1. Introduction
Biodetoxification of industrial effluent containing hazardous compounds is an important process used in wastewater treatment technology. Such processes are characterized by (i) low capital costs, (ii) environmental benignity and (iii) allows for the complete remediation of the wastewater, unlike chemical and physical processes that produce by-products which contribute to environmental deterioration. These chemical and physical processes are utilized in the degradation of cyanide and related compounds found in metallurgical and mineral processes where free cyanide (CN−) is utilized as a lixiviant for the recovery of precious metals from gold bearing ores in a process known as the cyanidation process [1]. Due to the environmental severity of such processes, biodegradation of cyanide and related compounds is preferable as it is more environmentally benign. However, this process results in the production of ammonium (Eq. (1) and (2)), which is regarded as a pollutant. Furthermore, wastewater containing elevated concentrations of ammonium can contaminate potable and surface waters resulting in eutrophication.
Biological nitrogen removal (BNR) is commonly utilized for the effective removal of nitrogenous compounds in wastewater. Traditional BNR systems consist of two steps: Nitrification by autotrophs under aerobic conditions, followed by denitrification by heterotrophs under anoxic conditions [2]. However, this system is associated with the following shortcomings: (i) the nitrification step is slow due to the low growth rate of autotrophs; (ii) autotrophs are vulnerable to high concentrations of ammonium and organic matter; and (iii) nitrification and denitrification reactors are separated due to the aerobic and anaerobic nature of the organisms employed in nitrification and denitrification, respectively [3, 4]. However, recently, heterotrophic organisms capable of nitrification and denitrification under aerobic conditions have been reported. Novel bacterial species such as Bacillus methylotrophicus L7 [5], Pseudomonas stutzeri YZN-001 [6], Rhodococcus sp. CPZ24 [7], Alcaligenes faecalis C16 [8] and other species have been reported to undergo heterotrophic nitrification and aerobic denitrification. These systems are associated with high nitrogen removal efficiencies due to the high growth rate of the microorganisms used in such systems. Utilizing these microorganisms, in comparison to traditional autotrophic nitrifying and anoxic denitrifying organisms, has the following advantages: (i) Heterotrophs grow rapidly, therefore microbial recycling is not required, (ii) Nitrification and denitrification can be achieved simultaneously, (iii) Minimal acclimation problems are observed, (iv) There is no need for pH-control, as the acidity that is generated during heterotrophic nitrification would be offset by the alkalinity produced during aerobic denitrification, (v) Heterotrophic nitrifiers are tolerant to low temperatures as compared to autotrophic organisms [2, 4, 8].
However, limited research has been conducted to evaluate the susceptibility of heterotrophic nitrifiers and aerobic denitrifiers to CN− and thiocyanate (SCN−) compounds in relation to the overall treatment of cyanide species found in cyanidation wastewater. A similar system where the presence of CN−, SCN− and phenol on autotrophic nitrifying and denitrifying organisms has been reported; however such a system was related to the remediation of cokes and gasification wastewaters [9], which have disparate contaminants compared to cyanidation wastewater. Therefore, there is a need for the evaluation of CN− and SCN− degrading organisms to conduct heterotrophic nitrification and aerobic denitrification under cyanogenic conditions since the bio-degradation of CN− and SCN− is rarely complete, especially during the cold season. Hence, this study was aimed at evaluating the heterotrophic nitrification and aerobic denitrification propensity of CN− and SCN− degrading organisms, with the overall aim of applying the same organisms in the biodegradation of CN− and SCN− and its related complexes.
2. Materials and Methods
2.1. Inoculum Preparation: Thiocyanate Degrading Organisms (TDO)
The thiocyanate degrading microbial consortia used in this study were sampled from an active lab-scale SCN− degradation system operated at the Bioresource Engineering Research Group (BioERG) laboratories, Cape Town, South Africa. The TDO’s were analyzed using the 16S rDNA amplicon gene sequencing approach to reveal the microbial species that were present within the microbial community and this data is available in Mekuto et al. [10].
For inoculum development, the reactor used had a height of 250 mm and a diameter of 104 mm with an operating volume of 1 L. Aeration was set to 0.4 L min−1 and the system was operated at room temperature (20 to 22°C). Mixing was achieved using an overhead stirrer fitted with 2-x-four bladed Rushton-type impellers, set at 250 rpm. The sample was centrifuged at 14,000 rpm for 5 min to concentrate the microbial cells. The cells were washed twice with a phosphate buffer solution (pH = 7.0) and thereafter, the cells were re-suspended in sterile distilled water subsequent to inoculation (1% v/v) in a nitrogen-free minimal media (MM) (pH = 9.9) as described previously by Mekuto et al. [11], with glucose being used as a carbon source at a concentration of 1 gL−1. The cells were grown for a period of 48 h at a temperature of 30°C in 250 mL Erlenmeyer flasks containing 200 mL of MM. This culture was then used to study the potential of the organisms to conduct heterotrophic nitrification and aerobic denitrification under cyanogenic conditions.
2.2. Inoculum Preparation: Cyanide Degrading Organisms (CDO)
Similarly, the CN− degrading consortium was sampled from a stock reactor treating CN− containing wastewater operated at the BioERG laboratories, Cape Town, South Africa. The configuration of the reactor was as described in section 2.1. This mixed microbial community were identified using 16S rDNA amplicon gene sequencing and the data is available in [10]. These bacterial species were previously isolated from CN− containing wastewater and were previously found to be cyanide degraders by [12], but were unable to degrade thiocyanate (data not shown). The inoculum was derived from the reactor using the procedures highlighted in section 2.1.
2.3. Experimental Design
2.3.1. CN− and SCN− biodegradation by CDO’s and TDO’s
To prove CN− and SCN− biodegradation by CDO’s and TDO’s, respectively, MM was adjusted to a pH of 9.9 and the CN− and SCN− concentrations were set at 250 mg CN−.L−1 and 200 mg SCN−.L−1. Furthermore, the CDO’s and TDO’s were co-cultured to assess dual SCN− and CN− biodegradation capacity in the same media using the concentrations stated. The CN− biological removal efficiency (BRE %) was calculated as defined in [11]. All experimental work containing CN− were conducted in airtight multiport shake flasks fitted with a syringe port to allow for sampling without opening the flask contents. This was done to prevent the volatilisation of CN− as hydrogen cyanide gas. Uninoculated flasks served as controls. The experimental error was calculated as the standard error of mean using the standard deviation obtained from a duplicate set of data (n = 2).
