1. Introduction
Diesel engine has gained much attention in recent years due to its higher fuel efficiency compared to gasoline engine. However, the NOx emission is one of the drawbacks that have been popularly discussed for diesel engine. NOx emission will cause some environmental pollution like photochemical smoke and acid rain [1, 2]. In order to meet the emission control regulations, exhaust gas after-treatment system is necessary for most diesel engine powered vehicles [3–5]. Nowadays, selective catalytic reduction (SCR) is one of the most promising after-treatment technologies that reduce NOx emission efficiently [6–8]. This system uses ammonia as the reductant to convert NOx in the exhaust to N2 and H2O by catalyst [9]. Since ammonia cannot be directly carried in vehicles, 32.5% aqueous urea solution (AdBlue) has been used as the standard reductant of SCR at present [10]. However, high urea solution injection would lead to ammonia slip that makes secondary pollution [11, 12]. To achieve a balance of high NOx conversion efficiency and low ammonia slip, several studies has been reported recently and has achieved some outcomes [13–16]. Nevertheless, SCR control is still a great challenge in present, due to its complicated dynamics and limited feedback information. Moreover, worldwide emission regulations require onboard diagnostic systems to be implemented for monitoring the emission component to avoid unexpected failures of the aftertreatment systems. Currently NOx sensor has been popularly used in vehicles [17]. However, it has been reported that it is cross-sensitive to ammonia. Some studies have been done to avoid ammonia cross-senitivity. Ming-Feng Hsieh and Junmin Wang used the EKF-based approach to significantly improve the accuracy of NOx concentration measurements from the original NOx sensor readings [18]. Jie Hou et al. proposed a method using the extended Kalman filter to predict the actual NOx concentration downstream of the catalyst [19]. Chih-Cheng Chou also proposed a method to solve the cross-sensitivity of the SNS to ammonia [20]. NH3 sensor for vehicle emission applications has not been popularly used in real applications and the studies on this sensor are limited though it has been developed recently. In this paper, a double NOx sensor system based on the ammonia cross-sensitivity was arranged on the downstream of the SCR catalyst to realize closed-loop control in order to control urea solution injection in optimal range accurately and some research was studied.
The rest of this paper is organized as follows. Firstly, the ammonia cross-sensitivity of the NOx sensor is introduced briefly. Following that, a description of the experimental setup and measuring method are provided. Then, experimental results of different tests are analyzed and studied. Finally, conclusive remarks are summarized.
2. Ammonia Cross-Sensitivity of NOx Sensor
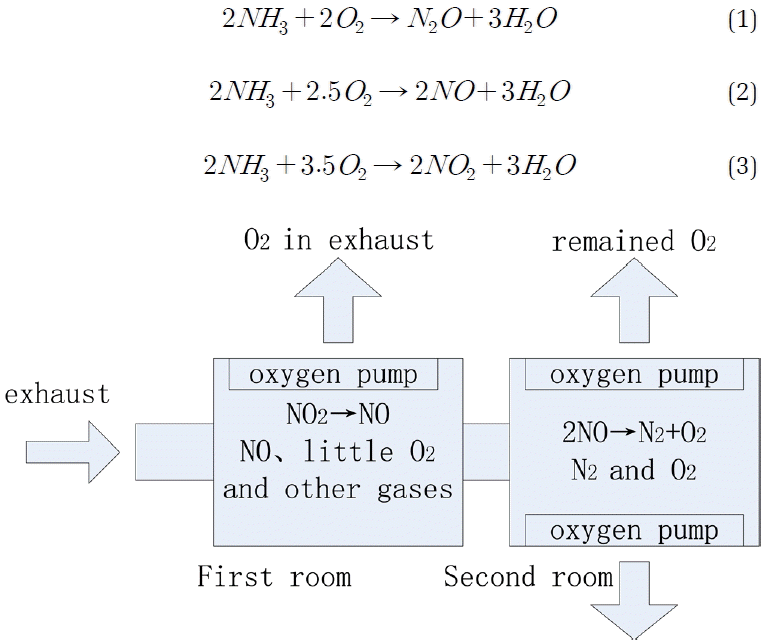
The operating principle of the NOx sensor used recently is shown in Fig. 1. It consists of two measuring rooms. Firstly, O2 is discharged by ZrO2 matrix oxygen pump and NO2 is restored to NO in the first measuring room. The O2 concentration is in a low concentration range in this time. Then O2 is discharged further and NO is restored to N2 and O2 by the catalyst in the second measuring room. The NOx concentration can be measured by measuring the O2 concentration in the second measuring room with oxygen sensor. The NOx concentration in the exhaust is twice of the O2 concentration measured.
