Assessment of the potential of duckweed (Lemna minor L.) in treating lead-contaminated water through phytoremediation in stationary and recirculated set-ups
Article information
Abstract
The problems of heavy metal contamination in water have become alarming and necessitate efficient remediation. However, conventional water and wastewater treatment techniques are considered costly, and some are even not environment-friendly. These problems trigger the idea of utilizing plants in the treatment process of metal-contaminated water. The current work investigated the potential of duckweed (Lemna minor L.) in treating lead-contaminated water through phytoremediation. The duckweed was used as bioaccumulator of lead (Pb) in the prepared stationary and recirculated set-ups at 3, 6, and 9 d. The physicochemical characteristics such as pH, BOD5, DO, turbidity, and temperature of the influent and effluent were compared. The highest bioaccumulation of 62.8% was achieved at 3 d in the recirculated set-up. The result of the analysis showed that duckweed has the potential in phytoremediation considering better quality effluent. The concentration of Pb in the effluent of 0.93 mg/L in the recirculated set-up with duckweed in 3 d was much lower compared to the initial concentration in the influent at 2.5 mg/L. This study demonstrated that duckweed could be a suitable plant for Pb removal from water with big implications in remediating heavy metal-contaminated water from various industries.
1. Introduction
Rapid urbanization and fast booming of industries in the present time consequently produce wastewater that carries contaminants with associated risks and hazards to humanity and the environment [1]. Approximately 50% of these contaminants can be in the form of heavy metals that are continually discharged to the environment at high concentrations. Also, 20 kinds of these metals are toxic, including lead (Pb) that can cause kidney problems and can affect the nervous system when directly absorbed and ingested by a human. This contaminant can also make the habitual environment of some organisms inefficient [2]. In fact, the presence of Pb in the environment primarily affected the 1,300 species of wildlife and aquatic organisms including scavengers that consume contaminated animals [3] Henceforth, this explains bioaccumulation wherein contaminants ingested by the primary consumer transfers to other hierarchal consumers. Also, the Pb had contaminated the deep wells and tap water resources in Manila, Philippines, with the concentrations of 0.45 mg/L and 0.61 mg/L, respectively [4]. It is, therefore, imperative to remove this contaminant in wastewater before disposal to eliminate risks and hazards to humans and the aquatic environment.
With the alarming concentration of metals and the harm that it brings to man and the environment, several techniques for the treatment of wastewater contaminated with Pb were introduced. The Pb removal has been previously studied through chemical precipitation [5], coagulation and flocculation [6], electrochemical treatments [7], membrane filtration [8], and ion-exchange [9]. However, most of these techniques produce another environmental problem including toxic sludge that remains unsettled within industries as well as its associated high cost of disposal [6]. In the continuous search and studies to lessen further the cost of treating Pb-contaminated water, the use of plants still poised effective in the removal of a contaminant in the process called phytoremediation [10].
Phytoremediation is a biological method of removing heavy metals and other contaminants naturally like in wastewater through the direct use of plants. Plants in this technique accumulate the pollutants from the source. This natural wastewater remediation contributes to the eco-friendly disposal of wastewater and a low-cost strategy to remove contaminants before releasing to water bodies [10]. Unlike those conventional techniques previously studied; phytoremediation is less costly, shows a good impact on the environment, and is aesthetically pleasing [2, 11, 12]. Duckweed, scientifically known as Lemna minor L., is one of the aquatic plants that has the potential for the removal of contaminants from wastewater owing its high passive uptake via micropores in the root cell walls (the apoplastic pathway) where sequestration or degradation takes place. Pollutants transport with water to the different parts of the plants, and the diffusive mechanism of the accumulated Pb in the duckweed enhances efficiency [13]. However, the potential of duckweed as an agent of contaminant removal focused mainly on a regular biotic stationary setting (wetland). This set-up is without the inclusion of any mechanism that may lessen the time needed for treatment process and further enhance its efficiency in treating wastewater [5, 14, 15].
This study evaluates the potential of duckweed in the removal of Pb in both stationary and recirculated set-ups. The dynamic mechanisms were applied in the recirculated set-up to improve the removal efficiency of duckweeds as a bio-accumulator [16]. The physico-chemical characteristics of the prepared Pb-contaminated influent and the subsequent effluent in both stationary and recirculated set-ups, as well as the plant growth rate, were also measured to determine the effects of contact time and set-up variation in the accumulation of Pb [17].