2.3.2. Heterotrophic nitrification and aerobic denitrification by CDO and TDO: Effect of CN− and SCN−
To assess the capacity of TDO’s and CDO’s to conduct heterotrophic nitrification and aerobic denitrification, the cultures were inoculated in 200 mL flasks with MM, containing an initial concentration of ammonium (as NH4Cl) and nitrate (as NaNO3) of 250 mg NH4 +-N.L−1 and 100 mg NO3 −-N.L−1, respectively. The effect of SCN− and CN− on nitrification and denitrification was assessed by supplementing the required concentrations of SCN− (25 and 50 mg SCN−.L−1) (as KSCN) and CN− (5, 10 and 15 mg CN−.L−1) (as NaCN) (Merck, Germany). The dual effect of both SCN− and CN− was also assessed at 25 mg SCN−.L−1, while CN− was varied as stated previously. The effect of CN− and SCN− on CN− and SCN− degraders was evaluated separately and in co-cultures. Oxygen sparging was not used to oxygenate the nutrient media, thus the organisms relied solely on the dissolved oxygen (DO) in the wastewater, which was equivalent to ~5 mg.L−1 (BANTE820 portable dissolved oxygen meter, BANTE instruments, China). The DO was measured prior to the commencement of the experimental work and was not monitored thereafter. The pH of the media was set at 9.9 and at a temperature of 30°C for both nitrification and denitrification studies. These conditions were based on (i) the fact that cyanide containing wastewaters are mostly alkaline [13, 14] and (ii) previous optimization studies on cyanide degrading organisms observed these conditions as being most suitable for successful CN− biodegradability [15]; hence these conditions were chosen on a pragmatic basis. The nitrification and denitrification rates were reported as averaged rates. Uninoculated flasks served as controls while the experimental error was calculated as the standard error of mean using the standard deviation obtained from a duplicate set of data (n = 2).
2.4. Analytical Methods
The collected samples were centrifuged at 14,000 rpm for 5 min and then analysed for ammonium, nitrate, nitrite, CN− using Merck ammonium (00683), nitrate (14773), nitrite (14776), cyanide (09701) and sulphate (00617) test kits. The basis behind the mechanism of determination of these compounds using Merck test kits has been elucidated by Mekuto et al. [11]. Thiocyanate was determined using the ferric method [16]. The pH was measured using a Crison Basic20 pH meter, which was calibrated daily.
3. Results and Discussion
3.1. Thiocyanate and Cyanide Biodegradation by CDO and TDO
