1. Introduction
Crude oil is a complex mixture of aliphatic, alicyclic and aromatic hydrocarbons. In oil mining areas and transportation routes, large quantities of crude oil are released into the environment, making crude oil one of the main sources of environmental pollution. Soil contaminated with crude oil has thus become a major concern due to the risks to health and the environment. So far researchers have conducted numerous remediation experiments for soil contaminated with crude oil, including bioremediation [1, 2], phytoremediation [3, 4], chemical oxidation [5], thermal remediation [6]. Also, solvent extraction [7], supercritical fluid extraction [8], and ultrasonic extraction [9] studied for crude oil remediation, but these processes extensively used for extraction of PAHs or aliphatic hydrocarbons from soils. Lim et al. [10] stated the advantages, limitations, average cost, and sustainability of all these remediation methods, and concluded that any single method was not adequate to remediate soil contaminated with crude oil. In this regard it is required to develop a new remediation approach which would be effective to remediate crude oil contaminated soil.
Subcritical water extraction (SCWE) is an environmental-friendly extraction method that can be used to remediate soil contaminated with different organic compounds. In the SCWE process, subcritical water (100°C ≤ T ≤ 374°C, pressure < 22.1 MPa) is used as a solvent instead of any organic solvent [11]. When the temperature increases from 25 to 350°C, the dielectric constant of water decreases from 78 to 2 at 100 bar, and thus the water acts as a solvent. For example, the dielectric constant for ethanol is 24 under ambient conditions, which is close to the subcritical water at 250°C and 100 bar. The efficacy of subcritical water in remediating various contaminated soils has been proved, including for those with PCBs [12], PAHs [11, 13], explosives [14], lubricating oil [15], and diesel [16]. In addition, Sushkova et al. [17] explained the possibility of benzo[a]pyrene extraction from soil and plants using subcritical water extraction method, and benzo[a]pyrene accumulation, migration, and transformation in soil-plant [18]. Therefore, Subcritical water extraction could be used as a well-developed instrumental analysis method as well for monitoring studies of oil pollution [19]. Although numerous studies have been carried out with subcritical water extraction, no data is available on the remediation of soil contaminated with crude oil. This study assessed the subcritical water extraction process in terms of its removal and recovery efficiencies to remediate soil contaminated with crude oil.
Most of the previous study focused only on the oil removal rather than the oil recovery of contaminated soil [10,15,16]. In this study, the oil recovery via subcritical water extraction has been explored. Furthermore, other authors optimize the operating conditions applied conventional methods where the effect of the variables was observed by changing a single variable and keeping the others constant except Islam et al. [16]. In this study, Box-Behnken Design (BBD) under response surface methodology (RSM) was applied to remove and recover the crude oil, and the feasibility and efficiency of RSM have already explored by researchers to optimize the process variables in the different field of science. For example, Gratuito et al. [20] synthesized activated carbon using coconut shell where process parameters optimized applying response surface methodology. Thus, it would be a better opportunity to remove and recover the oil form soil and observe the extracted oil composition using subcritical water.
This study applied BBD under RSM to optimize the process variables (namely temperature, time and flow rate) and assess the removal and recovery efficiencies of crude oil from contaminated soil through the use of subcritical water extraction.
2. Materials and Methods
2.1. Soil Contamination Procedure
A fraction of 250 g air-dried, homogenized, and sieved (< 2 mm) non-polluted soil was contaminated by mixing with crude oil. The physiochemical properties of the soil sample are shown in Table 1. To spike the soil, 10 g of used crude oil were liquefied in 250 mL of dichloromethane in a beaker, and 250 g of fresh soil were then added. The mixture of soil-solution slurry was aged for 24 h under rotation. After aging, the dichloromethane was separated via evaporation, and the soil was then air-dried until reaching a constant weight. The soil was accumulated in a glass bottle at room temperature, and the bottle was closed tightly and kept for more than a month prior to use. In the terms of total petroleum hydrocarbon (TPH), the initial concentration of the crude oil in the spiked soil was 8,401 mg/kg.
