1. Introduction
Constructed wetlands (CWs) are an efficient technology designed for the wastewater treatment by imitating natural principals occurring in natural wetlands, marshes, and lagoons. Wetland technology is simple, inexpensive, efficient, easy to operate and maintain as compared to the traditional methods of wastewater treatment [1]. The use of these systems is becoming very popular in many countries owing to lower energy consumption and simple design. Several studies have shown the overall effectiveness of CWs for municipal wastewater treatment [2,3].
The pollutant removal in CWs is achieved by the intensive action of its biotic and abiotic components such as bacteria, fungi, algae, macrophytes, media type, filtration, adsorption and sedimentation [3]. These organisms contribute to the reduction and removal of organic and inorganic pollutants, and pathogens by various treatment mechanisms such as microbial interactions and uptake by vegetation. Abiotic mechanisms include sedimentation, filtration, chemical precipitation, and adsorption [4]. Organic matter is removed in a subsurface flow (SF) constructed wetland by the aerobic bacteria attached to the porous media and plant roots. Plant roots, not only provide the necessary surfaces for bacteria to grow, but also provide oxygen. Nitrogen removal is essentially achieved by microbial interaction, plant uptake, and adsorption. Phosphorus removal in SF constructed wetlands is a result of bacteria removal, plant uptake, adsorption by the porous media and precipitation, wherein phosphorus ions reacts with the porous media [3]. The main characteristics responsible for the removal efficiency of CWs are hydraulic residence time, temperature, vegetation type, porous media, and microbial activity [5].
The support medium in the subsurface flow constructed wetlands has a fundamental role in the growth and development of plants and microorganisms and pollutant removal. In addition to providing physical support, the materials can directly interact with the pollutants mainly through sorption processes. The extent of these interactions may strongly influence the system’s behavior and performance, and an appropriate selection of the materials to be used as a medium is therefore an important step in the CW optimization [4]. Various artificial media have been tested to improve the nutrient removal in the subsurface-flow CWs, factory made light-weight expanded clay aggregates (LECA), granulated laterite, shale, crushed marble and sepiolite [6]. However, finding a suitable and low-cost filter medium in constructed wetland systems is a critical issue.
Biochar derived from the agricultural biomass waste is increasingly recognized as a multifunctional material for agricultural and environmental applications. Biochar are carbon rich material, valuable as soil amendments by augmenting the retention of fertilizers [7], and encourages beneficial microorganism [8]. They also have the ability to remediate organic and inorganic contaminants [9]. Biochar are known to have a highly porous structure, containing various functional groups and shown to be effective in the adsorption of heavy metals, particularly in aquatic systems [10]. Biochar is well known for its capability for carbon storage and reducing or suppressing CO2, CH4, and N2O production in soil and may contribute to the reduction in atmospheric greenhouse gases [11–13]. Although activated carbon is ideal for removing contaminants from water but are not cost effective. On the other hand, “sustainable” biochar requires less investment. A typical biochar is less carbonized than activated carbon. However more hydrogen and oxygen remain in its structure along with the ash originating from the biomass and can absorb hydrocarbons, organics, and some inorganic metal ions [14, 15], exhibiting potential for water purification and soil amelioration. Biochar have potential to replace traditional activated carbons such as wood, coconut shell, and coal as a low cost sorbent for contaminants and pathogens. Biochar might be used for removing contaminants from water while also being loaded with nutrients for subsequent use as a soil amendment, providing long-term sorption capacity and a fertilizer [16]. Biochar derived from pine needle was reported to effectively adsorb naphthalene, nitrobenzene and m-dinitrobenzene. Such biochar can be used as an environmental engineered sorbent for aqueous organic contaminants removal and soil immobilization [17]. Biochar obtained from straw was reported as a substitute for activated carbon to remove reactive brilliant blue and rhodamine B [18]. Moreover, sand amended with varying proportions of biochar in vertical flow wetland mesocosm were effective in removing BOD5, TSS, TVS and coliforms [19]. Biochar mixed into the substrate of constructed wetlands has been shown to immobilize toxic metals such as cadmium (Cd) [20]. Biochar derived from dairy manure was found to effectively adsorb lead and atrazine simultaneously with a slight competition effect [21].
Although, biochar has been investigated widely for its environmental remediation capability, however, the application of biochar as a media in CWs systems has not been systematically investigated to any significant extent. Therefore, this study aims to evaluate the efficiency of CWs by combining it with biochar as a porous media for the removal of organic matter, nitrogen, and phosphorus by using mesocosm scale horizontal surface flow CWs.