However, ammonia will generate oxidizing reaction with oxygen under the effect of ZrO2. It is influenced to measure NOx concentration in the exhaust. The main reactions of ammonia and oxygen are shown in the following equations:
The sequence of the three reactions depends on the temperature of the exhaust. In other words, the concentrations of N2O, NO and NO2 generated from ammonia is in different ratios. A NOx sensor test model is provided according to the reported studies:
The ammonia cross-sensitivity coefficient of NOx sensor is in a range of 0.5 and 2 depended on the temperature according to the equations mentioned before. The ammonia slip can be studied by using this characteristic.
3. Materials and Methods
3.1. Experimental Setup
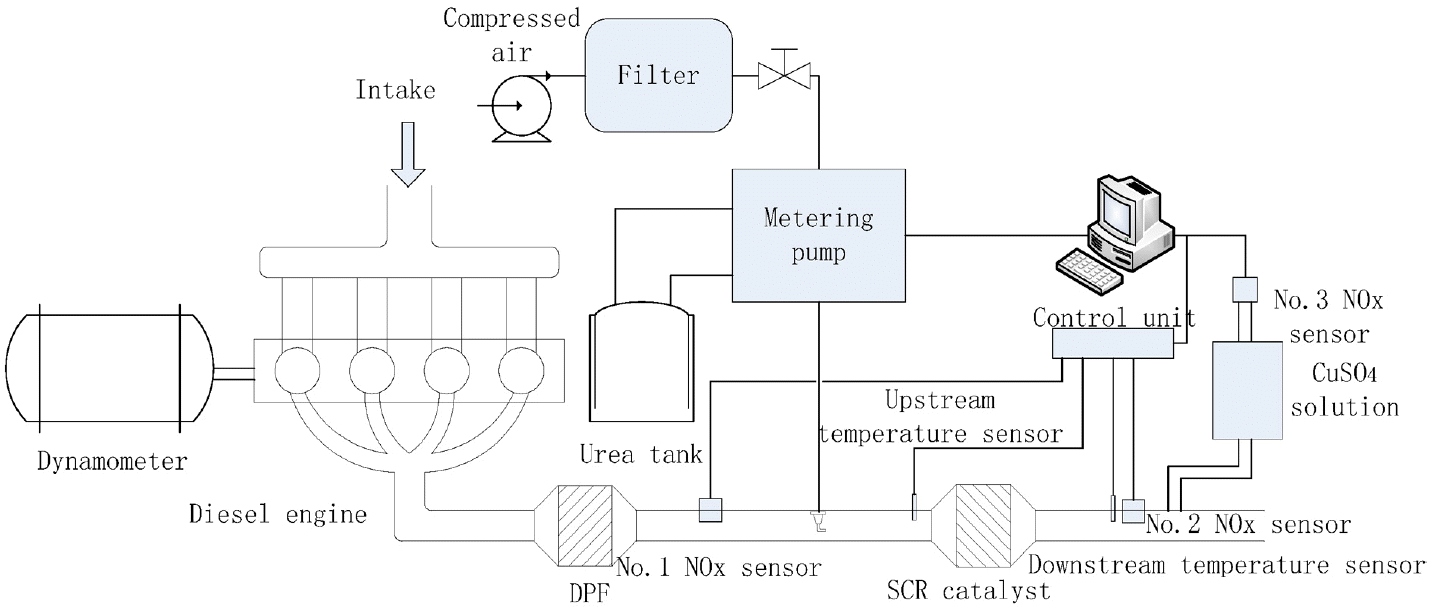
The general arrangement of experimental setup is shown in Fig. 2, mainly including diesel engine, dynamometer and SCR after-treatment system. The main parameters of experimental used diesel engine are shown in Table 1, and the SCR control and test system is composed of compressed air auxiliary metering pump, urea tank, control and measurement unit, SCR catalyst, temperature sensors and NOx sensors. The used catalyst is a vanadium catalyst which is Φ190 × 155 mm, 400 cpsi. The mass concentration of urea solution is 32.5%. The No.1 NOx sensor is used to measure the NOx concentration in the upstream of the catalyst while the No.2 NOx sensor is used to measure the NOx concentration in the downstream of the catalyst. The No.3 NOx sensor is used to measure the NOx concentration in the downstream of the catalyst while the gas has gone through the CuSO4 solution. NH3 is easily soluble in the water and can react with CuSO4 to generate complex. While NO is not soluble in the water and the rate of NO2 in NOx is few. In order to remove the influences of ammonia slip, CuSO4 solution is arranged before No.3 NOx sensor. The Diesel particulate filter (DPF) is used to reduce the influences of PM on nozzle clogging and catalyst deactivation.
