Study on treatment of acid mine drainage by nano zero-valent iron synergistic with SRB immobilized particles
Article information
Abstract
In view of the serious pollution and high cost of treatment of acid mine drainage (AMD) in coal mine, the polyving akohol (PVA) and boric acid embedding cross-linking method was used to prepare the immobilized particles for treatment of AMD with sulfate-reducing bacteria (SRB) and nano zero-valent iron (nano-Fe0) as the main body. In order to explore the specification and dosage of each matrix component of immobilized particle, a series of single factor tests and orthogonal tests were carried out to determine the optimal ratio of each matrix component. The results shows that when the SRB quality additive percentage was 30%, the nano-Fe0 dosage was 4%, the corn cob particle size was 60 mesh and the dosage was 3%, the SO42−, Cr6+ and Cr3+ removal rates were 82.99%, 99.78% and 38.78%, respectively, the TFe and COD release rates were 4.26 mg·L−1 and 1,033.4 mg·L−1, respectively, and the pH value was 8.04, and the treatment effect was the best.
1. Introduction
AMD is characterized by low pH, high sulfate content, and a variety of highly toxic heavy metal ions [1]. Such water, if discharged directly into the natural water, will cause serious pollution to the environment and greatly endanger the safety of animals, plants and human life. Therefore, it is urgent to repair the AMD produced by coal mining.
In recent years, domestic and foreign scholars have carried out a lot of research on treatment and remediation. At present, the basic treatment methods include neutralization method [2], wetland method [3] and microbial method [4]. However, the neutralization method will produce a large amount of sediment rich in heavy metals, which not only needs to be cleaned regularly, but also can easily lead to secondary pollution due to improper disposal [5]. Although the wetland method is easy to maintain and manage, its application is also restricted because it covers a large area and has a low treatment load, so H2S produced by microorganisms cannot be thoroughly treated, and the treatment effect fluctuates greatly with the season [6]. In recent years, microbial method has become a research hotspot in the world due to its advantages such as multiple types of heavy metals, thorough treatment, low treatment cost and environmental friendliness [7]. SRB is a prokaryotic microorganism living in an anaerobic environment. It can reduce SO42− and S2− by organic matter, as shown in Eq. (1)–(2) [8, 9]. The S2− produced by SRB metabolism is easy to form stable metal sulfide precipitation with heavy metal ions [10]. SRB has good regulation effect on sulphate, heavy metal and pH value in AMD. Therefore, SRB has become the representative microorganism in microbial method.
The growth and metabolism of SRB requires the use of carbon sources. However, traditional carbon sources cannot release organic matter slowly, and SRB metabolism and utilization of organic matter are limited, which can easily lead to excessive COD concentration in the water in the early stage of the experiment and insufficient organic matter in the later stage [11], which greatly affects the effect of treating AMD by biological method.
To a great extent, it affects the effect of treating AMD by biological method. In contrast, abandoned biomass materials commonly found in agriculture, such as corn cobs and peanut shells, can continuously and stably provide carbon sources for SRB through the slow release of carbon sources under the action of hydrolyzed microorganisms [12]. In order to make the carbon source be better used by SRB metabolism without causing secondary organic pollution of water, and considering the huge yield of corn crops in northern China, which is easy to obtain, and the high content of glucose and galactose in the corn crop [13], this experiment adopts the cohesive carbon source method and adopts the corn cob as the immobilized grain nutrient.
In addition to sufficient carbon sources, SRB also needs enough electron donors to meet metabolic activities during its growth [14]. Zero-valent iron is chemically active and has a low electrode potential (E0(Fe2+/Fe) = −0.440 V; E0(Fe3+/Fe2+) = 0.771 V), therefore, it has a high reduction capacity and can reduce ions, compounds and some organic compounds with strong oxidation properties [15]. The oxidation of Fe0 particles produces the low redox potential environment required by SRB, which achieves the optimal growth of SRB, and SRB can use the H2 produced by iron oxidation as an effective electron donor [16]. Nano-Fe0 is an ultrafine iron powder with particle diameter of order of magnitude between 1–100 nm. Compared with conventional zero-valent iron particles, nano-Fe0 has a larger specific surface area, which can provide more active sites for pollutants, thus improving treatment efficiency. At the same time, the enhanced surface activity can handle more types of pollutants, which is more conducive to the synchronous removal of multiple pollutant ions in AMD [17]. nano-Fe0 can effectively degrade various pollutants such as nitrate, heavy metal and dye, and is widely used to treat pollutants in aqueous solutions [18]. It is reported that the SRB-Fe0 system has a synergistic effect on the removal of heavy metals. Fe0 can effectively remove metals, and finally the SRB can form relatively stable metal sulfides [9]. In the SRB-Fe0 system, SRB and Fe0 are directly in contact with pollutants, and low acidity and high concentration of heavy metals are not conducive to the growth of SRB. Microbiological immobilization technology can effectively avoid this problem by embedding SRB and nano-Fe0 into particles, promoting the continuous growth of SRB and its adaptability to harsh environment.
Based on this, this paper proposes to prepare nano-Fe0 SRB immobilized particles by using microbial immobilization technology, using nano-Fe0 and SRB as cohesive carbon source, and using corn cob as cohesive carbon source for the treatment of AMD. Single factor test and orthogonal test were carried out to determine the optimal ratio of SRB dosage, nano-Fe0 dosage, corn cob dosage and corn cob particle size, so as to obtain a kind of immobilized grain with high treatment efficiency and strong adaptability. On this basis, EDS, XRD and SEM were used to analyze the composition, surface and internal structure of the particles before and after the reaction. Further research on the internal mechanism of immobilized particles to remove pollutants is expected to provide some theoretical reference for the practical application of nano-Fe0 SRB immobilized particles.
2. Material and Methods
2.1. Experimental Materials and Water Samples
The corn cob used in the experiment was taken from the local farmland in Fuxin, Liaoning province. After washing, drying, crushing and sieving, the corn cob was made into 60 mesh, 100 mesh and 200 mesh particles respectively. The nano-Fe0 particles selected for the experiment were purchased from Beijing Deke Daojin Science And Technology Co., LTD., with a particle size of 100 nm and a purity of 99.99%. Nano-Fe0 is sealed and stored in a dry and cool environment. It is not suitable to be exposed to the air for a long time to prevent agglomeration caused by moisture.
The SRB were cultured in the laboratory. The surface moist soil at the foot of gangue hill, Xinqiu District, Fuxin city, Liaoning Province was used as SRB seeding mud. Wipe the inside of the zipper bag with 75% ethanol, get the zipper bag after sterilization, and put the soil into the zipper bag to seal. The soil samples retrieved from the outdoor were immediately added to sterile deionized water at a solid-liquid ratio of 20 g/100 mL. Under aseptic conditions, 100 mL of the above liquid was added to the 400 mL modified Starkey medium, and the SRB was obtained by enrichment semi-continuous culture in an (37 ± 1)°C constant temperature CO2 anaerobic incubator. The above SRB was inoculated into the new medium at 10% of the inoculation amount every 5 days, and inoculated for 4 times continuously, until the culture medium produced a large amount of black precipitation, and the smell of rotten eggs when the bottle mouth was opened, indicating that a large amount of SRB had been enriched in the medium. The concentration of the cultured SRB was 1 × 106 mL−1.
The test water sample is a simulated water sample configured according to the water quality characteristics of the local mining area. Among them, the water quality test results of the local mining area were pH = 4.0 ± 0.2, and the mass concentrations of SO42−, Cr6+, Cr3+, Mn2+, Cu2+ and Zn2+ were 816 mg·L−1, 9.9 mg·L−1, 20.1 mg·L−1, 1.6 mg·L−1, 0.8 mg·L−1 and 1.2 mg·L−1, respectively. Therefore, the mass concentrations of the SO42−, Cr6+ and Cr3+ in the simulated water samples were 816 mg·L−1, 10 mg·L−1 and 20 mg·L−1, respectively, with a pH value of 4.0.
