1. Introduction
More than 100,000 dyes are available commercially and in use in various industries. Approximately 0.7 million tons of synthetic dyes are generated annually worldwide. However, nearly 15% of the dye is lost during the dyeing process, becoming released into the environment via industrial effluent-discharge processes [1]. Among all dyes, azo dyes with nitrogen double bonds (-N = N-), characterize the major group of synthetic dyes. These types of dyes have been applied in the cosmetics, textile, paper-making, and printing industries [1–2]. They are a waste material in the printing and dyeing processes and are accordingly discharged in the water system. Hence, the removal of azo dyes has been attracting extensive consideration worldwide [3].
Biological and physical techniques for the removal of azo dyes are not very effective, because of the presence of aromatic rings and the stability of the azo dyes’ molecules [4–5]. In addition, wastewater from textile-dyeing facilities is difficult to treat because of its highly fluctuating composition and color variability, depending on the dyestuff, fabric, and concentration of added fixing compounds [6]. However, effective and environmentally friendly techniques are necessary for the proper treatment of azo dyes in wastewater. Fenton-like degradation is a rapid and low-cost technique that is easy to apply on an industrial scale to remove the azo dyes from wastewater [7]. In the Fenton-like process, hydroxyl radicals are generated by oxidation using hydrogen peroxide [8–9]. Currently, nanoparticles are applied to remove azo dyes; these particles include zero-valent iron (Fe0) [10–11], zero-valent copper [1], zero-valent zinc [12], and hollow cobalt nanoparticles [2]. Recently, nano-metallic particles (NMPs) have emerged as a promising agent for treating phenol in wastewater [8, 13–14].
Advanced oxidation processes (AOPs) are processes in which hydroxyl radicals are generated in a liquid system and then attack organic pollutants [13]. Ultrasonic cavitation, also considered as an AOP, has also been applied for the degradation of azo dyes [1, 11]. An application of an ultrasonic process with nano-catalysts in the presence of hydrogen peroxide increases the rate of the oxidative degradation of azo dyes [14]. Extremely reactive hydroxyl radicals are generated in a liquid medium because of cavitational effects [15]. However, no study of the application of NMPs to the degradation of azo dye (methyl orange, MO) has been published earlier.
Therefore, this study was done to evaluate the degradation of MO by NMPs under a heterogeneous Fenton-like process. NMPs were recovered from the fine fraction (< 0.25 mm) of automobile-shredder residue (ASR), which is highly contaminated with heavy metals [16–17]. In many countries, ASR waste is dumped into landfills, increasing the degree of groundwater pollution [16, 18]. In this study, the fine fraction of ASR was used, because it consists of highly hazardous materials because of the availability of high levels of heavy metals [16]. In this study, the degradation efficiency of MO by NMPs is established, and we investigate the effects of the initial MO concentration, the amount of H2O2, the initial solution pH, the ultrasonic treatment time, and the NMP dose on the degradation efficiency of the MO removal process.
2. Materials and Methods
2.1. Preparation of NMPs from the Leaching Liquor of ASR
The details of the leaching conditions are as follows: 1.0 M of HNO3, a liquid-to-solid ratio of 10 mL/g, and an ultrasound power level of 500 W. To increase heavy-metal extraction from the ASR, an ultrasonic generator (VCX-500, Sonics & Materials, Inc., USA) was used for 1 h. A leaching solution (100 mL) was used for the synthesis of NMPs, and the pH of the solution was adjusted to 7.0 with 1.0 M of NaOH. The synthesis of the NMPs was completed according to Singh and Lee [18]. A 0.1 M solution of NaBH4 was added dropwise to a leaching solution at a pH of 7.0. Subsequently, the mixture was stirred using a temperature-controlled magnetic stirrer at 25 ± 0.5°C. The solution mixture became black in color, indicating that the metal ions were converted into NMPs. The separation of the NMPs was done by centrifugation at 4,000 rpm for 30 min. The recovered NMPs were washed several times with distilled water and then cleaned with ethyl alcohol to remove the excess NaBH4. The NMPs were afterward dried in a vacuum at 50°C. Fig. 1 shows a flowchart of the NMP preparation process and their application to remove MO from water.