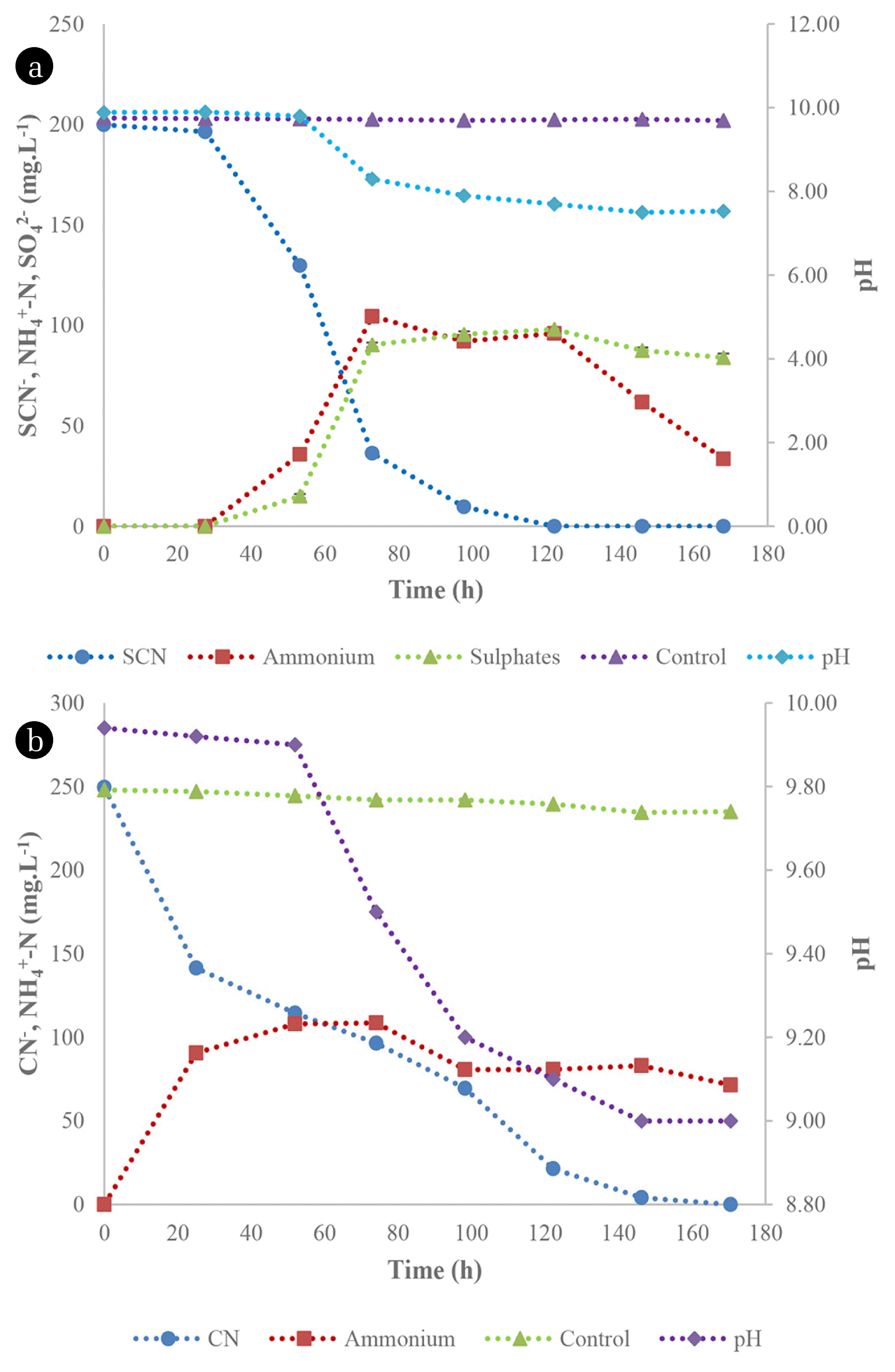
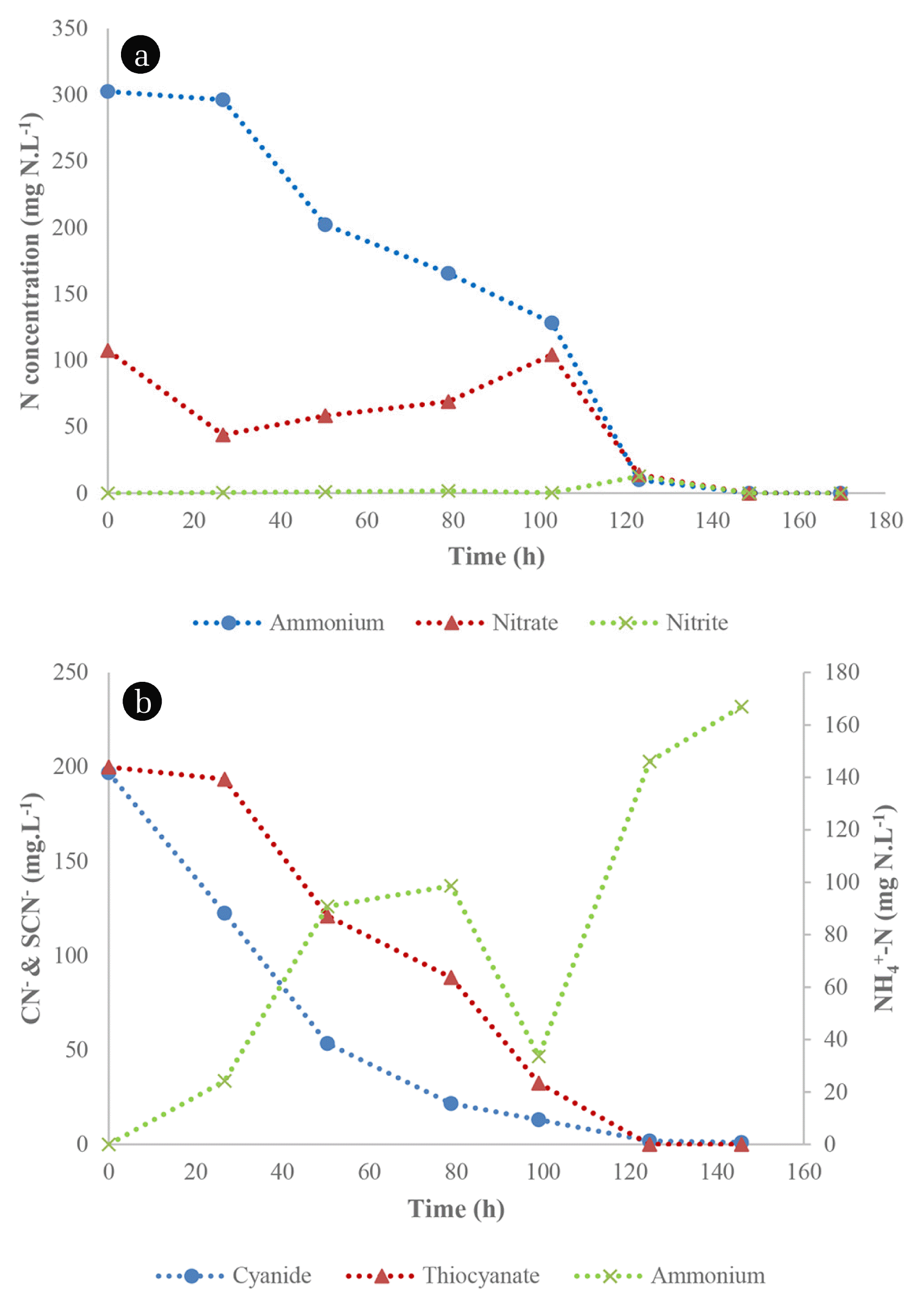
The degradation of SCN− and CN− by TDO and CDO was assessed in batch systems under alkaline conditions. The TDO were sampled from an active SCN− biodegradation system and were observed to have a SCN− degradation efficiency of 100 % after 96 h (Fig. 1(a)), resulting in ammonium nitrogen and sulphate formation. The sulphate concentration increased to 90 mg SO4 2−L−1 at 72 h and thereafter, plateaued. Additionally, ammonium nitrogen increased logarithmically after 27 h to 104 mg NH4 +-N.L−1. There was an observed pH decrease during SCN− degradation, which declined from an initial pH of 9.9 to 7.53. This was due to the acid produced from the oxidation of reduced sulphur species, thus resulting in a pH decrease. This is one of the few studies reporting on the degradation of SCN− under alkaline conditions since most studies are focused on the degradation of SCN− under neutral pH conditions [17, 18]. Wastewaters generated from metallurgical processes are mostly alkaline [19]; hence, studies undertaken in such conditions are vital. Sorokin et al. [20] demonstrated the capacity of the Halomonas and Thioalkalivibrio species to biodegrade SCN− under alkaline conditions and observed that these microbial species were able to degrade SCN−, producing cyanate, which was subsequently utilised by the organisms. Furthermore, Thioalkalivibrio thiocyanodenitrificans degraded SCN− aerobically and anaerobically under alkaline conditions, demonstrating the effectiveness of the applied microbial organisms [21].
The CDO were maintained in CN− containing media prior to preparation of the inoculum, which resulted in increased capacity of the organisms to biodegrade as they were able to achieve a BRE of 99.9% after 170 h (Fig. 1(b)). CN− decreased drastically during the initial 24 h of incubation and this observation may be due to the secretion of extracellular cyanide-degrading enzymes, a phenomenon which was observed by [12]. From the same study [12], similar microbial species which were not adapted to CN−, resulted in a BRE of only 57% from an initial concentration of 300 mg CN−.L−1. This observation demonstrated the effectiveness of microbial adaptation for improved capacity of the consortia to biodegrade. However, the CDO were observed to be unable to degrade SCN− (data not shown). Overall, there was an insignificant decrease in pH during the biodegradation process.