2.2. Preparation Method of Immobilized Particles
The immobilized particles were prepared by using the previous research results of the research group [19]. The mass percentage of 9% PVA and 1% sodium alginate were dissolved in distilled water and placed in a sealed container at room temperature at 25°C. After 24 h, the product was fully bloated and put into a thermostatic water bath. The product was heated at 90°C for 1.5 h and stirred continuously until it was bubble-free. The measured nano-Fe0 material and the corn cob powder were slowly added into the gel, fully stirred and evenly removed, and sealed and cooled to 37 ± 1°C at room temperature. 1–2 The gel mixture was absorbed by peristaltic pump and directly dropped into 2% CaCl2 saturated boric acid solution. During the process, the agitator was used for cross-linking at a stirring rate of 100 rpm. After 4 h, take out the particles, rinse them with 0.9% normal saline, and then suck up the surface water, and reciprocate for 3 times. The particles were activated in an anaerobic environment for 12 h using an improved Starkey culture medium solution with no organic components. The immobilized particles were prepared by this method in subsequent experiments.
2.3. Water Quality Monitoring Methods
SO42−: barium chromate spectrophotometry; Cr6+: diphenylcarbazide spectrophotometry; Cr3+: potassium permanganate-diphenylcarbazide spectrophotometry; TFe: phenanthroline spectrophotometry; COD: Rapid digestion spectrophotometry; pH: glass electrode method [20].
3. Results and Discussion
3.1. Single-Factor Experimental Study
3.1.1. Determination of SRB dosage
According to the preparation method of immobilized particles mentioned above, 200 mesh corn cobs with 5% mass fraction were added into the mixture gel, stirred and cooled to room temperature evenly. Then, SRB microbial community fluids with mass fraction of 10%, 20%, 30%, 40% and 50% were added and fully stirred evenly for the preparation of 5 kinds of immobilized particles, which were then added into a series of conical bottles according to the ratio of solid to liquid 1:10, and each experiment was repeated for three times. Sampling was conducted regularly every day to determine the concentration of characteristic pollutants and control the amount of pollutant release, calculate the removal rate of pollutants, and draw images from the average value of repeated experiments, so as to seek the best SRB bacterial colony liquid input. The test results are shown in Fig. 1.
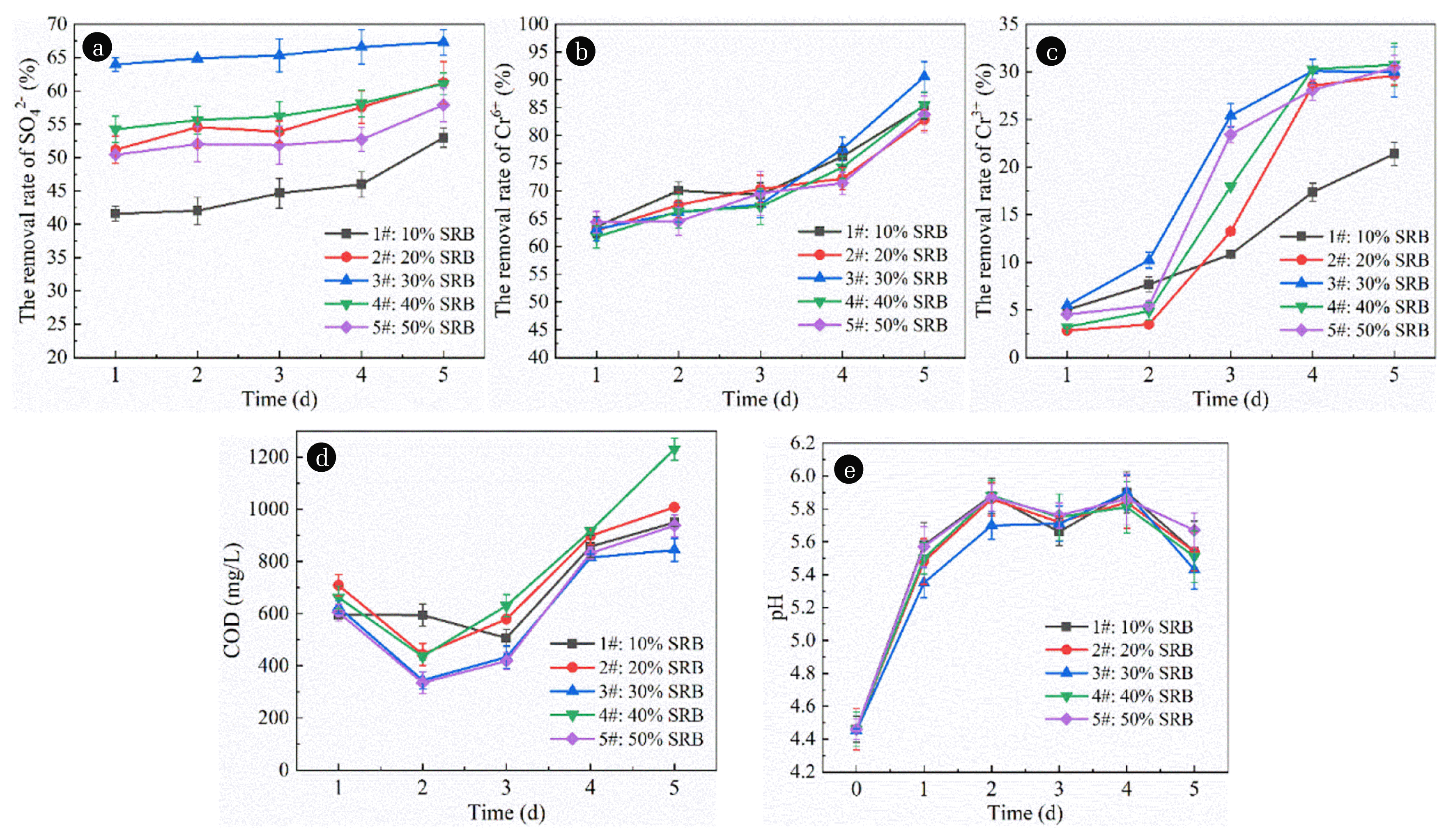
Effect of SRB dosage on immobilized particles treatment of AMD. (a) Effect of SRB dosage on the removal of SO42− by immobilized particles. (b) Effect of SRB dosage on the removal of Cr6+ by immobilized particles. (c) Effect of SRB dosage on the removal of Cr3+ by immobilized particles. (d) Effect of SRB dosage on COD release of immobilized particles. (e) Effect of SRB dosage in immobilized particles on pH in AMD.
3.1.1.1. The analysis of SO42− removal effect
As shown in Fig. 1(a), with the extension of time, the removal rate of SO42− shows an obvious upward trend. When the SRB dosage was 10%, 20%, 30%, 40% and 50%, respectively, the average removal rate of SO42− was 45.44%, 55.72%, 65.61%, 57.09% and 53.04%, respectively. This is because when the dosage of SRB is low, too few strains cannot well adapt to the inhibition of its activity by the strongly acidic environment in the wastewater, resulting in its limited activity and its ability to dissimilate and reduce SO42− to form S2− [21]. However, when the dosage of SRB is too large, due to the competitive growth relationship among strains within the bacteria group, too many strains will make the competitive effect more intense and significant [22], thus reducing the metabolic activity of part of SRB, thus affecting the removal effect of SO42−.
3.1.1.2. The analysis of Cr6+ removal effect
As shown in Fig. 1(b), the removal rate of Cr6+ shows an obvious rising trend with the extension of time. When the SRB dosage was 10%, 20%, 30%, 40% and 50%, the average removal rate of Cr6+ was 72.86%, 71.14%, 73.01%, 71.00% and 70.75%, respectively. This is because when the dosage of SRB is low, the amount of biological organic carbon source used by the strain is limited, so the remaining unhydrolyzed corn cob can still be used as an adsorbent to adsorb and remove Cr6+. Therefore, the removal rate of Cr6+ is at a high level. However, when the SRB dosage is high, the pH value of the system is still in weak acidity, which is not conducive to the precipitation of chromium hydroxide. It has been reported that the appropriate pH value is the key to the effective removal of Cr6+ by SRB, and the slightly acidic solution will limit the growth of SRB, resulting in the reduction of Cr6+ removal rate [23]. In addition, it can be seen from Fig. 1(a) that when the SRB dosage is high, the removal effect of SO42− is poor, so there are less H2S and S2− free in the solution. However, H2S and Cr6+ can undergo a redox reaction to convert Cr6+ to low toxicity Cr3+ [24]. S2− can be combined with Cr6+ and Cr3+ to form metal sulfide precipitation. Therefore, the reduction of H2S and S2− also leads to a decrease in the Cr6+ removal rate.