2. Materials and Methods
2.1. Mesocosm Experiment Unit Description
Four rectangular mesocosm tanks with horizontal subsurface flow CWs were constructed and labeled as A, B, C, and D (Fig. 1). A schematic of the experimental layout is shown in Fig. 1(c). The polyacrylic rectangular tanks had length, width, and depth of 1 m, 0.33 m, and 0.3 m, respectively. To exclude the parameter length-to-width ratio from the study and have common conditions for comparison, the tanks were constructed of the same shape and dimensions, even though in real operating CW systems the length-to-width ratio is determined from the hydraulics of the system and depends on the substrate material size. The relatively small width of the CW was chosen in order to ensure plug flow conditions in the units and the relatively large length was chosen in order to examine the pollutant removal along the units. Each tank has inlet and outlet connected to a valve for controlling the inflow and outflow. The wetlands substrate held 32 L of water therefore a batch volume of 30 L was passed through each wetland with a constant flow rate of 1.2 × 10−7 m3/sec, achieving an overall hydraulic retention time (HRT) of 3 days (Table 1). The wastewater was passed through a perforated acrylic pipe (diffuser, r = 0.75 cm) and placed across the entire width at the upstream side of the tank. These diffusers were placed horizontally, so that the wastewater flow has a uniform distribution across the tank. Similarly, an acrylic pipe was placed at the downstream end to collect the effluent sample for analysis (r = 0.25 cm). Three different porous media with various radius (D50) were used (Fig. 1(d)), e.g., Cobbles (D50 = 90 mm, range 30–180 mm) placed at a bottom depth of 5 cm, medium gravel (D50 = 15.0 mm, range 4–40 mm) placed at a height of 17 cm, and pea gravels (D50 = 10.0 mm, range 4–25 mm) placed at a height of 5 cm (Fig. 1(c)). Wetlands A and B consist of gravels as media, whereas biochar was used in Wetland C (33.15 Kg) and Wetland D (16.57 Kg) (see Table 1). The biochar used in this work were obtained from woody materials of oak tree (Quercus sp). The dried woody biomass was charred for 10 h at temperature (~600°C) at a heating rate of 10°C/min. Each Wetland was planted with Canna seedlings at equal distance from each other (Fig. 1(a) and 1(b)). Wetland A was the control and left unplanted, whereas wetlands B, C, and D were planted with the Canna seedlings. The experiments were performed after successful acclimatization and establishment of the plants and microbial community (2 months). Studies were conducted in various batches of 3 days over a period of 7 weeks. Samples were collected on every 3rd day. A modified OCED synthetic wastewater containing peptone (160 mg/L), meat extract (110 mg/L), kaolin clay (100 mg/L), urea (30 mg/L), NaCl (7 mg/L), CaCl2 (4 mg/L), MgSO4 (2 mg/L), K2HPO4 (28 mg/L), and potassium hydrogen phthalate (425 mg/L), at pH 6.7 was used to simulate the best characteristics of domestic wastewater [22]. Although synthetic wastewater does not fully simulate the domestic wastewater but use of synthetic wastewater for such experimental studies has advantages of minimizing variations of influent characteristics and is also safer for people in the laboratory.
2.2. Water Quality Monitoring
Synthetic wastewater was passed within wetland beds with hydraulic retention time (HRT) of 3 days in the temperature range 25 ± 2°C with average flow rate of 1.2 × 10−7 m3/sec in each wetland unit. Water samples were collected at the end of third day from the inlet and outlet over a period of seven weeks. The water samples were analyzed immediately in the laboratory for pH, total suspended solids, turbidity (2100N Hatch Turbidimeter, USA), COD, total nitrogen, NH3, NO3-N, and total phosphorus, PO4-P. Water quality analysis was performed according to the respective APHA standard methods [23].
2.3. Statistical Analysis
Average percentage removal was calculated from the data obtained for a period of seven weeks. The datasets were analyzed using one-way analysis of variance (ANOVA) using Microsoft Office Excel 2007 (Microsoft, USA). A significant difference was considered at the level of p < 0.05. Subsequent pair-wise comparisons were performed using Tukey post hoc tests (see supplementary information).
