1. Introduction
Municipal solid waste (MSW) management through open dumping and open burning is a common practice in Nigeria, and this has resulted in severe ecological, environmental and health problems. Also, open dumping or indiscriminate disposal of waste materials is a prevalent practice among the low-income and upper middle-income earners in many developing countries [1]. Illegal citation of dumpsites in recent times has become one of the key factors bedevilling several countries in the world particularly developing countries (such as Nigeria) where over 40% of the waste materials generated in advanced countries end up in open dumpsites in these countries [2]. Due to the increasing trend in population, consumption rate and the growing GNI/capita in the developing world, additional amounts of municipal, industrial and hazardous waste are entering into the waste streams every day. It has been estimated that globally, urban food waste has recently increased tremendously by 44% from 2005 to 2025 [3]. As urbanization and population growth continues to be on the increase, it is expected that at least several hundreds of million more people will be served by dumpsites in Nigeria. In other words, considering that there is a growing gap between increase in Nigerian population and growth of urbanization, it is almost certain that within the next 10–15 y much more waste will be driven to dumpsites and some additional hundreds of Nigerian population will be served by dumpsites [4]. However, if the present waste management trends are maintained in Nigeria, food waste disposal in dumpsites is expected to increase the dumpsite proportion of Nigerian anthropogenic greenhouse gas (GHG) emissions as well as contaminating its underlying soil and groundwater. The term “open dumpsite” is used to characterize a land disposal site where indiscriminate deposition of solid waste takes place with either no - or at best - very limited measures to control the rate and/or to protect the surrounding environment. In addition, it is typical that no planning (such as location sensitivity) or engineering measures (such as a liner system) have been implemented prior to the delivery of waste [5]. Leachate is the liquid substance produced when organic wastes undergo decomposition, and when water (due to rainfall, surface drainage, groundwater, etc.) percolates through solid waste undergoing decomposition. Leachate is a liquid that contains dissolved and suspended materials that, if not properly controlled, may pass through the underlying soil layers and contaminate surface and ground water. The composition of leachate depends on the stage of degradation, age of the dumpsite and the type of wastes within the disposal dumpsite [6–8]. Soil pollution is another environmental problem caused by dumpsites. MSW contains different heavy metals and organotin compounds which can be found in dumpsite leachate, air and soil produced either from plastic burning or smelting of scrap metals and e-waste. Depending on the category of contaminants, they end up either in water held in the soil or leached into ground water. Other contaminants like Hg, cadmium (Cd), Cu, Ni, lead (Pb), Zn, etc. present in leachate can alter the soil properties and also have some degree of impact on soil organisms and plants grown on such soils [9, 10]. Hammed et al. [11] investigated the physiochemical and heavy metal levels of the underlying soil at Awotan and Ajakanga dumpsites in Ibadan. The result indicated an increase in the concentration of heavy metals (particularly Cd, Cu, iron (Fe), Ni, Pb, and Zn) in the underlying soil of these two dumpsite than that of the soils at the control sites. Several studies have shown evidence of serious hazards caused by open waste dumping ultimately affecting the life cycles of plants [12], soil properties and significantly altering the behaviour of its underlying soil [13]. Ramakrishnegowda et al. [14] investigated the interaction effect of alkali on the geotechnical properties of shedi soil. It was observed that although plasticity index (PI) of the soil decreased and optimum moisture content increased with increasing concentration of alkali, the shear strength of the soil decreased significantly due to decrease in the cohesion of the soil particles. This correlated with the investigation carried out by Amadi et al. [15], where dumpsite underlying soils in Avu dumpsite in Imo state and Ihie dumpsite in Abia state contained several contaminants higher in manganese (Mn), Pb, Fe, Cd, etc. with pH relatively lower (3–4) than soil samples collected few kilometres from the waste dump. Studies conducted by Sruti et al. [16] revealed that upon the addition of leachate, the soil hydraulic conductivity decreased significantly when the permeating liquid which is the synthetic leachate containing microorganisms interacted with the soil grain particles. The decrease in hydraulic conductivity was caused by reduction of the effective porosity due to clogging effect of pore space as a result of increasing amount of microorganisms in the leachate. The soil’s PI showed an increasing trend upon addition of leachate, contrary to investigation by Ramakrishnegowda et al. [14], where the contaminated soil had higher values of hydraulic conductivity than the uncontaminated soils. Thus the authors concluded that this may be due to particles’ flocculation as a result of contamination with MSW. The flocculation process may have altered the behaviours of the fine particles from clay-like to silt-like formation and consequently, resulting in high permeability of the contaminated soils. Many contaminants (particularly heavy metals) are trapped in the soil beneath dumpsites, resulting in long term contamination of the underlying soil in terms of pH and geotechnical properties. This forms part of the investigation in this study to determine the effect of dumpsite on the hydraulic conductivity, PI and pH of the underlying soil as well as public health.