2.2. MO Degradation under Ultrasonic Irradiation
The ultrasonic irradiation of the MO solution was carried out in an air atmospheric condition using an ultrasonic generator equipped with a titanium horn transducer. The test of the degradation of MO by NMPs was carried out in 250 mL glass beaker under ultrasonic power of 500 W and a frequency of 20 kHz. Subsequently, 100 mL of the MO solution (at various concentrations) was taken in a beaker holding appropriate amounts of NMP particles; in the same mixture proper amounts of H2O2 were added. The effects of the different parameters, such as initial MO concentration, the amount of H2O2, the NMP dosage, and the pH value of the MO solution. On the degradation of MO were considered. The pHs of the MO solution were adjusted at 2, 2.5, 3, 3.5, and 4 with 0.1 M HCl and 0.1 N NaOH. The samples were taken after reaction times of 5, 10, 15, 20, 25, 30, 35, and 40 min. The residual MO concentrations were then analyzed after the samples were filtered using 0.45 μm filter paper. The degradation rate of MO by the NMPs was also measured without the addition of H2O2 under ultrasonic irradiation. A calibration curve was obtained using the standard MO solution with different known concentrations. All experiments were carried out at least three times.
The dye degradation efficiency of MO was calculated using Eq. (1):
where C0 and Ct denote the concentrations of MO at time 0 and t, respectively.
2.3. Analysis
The concentrations of elements in the leaching liquor of ASR were measured by means of inductively coupled plasma atomic emission spectroscopy (ICP-AES) (Perkin Elmer Inc., Optima 2000 DV). A UV–visible spectrophotometer (UV 1601, Shimadzu) was used to measure the residual MO concentration at a maximum wavelength (λmax) of 465 nm. A pH meter (Orion 3 Star, Thermo Scientific) was used to measure the solution pH. The NMPs were analyzed by a scanning electron microscope (SEM; S–4300CX; Hitachi, Japan). X-ray photoelectron spectroscopy (XPS) and Fourier-transform infrared spectrophotometry (FT-IR) analyses of the NMPs before and after the reaction were carried out using an Escalab–210 electron spectrophotometer (Spain) and an ISF 66/S spectrophotometer (Bruker), respectively. The XPS system was used to define the elemental states of various metals, and FT-IR was applied to describe the functional groups available on the surfaces of the NMPs.
2.4. Kinetics of MO Degradation
The degradation of MO by NMPs under ultrasonic irradiation was demonstrated by a pseudo-first-order reaction [13, 19],
where C0 signifies the initial concentration of MO (mg/L) and Ct is the concentration of MO at time t (min), with k representing the pseudo-first-order constant (min−1).
The degradation of the phenol by NMPs was also analyzed by pseudo-second-order reaction kinetics [20],
The slope of the plot between 1/Ct and time yields the pseu-do-second-order rate constant k.
3. Results and Discussion
3.1. Characterization of the NMPs
The ICP analysis indicated that the concentrations of Fe, Mn, Ni, Cu, Zn, Cr, and Pb in the ASR leaching liquor were 639.0, 35.0, 3.9, 41.5, 281.2, 2.5, and 41.2 mg/L, respectively. An SEM analysis of NMPs is given elsewhere [13]. The SEM image of the nanoparticles was found to be spherical and structurally rough on the surface, signifying that abundant reactive sites are available on the surfaces of the NMPs. The spherical NMPs forming an aggregate structure may result from magnetic interactions between the nanoparticles [8].
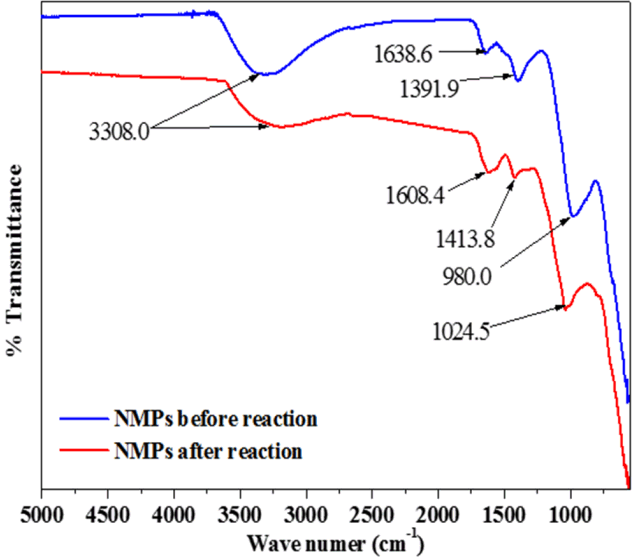
Fig. 2 shows the FT-IR spectra of the NMPs as measured before and after the reaction. Band peaks at 980.0 and 1,391.9 cm−1 were observed on the surfaces of the NMPs; these are confirmation of M-O stretching connected to metal oxides (such as MO, M2O3, and M3O4.), characteristic of the accessibility of metal oxides on the surfaces of the NMPs [13, 21]. The FT-IR spectra of the NMPs showed broad bands at 3,308.0 cm−1, which could be assigned to the hydroxyl group (O-H). After the reaction, the intensity of this band was reduced and the peak was found to be broader. After the reaction, the peaks of the bands were shifted from 1,638.6 to 1,608.4 cm−1, from 1,391.9 to 1,413.8 cm−1, and from 980.0 to 1,024.5 cm−1, likely corresponding to M-O stretching and representing oxidized form of NMPs in the reaction [13].
