1. Introduction
Atmospheric particles are source of several public health problems in the world and in the developing countries particularly, where urban environment protection policies had not followed the high demographics and urbanization of cities. Particulate matter (PM) is a mixture of solid and liquid substances of organic and inorganic character suspended in air. Those common air contaminant vary in terms of origin, chemical composition and size. Particles, especially PM < 2.5, often contain highly toxic polycyclic aromatic hydrocarbons (PAH), polychlorinated dibenzo-p-dioxins and dibenzofurans (PCDD/Fs), polychlorinated biphenyls (PCBs) and heavy metals, making them more hazardous and carcinogenic [1, 2]. Indeed previous studies point to a causal association between population exposure to PM in air and cardiovascular and lung cancer mortality [3, 4]. Previous studies performed in West African countries like Ghana [5], Guinea [6], Senegal [7], Benin [3] and Cape Verde [8] showed an important atmospheric PM concentration and by far exceeded the WHO limits (25 μg·m−3 daily for fine PM and 50 μg.m−3 daily for PM diameter > 10 μm) [9].
Plants play an important role in filtering ambient air by adsorbing PM onto leaf surfaces. Trees have larger leaf areas than herbaceous; moreover, their woody structure (stem, branches) and height result in a larger roughness, and thus deposition, than on herbaceous plants [10, 11]. The structure of tree crowns leads to turbulent air movements, which increase PM deposition on leaves [10]. Therefore, trees are generally more effective in capturing atmospheric particles than herbaceous plants [12]. Some species-specific features of leaves may strengthen this air filtration process, e.g., trichomes [13] and the chemical composition and structures of epicuticular waxes [14].
Several studies have shown a high correlation between leaf Saturation Isothermal Remanent Magnetization (SIRM) and atmospheric PM [15–17, 12]. Biomagnetic PM monitoring of leaves can, therefore, provide an easy and inexpensive way for monitoring the spatial and temporal distribution of atmospheric PM in urban environments [17], and thus offer an ideal tool for (spatial) PM assessment in low income countries [12]. Little work has been devoted to the differences between species in PM accumulation in tropical area. Such information is important, especially in view of the large number of tree and shrub species and cultivars being used in urban areas. The choice of species and planting design could have a major influence on the PM filtering performance of urban vegetation. For instance, leaf hydrophobicity, the repellency of a water droplet on a leaf surface is a functional trait that allows the plant to shed water from leaf surfaces to enhance photosynthesis, to decrease disease, and to prevent leaf damage from pollution [18–20]. Leaf surface wettability plays an important role in a plant’s ability to capture particulate pollution [21, 22]. Waxy cuticles and outgrowths on the leaf surface, such as trichomes, increase their hydrophobicity and facilitate the removal of water [23, 24]. The relation between leaf surface contamination with particles and leaf wettability was investigated in temperate regions. Kardel et al. [25] showed a significant influence of habitat on leaf wettability of Quercus robur and Sambucus nigra; leaf wettability was significantly lower in the industrial than in the semi-natural areas. For Neinhuis and Barthlott [21] leaf surfaces of Ginkgo biloba, having a low wettability, accumulate fewer particles compared to Fagus sylvatica and Q. robur, both have high-wettability.
Tropical areas are regions of high rain even often unevenly distributed. Some particles that are captured can later be washed off from leaves by rain. The use of PM into and onto leaves through leaf SIRM as an indicator of air quality may be biased by species choice and by rain preceding sampling.
The aim of this study is to evaluate: (1) the total leaf PM and the leaf-encapsulated PM; (2) the quantitative relationship between the amount of PM captured and leaf wettability. For this, leaves were sampled in two contrasting areas (Main roads and Parks). The total leaf SIRM and leaf SIRM encapsulated were specified by means of contact angle of water drop and sessile leaf surface. Finally, the relationship between leaf SIRM (total and encapsulated) and leaf wettability was found.
2. Materials and Methods
2.1. Study Area
The study was conducted in Abidjan, the largest city in Ivory Coast or Côte d’Ivoire (5°00′-5°30′ N, 3°50′- 4°10′W) in West Africa. The city has a main automobile fleet constituted in majority of second hand vehicles. All official transport vehicles (about 30,000 in 2010) had diesel engines contain a significant amount of sulphur compared to industrialized countries [25]. The city of Abidjan also contains several parks of which a national park (Banco National Park), a botanical garden, a municipality plants nursery and a floristic center. In these green areas, human influence was relatively weak and activities of pollution were more controlled relatively to road traffic. Climate is tropical, hot and humid with a long rainy season from May through July, a small rainy season (September–November) and two dry seasons in between.