3.1.1.3. The analysis of Cr3+ removal effect
As shown in Fig. 1(c), the removal rate of Cr3+ shows an obvious upward trend with the extension of time. When the SRB dosage was 10%, 20%, 30%, 40% and 50%, respectively, the average removal rate of Cr3+ was 12.44%, 15.52%, 20.24%, 17.42% and 18.40%, respectively. Thus, the overall average removal rate of Cr3+ is at a low level, only about 20%. This is because the Cr6+ in the wastewater will be reduced to Cr3+ by some reducing materials or microorganisms [25]. When the removal effect of Cr6+ is good, the amount of it will be reduced to Cr3+ is large. However, due to the limited adsorption capacity of the material to Cr3+, the removal effect is poor, namely, the better the removal effect of Cr6+ is, the worse the corresponding removal effect of Cr3+ will be. However, for 3#, due to its best removal effect on SO42− and the presence of a large number of free S2− in the wastewater, Cr3+ and its ability to form metal sulfide precipitation removal are enhanced, so its removal rates of Cr6+ and Cr3+ are at a high level.
3.1.1.4. The analysis of COD release
As shown in Fig. 1(d), with the extension of time, COD release shows a trend of first increasing, then decreasing and then increasing. This is due to the presence of some fine particles on the surface of the cob at the beginning of the reaction. These fine particles can be rapidly hydrolyzed into small molecular organic compounds in water. However, in the early stage, the activity of SRB is relatively low and the amount of carbon source used is limited, so a large number of organic compounds are accumulated and released into the water, resulting in a significant increase in COD release. Then, when SRB adapts to the wastewater environment, its metabolic activity is significantly enhanced and carbon source utilization rate is correspondingly increased, so COD shows an obvious downward trend. With the continuous progress of the reaction, the corn cob with a larger particle size began to hydrolyze, releasing more COD, so the COD release amount in the later period increased significantly. When the SRB dosage was 10%, 20%, 30%, 40% and 50%, respectively, the average COD release amount was 700.2 mg·L−1, 727.4 mg·L−1, 612.8 mg·L−1, 776 mg·L−1 and 626 mg·L−1, respectively. This is because when SO42− removal effect is good, COD/SO42− should be at a higher level [26], and the COD release amount should be correspondingly higher, so the COD release amount of 4# and 2# particles is significant. For particles 1# and 5#, due to the small dosage of SRB of particles 1# and large dosage of SRB of particles 5#, and the limited capacity of using carbon source in a small dosage of SRB, the COD amount of effluent of 5# is lower than 1#. The removal effect of 3# particles on SO42− and Cr is good, indicating that the SRB activity in 3# particles is strong, the COD consumed by SRB metabolism is high, and the COD released in water is relatively low.
3.1.1.5. The analysis of pH effect
As shown in Fig. 1(e), with the extension of time, pH shows a trend of fluctuation and slight decline in the later stage of rise. This is because the corn cob has not been massively hydrolyzed in the early stage of the reaction, and the active groups on the surface of the corn cob, such as hydroxyl, alcohol, carboxyl and amino groups, can adsorb H+ to the surface of the corn cob [27, 28], so the pH significantly increased in the early stage. The hydrolyzed corn cob, as a carbon source, enhances the metabolic activity of SRB, which can release a certain alkalinity [29] during metabolism, contributing to the increase of pH. However, the effect is not significant, so the pH is only maintained at a certain level. When the SRB dosage was 10%, 20%, 30%, 40% and 50% respectively, the average pH value of effluent was 5.72, 5.69, 5.62, 5.70 and 5.76, with little difference.
To sum up, by comparing the removal effects of the immobilized particles on SO42−, Cr6+ and Cr3+ and the ability to improve COD release and pH of the 5 groups, and taking into account the changes of the indicators of the 5 groups, the optimal SRB dosage was finally selected as 30% in the test.
3.1.2. Determination of Nano-Fe0 dosage
According to the preparation method of immobilized particles mentioned above, 200 mesh corn cobs with mass fraction of 5% and nano-Fe0 with mass fraction of 1%, 2%, 3%, 4% and 5% were added into the mixture gel. Then the SRB microflora solution with mass fraction of 30% was added to prepare 5 kinds of immobilized particles. The prepared immobilized particles were placed in AMD and each experiment was repeated three times. Sampling was conducted regularly every day to determine the concentration of characteristic pollutants and control the amount of pollutant release, calculate the removal rate of pollutants, and draw images from the average value of repeated experiments in order to seek the best nano-Fe0 dosage. The test results are shown in Fig. 2.
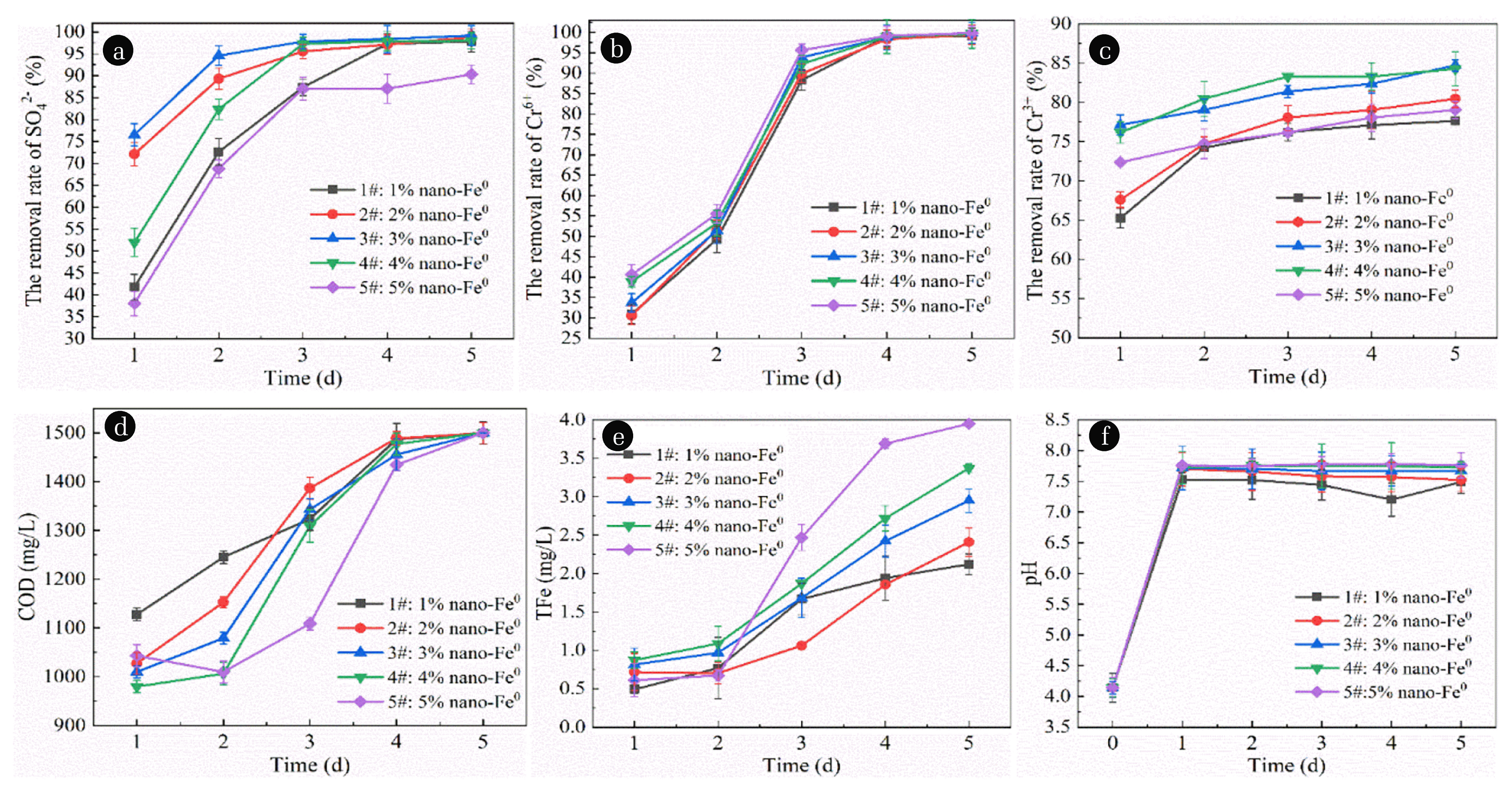
Effect of nano-Fe0 dosage on immobilized particle treatment of AMD. (a) Effect of nano-Fe0 dosage on the removal of SO42− by immobilized particles. (b) Effect of nano-Fe0 dosage on the removal of Cr6+ by immobilized particles. (c) Effect of nano-Fe0 dosage on the removal of Cr3+ by immobilized particles. (d) Effect of nano-Fe0 dosage on COD release of immobilized particles. (e) Effect of nano-Fe0 dosage on TFe release of immobilized particles. (f) Effect of nano-Fe0 dosage in immobilized particles on pH in AMD.