3. Results and Discussion
3.1. Characterization of Media
Biochar used in this experiment was purchased from a local market. Scanning electron microscopy/energy dispersive X-ray spectroscopy (SEM/EDX) was used to characterize the biochar (main elements: C:90%; O2:8%; P:0.54%; S:0.1%; K:0.38%; and Ca:0.38%). The SEM images (Fig. 2.) clearly show that the biochar was composed of irregular forms and varying particle sizes with very coarse and heterogeneous surfaces. Moreover, the SEM image also shows shallow channels of various diameters, originating from tracheid and vessel cells. The average pore size calculated from the SEM image ranged from 1 to 10 μm. These structures may be important for the high internal surface area and adsorption ability as an excellent absorbent. The pHpzc (point of zero charge) was determined using the known method as described in [24]. The pHpzc of biochar was 9.55. This inferred that below pH 9.55 the surface of these solids carried a net positive charge and beyond which it contained with net negative charge. The biochar had 9% moisture and 6% ash content which was determined by gravimetric method. The mineral content of the gravel was characterized using Xray fluorescence (XRF) the main elements were CaO 78.21%; SiO2 9.95%; MgO 5.46%; Al2O3 3.51%; Fe2O3 1.6%.
3.2. Suspended Solids and Turbidity
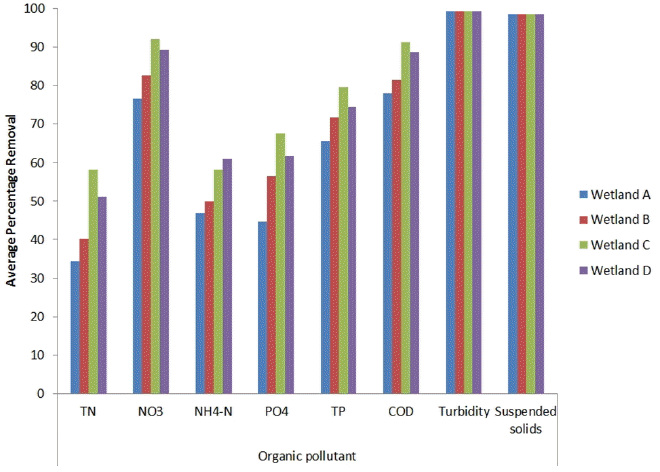
The average concentration and percentage removal of suspended solid are shown in Table 2 and Fig. 3. The average suspended solid removal was 98.62%. However, there was no significant difference in the removal percentage of suspended solids and turbidity among all the four wetlands. Moreover, there was no noticeable difference observed between the performance of the planted and unplanted wetlands. Other gravel-bed wetland studies using unplanted controls have reported no significant differences in their suspended solids removals compared to the planted and unplanted systems, indicating that the removal is primarily because of physical processes which are hardly affected by the plant growth and gravel type [25, 26]. Moreover the removal of suspended solids can be attributed as merely a mechanical filtering effect. Microbial breakdown of the organic portion of suspended solid however is an important slower mechanism of removal in operational wetlands. Turbidity reduction was more efficient than the suspended solid removal. The average turbidity removal was 99.33% among all the wetlands (Fig. 3) and observed within 3 days. The wetland-treated effluent was highly clarified with turbidity level < 0.5 NTU (Table 2).
3.3. pH
The pH is an important parameter in nutrient uptake from the aqueous solutions as it determines the surface charge of the adsorbent, degree of ionization, and speciation of the adsorbate in the aqueous media [27]. The initial influent pH was 7.28. After passing the wastewater in wetland beds, pH increased to 8.0 and 7.7 in wetlands C and D, respectively (Table 2). The increase in the pH in wetland C can be explained due to high pHpzc (~9.55) and highly alkaline nature of biochar contributed by the ash content (~6%). The observed pH ranges among all the four wetlands were within the recommended range (4.0 < pH < 9.5) for the existence of many treatment bacteria, denitrifiers, and nitrifiers. Moreover, the variation in pH occurred, can also be attributed to high concentrations of hydroxyls in the environment, carbonates, bicarbonates, sulfates, ammonia, and other ions from the biodigestion of organic matter [3].
