1. Introduction
Arsenic is widely distributed in all segments of natural environment viz., air, soil and water [1, 2]. Arsenic exists mainly in two different oxidation states i.e., As(III) (arsenite) and As(V) (arsenate) having various chemical species. The speciation of these species largely depends upon the pH/pE conditions of aquatic environment. The direct exposure of arsenic is highly toxic to the entire ecosystem [3, 4]. Although the elemental arsenic possesses no toxicity however, the inorganic arsenic compounds are highly toxic. Therefore, based on the adequate studies, arsenic and its compounds are eventually classified to Group 1 carcinogen [5, 6]. Arsenic toxicity leads to lung, skin, and bladder cancer and kidney related diseases [7–10]. The ground water at different places in India, China, Bangladesh, Mexico, Argentina, USA and many other countries around the globe are contaminated with arsenic. Moreover, at places, the situation is greatly alarming. Moreover, a rough estimate accounts that above 100 million people are affected with arsenic poisoning at different regions of world [11–13]. Therefore, the Environmental Protection Agency (EPA) and the World Health Organization (WHO) minimized the maximum contamination level (MCL) of arsenic from 50 μg/L to 10 μg/L in drinking water supply [14]. A low permissible limit of arsenic in water bodies compels to introduce a reliable and efficient sensing device for trace to sub-trace level detection of arsenic from aqueous solutions. Further, an on-site detection with required selectivity is an important aspect needs to be studied in sensor development.
In a line, electrochemical sensors find wide attention in the low level detection of several water pollutants in recent past [15–18]. Actually, it provides rapid, accurate, cost effective and robust instrumentation [19–23]. Literature survey reveals that several studies were conducted for the low level detection and measurements of arsenic from aqueous solutions using the electrochemical approach [24–30]. These studies are widely conducted for the detection of As(III) at highly acidic conditions from aqueous solutions. Further, it was reported that the As(V) is relatively stable and found difficult in direct electrochemical detection [31–32]. Therefore, As(V) was reduced chemically in situ or off situ to As(III) and then detected from aqueous solutions [31, 33–36]. Further, the carbon paste electrode (as working electrode) is a useful device for the easy modification and suitability for target species detection [16, 31]. Therefore, keeping in view the present communication aims to modify the carbon paste electrode using novel hybrid materials and further applied for direct low level detection of As(V) from aqueous solutions at neutral pH conditions. The selectivity of the sensor was well assessed carrying the measurements for real tap water samples spiked with arsenic(V).
2. Materials and Methods
2.1. Materials
Bentonite was procured commercially which was mined at Bhuj, Gujarat, India. It was crushed and sieved to obtain 100 BSS (British Standard sieve i.e., 0.150 mm) mesh size. The cation exchange capacity (CEC) of bentonite was found to be 69.35 meq/100 g of bentonite. Hexadecyltrimethylammonium bromide (HDTMA), carbon powder (Glassy Spherical Powder 2–12 micron) and titanium wire (0.81 mm) were procured from the Sigma-Aldrich, USA. Cadmium nitrate tetrahydrate, copper(II) sulphate pentahydrate, lead nitrate and manganese nitrate were obtained from Merck India. Ferric nitrate nonahydrate (AR), sodium chloride (AR), sodium nitrite (AR), glycine (AR) and paraffin oil were procured from HiMedia Chemicals, India. Sodium arsenate dibasic heptahydrate AR (98.5% Purity) was obtained from HiMedia Chemicals, India. Sodium metaarsenite was obtained from Wako Pure Chemical Industries Ltd., Japan. Ethylenediamine tetraacetic acid disodium salt was procured from the Loba Chemie, India. The deionized water is further purified (18 MΩ-cm) using a Millipore water purification system (Milli-Q+).
Further, the bentonite was modified organically using the hexadecyltrimethylammonium bromide by the wet cation exchange process summarized elsewhere [37]. The modified bentonite was named as BH material.
2.2. Instrument
The electrochemical studies were carried out using Potentiostat/Galvanostat (Model: SP 50; BioLogic Science Instruments, France). The electrochemical output data was analysed using the ECLab® computer software. Single compartment three electrode cell assembly was employed having Ag|AgCl reference electrode (Model: RE-5B; BASi, USA) and platinum electrode as an auxiliary electrode. The carbon paste electrode was fabricated in the laboratory and then it was used as a working electrode. The electrical circuitry was made using the titanium wires.