3.2. Measuring Method
The working condition of the diesel engine was adjusted to get the required catalyst temperature and space velocity. The urea solution was injected after the working condition of the diesel engine had been stable over 10 minutes. The injection quantity of the urea solution was determined by NOx concentration that measured by No.1 NOx sensor. The influences of NH3/NOx on NOx conversion and the situation of ammonia slip and storage were studied by observing the readings of No.1 and No.2 NOx sensors.
4. Results and Discussion
4.1. Influences of NH3/NOx on NOx Conversion Efficiency and Ammonia Slip
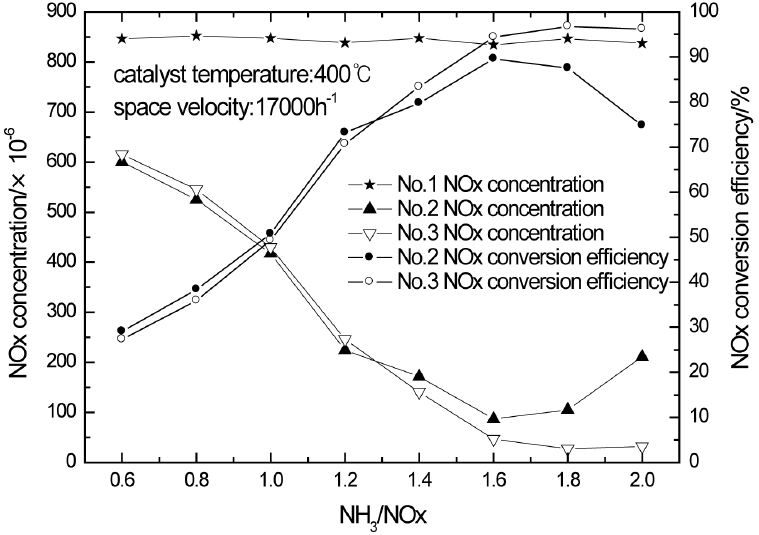
The change of the NOx concentrations measured by different sensors and the NOx conversion efficiency along with NH3/NOx is shown in Fig. 3 when the catalyst temperature is 400°C and space velocity is 17000/h. As Fig. 3 illustrates, the NOx concentrations measured by No.2 and No.3 sensor reduce as NH3/NOx increases while the NOx conversion efficiency rises. When NH3/NOx increases to about 1.4, the curve of the NOx concentrations measured by No.2 and No.3 sensor starts to separate, the same as the NOx conversion efficiency. And the ammonia slip in the downstream of the catalyst comes up. When NH3/NOx increases to more than 1.6, the NOx conversion efficiency begins to fall and ammonia slip in the downstream of the catalyst becomes more serious. This phenomenon is due to ammonia cross-sensitivity of the NOx sensor. The NOx concentration measured by No.2 sensor is higher than the actual value while the NOx conversion efficiency is lower when ammonia slip in the downstream of the catalyst comes up. When NH3/NOx continues to increase, the NOx conversion efficiency even falls. However, the influence of ammonia slip is eliminated in the measurement of No.3 sensor by CuSO4 solution, so the measured NOx concentration and conversion efficiency are closer to the true value.