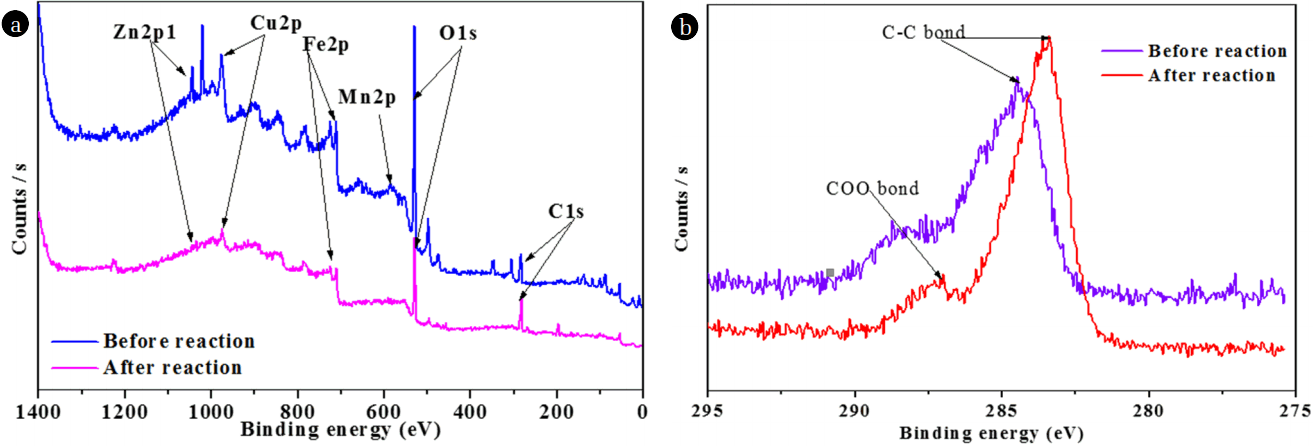
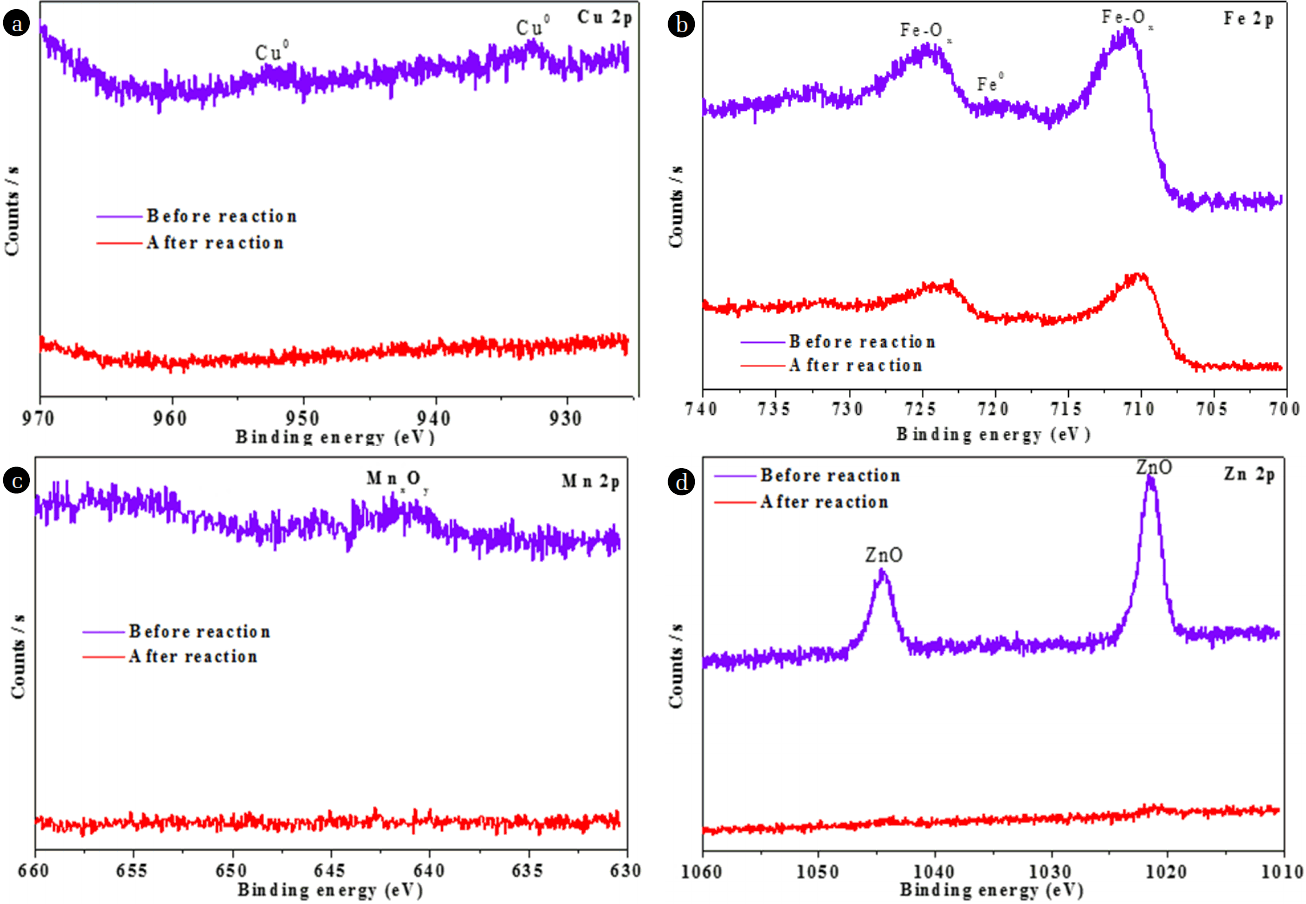
XPS was further applied to study the surface chemical compositions of NMPs. As shown in Fig. 3(a), the XPS analyses showed that C, O, and few metals (Fe, Cu, Zn, and Mn) existed on the surfaces of the NMPs. The presence of O likely stems from the fact that the NMPs were easily oxidized partially by oxygen when exposed to air. Two C (1s) peaks were identified at 284.5 and 287.6 eV, allotted to C-C/C-H and COO bonds, respectively [22–23]. However, after the reaction, these peaks were shifted to 283.4 and 286.99 eV, respectively. The O (1s) peak at 528.4 eV was recognized as showing the availability of oxides on the surfaces of the NMPs, whereas after the reaction, the intensity of this peak was reduced, possibly due to the conversion of oxides into metallic ions [14]. Metals existed on the surfaces of the NMPs in the form of elements, oxides of metals, and carbonates of metals. The peak of the binding energy (BE) of Cu was observed at 932.5 eV, representative of Cu0 [24]; moreover, this peak did not appear after the reaction with MO (Fig. 4(a)), indicating that Cu0 was oxidized to Cu+/Cu2+ [25]. The XPS spectra of Fe (2p) in Fig. 4(b) show two peaks, with BE values of 711.1 and 724.5 eV. These broad peaks could be assigned to iron oxides (Fe2O3), indicating that the surfaces of the NMPs were covered with an iron-oxide film which likely formed during the synthesis process [1, 8]. The intensities of both peaks decreased after the reaction, confirming that oxides of Fe were involved in the reaction. The BE of the Mn 2p before the reaction showed a peak at 641.9 eV, assigned to MnO and MnO2 [26]. However, after the reaction, this peak was not seen, signifying that the oxides of Mn contributed to the degradation of MO (Fig. 4(c)). The XPS spectra of Zn (2p) in Fig. 4(d) shows two peaks, at BEs of 1021.6 and 1044.6 eV, which were assigned to ZnO [27]. These peaks were not seen after the reaction, confirming the participation of ZnO in the degradation of MO.