2.3. Methodology
The carbon paste electrode was modified with the hybrid material BH and employed as working electrode. The carbon paste was prepared as described elsewhere [38]. Moreover, the release of carbon paste into the bulk solution was also checked by filtering the test solution using 0.25 μ filter paper. This showed that no carbon powder was found onto the filter paper. This indicated that the electrode surface was intact.
Stock solution (1.0 mg/L) of sodium arsenate (As(V)) was prepared dissolving an accurate amount of arsenic salt into the 1.0 mol/L KNO3 solutions. With the successive dilutions, using 1.0 mol/L KNO3 solutions whose pH was pre-adjusted by the drop-wise addition of conc. HNO3/NaOH solutions. Therefore, a required concentration of As(V) was used for electrochemical studies. The effect of interfering ions on As(V) detection was carried out using several cations or anions (150.0 μg/L) and As(V) (32.0 μg/L) solution. Prior conducting the electrochemical experiments, the As(V) solutions were degassed by bubbling high purity nitrogen gas (99.99%), at least, for 10 min.
The cyclic voltammograms were recorded exciting the working electrode against Ag|AgCl reference electrode at a constant or varied scan rate. Bentonite and BH-modified carbon paste electrodes along with the bare carbon paste electrode were employed as working electrodes. The working electrode was excited at an applied potential ± 1.0 V with a scan rate of 100 mV/s having 50.0 μg/L As(V) solution. The samples were scanned at least for 5 cycles and always the third cycle was considered for the measurements. In order to optimize the BH-modified carbon paste electrode function towards the redox behaviour of As(V), a scan rate dependence study was conducted varying the scan rates from 20 to 200 mV/s at a 50.0 μg/L As(V) and at pH 6.0 in 1.0 M KNO3 background electrolyte. The concentration dependence studies were conducted varying the As(V) concentration from 1.0 μg/L to 35.0 μg/L at pH 6.0 using 1.0 M KNO3 and at a constant scan rate of 100 mV/s. The electrochemical data was obtained for six replicate samples and an error is mentioned with the ± S.E. values.
3. Results and Discussion
3.1. Effect of Scan Rate
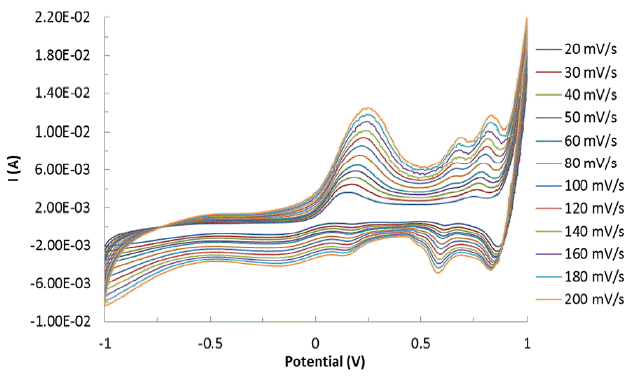
The CVs are presented as a function of scan rate in Fig. 1. The figure clearly shows that an increase in scan rates is apparently caused to increase in electrochemical signals (i.e., both cathodic and anodic peak currents).
Further, the electrochemically active surface area is obtained using the Randles-Sevik equation [37] (Eq. 1):
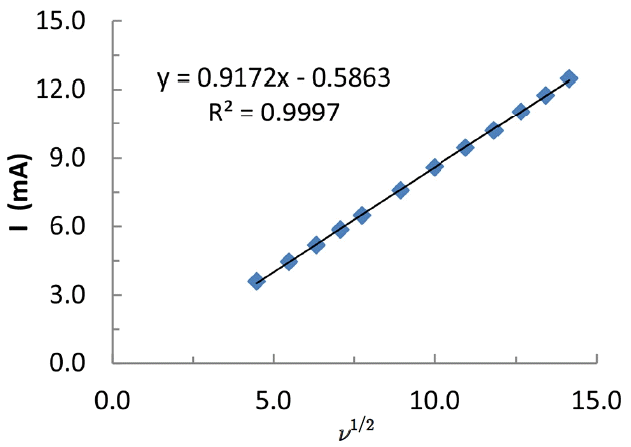
where I is the peak cathodic current (A); n is the number of electrons involved in reduction; D is the diffusion coefficient of arsenic (cm2/s); ν is the scan rate (V/s); C is the concentration of arsenic (mol/L); and A is the electroactive surface area of working electrode (cm2). The anodic peak current was plotted against the square root of scan rates (Fig. 2). A good linearity is obtained between the oxidative current (I) vs ν1/2 (ν: scan rate) (cf Fig. 2). The slope of the straight line (Fig. 2) is utilized to obtain the electroactive surface area of BH-modified carbon paste electrode. The diffusion coefficient of arsenic in water was taken 8.12×10−6 cm2/s for the As(III) [39], perhaps, as a reference value. The calculated surface area of the BH-modified electrode was found to be 3.763 mm2. This value apparently relates the number of specific sites available onto the electrode surface for efficient redox process to occur [40].
