AbstractMembrane bioreactor (MBR) technology has previously been used by water industry to treat high salinity wastewater. In this study, an anoxic-oxic biofilm-membrane bioreactor (AOB-MBR) system has been developed to treat mustard tuber wastewater of 10% salinity (calculated as NaCl). To figure out the effects of operating conditions of the AOB-MBR on membrane fouling rate (KV), response surface methodology was used to evaluate the interaction effect of the three key operational parameters, namely time interval for pump (t), aeration intensity (UGr) and transmembrane pressure (TMP). The optimal condition for lowest membrane fouling rate (KV) was obtained: time interval was 4.0 min, aeration intensity was 14.6 m3/(m2·h) and transmembrane pressure was 19.0 kPa. And under this condition, the treatment efficiency with different influent loads, i.e. 1.0, 1.9 and 3.3 kg COD m−3d−1 was researched. When the reactor influent load was less than 1.9 kg COD m-3d−1, the effluent could meet the third discharge standard of “Integrated Wastewater Discharge Standard”. This study suggests that the model fitted by response surface methodology can predict accurately membrane fouling rate within the specified design space. And it is feasible to apply the AOB-MBR in the pickled mustard tuber factory, achieving satisfying effluent quality.
1. IntroductionThe pickled mustard tuber is a local Chinese food fermented from fresh mustard tuber. It has gained wide popularity over the long history in China. It also has a huge market in the world. In recent years, Chinese pickled mustard tuber industry has really boosted in the Three Gorge Reservoir Region. Wastewater from pickled mustard production has reached 3.5 million m3 per year [1]. Mustard tuber wastewater has unique feature that it is of high salinity and high organic content. Salinity in mustard tuber waste-water ranges from 2 to 15 g/L, while chemical oxygen demand (COD) ranges from 0.3 to 10 g/L. When discharged directly to water body, the high salinity wastewater can severely impair water and ecologic quality of the Three Gorge Reservoir. Besides this, the soluble salts in the effluent would accumulate in the soil, causing land degradation and lessen of soil productivity [2].
The treatment of high salinity wastewater always poses a challenge in water industry. Physicochemical and biologic methods are two conventional techniques to treat salinity wastewater [3]. However, physicochemical technologies require high energy and could become a financial burden for routine operation and maintenance [4]. The biggest challenge in the high salinity wastewater treatment lies in the difficulty of operating an efficient biologic treatment system. Previous researches have showed that waste-water with high salinity affects the efficiency of biological treatment due to the following reasons. First, high salinity can increase osmotic pressure of cell, resulting in impairment of cell metabolism and biodegradation, and finally leading to plasmolysis [5–7]. Second, high salinity imposes adverse impacts on the microbial flocculation and lowers sedimentation performance of sludge [8, 9]. Third, high salinity reduces organic removal rate and denitrification rate [10, 11]. Finally, high salinity can cause inhibition of both aerobic and anaerobic biological treatment [5, 12].
In order to treat this kind of high salinity wastewater, various aerobic and anaerobic biological processes have been investigated worldwide. Zhou et al. [13] used an aerobic sequencing batch biofilm reactor (ASBBR) to treat mustard tuber wastewater. In their work, when the reactor was operated at the biofilm density of 50% and volumetric load of 0.22 kg COD m−3d−1, the effluent COD reached 95 mg/L. Sequential anaerobic-aerobic processes were investigated for the treatment of pharmaceutical wastewater with high salinity. A COD removal efficiency of 41.3% was achieved by an up-flow anaerobic sludge blanket (UASB), with an optimized organic loading rate of 8.11 g COD/L/d at a hydraulic retention time (HRT) of 48 h, then the UASB effluent was further treated by a membrane bioreactor (MBR). UASB + MBR achieved a removal efficiency of 94.7%, 51.1% for COD and TN [14]. Lu et al. [15] treated oilfield-produced water with high salinity with a hydrolysis acidification/bio-contact oxidation system (HA/BCO). By operating the biodegradation system with a 32 h HRT and a volumetric load of 0.28 kg COD/(m3·d), the treatment process achieved 63.5, 45 and 68.0% removal efficiencies of COD, NH3-N, and total petroleum hydrocarbon, respectively. However, most of those treatment methods are still at experimental stage and are not applicable to production yet.