3.1.2.1. The analysis of SO42− removal effect
As shown in Fig. 2(a), with the extension of time, the removal rate of SO42− shows an obvious upward trend. When the nano-Fe0 dosage was 1%, 2%, 3%, 4% and 5%, the average SO42− removal rate was 79.35%, 90.62%, 93.32%, 85.53% and 74.28%, respectively. This is because the nano-Fe0 particle size is so small that it can penetrate the cell wall and enter into the microbial cell, showing certain toxicity to microbial protease and inhibiting its metabolic activity [30]. Therefore, due to the large dosage of nano-Fe0 added to 5# particles, the excess nano-Fe0 in the system enters into the cell and has a strong inhibitory effect on SRB activity. However, because the dosage of 1# particle is too small, nano-Fe0 cannot play a good role in adsorption and reduction, and the SRB activity is low, so the SO42− removal effect is also at a low level. The dosage of nano-Fe0 in particles 2#, 3# and 4# was appropriate. The adsorption and reduction of nano-Fe0 promoted the growth of SRB, and the remaining nano-Fe0 did not produce toxicity to microbial protease. Therefore, the SO42− removal effect of particles 2#, 3# and 4# was significant, with an average removal rate of about 90%. Studies have shown that the removal rate of SO42− in AMD by SRB in the expanded granular sludge bed reactor is 60.8% [31]. Previous research results showed that [32] the average removal rate of SO42− from the immobilized particles prepared with bagasse, medicinite and SRB was 70.13% at pH = 4. Compared with the above studies, the removal rate of SO42− of the five particles prepared under this condition was higher, indicating that the addition of nano-Fe0 to the immobilized particles was conducive to the growth of SRB.
3.1.2.2. The analysis of Cr6+ removal effect
As shown in Fig. 2(b), with the extension of time, the removal rate of Cr6+ shows an obvious rising trend, reaching nearly 100% removal rate after 12 hours. When the nano-Fe0 dosage was 1%, 2%, 3%, 4% and 5%, the average removal rate of Cr6+ within 24 hours was 73.31%, 73.99%, 75.52%, 76.65% and 78.16%, respectively. This is because with the increase of nano-Fe0 dosage, more raw materials enhance their ability to reduce Cr6+ and transform it into Cr3+, so the removal effect of Cr6+ is significantly improved. However, compared with the 5 groups of particles, there was no significant difference in the removal rate of Cr6+, which further proves that nano-Fe0 has extremely strong activity, and a small dosage of nano-Fe0 can achieve excellent removal effect of Cr6+.
3.1.2.3. The analysis of Cr3+ removal effect
As shown in Fig. 2(c), the removal rate of Cr3+ shows an obvious upward trend with the extension of time. The average removal rate of Cr3+ was 74.07%, 75.98%, 80.93%, 81.51% and 76.07%, respectively, when the nano-Fe0 dosage was 1%, 2%, 3%, 4% and 5%. This is due to the 1#, 2# particles nano-Fe0 dosing quantity is less, the adsorption activity of Cr3+, points less, so the Cr3+, adsorption ability is poorer, at the same time, the reduce of nano-Fe0 dosing quantity SO42− removal effect is reduced, thus reduce the water free of S2− content, Cr3+, and metal sulfide precipitation probability corresponding lower formation, therefore, the removal rate of Cr3+ is low. For 5# particles, due to the biological toxic effect of nano-Fe0, the introduction of too much nano-Fe0 will inhibit the metabolic activity of SRB and reduce the probability of precipitation removal of Cr3+, so its removal effect on Cr3+ is weaker than that of 3# and 4# particles.
3.1.2.4. The analysis of COD release
As shown in Fig. 2(d), with the extension of time, COD release shows a trend of significant rise and then stabilization. When the nano-Fe0 dosage was 1%, 2%, 3%, 4% and 5%, the average COD release was 1,336.6 mg·L−1, 1,311.2 mg·L−1, 1,277.4 mg·L−1, 1,254.6 mg·L−1 and 1,219.4 mg·L−1, respectively. This indicates that there is a certain negative correlation between the dosage of nano-Fe0 and the amount of COD release. Although the cause of this abnormal phenomenon is mainly due to the nano-Fe0 decomposition of corn cob has strong catalytic effect, but when nano-Fe0 dosing quantity is higher, the nano-Fe0 destroys the internal structure of corn cobs, make its hydrolysis not well generated for SRB small molecule organic matter [33], so the content of COD in water decreases. At the same time, when the dosage of nano-Fe0 is high, due to its certain biotoxicity, the activity of hydrolyzed microorganisms is inhibited and the decomposition ability is weakened, so the COD release shows a downward trend.
3.1.2.5. The analysis of TFe release
As shown in Fig. 2(e), with the extension of time, TFe release shows a trend of continuous increase. When the nano-Fe0 dosage was 1%, 2%, 3%, 4% and 5%, the average TFe release was 1.40 mg·L−1, 1.35 mg·L−1, 1.77 mg·L−1, 1.98 mg·L−1 and 2.28 mg·L−1, respectively. This is because at the initial stage of the reaction, with the increase of nano-Fe0 dosage, the degree of the reaction with H+ in the AMD is correspondingly enhanced, and more iron ions are generated and free in the AMD, so the release amount of TFe in the solution increases. However, as the reaction progresses, the free iron ions will react with OH− in the solution to form hydroxide precipitation, reducing or leveling the TFe release in the solution. This conclusion is consistent with the research results of Homhoul et al. [34]. Homhoul et al. [34] showed that the formation of iron ions by the oxidation of Fe0 at the initial stage of the reaction would lead to an increase in the content of iron ions in the AMD. The iron ions generated could react with the reduction product OH− of water, and precipitate from the waste water to reduce the concentration of iron ions.
3.1.2.6. The analysis of pH effect
As shown in Fig. 2(f), with the extension of time, pH value increases rapidly in the early stage, but changes in the later stage by a small extent, showing a fluctuating state. This is because nano-Fe0 can react quickly with H+ in the early stage, significantly raising pH to neutral level. Then, due to the decrease of H+ content in AMD, the reaction degree was weakened and the improvement effect was not significant. When the nano-Fe0 dosage was 1%, 2%, 3%, 4% and 5%, respectively, the average pH value of the effluent was 7.45, 7.60, 7.72, 7.75 and 7.77, with little difference. Thus, it can be seen that nano-Fe0 has extremely strong activity, and a small dosage of nano-Fe0 can achieve a good effect on pH, increasing the pH value from 4.0 to neutral.
To sum up, by comparing the removal effects of the immobilized particles on SO42−, Cr6+ and Cr3+, as well as the lifting capacity of COD and TFe release and pH of the 5 groups, and taking into account the changes of the 6 groups of indicators, the optimal dosage of nano-Fe0 was selected as 3% in the final test.