2. Materials and Methods
The methodology adopted in this study is presented in this section. This also includes the experimental procedures and materials used to achieve the objective of this study.
2.1. Design of Experiment
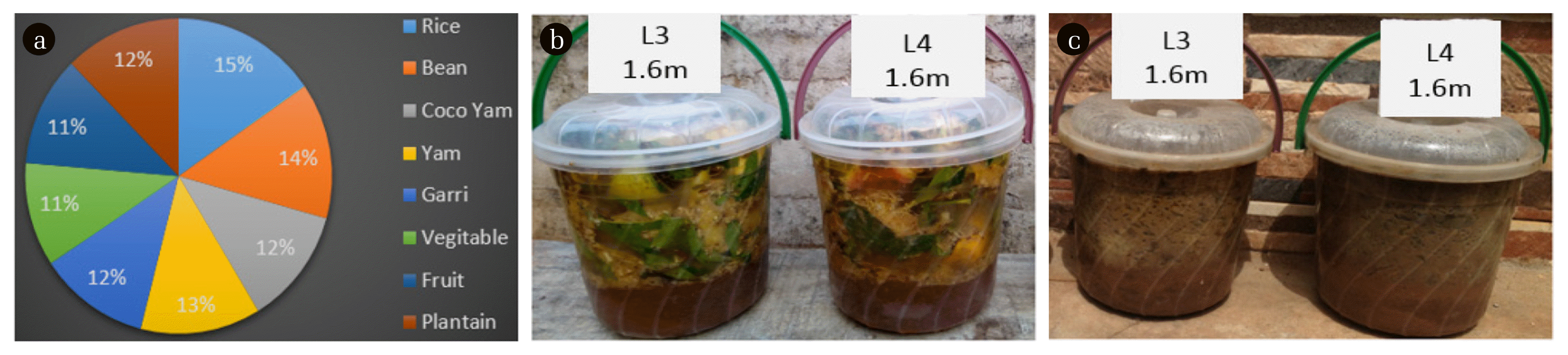
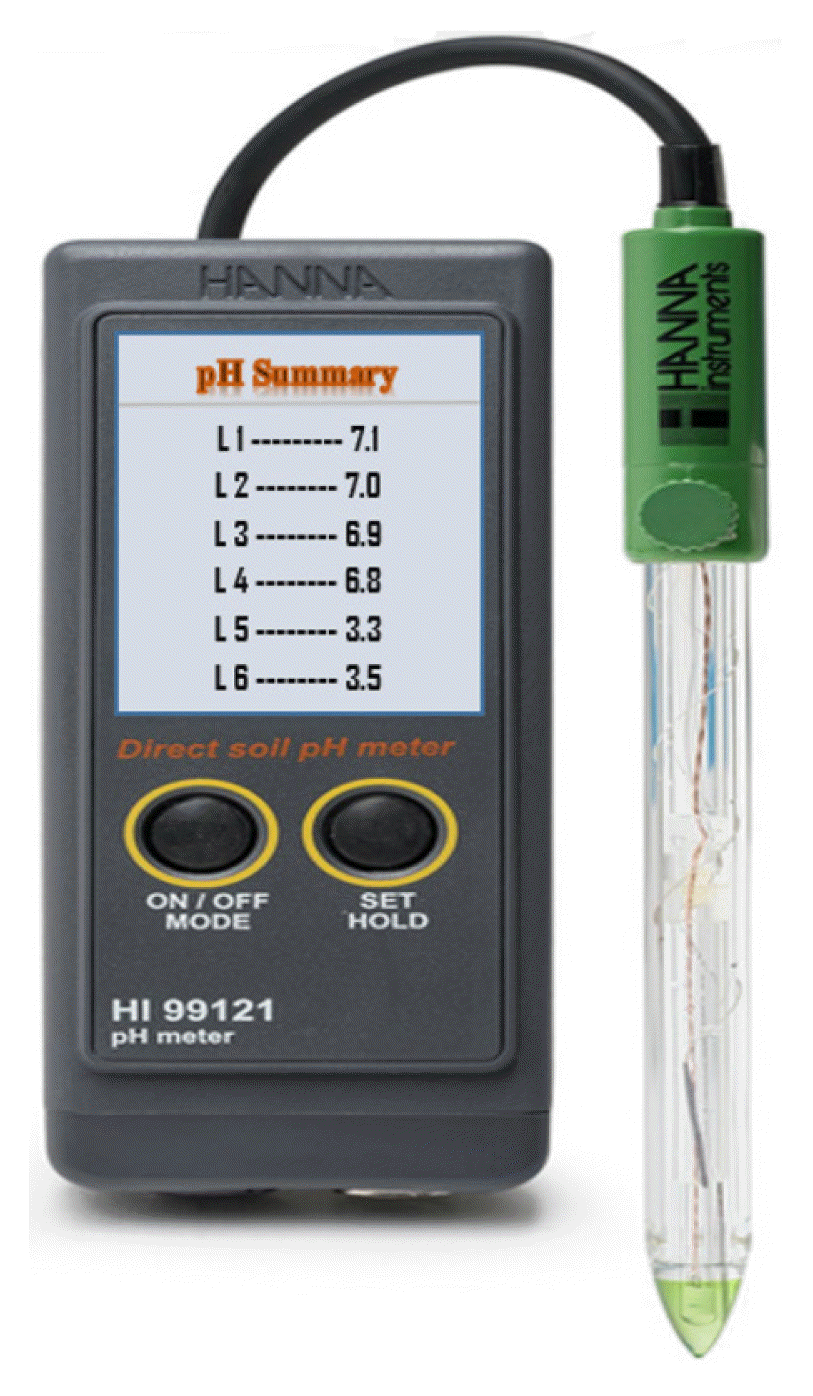
A total number of six soil samples collected from different locations in Benin City were analyzed to determine their various compositions. Two of the samples were collected differently from Uselu Market dumpsite and New Benin dumpsite, while the other two samples were collected 1.6 m from each of the dumpsite, whereas, the last two samples were combination of food waste with soil samples collected 1.6 m from each of the dumpsite. All samples were collected from a depth of 1.2 m using shovel. The respective soil samples after collection were tie properly in polyethylene bags to prevent air ingress. The soil samples were subjected to Atterberg limit test and consolidation teste in the laboratory. The chemical composition of the soil samples were analyzed using X-ray Fluorescence handheld device (X MET 7000) while pH of each soil samples were determined using digital soil pH meter (HI 99121 soil pH meter). As presented in Fig. 1, the soil food-waste combination was allowed for a period of three months for effective decomposition before analysis. a b c
2.2. Geotechnical Analysis
Moisture content (MC), liquid limit (LL), PI and hydraulic conductivity (coefficient of permeability) are the parameters determined for the aforementioned soil samples in this section. Standards, codes and formulas employed in determining each parameter are also encapsulated in this section.