3.2. Effect of Thiocyanate and Free Cyanide on Heterotrophic Nitrification by TDO and CDO
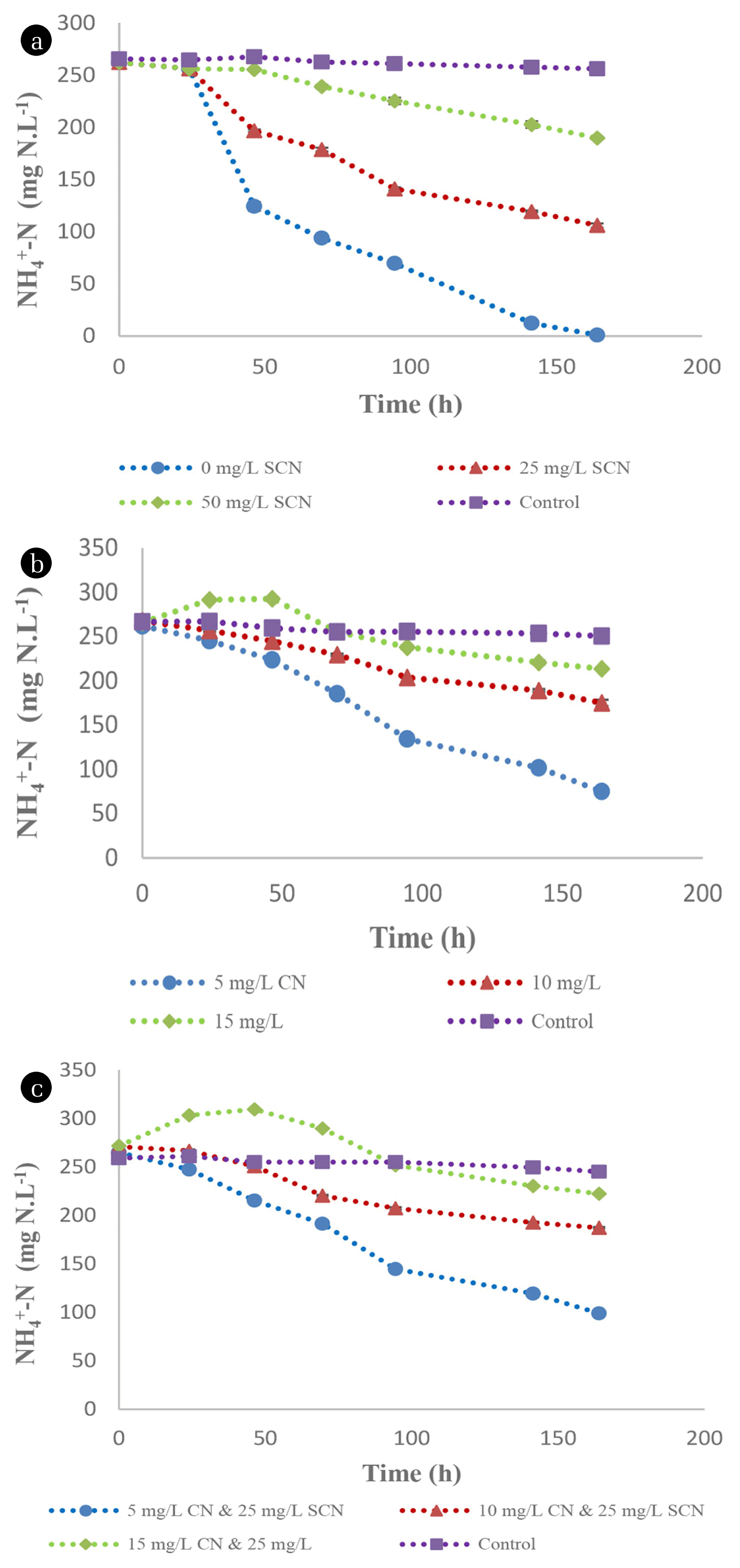
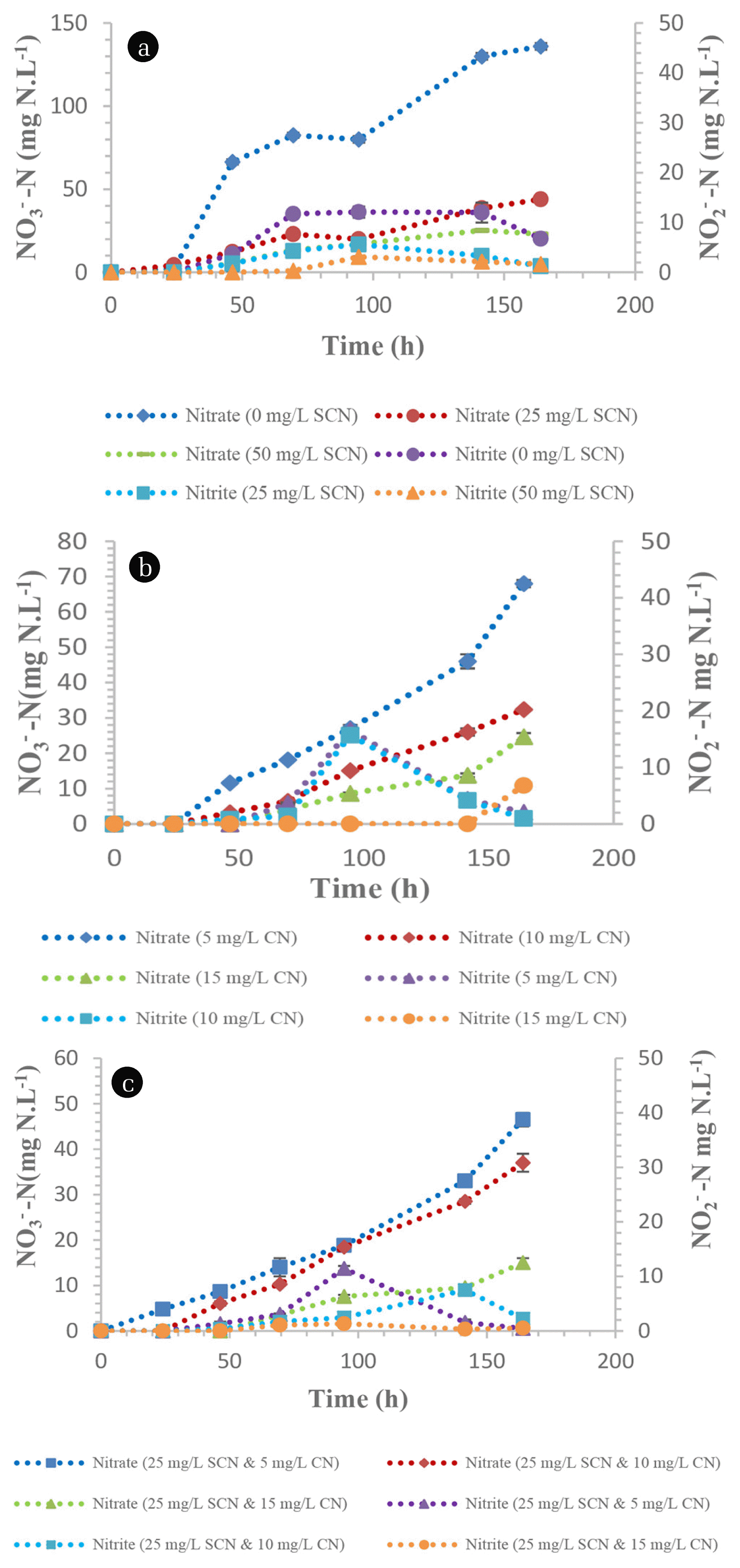
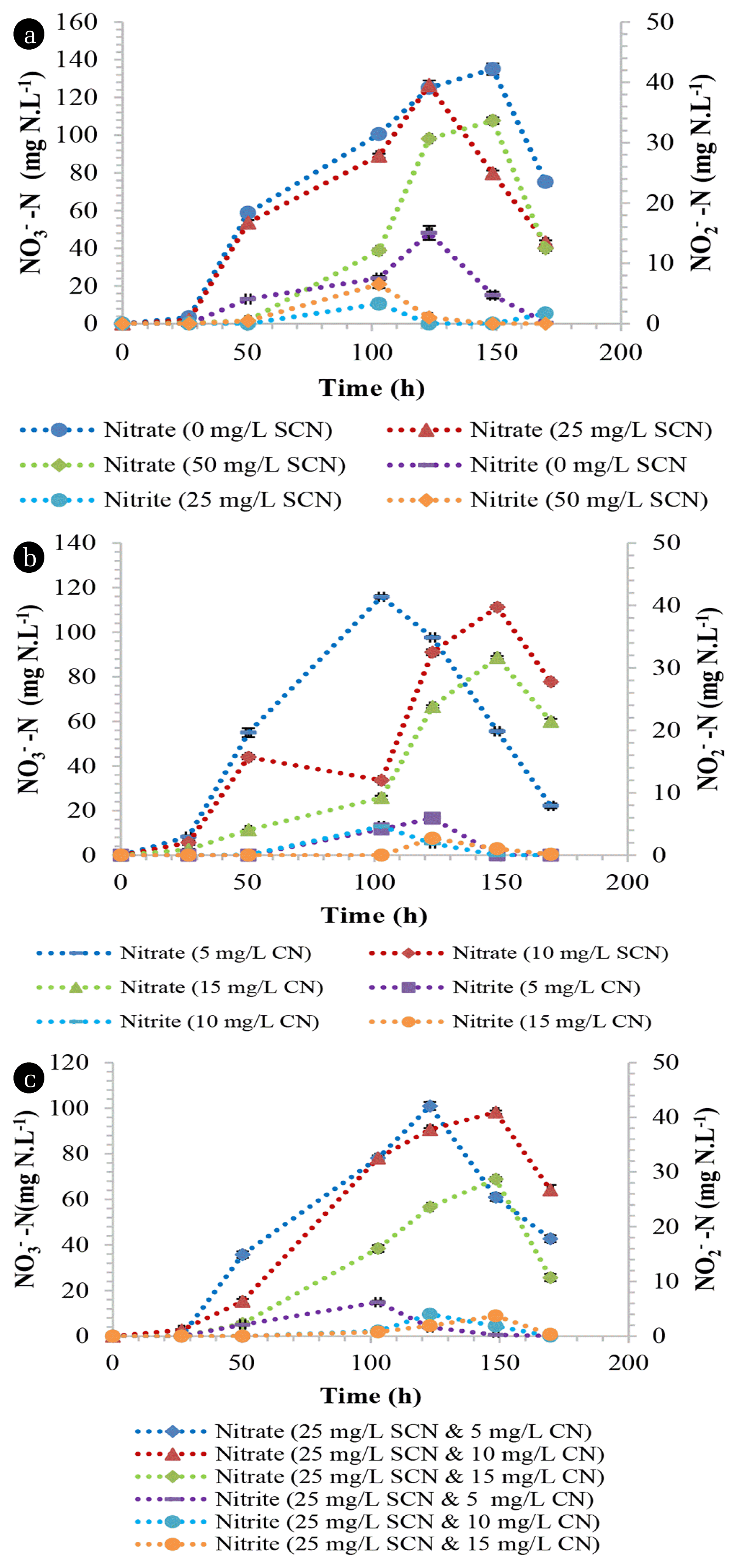
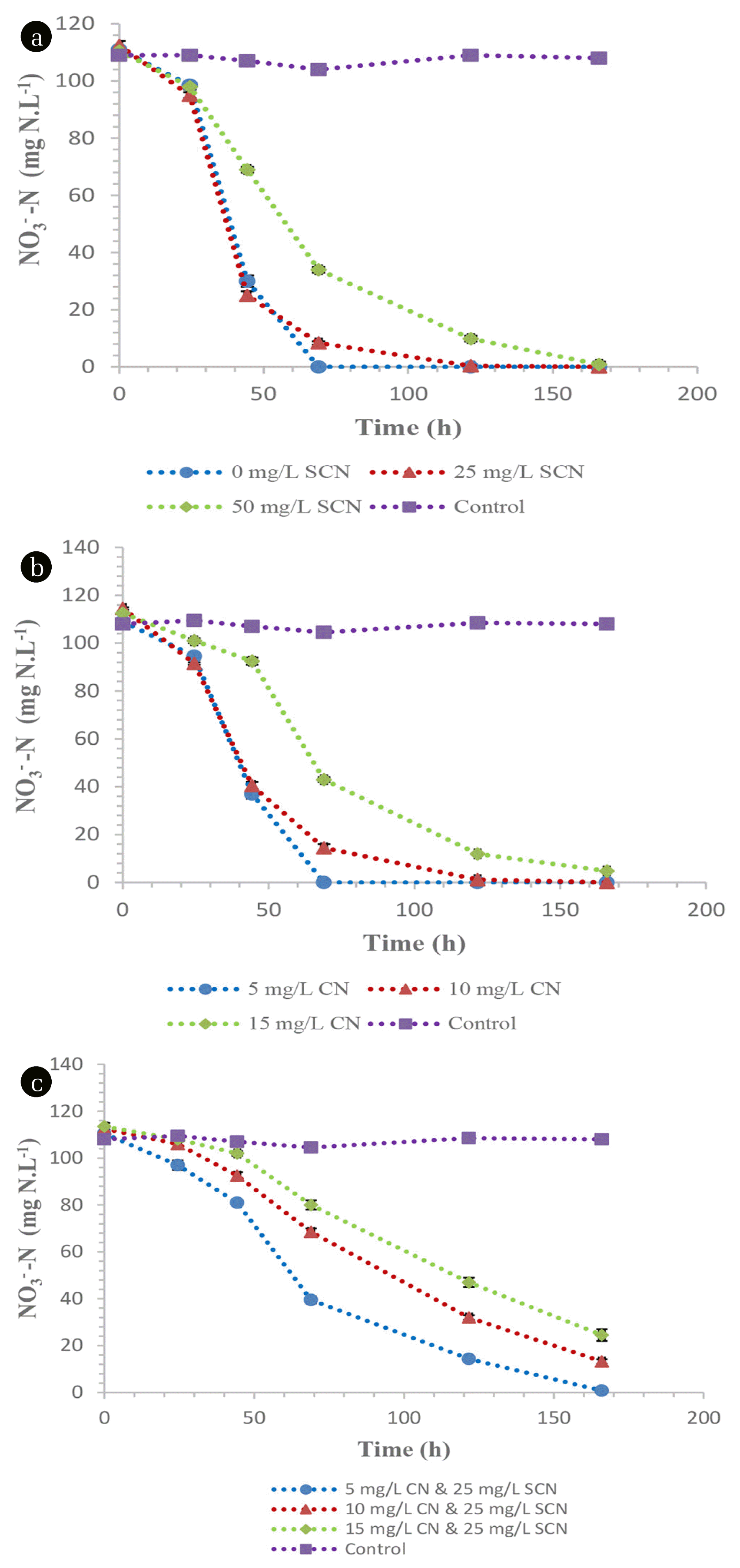
Heterotrophic nitrification under non- SCN− containing conditions by the members of the TDO community resulted in a nitrification rate of 1.59 mg NH4 +-N.L−1.h−1 while there was an observed inhibition on heterotrophic nitrification, where the nitrification rate decreased to 0.95 and 0.44 mg NH4 +-N.L−1.h−1 in media containing 25 and 50 mg SCN−.L−1, respectively (Fig. 2(a)). After nitrification, SCN− was not detected in the media (data not shown), suggesting its complete biodegradation. Nitrification rates under CN− concentration of 5, 10 and 15 mg CN−.L−1 were observed to be 1.14, 0.57 and 0.32 mg NH4 +-N.L−1.h−1, respectively (Fig. 2(b)). This demonstrated the acute toxicity and inhibition of nitrification by CN−. This phenomena was observed elsewhere [22], where 0.11 mg CN−.L−1 was suggested to be an accepted concentration for nitrification to transpire. The presence of both CN− (varying concentration) and SCN− (25 mg SCN−.L−1) proved to be detrimental on nitrification by TDO’s as the nitrification rates decreased drastically. The observed nitrification rates were 1.0, 0.51 and 0.301 mg NH4 +-N.L−1.h−1 from the initial concentrations of 5, 10 and 15 mg CN−.L−1 while the SCN− concentration was kept at 25 mg SCN−.L−1, respectively (Fig. 2(c)). The utilisation of ammonium nitrogen by the TDO’s resulted in the production of both nitrates and nitrites within the media. The maximum concentration of nitrates produced from the media supplemented