2.2. Subcritical Water Extraction Process
Distilled water was used as an extraction solvent, and helium gas was purged for 30 min in water to remove the dissolved oxygen. A lab-scale subcritical water reactor was used, and the experiments were carried out in the dynamic mode. The detailed extraction process is described in the literature. In short, 10 g of contaminated soil were inserted into an extraction cell (1 cm i.d. × 10.8 cm length), and both the inlet and the outlet of the cell were capped with a 0.2 μm stainless steel microfilter that was installed into the heating chamber. Outside the heating chamber, a cooling unit and a pressure control valve were linked with the outlet of the eduction reactor using a stainless-steel tube. The inlet of the eduction cell unit was linked using stainless steel tube from the high-pressure pump (Series II, Chrom Tech, Inc.). Two thermocouples (West 6100+ temperature controller) were connected to the apparatus to monitor the water temperature at the pre-heater unit and extraction cell. After setting the desired condition, the water was pumped vertically, and the heating system was turned on. After attaining the desired temperature, timing began to take into account the desired extraction period, and the water pump and heating were switched off upon reaching the desired extraction. The pressure was then released to ambient condition, and the reactor was left to cool to room temperature. The soil sample was then collected from the extraction cell to analyze the remaining concentration of crude oil (via TPH), and the effluent water sample was collected to recover the extracted oil.
2.3. Experimental Design and Optimization Analysis
This study used BBD under RSM to optimize the removal and recovery efficiencies of crude oil. RSM is a combined mathematical and statistical technique, and the feasibility of using this technique has been proven in various areas of science. Three major operating parameters were controlled for the SCWE process of crude oil from contaminated soil. Table 2 illustrates the coded and actual operating conditions of the factors, namely the temperature (x1), time (x2), and water flow rate (x3). Three factors-three levels BBD design was applied using BBD with three center runs, and a total of 15 experimental runs were given using software, as shown in Table 3. Design Expert 10 software and ANOVA were performed to analyze the experimental results and statistical analysis. The second order polynomial model was used to fit the response variables of crude oil extraction and recovery, and it is presented as follows:
where Y is the estimate response; β0 is a constant; β1, β2, and β3 are the linear coefficients; β12, β13, and β23 are the interaction coefficients between the three factors; β11, β22 and β33 are the quadratic coefficients.
2.4. TPH Analysis
The standard Korean test method was employed in the TPH analysis, following the methodology detailed by Park et al. [21]. A GC-FID was used to analyze the TPH concentration. The crude oil removal and recovery efficiencies were quantified using the following equations.
where C0 is the primary concentration of TPH in soil (mg/kg) and Cr is the remaining concentration in the treated soil (mg/kg).
3. Result and Discussion