3.2. Removal of MO by NMPs
3.2.1. Effect of the dosages of NMPs
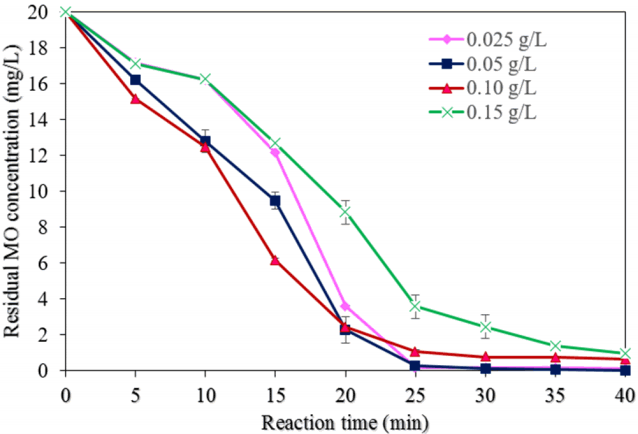
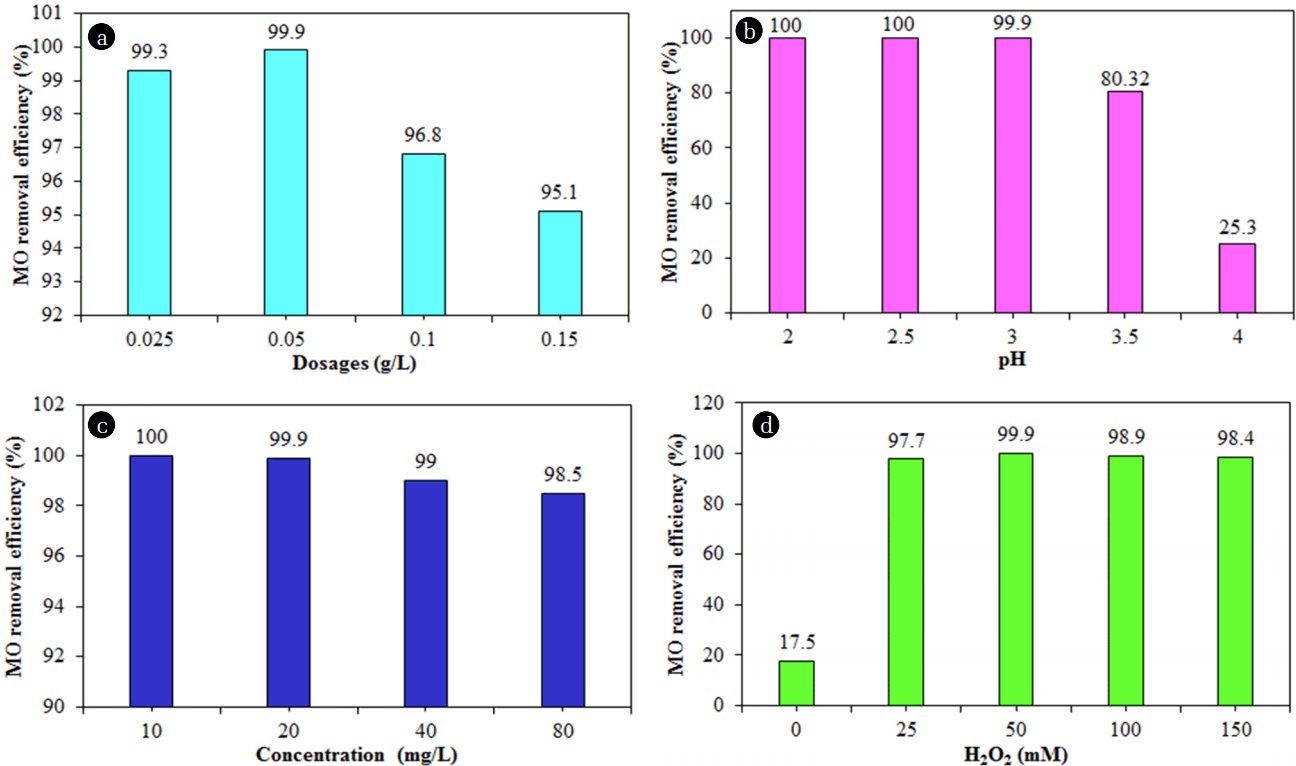
Fig. 5 displays the effect of different NMP dosages (0.025, 0.05, 0.10, and 0.15 0.20 g/L) on the degradation of MO (experimental conditions: pH of 3.0, the initial MO concentration of 20 mg/L, H2O2 concentration of 50 mM, a reaction time of 40 min, and ultrasound power of 500 W). As shown in Fig. 6(a), the degradation of MO was found to be 99.9% with a dose of 0.050 mg/L at a reaction time of 40 min, which can be explained as that, when the dose of NMPs increased, then the number of reaction sites also increased [8]. However, upon a further increase of the dose, the MO degradation efficiency was reduced to 95.1%, most likely because of the scavenging effect of hydroxyl radicals and the agglomeration of NMPs in the reaction system [28].