3.2. Cyclic Voltammetric Results
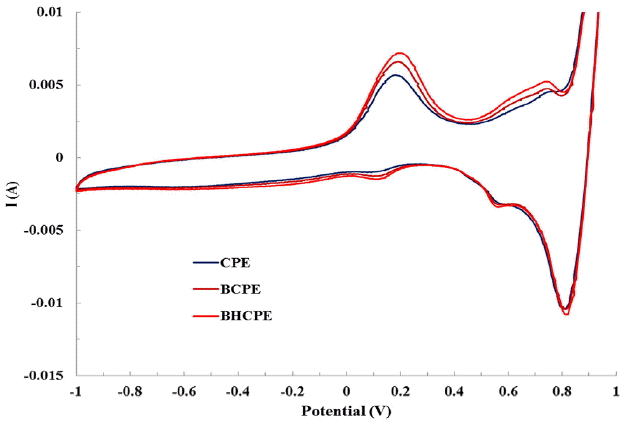
Cyclic Voltammograms for the redox behavior of As(V) using various CPEs is presented graphically in Fig. 3. Fig. 3 clearly shows that no significant change in CV pattern is occurred both in the bentonite- or BH-modified carbon paste electrodes. Nevertheless, a substantial increase in electrical signal is obtained; in particular, the anodic current is increased using the bentonite- or hybrid material-modified carbon paste electrodes comparing to the pristine carbon paste electrode. It is also noted that an additional increase in anodic current was obtained using the BH-modified electrode than the pristine bentonite-modified carbon paste electrode. These results clearly showed that the As(V) possessed relatively stronger affinity towards the hybrid material than the pristine bentonite. This is in accordance to the sorption data that obtained separately and presented elsewhere [41]. Similarly, the ΔEp was found to be 0.0154 V for the BH-modified electrode. Relatively low value of ΔEp was indicated to reversible nature of arsenic at the electrode surface [19]. Moreover, it also inferred that the arsenic was sorbed onto the electrode surface more strongly and giving out an enhanced electrical signal which therefore, be utilized for efficient sensor development [41–42].
Further, Fig. 3 clearly demonstrated that the voltammograms were found to be identical for all the three electrodes employed. It is also observed that a stepwise oxidation/reduction take place at the electrode surface. A dominant peak at an applied potential of Ca + 0.15 is due to the As(0) which is oxidized to As(III) at the potential + 0.71 V (vs Ag|AgCl) and at very high potential it is further oxidized to As(V) showing a significant high current. The reverse scan showed that the As(V) is reduced to the As(III) with a 2 electron reduction process at an applied potential of Ca + 0.83 (vs Ag|AgCl). As(III) is then reduced to As(0) with 3 electron reduction is observed at an applied potential of Ca + 0.16 V (vs Ag|AgCl). The reversible 3 and 2 electron redox processes are demonstrated as:
3.3. Concentration Dependence Studies
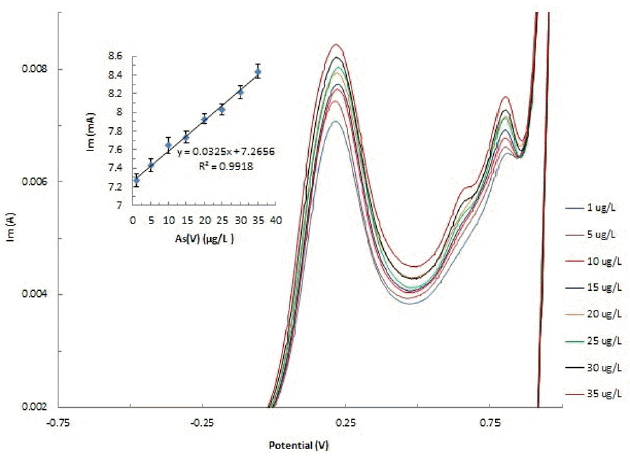
The concentration dependence CVs are obtained at an applied potential of ± 1.0 V and illustrated in Fig. 4. The cyclic voltammograms for As(V) exhibited a characteristic oxidative peak at Ca 0.21 V and 0.22 V for the pristine bentonite- and hybrid material-modified electrodes, respectively at pH 6.0 (cf Fig. 4 shown for BH-modified electrode). The anodic peak current was utilized to obtain the calibration line. A reasonably good linearity between the oxidative peak current and the concentration of As(V) was obtained for the studied concentration range (1.0 μg/L to 35.0 μg/L) using the BH-modified electrode. The calibration line equation is presented as: I = 0.0325 x C (μg/L) + 7.266 (R2 = 0.991) (Inset Fig. 4). The straight line equation, I = 0.029 x C (μg/L) + 7.142 (R2= 0.855) was obtained for the pristine bentonite-modified electrode. It is therefore indicated that pristine bentonite modified carbon paste electrode possessed with distorted relationship between the anodic peak current and concentration of As(V). An useful linear relationship between the anodic peak current with the As(V) concentration could therefore be utilized in the successful low level detection of As(V) from aqueous solution. This further be employed for the development of electroanalytical tool for low level As(V) detection.