3.1. Crude Oil Removal Using the RSM Approach
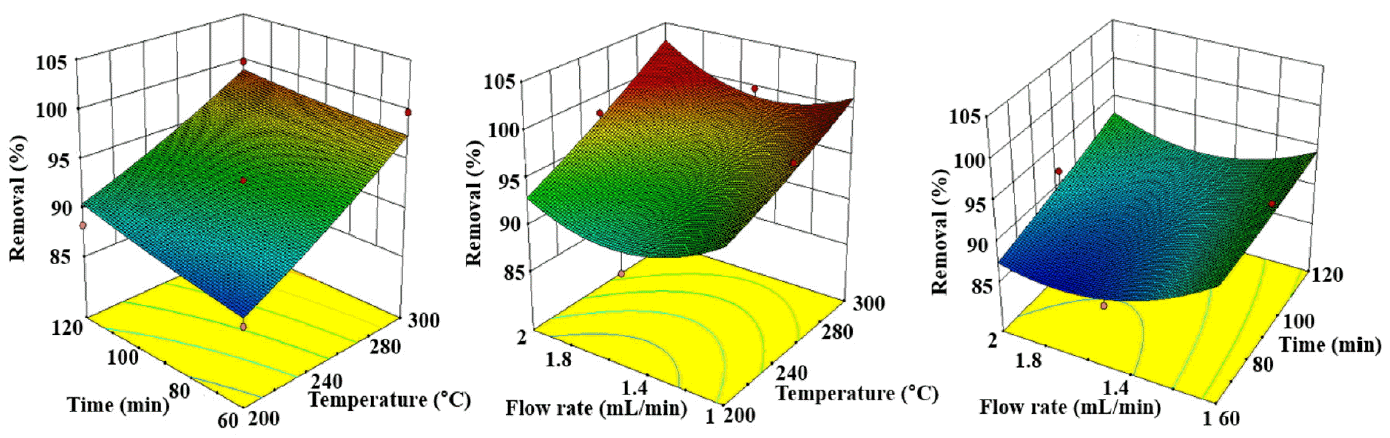
Table 3 illustrates the experimental runs from BBD and the removal and recovery percentages of crude oil. The maximum removal and recovery efficiencies of crude oil were 99.8% and 94.46%, respectively. Table 4 presents the results of the ANOVA for the quadratic model of the oil removal, and these results imply that the model was significant at p < 0.05. The regression model shows a good correlation between the temperature and response (p = 0.0017). However, the extraction time (p = 0.1753) and water flow rate (p = 0.28) are not well correlated with the response. The coefficient of determination (R2 value) and adjusted R2 value were 0.91 and 0.74, respectively. As a result of the ANOVA analysis, a second-order polynomial equation was determined as follows:
Fig. 1(a) illustrates the effect of temperature and time on crude oil removal. The effect of temperature on crude oil removal can be seen to be strongly significant (p = 0.0017), but the interactive effect of these two factors is not significant (p = 0.6568) (Table 4). The removal efficiency of crude oil increased as the temperature increased. For example, in the condition at 250°C for 60 min, 96.48% of crude oil was removed, but the removal efficiency increased to up to 99.8% at 300°C for 60 min. The high temperature reduces the surface tension and cohesive energy of water, therefore reducing the dielectric constant and viscosity and thus allowing for more extraction and dissolution of crude oil in water under the subcritical condition. These results are in agreement with previous studies conducted by several authors [11, 16, 22].
Fig. 1(b) shows the effect of the water flow rate and temperature on crude oil removal. The water flow rate had a slight impact on the crude oil removal compared to the effect of the temperature. At high temperatures, the effect of the flow rate was negligible. For example, 99.8% removal was observed at 1.5 mL/min, and a similar result (99.59%) was achieved at 1 mL/min, as shown in Table 3. Therefore, the extraction of crude oil can be concluded to be solely dependent on the water temperature. In runs 9 and 12 (Table 3), the removal efficiencies decreased from 96.48% to 92.12% as the water flow rate increased from 1 mL/min to 2 mL/min at 250°C. This is might be due to the lack of internal diffusion for a higher flow rate. The removal of organic compounds from soil matrix has been reported to be controlled by internal diffusion rather than external mass transfer [16].
The effect of the water flow rate and removal time are shown in Fig. 1(c), which illustrates that crude oil removal increased little as time increased. For instance, the removal rate increased from 96.48 to 98.15% when the extraction run time was extended to 120 min from 60 min. It would be the worst decision to consider increasing the time to more than 60 min regarding the effective removal of crude oil. An increase in the flow rate decreased the removal efficiency because a different mass transfer zone formed due to channeling or lack of residence time of the water for optimal diffusion. However, at a high temperature, the flow rate within the design boundary of 1 to 2 mL/min did not play a vital role in the removal of crude oil.