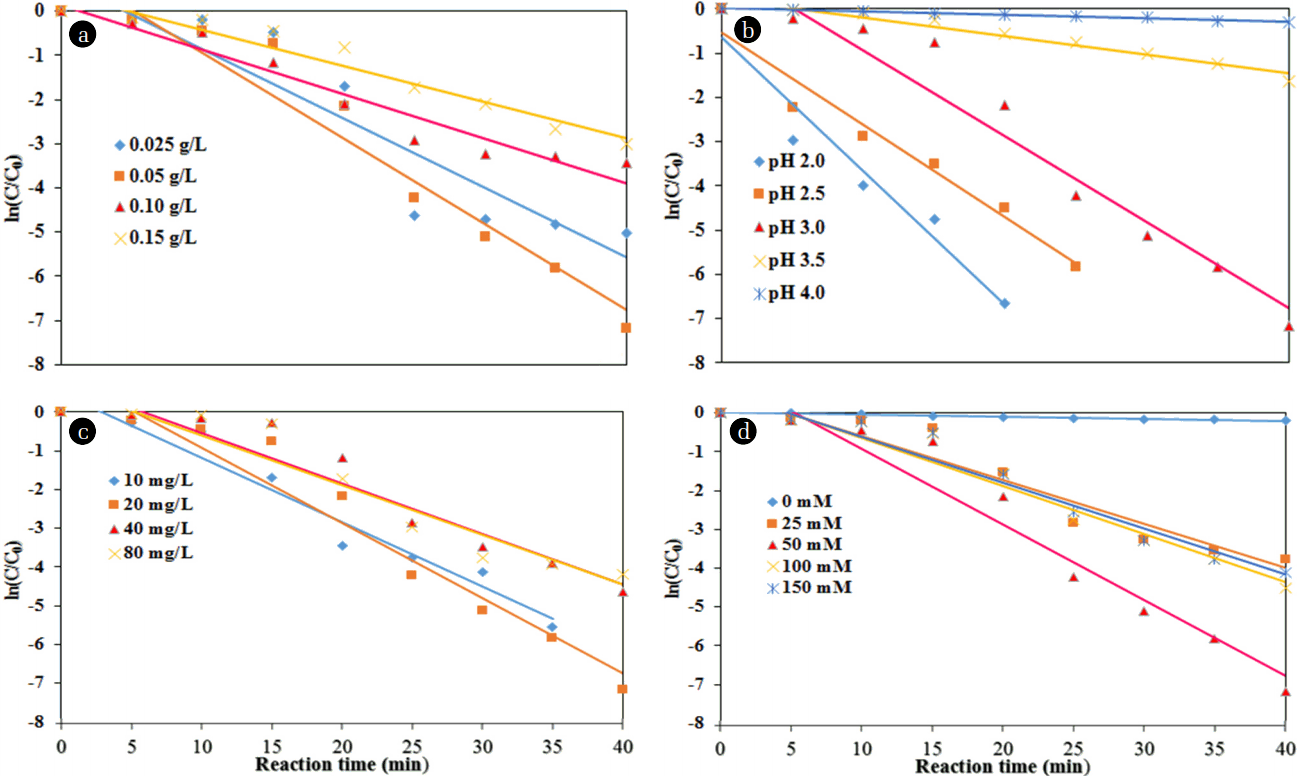
The values of the pseudo-first-order reaction rate constant (k) and correlation coefficient (R2) for the MO degradation rate with different dosages of NMPs were found from the plot of ln(C/C0) vs. the reaction time (Fig. 7(a)). Table 1 shows the values of k1 and R2 for diverse dosages of NMPs. When the quantity of NMPs was increased up to 0.050 g/L, the values of k1 and k2 were increased. The value of k1 was reduced approximately 47.8%; however, the value of k2 was reduced to 89.3% with a further increase in the dosage of NMPs.
3.2.2. Effect of the solution pH on MO degradation by NMPs
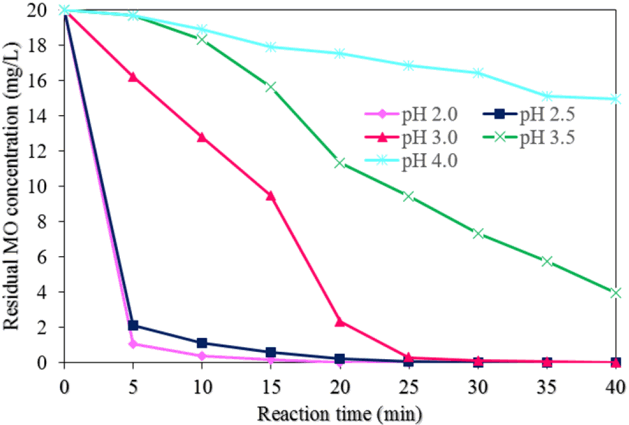
Fig. 8 displays the effects of the pH values on the MO degradation process by NMPs (experimental conditions: MO concentration of 20 mg/L, NMP dose of 0.05 g/L, concentration of H2O2 of 50 mM, reaction time of 40 min, and an ultrasound power level of 500 W). The highest and most rapid MO degradation rate was achieved at pH levels of 2.0 and 2.5. The degradation efficiency of MO was found to be approximately 100 % at reaction times of 25 and 30 min at pH levels of 2.0 and 2.5, respectively (Fig. 6(b)).