2.2. Sampling Design
Leaf sampling was performed during two campaigns in April and May 2014 in two habitats: Main roads and Parks. No rain events occurred 5 days prior to the sampling campaign and during the campaign itself. Main roads were composed of two busy roads ie: Lagoon Boulevard and North Highway, where traffic intensity was larger than 6000 vehicles per hour in rush-hour traffic [12].
Two sampling location were on each road. The coordinates of these sites are:
05°20.944′N - 04°00.828′W and 05°19.866′N - 04°01.127′W on Lagoon Boulevard, and 05°21.813′N - 04°04.982′W and 05°21.559′N - 04°03.984′W on North Highway. Leaf sampling was also conducted in two parks: municipal plant nursery (05°26.24′N - 03°59.352′ W and 05°26.218′N - 03°59.361′W and botanical garden (05°22,18′N - 03°53.28′W and 05°21.85′N - 03°53.01′W). In these green areas, human influence is relatively weak and pollution activities were most controlled relatively to the road traffic.
2.3. Plant Species Characteristics
The study was performed on three ornamental species used in Abidjan, i.e. Barleria prionitis L. (Acanthaceae), Ficus benjamina L. (Moraceae). Jatropha interrigima Jacq. (Euphorbiaceae). These plants were grown in 13 litres pots with compost and soil during 3 months in a municipal garden (municipal plant nursery) relatively far from any source of motor vehicle and industrial pollution. After three months of growth, two pots of each study species, which reached a height of about 1 m, were placed side-by-side in the selected sites on Main roads and Parks, separated from one another of 1 m. In each habitat, these plants have remained exposed to ambient air for three consecutive months.
Principal leaf surface characteristics are summarized in Table 1. B. prionitis is an erect, prickly shrub, usually single-stemmed, growing to about 1.5 m tall. The stems and branches are stiff and smooth and light brown to light grey in colour. Leaves are elliptic to oblong, 3 to 10 cm long and 1.5 to 4 cm broad. The base of the leaves is protected by three to five sharps, pale coloured spines. F. benjamina is a tropical evergreen tree reaching a height rarely exceeds 3 m when used as an ornamental plant. Leaves are glossy, oval of 6–13 cm long, with an acuminate tip. J. interrigima is an evergreen shrub or small tree with glossy leaves and densely hairy leaves when young. The plant has a rounded or narrow domed form and gets up to 4 m tall with a spread of 3 m or so, although in cultivation it is usually smaller. Leaves are extremely variable; they may be entire and elliptic or oval, or they may be fiddle shaped, or they may have three sharp pointed lobes.
2.4. Biomagnetic Monitoring
2.4.1. Leaves sampling
At each sampling location, and for each species, 6 mature and 6 young undamaged leaves were collected on the same plant and carefully placed in paper envelopes. Each group of leaves was separated into halves (2 × 3-mature leaves and 2 × 3-young leaves). Leaf area of each part was quantified with Image J software after scanning the leaves in the laboratory soon after sampling. One group of mature and young leaves was directly dried at ambient temperature. The other groups of mature and young leaves were washed manually with distilled water and dried at ambient temperature. Samples were thus divided in washed and unwashed leaves to distinguish leaf-accumulated and leaf-encapsulated particles.
In total, at each sampling location, and for each species, 4 groups of leaves were collected and consisted of 3 mature unwashed leaves, 3 mature washed leaves, 3 young unwashed leaves and 3 young washed leaves.
2.4.2. Leaf SIRM determination
After drying, group of 3 dried leaves were tightly packed together by cling film, avoiding the movement of any leaf parts and pressed in a 10 cm3 plastic container. Following the protocol of Matzka and Maher [27] and Kardel et al. [17], these containers were magnetized with a pulsed magnetic field of 1 T using a Molspin pulse magnetiser (Molspin Ltd, UK) and the “Saturated Isothermal Remnant Magnetism” (SIRM) was determined using a Molspin Minispin magnetometer with high sensitivity (0.1 × 10−10 A·m2). Each sample was measured twice to avoid measurement errors. After each ten measurements, the magnetometer was calibrated using a magnetically-stable rock specimen, as described by Mitchell et al. [16]. The measured SIRM values were normalized for the sampling pot volume (10 cm3) and the washed and unwashed leaf area (cm2) which leads to a SIRM-value expressed as A.