MBR technology provides a solution for the efficient treatment of high salinity wastewater by biological method. Several studies regarding treating salinity wastewater using MBR methods have been carried out to evaluate the effects of membrane performance and fouling behavior [14, 16]. However, membrane fouling is an inevitable consequence of membrane operation and a key problem that hinders the utilization of membrane bioreactor process. Membrane fouling could be caused by several factors, for example, the compositions of the biological system. Among them, operating conditions are key aspects in determining the economic feasibility of MBR system [17].
In this study, an anoxic-oxic biofilm-membrane bioreactor (AOB-MBR) is used to treat mustard tuber wastewater of 10% salinity, aiming to develop an optimal condition to obtain the highest treatment efficiency at lowest membrane fouling rate. Results of this study provide a scientific basis for AOB-MBR, which is a new technical process, to be applied to treat wastewater of high salinity and high COD. The new technique is low in energy cost as well as maintenance. The application of this technique to mustard tuber industry has great meanings in China as well as the rest part of world, where low-carbon, sustainable industry operation is promoted. The operating conditions of the reactor were optimized according to the mathematic model built by Box-Behnken design in Design Expert Software based on response surface methodology. After the optimal operating condition was obtained, the wiping off effects of COD, ammonia nitrogen (NH3-N), total nitrogen (TN) and suspended solids (SS) with different influent loads were studied.
2. Materials and Methods2.1. AOB-MBR and Its Operation
Fig. 1 demonstrates the installation of the AOB-MBR system. The AOB-MBR is welded by polyvinyl chloride (PVC) plate, which is stable and reliable. The volume of the reactor is 0.65 m3, with a size of 1.0 m × 0.5 m × 1.38 m, effective water depth in the reactor is 1.3 m. The reactor consisted of three units: anoxic zone, aerobic zone and membrane zone, the ratio of the length was 2:5:3. Anoxic zone is fitted with stirring devices to ensure homogeneous distribution in the reactor. Aerobic zone is stuffed with semi-soft fiber filler, and the biofilm density was 15%. Through micro porous aeration, dissolved oxygen in aerobic zone is maintained 3–5 mg/L. This provides adequate oxygen level for microbial growth. Aerator pipes with orifice diameter of 2 to 3 mm are placed in the membrane zone. This plays a main role in providing air flow and assuring a rotational flow state in the reactor. Hollow polyvinylidene fluoride (PVDF) fiber membrane modules (Motianmo Co. Ltd., Tianjin, China), with a membrane area of 2.0 m2, are set inside the membrane zone. PVDF membrane has a 0.2-micron pore size and possesses a good hydrophobic property.
Wastewater used in this experimental study came from Fuling Mustard Tuber Group Co. Ltd, Chongqing, China. The NaCl mass fraction in the sewage ranged from 9.7% – 10.3%. The sewage typically contained COD concentrations of 3300–3900 mg/L, NH3-N at 220–240 mg-N/L and TN at 390–450 mg-N/L and SS at 445–485 mg/L, respectively.
Before the test of the performance of the AOB-MBR system, a sludge saline adaptation was performed first. This is to adapt sludge to stand the object high salinity wastewater. The inoculated sludge was taken from an aeration tank in the wastewater treatment unit in Fuling Mustard Tuber Group Co. Ltd. The adaption was completed using a stepwise process. The influent salinity was controlled at 4% at the beginning of the domestication process to reduce the shock to the sludge system. When the COD removal rate reached 80%, the system is maintained stable for 10 days. Then the influent salinity was increased by 0.5% in the next stage [18]. This process is till the influent salinity reached 10%, which is the experiment concentration in this study.
