1. Introduction
The current global energy issue of the depletion of the world’s fossil fuel reserves due to the global consumption of coal has become a serious problem and is expected to become even more serious in the near future, with the impact of the global carbon dioxide emissions [1–2]. Renewable energy sources as biomass to bioenergy can provide a solution to this problem [2–5]. The biomass resources that can be used to produce bioenergy include various natural and derived materials such as including woody and herbaceous species, wood wastes, agricultural and industrial residues, waste paper, municipal solid waste (MSW), sawdust, bio-solids like sludge, grass, waste from food processing, animal wastes, aquatic plants, and algae [1–6].
Among the different biological treatment modalities, solid-state anaerobic digestion (SS-AD) has helped promote the development and application for biogas production from biomass. SS-AD is used in more than 60% of the existing anaerobic digesters in Europe [7–8]. It can operate a high solid content feedstock, as higher than 15% of total solids (TS). Consequently, SS-AD has the following advantage: a higher organic loading rate, a smaller reactor volume, a lower energy demand for heating, higher volumetric methane productivity, and less wastewater generation [8–10]. The result reported that SS-AD achieved a maximum methane yield of approximately 200 L/kg-volatile solids (VS) for the digestion of biomass containing 22% TS [11]. The digested byproduct of SS-AD can be used as a fertilizer or as solid fuel. It has serious problems, as a high moisture content and a poor dewaterability [7, 12]. The direct conventional thermal treatment methods, such as drying and combustion of byproduct need the high energy cost. The dewatering of the byproduct has to be improved to solve the aforementioned problem to make a useful alternative energy source.
Hydrothermal carbonization (HTC) is one of the thermo-chemical treatment methods that are currently being used to convert organic materials and to improve their dewatering and upgrade their properties so that they can be used as cost effective and environment-friendly alternative fuels [13–14]. HTC has prompted the production of hydrochar and has improved the dewatering of several kinds of biomass resources [13–16]. In this study, after hydrothermal conversion, the fuel properties of the obtained products were investigated, including their calorific value and fuel properties. The aforementioned technique can apply to produce a homogeneous solid fuel, such as coal, which can be easily dewatered into a powdery fuel with a 10% moisture content [14–17]. Therefore, the moisture content of the sludge can be reduced to approximately 20% due to the breakdown of the physical structure [16–19]. This research was conducted to develop the HTC process that can convert SS-AD byproduct to an alternative fuel. The specific goals of this study were to investigate the effects of the reaction temperature on dewatering performance, to improve the fuel properties of the SS-AD byproduct through the HTC, and to determine the optimal reaction temperature of the HTC process.
2. Materials and Methods
2.1. Materials
The organic residue that was used in this study was derived from the lab-scale SS-AD of livestock waste. The lab-scale SS-AD test was conducted for one-stage mode, batch reactors in the form of 300 mL glass bottles. All experiments were performed in duplicate (a total of 30 samples). The initial TS of all SS-AD reactors were set to 20%. The bottles were incubated at a mesophilic temperature (37 ± 1°C) for 30 d. The biogas yield of the system produced 0.3–0.5 m3/kg-volatile matter. Table 1 provides the characteristics of raw feedstock as livestock waste and organic residue.
2.2. Lab-scale Hydrothermal Carbonization Reactor
A lab-scale HTC reactor was used to investigate the effects of the hydrothermal reaction temperatures. The experiments were performed using a 2,000 mL reactor consisting of a reactor body, a heater, and a steam condenser that operated under nitrogen gas. A 1,000 mL of SS-AD byproduct was mixed with a 300 mL of water and then loaded onto the reactor. The operating temperatures of the HTC reactor were 180–240°C, and the reaction time was set to 30 min, with a 200 rpm agitation speed. After the completion of the hydrothermal reaction, the pressure and temperature were allowed to decrease to atmospheric pressure and room temperature, respectively, and the products were taken out of the reactor.
2.3. Analytical Procedures
The dewaterablity of the product of HTC was evaluated by measuring the capillary suction time (CST) and time to filter (TTF). The CST was determined using an improvised CST apparatus (model 319 Multi-purpose CST, Triton Electronics Ltd.) with single-radius test and CST paper (size: 79 cm, quantity: 200 approx., Triton Electronics Ltd.). The TTF was measured according to the Standard Methods [20]. Organic residue of SS-AD and HTC hydrochars were prepared as powder-material which were sieved, and a fraction with a particle size between 177 μm and 250 μm was set aside for analysis. Elemental composition analysis of the sample was carried out using an EA 2400 Series II CHNS/O organic elemental analyzer (PerkinElmer, Waltham, MA, USA). Proximate analysis was conducted using a 5E-MAG6700 automatic proximate analyzer. The calorific value was determined using the bomb calorimetric standard method. The combustion characteristics were determined by using a thermogravimetric analyzer (TGA-Q500, TA Instruments). Non-isothermal combustion of the samples was conducted in the furnace of the TGA system at atmospheric pressure. The sample weight loss (TG) and the weight loss rate (DTG) were continuously recorded under a temperature range of 50–800°C and at a heating rate of 10°C/min.