2.5. Leaf Wettability
Contact angles (in degree) of standardized water droplets with a leaf surface (or drop contact angle or DCA) can be used as a proxy for leaf wettability. This parameter indicates the hydrophobicity of the leaf surface determined by the physical and chemical composition of the cuticle [28]. A larger contact angle signifies a more hydrophobic leaf surface. In practice, following criteria picked up by Aryal and Neuner [29], leaves were termed; “super-hydrophilic” if DCA < 40°; “highly-wettable” if 40° < DCA < 90°; “wettable” if 90° < DCA < 110° [30]; “non-wettable” if 110° < DCA < 130°; “highly non-wettable” if 130° < DCA < 150° [31]; and super-hydrophobic if DCA > 150° [32]. Eight mature and undamaged leaves of B. prionitis, F. benjamina and J. interrigima were collected at Main roads and in Parks and transported to the laboratory with the petiole wrapped in wet paper. Images were taken in laboratory conditions with a Canon EOS 550D digital camera and macro objective (EF-S 18–55 mm f/3,5-5,6 IS) after placing a drop of 7.5 μl distilled water with a micropipette on leaf surface. This operation was repeated on the right and left sides and on the adaxial and abaxial surfaces of leaves. Drop contact angles were measured using a manual method described by Kardel et al., [25] with Image software as the average of the contact angles on the left and right side of the drop from the adaxial (DCAad) and abaxial (DCAab) leaf surface.
2.6. Drop Asymmetry
Leaf surface heterogeneity, which may be related to heterogeneity in leaf surface topology, wax deterioration or erosion [25], was estimated by the dimensionless drop asymmetry (DA) as a proxy for intra-leaf variability in DCA:
DCAR and DCAL are the angles measured at the right and left side of the drops, respectively.
2.7. Statistical Analysis
Statistical analysis was performed with Statistica 6.1 (Stat Soft. Inc, France). Leaf SIRM comparison between young and mature leaves, washed and unwashed leaves as well as comparison of DCA and DA on the adaxial and abaxial leaf surface and between Main roads and Parks were made using Student t test. Analyses of variance (ANOVA) with Tukey’s honestly significant difference (Tukey-HSD) were applied to determine significant differences in DCA and DA on adaxial and abaxial surface of three study species. Pearson’s correlation analysis was conducted to test the relationship of leaf SIRM with DCA and DA. A given effect was assumed significant at p < 0.05. Magnetic measurements were carried out at the Laboratory of Environmental and Urban Ecology of the Department of Bioscience Engineering (University of Antwerp, Belgium).
3. Results
3.1. Leaf SIRM in Habitats
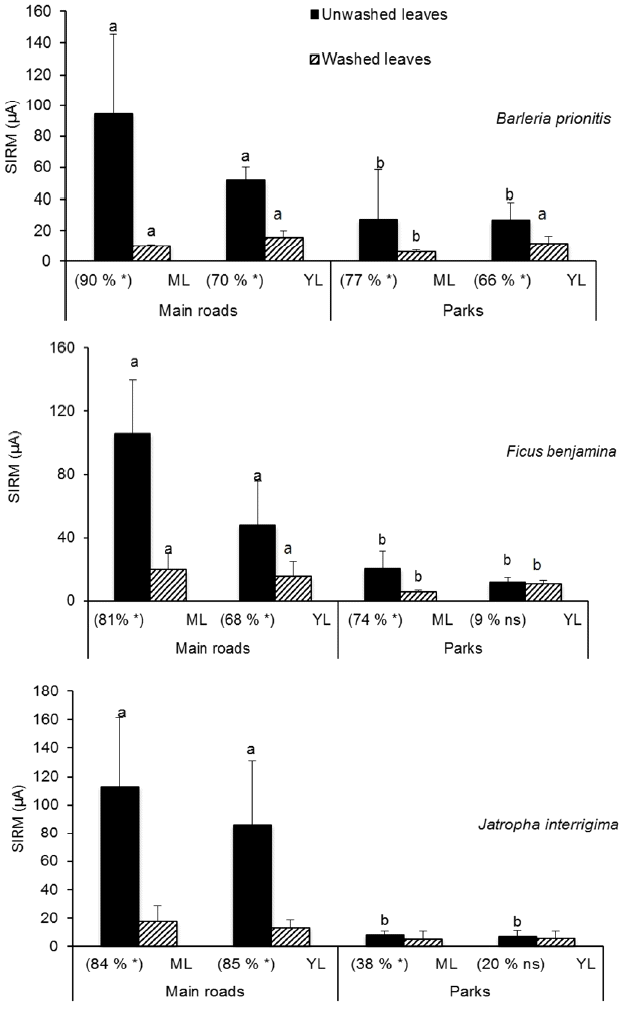
Fig. 1 shows leaf PM amounts from B. prionitis, F. benjamina and J. interrigima at Main roads and in Parks. In all species, SIRM of mature and young leaves was higher on Main roads than in Parks (p < 0.05). Thus, leaf SIRM of mature leaves ranged from 94 μA, 106 μA and 113 μA at roadsides respectively from B. prionitis, F. benjamina and J. interrigima, against 27 μA, 20 μA and 8 μA in Parks. In young leaves, leaf SIRM varied between 86 μA (J. interrigima) and 48 μA for F. benjamina at roadsides whereas their maximum was only 26 μA in Parks.