4.2. Influences of the Catalyst Temperature and Space Velocity on SCR Reaction Rate
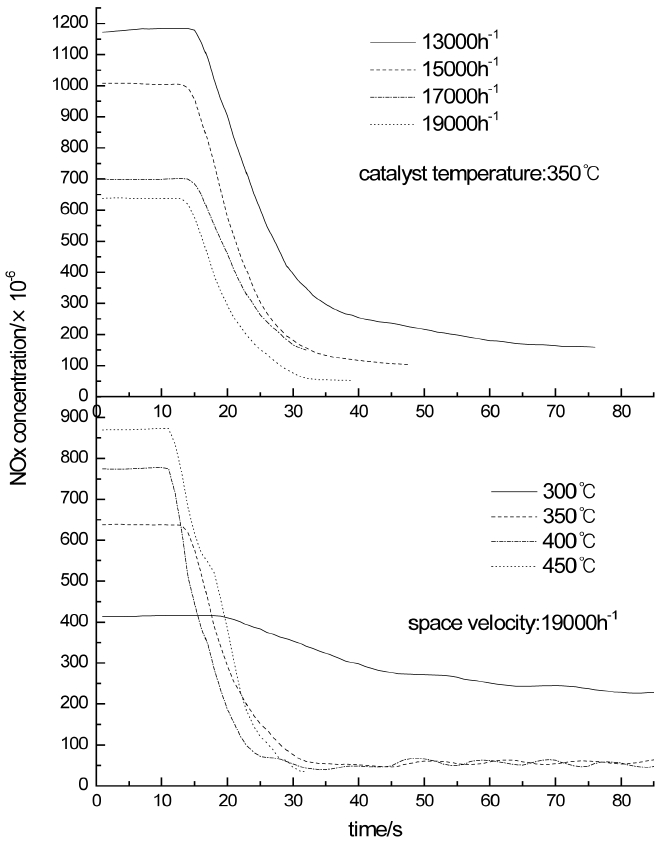
After the working condition of the diesel engine is adjusted to get the selected catalyst temperature and space velocity stably for 10 minutes. The urea solution is injected as NH3/NOx is controlled to 2 and the NOx concentrations measured by No.3 sensor is recorded continuously. The changes of the NOx concentrations are shown in Fig. 4 as the catalyst temperature is 300°C, 350°C, 400°C, 450°C and space velocity is 19000/h. As Fig. 4 illustrates, the necessary times that NOx concentrations get the 95% decline are 62 s at 300°C, 33 s at 350°C, 22 s at 400°C, and 14 s at 450°C respectively.
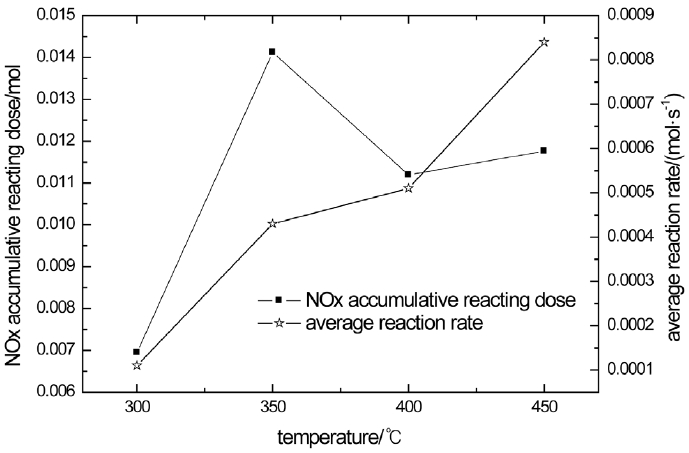
The NOx accumulative reacting dose and average reaction rate is analyzed in Fig. 5. From Fig. 5, the reacting doses of 350°C, 400°C, 450°C is close and is about twice of 300°C. Moreover, the reaction rate rises as the catalyst temperature rises. The reaction rate of 450°C is triple of 300°C. This shows that the rise of temperature not only rises the NOx conversion efficiency but also improve the reaction rate.