3. Results and Discussion
3.1. Improvement of the Dewaterability
The SS-AD byproduct retained high moisture content, which is the primary challenge in the isolation of the organic materials after SS-AD. Interstitial water and surface water as bound water are held by capillary forces between the particles and cannot be easily separated by normal mechanical methods. In contrast, the free water surrounding the flocks can be easily removed. The improvement of the deweterability means that the bound water is converted to free water to remove the water content the organic residue of SS-AD. The organic residues of SS-AD consist of many particles in the flock as a single large particle, such as that with a sludge flock shape. The HTC process breaks up the physical structure of the slurry phase included in the sludge and biomass [17–19].
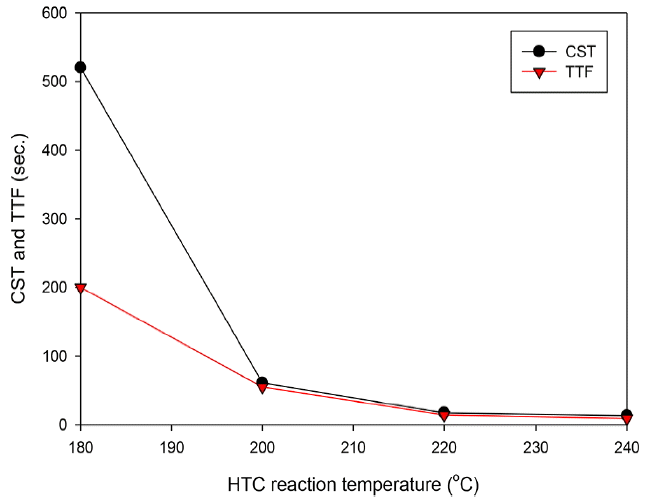
In this study, the CST analysis estimated the effects of the HTC in improving the dewaterability of the organic residues of SS-AD. The results of the CST and TTF analyses are shown in Fig. 1. CST and TTF analyses of raw organic residues of SS-AD are difficult to conduct because the slurry phase of the material is less accurate. In this study, the CST and TTF of the HTC product at 180°C were more than 520 and 200 s, respectively. At above the 200°C of HTC reaction temperature, the CST and TTF were 61 and 55 s at 200°C, and 17 and 14 s at 220°C, respectively. The CST and TTF results closely corresponded to the presence of bound water content and hydrophobicity [17–18, 21]. HTC converted the physical structure of the organic residues of SS-AD, which were broken down to convert the bound water to free water. The decreasing CST and TTF owing to these influences confirmed that the HTC process increased the sludge dewaterability.
3.2. Upgrading the Fuel Properties
The properties of the organic residue of SS-AD and the HTC hydrochar are shown in Table 2. The raw organic residue of SS-AD had a high volatile matter content (68.5%). The volatile matter decreased as the reaction temperature increased, due to the chemical dehydration and decarboxylation reactions [14–16]. The raw organic residue of SS-AD indicated 10.9% of fixed carbon content and 68.5% of volatile matter content. After the HTC process, the fixed carbon content increased to 13.5% and 14.8%, and the volatile matter content decreased to 59.3% and 54.2% at 200°C and 220°C, respectively. As a result, the fuel ratio (fixed carbon/volatile matter) increased due to the hydrothermal carbonization reactions as 0.16 to 0.23 and 0.27 at 200°C and 220°C, respectively. These results confirm that the HTC process was successful.
The elemental composition confirmed the improved fuel properties of the hydrochar from HTC. The carbon content of the hydrochar increased from 44.9% to 47.1% and 48.0% as the HTC reaction temperature increased at 200°C and 220°C, respectively. The oxygen and hydrogen contents of the products were also reduced.
Furthermore, the reduction in the nitrogen content was predicted to eliminate the potential risk of forming chemicals such as NOx. The nitrogen content decreased from 1.4% to 0.8%, respectively. In the HTC reaction, the volatile-N was converted to the gas or liquid phase through devolatilization [15–17]. Moreover, this effect can reduce the NOx emission potential, thus producing a clean energy resource.
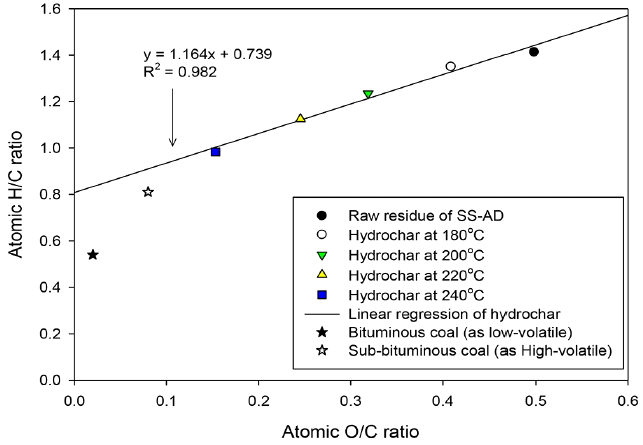
The Van Krevelen diagram confirmed that the coalification reaction of HTC has a significant effect on the elemental composition of the hydrochar as shown in Fig. 2. In this study, the atomic H/C and O/C ratios decreased as the HTC reaction temperature increased from 180°C to 240°C.