2. Materials and Methods
2.1. Chemicals and Duckweed Sources
A Certipur Lead ICP standard solution of 1000 mg/L from the Department of Science and Technology (DOST) – Cagayan de Oro City, Philippines was used in this study.
The duckweed was purchased from a farm that grows and distributes duckweed and some aquatic plants for feedstock and laboratory experiments in San Pedro, Laguna, Philippines. Due to financial constraints, pretreatment of duckweed before it was subjected to the Pb-contaminated water was not made. However, the supplier of the duckweed assured that it was cultured in a condition with no possible contamination of heavy metals since the water used in pre-growing was potable water.
2.2. Preparation of the Set-up
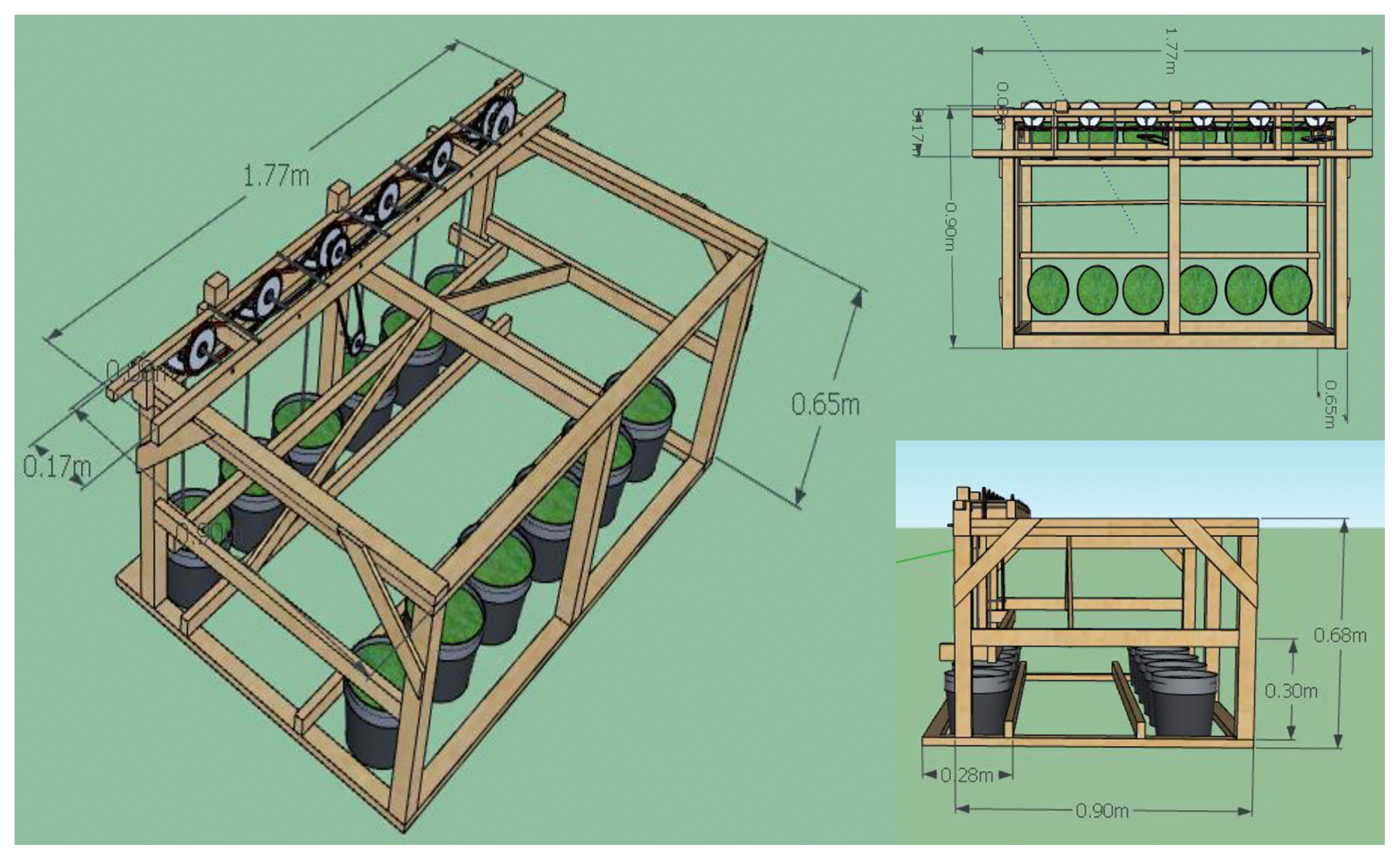
This study employed two set-ups: stationary and recirculated. The stationary set-up in means no agitation of Pb-contaminated water were duckweed grew in for 3, 6 and 9 d. Meanwhile, the recirculated set-up means introduction of minimal agitation (50 rpm) of Pb-contaminated water were duckweed grew in 3, 6, and 9 d. The recirculated set-up was run by a 12 V, 3 A direct current wiper motor using a step-down center-tapped voltage transformer at 220 V to 12 VAC. The motor was attached to the rectangular frame structure made of wood, round bars, sprockets, bearings, and chain. The propellers were assigned to each container for agitation with 2 cm height and 5 cm width and placed vertically at 2.5 cm from the bottom of the container. The uniformity of revolution was ensured by using the same kind and size of sprocket in containers. Six (6) HDPE rounded containers were used as tanks (20 cm diameter and 28 cm height). The experimental set-up is presented in Fig. 1.