3.1.3. Determination of corn cob particle size
According to the preparation method of immobilized particles mentioned above, 60 mesh, 100 mesh and 200 mesh cobs with mass fraction of 5% and 3% nano-Fe0 were added into the mixture gel respectively. Then the SRB microflora solution with mass fraction of 30% was added to prepare three kinds of immobilized particles. The prepared immobilized particles were placed in AMD and each experiment was repeated three times. Sampling was conducted regularly every day to determine the concentration of characteristic pollutants and control the amount of pollutant release, calculate the removal rate of pollutants, and draw images from the average value of repeated experiments to seek the best corn cob particle size. The test results are shown in Fig. 3.
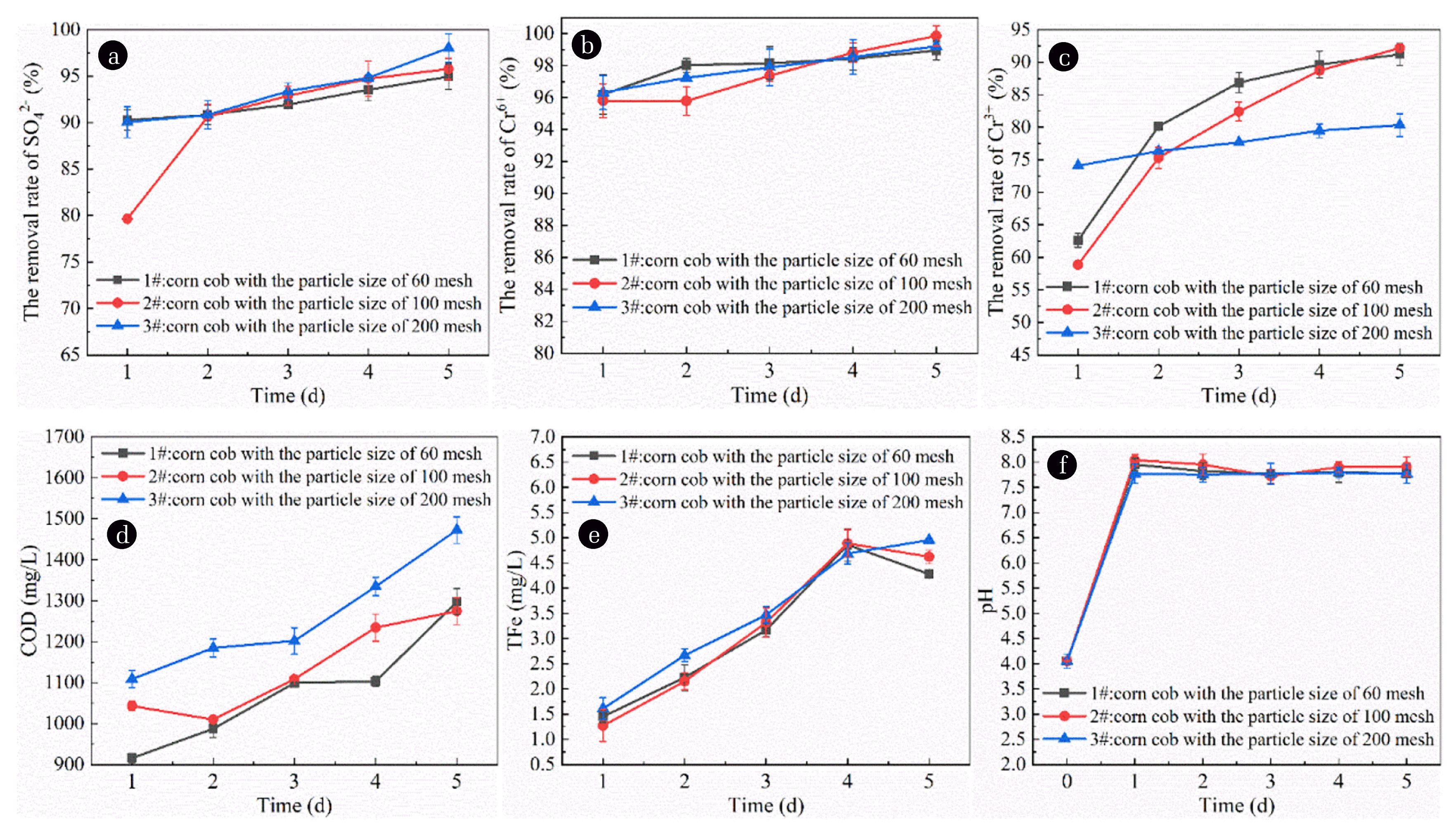
Influence of corn cob particle size on immobilized particle treatment of AMD. (a) Effect of corn cob particle size on removal of SO42− by immobilized particles. (b) Effect of corn cob particle size on the removal of Cr6+ by immobilized particles. (c) Effect of corn cob particle size on the removal of Cr3+ by immobilized particles. (d) Effect of corn cob particle size on COD release of immobilized particles. (e) Effect of corn cob particle size on TFe release of immobilized particles. (f) Effect of corn cob particle size in immobilized particles on pH in AMD.
3.1.3.1. The analysis of SO42− removal effect
As shown in Fig. 3(a), with the extension of time, the removal rate of SO42− shows a significant upward trend. When the cob particle size was 60, 100 and 200 mesh, the average SO42− removal rate was 92.32%, 90.74% and 93.42%, respectively. It can be seen that the removal rate of SO42− decreased first and then increased with the decrease of corn cob particle size. The removal of SO42− by the three particles is mainly dependent on the SRB metabolism of SO42− to produce S2−. It has been reported that SRB can convert sulfate into sulfide using organic matter under anaerobic conditions [35]. The results of previous studies [36] showed that in the immobilized particles filled with corn cob, SRB and salt-modified Wheat meal stone, the hydrolyzed products of corn cob could be used for SRB growth. When the removal area of SO42− is stable, the removal rate of the three kinds of particles has a small difference. The main reason for this difference is that when the grain size of the corn cob is large, there are pores inside the corn cob with large grain size, and part of SO42− will diffuse into the void. When the particle size of the corn cob decreased, the internal macropores of the corn cob decreased, and the removal of SO42− only depended on SRB metabolism. When the grain size of the corn cob is very small, the inner hole of the corn cob is easy to block, but the corn cob with very small grain size is easy to hydrolyze, which makes the COD content in the water higher and provides sufficient carbon source for the growth of SRB. Therefore, the metabolic activity of SRB is strong, so the SO42− removal effect is better.
3.1.3.2. The analysis of Cr6+ removal effect
As shown in Fig. 3(b), the removal rate of Cr6+ shows a significant upward trend with the extension of time. The Cr6+ removal rate was 97.95%, 97.53% and 97.84% when the cob particle size was 60, 100 and 200 mesh, respectively. It can be seen that the removal rate of Cr6+ decreased slightly with the decrease of corn cob particle size. This is because when the corn cob particle size is large, the hydrolysis of the corn cob is not sufficient, and the unhydrolyzed corn cob has a certain adsorption effect on Cr6+, so the removal rate of Cr6+ is slightly higher. It is reported that active groups such as hydroxyl group, alcohol group, carboxyl group and amino group in corn cob can be used as adsorbents for heavy metal ions [27, 28].When the particle size of the corn cob decreases, the adsorption capacity of the corn cob decreases due to more thorough hydrolysis, but the organic matter formed by hydrolysis can better promote the metabolic activity of SRB and generate more S2− and form precipitation with heavy metals. Therefore, the removal effect of the corn cob with small particle size is also at a good level.
3.1.3.3. The analysis of Cr3+ removal effect
As shown in Fig. 3(c), the removal rate of the Cr3+ shows a significant upward trend with the extension of time. The Cr3+ removal rate was 82.09%, 79.46% and 77.59% when the cob particle size was 60, 100 and 200 mesh, respectively. It can be seen that the removal rate of Cr3+ shows a certain downward trend with the decrease of corn cob particle size. The removal of Cr3+ by the three kinds of particles mainly depends on SRB metabolism to produce S2−, S2− and Cr3+ form precipitation, so that Cr3+ is removed. Secondly, the active groups on the surface of corn cob can also adsorb Cr3+. When the grain size of the corn cob is large, the corn cob is not completely hydrolyzed. The active groups on the surface of the corn cob, such as hydroxyl, alcohol, carboxyl and amino groups [27, 28], can adsorb Cr3+. The hydrolyzed corn cob provides carbon source for SRB and promotes the formation of sulfide precipitation between Cr3+ and S2−, so the removal effect of Cr3+ is better. When the particle size of the corn cob decreases, the removal of Cr3+ mainly depends on the formation of sulfide precipitation. At the same time, small particles of the corn cob will block the particle channel, affecting the ion mass transport, so the removal effect is reduced.