The analytical methods are validated with the useful quantities viz., limit of detection (LOD) and limit of quantification (LOQ) which are estimated using the standard equations described previously [37]. The LOD and LOQ values were found to be 1.61 μg/L and 5.38 μg/L, respectively. Interesting to note that the value of LOD was found to be reasonably low comparing to value prescribed for arsenic level in drinking water i.e., 10.0 μg/L. This further indicated the possible implication of electrochemical device using the BH-modified carbon paste electrode in the trace level detection of As(V) from aqueous solutions. It was reported previously that the carbon Nanotube with silicone showed significant enhanced detection limit comparing to the mineral oil. The LOD and LOQ values were reported 10.3 and 34.5 μg/L (for mineral oil); 3.4 μg/L and 11.2 μg/L (for silicone), respectively [44]. Similarly, the detection limit was obtained 4.4 μg/L for As(V) with the determination limit 11 μg/L [45]. A direct electrochemical determination of As(V) in neutral pH waters was conducted using the Mn-coated gold electrode under the anodic stripping voltammetric (ASV) studies. As(V) was reduced to As(III) in presence of elemental Mn onto the working electrode surface. Further, the micro-wire (Mn coated gold) electrode was vibrated during the deposition step to improve the sensitivity and the detection limit was found to be 0.2 nM As(V) at a deposition time of 180 s [35].
However, the other studies indicated very low detection limit of As(III) i.e., 0.1 μg/L was obtained using the Au-Nanoparticle modified carbon nanotubes [46]. On the other hand, the functionalized Au-Nano-particles showed the LOD and LOQ values of 2.5 and 8.4, respectively as obtained by the visible spectroscopic technique [6].
3.4. Effect of Cations and Anions Interference in the Detection of As(V)
The presence of co-existing ions viz., Cu(II), Pb(II), Cd(II), Mn(II) and Fe(III) and anions viz., Cl−, NO3−, PO43−, EDTA and glycine, having 150 μg/L of each, in the detection of As(V) is an useful parameter to simulate the studies for the real matrix complex system. Therefore, the estimation of As(V) (32 μg/L) at pH 6.0 was conducted in presence of these ions and results are illustrated in Table 1. It is noted that the CVs obtained for As(V) in presence of these co-existing ions are almost unaffected within the exciting potential ± 1.0 V. This further reaffirms that the detection of As(V) is possible even in presence of these ions. It is clearly evident from the Table 1 that the concentration of As(V) is not much affected in presence of co-existing ions (except Fe(III)). Fe(III) slightly affected the As(V) determination. This is possibly due to the oxidation of Fe(0) to Fe(III) around the same applied potential. It was reported previously that the use of constant current stripping analysis (CCSA) was successfully employed in the determination of As(III) and As(V) from aqueous solutions in presence of Cu(II). The detection limit was reported to be 3 ppb and 0.5 ppb for As(III) and As(V), respectively [47]. Similarly, the Cu(II) was not interfered the As(III) determination from aqueous solutions using the Pt-Nanoparticle modified glassy carbon electrodes [48].