2.2.1. Atterberg limit
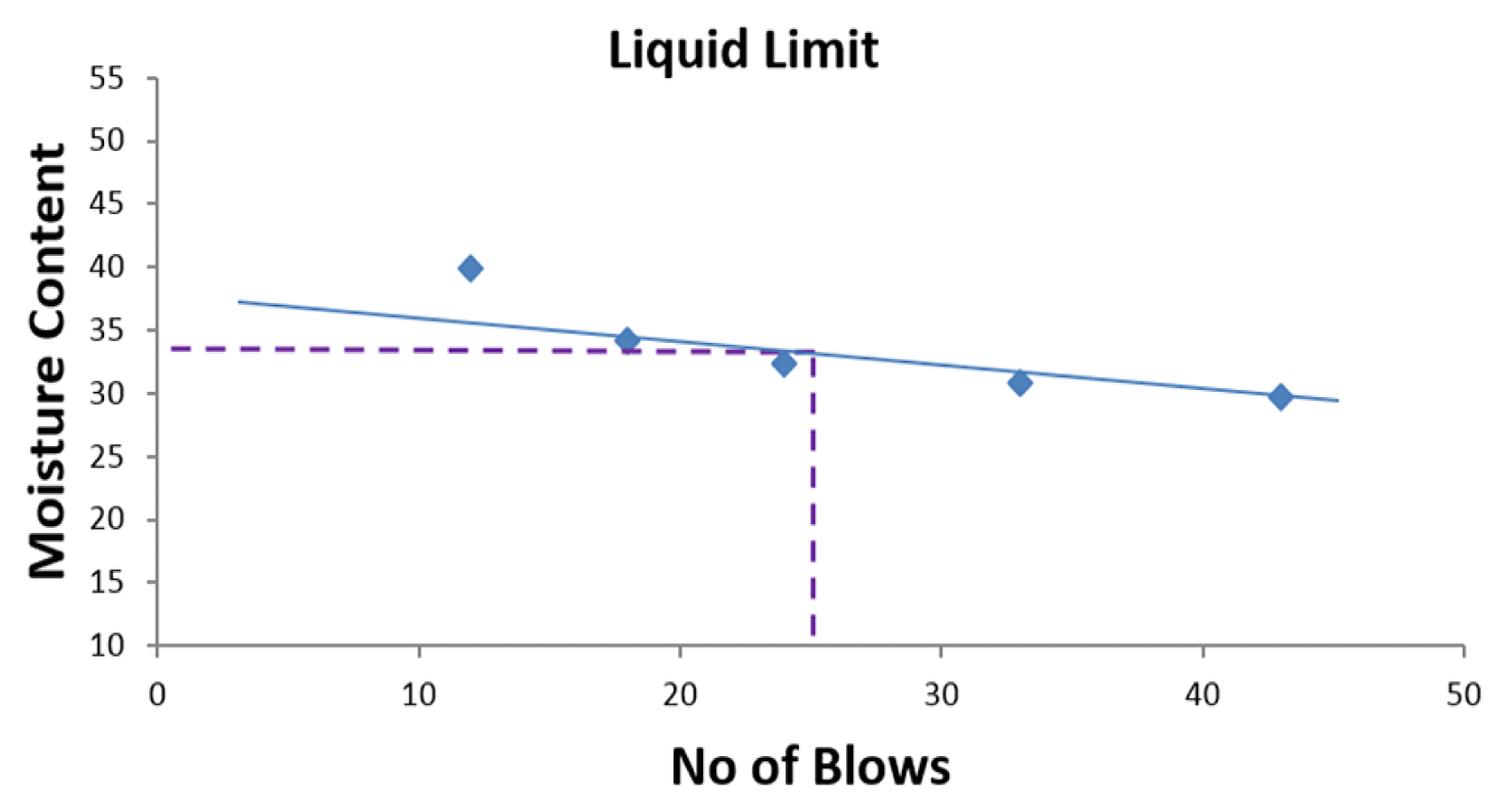
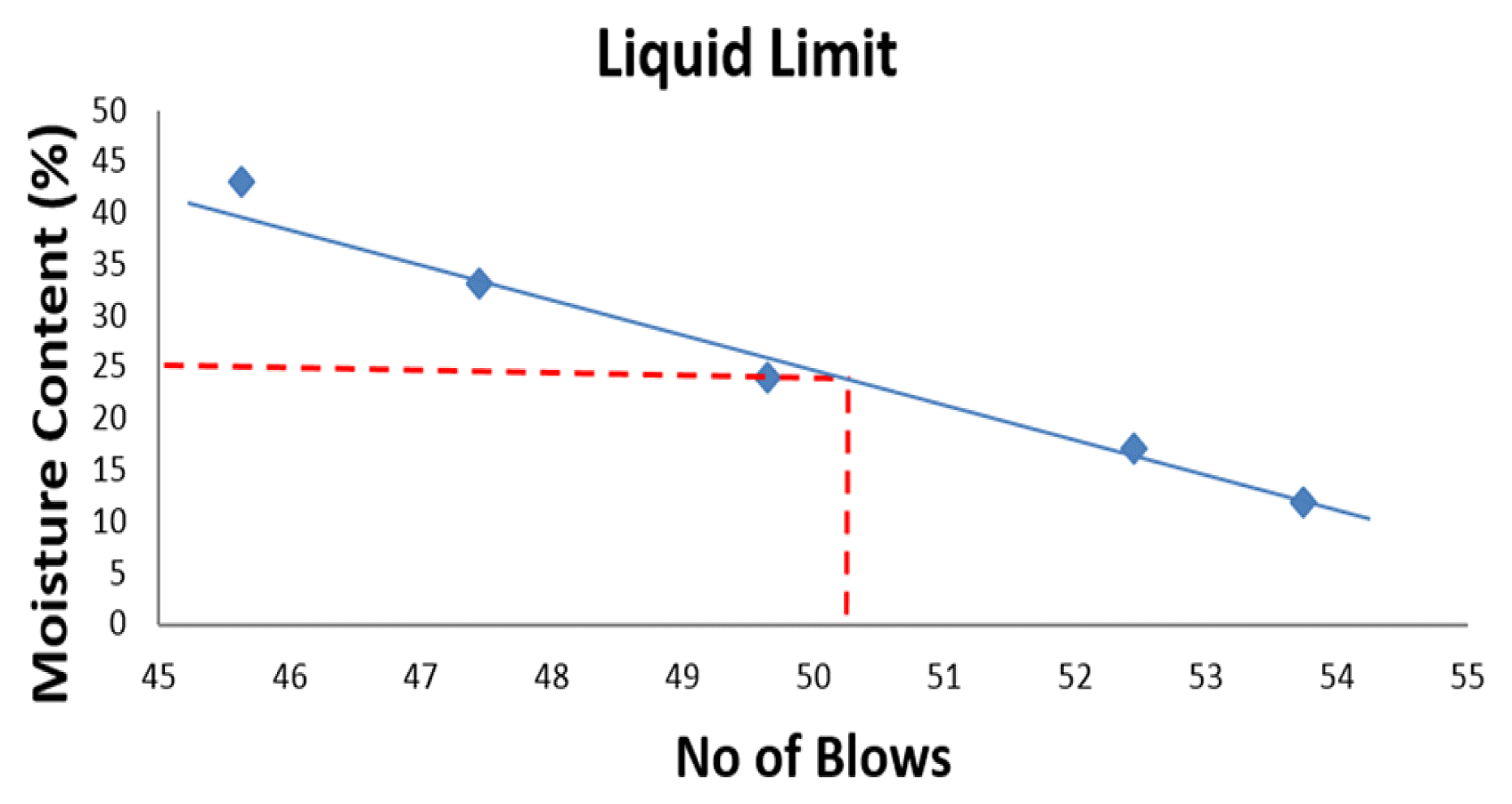
Atterberg limit is a soil test that can be used to determine the critical MCs of a fine-grained soil such as plastic limit (PL), LL, PI as well as the shrinkage limit which can be used to determine the shear strength and behaviour of a soil as the water content increases. LL is the amount of water content in which the state of a given soil sample changes from liquid state to plastic state or the minimum water content in which a soil begins to flow in the Casagrande cup and closes the grove when small share force is applied is also known as LL. Having carried out the Atterberg test according to the British standard (BS) code 1377: Part 2, Sections 4, 5: 1990, LL was determined by plotting MC against no. of blows and drawing a straight line through each of the plotted points. MC is the ratio of the mass of water present in the soil to the mass of solid particles that make up the soil sample, and the ratio is expressed in percentage given by Eq. (1);
Therefore,
Where W1 = Weight of empty can (g), W2 = Can weight + wet soil (g), W3 = Can weight + dry soil (g).
PL relates to the MC at which a given soil changes from plastic state to semisolid state and it is often expressed in percentage.
PI relates to the numerical difference between LL and PL. Plastic index is denoted as IP and it is mathematically expressed by Eq. (3);
Where, PL = Plastic limit, LL = Liquid limit.
In addition, the shrinkage limit is referred to the maximum MC in which reduction in water content of a given soil will not result in decrease in the soil’s total volume but an increase in water content will result in further increase in the water content of the soil.