with 0, 25, 50 mg SCN−.L−1 and 5, 10, 15 mg CN−.L−1 was 136, 44, 1.65 (Fig. 3(a)) and 68, 32.4 and 24.7 mg NO3 −-N.L−1, respectively (Fig. 3(b)). Similarly, the maximum nitrate nitrogen concentration from the dual impact of SCN− and CN− was 46.5, 37 and 15 mg NO3 −-N.L−1, respectively (Fig. 3(c)). The nitrite nitrogen concentration in all the tested media was less than 20 mg NO2 −N.L−1. In this study, it was observed that the nitrate nitrogen formation rate from ammonium oxidation was not in a 1:1 ratio as defined by Eq. (3) This was due to the utilization of the ammonium as a nitrogen source by the consortium; hence, a portion of the ammonium nitrogen was used intracellularly by the employed microbial organisms, resulting in low nitrate nitrogen detection in the media. This was also observed when Alcaligenes faecalis (A. faecalis) No.4 heterotrophically oxidised ammonium nitrogen, resulting in the lower detection of nitrates within the media [3]. The authors observed that 50% of the ammonium nitrogen was utilised intracellularly by A. faecalis No.4 and almost 39 to 48% was denitrified simultaneously and/or immediately after formation. It is imperative to note that the percentage of ammonium nitrogen that is utilised intracellularly varies between species and strains. Additionally, heterotrophic nitrifying organisms have been observed to have the ability to simultaneously nitrify and denitrify aerobically in the same media [7, 23, 24]. Therefore, the low detection of nitrates in the media was attributed to its utilisation by the microbial species.
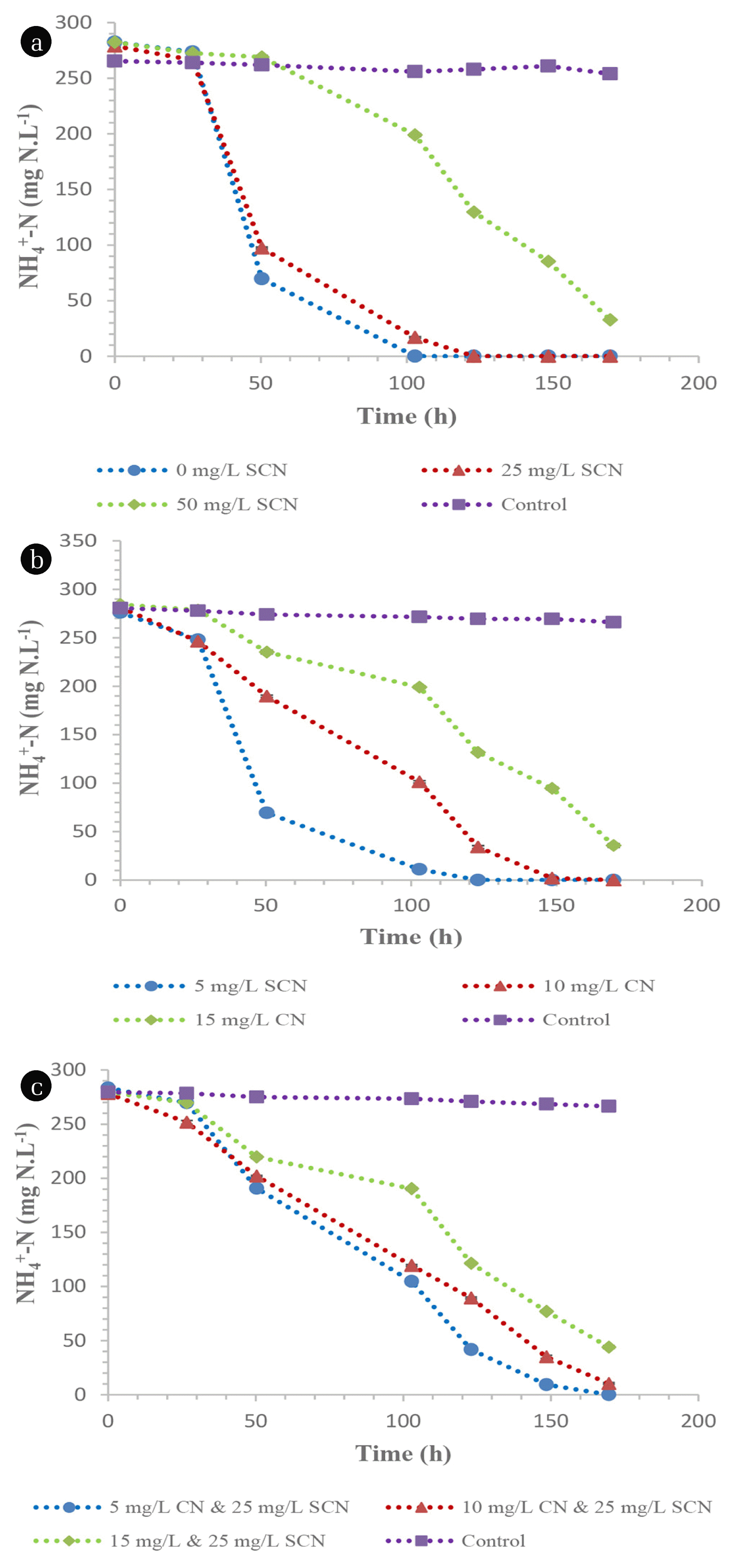