After the adaption process is completed, the reactor was then fed with mustard tuber wastewater in a timely manner. The volume of each feed event is exactly one reactor volume (0.65 m3). During the test, the transmembrane pressure is fixed. Dump pump worked 8 min continuously in each filtration cycle, which was recommended by the membrane modules manufacturer, and time interval for pump was set according to the experimental requirements. The outflow pipe of the dump pump is connected to the bucket directly. Hence, the treated water returned to the bucket in order to stabilize the water level in the reactor. Meanwhile, mixed liquid was refluxed from the aerobic zone to the anoxic zone with a reflux ratio of 200%. In addition, the mixed liquid suspended solids (MLSS) concentration of membrane zone was kept at 7000 mg/L. The change range should be no more than 10%. When the reactor ran steadily in different influent loads, continuous sampling schemes were carried out to record the concentrations of COD, NH3-N, TN and SS in the effluent.
2.2. Experiment DesignA single factor experiment was conducted previously to investigate the effect of AOB-MBR operating conditions on membrane fouling. Research found that there are three key operational parameters that affect membrane fouling rate (KV), namely time interval for pump (t), aeration intensity (UGr) and transmembrane pressure (TMP). The membrane fouling rate (KV) decreased at first, then increased with the increasing of time interval (t, 0–4 min), aeration intensity (UGr, 6–15 m3/(m2·h)), and transmembrane pressure (TMP, 10–20 kPa). The optimal values of the three parameters mentioned above were t = 3 min, UGr = 12 m3/(m2·h), TMP = 15 kPa respectively [19]. The optimal value of the parameters was obtained in a single factor experiment. Due to the interaction effect in the operating conditions, the optimal operating condition of the overall system was not just a simple combination of the optimal value of the three parameters. Response surface methodology, which is an optimization method, explores the relationships between multiple explanatory variables and one or more response variables. This is accomplished by using a sequence of designed experiments to obtain an optimal response for the overall system [20, 21].
In this study, a Box-Behnken design with three factors at three levels was applied using the Design-Expert 8.0.7.1 software (Stat-Ease Inc., Minneapolis, USA). The ranges of the three factors are shown in Table 1, where A, B, C represents t, UGr, and TMP respectively. A level of the +1, 0, −1 are used to represent the level of the three factors.
2.3. Calculation of KVThe accumulation and removal of contaminations on membrane surface is a dynamic process with lots of random influence factors. Hence it is difficult to monitor the resistance of membrane filtration at a given time. However, variation of the average filtration resistance with the cumulative filtering volume (∑V) could be considered as an index to assess membrane fouling rate (KV, m−1·L−1). The average membrane flux (Jave, L/m2·h) is calculated according to Eq. (1)
where V= cumulative filtering volume in a filtration cycle (L); A= total membrane area (m2); T= filtration cycle time (hour). Furthmore, based on Darcy’s law, average filtration resistance (Rave, m−1) could be calculated from Eq. (2):
where η= the viscosity of mixed liquor (mPa·s), measured with a NDJ-7 rotary viscosity meter. The relations
were plotted with Rave as ordinate against ∑V as abscissa, and KV was obtained as slope of the straight line.
2.4. Analytical MethodFor this study, parameters such as influent and effluent concentrations of NH3-N and TN were tested periodically and analyzed according to the standard methods in Analysis in Water and Wastewater [22]. Chlorides in the wastewater could seriously interrupt COD analysis. To reduce the impacts, an accurate and precise COD determination method was adopted. In this method, at first, AgNO3 is added equivalently according to the concentration of Cl−, and then a few amount of HgSO4 was added to prevent the free Cl− in wastewater [23]. COD was measured by potassium dichromate method using digest instrument and spectrophotometer. The concentrations of NH3-N and TN were measured by Nessler reagent method and alkaline potassium persulfate digestion ultraviolet spectrophotometric method, respectively, using a spectrophotometer. The pH was recorded using a pH analyzer. MLSS and SS concentrations were measured according to standard methods.
3. Results and discussion3.1. Response Surface AnalysisTaking the membrane fouling rate (KV) as a responsive index, a Box-Behnken test with three factors and three levels was conducted. Box-Behnken experiment plan and results are presented in Table 2. Among tests 1 to 15, repeated tests 13–15 were carried out under the same condition to evaluate errors.