2.2.2. Consolidation test
The application of compressive load on a laterally confined layer of soil usually results in vertical deformation of the soil. The rate of change in thickness of the soil under such compressive load depends on the soil’s permeability as well as distance the water will flow to reach a drainage surface [17]. The consolidation properties were determined from disturbed samples which were compacted to about 4–6% water content with 25 blows in a mould. The test was carried according to BS code 1377: Part 6, Section 6: 1990. During the experimental test, the specimen was allowed to consolidate under several incremental loads such as 1 kg, 2 kg, 4 kg, 8 kg, 16 kg and 32 kg and each incremental load was maintained until the compression effect diminishes. Using dial gauge indicator, readings were taken for 5, 10, 15, 30 s, 1, 2¼, 4, 9, 16, 25 min and 1 h on application of each load. The specific gravity (GS) of a material is the ratio by weight of a given volume of soil at room temperature to the mass of equal volume of water at the same temperature, in effect, it implies how heavier or lighter the weight of such material is than water. Using the general equation, GS is given by Eq. (4);
Where W1 = Empty bottle weight, W2 = Bottle weight + oven dried soil, W3 = Bottle weight + oven dried soil + water, W4 = Bottle weight + water.
To determine the buck density which is the individual particle weight that constitutes a given soil divided by the total volume (internal pore volume of soil, volume of soil particles as well the soil inter-particle void volume) at which they occupy in the mould, Eq. (6) was used.
Where M = Mass of wet compacted soil (g), V = Volume of the mould (cm3).
The volume in this case is the total volume occupied by soil particles in the mould and was measured as 1,000 cm3. Furthermore, the dry density which relates to a given soil sample subjected to a temperature of 105° to remove the MC was determined using Eq. (7);
Where ρb = buck density, M.C = Moisture Content expressed in percentage.
However, the bulk density and the corresponding dry density for the compacted soil sample are both expressed in g/cm3. Coefficient of compressibility (m2/KN) given by Eq. (8);
Where, coefficient of volume change (m2/KN) is given by Eq. (9);
ef = Final void ratio
Coefficient of consolidation (m2/y) given by Eq. (10);
Where d = Drainage path length, t = Time factor.
Coefficient of permeability (m/s) given by Eq. (11);
Where Mv = Coefficient of volume change, Cv = Coefficient of consolidation, γw = Density of water.
During computation, initial voids ratio is given as shown in Eq. (12);
Where ei = Initial void ratio, GS = Specific gravity, ρd= Dry density of soil solids.
Degree of saturation (So) expressed in percentage is the ratio of liquid content to the total volume of voids present in a given soil mass as shown in Eq. (13);
The void ratio change factor (F) is given by Eq. (14);
Where, e = Void ratio, Ho = Initial height of specimen. At the end of the experimental test, the following parameters were calculated using standard formulas mentioned earlier. Void ratio at the end of the experimental test is given as shown in Eq. (15);
Where, ef = Final void ratio at the end of the test. Substituting ef into ei in Eq. (12), degree of saturation at the end of the test becomes;
3. Results and Discussion
Table 1 represents a summary of consolidation test carried out on soil samples used in this study, Tables 2–5 represent the results of Atterberg limit test for the same soil samples in this study whereas, the percentage composition of soil samples in this study is presented in Table 6. Fig. 2–5 are the graphical representation of LL for each of the samples used in this study, while Fig. 6 shows the pH for each of the soil samples used in this study.
Considering the six (6) samples used in this study, four (4) samples were subjected to geotechnical test to analyse their LL, PI and hydraulic conductivity. The four samples included L1-Soil sample collected 1.6 m from New Benin dumpsite, L2-Soil sample collected 1.6 m from Uselu Market dumpsite, L5-Soil sample collected from New Benin dumpsite, L6-Soil sample collected from Uselu Market dumpsite. However, sample L3 was soil sample collected 1.6 m from New Benin dumpsite intermixed with food waste whereas, sample L4 was soil sample collected 1.6 m from Uselu Market dumpsite combined with food waste. The other two samples were combined with food waste and allowed for a period of three months for effective decomposition, after which, their pH were examined