3.5. Real Matrix Analysis
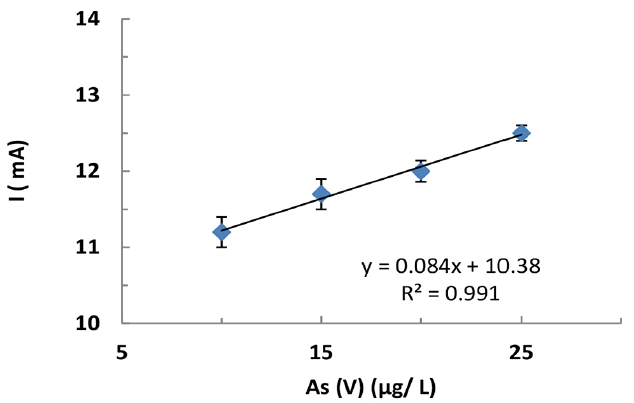
Detection of As(V) is investigated in As(V) spiked tap water samples. Standard additions of As(V) was conducted in solution preparations using the available tap water. The tap water was subjected for the total organic carbon (TOC) and inorganic carbon (IC) analysis using a TOC analyzer (Model: TOC-VCPH/CPN; Shimadzu, Japan). The TOC and IC values were found to be 1.198 mg/L and 6.478 mg/L, respectively. This clearly showed that the tap water was contained with significant amount of inorganic carbon i.e., possibly due to the presence of carbonates or bicarbonates of alkali metals. Moreover, it also possessed with some organic carbon. The electrochemical response of As(V), prepared in tap water, onto the BH modified-CPE is almost identical comparing to the purified water scan. No obvious change in the CV pattern of As(V) is observed in the water sample indicated the potential application of working electrode in real water sample analysis. It is obvious from the results that the sensitivity of the analytical data is slightly suppressed in the tap water analysis. However, an optimum and required linearity between the As(V) concentration and anodic peak current was obtained which could help in real water sample analysis and possibly be employed in the real water sample analysis (cf Fig. 5). A similar observation was obtained for the low level detection of Cd(II) from aqueous solutions using the modified carbon paste electrodes [49].
3.6. Presence of As(III) and As(V)
The concentration of As(III) was varied from the 5.0 μg/L to 45.0 μg/L but the total concentration of As(III) and As(V) was kept constant to 50.0 μg/L. The cyclic voltammograms were obtained in 1.0 mol/L KNO3 solutions and at a scan rate 100 mV/s pH 6.0. The results obtained are demonstrated in Fig. 6. It is observed that the cathodic or anodic peak current is not significantly changed varying the As(III) concentration from 5.0 μg/L to 45.0 μg/L.
The results, therefore, indicated that the total concentration of arsenic was obtained as constant. The average oxidative peak current is found to be 7.866 ± 0.573 μA (3.σ) at pH 6.0. These results are in good approximation of estimating total arsenic in solutions.
4. Conclusions
The cyclic voltammetric studies exhibited characteristic reversible behavior of As(V) in aqueous solution using 1.0 mol/L KNO3 solutions and employing the hybrid material modified carbon paste electrode. A significant enhanced electrochemical signal was obtained with the hybrid material (BH)- or pristine bentonite-modified carbon paste electrode compared to the unmodified carbon paste electrode. The concentration dependence study unveiled the higher affinity of As(V) towards the BH-modified electrode compared to the pristine bentonite-modified electrode for the As(V) detection. The presence of co-existing ions viz., Cu(II), Cd(II), Mn(II), Pb(II), Cl−, NO3−, PO43−, EDTA and glycine was not affected significantly the detection of As(V) from the aqueous solution. However, the presence of Fe(III) slightly influenced the As(V) determination. The limit of detection and limit of quantification was found to be 1.61 and 5.38 μg/L, respectively using the BH-modified carbon paste electrode. The electrochemical detection of As(V) from tap water samples using the hybrid material-modified carbon paste electrode was found reasonably well hence, possibly, be employed for the trace detection of As(V) from aqueous solutions. Moreover, a single voltammogram could be used for the detection of total arsenic i.e., As(III) + As(V) from aqueous solutions.