The influences of space velocity on SCR reaction rate are also studied. The changes of the NOx concentrations are also shown in Fig. 4 as space velocity is 13000/h, 15000/h, 17000/h, 19000/h and the catalyst temperature is 350°C. As Fig. 4 illustrates, the necessary times that NOx concentrations get the 95% decline are 62 s at 13000/h, 39 s at 15000/h, 34 s at 17000/h, and 33 s at 19000/h respectively.
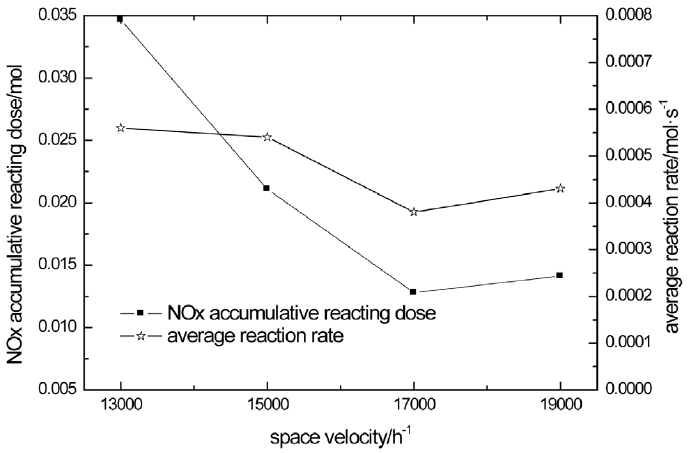
The NOx accumulative reacting dose and average reaction rate is analyzed in Fig. 6. From Fig. 6, higher space velocity leads to less reacting dose but the accumulative reacting dose increases at 19000/h. It may be an error or because of some other reasons. The average reaction rate does not have obvious change while the space velocity rises from 13000/h to 19000/h. This shows that space velocity has no effect on SCR reaction rate.
The temperature is one of the most important elements of chemical reaction rate. The rise of the temperature leads a part of the molecule of low energy to become activated molecules. It increases the proportion of the activated molecules and the number of effective collision increases. Moreover the rise of the temperature makes the movement of the reactant molecules rate become accelerated. So the reaction rate is increased. The space velocity is not the main element of chemical reaction rate, so there is no obvious change in reaction rate.