However, the lowest MO degradation efficiency was found to be approximately 25% at a pH of 4.0 at a reaction time of 40 min. The MO degradation rate decreased with an increase in the pH from 2.0 to 4.0. This arose due to the decay of H2O2 with the decrease in catalytic activity of the NMPs [29]. The values of k1 and R2 for the MO degradation rate with different pH values were found from the plot of ln(C/C0) vs. the reaction time (Fig. 7(b)). Table 1 shows the values of k1 and R2 with different pH values. The value of k1 was reduced about 97% when the value of pH was increased from 2.0 to 4.0. However, the value of k2 was increased with the increase in the pH of the solution.
3.2.3. Effect of the initial MO concentration on MO degradation
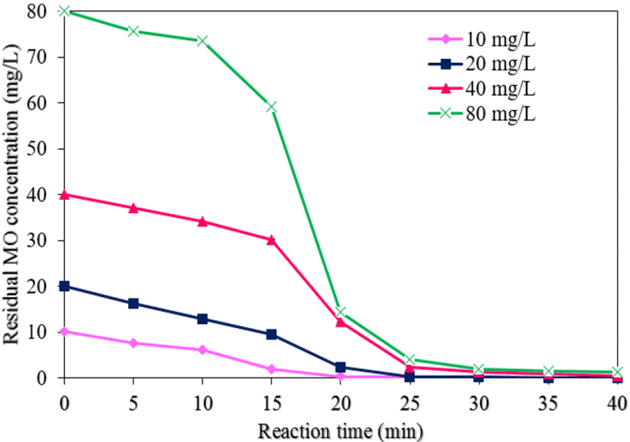
Fig. 9 exhibits the effect of different initial MO concentrations on the degradation of MO by NMPs (experimental conditions: a NMP dose of 0.05 g/L, a pH of 3.0, a H2O2 concentration of 50 mM, a reaction time of 40 min, and ultrasound power of 500 W). The degradation efficiency of MO was reduced from 100 to 98.5% with an increase in the MO concentration from 10 to 80 mg/L at a reaction time of 40 min (Fig. 6(c)). This result indicates rapid occupying of all the active sites of the NMPs by the MO molecules at a high level of MO, resulting in a decrease in the production of hydroxyl radicals in the sono-catalysis process [13, 27]. The residual concentrations of MO achieved were found to be 0, 0.016, 0.39, and 1.21 mg/L at a reaction time of 40 min with initial MO concentrations of 10, 20, 40, and 80 mg/L, respectively. The values of k1 and R2 for the MO degradation rate with different concentrations of MO were found from the plot of ln(C/C0) vs. the reaction time (Fig. 7(c)). Table 1 shows the values of k1, k2, and R2 with different concentrations of MO. When the MO concentration was increased from 10 to 80 mg/L, the values of k1 and k2 were decreased to 97.4% and 99.8%, respectively.
3.2.4. Effect of the amount of H2O2
Fig. 10 demonstrates the effects of different concentrations of H2O2 on the MO degradation rate (test conditions: a NMP dosage of 0.05 g/L, a pH of 3.0, an initial MO concentration of 20 mg/L, a reaction time of 40 min, and ultrasound power of 500 W). The degradation of MO by NMPs was very slow in the absence of H2O2, because there was insufficient production of hydroxyl radicals in the system. As displayed in Fig. 6(d), the degradation efficiency of MO increased considerably, from approximately 17% to 100% by increasing H2O2 concentration from 0 mM to 50 mM, perhaps because of enough hydroxyl radicals were produces [27]. MO degradation was achieved very rapidly with 50 mM of H2O2. The degradation of MO was increased by an increasing concentration of H2O2, to 50 mM. However, upon a further increase in the amount of H2O2, the degradation efficiency of MO was reduced slightly. The MO degradation may be possible with H2O2 with ultrasound (without NMPs); however, its degradation with only H2O2 is somewhat difficult, because there is no external agent to break down the H2O2. The values of k and R2 for the MO degradation rate with different concentrations of H2O2 were obtained from the plot of ln(C/C0) vs. the reaction time (Fig. 7(d)). Table 1 shows the values of k1, k2 and R2 with different amounts of H2O2. The values of k1 and k2 were increased with an increase in the amount of H2O2, from 0 mM to 100 mM; however, these values were decreased with a further addition of H2O2.