3.2. Crude Oil Recovery Using the RSM Approach
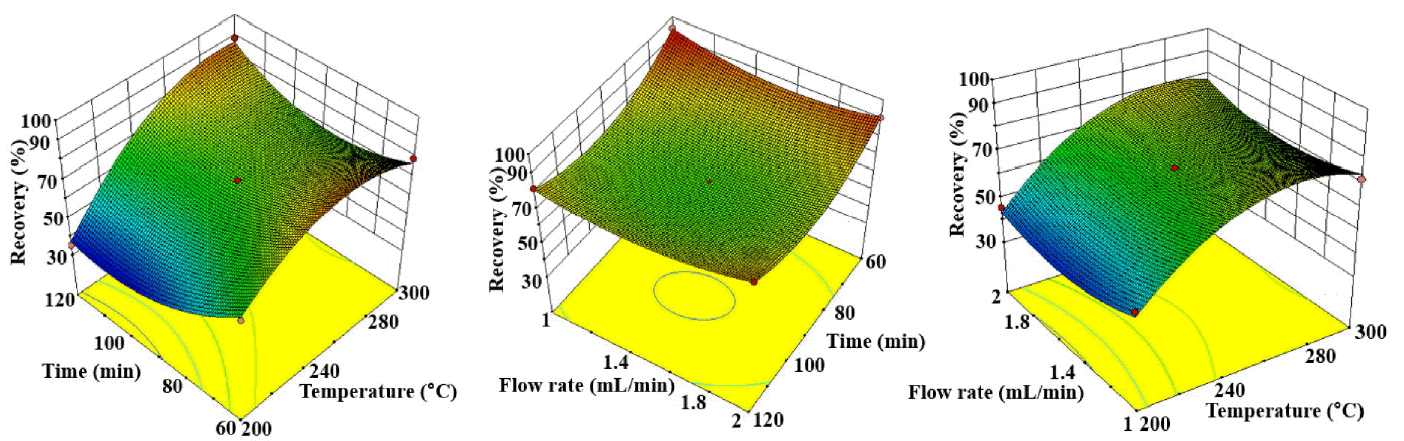
Table 5 shows the ANOVA results for the second order quadratic model for the crude oil recovery, and the results indicate that the model is significant at p < 0.0001. For a 95% confidence interval, the P values of the ANOVA results illustrate that the extraction temperature (p < 0.0001) and time (p = 0.0058) had a larger effect on crude oil recovery than the extraction flow rate (p = 0.7445) for crude oil recovery (Table 5). The coefficient of determination (R2 = 0.99) and the adjusted R2 value (0.97) indicate that the model was well correlated. The results of the ANOVA analysis produced the following second order polynomial equation:
Fig. 2 shows the interactive effects of the process variables in three-dimensional response surface plots for crude oil recovery. The highest recovery of 94.46% was observed at 250°C for 60 min. In contrast with oil removal, crude oil recovery was low. For example, the highest removal efficiency for crude oil of 99.8% was observed at 300°C for the 60 min extraction, and the recovery was found to be 88.6% at the same operating condition. This means that the high temperature reduced the recovery efficiency, and this might have increased the crude oil degradation. Many organic compounds have been reported to be degraded in subcritical water during hydrothermal treatment of contaminated soil due to hydrolysis or acid/base-catalyst reactions [14, 23]. In this study, several degraded products, such as phenol, 3-methyl-2-cyclopentanone, 3,4-dihydroxy-5-amino-pyridazine and 1,4-dimethoxy-2,6-dimethylbenzene were identified via GC-MS analysis in the treated soil residue, and these had not been present in the contaminated soil. Although the quantity of degradation products was not determined, degradation at high temperature could be assumed to be responsible in reducing the oil recovery. However, it is difficult to explore the detailed mechanism of the crude oil degradation since crude oil is composed a wide range of hydrocarbons. The interactive effects between flow rate and temperature (p = 0.2951) and between flow rate and time (p = 0.1676) were not significant for crude oil recovery (Table 5).
3.3. Confirmation Experiment
To obtain the best removal and recovery efficiencies of crude oil (setting the target removal efficiency to 99.9), experiments were performed by resolving the regression equation within the same range of operating parameters. A total of 63 suitable solutions were listed using software (Design-Expert 10) according to the desirability factor. Among these, two suitable solutions were selected to ensure the accuracy of the regression solutions from the experiments. Table 6 shows suitable solutions for A and B and of the results that were obtained. An oil removal of about 98.7% and oil recovery of 83.3% were obtained for a while a similar removal (98.7%) but lower recovery (82.0%) was observed for B. In contrast to oil recovery, the observed oil removal efficiencies should be noted to be close to the predicted value (99.9%).
4. Conclusions
The BBD under RSM was used to optimize the process variables namely, temperature, extraction time and water flow rate for crude oil removal and recovery in the subcritical water extraction process. It showed that the temperature is the most crucial variable for subcritical water extraction process. As can be seen from this study results that the removal efficiency of crude oil was increased with increasing temperature within the range studied (200–300°C); however, in the case of oil recovery, it was decreased at 300°C, might be due to degradation process. The optimum conditions for maximum crude oil removal and recovery were found to be 250°C, 120 min, and 1.5 mL/min according to the quadratic model. Under these experimental conditions, crude oil removal and recovery were found to be 99.69% and 87.33%, respectively.