4.3. Influences of the Catalyst Temperature and Space Velocity on Ammonia Slip and Ammonia Saturated Storage
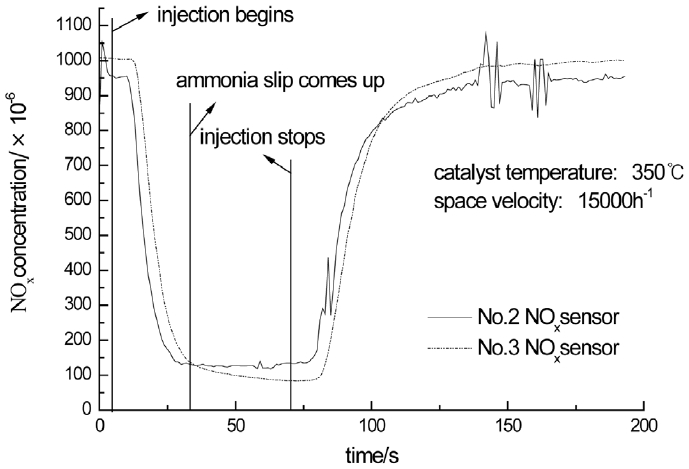
The working condition of the diesel engine is adjusted to make the catalyst temperature and space velocity to be stable. The urea solution begins to be injected as NH3/NOx is controlled to 2. The injection is stopped when the NOx concentrations measured by No.2 and No.3 sensor are stable to certain values. The change of the NOx concentrations measured by No.2 and No.3 sensor are shown in Fig. 7 as the catalyst temperature is 350°C and space velocity is 15000/h. As Fig. 7 illustrates, the curves of NOx concentration measured by No.3 sensor is relatively smooth because the CuSO4 solution has a certain effect to stabilize exhaust pressure. The NOx concentration measured by No.2 sensor becomes stable to about 133×10−6 gradually when the injection begins and returns to the initial value gradually when the injection stops. The NOx concentration measured by No.3 sensor becomes stable to about 84×10−6 gradually when the injection begins and returns to the initial value gradually when the injection stops. In addition, the ammonia slip is thought to begin when the NOx concentration measured by No.2 sensor surpasses the value measured by No.3 sensor removing the initial difference of the sensors. And the time of the ammonia slip is 36 s.
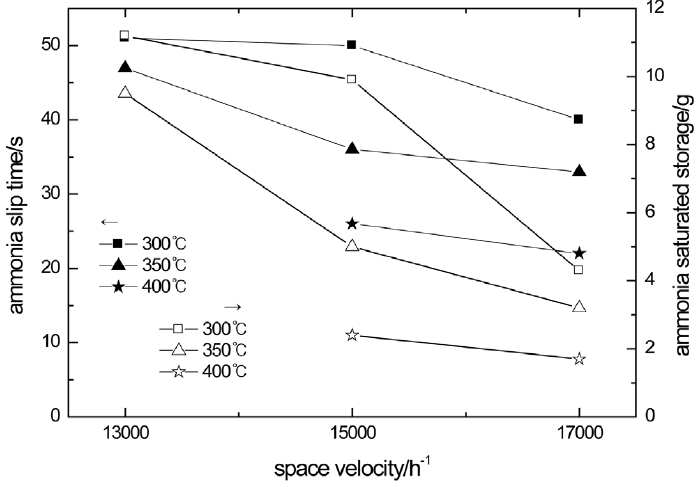
The moments that the ammonia slip occurs along with the change of space velocity in the downstream of the catalyst when the catalyst temperature is 300°C, 350°C, and 400°C are shown in Fig. 8. As Fig. 8 shows, the moment of ammonia slip brings forward as the catalyst temperature or space velocity rises. The ammonia slip brings forward because the rise of the catalyst temperature leads to the reducing of ammonia saturated storage. As space velocity rises, it is difficult for NH3 to diffuse adequately in the interior of the catalyst. Thus the probability of adsorption, desorption and SCR reaction that occur in the active site of the catalyst by NH3 reduces. As a result, NH3 is took away by the exhaust while NH3 has not diffused to the active site adequately and reacted fully.
The NOx concentration quantity that has not reacted is gained by integrating the NOx concentration measured by No.3 sensor from the moment that begins injecting to the moment that ammonia slip occurs. Thus the NOx concentration quantity that has been restored is the difference of the NOx concentration quantity in the exhaust and the NOx concentration quantity that has not reacted. It is assumed that all the reactions carry out according to the standard SCR reaction. The NH3 concentration quantity that has reacted can be gained by chemical reaction mechanism. Then the ammonia saturated storage can be obtained. The ammonia saturated storage is the difference of the NH3 concentration quantity injected and NH3 concentration quantity that has reacted.
The change rule of the ammonia saturated storage along with space velocity at 300, 350 and 400°C is shown in Fig. 8. As Fig. 8 shows, the ammonia saturated storage of the catalyst reduces while the temperature rises. The ammonia saturated storage is more at low temperature. And it reduces while space velocity rises. When the temperature becomes higher, it is harder the ammonia to be adsorbed in the catalyst. The higher space velocity also leads the ammonia adsorption to be less.