3.4. Total Nitrogen
The mechanisms involved in the nitrogen removal in CWs include volatilization, ammonification, nitrification/denitrification, plant uptake, and matrix adsorption [3, 28]. Moreover several studies also have shown the ability of biochar to retain nitrogen [29]. Wetland C containing biochar exhibited a higher removal percentage of TN of 58.2%, which was significantly greater (p < 0.05) than those of wetlands B (40.10%) and A (34.31%) (see Fig. 3 and supplementary information). The values of the TN removal were lower as compared to organic matter. This particular result was observed in most of the wetland systems, probably because total nitrogen and total phosphorus removal require longer HRTs. Furthermore, poor nitrogen removal in wetlands is generally due to limited availability of oxygen, as the removal of organic matter consumes most of the oxygen released by the plant roots [30]. Moreover, organic nitrogen (amino acids, peptides, urea, etc.) removal mechanisms also depend upon the microbial degradation process, which also requires longer retention time and extent of microbial activity. Furthermore, the conditions in the vegetated beds of HSF CWs are usually anoxic and/or anaerobic, so that the major obstacle for higher removal of nitrogen is a low rate of nitrification [28]. There was no significance variance (p > 0.05) observed between the unplanted wetland A (34.31%) and planted wetland B (40.10%) (see supplementary information). Nevertheless, several other studies confirmed the effect of vegetation on the removal of total nitrogen [2, 3].
3.5. Nitrates
Denitrification is believed to be the dominant and long-term mechanism, particularly when nitrate loading rates are high [3]. Several studies have demonstrated that biochar facilitates denitrification and enhances microbial activity in soil [31]. Wetland denitrification occurs in the anoxic zones of the sediments beneath an aerobic water surface layer or in anoxic microsites of a biofilm attached to plant tissue or substrata. The highly porous biochar contains a high surface area, providing anoxic condition for the microbial biofilm formation and could be the possible reasons for the significant reductions (p < 0.05) in nitrate in the case of wetland C (92.08%) as compared to wetland B (82.8%) (Fig. 3). No significance variance (p > 0.05) observed between the unplanted wetland A (76.7%) and planted wetland B (82.8%) (see supplementary information). However, several other studies confirmed the effect of vegetation on the removal of nitrates.
3.6. Ammonia
In CWs ammonia nitrogen removal is due to adsorption, nitrification, plant uptake and volatilization. The adsorption of ammonia on to biochar is well known [12, 32]. Moreover, ammonia adsorbed onto biochar is bioavailable and provides a source of nitrogen for plants when the biochar-NH3 complex is placed in the soil-plant matrix [32]. Considering the net positive charge on biochar surface at the prevailing pH due to high pHpzc (~8.55), ammonium adsorption is less evident but cannot be ruled out completely. It can be assumed that ammonia removal could be due to combine effect of adsorption, nitrification-denitrification, and volatilization (to marginal extent). The biochar containing wetland demonstrated 58.3% ammonia removal in wetland C as compared to 50.01% in wetland B (Fig. 3). In addition, the pores inside biochar and biochar particles may provide microenvironment conditions and favor the growth of microbial communities such as ammonia-oxidizing bacteria [33]. Other studies demonstrated the role of plant in the removal of ammonia; however, in this study, no significant results (p > 0.05) were obtained between the planted and unplanted beds, and this was attributed due to the difference in influent characteristics, size and shape of the mesocosm unit, and operating conditions as compared to the other studies.
3.7. Total Phosphorus
Biochar has the potential to adsorb phosphorus or affect the precipitation of phosphorus [34]. The phosphorus removal is strictly associated with the physiochemical and hydrological properties of the filter material, whereas phosphate is mainly adsorbed or precipitated in filter media [3]. The efficiency of HSF CWs for phosphorus removal is shown in Fig. 3. There was a significant removal (p < 0.05) of total phosphorus in wetland C (79.5%) as compared to wetland B (71.8%). This phenomenon can be explained by point of zero charge (pHpzc) of biochar. The pHpzc was found to be ~9.5; therefore, the biochar surface was expected to be positively charged in most natural aqueous conditions and electrostatically attract the negatively charged phosphate species. Although the initial solution pH values in this study were ~7, the reductions of aqueous phosphate during the experiments affected the dynamics of solution pH [34]. Moreover, the vegetation plays an important role as they provide temporary storage of phosphorus. The assimilation by plants and microorganisms mainly supports the phosphorus removal in CWs [35]. The lack of vegetation in wetland A (65.6%) could be also responsible for the lower phosphorus removal as compared to the vegetated wetland B (71.8%) (p < 0.05).