Heterotrophic nitrification by the CDO’s proved to be robust as these species were unaffected by the presence of both SCN− and CN−. These organisms demonstrated high nitrification rates of 1.66, 1.67 and 1.47 mg NH4 +-N.L−1.h−1 in media spiked with 0, 25 and 50 mg SCN−.L−1, respectively (Fig. 4(a)). Similarly, CN− concentration did not have any inhibitory effect on CDO’s as they were able to achieve nitrification rates of 1.63, 1.66 and 1.47 mg NH4 +-N.L−1.h−1 from media spiked with 5, 10 and 15 mg CN−.L−1, respectively (Fig. 4(b)). Furthermore, the organisms demonstrated their effectiveness as these species were not inhibited by the dual presence of SCN− and CN−, achieving nitrification rates of 1.67, 1.58 and 1.39 mg NH4 +-N.L−1.h−1 from the initial concentrations of CN− and SCN− of 5, 10 and 15 mg CN−.L−1 while the SCN− concentration was kept at 25 mg SCN−.L−1, respectively (Fig. 4(c)). Although there is a scarcity of information on alkaline nitrification, it has been observed that traditional nitrifying organisms are unable to perform nitrification efficiently under highly alkaline conditions [25, 26] but high nitrification efficiencies are at pH ranges of 7.0 to 8.5 [27]. This study has shown that both the TDO’s and CDO’s were able to nitrify under alkaline conditions. The maximum concentrations of nitrate nitrogen produced from the media supplemented with 0, 25, 50 mg SCN−.L−1 and 5, 10, 15 mg CN−.L−1 were 135, 126.5, 107.7 (Fig 5(a)) and 115.9, 111.2 and 88.7 mg NO3 −-N.L−1, respectively (Fig. 5(b)). Similarly, the maximum nitrate nitrogen concentrations in cultures containing both SCN− and CN− were 100.95, 98.3 and 68.9 mg NO3 −-N.L−1 (Fig. 5(c)). The nitrite concentration in all the tested media was less than 20 mg NO2 −-N.L−1. These organisms, i.e. CDO’s, were also observed to conduct nitrification and aerobic denitrification in a continuous CN− biodegradation process elsewhere [15], demonstrating their effectiveness as cyanide degraders, nitrifiers and denitrifiers.