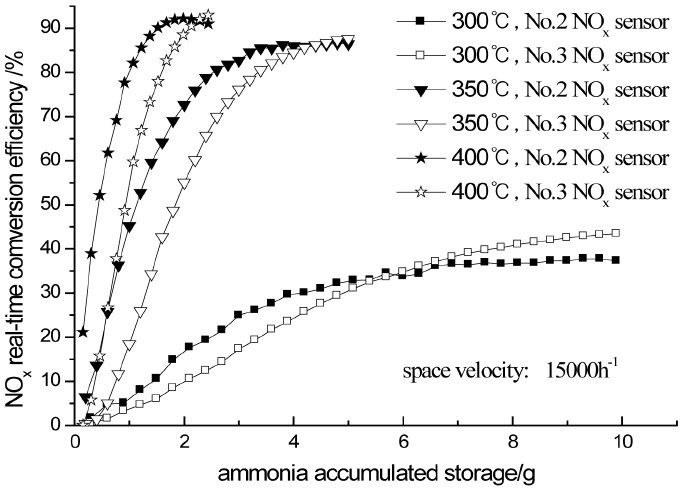
4.4. Influences of Ammonia Storage on NOx Real-Time Conversion Efficiency
The NOx real-time conversion efficiency along with ammonia accumulative storage at different catalyst temperatures when space velocity is 15000/h is shown in Fig. 9. It is assumptive that the ammonia storage increases equably. As Fig. 9 shows, the NOx real-time conversion efficiency rises as ammonia accumulative storage increases and stabilizes gradually. The NOx conversion efficiency measured by No.2 sensor is higher than No.3 sensor at the beginning due to the existence of initial error and the installation site while becomes lower in the end. No.2 sensor is closer to the catalyst than No.3 sensor that makes the response time shorter so the NOx conversion efficiency measured becomes higher.
The reaction rate is slower at low temperature because the catalyst activity is lower. The concentration of the ammonia on the surface of the catalyst increases when the ammonia storage increases, so the reaction rate is becoming higher. The NOx real-time conversion efficiency is becoming higher gradually. When the temperature rises, the ammonia saturated storage reduces. However, the increase of catalyst activity becomes more significant. So the chemical reaction rate accelerates and the NOx real-time conversion efficiency rises gradually. When the NOx conversion efficiency has reached the maximum, if the urea solution continues to be increased, the ammonia accumulative storage increases while the NOx conversion efficiency does not rise. After the ammonia storage has exceeded the saturation value, ammonia slip begins. The NOx conversion efficiency measured by No.2 sensor starts to reduce and be lower than No.3 sensor measured at this time.
It is appropriate to increase ammonia storage in the condition of low temperature in order to increase NOx conversion efficiency by using this characteristic of the catalyst. However, it is necessary to control ammonia in a certain range considering security. Otherwise, ammonia saturated storage will reduce after the temperature rises quickly. It will cause ammonia slip and become secondary pollution.
5. Conclusions
In this study, a double NOx sensor system was arranged on the downstream of the SCR catalyst based on ammonia cross-sensitivity. The influences of NH3/NOx on NOx conversion efficiency and ammonia slip were analyzed. The influences of catalyst temperature and space velocity on SCR reaction rate, ammonia slip, ammonia storage and the changing rule of NOx real-time conversion efficiency with ammonia storage were investigated. The following conclusions were obtained
Firstly, the NOx conversion efficiency increased with the increase in NH3/NOx and ammonia slip begins from the NH3/NOx equal to 1.4. Secondly, the increase of temperature caused high improvement of the SCR reaction rate while the increase of space velocity had no obvious change. Thirdly, the ammonia slip was in advance as catalyst temperature or space velocity increase. Then, the ammonia storage reduced with the increase in catalyst temperature or space velocity. Finally, the NOx real-time conversion efficiency increased with the increase in ammonia accumulative storage and reached the maximum value gradually.