Design-Expert was used to analysis results of the Box-Behnken design. A modified model equation on operating conditions can be written for the significant effects and interactions. The regression model is reduced to the significant terms and a prediction equation is written for membrane fouling rate:
As reported in Table 3, the analysis of variance (ANOVA) results indicate that A, B, C, AB, AC, BC, A2, B2, C2, A2B, A2C, AB2 are significant model terms, as indicated by the low p values (p < 0.05). Moreover, the lack-of-fit F-test indicates the variation of the data around the fitted model. If the model does not fit the data well, the lack-of-fit F-test will be significant, which suggests that there may be some systematic variation unaccounted in the hypothesis model [24]. In this case, the lack-of-fit F-test is not significant (p = 0.2432 > 0.05), implying a significant model correlation between the variables and membrane fouling rate. R2 = 0.9995 ensured a good model fit and adjusted R2 = 0.9996 showed the strong significance of the model, indicating that the equation could be used to predict KV and optimize operating conditions [25]. From the Table 3, the evident interaction effect of the AB, AC and BC on membrane fouling rate were accurately described by P value, the P value of three factors were all less than 0.01.
The regression equation derived from the statistical analysis was used to generate three-dimensional plots shown in Fig. 2. These figures demonstrate the interaction between time interval (t) to aeration intensity (UGr), time interval (t) to transmembrane pressure (TMP), and aeration intensity (UGr) to transmembrane pressure (TMP), respectively.
The three-dimensional graph shown in Fig. 2(a) was developed as a function of the UGr and t, while the TMP was held constant at 15 kPa, which was the middle value. The membrane fouling rate (KV) decreased at first, and then increased with the increasing of time interval (t). A similar trend was observed when UGr increased, indicating there is a strong correlation between UGr and t. With increasing aeration intensity (UGr), the cross flow velocity on the membrane surface increased. It could be observed that bubble flow scours membrane foulants under this condition. This reduced filtration resistance within the membrane which promotes good membrane flux. However, if the aeration intensity is too high, it may disrupt the flocs due to shear stress. This leads to plugged membrane pores. At the same time, aeration time showed similar trend with membrane flux. Thus, it is necessary to find a good equilibrium between UGr and t, which could control pollution and decrease cost simultaneously [26].
Based on an overall analysis of the operational conditions, an optimal value was obtained using the Design-Expert, KV reached a minimum of 1.49 × 1011 m−1L−1 at t = 3.90 min, UGr = 14.57 m3/(m2·h), and TMP = 19.09 kPa. To simplify the operation process, the original three optimal values were adjusted to t = 4.0 min, UGr = 14.6 m3/(m2·h), TMP = 19.0 kPa, the corresponding predicted value of KV was 2.07 × 1011. Three parallel tests were carried out under the adjusted optimal operating condition. Then the predicted and actual KV were compared to verify the correlation of regression equation. The average KV of the parallel tests was 1.87 × 1011 ± 1.9 × 1010, which agrees well with the calculated result. Hence the regression Eq. (4) could be used to predict KV value in the range of experimental variables.
3.2. Treatment Efficiency of AOB-MBRIndicators of treatment efficiency of AOB-MBR include removal rate of COD, NH3-N, TN and SS. These indicators were tested when the reactor reached a steady state under the optimal condition, i.e. t = 4.0 min, UGr = 14.6 m3/(m2·h), TMP = 19.0 kPa. Then the treatment efficiency of AOB-MBR under different influent loads, controlled by changing HRT were compared (Table 4).
COD removal rateWhen the organic load was set at 1.0 and 1.9 kg COD/(m3·d) separately, with an influent COD concentration of 3600 mg/L, the average effluent COD concentration was 245.8 mg/L and 350.0 mg/L respectively. The effluent water quality met the third discharge standard of “Integrated Wastewater Discharge Standard”, which was lower than 500 mg/L. Table 4 compared COD removal efficiency under influent load 1.0 kg COD/(m3·d) and 1.9 kg COD/(m3·d). It shows that the removal rate under 1.9 kg COD/(m3·d) declined slightly by 2.9%. This shows AOB-MBR has a high flexibility with respect to fluctuating loading rates. Wang et al. [27] used SBR process to treat seawater with 10.5% salinity, when HRT was 12 h and the organic load was 0.3–1.0 kg COD/(m3·d), the corresponding COD removal efficiency was about 87%. In contrast, membrane bioreactor has an ability to withstand relatively high loading rates. Moreover, comparing to the conventional activated sludge process (ASP), the high number of microorganism and the effectiveness of the membrane process in MBR contribute to the overall depurative process [16].