3.3. Effect of Thiocyanate and Free Cyanide on Aerobic Denitrification by TDO and CDO
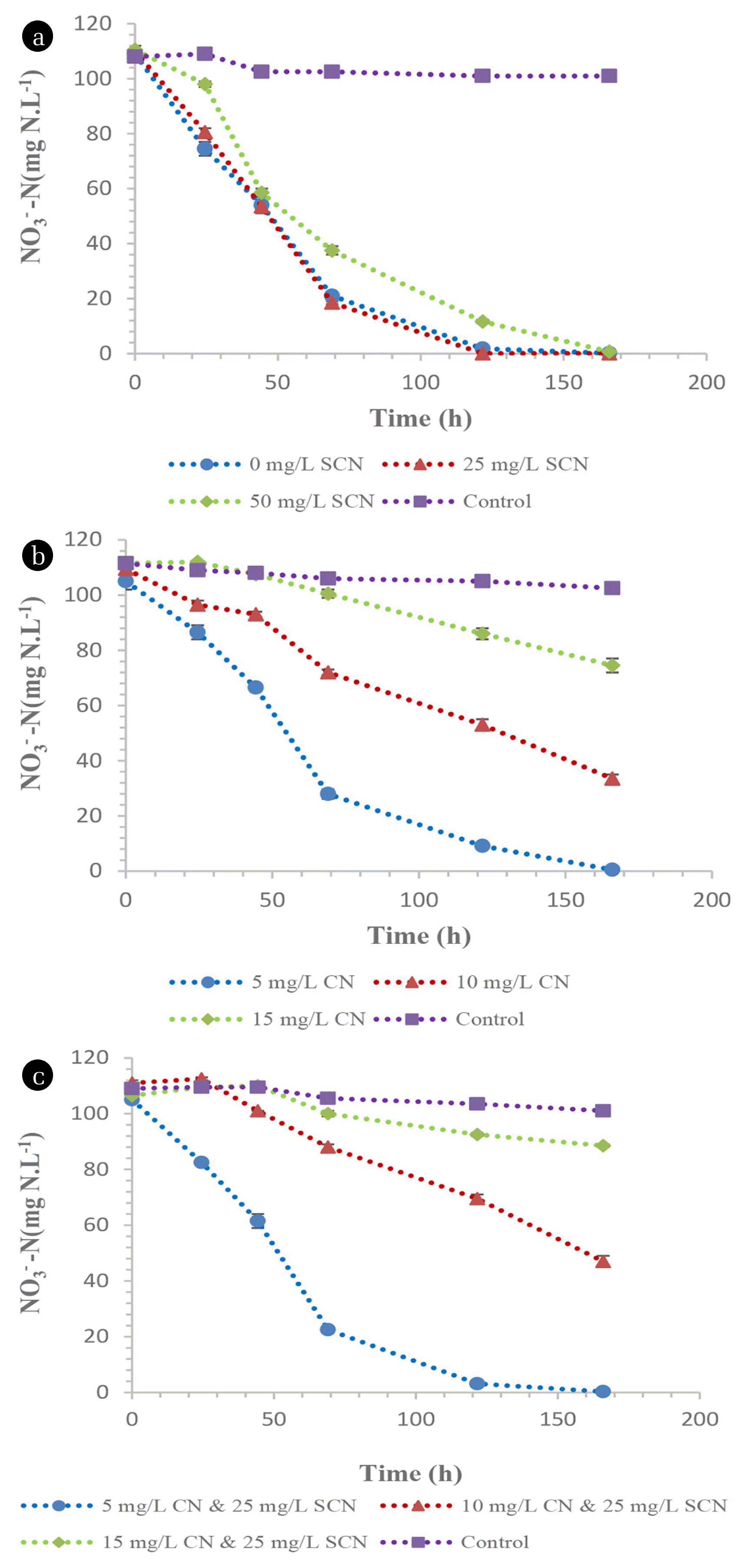
The aerobic denitrification potential of the TDO’s and CDO’s was evaluated under cyanogenic conditions, from an initial nitrate nitrogen concentration of 100 mg NO3 −-N.L−1. The TDO demonstrated the ability to denitrify aerobically as these species were able to achieve a complete denitrification rate of 0.66 mg NO3 −-N.L−1.h−1 in media supplemented with 0, 25, 50 mg SCN−.L−1, respectively (Fig. 6(a)) with minimal detection of nitrites within the media (≤ 0.04 mg NO2 −-N.L−1) (data not shown). Similarly, 5 mg CN−.L−1 did not inhibit aerobic denitrification by these species since the denitrification rate exceeded 0.64 mg NO3 −-N.L−1.h−1. However, denitrification was inhibited by the presence of 10 and 15 mg CN−.L−1, resulting in denitrification rates of 0.46 and 0.22 mg NO3 −-N.L−1.h−1, respectively (Fig 6(b)). Additionally, the dual effect of CN− and SCN− proved to be detrimental, especially when the media was spiked with 25 mg SCN−.L−1 and 10 mg CN−.L−1, as well as 25 mg SCN−.L−1 and 15 mg CN−.L−1, achieving denitrification rates of 0.39 and 0.11 mg NO3 −-N.L−1.h−1, respectively, while denitrification was unaffected by the presence of 25 mg SCN−.L−1 and 5 mg CN−.L−1 (Fig. 6(c)). The denitrification trends illustrated in Fig. 6(c) were similar to those observed in Fig. 6(b), which demonstrated the inhibitory effect that CN− had on the denitrification by the TDO’s.