3.8. Orthophosphates
The orthophosphate removal was significantly similar (p < 0.05) to the total phosphorus removal with the highest removal in wetland C (67.7%) (Fig. 3). As the biochar surface is expected to be positively charged at low pH owing to high pHpzc value and significantly able to adsorb the free PO4 ions. In addition, significant orthophosphate removal was also observed in wetland B (56.5%), because of the presence of high calcium oxides in gravels, causing sorption of orthophosphate ions onto the surface of gravels and/or by the precipitation of orthophosphate ions with calcium ions [36]. The presence of aluminum and iron oxalates present in gravels also affects the reduction of the orthophosphate [34]. With regard to the PO4 removal, this study shows a significant variation (p < 0.05) in the percentage removal between wetlands A (44.6%) and B (56.5%) for the unplanted and planted units, respectively. Several studies reported the significant variation in the planted and unplanted wetlands with respect to the phosphate removal, demonstrating that plants play a role in the removal of inorganic phosphorus [37, 38].
3.9. Chemical Oxygen Demand (COD)
Higher removal efficiency among the various constituents for all the units was observed for COD. Higher removal rates were observed in wetlands C (91.3%) comprising biochar as media as compared to wetland B (81.5%) (Fig. 3). The π–π bond interactions between molecules and biochar, direct molecular-biochar electrostatic attraction/repulsion, and intermolecular hydrogen bonding are some of the proposed combined mechanisms for the COD removal. Apart from the adsorption, mechanism such as precipitation, oxidation, and anaerobic digestion are also responsible for the reduction of COD [3].With regard to the organic matter (COD), several studies show a difference in the removal activity between the planted or unplanted wetlands [2]. This study also shows significant removal difference between wetlands A (78.07%) and B (81.47%), the results show that the plants play a role in the organic matter removal [3].
3.10. Effect of HRT on Nutrient Removal
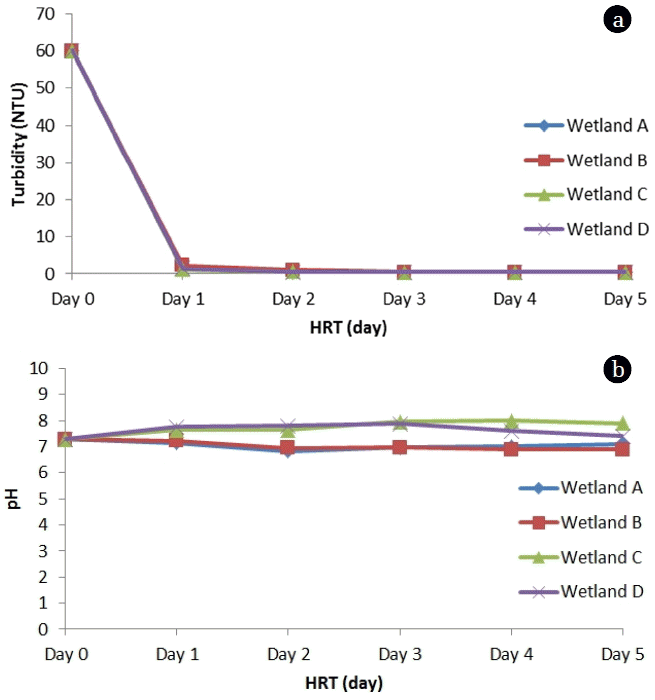
The effect of Hydraulic Retention time (HRT) was studied at interval of 24 hours up to 5 days. The effect of HRT variation was observed on nutrient removal among all the four wetlands. Turbidity removal (99%) was achieved among all the wetlands within the 24 hours after introducing the wastewater. The turbidity was reduced from 60.1 NTU to 1.4 NTU within 24 hours in all the wetlands (Fig. 4(a)).