When the organic load was set at 3.3 kg COD/(m3·d), the average effluent COD concentration was 518.5 mg/L which results in a COD removal efficiency of 85.6%. However, the effluent quality fluctuated obviously and exceeded the third discharge standard of “Integrated Wastewater Discharge Standard”. This observation implies that with the increase of substrate concentration, the refractory compounds in the mustard tuber wastewater were increased. This could probably have affected the biodegradation efficiency. Thus the excessive organic matter was not degraded effectively, which leads to a decline of effluent water quality.
In the Three-Gorge Reservoir, pickled mustard tuber factories are usually located in a food industrial park. Therefore, according to the Chinese Government, wastewater from a food industrial park needs centralized collection and treatment before discharge. To meet the water quality standards for discharge to municipal sewers, which is the third discharge standard of the “Integrated Wastewater Discharge Standard”. The AOB-MBR described in this paper could be implemented in the pickled mustard tuber factory, with at an organic load less than 1.9 kg COD/(m3·d), The effluent could then be discharged to municipal sewers system and then treated in a wastewater treatment plant.
NH3-N removal rate
Table 4 shows the wiping off effect of ammonia nitrogen with different influent load. When the organic load was set at 1.0 kg COD/(m3·d). The average effluent ammonia concentration was 19.1 mg/L, which results in an ammonia removal efficiency of 91.7%. The removal of ammonia in AOB-MBR was a result of microbial metabolism and biodegradation more than membrane interactions. A variety family of microorganism was discovered in the reactor by microscope. A long sludge retention time (SRT) would satisfy the growth of nitrobacteria. The heterotrophs in suspension had the advantage of competition growth to nitro-bacteria, while in biofilms the heterotrophs growth was at a disadvantage, due to the diffusion limitations of substrates in the biofilms. Therefore, nitrobacteria were not out-competed in the biofilms by heterotrophs. Thus, biofilms with high nitrifying activity could be obtained [28]. Moreover, mixed liquid from aerobic zone was back flowed to anoxic zone. This would increase the efficiency of nitrification reaction by reducing nitrates and nitrites concentrations accumulated in the aerobic zone.
When the influent load was at 1.9 and 3.3 kg COD/(m3·d), the average effluent ammonia concentration was 17.4 and 20.2 mg/L separately. The corresponding removal rate of NH3-N was 92.4% and 91.2% respectively. As shown in Table 4, no significant difference in the ammonia removal efficiency was observed between the three cases. This means the increase of the influent load has little influence on the ammonia removal rate. Our analysis suggests that a new biofilm MBR system, which is a biofilm reactor combined with a MBR, has a high nitrogen removal capacity and efficiency. This result is in good agreement with previous experimental investigations, reporting the biofilms with high nitrifying activity [17, 29]. Also, inorganic salts (e.g., Cl−, SO42−, Na+, Ca2+) lower settling performance of sludge, which is good for oxygen transmission and ensures an oxygen-affluent environment for the growth of nitrobacteria.
TN and SS removal rateThree influent loads, i.e. 1.0, 1.9 and 3.3 kg COD/(m3·d), were experimentally compared at the same influent TN concentration of 420 mg/L. As shown in Table 4, the average effluent TN concentration of these three cases are nearly the same, with the effluent TN concentration 161.7, 161.1, 166.3 mg/L and the corresponding TN removal efficiency was 61.5%, 61.6% and 60.4%.
A high effluent TN concentration indicated that a weak denitrifying ability of AOB-MBR. Considering the refractory compounds in the mustard tuber wastewater, a relatively high effluent TN concentration was probably caused by insufficient carbon that is required for denitrification in anoxic zone [30]. In addition, the reflux ratio of mixed liquid from aerobic zone would remarkably affect nitrogen removal efficiency. In general, it is more efficient when the reflux ratio at the high ration condition [31]. Therefore, research regarding the influences of mixed liquid reflux ratio and the external carbon supply in the AOB-MBR should be a priority.
