treatment of waste and wealth from waste. As nano-technology is the fast growing field and by considering its applications in various field, nanomaterial synthesis from waste is considered to be an effective way which offers valuable recovery of elements and economic advantages. However, to solve the problem arising due to increased generation of waste and exploitation of primary resources research needs to be done to improve and develop the recovery process. Industrial waste such as Tannery waste, electroplating industry waste, papermill waste, steel industry waste, fly ash, jute mill industrial waste, silicon waste etc., have been utilized in synthesizing waste derived nanomaterials and each method of synthesis has its own advantage. More specifically, the pretreatment of waste influences the properties of the nanomataerial by affecting the surface chemistry. It is clear that the physical and chemical factors during synthesis process affect the formation of nanomaterial. Therefore, the practicability of synthesis methods, for large scale production of waste derived materials should be taken in to consideration. Specifically, further studies needs to be carried to manage the toxic gases that are released during the thermal synthesis method. The waste derived nanomaterial synthesized by physico chemical methods have wide application in environmental remediation. The nanomaterials synthesized from industrial waste include Fe3O4, MFe2O4 (M− Mn, Cu, Zn, Ag), magnetic biochar, MgCr2O4, Cr2O3, CuO, Copper ferrite, etc., Among which iron rich waste is widely used and has been proven to be effective in removal of pollutant by fenton degradation, photocatalytic degradation and adsorption method. Despite the application in pollutant remediation, it is important to study the hazard that may cause by the by-products during reaction or leaching back of original pollutants. However, for further large application of waste derived nanomaterials, LCA needs to be done by focusing on energy consumption, pollution reduction and cost efficiency. Additionally, by increasing stability and activity of the nanomaterials, sustainable zero waste can be achieved.
Notes
Author Contributions
J.V.J. (Research Scholar) wrote the manuscript. N.V. (Professor) revised the manuscript.