2.3. Preparation of the Pb-contaminated Water
Using the standard procedure of DOST, 47.5 mL of the 1000 mg/L standard Pb solution was diluted in 19 L of distilled water to achieve the desired concentration of 2.5 mg/L. The mixing was handled with safety precautions due to the possible health effects of Pb on humans.
2.4. Experimental Set-up
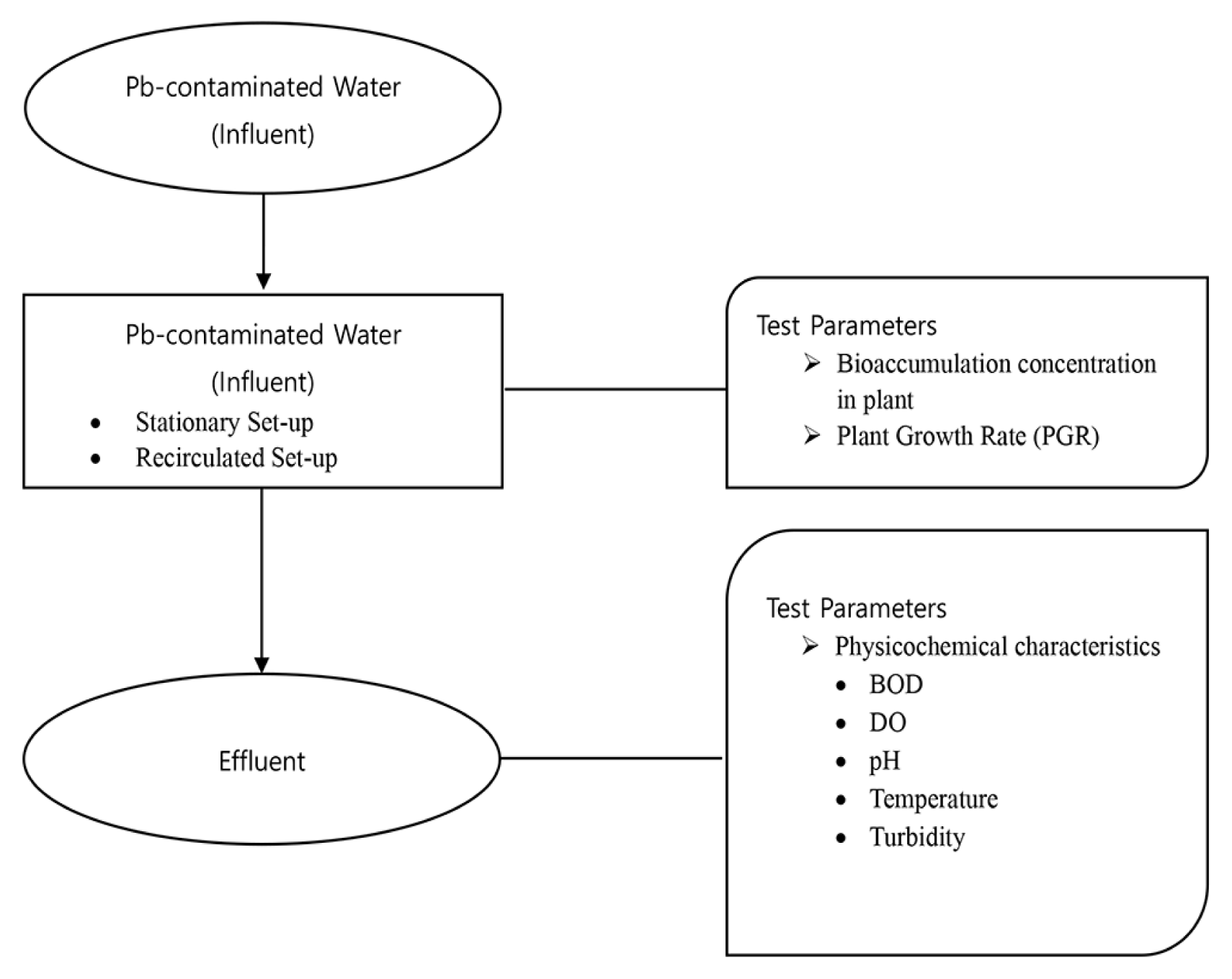
The prepared Pb-contaminated water was poured into the container. The 150 g duckweed was then introduced to each container and appropriately labeled with its respective set-up and contact time (3, 6, and 9 days). At the end of the contact time of each run, the duckweed was harvested, weighed and kept in a 1-L glass container with proper label and seal. In compliance with the guidelines from the testing laboratories, the samples were placed in a bucket with ice and brought to the testing laboratories within 4 h after harvest to evade the discrepancy in the analyses and determination of the required characteristics. Presented in Fig. 2 is the schematic plan of the study.
Plant growth rate, as performed by weighing duckweed before and after phytoremediation, was computed using Eq. (1).
2.5. Analytical Methods
The concentration of Pb in the sample was analyzed at F.A.S.T. Laboratories in Cagayan de Oro City, Philippines using an Atomic Absorption Spectrophotometer. The other parameters like pH, temperature, and turbidity were identified and analyzed in the same laboratory using 4500 H+ Electrometric Method and 2130 B Nephelometry, respectively. Physico-chemical characteristics such as the BOD5 and DO were determined and analyzed at Aeronics Incorporated through Azide Modification Method (Dilution Technique) and Azide Modification (Winkler Method), respectively.