3.2.5. Synergistic effects of combined sonication with Fenton’s reagent
The degradation efficiency of MO was found to be approximately 100% for NMPs combined with ultrasonic irradiation and H2O2. The degradation efficiency of MO by NMPs with a combination of H2O2 and ultrasound represents the great potential of MO degradation in a very short period of time as compared to only the NMP/ultrasound power or H2O2/ultrasound power combinations. The oxidation of MO by ultrasonically dispersed NMPs in the absence of H2O2 required more sonication time for the complete degradation of MO. In the ultrasound/NMP system, the MO degradation efficiency was low (16.5%) at a reaction time of 40 min. However, in the NMP/ultrasound/H2O2 system, a MO degradation efficiency rate of 100% was achieved. The removal of MO was low because too few hydroxyl radicals were produced during the sono-advanced Fenton process (AFP). The ultrasonic irradiation effect was more noticeable at room temperature, because most of the bubbles were filled with gas. The increase in the reaction temperature reduced the degradation efficiency, perhaps because the cavitation threshold was lowered. Therefore, more water vapor was produced by the cavitation bubbles; this water vapor could decrease the ultrasonic energy produced during cavitation [30].
3.3. Possible Mechanism for the Degradation of MO by NMPs
During the ultrasonic irradiation, both physical and chemical processes, such as configuration, expansion, and adiabatically implosive breakdown of microbubbles in the aqueous system, are involved. These processes could be triggered by the propagation of the ultrasonic wave through acoustic cavitation. The quick breakdown of cavitation bubbles may be coupled with adiabatic heating of the vapor phase of the bubble, which gains great local transitory temperatures and pressures [31]. Under these exciting conditions, water molecules break down through heat energy to generate the extremely reactive radicals oH and oOH, and yields HO2o in the occurrence of O2 in the aqueous system. Therefore, organic compounds such as MO in close proximity to the bubble/water interface can be exposed to thermal decomposition and/or secondary reactions between the reactive radicals and MO, resulting in their degradation or mineralization [32]. The oxidation of MO by hydroxyl radicals in the sono-AFP is highly probable. Many hydroxyl radicals are generated in an ultrasound/NMP/H2O2 system [8, 13, 33]. In the sono-AFP, a pressure pulse is created during the breakdown of a solo cavity, leading to a high rate of production of hydroxyl radicals [15]. The ultrasound irradiation effects during the MO degradation process led to the production of many hydroxyl radicals, which mainly target MO molecules in an aqueous system. Ultrasonic irradiation improves the corrosion of NMPs and successively releases many metal ions. Appropriate amounts of metals cations (Mn+) and H2O2 are required to stimulate the AFP. In the presence of H2O2, the ultrasound irradiation produces many hydroxyl radicals in an aqueous system (Eq. (4)). The NMPs can react with the added H2O2, causing the generation of further hydroxyl radicals [8, 33]. The highest rate of MO degradation was achieved at a pH of 3.0, which can be attributed the presence of several metal oxides on the surfaces of NMPs. These metal oxides were released shortly at an acidic pH, after which many active sites existed for reaction with MO.
Here, M is the metal, n is the number of valences, O denotes the oxides, ‘)))))’ represents the ultrasound waves, and x and y correspondingly represent the number of moles.
4. Conclusions
The degradation of MO by ultrasonically dispersed NMPs was examined with and without the addition of hydrogen peroxide. The removal of MO was found to be affected by the pH value of the solution and by the NMP and hydrogen peroxide dosages. This study particularly demonstrated that NMPs are highly efficient for MO degradation in the presence of H2O2 and ultrasonic power. A decrease in the pH value and the initial concentration of MO increased the degradation efficiency of MO; however, an increase in the NMP and H2O2 dosages increased the MO degradation efficiency level only up to a certain limit. Excess amounts of NMPs and H2O2 decreased the MO degradation efficiency. The optimum conditions for the degradation of MO were a NMP dosage of 0.05 g/L, an H2O2 concentration of 50 mM, an MO concentration of 40 mg/L, a pH of 3.0, ultrasonic power of 500 W, and a reaction time of 40 min. The pseudo-first-order kinetics were found to be appropriate for the degradation of MO by NMPs. XPS and FT-IR analyses confirmed that NMPs were oxidized and corroded after the reaction. The results of the present study provide ample evidence of the removal of azo dye from wastewater via the application of NMPs under the sono-AFP.