3.1.3.4. The analysis of COD release
As shown in Fig. 3(d), COD release shows a certain upward trend with the extension of time. When the grain size of corn cob was 60, 100 and 200 meshes, the average COD release was 1,080.2 mg·L−1, 1,134.2 mg·L−1 and 1,260.6 mg·L−1, respectively. It can be seen that the average COD release shows an increasing trend with the decrease of corn cob particle size. This is because the cob with oversize particle size is more prone to hydrolyze under the catalytic action of nano-Fe0, releasing more organic matter into the water, so the COD release amount is higher.
3.1.3.5. The analysis of TFe release
As shown in Fig. 3(e), with the extension of time, TFe release shows a certain upward trend. When the cob particle size was 60, 100 and 200 mesh, the average TFe release was 3.198 mg·L−1, 3.250 mg·L−1 and 3.478 mg·L−1, respectively. It can be seen that the average TFe release shows an overall trend of increasing with the decrease of corn cob particle size. This is because when the particle size of corn cob is small, its hydrolysis in AMD is more thorough, and the SRB activity is significantly increased due to the higher COD content. As the reduction of SO42− by SRB requires the participation of reducing electrons, nano-Fe0 will release a large amount of Fe2+ and Fe3+ into the water body while replenishing the reduced electrons [37], resulting in a high TFe content.
3.1.3.6. The analysis of pH effect
As shown in Fig. 3(f), pH value shows a certain upward trend with the extension of time. When the grain size of corn cob was 60, 100 and 200 mesh, the average pH value of water was 7.83, 7.90 and 7.77, respectively, with little difference, and the water output was in a weakly alkaline state. This further indicates that nano-Fe0 is highly active and can rapidly increase the pH value of the wastewater to a neutral state. However, because it is prone to corrosion in acidic water environment, hydroxide precipitation or fine flocculants are generated and dispersed in the water, the wastewater showed a certain weak alkalinity [38].
To sum up, by comparing the removal effects of immobilized particles on SO42−, Cr6+ and Cr3+, and the ability to improve COD release and pH, considering that the COD and TFe release should not be too large, and considering the changes of the six groups of indicators, the optimal particle size of corn cob was finally selected as 60 mesh.
3.1.4. Determination of cob dosage
According to the preparation method of immobilized particles mentioned above, 60 mesh corn cobs with mass fraction of 1%, 3% and 5% and nano-Fe0 with mass fraction of 3% were added to the mixture gel respectively. Then the SRB microflora solution with mass fraction of 30% was added to prepare three kinds of immobilized particles. The prepared immobilized particles were placed in AMD and each experiment was repeated three times. Sampling was conducted regularly every day to determine the concentration of characteristic pollutants and control the amount of pollutant release, calculate the removal rate of pollutants, and draw images from the average value of repeated experiments to seek the best corn cob dosage. The test results are shown in Fig. 4.
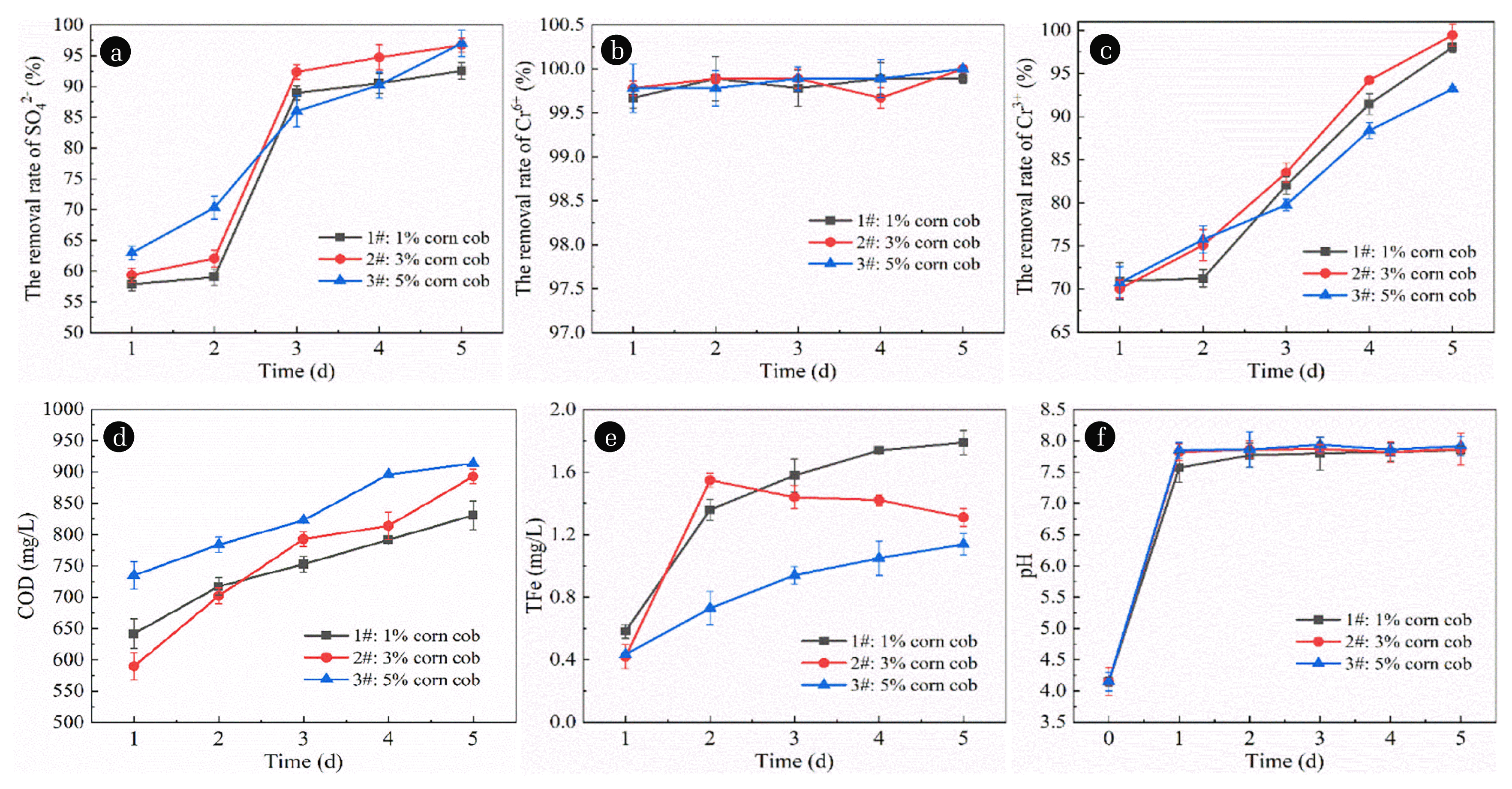
Effect of corn cob dosage on immobilized granules treatment of AMD. (a) Effect of corn cob dosage on the removal of SO42− by immobilized particles. (b) Effect of corn cob dosage on the removal of Cr6+ by immobilized particles. (c) Effect of corn cob dosage on the removal of Cr3+ by immobilized particles. (d) Effect of corn cob dosage on COD release of immobilized particles. (e) Effect of corn cob dosage on TFe release of immobilized particles. (f) Effect of corn cob dosage in immobilized particles on pH in AMD.