3.2. SIRM from Young and Mature Leaves
Leaf SIRM is higher on mature leaves than on young ones on Main roads. SIRM was thus 1.80, 2.21 and 1.31 times higher in mature leaves than in young leaves respectively in B. prionitis, F. benjamina and J. interrigima at roadside (Fig. 1). However, there are no significant differences (p > 0.05) between SIRM of young and mature leaves in Parks in samples that have undergone the same treatment (washed or unwashed) (Fig. 1, Table 2).
Table 3 showed p values of unwashed and washed leaves compared in different habitats. For example in Main roads, SIRM of unwashed mature leaves of F. benjamina was always statistically higher (p < 0.05) than mature washed ones and young leaves (washed or unwashed) also. By contrast, young washed leaves of J. interrigima sampled in Parks were not statistically different (p > 0.05, Table 3) than mature and young unwashed ones.
3.3. Wash-off of Leaf SIRM
The wash-off of leaf SIRM varied strongly between species and between leaf age and habitat (Fig. 1). In Main roads, leaf SIRM is statistically lower after the washing (p < 0.05); The wash-off rates were generally over 70%. 19% in mature leaf SIRM and 32% in young leaves. F. benjamina was the plant species that encapsulates most leaf SIRM among study species at roadsides. In Parks, SIRM of washed and unwashed leaves were statistically different in mature leaves. The largest loss of leaf SIRM was observed with B. prionitis (77%, p < 0.05) and the lowest with J. interrigima (38%, P < 0.05) from mature leaves. However, from young leaves in Parks, there was no significant difference between SIRM of washed and unwashed leaves from F. benjamina and J. interrigima.
3.4. Interspecific SIRM Variation
There were no significant differences between interspecific leaf SIRM of unwashed mature leaves (Table 2). Regarding unwashed young leaves, J. interrigima leaf SIRM (86 μA) was statistically higher than that obtained with B. prionitis (52 μA). When leaves were washed, the encapsulated SIRM of B. prionitis (5 μA) and F. benjamina (5 μA) in Parks, were statistically higher than those encapsulated by J. interrigima (2 μA) (hairy species) (Table 2). For other treatments and other types of leaves, no significant differences were found between leaf SIRM encapsulated by the three species.
3.5. Drop Contact Angle
The lowest mean of DCA for adaxial (71.0°) and abaxial (65.9°) leaf surface were observed with B. prionitis at roadsides and Parks (ANOVA Tukey test) (Table 4). The highest DCA were found on F. benjamina adaxial and abaxial leaf surface in both investigated habitats. On adaxial surface, these angles vary from 80.9°–83.4° in Main roads and 63.2° – 65.7° in Parks. On abaxial surface, DCA were ranged from 83.2° – 84.6° in Main Roads and 65.6° – 76.5° in Parks. Any significant differences (t test; p > 0.05) were obtained between DCA mean of these two species from adaxial and abaxial leaf surface considered.
At the intraspecific level, DCA at roadsides were always higher than those obtained in Parks (t test; p < 0.05). Any significant change was found in adaxial and abaxial surface DCA at Main roads (t test; p > 0.05). It is the same trend in Parks in B. prionitis and J. interrigima. However, in this habitat, DCA on the abaxial surface is greater than on adaxial surface (p < 0.05) (Table 4).
3.6. Drop Asymmetry
The highest values of Drop Asymmetry (DA) were observed on leaf surfaces of B. prionitis (Table 5): 17.2 and 26.0 respectively from adaxial and abaxial surfaces on Main roads and 7.6 and 18.5 in Parks. Lower DA values were obtained on both waxy surface of F. benjamina vary between 7.6–11.2 at Main roads and 10.8–16.6 in Parks.
Habitat significantly influenced the adaxial DA of B. prionitis and the abaxial DA of J. interrigima (test t; p < 0.05) (Table 5). Any habitat influence was observed on both surface of F. benjamina leaves and no significant differences (t test; p > 0.05) were also obtained between mean adaxial and abaxial DA for all species in habitats (Table 5).