3. Results and Discussion
3.1. Physico-chemical Characteristics of Influent and Effluent
Physico-chemical characteristics of the influent before the application of duckweed were 0.23 mg/L BOD5, 7.8 mg/L DO, 6.6 pH, 22.2°C, and 1.3 NTU turbidity (Table 1). These results were considered as the best condition for plant introduction to proceed phytoremediation, which was similarly observed in the study of Chaudhary and Sharma [18].
As to the analysis results of the effluent, the anticipated increase of BOD5 from 0.23 mg/L in influent to 112 mg/L was due to the formation of some organic matters after some duckweed died during the process. The increase of BOD5 values was also attributed to the decrease of DO from 7.8 mg/L in the influent to 1.1 mg/L in the effluent, which indicates that the two properties are inversely proportional without any intruding factors [19, 20].
Meanwhile, the temperature of the influent and effluent remained constant at 22.2°C, signifying that no effect in the treatment process as similarly observed in another study [21]. The pH in both influent and effluent remained constant at 6.6 which is just near to the neutral pH value. On the other hand, the turbidity of the effluent of 3.74 NTU is greater than in the influent of 1.3 NTU. The increase in turbidity was anticipated due to the formation of organic matters as some small duckweeds died during the experiment.
The changes in the physico-chemical characteristics of the effluent are good manifestation of the potential of duckweed in the phytoremediation of lead-contaminated water. However, there is a need to incorporate additional treatment of the effluent to decrease the BOD5 and turbidity concentrations according to what is acceptable in statutory and regulatory requirements.