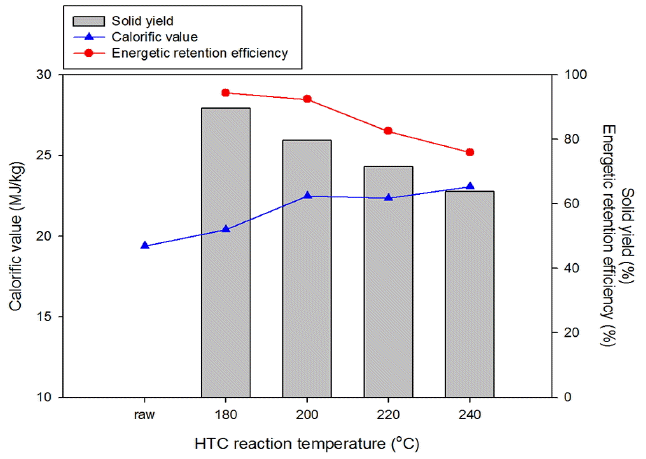
The atomic H/C and O/C ratios decreased from 1.3 and 0.5 to 1.1 and 0.3, respectively, as the reaction temperature increased from 180°C to 240°C. The slope of the regression line was 1.164, indicating reduced atomic H/C and O/C ratios, which confirmed the occurrence of dehydration and decarboxylation reactions during HTC. The results of hydrochar showed properties approaching between lignite and sub-bituminous coal properties as the HTC reaction temperature increased [22–23]. These results suggest that HTC can improve the properties of hydrochar from organic residue of SS-AD. As a result, the elevated carbon and fixed carbon contents can be said to have a strong influence on the calorific value [24–25]. The calorific values of the hydrochar from the organic residue of SS-AD were improved from 19.4 to 20.4 MJ/kg at 180°C, 22.4 MJ/kg at 200°C, 22.5 MJ/kg at 220°C, and 23.1 MJ/kg at 240°C, respectively (as shown in Fig. 3), and the influence of HTC was estimated by calculating the energy-related properties associated with the solid yield, including the energetic retention efficiency as follows Eq. (1) to (2):
The energetic retention efficiency can provide the optimum condition of the HTC reaction to the hydrochar production solid fuel. The increasing reaction temperatures influenced the rising calorific value of the hydrochar, but the product yield of the hydrochar decreased due to the dehydration and decarboxylation (as converted to CO2 and H2O) reactions [14–16]. As shown in Fig. 3, the highest energetic retention efficiency of a given material was 94.3% at 180°C of HTC. However, this HTC reaction temperature was not enough to improve the dewaterability. Therefore, the optimum reaction temperature of HTC for the organic residue of SS-AD was found to be 200°C, which was 91.9% of the energetic retention efficiency with which the improvement of dewaterability can start.
3.3. Combustion Behavior of Organic Residue of SS-AD and Hydrochar
Fig. 4 compares the TG and DTG profiles of the organic residue of SS-AD and the hydrochar of HTC at 200°C as the optimum temperature. The combustion characteristics were compared based on three characteristic temperatures: the ignition temperature (Ti), the maximum combustion rate temperature (Tm), and the burnout temperature (Tb) as determined from the TG and DTG curves. Ti provides the temperature at which fuels start to burn; Tm corresponds to the highest DTG peak temperature; Tb provides the temperature at which the weight loss diminishes at a rate of 1% /min [26–27]. Ti increased from 232.7°C to 257.3°C, and Tm also increased from 274.3°C to 303.8°C due to decreased volatile matter owing to the HTC reaction. Tb decreased from 502.8°C to 495.1°C. The different combustion behaviors between the organic residue of SS-AD and hydrochar were shown by several stages depending on the rate of weight loss of the DTG curve (i.e., dehydration stage, the devolatilization stage, and combustion stage). As a result, the combustion range of the hydrochar was extended at the devolatilization and combustion stages due to the increased fixed carbon content. Moreover, the dehydration range decreased from 181.1°C to 157.4°C, which resulted in the improvement of the chemical drying performance of the organic residue of SS-AD.
4. Conclusions
HTC was used to convert the organic residue of SS-AD from livestock waste into an alternative solid fuel with high energy efficiency. After the completion of HTC, further treatment was performed to break down the physical structure of the organic residue of SS-AD to improve the dewaterability as released free water. Moreover, the organic residue of SS-AD obtained through HTC significantly increased the carbon and fixed carbon contents with respect to the calorific values due to the dehydration and decarboxylation reactions. The Van Krevelen diagram, which provided the H/C and O/C ratios decreased, in correlation with the primary reactions of coalification. Furthermore, the optimum reaction temperature of HTC for the organic residue of SS-AD was found to be 200°C, which was 91.9% of the energetic retention efficiency with which the improvement of the dewaterability can be started. The thermogravimetric analysis also confirmed the improvement of the combustion characteristics through HTC.