3.7. Relationship between Total Leaf SIRM, Leaf-Encapsulated SIRM, DCA and DA
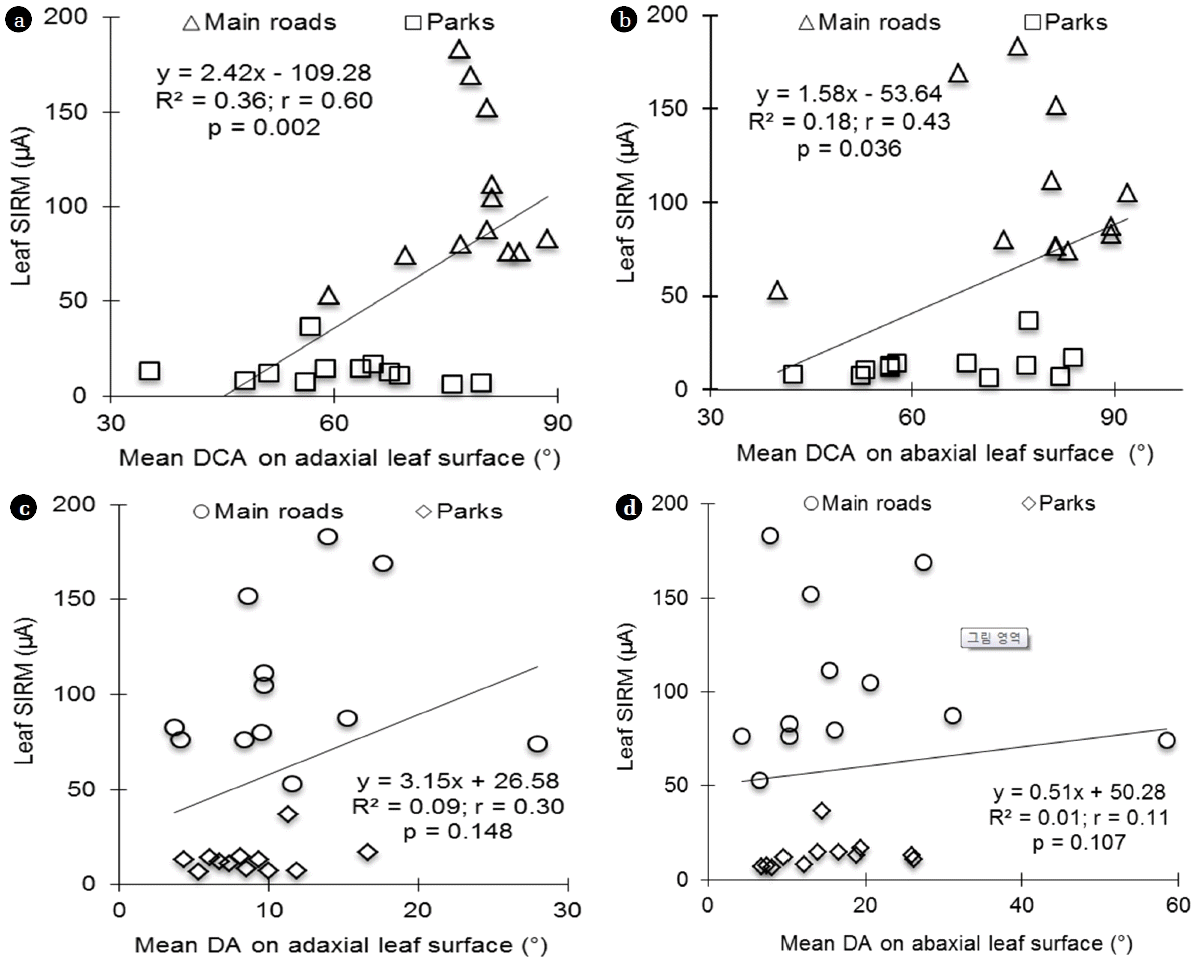
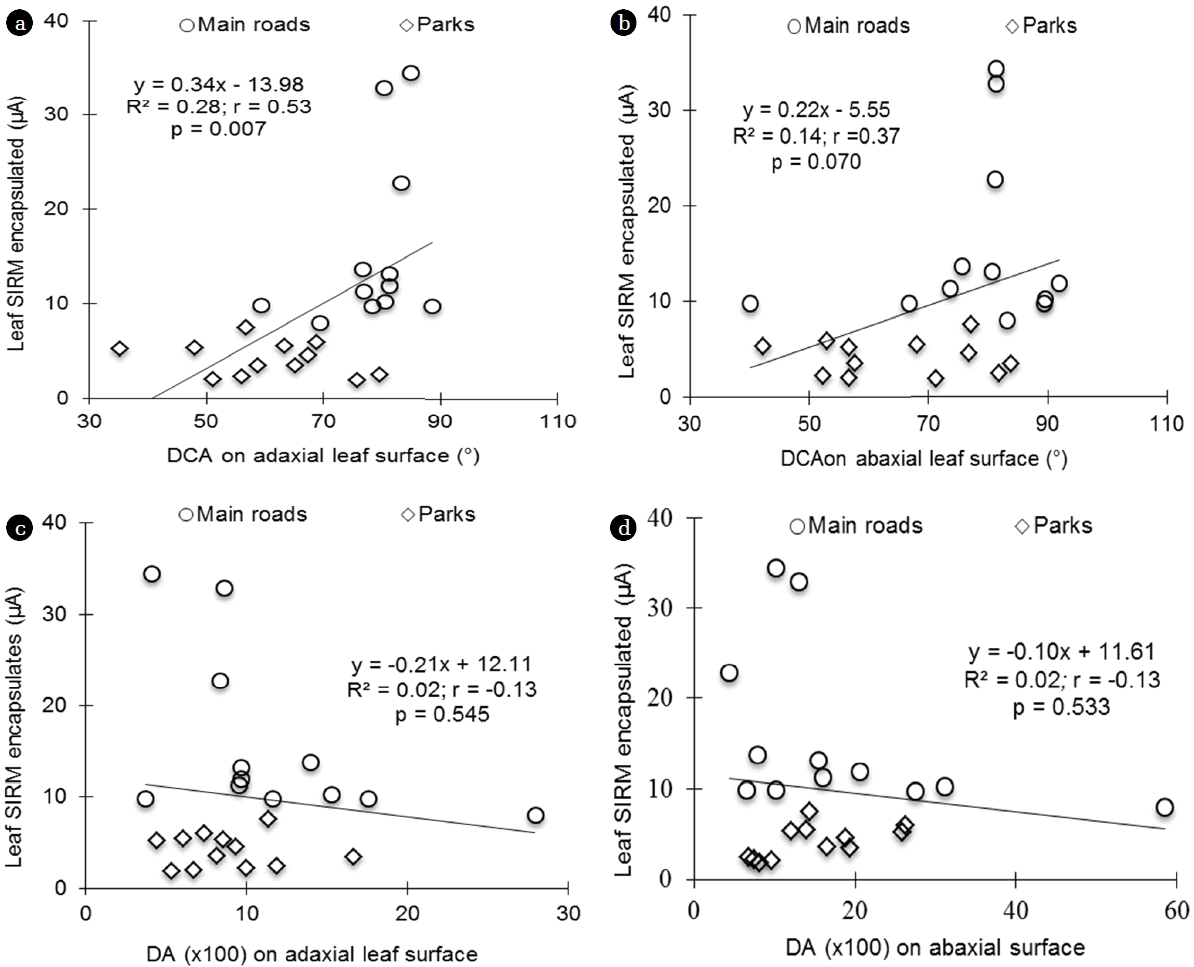
From the three investigated plant species, a significantly positive correlation was found between drop contact angles on adaxial and abaxial surfaces and leaf SIRM (Fig. 2, (a) and (b)) and leaf-encapsulated SIRM (Fig. 2, (a) and (b)). Pearson’s correlation coefficients were r = 0.60 and r = 0.43 respectively in adaxial (Fig. 2(a), p = 0.002) and abaxial (Fig. 2(b); p = 0.036) leaf SIRM. In leaf-encapsulated SIRM, r = 0.53 and r = 0.37 were respectively on adaxial (Fig. 2(a), p = 0.007) and abaxial (Fig. 2(b); p = 0.070) surfaces. No significant correlations were found between total leaf SIRM or leaf-encapsulated SIRM and the drop asymmetry (Fig. 3, p > 0.05).
4. Discussion
4.1. Effect of Habitat on Leaf SIRM
Results of this study showed leaf SIRM was higher at roadsides than in Parks (Fig. 1, Table 2). These results confirm that Main roads were potentially more polluted than Park as it has been already demonstrated in a previous study in Abidjan [12]. This result obtained in a tropical country also confirms those obtained in temperate areas by Weijers et al. [33]; Serbula et al. [34]; Kardel et al. [17]; Hofman et al. [35]. These authors showed that air quality was better within parks and worsened when approaching roads suggesting that the main sources of pollution determined with SIRM were car exhaust [36, 25]. The habitat quality in Main roads might, therefore, be considered to be low compared to Parks as already demonstrated in several studies like Cavanagh et al. [37], Mitchell and Maher [38], Serbula et al. [34], Dias et al. [39] and Koffi et al. [40]. As a matter of fact, air quality was better within parks (urban background) due to the absence of motorised vehicles [12].