3.2. Bioaccumulation Concentration
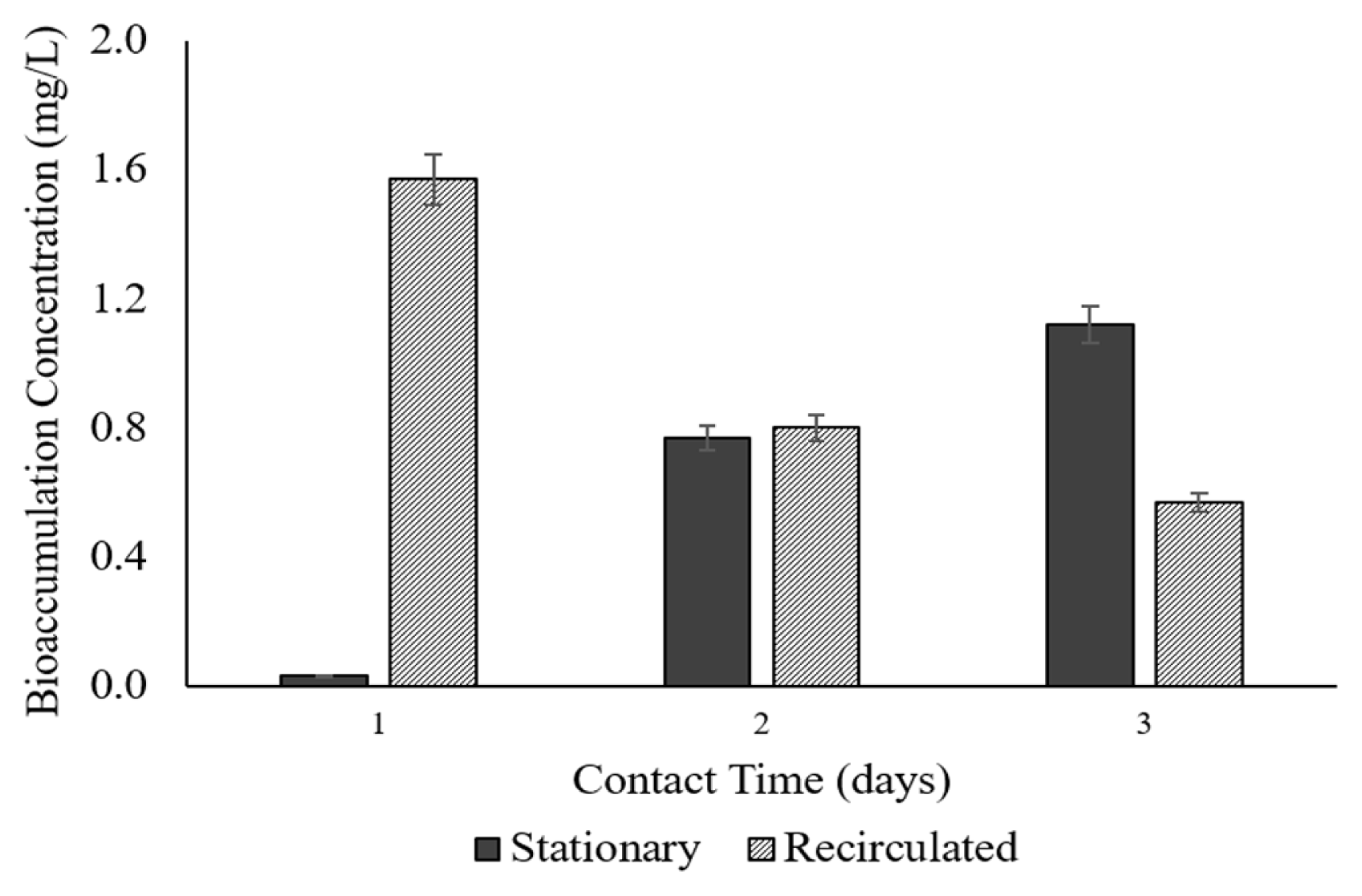
The potential of duckweed as an accumulator was dependent on the amount of Pb it absorbed. The concentration of the accumulated Pb by duckweed in various contact times (3, 6, 9 d) both in the stationary and recirculated set-ups is presented in Fig. 3.
In the data gathered, the recirculated set-up showed a high bioaccumulation concentration of Pb. In just 3 d, a considerable bioaccumulation concentration of 1.57 mg/L (62.8%) was attained. This result implies the efficient accumulation capabilities of duckweed as influenced by the recirculation mechanism.
However, as time went by, after 6 – 9 d, the bioaccumulation concentration was decreased at 0.80 mg/L and 0.57 mg/L, respectively. This is because, at longer period, the bioaccumulation would eventually decrease due to the prolonged period with high agitation. The increase in time also resulted in the death of tiny new-sprout and those less tolerable duckweeds [22–24].