The strong adsorption filtration capability of AOB-MBR is verified by the low effluent SS. Table 4 shows that the average effluent SS concentration in the three cases was relatively steady without significant change. The effluent SS concentration is 8.4, 9.0, 11.0 mg/L respectively.
4. ConclusionsOperating conditions of the anoxic-oxic biofilm-membrane bio-reactor (AOB-MBR) treating 10% salinity mustard tuber wastewater was optimized by using the response surface methodology. The treatment efficiency under the optimal operating condition was investigated.
The optimal condition can be defined as follows: t = 4.0 min, UGr = 14.6 m3/(m2·h), TMP = 19.0 kPa and the corresponding KV value was 2.07 × 1011. It could alleviate membrane fouling and prolong the lifespan of membrane in the system. Treatment efficiency study found: when the reactor influent load was less than 1.9 kg COD/(m3·d), the effluent can meet the third discharge standard of “Integrated Wastewater Discharge Standard”, and the removal rate of COD, NH3-N, TN and SS was more than 90.3%, 92.4%, 61.6% and 98.1% respectively. The effluent could be discharged into sewers then treated in a wastewater treatment plant.
AcknowledgmentsThe work reported here was financially supported by the China National Science Foundation Program (Grant Number: 51008318) and the 111 Project, No.B13041.
References1. Chai H, Kang W. Influence of biofilm density on anaerobic sequencing batch biofilm reactor treating mustard tuber wastewater. Appl Biochem Biotechnol. 2012;168:1664–1671.
2. Park JH, Li XF, Edraki M, Baumgartl T, Kirsch B. Geochemical assessments and classification of coal mine spoils for better understanding of potential salinity issues at closure. Environ Sci Proc Impacts. 2013;15:1235–1244.
3. Hu Q, Hu S. Effects of salinity on performance of membrane bioreactor for wastewater treatment. Environ Pollut Contr. 2012;34:60–63. 71
4. Jang D, Hwang Y, Shin H, Lee W. Effects of salinity on the characteristics of biomass and membrane fouling in membrane bioreactors. Bioresource Technol. 2013;141:50–56.
5. Lefebvre O, Moletta R. Treatment of organic pollution in industrial saline wastewater: A literature review. Water Res. 2006;40:3671–3682.
6. Vallero MVG, Hulshoff Pol LW, Lettinga G, Lens PNL. Effect of NaCl on thermophilic (55C) methanol degradation in sulfate reducing granular sludge reactors. Water Res. 2003;37:2269–2280.
7. Uygur A. Specific nutrient removal rates in saline wastewater treatment using sequencing batch reactor. Process Biochem. 2006;41:61–66.
8. Jeison D, Kremer B, van Lier JB. Application of membrane enhanced biomass retention to the anaerobic treatment of acidified wastewaters under extreme saline conditions. Sep Purif Technol. 2008;64:198–205.
9. Boopathy R, Bonvillain C, Fontenot Q, Kilgen M. Biological treatment of low-salinity shrimp aquaculture wastewater using sequencing batch reactor. Int Biodeterior Biodegrad. 2007;59:16–19.
10. Fontenot Q, Bonvillain C, Kilgen M, Boopathy R. Effects of temperature, salinity, and carbon: nitrogen ratio on sequencing batch reactor treating shrimp aquaculture wastewater. Bioresource Technol. 2007;98:1700–1703.
11. Tsuneda S, Mikami M, Kimochi Y, Hirata A. Effect of salinity on nitrous oxide emission in the biological nitrogen removal process for industrial wastewater. J Hazard Mater. 2005;119:93–98.
12. Aloui F, Khoufi S, Loukil S, Sayadi S. Performances of an activated sludge process for the treatment of fish processing saline wastewater. Desalination. 2009;246:389–396.
13. Zhou J, Gan C, Long T, Chai H. Research on efficiency of anaerobic sequencing batch biofilm reactor for hypersalt mustard tuber wastewater treatment. China Water Waste. 2006;22:77–80.