3.1.4.1. The analysis of SO42− removal effect
As shown in Fig. 4(a), with the extension of time, the removal rate of SO42− shows a significant upward trend. When the cob loading was 1%, 3% and 5%, the average SO42− removal rate was 77.81%, 81.03% and 81.31%, respectively. It can be seen that the removal rate of SO42− shows an increasing trend with the increase of corn cob dosage. This is because with the increase of the dosage of corn cob added, the hydrolysis of corn cob will be further enhanced under the catalytic action of nano-Fe0, and the amount of available carbon source of SRB is sufficient, so the SO42− removal rate will be improved. However, when the added dosage of corn cob continues to increase, more organic matter will cause secondary pollution of water body. Meanwhile, due to the limited metabolic capacity of SRB, the SO42− removal rate does not increase significantly. Das B et al. [39] analyzed the influence of COD/SO42− on the removal of sulfate from SRB. The results showed that when COD/SO42− = 1 and 2, the average removal of SO42− was 61% and 77%, respectively, and when COD/SO42− = 4, the maximum removal rate of SO42− was 99%. It has been reported that the removal efficiency of SO42− increases with the increase of COD/SO42−, and decreases with the further increase of COD/SO42− after reaching its maximum value [39–41]. The results of this study are consistent with the above reports.
3.1.4.2. The analysis of Cr6+ removal effect
As shown in Fig. 4(b), the removal rate of Cr6+ shows a significant upward trend with the extension of time. The average removal rate of Cr6+ was 99.67%, 99.78% and 99.82%, respectively, when the dosage of corn cob was 1%, 3% and 5%. It can be seen that the removal rate of Cr6+ shows a slightly increased trend with the increase of the dosage of corn cob added, with little difference. The removal rate of the three groups of grain could reach more than 99%. This is because nano-Fe0 has strong reductibility, which can rapidly reduce the Cr6+ in water to Cr3+, and then remove it by precipitation or adsorption. Compared with that, the adsorption effect of corn cob is weak, so the dosage of adding corn cob has little influence on the removal of Cr6+.
3.1.4.3. The analysis of Cr3+ removal effect
As shown in Fig. 4(c), the removal rate of Cr3+ shows a significant upward trend with the extension of time. The average removal rate of Cr3+ was 82.74%, 84.45% and 81.57% when the cob loading was 1%, 3% and 5%, respectively. It can be seen that the removal rate of Cr3+ shows a trend of first increasing and then decreasing with the increase of cob dosage. This is because under the catalysis of nano-Fe0, more corn cobs can provide more carbon sources for SRB, and SRB is more active, which can release more S2− and Cr3+ to form precipitation, so the removal rate is increased. In addition, with the increase of corn cob dosage, its ability to adsorb Cr3+ as an adsorbent was also enhanced, which also contributed to the removal rate to some extent. However, when the dosage of corn cob continues to increase, due to the limited capacity of SRB to utilize carbon sources, the COD content in water is relatively high. Relevant studies have shown that a high carbon-sulfur ratio inhibits the growth activity of SRB [42], and the content of S2− generated by reduction decreases. Therefore, the removal efficiency of Cr3+ precipitation decreases and the removal effect becomes worse.
3.1.4.4. The analysis of COD release
As shown in Fig. 4(d), COD release shows a certain upward trend with the extension of time. When the dosage of corn cob was 1%, 3% and 5%, the average COD release amount was 746.8 mg·L−1, 758.2 mg·L−1 and 830.4 mg·L−1, respectively. It can be seen that the COD release amount presents an increasing trend with the increase of cob dosage. This is because under the catalytic action of nano-Fe0, more corn cob hydrolyzation will release a large amount of organic matter, while SRB’s ability to utilize carbon source is limited, so the COD content of the effluent is relatively high.
3.1.4.5. The analysis of TFe release
As shown in Fig. 4(e), with the extension of time, TFe release shows a certain upward trend. When the dosage of corn cob was 1%, 3% and 5%, the average TFe release amount was 1.410 mg·L−1, 1.228 mg·L−1 and 0.858 mg·L−1, respectively. Therefore, TFe release shows a decreasing trend with the increase of cob dosage. This is because with the increase of corn cob dosage, it has a good adsorption effect on H+ in wastewater, and the process of SRB dissimilar reduction of SO42− also releases alkalinity. Therefore, the free OH− in the water can form precipitation and deposit on the surface of particles with Fe2+ and Fe3+ generated by the hydrolysis of nano-Fe0, so the TFe release in the water is reduced.
3.1.4.6. The analysis of pH effect
As shown in Fig. 4(f), pH value shows a certain upward trend with the extension of time. When the cob dosage was 1%, 3% and 5%, the average pH value of water was 7.77, 7.86 and 7.90, respectively. It can be seen that the pH value of water shows a certain trend of increasing with the increase of cob dosage. This is because, on the one hand, with the increase of the dosage of corn cob, its adsorption capacity of H+ was enhanced. On the other hand, more cobs cause the SRB metabolism to release more alkalinity, thus increasing the pH.
To sum up, by comparing the removal effects of immobilized particles on SO42−, Cr6+ and Cr3+, as well as the lifting capacity of COD release and pH of the 3 groups, considering that the COD and TFe release should not be too large, and considering the changes of the 6 groups of indicators, the optimal dosage of corn cob was finally selected as 3%.
3.2. Orthogonal Experimental Study
Based on the composition of immobilized particles in the previous study, the orthogonal test of L9(34) was carried out by selecting three levels of four factors according to the single factor test results, which were SRB dosage of 20%, 30% and 40%, nano-Fe0 dosage of 2%, 3% and 4%, corn cob particle size of 60 mesh, 100 mesh and 200 mesh, corn cob dosage of 1%, 3% and 5%. Immobilized particles were prepared according to the orthogonal experimental design, and immobilized particles were placed in AMD to determine the indicators of characteristic pollutants. Three replicates were performed for each experiment, and the average value of the replicates was taken for the analysis of the results, as shown in Table 1. By means of range analysis, the optimal ratio of SRB bacterial liquid, nano-Fe0 and corn cob in immobilized particles was determined.
3.2.1. Analysis of SO42− test result
The greater the range of a certain influencing factor, the greater the influence on the experimental results. As can be seen from table S2, the influence of the four factors on the removal of SO42− is not significant. According to the range of table S1, the order of influence on SO42− removal rate is: A > D > C > B. Therefore, according to the mean size, the optimal combination of factors to determine the SO42− removal rate is A3B3C2D2, that is, the SRB dosage is 40%, the nano-Fe0 dosage is 4%, the corn cob size is 100 mesh, and the corn cob dosage is 3%. The SO42− removal rate of particles is the best under this composition ratio.
3.2.2. Analysis the experiment results of Cr6+
It can be seen from table S4 that the four factors have insignificant effects on the removal of Cr6+. According to the range of table S3, the order of influence on the removal rate of Cr6+ is D > B > A > C. Therefore, according to the mean value, the optimal combination of factors to determine the Cr6+ removal rate is A2B2C1D3, that is, the SRB is 30%, the nano-Fe0 is 3%, the corn cob is 60 mesh, and the corn cob dosage is 5%. The Cr6+ removal rate of particles is the best under this composition ratio.
3.2.3. Analysis the experiment results of Cr3+
It can be seen from table S6 that the four factors have insignificant effects on the removal of Cr3+. According to the range of table S5, the order of influence on the removal rate of Cr3+ is B > C > A > D. Therefore, according to the mean size, the optimal combination of factors to determine the Cr3+ removal rate is A1B3C2D2, that is, SRB is 20%, nano-Fe0 is 4%, corn cob is 100 mesh, and the corn cob dosage is 3%. The Cr3+ removal rate of particles is the best under this composition ratio.
3.2.4. Analysis the experiment results of COD
It can be seen from table S8 that, among the four factors, SRB has a significant influence on COD released by particles. According to the range of table S7, the order of influence on COD release is: A > C > D > B. Therefore, the optimal combination of factors to determine the COD release amount according to the mean value is A3B1C2D2, that is, SRB is 40%, nano-Fe0 is 2%, corn cob is 100 mesh, and the corn cob dosage is 3%. The COD release amount of particles is the best under this composition ratio.
3.2.5. Analysis the experiment results of TFe
It can be seen from table S10 that the four factors have insignificant effects on the removal of TFe. According to the range of table S9, the order of influence on TFe release is: B > D > A > C. Therefore, the optimal combination of factors to determine the TFe release amount according to the mean size is A2B1C1D1, that is, the SRB is 30%, the nano-Fe0 is 2%, the corn cob is 60 mesh, the corn cob dosage is 1%, and the TFe release amount of particles is the best under this composition ratio.