4.2. SIRM of Mature and Young Leaves
Leaf SIRM is higher on mature leaves than on young leaves in Main roads. This result may suggest a particle accumulation in leaves over time as already observed by Mitchel et al.[16], Kardel et al. [17], Rodriguez-Germade et al. [41] and Hofman [42]. All things considered, pollutants particles (determined from the SIRM) appear gradually settled on the leaf surface until dynamic equilibrium between particle deposition and particle loss is reached; this equilibrium depends on the species [16]. J. interrigima leaf area (34.29 cm2) and hair density (120 hairs·cm−2) were the highest among species tested (Table 1); leaf SIRM was (mathematically) higher than the other species studied (Table 2), even if these differences were not significant. The complexity of J. interrigima leaf surface would make this species most likely to intercept air pollutants than B. prionitis and F. benjamina. Indeed, studies have shown that leaves with complex shapes, ridged surface fine hairs or emitting sticky substances may accumulate particles efficiently [43, 17, 44, 45]. However, within these characteristics, atmospheric PM amount a species with ridged leaf surfaces, was significantly higher than species with waxy leaf surfaces [45]. However, F. benjamina leaves, although having no roughness, had SIRM comparable to those obtained with J. interrigima probably because of its wax layer cuticle. As a matter of fact, studies have shown that some waxy species, during the growth, accumulates particle in wax formation [46, 2, 47]. In parks, given the low presence of pollutants unlike roads, the difference between young and mature (Fig. 1, Table 3) leaves was not clear.
4.3. Wash-off Effect on Leaf SIRM
Results showed leaf SIRM varied strongly between species and between leaf age and habitat (Fig. 1). The fact that the largest losses were obtained at roadsides makes sense, because this habitat is the most polluted compared to Parks, as we explained in the section above. At Main roads, washed leaf SIRM was statistically lower than unwashed leaves; erosion rates were generally over 70%. The SIRM detected after washing leaves is derived from cuticular encapsulation of surface-deposited particles as already demonstrated by Kardel et al. [17], Dzierżanowski et al. [2], Lehndorff et al. [46], Terzaghi et al. [47] and Hofman et al. [42]. All things considered, the particulate matter responsible for the magnetic signal is not found only on the leaves’ surface but is rather incorporated into their structure through the stomata cavities or their cuticle waxy protective layer [47, 49, 50]. Dzierżanowski et al. [2] and Terzaghi et al. [47] showed that cuticular encapsulation occurs mainly in small particles (< 10 μm) and is negligible in particles larger than 10.6 μm which were removed by a washing procedure.
With 20% of encapsulated SIRM in mature leaves and 32% in younger, F. benjamina (waxy) seems to be more particle encapsulated than other two species tested. The presence of leaf hair could constitute a sort of particle barrier preventing particles to penetrate the cuticle. On such surfaces, the contact area between a particle and the underlying leaf surface is reduced [45]. Since the particles trapped in, hair network was therefore easily washed off. The high encapsulation in F. benjamina leaves may suggest an accumulation of PM during wax formation as already demonstrated by Dzierżanowski et al. [2] on Acer campestre, Physocarpus opulifolius, Spiraea japonica and Tilia cordata. In our study, SIRM encapsulated by the young leaves from F. benjamina was 32% higher than those encapsulated by mature leaves (Fig. 1) confirming that the encapsulation of magnetic particles into the leaf tissue mainly occurs during the growth phase (compared to mature) probably due to higher wax regeneration or formation during this step [16, 17].
4.4. Leaf Wettability
4.4.1. Drop contact angle between species
In this study, drop contact angle varied between species, habitat and between the adaxial and abaxial surface of a leaf (Table 4). DCA were higher from F. benjamina (wax layer cuticle) and J. interrigima (most complex surface in term of hair and vein densities) than on B. prionitis. According to Kardel et al. [17], a species with wax, hydrophobic leaf surface, expressed by a large drop contact angle or small leaf wettability, is unable to accumulate many magnetic particles on its surface like species with a hydrophilic leaf surface (large leaf wettability). However, no significant differences were found between the species leaf SIRM even arithmetically, F. benjamina and J. interrigima leaf SIRM were generally higher than B. prionitis leaf SIRM on roadsides. The absence of significant difference is due to the inhomogeneity of studied road characteristics resulting in a large standard deviation (Table 2). For example, traffic density is higher on the Lagoon Boulevard than on North Highway during rush hour. Traffic jams, and thus the high production of combustion residues from engine vehicle, on Lagoon Boulevard is more important than on North Highway. In addition, North Highway is wider than Lagoon Boulevard.