14. Shi XQ, Lefebvre O, Ng KK, Ng HY. Sequential anaerobic-aerobic treatment of pharmaceutical wastewater with high salinity. Bioresource Technol. 2014;153:79–86.
15. Lu M, Zhang Z, Yu W. Biological treatment of oilfield-produced water: A field pilot study. Int Biodeterior Biodegrad. 2009;63:316–321.
16. Di Bella G, Di Trapani D, Torregrossa M, Gaspareet V. Performance of a MBR pilot plant treating high strength waste-water subject to salinity increase: Analysis of biomass activity and fouling behaviour. Bioresource Technol. 2013;147:614–618.
17. Di Trapani D, Di Bella G, Mannina G, Torregrossa M, Gaspare V. Comparison between moving bed-membrane bioreactor (MB-MBR) and membrane bioreactor (MBR) systems: Influence of wastewater salinity variation. Bioresource Technol. 2014;162:60–69.
18. Zhou J, Wu Q, Long T, Wang X. Establishment of microbiological system for treatment of mustard tuber wastewater with high salinity. China Water Waste. 2007;23:17–20. 25
19. Wei Y. Study on the membrane fouling characteristics and treatment efficiency of the membrane bioreactor treating mustard tuber wastewater with high salinity. MASTER Chongqing U. 2013;
20. Zheng H, Jiao S, Deng X, Feng L, Zhang H, Chen R. Optimization of preparation and application of PPFS by response surface methodology. Chinese J Environ Eng. 2012;6:9–14.
21. Rokhina EV, Sillanpaa M, Nolte MCM, Virkutyte J. Optimization of pulp mill effluent treatment with catalytic adsorbent using orthogonal second-order (Box-Behnken) experimental design. J Environ Monit. 2008;10:1304–1312.
22. APHA. Standard Methods for Water and Wastewater Examination. Washington: American Public Health Association; 2005.
23. Guo J, Lin J, Fang F, Zhu Y, Bao Z. The chloride mask in COD determination of pickled mustard wastewater with high salt. J Chongqing U. 2014;37:117–122.
24. Muhamad MH, Abdullah SRS, Mohamad AB, Rahman RA, Kadhum AAH. Application of response surface methodology (RSM) for optimisation of COD, NH3-N and 2,4-DCP removal from recycled paper wastewater in a pilot-scale granular activated carbon sequencing batch biofilm reactor (GAC-SBBR). J Environ Manage. 2013;121:179–190.
25. Qiu Z, Aita GM, Mahalaxmi S. Optimization by response surface methodology of processing conditions for the ionic liquid pre-treatment of energy cane bagasse. J Chem Technol Biot. 2014;89:682–689.
26. Yang X, Wang S, Lu N. Optimum aeration strength and its influencing factors for membrane fouling controll in an integrated membrane bioreactor. Technol Water Treat. 2006;32:17–19.
27. Wang Z, Wu Z. Present situation and prospect of the biological treatment of wastewater with high salinity. Ind Water Treat. 2002;22:1–4.
28. Artiga P, Toriello GG, Mendez R, Garrido JM. Use of a hybrid membrane bioreactor for the treatment of saline wastewater from a fish canning factory. Desalination. 2008;221:518–525.
29. Oyanedel V, Campos JL, Garrido JM, Lazarova V, Méndez R. Development of a membrane-assisted hybrid bioreactor for ammonia and COD removal in wastewaters. J Chem Technol Biot. 2005;80:206–215.
30. Chai H, Chen W, He Q, Zhou J. Effects of volumetric load in an anaerobic sequencing batch biofilm treating industrial saline wastewater. Environ Technol. 2015;36:648–653.
31. Li Y, Zhou S, Qiu Y, Wu S. Effect of Mixed-liquid Return Ratio on A2/O Process Performance. Chinese Environ Sci Technol. 2010;33:142–145.
Fig. 1The diagram of process flow.
1: Bucket, 2: Feed pump, 3–4: Flowmeter, 5: Stirrers, 6: Semi-soft filler, 7: Reflux pump, 8: Membrane module, 9: Vacuum gauge, 10–11: Air pump, 12: Dump pump.