3.2.6. Analysis the experiment results of pH
It can be seen from table S12 that nano-Fe0 has a significant influence on the pH regulation ability of particles among the four factors. According to the range of table S11, the order of influence on pH is B > A > D > C. Therefore, according to the mean value, the optimal pH combination is A2B3C1D3, that is, the SRB is 30%, the nano-Fe0 is 4%, the corn cob is 60 mesh, and the corn cob dosage is 5%. The pH raising effect of the particles is the best under this composition ratio.
3.2.7. Determine the result of orthogonal experiment
Based on the orthogonal experimental results from 3.1 to 3.6 (A3B3C2D2, A2B2C1D3, A1B3C2D2, A3B1C1D1, A2B3C1D3), the optimal composition ratio of nano-Fe0 immobilized particles is A2B3C1D2, that is, 30% of SRB, 4% of nano-Fe0, 60 mesh corn cob particle size, 3% of corn cob dosage, and the best performance of nano-Fe0 immobilized particles under this composition ratio was determined.
3.3. Instrument Characterization of Particles
3.3.1. EDS analysis
Nano-Fe0 SRB immobilized particles were prepared according to the optimal ratio determined by the above orthogonal test. The particles before and after the reaction were dried and EDS energy spectrum analysis was carried out. The experimental results were shown in Fig. 5(a) and (b).

EDS, XRD and SEM analysis of AMD before and after particles treatment. (a) EDS analysis before reaction of particles. (b) EDS analysis after reaction of particles. (c) XRD analysis before reaction of particles. (d) XRD analysis after reaction of particles. (e) SEM in surface before reaction of particles. (f) SEM in surface after reaction of particles. (g) SEM in cross-section before reaction of particles. (h) SEM in cross-section after reaction of particles.
It can be seen from Fig. 5(a) that, before the reaction, the Fe0 SRB immobilized nanoparticles mainly contained C, O, Cl, Ca and Fe elements, and their weight percentages were 10.09%, 21.90%, 1.09%, 1.58% and 65.34%, respectively. After the reaction, the immobilized particles mainly contained C, O, S, Ca, Cr and Fe elements, and their weight percentages were respectively 9.22%, 20.19%, 5.10%, 0.44%, 4.29% and 60.57%. After the reaction, S and Cr elements obviously appeared in the particles. For element S peak, this is because the SRB in the particle dissimilar reduces SO42− to form S2− during the reaction process, and then forms metal sulfide with heavy metal ions or adsorbs the biomass material of corn cob to deposit it on the surface and inside of the particle. Therefore, obvious element S peak appears after the reaction. For the Cr element peak, the reason is that the particles are immersed in the waste water containing chromium in the reaction process. Under the strong reduction effect of nano-Fe0, the Cr6+ in the water is rapidly transformed into Cr3+. Part of Cr3+ is adsorbed on the particle surface by corn cob and other adsorption materials, while the other part forms metal sulfide precipitation and deposition on the particle surface and internal pores with S2−, resulting in obvious Cr element peak after the reaction. In addition, compared with before and after the reaction, Fe peak content decreased from 65.34% to 60.57%. This is because part of the elemental nano-Fe0 in the particle reacts with H+ in the wastewater to form Fe2+, and then dissociates into the wastewater, so the Fe element peak in the particle decreases. The peaks of other elements did not change much before and after the reaction, indicating that the particles were stable and did not cause a large amount of matrix leakage.
3.3.2. XRD analysis
Nano-Fe0 SRB immobilized particles were prepared according to the optimal ratio determined by the above orthogonal test. The particles before and after the reaction were dried and ground into 200 mesh powder for XRD analysis. The test results are shown in Fig. 5(c) and (d).
It can be seen from Fig. 5(c) that Fe elements in the nano-Fe0 SRB immobilized particles before the reaction mainly exist in elemental iron and Fe2O3. The existence of Fe2O3 is mainly due to the fact that the nano-Fe0 in the surface layer of particles is easily oxidized by air. Since it is difficult to completely isolate the air in the experiment, there will be a small amount of Fe2O3 in the surface layer of particles. Through observation, we found that there were no other elements in the particles except organic compounds. This indicates that the particle purity of the finished product is higher.
After the reaction, two new elements S and Cr appear in the particles, and the phase characterized by them by XRD is Cr2S3. This further confirms that the removal of Cr3+ by particles is dependent on the metal sulfide precipitation formed by S2− which is free from the water. In addition, the main existence form of Fe element is FeS. This indicates that the S2− generated by SRB foreignizing reduction of SO42− can form precipitation with heavy metal ions on the one hand, and form FeS with Fe2+, on the other hand, with a more thorough removal effect. Therefore, in the later stage of this experiment, some sulfide precipitation such as FeS can be considered to recover and purify the elemental sulfur, so as to further improve the available potential of elements.
3.3.3. SEM analysis
After drying the particles before and after the above reaction, SEM was used to scan the surface and section structure of the particles with a magnification of 200 times. The microscopic changes of the particle structure before and after the reaction were observed, and the reaction process of the particles was analyzed to further reveal the mechanism of the coordinated treatment of AMD by particles. SEM microscanning is shown in Fig. 5(e) and (f).
It can be seen from Fig. 5(e) that the particle surface and section surface structure changed greatly before and after the reaction. Before the reaction, the surface texture of particles was uniform and neat, the pores were unobstructed and the size was appropriate. The porosity inside the particle is developed and the permeability is good. This indicates that the prepared finished particles can meet the requirements of adsorption and removal of pollutant ions, and at the same time can better meet the contact of nano-Fe0 material with H+ in AMD, and improve the pH value of AMD.
After the reaction, there was almost no particle deposition on the particle surface, and obvious folds and bulges and large pores and cracks appeared. This is because the finished product particles have large pore structure and strong SRB biological activity, and the heavy metal ions in the waste water can enter into the particles smoothly and react to deposit, so there is no obvious particle deposition on the surface of the particles. As can be seen from the particle cross section, after the reaction, the particle channel becomes narrower, a large number of particles are deposited on the inner wall of the channel, and filaments appear in the channel. This is because the FeS and Cr2S3 generated by the particle reaction enter into the particle through abundant channels and eventually deposit on the inner wall of the channels, causing the obstruction of the channels.
4. Conclusions
By conducting a single factor test to determine that the optimal dosage of SRB and nano-Fe0 were 30% and 3%. The optimal size of corn cob was 60 mesh and the optimal dosage of corn cob was 3%.
Through the orthogonal experiment to determine that when the mass percentage of SRB was 30%, the dosage of nano-Fe0 was 4% and the dosage of 60 mesh corn cob was 3%, the removal rates of SO42−, Cr6+ and Cr3+ were 82.99%, 99.78% and 38.78%, respectively. The release of TFe, COD were 4.26 mg·L−1 and 1,033.4mg·L−1, respectively. The pH value was 8.04. Under these conditions, it can achieve the best treatment.
The analysis by EDS confirmed that the particles mainly contain C, O, Cl, Ca, Fe elements. After the reaction, there were two new elements of S and Cr appeared in the particles, and the peak value of Fe decreased. The analysis by XRD showed that the elements S and Cr of the particles mainly existed in the form of FeS and Cr2S3 after the reaction. The analysis by SEM showed that the surface and internal structure varied greatly before and after the particle reaction. In summary, particles in the process of dealing with AMD occurred a series of complex reactions.
Supplementary Information
Acknowledgements
This research was supported by the National Natural Science Foundation of China (41672247, 41102157, 51304114), Liaoning Province’s “Program for Promoting Liaoning Talents” (XLYC1807159).
Notes
Author Contributions
J. D. (Professor) made the experiment plan and guided the experiment in the whole process. X. W. (Ph.D. student) was in charge of data analysis and paper writing. B. L. (Professor) and Y. Y. (Professor) was in charge of experiment guidance and paper revision. Y. D. (Ph.D. student) and M. W. (Master student) was responsible for water quality analysis and detection.