alongside the aforementioned four samples. All samples used in this study were collected from a depth of 1.2 m. During the laboratory test, it was observed that the soil samples embedded within a ring in the consolidation machine decreased as load on the consolidometer increased, and the trend got to a point where the consolidated sample stabilized and was no longer decreasing. A dial gauge was used to take readings at different time intervals as the sample decrease in volume. At each point of stabilization where the load was no longer causing further decrease to the sample, the consolidometer was disengaged and the wet consolidated sample with ring was measured using weighing balance. Consequently, the wet consolidated sample was placed in the oven (105–110°C), and dried consolidated sample with the ring was measured after 24 h. As shown in Table 1, hydraulic conductivity for sample obtained 1.6 m from Uselu Market dumpsite was quite high 2.42 × 10−3 m/s, whereas, reduction was observed for hydraulic conductivity with a value of 1.0 × 10−6 m/s for sample obtained from Uselu Market dumpsite. However, the result of hydraulic conductivity obtained for samples collected 1.6 m from New Benin dumpsite was very high with a value of 2.14 × 10−2 m/s, whereas, the sample collected from the same New Benin dumpsite was 1.45 × 10−6 m/s. Geotechnical assessment of open dumpsite in Illorin, Nigeria by Ige [18], revealed hydraulic conductivity of 1 × 10−9 m/s which is highly consolidated than the values obtained in this studies. However, Evangelin et al. [19] investigated the impact of MSW on the geotechnical properties of soil and obtained hydraulic conductivity between the range of 2.11 × 10−5 and 3.15 × 10−5 which is not too low and not too high. The low hydraulic conductivity obtained in this study may have been due to the effect of clogging by organic waste which can occur as a result of the growth of microorganisms and biological material build-up during waste decomposition or particulate transport present in leachate generated by the waste. Oftentimes, clogging may result in very low hydraulic conductivity due to interaction with leachate which has the potentials of hampering the underlying soil drainage layer by making pore spaces within the soil over saturated as a result of microorganisms and biological material build-up within the waste stream. This correlates with the investigation carried out by Sruti et al. [16] where hydraulic conductivity of soil interacting with leachate decreased significantly. However, if soil hydraulic conductivity is too low, it indicates low clay content in the soil which implies that the rate of leachate percolation into soil and ground water will be delayed and vice versa. PI for sample obtained 1.6 m from Uselu Market dumpsite was quite low (6.9%) with LL of 36.6%, whereas, an increase was observed for PI (18.53%) with LL of 34% for sample obtained from Uselu Market dumpsite. This however was similar to results obtained for samples collected 1.6 m from New Benin dumpsite where PI of 6.0% and LL of 25% was obtained while PI of 13.8% and LL of 34% were obtained for samples collected from New Benin dumpsite. In similar studies carried out by Ige [18], results of LL from dumpsite underlying soils were in the range of 35.34% and 40.56% and PI between the range of 17.5% and 20.55%. LL between the range of 30% and 35% and PI between the range of 9% and 20% was obtained by Ayininuola [20]. Furthermore, LL between the range of 24% and 38% and PI between the range of 4% and 9% was obtained by Harshani et al. [21]. The low PI obtained in this study may have been due to the limited amount of clay present in the soil particles, whereas, increase observed in PI of the samples may have been caused by reduction of the effective porosity due to clogging effect of pore space as a result of increasing amount of microorganisms in the leachate.
As mentioned earlier, compositions of the soil samples were analysed using X-ray florescence analyser (X-MET 7000) and the results are tabulated in Table 6, while direct soil pH meter was used to measure the pH of the soil samples. Each of the samples were assigned a label as follows; L1-Soil samples collected 1.6 m from New Benin dumpsite, L2-Soil sample collected 1.6 m from Uselu Market dumpsite, L3-Soil sample collected 1.6 m from New Benin dumpsite with decomposed food, L4-Soil sample collected 1.6 m from Uselu Market dumpsite with decomposed food, L5-Soil sample collected from New Benin dumpsite, L6-Soil sample collected from Uselu Market dumpsite.