However, the CDO’s demonstrated a high denitrification potential, as these species were not affected by the presence of SCN−, CN− or the dual effect of SCN− and CN−. The denitrification rates were in excess of 0.67 mg NO3 −-N.L−1.h−1 irrespective of toxicant concentration (Fig. 7(a) & (b)). Aerobic denitrification was slightly inhibited when the media was spiked with 25 mg SCN−.L−1 and 10 mg CN−.L−1, and 25 mg SCN−.L−1 and 15 mg CN−.L−1, achieving denitrification rates of 0.6 and 0.54 mg NO3 −-N.L−1.h−1 (Fig. 7(c)). Aerobic denitrification has been previously reported to produce nitrous oxide as an intermediate prior to the formation of nitrogen gas as summarised in Eq. (4). The enzymology involved in this process was explained elsewhere [28]. However, the production of nitrous oxide from aerobic denitrification systems has been largely investigated in axenic cultures [29, 30], which is not representative of microbial populations. The description of pathways and the enzymology associated with the production of nitrous oxide from aerobic denitrification systems in microbial populations has been reported to be challenging, since individual organisms within the population utilise different bio-chemical pathways for the utilisation of nitrates [31].
3.4. Simultaneous Heterotrophic Nitrification and Aerobic Denitrification by a Mixed Culture of TDO and CDO
CDO’s were co-cultured with the TDO’s and evaluated for simultaneous nitrification and aerobic denitrification in the presence of 25 mg SCN−.L−1 and 15 mg CN−.L−1. The co-culture was able to simultaneously nitrify and aerobically denitrify in the presence of SCN− and CN−, achieving nitrification and denitrification efficiencies of 100% (1.83 mg NH4 +-N.L−1) (Fig. 8(a)). There was an observed nitrate nitrogen increase to 104 mg NO3 −-N.L−1 between 20 h to 100 h; thereafter, the nitrate nitrogen concentration decreased.
The co-culture was further evaluated for the simultaneous bio-degradation of CN− (200 mg CN−.L−1) and SCN− (200 mg SCN−.L−1), resulting in complete degradation of SCN− after 124 h while the BRE of CN− exceeded 99% (Fig. 8(b)). The ammonium nitrogen accumulated in the cultures was 98.7 mg NH4 +-N.L−1 after 78 h which decreased to 33.6 mg NH4 +-N.L−1 at 100 h. However, there was an observed ammonium accumulation within the cultures after 100 h, escalating to 167 mg NH4 +-N.L−1 at 146 h. This increase was due to ammonium production from the bio-degradation of CN− and SCN− as described in Eq. (1) and (2). Simultaneous nitrification and aerobic denitrification has been reported in various studies [6, 32]. However, these studies were undertaken under SCN− and CN− free systems. Nitrification under CN− laden conditions was investigated using activated sludge obtained from a coking wastewater treatment plant and in this study, nitrification was inhibited when the media was supplemented with 0.2 mg CN−.L−1 [9], with SCN− having a minimal inhibitory effect on nitrification. While in a separate study, the Enterobacter, Yersinia, and Serratia species were able to carry out nitrification under cyanogenic conditions (66 mg CN−.L−1), with minimal inhibition [33]. The high CN− tolerance was attributed to the differences in composition, strength and overall quality of the microbial consortia used [34]. This study demonstrated the robustness of heterotrophic nitrification and aerobic denitrification under CN− and SCN− laden conditions, when subjected to a mixed culture of TDO’s and CDO’s.
4. Conclusions
Heterotrophic nitrification and aerobic denitrification under SCNand CN− laden conditions by SCN− and CN− degrading organisms was successfully demonstrated, particularly in co-cultures. CN− (≥ 10 mg CN−.L−1) inhibited nitrification and denitrification in media inoculated with SCN− degrading organisms (TDO’s), while the CN− degrading organisms (CDO’s) were not inhibited by SCNnor CN−, irrespective of the concentration load. Additionally, the presence of both CN− (≥ 10 mg CN−.L−1) and SCN− (25 mg SCN−.L−1) inhibited nitrification and aerobic denitrification by the thiocyanate degraders while the cyanide degraders were not inhibited. A mixed culture containing both SCN− and CN− degraders was able to nitrify and aerobically denitrify without being inhibited by the presence of both SCN− and CN−, irrespective of the toxicant concentration load. This demonstrated the robustness and effectiveness of these organisms to carry out heterotrophic nitrification and aerobic denitrification under cyanogenic conditions – a process which can be applied to the nitrification and denitrification of ammonium nitrogen and nitrates formed from CN− and SCN− biodegradation systems. The data signifies that the influx of these toxicants into secondary processes (nitrification and denitrification) will not have an inhibitory effect on nitrification and denitrification since these organisms are able to degrade, nitrify and denitrify. Therefore, these organisms can be utilized for the treatment of cyanide-laden wastewaters including the treatment of the by or end-products of the biodegradation process. However, future studies should focus on the biochemical mechanisms of heterotrophic nitrification and aerobic denitrification.