The trend of bioaccumulation concentration in the recirculated set-up was inversely proportional to that of the stationary set-up. There is an increasing bioaccumulation concentration with time observed in the stationary set-up. At 3 d, bioaccumulation concentration was at 0.03 mg/L; while a recorded value of 0.77 mg/L and 1.12 mg/L were obtained in 6 and 9 days, respectively. The increasing bioaccumulation concentration was due to the duckweed’s natural accumulating characteristics given an excellent environmental state with time. Fig. 4 shows the mass balance of bioaccumulated Pb and the dissolved Pb in the effluent.

Mass balance of lead at (a) 3 d stationary (b) 3 d recirculated (c) 9 d stationary (d) 9 d recirculated.
The findings of the experiment showed that the efficiency of duckweed to accumulate Pb from contaminated water could be enhanced by the application of a dynamic-moving mechanism as done in the recirculated set-up. Though bioaccumulation concentration decreased with time in the recirculated set-up, the result provided a favorable chance of duckweed in treating wastewater. Thus, consideration of the agitation speed would be among the exciting facet to work in the next stage of the project.
Lastly, the Pb content of the effluent in the 9 d stationary (1.38 mg/L) and 3 days recirculated set-up (0.93 mg/L) were much lower compared to the influent (2.5 mg/L). This result signifies that duckweed was able to accumulate Pb from the contaminated water. Moreover, the data also indicate that recirculated set-up has a higher potential with lesser time in enhancing the capabilities of duckweed as contaminant accumulator from wastewater compared to the stationary.
3.3. Plant Growth Rate (PGR)
The potential of duckweed to accumulate contaminants also depends on its survival in the contaminated media. From the recorded data of the mass of plants before and after phytoremediation, plant growth rate was calculated using Eq. (1). Table 2 presents the data gathered with regards to its PGR.
The plant growth rates in the two set-ups were marked negative due to the nutrient-free distilled water used in the experiment. The use of distilled water in this study contributed to the inhibitions of the plant to grow due to lack of nutrients [19, 25]. In real wastewater, the availability of nutrients would boost the growth of duckweed, thereby making it more rigorous in accumulating contaminants. This finding is supported by a previous study [26], claiming that most industrial wastewater is nutrient-rich, thus allowing duckweed to multiply, grow and survive when induced as a treatment agent and accumulator of contaminants in water. Moreover, the negative PGRs in the early stage of the treatment process were due to the adaptive phase of duckweed towards Pb exposure. The recovery of duckweeds’ growth during the 9th day in the recirculated set-up with 0.16% was due to the transfer of nutrients from the degraded duckweed that has died in the adaptive stage of the plant during phytoremediation.
4. Conclusions
The phytoremediation potential of duckweed as accumulator was best seen in the recirculated set-up at 3 d of contact time with 1.57 mg/L of Pb accumulation, a way higher compared to the 1.12 mg/L attained in stationary set-up after 9 long days. The inclusion of the dynamic mechanism in the recirculated set-up enhanced the treatment process by shortening the time needed to treat wastewater at only 3 d. It was shown in this study that the duration of phytoremediation was shortened due to the incorporation of agitation in the recirculated set-up compared to the usual phytoremediation process which requires a longer span of time for heavy metals to be accumulated by the plants. The plant growth rate was observed with time due to the natural adaptation of plants in the contaminated water and the mechanisms of the set-up.
Acknowledgment
The authors would like to convey thanks to the faculty and staff of the College of Engineering and Technology of the University of Science and Technology of Southern Philippines - Claveria for the support in conducting this study.
Notes
Author Contributions
L.J.A.U. (Envi. Engr.) is the lead researcher of this work. P.C.S.P. (Envi. Engr.) is the head in the conduct of the experiment. C.M.N.N. (Envi. Engr.) helped in the conduct of the experiment. J.T.T. (Envi. Engr.) helped in the conduct of the experiment. D.C.A. (Envi. Engr.) helped in the conduct of the experiment. J.C.T. (Envi. Engr.) helped in the conduct of the experiment. A.L.I. (Ph. D.) is the lead advisor of the project. V.I.F.M. (Envi. Engr.) is the assistant advisor and the corresponding author of this paper. R.O.A. (Ph. D.) is the overall consultant of this project and has helped in the preparation of the manuscript.