According to the criteria edited by Aryal and Neuner [29] all studied species were highly-wettable because DCA were greater than 40° and less than 90° (Table 4) confirming that most leaves from non-freezing tropical and subtropical origins were highly wettable while temperate climat leaves were non-wettable and subalpine or alpine leaves were highly non-wettable [29]. However, J. interrigima with its leaf trichomes of and F. benjamina with its leaf waxes were more wettable than B. prionitis. Previous studies showed that waxy cuticles and outgrowths, such as trichomes, increase the wettability or hydrophobicity and facilitate the removal of polluted particles from leaf surface [23, 24, 20].
4.4.2. Effect of habitat on leaf wettability
In our study area, drop contact angle was higher on Main roads than in Parks for all species and from adaxial and abaxial surfaces (Table 5). The higher leaf DCA on Main roads areas might be due to erosion of the epicuticular wax, which is related to pollution stress. These results confirm Kardel et al. [17] observations on scanning electron microscope images of Alnus glutinosa, Acer pseudoplatanus, Betula pendula, Quercus robur and Sambucus nigra. The potential of cuticular perturbations in unsuitable habitats is high, due to different interactions occurring at the leaf surface, such as gas exchange and invasion by pathogens and insects. Many studies indeed revealed that the epicuticular wax is affected by gaseous pollutants, through dry and wet deposition [51, 52, 53, 20]. Nevertheless, the degree to which habitat type affected leaf wettability depended on tree species and time of sampling.
4.4.3. Inter and intraspecific leaf heterogeneity
The highest values of Drop Asymmetry were observed at the adaxial and abaxial leaf surfaces of B. prionitis compared to F. benjamina. This result confirmed the high surface heterogeneity of the two first species (hairy and more vein density) than the waxy F. benjamina. Previous studies showed that for many species, an increased exposure to pollution leads to an increased damage of the leaf surface [25, 49, 54, 40]. Furthermore, particles deposition on the leaf surface can be heterogenic [55] and therefore, leaf surface heterogeneity for leaves exposed to air increase. This is what we observed in this study for the adaxial leaf surface of B. prionitis and abaxial leaf surface of J. interrigima.
4.5. Relationship between Leaf SIRM, Leaf SIRM Encapsulated and Leaf Wettability
For the three investigated species, a significantly positive correlation was found between Drop contact angles on adaxial and abaxial surfaces and leaf SIRM (Fig. 2) and leaf SIRM encapsulated (Fig. 3). This result could be due to the high-wettability of study leaves. As a matter of fact, stored water on leaf surfaces increases the potential for plant pathogens and the potential for leaf damage from pollutant particles [56, 57]. This leaf property induces a higher capacity of investigation leaf species to encapsulate more pollutants as the DCA increases, as was the case in this study.
If the leaves were not wettable, encapsulation particles would be difficult or impossible [58] because the contact area between a particle and the underlying leaf surface is considerably reduced. As a consequence, the physical adhesion forces between the particle and the surface will be reduced owing to leaf surface free energy characteristics [45]. If water rolls over such a hydrophobic surface, contaminating particles are picked up by water droplets, or they adhere to the surfaces of water droplets, and are then removed with the droplets as they roll off the leaves [45]. For wettable or high-wettable leaf surfaces with low contact angles (< 90°), the much larger contact area may lead to much stronger force between particles and leaf surfaces. Accordingly, mature leaves of B. prionitis, F. benjamina and J. interrigima, highly-wettable surfaces, which promote the accumulation and deposition of particles on leaf surfaces, making them appropriate species for air quality biomonitoring in humid environment such as African tropics.
5. Conclusions
This study attempted to evaluate the process of particles deposition and encapsulation on young and mature leaves of subtropical plants sampled at roadside and in parks.
Main roads were potentially more polluted than Park confirming that the main sources of pollution determined with SIRM were car exhaust. Leaf SIRM was higher on mature leaves than on younger at Main roads suggesting a particle accumulation in leaves over time especially for waxy species i.e., F. benjamina. With 20% of SIRM encapsulated in mature leaves and 32 % in youngers, F. benjamina seems to be more particle encapsulated than the other two hairy species tested (B. prionitis and J. interrigima). The particulate material responsible for the magnetic signal is not found only on the leaves’ surface but is rather incorporated into their structure through the stomata cavities or their cuticle waxy protective layer. All tested species were highly-wettable because of drop contact angle was greater than 40° and less than 90°. However, J. interrigima witn its leaf trichomes and F. benjamina with its leaf wax were more wettable than B. prionitis. A significantly positive correlation was found between wettability intensity and mature leaf SIRM. Accordingly, mature leaves of B. prionitis, F. benjamina and J. interrigima, highly-wettable surfaces, which promote the accumulation and deposition of particles on leaf surfaces, making them appropriate species for air quality biomonitoring in humid environment such as African tropics.