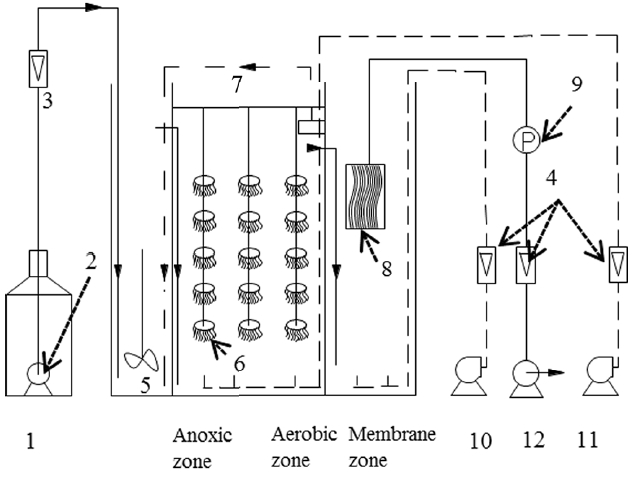
Fig. 2Response surface for KV as a function of (a) UGr and t (TMP=15 kPa), (b) TMP and t (UGr =12 m3/(m2·h)), and (c) TMP and UGr (t = 3 min). 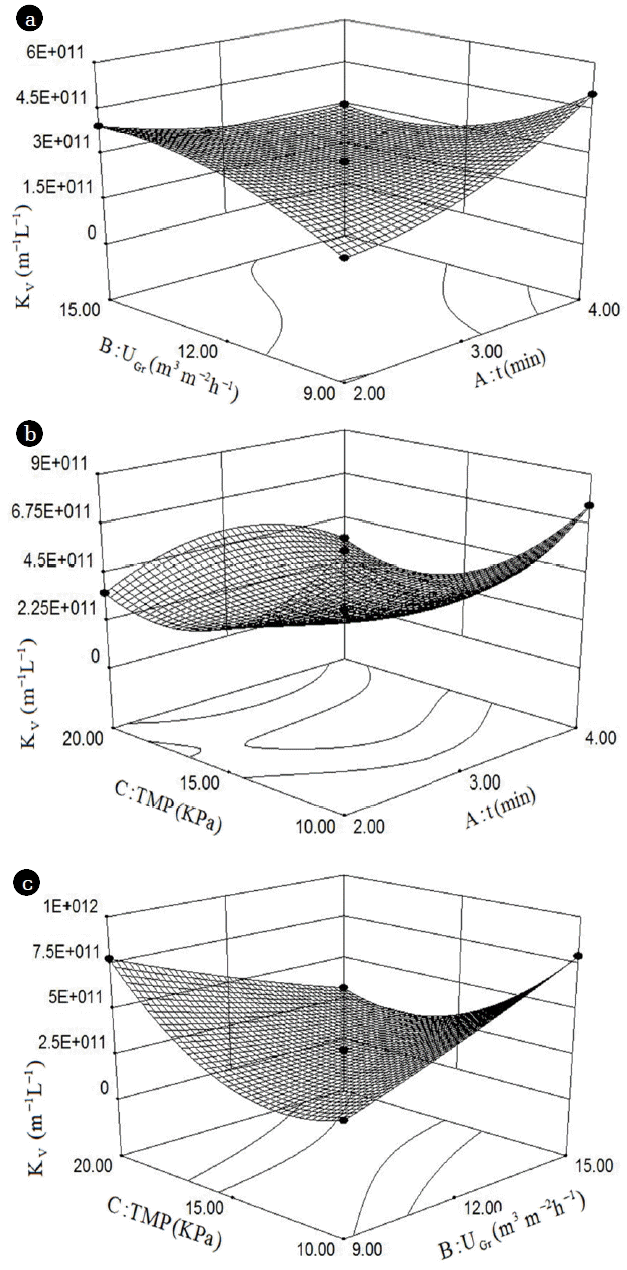
Table 1Levels of Factors
Table 2Box-Behnken Design and Experimental Results Table 3ANOVA for Membrane Fouling Rate (KV) Table 4Treatment Efficiency of AOB-MBR |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||