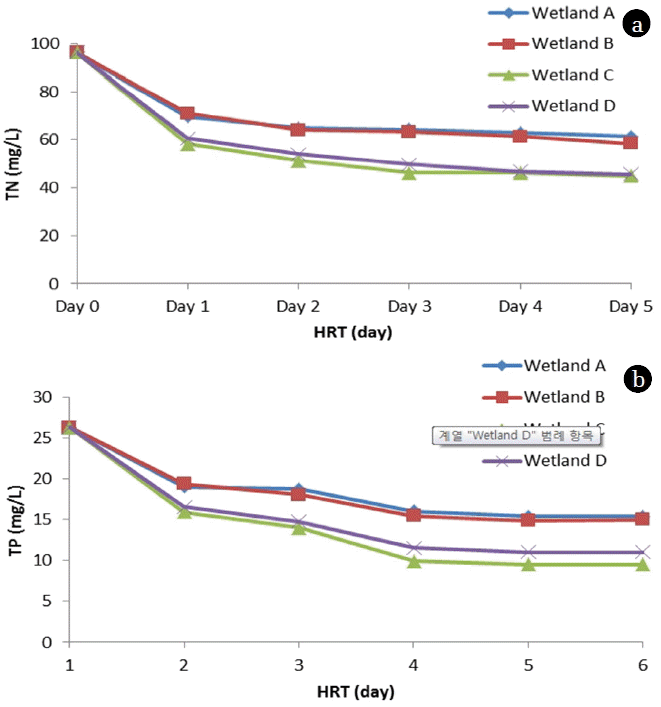
Moreover, turbidity level below 0.5 NTU was achieved within 3 days. pH variation was observed in all wetlands. The pH was observed to increase from initial value of 7.7 to final 7.9 and 7.4 in wetland C and D respectively. While a gradual decrease in pH was observed in wetland A and B to 7.1 and 6.9 respectively as retention time was increased up to 5 days (Fig. 4(b)). The increase in pH as observed in wetland C and D is likely due to alkaline nature of biochar contributed by carbonates, bicarbonates. A significant TN removal was achieved within 3 day among all wetlands (Fig. 5(a)). TN removal was observed to slow down on 4 and 5th day. Moreover, TN removal was 1.4 times higher in wetland C and D than those observed in wetland A and B on 3rd day. In case of TP, the best TP removal was achieved in wetland C and D on 4th Day (Fig. 5(b)). However, the TP removal slowed down after 4 days HRT, possibly suggesting that TP removal in wetland was relatively slower compared as compared to TN removal. Similar observation was seen in case of PO4-P, where PO4-P concentration was reduced from 16.3 mg/L to 5.9 mg/L in wetland C and 6.8 mg/L in wetland D on 4th day (Fig. 6(a)). While, PO4-P concentration was 9.5 mg/L in wetland A and 9.3 mg/L in wetland B on 4th day. The COD concentration was observed to reduce drastically from 421.3 mg/L to 56.9 mg/L in wetland C and 77.5 mg/L in wetland D on 4th day (Fig. 6 (b)). However, wetland A and B, exhibited slow removal of COD and the lowest concentration was observed to be 113.5 mg/L and 103.3 mg/L in spite of increasing HRT up to 5th Day. The prime reason for high COD removal is due to filtering, settling and adsorption process contributed due to biochar. Although, most of the nutrients were reduced up to HRT of 3 days, but for better removal efficiency it is recommended to either reduce the flow rate or increase the HRT up to 4 days.
3.11. Biomass Growth Studies
The macrophytes growing in constructed treatment wetlands have several properties in relation to the treatment processes that make them an essential component of the design [39]. All the planted wetlands (B, C and D) showed positive growth in the nutrient rich synthetic wastewater, without any obvious symptoms of toxicity or nutrient deficiency. After a period of 3 months, mean plant height ranged from 0.8 m to 1.53 m. Root and rhizome growth accounted to be 40 % of total plant growth with maximum depth of 15–23 cm below the ground. The biomass growth was varied among the mesocosm units and no significant effect was observed with respect to biochar addition on plant growth.
4. Conclusions
This preliminary study reveals that wetlands with biochar were more efficient as compared to the wetland with gravels alone in the reduction of various organic and inorganic pollutants. Highest removal rate were achieved in wetland C containing biochar with 58.27% removal of total nitrogen, 79.5% removal total phosphate, 68.1% removal of PO4-P, 92.1% removal of NO3-N, 58.3% removal of NH3-N and 91.3 % of COD removal. The removal of the pollutants by biochar depended on various factors such as adsorption, precipitation, filtration, sedimentation, microbial degradation, and plant uptake. Moreover, presence of high surface areas of the biochar may be a key factor to facilitate pollutant degradation. This preliminary study indicates that the wetlands amended with biochar can be primarily used for secondary treatment of domestic and municipal wastewaters. However this study is preliminary and once again, the researchers acknowledge that further investigation is needed with a more rigorous methodology before conclusions can be drawn. In general, the use of biochar in wetlands may provide an option of cost-effective sustainable wastewater treatment with lower energy footprint.