While soil samples obtained 1.6 m from each dumpsite and soil samples obtained 1.6 m and intermixed with food waste showed less toxicity in composition values, samples obtained from the two dumpsite showed higher percentage of heavy metals and toxic compounds which may have been caused by interaction of organic waste with hazardous waste (such as e-waste, battery waste, medical waste, etc.) in the dumpsite. This also may have been due to lack of waste segregation and characterization as well as indiscriminate disposal of waste in the dumpsites, including hazardous and non-hazardous waste materials. As shown in Table 6, the results of composition analysis obtained from the soil samples used in this study correlated with the investigation carried out by Hammed et al. [11], Ogunmodede et al. [22], and Azeez et al. [23] but slight variation was noted in the values. This is likely due to the difference in the category of waste materials disposed at the dumpsite investigated by these authors. These heavy metals and toxic compound if present in high quantities as shown in Table 6 can alter the properties as well as the soil pH. To justify this argument, it can be observed in Fig. 6, that soil samples collected from two dumpsites in this study are in the ultra-acidic range. This is because, these dumpsites receive all forms of waste including hazardous and non-hazardous waste materials, whereas, soil samples collected 1.6 m from each of the dumpsites and combination of soil samples collected 1.6 m with food waste were in the neutral range. This therefore implies that, organic materials (such as food waste) that have not been left to decompose and interact with other toxic materials in dumpsites can be used as a valuable product for compost manure. E-waste is chemically and physically distinct from other forms of municipal and industrial waste; it contains both valuable and hazardous materials that require special handling and recycling methods to avoid environmental contamination and detrimental effects on human health. E-waste is either landfilled, or exported from advanced countries to developing countries, where it may be recycled using primitive techniques and little concern for safety of worker and environmental protection. The chemical composition of e-waste varies depending on the age and type of the discarded item. However, the interior part of most e-wastes are composed of heavy metals (particularly Cu, Al, and Fe, etc.) enclosed with plastics or ceramic cover. Some heavy metals are used in the manufacture of electronic products, while polycyclic aromatic hydrocarbons (PAHs) are found to exist in significant quantities in the plastic cover. The low-temperature combustion of e-waste in dumpsites can generate PAHs while burning of insulated wires can generates a significant amount of dioxins than burning domestic waste [24]. Obsolete refrigerators, spray cans and air conditioning units found in open dumpsites contain ozone-depleting chlorofluorocarbons (CFCs) which can result in global warming. Studies have shown that the chemical composition of e-waste and other hazardous waste in dumpsites can leach into the soil or migrate into the atmosphere when combusted, thereby adverse health defects such as causing changes in thyroid function, adverse neonatal outcomes, inflammation and oxidative stress-precursors to cardiovascular disease, DNA damage and possibly cancerous ailment, respiratory disease, etc. Burning of plastics, or polyvinyl chloride (PVCs), can produce hydrogen chloride gas, or hydrochloric acid, which can cause fluid build-up in the lungs and possible ulceration of the respiratory tract [25, 26]. Sources of risk associated with dumpsites can be classified into three sectors: informal recycling, formal recycling and exposure to hazardous compounds generated from dumpsites. The smoke generated from burning these inorganic materials is composed of tiny particles (particulates), which contain toxic pollutants that can result in changes in earth’s temperature. If inhaled, these microscopic particles can result in one or more of the aforementioned health effects. However, an extensive assessment is necessary due to the numerous category of waste disposed in dumpsites and their complex compositions which cannot fully be exhausted in this paper.
4. Conclusions
Various analysis carried out in this study has shown that dumpsites are likely to contain highly hazardous materials, as such, a wide range of toxic substances can be released into the environment. Review of related studies have also indicated that many of these contaminants find their way into underlying soils of the waste disposal sites, groundwater as well as the atmosphere thereby affecting living things and the ecosystem in the long run. While the pH of soil samples collected from New Benin dumpsite and Uselu Market dumpsite ere in the ultra-acidic range (3.5, 3.5) the pH of soil samples obtained from the combination of food waste with soil samples collected at a distance of 1.6 m from each of the aforementioned dumpsites were observed to be in the neutral range (6.8, 6.9). This indicates that the interaction between toxic contaminant in dumpsites and their underlying soils can significantly alter the geotechnical properties of such soils, damage plant root, affect soil fertility, etc. whereas, pH obtained from the soil-food waste combination indicates that food waste can be used as a valuable product for compost manure. Depending on the toxicity of such soils, plant root can easily take up these contaminants which however instigate various ailments when consumed by human beings. Despite the methodological limitations in this study, existing scientific literature on the health effects of illegal dumpsite provides strong indication of existing linkages between dumpsites and the health effects of workers, informal recyclers and residents in open dumpsite situated areas. Besides municipal waste, healthcare waste, hazardous and e-waste are common streams found in dumpsites which further supports the various arguments on the negative effects of waste dumpsites on public health and environment. The problem is that in most dumpsites, previous waste materials are usually present in unknown quantities and unknown interaction level with the surroundings. Hence, integration of effective lining system such as High Density Polyethylene (HDPE) liners in waste dumpsites; design, utilization and recommendation of engineered landfill system, promotion of Energy to Waste technologies and waste to organic fertilizers can help minimize this challenges posed by the poor waste management techniques in Nigeria and developing countries at large.